Abstract
Background
Brominated flame retardants (BFRs)—polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCD)—belong to the group of relatively “new” environmental contaminants. The occurrence of these compounds in the Czech aquatic ecosystem was for the first time documented within the 3-year monitoring study initiated in 2001.
In 2002–2003 HBCD and the major PBDE congeners (28, 47, 49, 66, 85, 99, 100, 153, 154, and 183) were found in 136 freshwater fish samples collected from several sampling sites located at three Czech rivers (Vltava, Elbe, Tichá Orlice). Chub (Leuciscus cephalus), barbel (Barbus barbus), bream (Abramis brama), perch (Perca fluviatilis), and trout (Salmo trutta), representing the most common fish species, were examined by gas chromatography coupled with negative chemical ionization mass spectrometry.
Results
The presence of PBDE congeners and HBCD was detected in all analyzed samples (limits of detection for target analyts ranged from 0.015 to 0.1 ng/g lipid weight). Without exception the dominating congener was BDE-47. The most pronounced extent of fish contamination was found in the Vltava river at Klecany, downstream from the industrial agglomeration of Prague. As for fish species, the highest concentrations of PBDEs (sum of congeners) were measured in benthic species, represented by bream and barbel, up to 19.6 ng/g wet weight and 16.5 ng/g wet weight, respectively. The lowest accumulation occurred in predator fish (perch and trout). The highest levels of HBCD were detected in barbel from Srnojedy on the Elbe River (15.6 ng/g wet weight), downstream.
Keywords: aquatic ecosystem, brominated flame retardants, contamination, fish, hexabromocyclododecane, polybrominated diphenyl ethers
In the recent decade several monitoring studies have started to focus on not only “classic“ persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs), organo-chlorinated pesticides (OCPs), and/or polychlorinated dibenzodioxins/polychlorinated dibenzofurans (PCDDs/PCDFs) but also on other groups of halogenated xenobiotics such as brominated flame retardantds (BFRs). These chemicals are used mainly as additives in polymers to prevent them from catching fire (de Wit 2002; Hale et al. 2003). Generally, two types of BFRs can be distinguished: a) reactive compounds, for instance tetrabromobisphenol A (TBBPA), are incorporated by covalent binding into polymeric matrix and b) additive BFRs, represented by polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD), and/or polybrominated biphenyls (PBBs), are merely dissolved in polymeric material. Although TBBPA is used mainly in North America, the production of PBDEs prevails in Europe (de Wit 2002; Rahman et al. 2001).
Products based on penta-, octa-, and decabromodiphenyl ethers are currently the only commercially interesting PBDEs (de Boer et al. 2000a). They are used in the housing and electronic parts of television sets or personal computers and also in textiles [de Wit 2002; World Health Organization (WHO) 1994, 1997]. PentaBDEs are mainly applied in textiles and polyurethane foams, whereas decaBDEs are used in textile as well as in many other kinds of synthetic plastics such as poly-ester used for electronic circuit boards (de Wit 2002; Petterson and Karlsson 2001). HBCD is used in foams and expanded polystyrene and final products such as upholstered furniture, interior textiles, and packaging material (de Wit 2002).
The occurrence of BFRs in various environmental compartments is of great concern because of their high lipophility (log Kow is between 5 and 10) and/or high resistance to degradation processes (Haglund et al. 1997). Although the first reports on a presence of PBDEs in both abiotic and biotic matrices were published as early as the late 1970s [see Zweidinger et al. (1979) for early data on air particles] and the beginning of the 1980s [see Andersson and Blomkvist (1981) concerning fish from Swedish rivers], intensive investigation into their occurrence in the environment started a decade later. Various PBDE congeners were found in Dutch (de Boer et al. 2000b), Swedish (Haglund et al. 1997; Sellström et al. 1993, 1998), Japanese (Ohta et al. 2002; Watanabe et al. 1987), British (Allchin et al. 1999), and Canadian (Alaee et al. 1999) fish samples. Similarly, these brominated POPs were also detected in sediments, wastewaters, and air (Allchin et al. 1999; de Boer et al. 2003; Sellström et al. 1998). BFRs may be released into the environment from many sources such as a) landfills (additive types may leach out); b) emissions originated during incineration processes (brominated dioxins and furans may originate under these conditions) (de Wit 2002); and/or c) effluents from sewage treatment plants (STPs), and communal and industrial wastes.
