Abstract
Background
An array of environmental compounds is known to possess endocrine disruption (ED) potentials. Bisphenol A (BPA) and bisphenol A dimethacrylate (BPA-DM) are monomers used to a high extent in the plastic industry and as dental sealants. Alkylphenols such as 4-n-nonylphenol (nNP) and 4-n-octylphenol (nOP) are widely used as surfactants.
Objectives
We investigated the effect in vitro of these four compounds on four key cell mechanisms including transactivation of a) the human estrogen receptor (ER), b) the human androgen receptor (AR), c) the aryl hydrocarbon receptor (AhR), and d) aromatase activity.
Results
All four compounds inhibited aromatase activity and were agonists and antagonists of ER and AR, respectively. nNP increased AhR activity concentration-dependently and further increased the 2,3,7,8-tetrachlorodibenzo-p-dioxin AhR action. nOP caused dual responses with a weak increased and a decreased AhR activity at lower (10−8 M) and higher concentrations (10−5–10−4 M), respectively. AhR activity was inhibited with BPA (10−5–10−4 M) and weakly increased with BPA-DM (10−5 M), respectively. nNP showed the highest relative potency (REP) compared with the respective controls in the ER, AhR, and aromatase assays, whereas similar REP was observed for the four chemicals in the AR assay.
Conclusion
Our in vitro data clearly indicate that the four industrial compounds have ED potentials and that the effects can be mediated via several cellular pathways, including the two sex steroid hormone receptors (ER and AR), aromatase activity converting testosterone to estrogen, and AhR; AhR is involved in syntheses of steroids and metabolism of steroids and xenobiotic compounds.
Keywords: androgenic, aromatase, BPA, BPA-DM, endocrine disruption, estrogenic, nNP, nOP, nuclear receptors
Endocrine disruptors (EDs) are exogenous compounds that have the potential to interfere with hormonal regulations and the normal endocrine system and consequently cause health effects in animals and humans (U.S. Environmental Protection Agency 2000). EDs include environmental persistent organohalogens, pesticides, and industrial chemicals such as some plasticizers and surfactants. The involvement of EDs in disruption of development, reproduction, the immune system, and the neural system has been supported in a wide range of fish and animal species, whereas for human beings the ED hypothesis is still controversial (Bonefeld-Jorgensen 2004; Bonefeld-Jorgensen and Ayotte 2003; Owens and Koeter 2003; vom Saal and Hughes 2005). Most EDs are synthetic compounds, some of which were designed to act as estrogens (e.g., oral contraceptives), whereas many were designed for other purposes and accidentally possessed estrogenic activity, such as plasticizers (Krishnan et al. 1993; Soto et al. 1991). Naturally occurring xenoestrogens in the environment include phytoestrogens produced by plants reported to have pleiotropic effects, including antioxidative and apoptotic activity, inhibitors of kinases, and suggested anticancer actions on prostate and breast carcinomas (Basly and Lavier 2005; Mueller 2002; Sirtori et al. 2005). Other EDs such as the pesticides vinclozolin, procymidone, and p,p′-dichlorodiphenyl-dichloroethene (DDE) possess in vitro and in vivo androgenic actions and can affect male reproduction in animals (Gray 1998).
Bisphenol A (BPA) and BPA dimethacrylate (BPA-DM) are monomers used largely in polycarbonate plastic and polystyrene resins and as dental sealants. Halogenated derivatives of BPA, such as tetrabromobisphenol A (TBBPA), are widely used as flame-retardants for building material, paints, plastic products including epoxy resin, electronic circuit boards, and other electronic equipments. Depolymerization of these products results in BPA and its derivatives, which leach into foods (Brotons et al. 1995), into infant formula from plastic bottles (Biles et al. 1999), into saliva of patients treated with dental sealants (Olea et al. 1996; Pulgar et al. 2000), and in fresh food at the microgram to milligram per kilogram level (Vivacqua et al. 2003). BPA and TBBPA have been detected in the concentration range of 0.1–10 ppb in human blood, urine, and fetal tissues, and related BPA levels in blood and fat tissues have also been reported (Ikezuki et al. 2002; Schonfelder et al. 2002; Thomsen et al. 2001; vom Saal and Hughes 2005).
Alkylphenol ethoxylates (APEs) are widely used surfactants and detergents in domestic and industrial products and are commonly found in wastewater. In sewage treatment plant effluents, APEs are degraded to the more resistant alkylphenols such as 4-n-nonylphenol (nNP) and 4-n-octylphenol (nOP). Data from studies across many regions of the world have shown significant levels in samples of every environmental compartment examined, including fish muscle tissue (Ying et al. 2002), and they are generally ubiquitous in food as well (Guenther et al. 2002).
The sex steroid receptors such as the estrogen receptors (ER) α and ERβ and the androgen receptor (AR) belong to the nuclear receptor family and are ligand-dependent transcription factors (Bjornstrom and Sjoberg 2005; Schwabe and Teichmann 2004; Verrijdt et al. 2003). The genomic-mediated pathway of EDs via ER and AR includes steps such as binding of ligand to receptor, translocation into nucleus, and binding of the receptor–ligand complex to a specific DNA response element causing gene expression.
Several years ago BPA, BPA-DM, nNP, and nOP were reported to elicit estrogenic activity (Andersen et al. 1999; Krishnan et al. 1993; Soto et al. 1991; Steinmetz et al. 1998; White et al. 1994). Since then, numerous studies have been carried out to assess the endocrine disruption potentials of these industrial compounds in vitro and in vivo (Alonso-Magdalena et al. 2006; Choi and Jeung 2003; Ghisari and Bonefeld-Jorgensen 2005; Gutendorf and Westendorf 2001; Kazeto et al. 2004; Mosconi et al. 2002; Olsen et al. 2003; Rivas et al. 2002; Safe et al. 2002; Sonnenschein and Soto 1998; vom Saal and Hughes 2005; Williams et al. 2001). Many studies have focused on the estrogenic activities of the compounds in vitro by their potential to affect cell proliferation (E-SCREEN) or ER transactivation in human or yeast cells (e.g., Andersen et al. 1999; Legler et al. 2002; Van den Belt et al. 2004; Vivacqua et al. 2003; Wilson et al. 2004) or the binding capacity to steroid receptors (Scippo et al. 2004). In animals, the rodent uterotrophic bioassays have verified the estrogenic effects of BPA and nNP (Owens and Koeter 2003), and developmental studies have revealed toxic effects of BPA-DM on the reproductive system in mice (Darmani and Al-Hiyasat 2004) and of nOP in sows (Bogh et al. 2001).
Antagonistic effects on AR in vitro of BPA, nNP, and nOP have previously been reported (Lee et al. 2003; Paris et al. 2002; Roy et al. 2004; Sultan et al. 2001; Xu et al. 2005). Neonatal exposure to BPA and nOP affected development of the male reproductive system (Nagel et al. 1999) and plasma testosterone in infant rats (Williams et al. 2001), whereas BPA was reported to have no antiandrogenic effects on adult rats in the Hersberger assay (Nishino et al. 2006).
An androgen:estrogen balance disturbed by estrogenic compounds was suggested to influence premature activation of spermatogenesis in humans (Kula et al. 1996), being consistent with the ability of BPA and nOP to advance the onset of pubertal spermatogenesis in rats (Atanassova et al. 2000). The androgen:estrogen ratio is among other things determined by aromatase (CYP19) activity that is responsible for the irreversible estrogen biosynthesis from androgens (Jones et al. 2006; Seralini and Moslemi 2001; Simpson et al. 2002). Depressed ovarian aromatase activity in the red mullet was suggested to be caused by nNP and nOP (Martin-Skilton et al. 2006), whereas increased CYP19 gene expression was reported in nNP-exposed zebrafish (Kazeto et al. 2004), and nNP- or BPA-exposed medaka fish liver (Min et al. 2003). In rats, a decreased serum 17β-estradiol (E2) and aromatase mRNA level in Leydig cells was interpreted to play a role in inhibited testicular steroidogenesis by BPA (Akingbemi et al. 2004). Interestingly, although no effect of BPA was observed on CYP19 mRNA levels in human placental JEG-3 cells, a time- and concentration-dependent modulation of the aromatase activity was reported suggesting an interaction between the enzyme and BPA (Nativelle-Serpentini et al. 2003). In summary, effects on aromatase activity caused by the alkylphenols and BPA have been reported in fish, rodent, and human cell studies.
