Abstract
Objective
The aim of this study was to assess whether the joint effects of three androgen receptor antagonists (vinclozolin, flutamide, procymidone) on male sexual differentiation after in utero and postnatal exposures can be predicted based on dose–response data of the individual chemicals.
Methods
Test chemicals and mixtures were administered by gavage to time-mated nulliparous, young adult Wistar rats from gestational day 7 to the day before expected birth, and from postnatal days 1–16. Changes in anogenital distance (AGD) and nipple retention (NR) in male offspring rats were chosen as end points for extensive dose–response studies. Vinclozolin, flutamide, and procymidone were combined at a mixture ratio proportional to their individual potencies for causing retention of six nipples in male offspring.
Results
With AGD as the end point, the joint effects of the three anti-androgens were essentially dose additive. The observed responses for NR were slightly higher than those expected on the basis of dose addition. A combination of doses of each chemical, which on its own did not produce statistically significant AGD alterations, induced half-maximal mixture effects. At individual doses associated with only modest effects on NR, the mixture induced NR approaching female values in the males.
Conclusions
Effects of a mixture of similarly acting anti-androgens can be predicted fairly accurately on the basis of the potency of the individual mixture components by using the dose addition concept. Exposure to anti-androgens, which individually appears to exert only small effects, may induce marked responses in concert with, possibly unrecognized, similarly acting chemicals.
Keywords: AGD, anti-androgen, combination effect, developmental exposure, flutamide, mixture, nipple retention, procymidone, vinclozolin
Androgens are key regulators of male sexual differentiation during the in utero and early postnatal development. Exposure to chemicals that counteract androgen action at some stage in this period can permanently demasculinize male fetuses and lead to malformations of the reproductive tract. Examples of chemicals known to disrupt sexual differentiation in this way include pesticides and their metabolites, such as vinclozolin, procymidone, 1,1-dichloro-2,2-bis(4-chlorophenyl) ethylene (p,p′-DDE), and linuron, and certain phthalate esters such as di-ethylhexyl phthalate and di-butyl phthalate (Gray et al. 2000, 2001). Reduced anogenital distance, retention of nipples or areolas, hypospadias, agenesis of sex accessory tissues, and undescended testes have been described as consequences of disruption of androgen action in the developing rat. These effects are thought to arise through antagonism of androgens at the steroid receptor level and/or via suppression of testosterone synthesis in Leydig cells (Fisher 2004; Gray et al. 2001).
Many anti-androgenic chemicals have been found as mixtures in humans (Blount et al. 2000; Swan et al. 2005), including children (Brock et al. 2002; Main et al. 2006), and in wildlife (Guillette 2000). These findings have stimulated interest in exploring the consequences of combined exposures to anti-androgens, although relatively few studies have addressed the issue. There is good evidence that inhibition of androgen binding and other receptor-mediated events occur in an additive fashion (Birkhoj et al. 2004; Gray et al. 2001; Nellemann et al. 2003), but little is known about the developmental effects of in utero and early postnatal exposure to multiple anti-androgenic chemicals.
In this article, we present data from detailed investigations of the ability of combinations of androgen receptor (AR) antagonists to induce disruption of male sexual differentiation after long-term exposures in utero and postnatally. We selected a mixture of vinclozolin, procymidone, and flutamide for our experiments. Vinclozolin metabolites compete with androgens for AR binding (Kelce et al. 1994), suppress androgen-dependent gene transcription (Kelce et al. 1997), and affect reproductive development. Procymidone and flutamide also antagonize competitively the AR binding of androgens, with consequent inhibition of AR-mediated gene expression (Ostby et al. 1999; Simard et al. 1986). Common developmental effects of all three chemicals after in utero exposure of male rats include reduced anogenital distance (AGD), nipple retention (NR), hypospadia, diminished prostate weight, reduced testis and epididymal weights, and altered behavior in male offspring (Foster and McIntyre 2002; Gray et al. 1994; Hellwig et al. 2000; Hib and Ponzio 1995; Hotchkiss et al. 2002; McIntyre et al. 2001; Miyata et al. 2002; Ostby et al. 1999; Shimamura et al. 2002). There is no particular environmental relevance to this mixture. The choice of compounds was motivated by our interest to explore the predictability of combination effects caused by similarly acting anti-androgens rather than to emulate “real world” mixtures.
Conclusive answers to the question of combination effect predictability require quantitative comparisons between predicted and experimentally observed mixture effects. Experimentally, we have approached this task in a step-wise fashion: a) Dose–response curves for all single-mixture components were recorded. b) These data were used for the calculation of additivity expectations for a mixture of specific composition using “fixed mixture ratio design” (Altenburger et al. 2000; Hewlett and Plackett 1959). c) The mixture experiments were conducted. d) The observed combination effects were compared with the predicted responses.
The choice of an appropriate model for the calculation of additivity expectations is essential for assessments of mixture effects because it is in relation to these additivity expectations that combination effects are judged in terms of synergisms or antagonisms. Several concepts for the computation of expected additive effects of anti-androgens have been used. The simple method of summing the individual effects of chemicals in the combination, termed “effect summation,” has been drawn on previously (Gray et al. 2001) but produces unreliable results with sigmoidal dose–response curves (Kortenkamp and Altenburger 1998). The concept of dose addition, also referred to as “concentration addition” (Loewe and Muischnek 1926), is usually employed for combinations of chemicals with similar modes of action. It has previously given additivity expectations well in agreement with experimental observations for inhibition of AR binding and AR-mediated responses in vitro and in vivo (Birkhoj et al. 2004; Nellemann et al. 2003). In light of these observations, we reasoned that dose addition would also produce valid additivity expectations for developmental effects after prolonged in utero and postnatal exposures.