Like other POPs, the BFR group is the subject of a wide range of toxicologic and ecotoxicologic studies. Some of these studies classified these chemicals as endocrine disruptors. These substances may exhibit adverse effects on the regulation of thyroid hormone and induce immunotoxicity. They also induce neurotoxicity, causing interferences at sensitive periods of brain development (de Boer et al. 2000a; Rahman et al. 2001).
The goal of the present study, which is the first conducted in the Czech Republic, was to recognize the extent of contamination by PBDEs and HBCD in aquatic ecosystems. Several fish species common to the Czech rivers Vltava, Elbe, and Tichá Orlice were used as biomonitors for this purpose.
Materials and Methods
Sample collection
Five fish species—chub, barbel, bream, perch, and trout—were caught at several sampling sites at three Czech rivers (Vltava, Elbe, and Tichá Orlice) during 2001–2003 and were delivered to the laboratory in edible form (fillets). Before storage at −18°C, fish samples were pooled according to fish weight and length (parameters correlating with age of the fish). Typically one pooled sample was prepared from three to five individual fish. Lipid content was determined in each composite sample using extraction by n-hexane:dichlormethane (1:1, vol/vol). The characteristics of examined samples are summarized in Tables 1 and 2; sampling sites are shown in Figure 1.
Table 1.
Characteristics of analyzed set of fish samples from rivers Vltava and Elbe; mean value and coefficient of variation [CV (%)].
| Vltava River
|
Elbe River
|
|||||
|---|---|---|---|---|---|---|
| Fish | Hluboká n/V | Podolí | Klecany | Kunětice | Srnojedy | Hřensko |
| Chub | ||||||
| No. of samples | 10 | 7 | 4 | 7 | 7 | 10 |
| Age (year) | 5 (25) | 4 (31) | 5 (36) | 6 (24) | 6 (20) | 7 (19) |
| Weight (g) | 666 (44) | 454 (68) | 691 (58) | 440 (90) | 382 (54) | 643 (62) |
| Lipids (%) | 2.7 (13) | 2.1 (20) | 3.2 (24) | 1.9 (21) | 2.1 (36) | 3.1 (16) |
| Bream | ||||||
| No. of samples | 3 | 5 | 2 | 5 | 5 | 5 |
| Age (years) | 4 (39) | 7 (25) | 7 (19) | 8 (18) | 8 (14) | 8 (11) |
| Weight (g) | 415 (65) | 908 (45) | 1,267 (14) | 763 (44) | 637 (34) | 733 (34) |
| Lipids (%) | 4.0 (23) | 3.6 (14) | 3.6 (13) | 2.1 (17) | 2.1 (16) | 3.0 (18) |
| Barbel | ||||||
| No. of samples | NA | 2 | 5 | 6 | 3 | 5 |
| Age (years) | NA | 6 (10) | 5 (32) | 8 (24) | 7 (46) | 10 (3) |
| Weight (g) | NA | 1,120 (37) | 1,136 (55) | 786 (72) | 618 (105) | 1,642 (20) |
| Lipids (%) | NA | 3.4 (12) | 4.8 (20) | 4.0 (20) | 3.6 (87) | 3.0 (11) |
| Perch | ||||||
| No. of samples | 6 | 5 | 2 | 3 | 5 | 6 |
| Age (years) | 3 (33) | 3 (24) | 3 (31) | 4 (29) | 6 (22) | 5 (11) |
| Weight (g) | 126 (114) | 149 (55) | 71 (40) | 76 (65) | 248 (78) | 144 (38) |
| Lipids (%) | 0.7 (31) | 1.0 (21) | 0.63 (18) | 0.8 (27) | 0.9 (21) | 0.8 (13) |
NA, not analyzed.