Many EDs elicit multiple mechanisms of action; and apart from their cell and tissue-specific ER and AR agonist or antagonist activities, the involvement of other receptors such as the aryl hydrocarbon receptor (AhR) must be considered as well (Safe et al. 2002). The AhR is a transcription factor that mediates the effects of polyaromatic hydrocarbons, dioxins such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), polychlorinated biphenyls (PCBs) and other EDs such as certain pesticides (Fujii-Kuriyama and Mimura 2005; Long et al. 2003, 2006). As a heterodimer with AhR-nuclear-translocator (Arnt), AhR regulates the inducible expression of CYP1 and other CYP families and plays a crucial role in xenobiotic metabolism, teratogenesis (Thomae et al. 2006) and immune suppression (Novosad et al. 2002). In addition, studies with AhR-null female mice have shown that AhR plays a key role in female reproduction by activating ovarian CYP19 gene transcription (Baba et al. 2005). The potential of BPA and alkylphenols to affect the role of AhR is demonstrated by the following studies: Low-dose in utero exposure of mice embryos showed increased AhR mRNA expression in brain, testes, and ovaries (Nishizawa et al. 2005b). BPA also up-regulated the mRNA level of the AhR repressor (AhRR) and Arnt in mid- and late-stage mice embryos, disrupting the expression of AhR and related factors and xenobiotic metabolizing enzymes (Nishizawa et al. 2005a). In mice Hepa-1c1c7 cells, nNP suppressed CYP1A1 expression by antagonizing the dioxin-responsive element (DRE) binding of nuclear AhR (Jeong et al. 2001). In parallel with E2, an estrogenic effect of nNP was observed in marine fish, Gobious niger, and the P4501A1 inhibition by nNP was mediated through activation of the AhRR (Maradonna et al. 2004). Also, in Atlantic salmon nNP was suggested to have an impact on the metabolism of endogenous and exogenous substrates by modulation of hepatic CYP1A1 via AhR (Meucci and Arukwe 2006). In summary, BPA and nNP are both able to affect AhR action in cell cultures, in murine fetuses, and/or in fish.
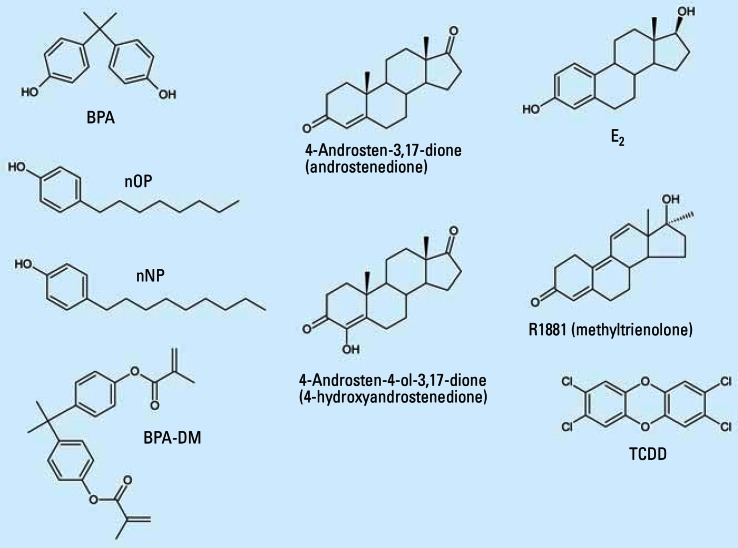
The aim of the present study was to analyze the relative ED potencies of BPA, BPA-DM, nNP, and nOP (Figure 1) in four key in vitro bioassays for ED effects including ER, AR, and AhR transactivation and aromatase activity.
Figure 1.
Structures of the chemicals used in the present study.
Materials and Methods
Materials
BPA was obtained from Sigma-Aldrich Co. (Birmingham, UK). BPA-DM was purchased from Aldrich Chemical Co. (St. Louis, MO, USA). nNP and nOP were purchased from Lancaster Synthesis Ltd. (Birmingham, UK) and Aldrich Chemical Co., respectively. The four chemicals (purity ≥ 98%), and TCDD, 98% (Cambridge Isotopes Laboratories Inc., Andover, MA, USA) were dissolved in dimethyl sulfoxide (DMSO) (BDH Laboratory Supplies, Pool, UK). The E2 (Sigma, St. Louis, MO, USA) was dissolved in 96% ethanol (extra pure; Merck, Darmstadt Germany). Luciferin and fluorescamine were from Amersham Pharmacia Biotech (Piscataway, NJ, USA) and Molecular Probes (Eugene, OR, USA), respectively, and bovine serum albumin from Promega (Madison WI, USA); [1β-3H]Androst-4-ene-3,17-dione was from PerkinElmer (Boston, MA, USA), and both 4-androstene-3,17-dione (4-AD) and 4-androsten-4-ol-3,17-dione (4-AOD) were from Sigma Aldrich (Milwaukee, WI, USA). Methyltrienolone (R1881) was purchased from Mikromol Gmbh (Luckenwalde, Germany). The structures of the test chemicals are shown in Figure 1.
ER-activated luciferase expression assay
We used the stable transfected MVLN cell line, derived from the human breast adenocarcinoma MCF-7 cell line (Pons et al. 1990), to assess effects on ER–luciferase transactivation as described by Bonefeld-Jorgensen et al. (2005). The luciferase data were corrected to cell protein, and the results are given as relative light unit (RLU)/microgram protein. Each compound was tested in triplicate in at least three independent assays alone and as co-treatment with 25 μM E2 [40% of the effect concentration (EC40) of E2]. An E2 dose–response control (0.05–500 pM E2) was performed in parallel each analysis day as described (Bonefeld-Jorgensen et al. 2005). The maximal (EC100) and half maximal (EC50) effective concentrations of E2 were 150 pM and 33 pM, respectively.
Aromatase activity
We performed the assay protocol as described by Drenth et al. (1998), with minor modifications. Human JEG-3 choriocarcinoma cells (no. HTB-36; ATCC, Manassas, VA, USA) were maintained in minimum essential medium (Invitrogen, Life Technologies, Glasgow, UK) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 64 mg/L garamycin, and 1 mM sodium pyruvate (Gibco BRL, Life Technology, Gaithersburg, MD, USA). Cells were seeded in 24-well culture plates (Nunc) at 4 × 104 cells/well in 1 mL culture medium for 2 days (∼ 50% confluence). Serum-free medium ± test compound and/or control were added (maximum 0.1% DMSO) and incubated (37°C, 5% CO2/95% O2) for 18 hr (∼ 90% confluence), and then medium was removed, cells were washed with phosphate-buffered saline (PBS), and 0.5 mL serum-free medium containing 0.2 μCi [1β-3H]androst-4-ene-3,17-dione and 10 nM unlabeled 4-AD, corresponding to the KM value of the enzyme, was added. After 2 hr of incubation, the aromatization was terminated by placing the 24-well plates on ice. We extracted 200 μL of the culture medium with CHCL3, and treated 100 μL of the aqueous phase with 100 μL dextran-charcoal in PBS (5%) (Sigma Aldrich). Finally, we mixed 150 μL of the water phase with 4 mL Hionic Fluor (Packard BioScience, Groningen, the Netherlands) in a 6-mL vial for scintillation (Packard BioScience) and assayed for radioactivity (Packard Liquid Scintillation Analyzer, model Tri-carb 2200; Packard Instrument, Meriden, CT, USA). The determined aromatase activity was subtracted from background level (data from wells with medium only), corrected to cell protein concentration, and related to the solvent control (0.1% DMSO). The protein was determined on cell lyses with 0.5 mL 0.1 M NaOH by the modified Lowry protein assay reagent (Pierce, Rockford, IL, USA) according to the manufacturer’s manual. In parallel, the 4-AOD was used as an aromatase inhibitor control at 10 μM (EC100) and 6 nM (EC50). Each compound was tested at 10−9, 10−8, 10−7, 10−6, 10−5, and 10−4 M in triplicate in at least three independent assays. In each assay, all data were related to the positive control (substrate; 4-AD), which were set to 100%.