Although a series of articles have been published describing the successful application of the fixed mixture ratio approach to in vitro systems (Altenburger et al. 2000; Backhaus et al. 2000; Payne et al. 2001; Rajapakse et al. 2002, 2004; Silva et al. 2002), there is comparatively little experience with in vivo assays. In the endocrine disruptor field, Brian et al. (2005) have recently demonstrated the usefulness of this method to the assessment of multicomponent mixtures of estrogenic chemicals in fish, but there are as yet no examples with mammalian assays in vivo. Thus, to make the assessment of developmental effects of mixtures of chemicals a viable proposition, a number of practical requirements had to be considered. Of particular importance were demands of minimal data variation and high reproducibility. When dealing with several mixture components and a large number of dose levels, the parallel testing of all agents and their mixtures is not a realistic option, especially not with in vivo experiments. Thus, reliance had to be made on historical data, in some cases recorded more than a year before commencement of the mixture experiments, and this placed great emphasis on the reproducibility of test outcomes. We considered that the high demands in terms of data variation were more likely to be met with developmental end points that lend themselves to straight-forward quantification. For these reasons, we selected changes in AGD and NR in male offspring of rats as main end points for our mixture experiments. Both these end points are sensitive to anti-androgen exposure.
The aim of our studies was to assess whether the joint effects of mixtures of AR antagonists can be predicted accurately over a large effect range on the basis of dose–response data of the individual components. We reasoned that if there are demonstrable consistent relationships between the potency of individual chemicals and the ways in which they act together, powerful tools for prospective risk assessment would become available. These tools could open the way to make productive use of existing single-chemical databases for the prediction of mixture effects. A second aim was to determine whether there would be joint effects when every mixture component was present at doses that individually do not produce observable responses.
Materials and Methods
Chemicals
The chemicals used were vinclozolin (CAS No. 50471-44-8, purity 99%, ChemService catalogue no. PS-1049; Bie & Berntsen, Herlev, Denmark), procymidone (CAS No. 32809-16-8, purity 99%, Chem-Service catalogue no. PS-2126; Bie & Berntsen), flutamide (CAS No. 13311-84-7, purity 99%, catalogue no. F9397; Sigma Aldrich, Brønby, Denmark), and corn oil used as vehicle (Bie & Berntsen).
Studies and dose levels
Before the mixture experiment, dose–response studies for each chemical were conducted. The dose ranges were chosen with the aim to cover the entire range of effects from no effect up to maximum effects, as determined by measurement of AGD and NR. At the same time, it was attempted to select doses that would not cause marked effects on body weights in the dams, and especially in the offspring, as this would complicate evaluation of the effects on AGD and NR. The dose levels selected for the dose–response studies were based on the reductions of AGD and increase of NR reported for vinclozolin (Gray et al. 1994, 1999; Hellwig et al. 2000; Hotchkiss et al. 2002; Shimamura et al. 2002), flutamide (Foster and McIntyre 2002; Hib and Ponzio 1995; Hotchkiss AK et al. 2002; McIntyre et al. 2001; Miyata et al. 2002), and procymidone (Ostby et al. 1999). As data on procymidone were relatively limited, a range-finding study was performed before the dose–response study. To gain information about variability of effects between studies, we ran selected doses of vinclozolin, flutamide, and procymidone in parallel with the mixture experiment. An overview of the studies including dose levels and number of animals is shown in Table 1. A similar study design was used for all studies (see below).
Table 1.
Studies, groups, doses, and number of time-mated animals per group.
| Study | Groups and doses | No. of animals per group |
|---|---|---|
| 1. Vinclozolin and flutamide, dose–response | Control: vehicle-dosed | 16 |
| 6 doses of vinclozolin: 5, 10, 20, 40, 80, or 160 mg/kg/day | 8 | |
| 6 doses of flutamide: 0.5, 1.0, 2.0, 4.0, 8.0, or 16 mg/kg/day | 8 | |
| 2. Procymidone, range-finding | Control: vehicle-dosed | 16 |
| 2 doses of procymidone: 25 or 200 mg/kg/day | 4 | |
| 3. Procymidone, dose–response | Control: vehicle-dosed | 16 |
| 6 doses of procymidone: 5, 10, 25, 50, 100, or 150 mg/kg/day | 8 | |
| 4. Mixture study of vinclozolin, flutamide, and procymidone | Control: vehicle-dosed | 16 |
| 5 doses of mixture: 7.87, 19.67, 39.33, 70.80, or 106.19 mg/kg/day | 16 | |
| 2 doses of vinclozolin: 24.5 or 95.9 mg/kg/day | 16 | |
| 2 doses of flutamide: 0.77 or 3.86 mg/kg/day | 8 | |
| 2 doses of procymidone: 14.1 or 61.8 mg/kg/day | 8 |
For the mixture study, a master mixture was prepared by combining doses of vinclozolin, flutamide, and procymidone that all induced a half-maximal degree of NR (six nipples) in male offspring. This approach was chosen to avoid one single chemical contributing disproportionately to the overall mixture effect. The resulting mixture ratio of vinclozolin, flutamide, and procymidone was 31:1:18 based on weight (Table 2), and the master mixture contained 22,026 mg vinclozolin, 696.6 mg flutamide, and 12,675 mg procymidon in 600 mL corn oil. It was diluted into five doses of the mixture (1:14, 1:5, 1:2, 6:4, and 9:1). These dose levels were chosen with the aim of covering the entire dose–response curve.
Table 2.