Table 2.
Characteristics of analyzed set of fish samples from the Tichá Orlice River; mean value and coefficient of variation [CV (%)].
| Tichá Orlice River
|
|||
|---|---|---|---|
| Fish | Lichkov | Králíky | Červená Voda |
| Trout | |||
| No. of samples | 6 | 6 | 6 |
| Age (years) | 3 (24) | 2 (27) | 3 (23) |
| Weight (g) | 143 (28) | 103 (49) | 148 (29) |
| Lipids (%) | 1.6 (10) | 1.5 (23) | 2.7 (9) |
Figure 1.
The sampling sites on the Czech rivers.
Characterization of fish used as biomonitors
The fish used as biomonitors in the present study represented a spectrum of freshwater species typically found in Czech aquatic ecosystems. Chub (Leuciscus cephalus) is a relatively abundant fish found in Czech rivers and is suitable as a bioindicator of contamination in aquatic ecosystems. This omnivorous species grows slowly, and its mean lipid content in muscle is about 2.5%. Barbel (Barbus barbus) and bream (Abramis brama) have high lipid content in muscle (up to 7%); for this reason they are able to bioaccumulate lipophilic organic pollutants to a large degree. These fish live in close contact with benthic sediments. Perch (Perca fluviatilis) and trout (Salmo trutta) belong to a group of predators, and their lipid content in fillet is relatively low (not more than 1%). The fish were treated humanely and with regard for alleviation of suffering.
Chemicals
Standard solutions containing PBDE congeners (concentration 50 μg/mL in nonane) are 2,4,4′-triBDE (BDE-28); 3,4,4′-BDE (BDE-37); 2,2′,4,4′-tetraBDE (BDE-47); 2,2′,4,5′-tetraBDE (BDE-49); 2,3′,4,4′-tetraBDE (BDE-66); 2,2′,3,4,4′-pentaBDE (BDE-85); 2,2′,4,4′,5-pentaBDE (BDE-99); 2,2′,4,4′,6-pentaBDE (BDE-100); 2,2′,4,4′,5,5′-hexaBDE (BDE-153); 2,2,4′,4,5′,6′-hexaBDE (BDE-154); 2,2′,4,4′,5′,6-BDE (BDE-183) and deca-BDE (BDE-209). All were obtained from Cambridge Isotope Laboratories (≥ 98% pure; CIL, Andover, MA, USA). Working standard solutions were prepared in isooctane and were stored in a refrigerator (5°C). The α-HBCD standard (50 μg/mL in toluene) with declared purity of 98% was supplied by CIL. A standard solution of PCB-112 (10 μg/mL in isooctane) was purchased from Gr. Ehrenstorfer GmBH (Augsburg, Germany).
The organic solvents (hexane, cyclohexane, isooctane) declared as organic trace analysis grade were supplied by Merck (Darmstadt, Germany). Ethylacetate and dichloromethane were obtained from Scharlau (Barcelona, Spain). Anhydrous sodium sulfate, supplied by Penta Chrudim (Chrudim, Czech Republic), was heated at 600°C for 5 hr, then stored in a desiccator before use. Styrene–divinylbenzene gel (Bio Beads S-X3, 200–400 mesh) was purchased from Biorad Laboratories (Hercules, CA, USA). Sulfuric acid (98%) was obtained from Merck.
Instruments
We used a homogenizer (model 2094; Foss Tecator, Hilleroed, Denmark) to homogenize fish samples. For the extraction step, we used the Soxhlet extractor Gerhart 173200 EV (Gerhart, Königswinter, Germany) with a cellulose extraction thimble (Whatman, Brentford, UK).
An automated gel permeation chromatography (GPC) system consisting of 350 MASTER pump, fraction collector, automatic regulator of loop XLI, microcomputer (software 731 PC via RS32C), dilutor 402 (GILSON, Villiers le Bel, France), and stainless steel column 500 × 8 mm inner diameter (i.d.) packed with Bio-Beads S-X3 (soft gel) was used for a cleanup of crude extracts.