AhR-CALUX
The stable transfected mouse hepatoma cell line Hepa1.12cR carrying the pGudLuc1.1 AhR-luciferase reporter gene was kindly provided by M.S. Denison (University of California, Davis, CA, USA). The AhR transactivation of luciferase (AhR-CALUX) was carried out as described by Long et al. (2003, 2006). The data are given as RLU/microgram cell protein. Each compound was tested in at least three independent assays in quadruplicate. The mean of the solvent control (0.1% DMSO) (basal activity) was set to 1, to which the activity of test compounds was related. Results are reported as mean ± SD.
Cell cytotoxicity
Cell cytotoxicity tests for the ER, AhR-CALUX, and aromatase assays were performed according to the CellTiter 96 Non-Radioactive Cell Proliferation assay from Promega (Madison, WI, USA) and cytotoxicity detection kit (LDH) from Roche (Mannheim, Germany) as described by Bonefeld-Jorgensen et al. (2005) and Long et al. (2003).
Statistical analyses for aromatase-, ER-, and AhR-transactivation analyses
The statistical analysis was performed in SPSS 10.0 (SPSS Inc., Chicago, IL). Because of inequality of variance and relatively few data points per concentration, nonparametric statistics was performed. We used the Kruskal-Wallis test to compare differences between different concentrations, and the Jonckheere-Tepstra test to analyze for a linear trend between concentrations and response. If one or both tests showed a significant difference (p ≤ 0.05), we used the Mann-Whitney test to compare the difference between each test concentration and the respective control.
We performed dose–response analysis in Sigma Plot 8.0 (SPSS) by fitting the curves to the four (ER) and three (aromatase, AhR) “parameter sigmoid curve” fit, and calculated EC100, EC50, IC100, or IC50 for each test compound.
AR-reporter gene assay
We tested AR transactivation in a luciferase reporter assay. Chinese hamster ovary (CHO) cells were transiently transfected with the expression vector pSVAR0 (human AR) and the MMTV-LUC reporter plasmid as described by Vinggaard et al. (2002), except that most pipetting procedures were performed using a Biomek2000 laboratory robot (Beckman Coulter, Fullerton, CA, USA) and that 0.1 nM R1881 was used as the AR agonist. Each test compound was tested at concentrations of 0.15, 0.3, 1.3, 2.5, 5, 10, 20, and 40 × 10−6 M in three independent assays as triplicates, and within each assay all data were related to 0.1 nM R1881, which was set to 100%. Using the SigmaStat program, we performed analysis of variance, and if data were statistically significant followed with a Dunnett’s test (p < 0.05). Dose–response analysis was performed in Sigma Plot fitting the curves to the “four parameter logistic curve” fit, and IC50 (half maximal inhibitory concentration) was calculated for each test compound. We determined cytotoxicity in parallel by transfecting cells with a plasmid (pSVAR13) encoding for a constitutively active AR, which lacks the ligand-binding domain (Vinggaard et al. 2002). These experiments were designed exactly as was the AR reporter gene assay except that the ratio between pSVAR13 and MMTV-LUC was 2:100.
Results
Effects on ER transactivity
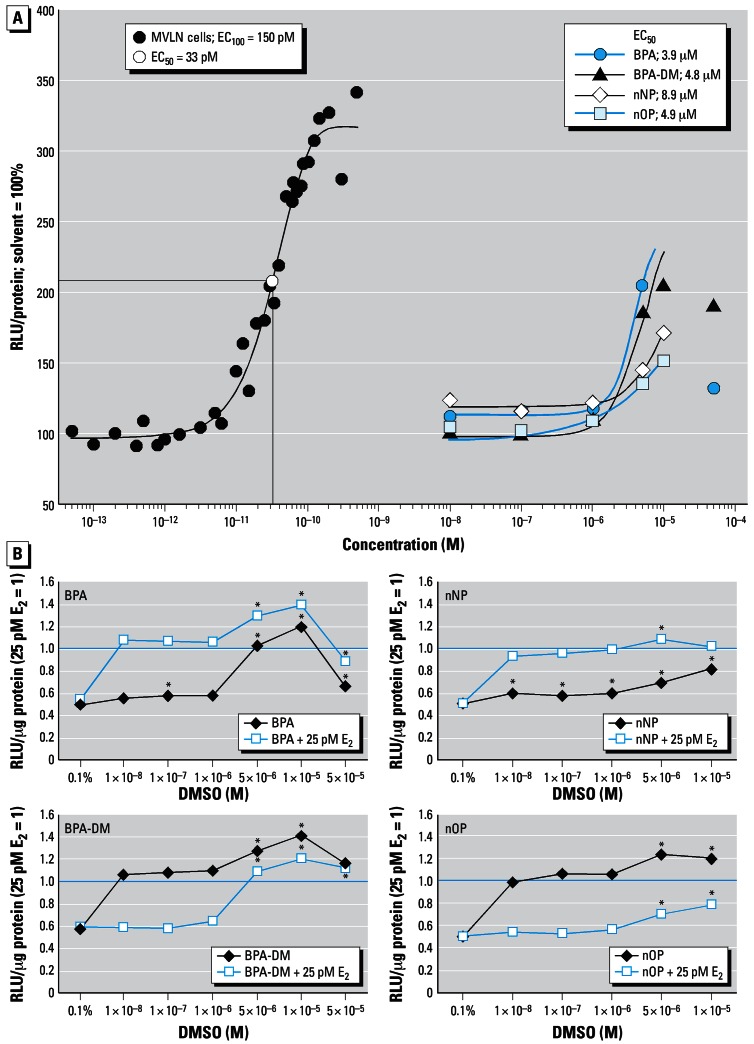
BPA and BPA-DM elicited ER-mediated dose–response luciferase activity in the concentration range of 10−8 to 5 × 10−5 M, with the maximum response being approximately 75% of the natural ligand E2- induced maximum response. The EC50 values were 3.9 μM for BPA and 4.8 μM for BPA-DM. (Figure 2, Table 1). Co-treatment of MVLN cells with 25 pM E2 (EC40) and each of the two compounds showed at 5 μM and 10 μM a further increased estrogenic effect above the E2 EC40 control, whereas the significant decreased response at 50 μM might be the beginning of cell cytotoxicity (Figure 2B). Although lower maximum induction was observed, the alkylphenols nNP and nOP alone also elicited a clearly significant estrogenic response in the concentration range of 10−8 M to 10−5 M and 10−6 M to 10−5 M, respectively, with EC50 values of 8.9 μM and 4.9 μM, respectively (Figure 2, Table 1). We observed further increase of the 25 pM E2-induced activity level for nNP and nOP at 5 × 10−6 and 5 × 10−6–10−5 M, respectively (Figure 2B); cytotoxicity was observed at 25 and 50 μM, respectively (Table 1).
Figure 2.
Dose–response ER transactivation of E2 and the four test chemicals. (A) The MVLN cells were exposed to E2 in the concentration range of 0.05–500 pM and to the test chemicals at 10−8–10−4 M for 24 hr. Solvent control was set to 100%. E2 EC100 and EC50 as well as EC50 for each of the test chemicals were determined by Sigma Plot 8.0. (B) Agonistic and antagonistic ER activity of test chemicals BPA, BPA-DM, nNP, and nOP. The chemicals were tested alone or on co-exposure with 25 pM E2, which was set to 1. Mean values are shown (n ≥ 3).
*Significantly different from the respective solvent controls (cells + 0.1% DMSO; 25 pM E2 + 0.1% DMSO).
Table 1.