Statistical dose–effect descriptors for single and mixture exposures.
| Dose–response function
|
Effect doses (mg/kg/day)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Substance | Fraction in mixtureb | RMc | θ̂1 | θ̂ 2 | θ̂3 | θ̂max | Medium | Low | NOAELa (mg/kg/day) |
| Nipple retention | ED6d (95% CI) | ED1d (95% CI) | |||||||
| Vinclozolin | 0.622301 | BP-probit | −1.76 | 0.025 | 1.024 | 12 | 67.18 (55.78–77.08) | 5.73 (0.67–25.58) | < 5.0 |
| Flutamide | 0.019558 | Probit | −0.61 | 2.01 | — | 12 | 2.02 (1.71–2.40) | 0.41 (0.28–0.60) | < 0.5 |
| Procymidon | 0.358141 | BP-logit | −5.09 | 1.21 | 0.1 | 12 | 33.91 (24.81–45.74) | 7.51 (> 0.1–10.76) | 10.0 |
| Mixture | bw-Weibull | −19.66 | 16.83 | −0.8 | 13 | 20.78 (17.76–23.52) | 8.21 (6.67–10.42) | < 7.87 | |
| AGD index | ED50e (95% CI) | ED90e (95% CI) | |||||||
| Vinclozolin | 0.622301 | Logit | −6.80 | 3.59 | — | 1 | 78.65 (67.43–93.32) | 9.21 (11.57–29.08) | 5.0 |
| Flutamide | 0.019558 | Weibull | −1.38 | 1.85 | — | 1 | 3.54 (2.84–4.37) | 0.34 (0.19–0.57) | < 0.5 |
| Procymidon | 0.358141 | bw-Weibull | −6.31 | 2.087 | −0.20 | 1 | 69.38 (56.69–86.38) | 1.84 (4.12–22.47) | 10.0 |
| Mixture | Glogit II | −9.24 | 7.21 | 0.29 | 1 | 39.77 (32.51–49.48) | 4.68 (6.79–20.73) | 19.67 | |
NOAEL – no observed adverse effect level, marked as “<” when the lowest tested dose already produced a significant effect.
Ratio of the dose of each compound to total mixture dose.
RM – regression models as defined by Scholze et al. (2001); for more details see “Material and Methods”; θ̂1, θ̂3, θ̂3,– statistical estimates of model parameters, given for doses expressed as mg/kg/day (rounded values); θ̂max– upper model asymptote.
ED6, ED1 – effect doses for 6 and 1 nipples, calculated from the respective dose–response function.
ED50, ED90 – effect doses for 50% and 90% normalized AGD index, calculated from the respective dose–response function; 95% CI – 95% confidence intervals for mean effect doses given in mg/kg/day.
Animals and dosing
The animals were treated humanely and with regard for alleviation of suffering. The studies were performed under conditions approved by the Danish Agency for Protection of Experimental Animals and by the inhouse Animal Welfare Committee.
Time-mated nulliparous, young adult Wistar rats (HanTac:WH, Taconic Europe, Ejby, Denmark; body weight approximately 200 g) were supplied at day 3 of pregnancy. The day after mating was designated gestational day (GD) 1, and postnatal day (PND) 0 was the day of birth. On the day after arrival (GD4), the dams were distributed pseudorandomly into groups of 16 or 8 animals with similar body weight (bw) distributions. They were housed in pairs until GD21 and alone thereafter under standard conditions in semitrans-parent plastic cages (15 × 27 × 43 cm) with Aspen bedding (Tapvei, Gentofte, Denmark) situated in an animal room with controlled environmental conditions (12-hr light–dark cycles with light starting at 2100 hours, light intensity 500 lux, temperature 21 ± 2°C, humidity 50% ± 5%, ventilation eight air changes per hour). A complete rodent diet for growing animals ALTROMIN 1314 (soy- and alfalfa-free; ALTROMIN GmbH, Lage, Germany) and acidified tap water (to prevent microbial growth) were provided ad libitum.
Test chemicals and mixtures were administered by gavage from GD7 to the day before expected birth (GD21) and from PND1 until PND16. The dosing volume of 2 mL/kg bw was calculated on the basis of the body weight of the animal on the day of dosing. The dose levels and group sizes are shown in Table 1. Animals were inspected for general toxicity twice daily. The studies were performed using four blocks (with 1 week in between), and all dose groups were equally represented in the blocks.
Anogenital distance and nipple retention
In all studies, AGD and NR were recorded by the same technician who was blinded with respect to exposure groups. After birth all live pups in the litter were weighed, sexed, and AGD was measured using a stereomicroscope. The sex of several of the pups in the highest dose groups could not be determined based on the AGD, as the AGDs were similar to female values in all pups in some litters. In these cases, the sex of the pups was determined later by internal inspection of reproductive organs with the presence of testes defining a male. The highest AGD values obtained in the litter were used as the values for male pups. This approach was chosen because these values were most likely to represent the males, as males normally have longer AGDs than females. For dose–response analysis, AGD data were analyzed by the calculated AGD-index, namely, AGD divided by the cube root of body weight. The cube root was used because this converts a three-dimensional end point (weight) into a one-dimensional such as the AGD (Gray et al. 1999; Robert et al. 1999). This ratio assumes that the relationship between AGD and transformed body weight is directly proportional and linear. To assess the validity of this assumption, we explored using the transformed body weight as a co-variable in statistical analyses. However, we did not detect any relevant difference between these two approaches, and in the interest of keeping the model parameters low, we decided to base all statistical analyses on the AGD index.
The body weights of all pups were recorded on PND 12 ± 1, together with the number of nipples/areolas, defined as a dark focal area (with or without a nipple bud) located where nipples are normally present in female offspring. Females normally have 12 nipples, but may in a few cases show up to 14.
Data normalizations and dose–response analysis
Slight differences in absolute control values between studies were controlled for by standardizing the absolute AGD indices to relative values between one (no effect on male AGD index) and zero (complete feminization). The mean AGD indices from unexposed male and female pups were used to define the minimum and maximum responses, respectively. Regression analyses were based on normalized AGD indices. In contrast, absolute AGD indices were used for estimations of no observed adverse effect levels (NOAELs). Because NR is a quantal end point, data normalization was not necessary in this case.
Statistical dose–response regression analyses for both end points were carried out by applying a best-fit approach (Scholze et al. 2001). Various nonlinear regression models (logit, probit Weibull, generalized logit), which all describe monotonic sigmoidal dose-response relationships, were fitted independently to the same data set and the best-fitting model was selected on the basis of a statistical goodness-of-fit criterion, the information criterion of Schwarz (1978).
To control for litter effects on AGD, dose–response data for the normalized AGD indices were analyzed by a generalized nonlinear mixed-model approach (Vonesh and Chinchilli 1996), with the litter as a random effect modifier for individual AGD data.