A vacuum evaporator (Büchi Rotavapor R-114) and water bath (B-480) (Büchi, Postfach, Switzerland) were used for concentration of extracts.
We used an Agilent 6890 gas chromatograph equipped with electronic pressure control (EPC), split/splitless injector, and coupled to a mass selective detector Agilent 5973 (Agilent Technologies, CA, USA). Capillary columns used were the a) DB-XLB column (30 m × 0.25 mm i.d. × 0.1-μm film thickness), and b) BD-XLB (15 m × 0.25 mm i.d. × 0.1-μm film thickness (all from J&W Scientific, Folsom, CA, USA) were employed for separation of PBDEs and HBCD.
Extraction of fish samples
Thirty grams of homogenous fish muscle were mixed with 120 g anhydrous sodium sulfate to form a flowing powder. The sample was transferred into a cellulose extraction thimble and stored in a desiccator for 12 hr to complete the desiccation process, then inserted into a Soxhlet apparatus and extracted for 8 hr (seven cycles per hour) with 340 mL solvent mixture n-hexane:dichlormethane (1:1, vol/vol). The crude extract was carefully evaporated by rotary vacuum evaporator, and the residual solvents were removed by a gentle stream of nitrogen. The lipid content was determined gravimetrically using the analytical balance A&D MH–300 (A&D Co., Tokyo, Japan) with 0.001-g accuracy.
Cleanup
Extracted lipids were dissolved in 10 mL of cyclohexane:ethylacetate mixture (1:1, vol/vol) containing 5 ng/mL PCB-112 (this congener is not present in commercial mixtures or environmental samples); this was considered the recovery standard. Two milliliters of this solution (corresponding to 6 g wet sample) were loaded onto a GPC column. The mobile phase was cyclohexane: ethylacetate (1:1, vol/vol) with a flow rate of 0.6 mL/min. The fraction corresponding to the elution volume of 14–30 mL was collected. The eluate was evaporated by rotary vacuum evaporator, and the residual solvents were carefully eliminated by a gentle stream of nitrogen to dryness. The residue was then dissolved in 1 mL isooctane containing 1 ng/mL BDE-37 (3,4,4′-BDE) as syringe standard and treated with concentrated sulfuric acid (approximately three drops) to remove residual lipids. After 10 min of compete phase separation, an aliquot of the upper organic (isooctane) layer was taken and transferred into a glass vial for subsequent gas chromatography (GC )analysis.
GC analysis
We used a high-resolution GC (HRGC) unit resolution mass-selective detector (MSD) for analyses of the PBDEs and HBCD in purified extracts. The GC conditions (column 1) were as follows: column temperature program, from 105°C (hold 2 min) to 300°C at 20°C/min (hold 5 min); carrier gas, helium (Linde, Prague, Czech Republic) with a constant flow of 1.5 mL/min; injection temperature, 275°C; injection volume, 1 μL using pulsed splitless injection mode (splitless time, 2 min). An MSD with quadrupole analyzer was operated in a selective ion-monitoring (SIM) mode in a negative chemical ionization (NCI). Monitored ions (m/z) were 79, 81, 159, and 161 (PBDEs); 79, 81, 158, and 160 (HBCD); and 326 and 328 (PCB-112, internal standard). Ion m/z 79 was used to quantify all target analytes. Methane was used as a reagent gas (purity 99.995%, Linde) and was set at a pressure 2 × 10−4 mbar. Ion source temperature was 150°C and quadrupole temperature 105°C.
We monitored the presence of decaBDE using the same GC coupled with negative chemical ionization mass spectrometry (GC/MS-NCI) employing a shorter column (column 2). The temperature program was as follows: from 80°C (hold 2 min) to 280°C at 20°C/min and to 320°C at 5°C/min (hold 5 min); carrier gas, helium with constant flow 3 mL/min; injection temperature, 285°C; injection volume, 1 μL using pulsed splitless injection mode (splitless time, 2 min). Monitored ions were m/z 485 and 487; the ion at m/z 487 was used for quantification.