ER, AR, AhR, and aromatase characteristics of the four test chemicals alone.
| Assay | LOEC | MOEC | Maximum %a | REP | EC50b /IC50c | Cytotox (M) |
|---|---|---|---|---|---|---|
| ER | ||||||
| E2 | 1 × 10−11 | 1.5 × 10−10 | — | 1 | 3.3 × 10−11b | — |
| BPA | 1 × 10−7 | 1 × 10−5 | — | 1 × 10−4 | 3.9 × 10−6b | — |
| BPA-DM | 5 × 10−6 | 1 × 10−5 | — | 2 × 10−6 | 4.8 × 10−6b | — |
| nNP | 1 × 10−8 | 1 × 10−5 | — | 1 × 10−3 | 8.9 × 10−6b | 2.5 × 10−5 |
| nOP | 5 × 10−6 | 1 × 10−5 | — | 2 × 10−6 | 4.9 × 10−6b | 5.0 × 10−5 |
| ARd | ||||||
| BPA | 0.6 × 10−6 | 2 × 10−5 | 90 | ND | 1.0 × 10−6c | > 4 × 10−5 |
| BPA-DM | 2.5 × 10−6 | 2 × 10−5 | 89 | ND | 2.3 × 10−6c | > 4 × 10−5 |
| nNP | 2.5 × 10−6 | 2 × 10−5 | 56 | ND | 1.4 × 10−5c | > 4 × 10−5 |
| nOP | 0.6 × 10−6 | 1 × 10−5 | 92 | ND | 1.1 × 10−6c | > 2 × 10−5 |
| Aromatased | ||||||
| 4-AOD | 1 × 10−9 | 1 × 10−4 | 100 | 1 | 6 × 10−9c | — |
| BPA | 1 × 10−4 | 1 × 10−4 | 59 | 1 × 10−5 | — | — |
| BPA DM | 1 × 10−4 | 1 × 10−4 | 40 | 1 × 10−5 | — | — |
| nNP | 1 × 10−9 | 1 × 10−5 | 71 | 1 | — | 1 × 10−4 |
| nOP | 1 × 10−7 | 1 × 10−5 | 47 | 1 × 10−2 | — | 1 × 10−4 |
| AhR | ||||||
| TCDD | 2 × 10−12 | 1 × 10−8 | — | 1 | 6.4 × 10−11b | — |
| BPAd | 5 × 10−5d | 1 × 10−4d | 54 | — | ND | > 10−4 |
| BPA-DM | 1 × 10−5 | 1 × 10−5 | — | 1 × 10−7 | ND | > 10−4 |
| nNP | 5 × 10−8 | 1 × 10−4 | — | 4 × 10−5 | 2.4 × 10−5 | > 10−4 |
| nOP | 1 × 10−8 | 2.5 × 10−8 | — | 1 × 10−4 | ND | > 10−4 |
| nOPd | 5 × 10−5 d | 1 × 10−4d | 46 | — | ND | > 10−4 |
Abbreviations: —, no data; LOEC, lowest effect concentration in molar (M); MOEC, maximal effect concentration in molar (M); ND, not determined; REP, relative potency.
Maximum down-regulation of the control inducer, which was set to 100% [0.1 nM R1881 (AR), 10 nM 4-AD (aromatase), 60 pM TCDD (AhR)]; REP-ER = LOECE2/LOECtest chemical; REP-aromatase = LOEC4-AOD/LOECtest compound.
EC50/cIC50: Molar concentration which exert 50% increase/50% inhibition compared to the max response of their respective control, respectively.
Inhibited activity.
Effects on AR transactivity
BPA, BPA-DM, nNP, and nOP elicited a significantly antiandrogenic effect on the R1881-induced AR activity in the range of 0.60–20 μM, with IC50 values of 1.0, 2.3, 14.1, and 1.1 μM, respectively, and a maximum inhibition (MI) of 90, 89, 56, and 92%, respectively. We determined cytotoxicity for BPA, BPA-DM, and nNP at concentrations > 40 μM and for nOP at concentrations > 20 μM (Figure 3 and Table 1).
Figure 3.
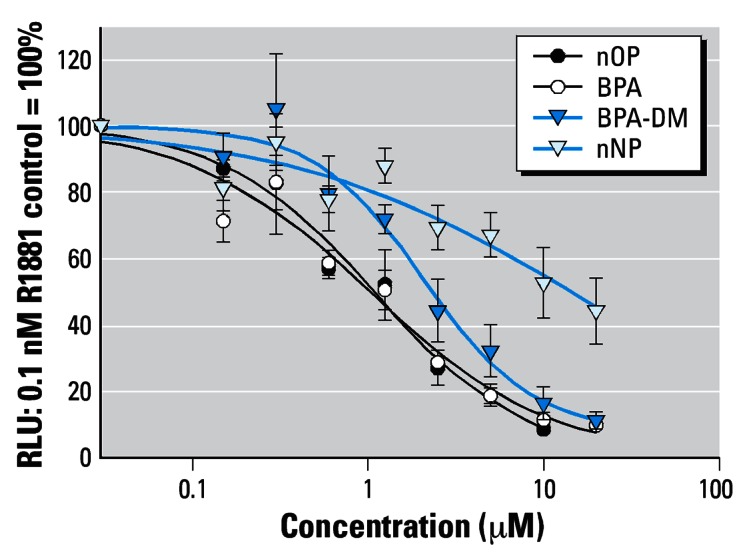
AR antagonism of the test chemicals on co-exposure with R1881 in transient transfected CHO-K1 cells. Cells were transiently transfected with the AR expression pSVAR0 and the reporter pMMTV-LUC vector.
Aromatase activity
The inhibitor control 4-AOD caused an aromatase inhibition in the concentration range of 10−9 M to 10−3 M, with an IC50 of 6 × 10−9 M and 100% inhibition at 10−4 M (Table 1). BPA and BPA-DM decreased the aromatase activity significantly at 10−4 M, with MI of 59% and 40%, respectively. nNP and nOP decreased the aromatase activity in the range 10−9–10−5 M and 10−7–10−5 M, respectively, with MI at 10−5 M of 71% and 47%, respectively. Cytotoxicity was observed at 10−4 M for both nNP and nOP (Table 1).
Effects on AhR transactivation
Effects on AhR transactivation data are shown in Table 1 and Table 2. Decreased AhR activity was observed for BPA alone and on co-treatment with TCDD-EC50 at 5 × 10−5 to 10−4 M and 10−4 M, respectively. BPA-DM alone weakly but significantly increased the CALUX activity at 10−5 M. Although weak compared with TCDD, nNP alone elicited significant increased (∼ 4-fold) dose–response AhR-CALUX activity in the range of 5 × 10−8 M to 10−4 M, with an EC50 of 24 μM. Moreover, nNP further increased and decreased the TCDD-induced AhR activity at 2.5–5 × 10−5 M and 10−4 M, respectively. For nOP alone, weak increased AhR-CALUX activity was observed at 10−8–2.5 × 10−8 M, whereas a decrease was observed at 5 × 10−5 M and 10−4 M. At 10−4 M, nOP decreased TCDD-induced activity by 60%. None of the compounds elicited any toxicity in Hepa1.1 2cR cells at the tested concentration range. Rather, each of the compounds caused a significantly increased cell proliferation at 10−4 M. However, we cannot exclude that the decreased CALUX activity observed at 10−4 M for all compounds, except nNP alone, might be a response due to beginning cytotoxicity.
Table 2.