To take account of uncertainties in the mean control estimates for AGD during the scaling of effects, we included an upper and lower asymptote in the regression models. However, neither for the individual compounds nor for the mixture were the resulting model parameters significantly different from 0 and 1, respectively. To avoid overparameterization, upper and lower asymptotes were therefore not estimated, but instead set a priori to 0 and 1 (see θmax = 1 in Table 2). Statistical analyses of AGD data were carried out using the SAS procedure PROC NLMIXED (SAS Institute Inc., Cary, NC, USA).
The number of nipples/areolas was assumed to follow a binomial distribution with a response range between 0 and θmax, with θmax being equal to the biologically possible maximal number of nipples in rats, either 12 or 13 (Table 2). The choice of θmax was decided on considering the global fit (information criterion of Schwarz). To account for litter effects on NR, correlation structures between number of nipples/areolas and litter were modeled by the generalized estimating equations method (Vonesh and Chinchilli 1996). All statistical analysis was performed using the SAS procedure PROC GENMOD (SAS Institute, Inc., Cary, NC, USA).
The analyses for single compounds were carried out using effect data pooled from the initial dose–response studies (Table 1, studies 1–3) and the repeat experiments run concurrently with the mixture study (Table 1, study 4). “Study run” was implemented as an additional model factor in data analysis. The effect doses (EDx) shown in Table 2 were selected for low and median response levels and were calculated from the functional inverse of the best-fitting model. Statistical uncertainties for the estimated effect doses were expressed as 95% confidence belts and approximately determined by applying the bootstrap method (Efron and Tibshirani 1993).
NOAELs were estimated using multiple contrast tests (Hothorn 2004). These tests were chosen as they are already implemented in the SAS procedures PROC NLMIXED and PROC GENMOD. Corresponding optimal contrasts were determined according to the best-fit regression model (Bretz et al. 2005).
Calculation of mixture–effect predictions using dose addition
To assess whether the joint effect of the three chemicals was dose additive, we predicted mixture effects based on information about the dose–effect relationships of all individual mixture components. These data were derived from the best-fit regression functions (Table 2) and used to calculate the expected responses of a mixture with defined mixture ratio over a large range of responses (“fixed mixture ratio design”) (Faust et al. 2001). The choice of doses was based on the concentration range described by the additivity prediction, which is defined for a multi-component mixture of three components as
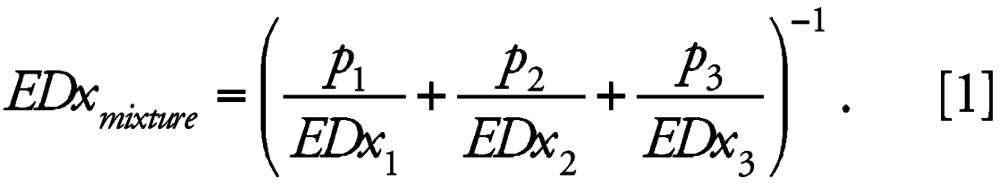 |
Here, EDx1, EDx2, and EDx3 are the effect doses of vinclozolin, flutamide, and procymidon that on their own produce the same quantitative effect x as the mixture, and p1, p2, and p3 are the relative proportions of the corresponding individual doses present in the total mixture dose (see Table 2, “Fraction in mixture”). The individual effect doses were derived from the dose–response functions for vinclozolin, flutamide, and procymidone by using their inverse functional form. Equation 1 allows calculation of any effect dose of a mixture under the hypothesis of dose additivity, provided the dose–response functions of all mixture components and the mixture ratio are known. Graphs of predicted mixture dose–response curves (Figure 1) were obtained by calculating numerous EDxmixture values, with x varying from 10 to 90% for the normalized AGD index and from 1 to 11 for nipples. The statistical uncertainty for the predicted mixture–effect doses EDxmixture was determined by using the bootstrap method (Efron and Tibshirani 1993) and expressed as 95% confidence intervals (CIs) for the predicted mean estimate. Differences between predicted and observed effect doses were deemed statistically significant when the 95% confidence belts of the prediction did not overlap with those of the experimentally observed mixture effects.
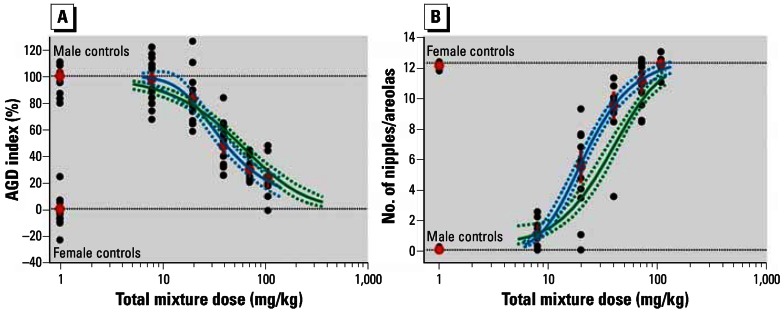
Figure 1.
Effects of mixed exposure to vinclozolin, flutamide, and procymidone on AGD (A) and NR (B). Results shown are group mean ± SE (red), litter means (black), mean dose–response curve ± 95% confidence belt based on regression analysis (blue) and mean predicted mixture effect ± 95% confidence belt (green). Dashed horizontal lines show male and female control values. See text for details.
Results
Pregnancy and litter data
No clinical signs of general toxicity were observed during the daily observations. The maternal body weight gain from GD7 to PND1 was significantly decreased (12.8 ± 14.6 g compared with 24.3 ± 9.4 g in the control group) in dams receiving the highest dose of vinclozolin (160 mg/kg/ day), but none of the other doses of vinclozolin provoked this effect. Pregnancy length, litter sizes, birth weight of male and female offspring, and sex ratios in the litters remained unaltered in all vinclozolin-dosed groups when compared to controls. None of the tested doses of flutamide induced reductions of maternal weight gain, or other signs of maternal toxicity. In the range-finding study with procymidone (Table 1, study 2), litter sizes were markedly decreased at the highest dose of 200 mg/kg/day. The dose–response study (Table 1, study 3) using 150 mg/kg/day as the highest dose did not show effects on pregnancy length, litter sizes, birth weights, or sex ratios in the litters. Maternal body weight gain from GD7 to PND1 was decreased in the dams exposed to 25 mg/kg/day procymidone and higher, but no clear dose–response relationship was apparent with these weight gain changes.
In dams exposed to the two highest doses of the mixture of vinclozolin, flutamide, and procymidone (Table 1, study 4), maternal body weight gain from GD7 to PND1 was decreased. Among the groups of pregnant rats that were dosed with the single agents in parallel with the mixture experiment (Table 1, study 4), those receiving the higher dose of procymidone (61.8 mg/kg/day) also had diminished weight gain. Decreased litter sizes were observed at the high dose of flutamide (3.86 mg/kg/day). As this was not found in the previous dose–response study at the similar dose level of 4 mg/kg/day, or at the higher doses of 8 and 16 mg/kg/day, this is considered a random finding unrelated to exposure to flutamide. None of the mixture doses caused significant effects on pregnancy length, litter sizes, birth weights, and sex ratios in the litters.
Effects of vinclozolin, flutamide, and procymidone on AGD and NR
All chemicals produced dose-dependent changes in AGD index and NR and the resulting dose–response curves were observed to be quite steep. The entire effect range from control levels to maximal responses could be covered by dose changes of only two orders of magnitude (Figure 2). While vinclozolin and procymidone were of similar potency, flutamide was effective at approximately 10-fold lower doses.
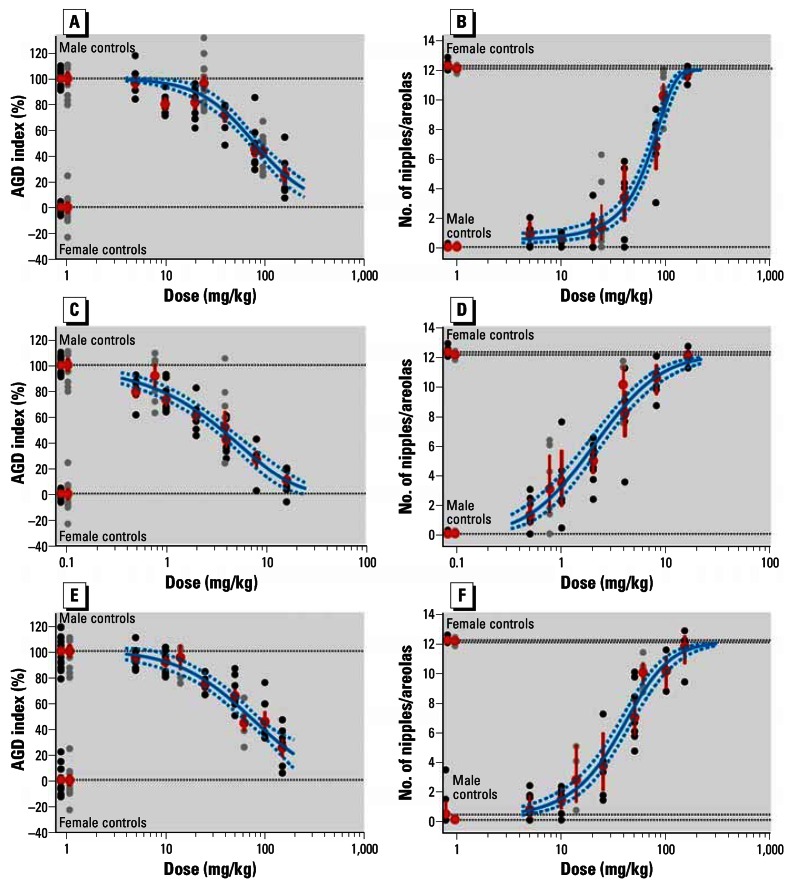
Figure 2.
Effects of vinclozolin (A,B), flutamide (C,D), and procymidone (E,F), given individually, on AGD (left) and NR (right). Results shown are group mean ± SE (red), litter means from the dose–response studies (black), litter means from the single doses within mixture study (grey), and the mean dose–response curve ± 95% confidence belt based on regression analysis (blue). Dashed horizontal lines show male and female control values. See text for details.
Compared with the AGD index, NR was generally the more sensitive end point. At the lowest tested doses of 5 and 0.5 mg/kg/day, respectively, vinclozolin and flutamide induced statistically significant changes in NR, whereas the respective AGD indices did not differ significantly from those of controls at these doses. NOAELs could therefore not be defined for vinclozolin and flutamide. For procymidone a NOAEL of 10 mg/kg/day was estimated.
To gain an impression of variability among studies, selected dose levels of all three chemicals were retested in parallel with the mixture experiment. The reproducibility of effects observed in the earlier studies (Table 1, studies 1–3) was generally good. At the lower doses, the animals in the repeat studies were slightly less responsive in terms of changes in AGD index, but the NR effects tended to be a little higher upon retesting (Figure 2; Table 3). Table 2 summarizes key parameters characterizing the dose–response relationships of each single substance. Generally, our data are in broad agreement with results published by others (Foster and McIntyre 2002; Gray et al. 1999; Hellwig et al. 2000; Hib and Ponzio 1995; Hotchkiss et al. 2002; McIntyre et al. 2001; Miyata et al. 2002; Ostby et al. 1999; Shimamura et al. 2002). However, because of the unprecedented level of detail in our dose–response analyses, more in-depth comparisons are not possible.
Table 3.