AhR agonistic and antagonistic effects of the 4 test chemicals.
| Concentration | BPA | BPA-DM | nNP | nOP |
|---|---|---|---|---|
| 0.1% DMSO | 1.03 ± 0.04 | 1.00 ± 0.05 | 0.98 ± 0.07 | 0.99 ± 0.04 |
| 60 pM TCDD | 14.84 ± 6.26* | 14.84 ± 6.26* | 13.89 ± 7.32* | 13.89 ± 7.32* |
| Compounds alone | ||||
| 1.0 × 10−8 M | 1.15 ± 0.21 | 1.15 ± 0.23 | 1.30 ± 0.30 | 1.34 ± 0.27* |
| 2.5 × 10−8 M | 1.07 ± 0.21 | 1.11 ± 0.23 | 1.17 ± 0.22 | 1.27 ± 0.21* |
| 5.0 × 10−8 M | 1.11 ± 0.14 | 1.10 ± 0.08 | 1.30 ± 0.12* | 1.18 ± 0.11 |
| 1.0 × 10−7 M | 1.07 ± 0.20 | 1.12 ± 0.25 | 1.19 ± 0.13* | 1.20 ± 0.20 |
| 1.0 × 10−6 M | 1.22 ± 0.36 | 1.02 ± 0.09 | 1.47 ± 0.20* | 1.08 ± 0.17 |
| 1.0 × 10−5 M | 1.43 ± 0.33 | 1.37 ± 0.27* | 3.91 ± 1.23* | 1.00 ± 0.29 |
| 2.5 × 10−5 M | 1.20 ± 0.37 | 1.15 ± 0.28 | 6.19 ± 2.22* | 0.79 ± 0.25 |
| 5.0 × 10−5 M | 0.73 ± 0.12* | 0.86 ± 0.16 | 7.99 ± 1.64* | 0.41 ± 0.03* |
| 1.0 × 10−4 M | 0.46 ± 0.15* | 0.97 ± 0.25 | 3.68 ± 0.20* | 0.54 ± 0.19* |
| Compounds + TCDD | ||||
| 60 pM TCDD | 1.08 ± 0.19 | 1.10 ± 0.25 | 0.98 ± 0.03 | 1.04 ± 0.06 |
| 1.0 × 10−8 M | 1.14 ± 0.15 | 1.44 ± 0.33 | 1.17 ± 0.24 | 1.18 ± 0.12 |
| 2.5 × 10−8 M | 1.13 ± 0.06 | 1.48 ± 0.60 | 1.13 ± 0.16 | 1.22 ± 0.19 |
| 5.0 × 10−8 M | 1.34 ± 0.33 | 1.53 ± 0.45 | 1.19 ± 0.13 | 1.12 ± 0.09 |
| 1.0 × 10−7 M | 1.23 ± 0.11 | 1.64 ± 0.62 | 1.14 ± 0.19 | 1.18 ± 0.15 |
| 1.0 × 10−6 M | 1.18 ± 0.27 | 1.57 ± 0.56 | 1.22 ± 0.22 | 1.15 ± 0.14 |
| 1.0 × 10−5 M | 1.40 ± 0.37 | 1.86 ± 0.76 | 1.34 ± 0.35 | 1.24 ± 0.20 |
| 2.5 × 10−5 M | 1.36 ± 0.42 | 1.44 ± 0.35 | 1.46 ± 0.38* | 1.34 ± 0.45 |
| 5.0 × 10−5 M | 0.93 ± 0.16 | 1.23 ± 0.21 | 1.41 ± 0.36* | 1.10 ± 0.39 |
| 1.0 × 10−4 M | 0.44 ± 0.13* | 0.56 ± 0.29 | 0.61 ± 0.22* | 0.41 ± 0.11* |
Concentrations up to 10−4 M were not cytotoxic to the cells, whereas concentrations > 10−4 M elicited cytotoxicity to the Hepa1.1 2cR cell. The results given refer to effects observed at concentrations eliciting no cytotoxicity (n ≥ 3).
p < 0.05, versus the respective control, which was set to 1.
Discussion
In the present study we demonstrated that BPA, BPA-DM, nNP, and nOP elicited an impact on most of the selected end points: ER, AR, AhR, and aromatase activity. Estrogenicity was observed for the four compounds where BPA and nNP showed the highest relative potency compared with E2, supporting previously reported in vitro and in vivo data. The four chemicals also antagonized AR transactivation in a concentration-dependent manner, with IC50 values in the order BPA ≤ nOP ≤ BPA-DM < nNP. In addition, all four chemicals inhibited the aromatase activity in JEG-3 cells. Furthermore, nNP activated AhR in a concentration-dependent manner; BPA-DM activated AhR at 10−5 M only, whereas BPA inhibited the AhR action at 5 × 10−5 to 10−4 M, and nOP elicited a weakly induced and a decreased AhR activity at lower and higher concentrations, respectively. Thus our in vitro data indicate that the four industrial chemicals have the potential to affect several cellular pathway systems, including gene expressions regulated via the steroid receptors ER and AR, the conversion of testosterone into estrogen by aromatase, and the function of AhR, involved in syntheses of steroids such as estrogens and metabolism of steroids and xenobiotic compounds.
MVLN cells, derived from MCF-7 cells (Pons et al. 1990), express both ERα and ERβ (Gaido et al. 1998; Grunfeld and Bonefeld-Jorgensen 2004; Hofmeister and Bonefeld-Jorgensen 2004) that can bind the ER response element vitellogenin, vit-tk, in front of the luciferase gene (Gruber et al. 2004). Thus the estrogenicity demonstrated for the four tested compounds in our analyses is mediated by transactivation of ERα and/or ERβ. Moreover, based on previous reports including E-SCREEN, ER transactivation, binding assays to the two ER subtypes, and effects observed with the ER antagonists ICI 182,780 and tamoxifen (Gutendorf and Westendorf 2001; Scippo et al. 2004; Vivacqua et al. 2003; Wilson et al. 2004), we can conclude that the estrogenic potential of these four compounds is accomplished through the ERs. However, we cannot exclude that other cellular mechanisms are involved, such as activation of the transcription factor cAMP-responsive element binding protein via binding to nonclassical membrane ERs (Quesada et al. 2002). In addition, BPA and nNP effects were exerted predominantly by the parent compounds and not by their metabolites (Legler et al. 2002).
We observed an antagonistic effect on R1881-induced AR transactivition of the four tested chemicals. To our knowledge, the AR antagonism of BPA-DM has not previously been reported. As we performed the present study, another study using transiently transfected [human AR (hAR) and MMTV-CAT] Africa monkey kidney CV-1 cells reported an antagonizing effect on AR by BPA, nNP, and nOP with similar IC50 values, except that nOP had an IC50 approximately 100 times higher (97.1 μM) (Xu et al. 2005) than ours. In contrast, in a recently established stably transfected CHO-AR-LUC cell line, only BPA but not nNP and nOP elicited anti-AR effects (Roy et al. 2004); similarly, BPA but not nNP antagonized AR action in stable transfected (hAR and MMTV-luciferase) PC-3 prostate cells (Paris et al. 2002; Sultan et al. 2001). Our AR antagonistic data for BPA and nNP are consistent with results obtained in yeast (Lee et al. 2003) and transient transfected (hAR and ARE2-luciferase) NIH3T3 cells (Kitamura et al. 2005). In contrast, BPA showed no effect on AR action in transient transfected (hAR and MMTV-luciferase) human hepatoma HepG2 cells (Gaido et al. 2000). Whole cell binding assays showed that BPA has the potential of binding to the AR (Paris et al. 2002). We suggest that the antiAR action observed in the present study for BPA, BPA-DM, nNP, and nOP indicates that the chemicals have the ability to bind the AR and thus compete with endogenous androgens for binding and regulating AR-dependent gene expression. However, indirect interference with other transcriptional factors involved in AR transactivation may also play a role. The discrepancy between the reported AR effects of the compounds might be caused by different sensitivities of the various assays, differences in co-factors in the different cell lines, and/or the use of different AR response elements (Sommer and Haendler 2003; Verrijdt et al. 2003). However, it is important to clarify the cause(s) of the discrepancies in the use of AR-reporter analyses to assess the potential actions of chemicals on androgenic processes in vivo.