Statistical uncertainty of predicted and observed effect doses for the mixture of vinclozolin, flutamide, and procymidone.
| Effect doses for the mixture (mg/kg/day)
|
|||
|---|---|---|---|
| Effect level | Observeda [Mean (95% CI)] | Predicted by DApooledb [Mean (95% CI)] | Predicted by DAconfirmationc [Mean (95% CI)] |
| Relative AGD index (%) | |||
| 90 | 14.68 (6.79–20.73) | 8.29 (5.06–11.17) | 14.98 (4.22–19.80) |
| 50 | 39.77 (32.51–49.48) | 53.75 (48.22–60.34) | 52.58 (44.17–75.98) |
| 20 | 112.45 (77.79–184.51) | 143.19 (113.05–193.54) | 129.34 (100.67–204.49) |
| Number of nipples/areolas | |||
| 1 | 8.21 (6.67–10.42) | 7.43 (0.11–9.36) | 7.45 (3.06–11.73) |
| 6 | 20.78 (17.76–23.52) | 33.88 (29.23–38.41) | 27.98 (22.04–34.07) |
| 10 | 45.33 (38.91–55.86) | 73.66 (67.25–82.05) | 57.31 (50.17–67.58) |
Effect doses as calculated from the dose–response functions given in Table 2.
DA, dose addition; predicted effect doses are based on pooled data from studies 1–4 for vinclozolin, flutamide, and procymidone and were calculated from the respective dose–response functions given in Table 2.
Predicted effect doses are based on data for vinclozolin, flutamide, and procymidone from study 4 only, where two doses each of these chemicals were run in parallel with the mixture experiment. Predicted mixture–effect doses were estimated by linear regression (model estimates not shown).
Combination effects of vinclozolin, flutamide, and procymidone
The mixture of vinclozolin, flutamide, and procymidone produced dose-dependent changes in AGD index and NR (Figure 1). The NOAEL for changes in AGD index was 19.67 mg/kg/day, but the lowest tested mixture dose of 7.87 mg/kg/day induced statistically significant changes in NR (Table 2). Therefore, the overall mixture NOAEL is lower than 7.87 mg/kg/day.
The dose–response data for the single agents, pooled from all studies (Figure 2; Table 2), were used to compute predicted dose-additive combination effects covering the entire range of effects (Figure 1, green curves). For both end points, the anticipated combination effects fell within the range of the effects that were observed experimentally.
Numerical comparisons between predicted and observed AGD index (Table 3) revealed fairly good agreement. Despite the long period that had elapsed between the recording of the effects of the individual mixture components and the mixture experiment itself, the predicted effect doses in the median and high effect ranges differed by only a factor of 1.3 from those experimentally observed. Whether the anticipated combination effects were calculated using the data from the concurrent studies or using the pooled data sets including the historical data had little influence on the quality of the prediction. The joint effects of vinclozolin, flutamide, and procymidone on reductions of AGD in male rats were essentially dose additive.
In contrast, the deviations between prediction and observation were generally larger for NR than for AGD index, with observed NR responses exceeding the predicted mixture effects (Table 3; Figure 1). The effect doses predicted on the basis of the pooled single agent data were higher than the observed mixture–effect doses in the median- and high-effect range (6 and 10 retained nipples/areolas). This was not the case for the low-effect range (1 retained nipple/areola). Predictions based on the responses seen with single agents run in parallel with the mixture study (Table 1, study 4) produced lower effect doses in the median- and high-effect range, in better agreement with the observed results.
Mixture effects at low doses of individual mixture components
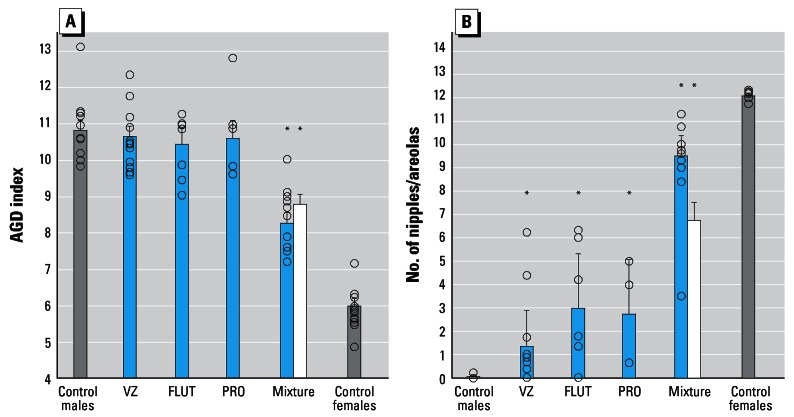
Because the doses of the single chemicals present in the mixture were quite low, we assessed whether there were significant combination effects when all components were present at doses that individually did not induce observable effects. At a dose of 39.37 mg/kg/day, the mixture induced a marked effect on the AGD index (around 50% reduction). This mixture contained 24.5 mg/kg/day vinclozolin, 0.77 mg/kg/day flutamide and 14.1 mg/kg/day procymidone, and individually these doses did not induce significant reductions in the AGD index (Figure 3). With NR as the end point, the single-chemical effects were small but statistically significant at these doses, whereas the combined exposure induced a marked effect.
Figure 3.
Mixture effects on AGD (A) and NR (B) at low doses of individual mixture components. Results shown are group mean ± 95% confidence belt for control males and females (gray), individual doses of 24.5 mg/kg vinclozolin (VZ), 0.77 mg/kg flutamide (FLUT), and 14.1 mg/kg prycymidone (PRO) (blue), the combined mixture dose of 39.37 mg/kg (blue), and the predicted mixture effect (white). Open circles represent litter means.
*p < 0.05 compared to control.
Discussion
Previous work with anti-androgens has focused mainly on events surrounding AR binding and activation and has shown that combinations of these chemicals are able to act together in an additive fashion (Birkhoj et al. 2004; Nellemann et al. 2003). These studies have prepared the ground for addressing the question as to whether there are also joint effects with responses further removed from receptor binding and activation, such as those related to male sexual differentiation. For the first time, we have addressed this question by using the fixed mixture ratio approach for studying combination effects on the disruption of male sexual development.
With reductions of AGD as the end point, the experimentally observed mixture effects were in good agreement with the dose additivity expectation calculated on the basis of the individual dose–response relationships for vinclozolin, flutamide, and procymidone.
Although the predicted mixture effects for NR also fell within the limits of the lowest experimentally recorded responses, the NR responses in the median and high end indicated that the mixture was more potent than predicted. The movement away from the anticipated combination effects can be partly attributed to the fact that the single agent responses seen concurrently with the mixture study were slightly higher than previously recorded, particularly in the high-effect range. When the mixture–effect prediction was based solely on the data from the concurrently run single agent studies, the differences between anticipated and observed effect doses for NR became smaller. This could indicate that the animals used for the mixture experiment showed subtle differences in their responses to the anti-androgens compared with the rats used for the earlier dose–response studies. The reason such differences should have become apparent only in terms of altered NR, but not in relation to AGD, may lie partly in the greater sensitivity of NR as an anti-androgenic end point. However, other as yet unrecognized factors may also have played a role. Seen in this light, we hesitate to interpret the joint effects of the mixture on NR as weakly synergistic, although the numerical discrepancies between observed and anticipated additive effects would support such a conclusion. Much larger studies would be required to resolve conclusively whether vinclozolin, flutamide, and procymidone exhibit a weak synergism with respect to NR. Nevertheless, in view of the complexity of the events leading to alterations in AGD and NR, and considering the experimental challenges in recording such effects reliably and reproducibly over a long period, we were surprised that the combined effects of the three anti-androgens could be predicted quite accurately. We therefore conclude that the dose addition approach provides an excellent basis for prediction of the joint effects of multicomponent mixtures of similarly acting anti-androgens.
Although the primary aim of our work was to assess the predictability of mixture effects of anti-androgens, the results of our study also allow assessments of the question as to whether there are joint effects when all mixture components are present at doses that individually do not induce detectable effects. This phenomenon, somewhat provocatively dubbed “something from ‘nothing’” (Silva et al. 2002), has been observed with multi-component mixtures of estrogenic agents in reporter-based assays (Rajapakse et al. 2002; Silva et al. 2002), the uterotrophic assay (Tinwell and Ashby 2004), and vitellogenin induction in fish (Brian et al. 2005). The basis of the something from nothing phenomenon derives from the theoretical assumptions that underlie the concept of dose addition. According to dose addition, every agent at any dose contributes, in proportion to its toxic unit, to the overall effect of a mixture. Because every mixture component can be replaced totally or in part by an equal fraction of an equi-effective dose of another, it does not matter whether the individual doses are also effective on their own. “Something from nothing” effects should occur even when individual toxicants are present at doses below effect thresholds, provided sufficiently large numbers of components sum up to a suitably high total-effect dose.
The results shown in Figure 3 support the idea that the “something from nothing” phenomenon also applies to alterations in the AGD of male rats exposed to anti-androgens during development. In this case a combination of 24.5 mg/kg/day vinclozolin, 0.77 mg/kg/day flutamide and 14.1 mg/kg/day procymidone induced half-maximal AGD alterations, but the effects induced by each chemical on its own did not reach statistical significance when compared with effects in untreated controls. However, whether the doses of the chemicals present in the mixture were indeed equivalent to nothing in the sense of zero effect levels is debatable. Regression analysis of the dose–response data for the three chemicals (Figure 2) showed that the effects associated with these doses were between 5 and 10% of a biologically possible maximal effect. In addition, in the earlier dose–response study, vinclozolin actually induced a significant effect on AGD at a lower dose than the 24.5 mg/kg/day present in the mixture. Generally, these results show that lack of statistical significance cannot be equated with an absence of biological effects.
Because of the apparently greater sensitivity of NR as an anti-androgenic end point, the something from nothing effect could not be evaluated with a combination of 24.5 mg/kg/day vinclozolin, 0.77 mg/kg/day flutamide and 14.1 mg/kg/day procymidone, because the individual doses induced NR that clearly reached statistical significance (Figure 3). The results, however, illustrate something not too dissimilar from the something from nothing phenomenon, which could be called “marked effects from small effects”: The mixture-induced NR approaching complete feminization of the males, whereas the individual doses caused only modest effects. In general, our findings do not contradict theoretical expectations and are consistent with the earlier observations made with mixtures of estrogenic chemicals (Brian et al. 2005; Rajapakse et al. 2002; Silva et al. 2002; Tinwell and Ashby 2004). The something from nothing phenomenon would most probably have been demonstrated also with NR as the end point, had lower doses been employed or had more mixture components been combined.
In conclusion, our results show that combinations of similarly acting anti-androgens are able to produce developmental effects in male offspring of rats. These effects can be predicted fairly accurately on the basis of information about the potency of the individual mixture components by using the dose addition concept. There are indications that anti-androgens act together to produce marked joint effects when combined at doses that individually produce small, statistically insignificant responses. The significance of these findings for human and environmental risk assessment cannot be overstated; doses of endocrine-active chemicals, which appear to exert only small effects when judged on their own, may induce marked responses when they act in concert with numerous, possibly unrecognized, similarly acting agents.
Footnotes
We thank D. Hansen and B. Herbst for their excellent technical assistance.
This article is part of the monograph “Endocrine Disruptors—Exposure Assessment, Novel End Points, and Low-Dose and Mixture Effects.”
This work is part of the European Union-supported EDEN-project “Endocrine Disrupters: Exploring Novel Endpoints, Exposure, Low Dose- and Mixture-Effects in Humans, Aquatic Wildlife and Laboratory Animal” (QLK4-CT-2002-00603), and financial support from the European Commission is gratefully acknowledged.