In the synthesis of steroid hormones from cholesterol, the aromatase enzyme is pivotal by its irreversible conversion of androgens to estrogens (Simpson et al. 2002). Aromatase, which is located in the endoplasmic reticulum membrane, is expressed in several tissues and cell types in humans, including adipose tissue, various sites of the brain, and testicular Leydig cells in males (Jones et al. 2006). Aromatase activity and endogenous estrogens (and ERs) are important in male reproduction (Carreau et al. 2006). In rat Leydig cells, BPA inhibited testicular steroidogenesis by a decrease in 17α-hydroxylase/17–20 lyase and CYP19 expression causing decreased testosterone and E2 synthesis (Akingbemi et al. 2004). After 18-hr exposure of JEG-3 cells to BPA, BPA-DM, nNP, or nOP, we observed a significant inhibition of aromatase activity. To our knowledge, this is the first report showing BPA-DM, nNP, and nOP effects on aromatase activity in mammalian cells. nNP and, to lesser extent nOP, caused a dose-dependent inhibition of aromatase activity in the range of 10−9–10−5M. Our data are supported by an earlier report examining BPA in JEG-3 cells, in which a significant effect was observed on aromatase activity but not on gene expression (Nativelle-Serpentini et al. 2003). Given the observation that short (2 hr) exposure increased and long (18 hr) exposure decreased aromatase activity, and that parallel transfections with CYP19 cDNA elicited similar effects, Nativelle-Serpentini et al. (2003) concluded that the xenobiotics acted at the aromatase protein level. In summary, the tested plasticizers seem to have the potential to affect the aromatase activity and thus the synthesis of estrogens in mammals as well as in fish (Kazeto et al. 2004; Martin-Skilton et al. 2006; Min et al. 2003).
The present study suggests that BPA, BPA-DM, nNP, and nOP have the potential for indirect AhR-mediated actions on xenobiotic metabolism, steroid synthesis, and metabolism. This is the first report, to our knowledge, showing the ability of BPA-DM and nOP to affect the transactivation of AhR. BPA and BPA-DM elicited in the 10−5 M range weak antagonistic and agonistic AhR effects, respectively. We observed a clear dose-dependent AhR activation for nNP, although it was weak compared with that for TCDD. In previous in vitro studies, BPA and nNP had no effects on their own, but antagonized TCDD-induced CYP1A1 mRNA and 7-ethoxyresorufin-O-deethylase (EROD) activity levels in Hepa-1c1c7 cells via interference with AhR:DRE binding and/or transport into the nucleus and/or the action of co-transcription factors (Jeong et al. 2000, 2001). Similarly to BPA and nNP, estradiol was shown to antagonize AhR:TCDD-induced CYP1A1 expression and EROD activity, and weakly induce EROD activity via AhR not involving ER (Jeong and Lee 1998). In vivo, the endocrine disruption potential of BPA on mice embryonic development was demonstrated at very low doses (0.02 μg/kg/day) (Nishizawa et al. 2005a, 2005b).
We observed weak increased and decreased AhR activity at lower (10−8 M) and higher (10−6 M) nOP concentrations, respectively, and nOP also induced ER activity at ∼ 10−6 M. We wonder whether this estrogenic mimic of nOP can be explained by the fact that the bifocal effects of estradiol on AhR can either induce or antagonize AhR function (Jeong and Lee 1998; Kharat and Saatcioglu 1996). That nOP elicited AhR agonism at lower concentrations and reduced AhR transactivation at higher concentrations might be attributed to inhibitory cross-talk of AhR and ER. However, the specific mechanisms involved must be further studied.
The demonstration in AhR knock-out mice that AhR is an important factor in female reproduction by regulating the expression of ovarian aromatase elucidates the physiologic role of AhR and also suggests AhR as a mediator of endocrine disruption (Baba et al. 2005). Antiestrogenic actions mediated via AhR are well described, in which AhR:ligand inhibits ER binding to ERE (Pocar et al. 2005) and increases proteasomal degradation of ER (Wormke et al. 2003). A further perspective of AhR as a factor in ED is given by AhR’s estrogenic potential in absence of estrogen via the agonist-activated AhR:Arnt interaction, with ERα and/or ERβ leading to transcriptional activation of ER-regulated genes (Ohtake et al. 2003). In addition, antiandrogenic actions of TCDD have pointed out two possible mechanisms: blocking of AR-induced gene expression and AhR–AR cross-talk possibly involving competition of co-regulators (Barnes-Ellerbe et al. 2004; Jana et al. 1999).
Hypothetically, AhR might play a role in regulating the cell ratio of androgens:estrogens via activation of CYP19, thereby affecting male and female reproduction, affecting estrogenic actions via ERs, inducing cell metabolism via, for example, CYP1A, and inhibiting AR functions.
In summary, our in vitro data demonstrated that the four tested chemicals have the potential to affect central endocrine pathways through their capacity to affect the function of the nuclear receptors ER, AR, AhR, and aromatase activity. BPA-related compounds and alkylphenols have been found in human fluids in 0.1–10 nM (vom Saal and Hughes 2005) and 0.4–13.9 ng/mL urine (Kawaguchi et al. 2004; Kuklenyik et al. 2003), respectively. In this study, the lowest observed effect concentrations for the BPA phenols and the alkylphenols were in the 100–1,000 nM and 10–1,000 nM ranges, respectively. Although the effective concentrations in vitro for the tested compounds were ≥ 1,000 times higher than the level found in humans, their ability to act via more than one mechanism might enhance the biologic effect in the intact organism, because the final response will likely be determined as a sum of the interactions of all pathways implicated. Furthermore, because most humans are exposed to several chemicals simultaneously and some EDs have been shown to act additively in vitro and in vivo (Birkhoj et al. 2004; Nellemann et al. 2003; Rajapakse et al. 2002), potential mixture effects should also be taken into consideration in the risk assessment.
Footnotes
We thank B.S. Andersen, B.M. Plesning, and A. Keblovski for excellent technical assistance.
This article is part of the monograph “Endocrine Disruptors—Exposure Assessment, Novel End Points, and Low-Dose and Mixture Effects.”
The study was supported by the European Union project ENDOMET, QLRT-2001-02637, and the Danish Research Council grant 2107–04–0006.