References
- Altenburger R, Bödeker W, Faust M, Grimme LH. Analysis of combination effects in aquatic toxicology. In: Corn M, editor. Handbook of Hazardous Materials. San Diego: Academic Press; 2000. pp. 15–27. [Google Scholar]
- Backhaus T, Altenburger R, Bödeker W, Faust M, Scholze M, Grimme LH. Predictability of the toxicity of a multiple mixture of dissimilarly acting chemicals to Vibrio fischeri. Environ Toxicol Chem. 2000;19:2348–2356. [Google Scholar]
- Birkhøj M, Nellemann C, Jarfelt K, Jacobsen H, Andersen HR, Dalgaard M, et al. The combined antiandrogenic effects of five commonly used pesticides. Toxicol Appl Pharmacol. 2004;201:10–20. doi: 10.1016/j.taap.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, et al. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz F, Pinheiro JC, Branson M. Combining multiple comparisons and modelling techniques in dose-response studies. Biometrics. 2005;61:738–748. doi: 10.1111/j.1541-0420.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, et al. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JW, Caudill SP, Silva MJ, Needham LL, Hilborn ED. Phthalate monoesters levels in the urine of young children. Bull Environ Contam Toxicol. 2002;68:309–314. doi: 10.1007/s001280255. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to the Bootstrap. London: Chapman & Hall; 1993. [Google Scholar]
- Faust M, Altenburger R, Backhaus T, Blanck H, Boedeker W, Gramatica P, et al. Predicting the joint algal toxicity of multi-component s-triazine mixtures at low-effect concentrations of individual toxicants. Aquat Toxicol. 2001;56:13–32. doi: 10.1016/s0166-445x(01)00187-4. [DOI] [PubMed] [Google Scholar]
- Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Foster PM, McIntyre BS. Endocrine active agents: implications of adverse and non-adverse changes. Toxicol Pathol. 2002;30:59–65. doi: 10.1080/01926230252824716. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol. 1994;129:46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JME, Kelce WR. Environmental anti-androgens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health. 1999;15:48–64. doi: 10.1177/074823379901500106. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Guillette LJ., Jr Contaminant-induced endocrine disruption in wildlife. Growth Horm IGF Res. 2000;10:S45–50. doi: 10.1016/s1096-6374(00)80009-x. [DOI] [PubMed] [Google Scholar]
- Hellwig J, van Ravenzwaay B, Mayer M, Gembardt C. Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul Toxicol Pharmacol. 2000;32:42–50. doi: 10.1006/rtph.2000.1400. [DOI] [PubMed] [Google Scholar]
- Hewlett PS, Plackett RL. A unified theory for quantal responses to mixtures of drugs: non-interactive action. Biometrics. 1959;15:591–610. [PubMed] [Google Scholar]
- Hib J, Ponzio R. The abnormal development of male sex organs in the rat using a pure antiandrogen and a 5 alpha-reductase inhibitor during gestation. Acta Physiol Pharmacol Ther Latinoam. 1995;45:27–33. [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenburgh JG, Gray LE., Jr Androgens and environmental antiandrogens affect reproductive development and play behavior in the Sprague-Dawley rat. Environ Health Perspect. 2002;110:435–439. doi: 10.1289/ehp.02110s3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn L. A robust statistical procedure for evaluating genotoxicity data. Environmetrics. 2004;15:635–641. [Google Scholar]
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Lambright CR, Gray LE, Jr, Roberts KP. Vinclozolin and p,p′-DDE alter androgen-dependent gene expression: in vivo confirmation of an androgen receptor-mediated mechanism. Toxicol Appl Pharmacol. 1997;142:192–200. doi: 10.1006/taap.1996.7966. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Altenburger R. Synergisms with mixtures of xenoestrogens: a reevaluation using the method of isoboles. SciTotal Environ. 1998;221:59–73. doi: 10.1016/s0048-9697(98)00261-7. [DOI] [PubMed] [Google Scholar]
- Loewe S, Muischnek H. Über Kombinationswirkungen I. Mitteilung: Hilfsmittel der Fragestellung. Naunyn-Schmiedebergs Arch Exp Pathol Pharmakol. 1926;114:313–326. [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Foster PMD. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol Sci. 2001;62:236–249. doi: 10.1093/toxsci/62.2.236. [DOI] [PubMed] [Google Scholar]
- Miyata K, Yabushita S, Sukata T, Sano M, Yoshino H, Nakanishi T, et al. Effects of perinatal exposure to flutamide on sex hormones and androgen-dependent organs in F1 male rats. Toxicol Sci. 2002;27:19–33. doi: 10.2131/jts.27.19. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol Sci. 2003;71:251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE., Jr The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol Ind Health. 1999;15:80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Payne J, Scholze M, Kortenkamp A. Mixtures of four organochlorines enhance human breast cancer cell proliferation. Environ Health Perspect. 2001;109:391–397. doi: 10.1289/ehp.01109391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Scholze M, Kortenkamp A. Deviation from additivity with estrogenic mixtures containing 4-nonylphenol and 4-tert-octylphenol detected in the E-SCREEN assay. Environ Sci Technol. 2004;38:6343–6352. doi: 10.1021/es049681e. [DOI] [PubMed] [Google Scholar]
- Robert H, Holson JF, Stump DG, Knapp JF, Reynolds VL. Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol. 1999;13:383–390. doi: 10.1016/s0890-6238(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Scholze M, Bödeker W, Faust M, Backhaus T, Altenburger R, Grimme LH. A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ Toxicol Chem. 2001;20:448–457. [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Shimamura M, Kodaira K, Kenichi H, Ishimoto Y, Tamura H, Iguchi T. Comparison of antiandrogenic activities of vinclozolin and camphorquinone in androgen receptor gene transcription assay in vitro and mouse in utero exposure assay in vivo. Toxicology. 2002;174:97–107. doi: 10.1016/s0300-483x(02)00044-6. [DOI] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”—eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Simard J, Luthy I, Guay J, Belanger A, Labrie F. Characteristics of interaction of the antiandrogen flutamide with the androgen receptor in various target tissues. Mol Cell Endocrinol. 1986;44:261–270. doi: 10.1016/0303-7207(86)90132-2. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Steward SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinwell H, Ashby J. Sensitivity of the immature rat uterotrophic assay to mixtures of estrogens. Environ Health Perspect. 2004;112:575–582. doi: 10.1289/ehp.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonesh E, Chinchilli VM. Linear and Nonlinear Models for the Analysis of Repeated Measurements. New York: Marcel Dekker; 1996. [Google Scholar]