References
- Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145(2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, et al. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999;107(suppl 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, et al. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000;141(10):3898–3907. doi: 10.1210/endo.141.10.7723. [DOI] [PubMed] [Google Scholar]
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, et al. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25(22):10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Ellerbe S, Knudsen KE, Puga A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol Pharmacol. 2004;66(3):502–511. doi: 10.1124/mol.104.000356. [DOI] [PubMed] [Google Scholar]
- Basly JP, Lavier MC. Dietary phytoestrogens: potential selective estrogen enzyme modulators? Planta Med. 2005;71(4):287–294. doi: 10.1055/s-2005-864092. [DOI] [PubMed] [Google Scholar]
- Biles JE, White KD, McNeal TP, Begley TH. Determination of the diglycidyl ether of bisphenol A and its derivatives in canned foods. J Agric Food Chem. 1999;47(5):1965–1969. doi: 10.1021/jf9810867. [DOI] [PubMed] [Google Scholar]
- Birkhoj M, Nellemann C, Jarfelt K, Jacobsen H, Andersen HR, Dalgaard M, et al. The combined antiandrogenic effects of five commonly used pesticides. Toxicol Appl Pharmacol. 2004;201(1):10–20. doi: 10.1016/j.taap.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bogh IB, Christensen P, Dantzer V, Groot M, Thofner IC, Rasmussen RK, et al. Endocrine disrupting compounds: effect of octylphenol on reproduction over three generations. Theriogenology. 2001;55(1):131–150. doi: 10.1016/s0093-691x(00)00451-9. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC. The Human Health Effect Programme in Greenland, a review. Sci Total Environ. 2004;331(1–3):215–231. doi: 10.1016/j.scitotenv.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Ayotte P. AMAP Assessment 2002: Human Health in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme; 2003. Toxicological properties of POPs and related health effects of concern for the Arctic populations; pp. 57–74. [Google Scholar]
- Bonefeld-Jorgensen EC, Grünfeld HT, Gjermandsen IM. Effect of pesticides on estrogen receptor transactivation in vitro: A comparison of stable transfected MVLN and transient transfected MCF-7 cells. Mol Cell Endocrinol. 2005;244:20–30. doi: 10.1016/j.mce.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S, Delalande C, Silandre D, Bourguiba S, Lambard S. Aromatase and estrogen receptors in male reproduction. Mol Cell Endocrinol. 2006;246(1–2):65–68. doi: 10.1016/j.mce.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Choi KC, Jeung EB. The biomarker and endocrine disruptors in mammals. J Reprod Dev. 2003;49(5):337–345. doi: 10.1262/jrd.49.337. [DOI] [PubMed] [Google Scholar]
- Darmani H, Al-Hiyasat AS. Reproductive toxic effect of bisphenol A dimethacrylate in mice. J Biomed Mater Res A. 2004;69(4):637–643. doi: 10.1002/jbm.a.30029. [DOI] [PubMed] [Google Scholar]
- Drenth HJ, Bouwman CA, Seinen W, Van den Berg M. Effects of some persistent halogenated environmental contaminants on aromatase (CYP19) activity in the human choriocarcinoma cell line JEG-3. Toxicol Appl Pharmacol. 1998;148(1):50–55. doi: 10.1006/taap.1997.8307. [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- Gaido K, Dohme L, Wang F, Chen I, Blankvoort B, Ramamoorthy K, et al. Comparative estrogenic activity of wine extracts and organochlorine pesticide residues in food. Environ Health Perspect. 1998;106(suppl 6):1347–1351. doi: 10.1289/ehp.98106s61347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58(4):852–858. [PubMed] [Google Scholar]
- Garrison PM, Tullis K, Aarts JM, Brouwer A, Giesy JP, Denison MS. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol. 1996;30(2):194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- Ghisari M, Bonefeld-Jorgensen EC. Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol Cell Endocrinol. 2005;244(1–2):31–41. doi: 10.1016/j.mce.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gray LE., Jr Xenoendocrine disrupters: laboratory studies on male reproductive effects. Toxicol Lett. 1998;102–103:331–335. doi: 10.1016/s0378-4274(98)00327-0. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol Metab. 2004;15(2):73–78. doi: 10.1016/j.tem.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Grunfeld HT, Bonefeld-Jorgensen EC. Effect of in vitro estrogenic pesticides on human oestrogen receptor alpha and beta mRNA levels. Toxicol Lett. 2004;151(3):467–480. doi: 10.1016/j.toxlet.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Guenther K, Heinke V, Thiele B, Kleist E, Prast H, Raecker T. Endocrine disrupting nonylphenols are ubiquitous in food. Environ Sci Technol. 2002;36(8):1676–1680. doi: 10.1021/es010199v. [DOI] [PubMed] [Google Scholar]
- Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166(1–2):79–89. doi: 10.1016/s0300-483x(01)00437-1. [DOI] [PubMed] [Google Scholar]
- Hofmeister MV, Bonefeld-Jorgensen EC. Effects of the pesticides prochloraz and methiocarb on human estrogen receptor alpha and beta mRNA levels analyzed by online RT-PCR. Toxicol In Vitro. 2004;18(4):427–433. doi: 10.1016/j.tiv.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Cross-talk between 2,3,7,8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochem Biophys Res Commun. 1999;256(3):462–468. doi: 10.1006/bbrc.1999.0367. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Kim JY, Choi CY. Down-regulation of murine Cyp1a-1 in mouse hepatoma Hepa-1c1c7 cells by bisphenol A. Biochem Biophys Res Commun. 2000;277(3):594–598. doi: 10.1006/bbrc.2000.3717. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Kim JY, Choi CY, You HJ, Hahm K. Suppression of CYP1A1 expression by 4-nonylphenol in murine Hepa-1c1c7 cells. Cancer Lett. 2001;165(1):95–101. doi: 10.1016/s0304-3835(01)00407-4. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Lee SS. Suppressive effects of estradiol on 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated transcriptional activation of murine Cyp1a-1 in mouse hepatoma Hepa 1c1c7 cells. Cancer Lett. 1998;133(2):177–184. doi: 10.1016/s0304-3835(98)00224-9. [DOI] [PubMed] [Google Scholar]
- Jones ME, Chin Boon W, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17(2):55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Inoue K, Sakui N, Ito R, Izumi S, Makino T, et al. Stir bar sorptive extraction and thermal desorption-gas chromatography-mass spectrometry for the measurement of 4-nonylphenol and 4-tert-octylphenol in human biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799(1):119–125. doi: 10.1016/j.jchromb.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kazeto Y, Place AR, Trant JM. Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat Toxicol. 2004;69(1):25–34. doi: 10.1016/j.aquatox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kharat I, Saatcioglu F. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin are mediated by direct transcriptional interference with the liganded estrogen receptor. Cross-talk between aryl hydrocarbon- and estrogen-mediated signaling. J Biol Chem. 1996;271(18):10533–10537. doi: 10.1074/jbc.271.18.10533. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, et al. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84(2):249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Ekong J, Cutchins CD, Needham LL, Calafat AM. Simultaneous measurement of urinary bisphenol A and alkylphenols by automated solid-phase extractive derivatization gas chromatography/mass spectrometry. Anal Chem. 2003;75(24):6820–6825. doi: 10.1021/ac0303158. [DOI] [PubMed] [Google Scholar]
- Kula K, Slowikowska-Hilczer J, Romer TE, Metera M, Jankowska J. [Precocious maturation of the testis associated with excessive secretion of estradiol and testosterone by Leydig cells] [in Polish] Pediatr Pol. 1996;71(3):269–273. [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75(1):40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Legler J, Dennekamp M, Vethaak AD, Brouwer A, Koeman JH, van der Burg B, et al. Detection of estrogenic activity in sediment-associated compounds using in vitro reporter gene assays. Sci Total Environ. 2002;293(1–3):69–83. doi: 10.1016/s0048-9697(01)01146-9. [DOI] [PubMed] [Google Scholar]
- Long M, Andersen BS, Lindh CH, Hagmar L, Giwercman A, Manicardi GC, et al. Dioxin-like activities in serum across European and Inuit populations. Environ Health. 2006;5:14. doi: 10.1186/1476-069X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Laier P, Vinggaard AM, Andersen HR, Lynggaard J, Bonefeld-Jorgensen EC. Effects of currently used pesticides in the AhR-CALUX assay: comparison between the human TV101L and the rat H4IIE cell line. Toxicology. 2003;194(1–2):77–93. doi: 10.1016/j.tox.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Maradonna F, Polzonetti V, Bandiera SM, Migliarini B, Carnevali O. Modulation of the hepatic CYP1A1 system in the marine fish Gobius niger, exposed to xenobiotic compounds. Environ Sci Technol. 2004;38(23):6277–6282. doi: 10.1021/es049786h. [DOI] [PubMed] [Google Scholar]
- Martin-Skilton R, Lavado R, Thibaut R, Minier C, Porte C. Evidence of endocrine alteration in the red mullet, Mullus barbatus from the NW Mediterranean. Environ Pollut. 2006;141(1):60–68. doi: 10.1016/j.envpol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Meucci V, Arukwe A. The xenoestrogen 4-nonylphenol modulates hepatic gene expression of pregnane X receptor, aryl hydrocarbon receptor, CYP3A and CYP1A1 in juvenile Atlantic salmon (Salmo salar) Comp Biochem Physiol C Toxicol Pharmacol. 2006;142(1–2):142–150. doi: 10.1016/j.cbpc.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Min J, Lee SK, Gu MB. Effects of endocrine disrupting chemicals on distinct expression patterns of estrogen receptor, cytochrome P450 aromatase and p53 genes in Oryzias latipes liver. J Biochem Mol Toxicol. 2003;17(5):272–277. doi: 10.1002/jbt.10089. [DOI] [PubMed] [Google Scholar]
- Mosconi G, Carnevali O, Franzoni MF, Cottone E, Lutz I, Kloas W, et al. Environmental estrogens and reproductive biology in amphibians. Gen Comp Endocrinol. 2002;126(2):125–129. doi: 10.1006/gcen.2002.7781. [DOI] [PubMed] [Google Scholar]
- Mueller SO. Overview of in vitro tools to assess the estrogenic and antiestrogenic activity of phytoestrogens. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777(1–2):155–165. doi: 10.1016/s1570-0232(02)00282-9. [DOI] [PubMed] [Google Scholar]
- Nagel SC, vom Saal FS, Welshons WV. Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J Steroid Biochem Mol Biol. 1999;69(1–6):343–357. doi: 10.1016/s0960-0760(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Nativelle-Serpentini C, Richard S, Seralini GE, Sourdaine P. Aromatase activity modulation by lindane and bisphenol-A in human placental JEG-3 and transfected kidney E293 cells. Toxicol In Vitro. 2003;17(4):413–422. doi: 10.1016/s0887-2333(03)00046-8. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol Sci. 2003;71(2):251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Nishino T, Wedel T, Schmitt O, Schonfelder M, Hirtreiter C, Schulz T, et al. The xenoestrogen bisphenol A in the Hershberger assay: androgen receptor regulation and mor-phometrical reactions indicate no major effects. J Steroid Biochem Mol Biol. 2006;98(2–3):155–163. doi: 10.1016/j.jsbmb.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Imanishi S, Manabe N. Effects of exposure in utero to bisphenol A on the expression of aryl hydrocarbon receptor, related factors, and xenobiotic metabolizing enzymes in murine embryos. J Reprod Dev. 2005a;51(5):593–605. doi: 10.1262/jrd.17026. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Morita M, Sugimoto M, Imanishi S, Manabe N. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. J Reprod Dev. 2005b;51(3):315–324. doi: 10.1262/jrd.16008. [DOI] [PubMed] [Google Scholar]
- Novosad J, Fiala Z, Borska L, Krejsek J. Immunosuppressive effect of polycyclic aromatic hydrocarbons by induction of apoptosis of pre-B lymphocytes of bone marrow. Acta Medica (Hradec Kralove) 2002;45(4):123–128. [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Meussen-Elholm ET, Samuelsen M, Holme JA, Hongslo JK. Effects of the environmental oestrogens bisphenol A, tetrachlorobisphenol A, tetrabromobisphenol A, 4-hydroxybiphenyl and 4,4′-dihydroxybiphenyl on oestrogen receptor binding, cell proliferation and regulation of oestrogen sensitive proteins in the human breast cancer cell line MCF-7. Pharmacol Toxicol. 2003;92(4):180–188. doi: 10.1034/j.1600-0773.2003.920408.x. [DOI] [PubMed] [Google Scholar]
- Owens W, Koeter HB. The OECD program to validate the rat uterotrophic bioassay: an overview. Environ Health Perspect. 2003;111:1527–1529. doi: 10.1289/ehp.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris F, Balaguer P, Terouanne B, Servant N, Lacoste C, Cravedi JP, et al. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit alpha and beta estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002;193(1–2):43–49. doi: 10.1016/s0303-7207(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129(4):379–389. doi: 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- Pons M, Gagne D, Nicolas JC, Mehtali M. A new cellular model of response to estrogens: a bioluminescent test to characterize (anti) estrogen molecules. Biotechniques. 1990;9(4):450–459. [PubMed] [Google Scholar]
- Pulgar R, Olea-Serrano MF, Novillo-Fertrell A, Rivas A, Pazos P, Pedraza V, et al. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ Health Perspect. 2000;108:21–27. doi: 10.1289/ehp.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada I, Fuentes E, Viso-Leon MC, Soria B, Ripoll C, Nadal A. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17beta-estradiol rapidly activate transcription factor CREB. Faseb J. 2002;16(12):1671–1673. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas A, Lacroix M, Olea-Serrano F, Laios I, Leclercq G, Olea N. Estrogenic effect of a series of bisphenol analogues on gene and protein expression in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2002;82(1):45–53. doi: 10.1016/s0960-0760(02)00146-2. [DOI] [PubMed] [Google Scholar]
- Roy P, Salminen H, Koskimies P, Simola J, Smeds A, Saukko P, et al. Screening of some anti-androgenic endocrine disruptors using a recombinant cell-based in vitro bioassay. J Steroid Biochem Mol Biol. 2004;88(2):157–166. doi: 10.1016/j.jsbmb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Safe SH, Pallaroni L, Yoon K, Gaido K, Ross S, McDonnell D. Problems for risk assessment of endocrine-active estrogenic compounds. Environ Health Perspect. 2002;110(suppl 6):925–929. doi: 10.1289/ehp.02110s6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe JW, Teichmann SA. Nuclear receptors: the evolution of diversity. Sci STKE 2004. 2004;(217):pe4. doi: 10.1126/stke.2172004pe4. [DOI] [PubMed] [Google Scholar]
- Scippo ML, Argiris C, Van De Weerdt C, Muller M, Willemsen P, Martial J, et al. Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal Bioanal Chem. 2004;378(3):664–669. doi: 10.1007/s00216-003-2251-0. [DOI] [PubMed] [Google Scholar]
- Seralini G, Moslemi S. Aromatase inhibitors: past, present and future. Mol Cell Endocrinol. 2001;178(1–2):117–131. doi: 10.1016/s0303-7207(01)00433-6. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, et al. Aromatase—a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37(6):423–438. doi: 10.1080/07853890510044586. [DOI] [PubMed] [Google Scholar]
- Sommer A, Haendler B. Androgen receptor and prostate cancer: molecular aspects and gene expression profiling. Curr Opin Drug Discov Devel. 2003;6(5):702–711. [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. 1998;65(1–6):143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonylphenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ Health Perspect. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139(6):2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- Sultan C, Balaguer P, Terouanne B, Georget V, Paris F, Jeandel C, et al. Environmental xenoestrogens, antiandrogens and disorders of male sexual differentiation. Mol Cell Endocrinol. 2001;178(1–2):99–105. doi: 10.1016/s0303-7207(01)00430-0. [DOI] [PubMed] [Google Scholar]
- Thomae TL, Stevens EA, Liss AL, Drinkwater NR, Bradfield CA. The teratogenic sensitivity to 2,3,7,8-tetrachlorodibenzo-p-dioxin is modified by a locus on mouse chromosome 3. Mol Pharmacol. 2006;69(3):770–775. doi: 10.1124/mol.105.019760. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J Environ Monit. 2001;3(4):366–370. doi: 10.1039/b104304h. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) Washington, DC: U.S. Environmental Protection Agency; 2000. [accessed 14 September 2006]. Available: http://www.epa.gov/scipoly/oscpendo/edspoverview/edstac.htm. [Google Scholar]
- Van den Belt K, Berckmans P, Vangenechten C, Verheyen R, Witters H. Comparative study on the in vitro/in vivo estrogenic potencies of 17beta-estradiol, estrone, 17alpha-ethynylestradiol and nonylphenol. Aquat Toxicol. 2004;66(2):183–195. doi: 10.1016/j.aquatox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Verrijdt G, Haelens A, Claessens F. Selective DNA recognition by the androgen receptor as a mechanism for hormone-specific regulation of gene expression. Mol Genet Metab. 2003;78(3):175–185. doi: 10.1016/s1096-7192(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Nellemann C, Dalgaard M, Bonefeld-Jorgensen E, Andersen HR. Antiandrogenic effects in vitro and in vivo of the fungicide prochloraz. Toxicol Sci. 2002;69(2):344–353. doi: 10.1093/toxsci/69.2.344. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Recchia AG, Fasanella G, Gabriele S, Carpino A, Rago V, et al. The food contaminants bisphenol A and 4-nonylphenol act as agonists for estrogen receptor alpha in MCF7 breast cancer cells. Endocrine. 2003;22(3):275–284. doi: 10.1385/ENDO:22:3:275. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135:175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Williams K, McKinnell C, Saunders PT, Walker M, Fisher JS, Turner KJ, et al. Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum Reprod Updata. 2001;7(3):236–247. doi: 10.1093/humupd/7.3.236. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LE., Jr Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci. 2004;81(1):69–77. doi: 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, et al. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol Cell Biol. 2003;23(6):1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, et al. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216(2–3):197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ying GG, Williams B, Kookana R. Environmental fate of alkylphenols and alkylphenol ethoxylates—a review. Environ Int. 2002;28(3):215–226. doi: 10.1016/s0160-4120(02)00017-x. [DOI] [PubMed] [Google Scholar]