We identified the target analytes by comparing their retention times with retention times of standards and by MS confirmation. For quantification, a multilevel calibration curve was used (at least 5 points for each congener).
Quality assurance
For each extraction batch (consisting of five fish samples), one procedure blank was processed. The results were corrected for blank interferences and for recovery (PCB-112 was added as surrogate before GPC cleanup). Limit of detection (LOD) was calculated as quantity of analyte that generates a response 3 times greater than the noise level of the detection system. Limits of quantification (LOQs) were the minimum concentrations of analytes possible to quantify with acceptable accuracy and precision. Under these conditions, the LOQ was the lowest calibration level and corresponded for particular analyte to 3 × LOD.
LOD values (nanograms per gram lipid weight) for fish were BDE-28, 0.015; BDE-47, 0.015; BDE-49, 0.015; BDE-66, 0.015; BDE-85, 0.02; BDE-99, 0.015; BDE-100, 0.015; BDE-153, 0.02; BDE-154, 0.015; BDE-183, 0.015; BDE-209, 2.0; and HBCD, 0.1.
For recovery testing of the overall analytical method, chub muscle was spiked at level 2 ng/g (of each analyte) by 100 μL standard mixture (500 ng/mL) in acetone. Real-life samples were also analyzed to obtain background levels of analytes. PBDE recoveries ranged between 83–101%, and recovery of HBCD was 91%. Acceptable recovery rate was 80–110%. We also determined the precision of the analytical method (repeatability) by analyzing six spiked fish samples; repeatability ranged from 4 to 12% (expressed as relative SD). Recovery of BDE-209 was 78 ± 3% (n = 6). Chub muscle samples spiked at 20 ng/g wet weight and were analyzed within the validation process. The method we used is fully validated. The repeatability of our results is documented by our participation in certification study BROC (biological reference materials for organic contamination) (van Leeuwen et al. 2006).
Results and Discussion
As mentioned previously, fish is widely used as a biomonitor of bioavailable POPs that occur in aquatic environments. However, interpretation of obtained data is not simple. Both bioaccumulation and depuration processes may take place in aquatic biota simultaneously, and the ratio of their intensities may differ widely among the fish species. It should be noted that the concentration of POPs measured in their bodies is dependent on many factors such as age, sex, and/or feeding habits of particular resident species. In practice it is difficult to obtain homogenous sets of biomonitors from an entire river. Differences exist among sampling localities in terms of food availability, causes of variations of fat content, and hence varying accumulation potential in fish. Table 3 is a summary of fish characteristics and the results (based on wet weight) of target PBDEs and HBCD (sum of isomers) in collected samples. It should be noted that technical HBCD mixtures consist of three diastereomers—α, β, andγ—the last being the typically dominating component (up to 80%) of this primary polluting material. In other words, biotransformation of γ-HBCD may occur in biota, resulting in a changed contamination pattern, which may lead under certain circumstances (e.g., biomagnification) to α-HBCD becoming the dominant component in the diastereomers profile. One should be aware that under GC conditions (hot injection), thermal conversion of γ-HBCD yielding α-diastereomer also may occur. Therefore, in most studies using GC for quantification, α-diastereomer is used as the calibration standard representing HBCD groups. For determination of all individual HBCD diastereomers, LC/MS must be used (Morris et al. 2004).
Table 3.
Mean concentration and coefficient of variation [CV (%)] of PBDE congeners and HBCD in fish sample (ng/g wet weight), aggregated data.
| Fish, locality | Lipids (%) | BDE-28 | BDE-47 | BDE-49 | BDE-85 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | HBCD |
|---|---|---|---|---|---|---|---|---|---|---|
| Trout | ||||||||||
| Králíky | 2.7 (14) | 0.03 (35) | 1.05 (50) | 0.03 (21) | 0.07 (66) | 0.72 (53) | 0.21 (46) | 0.08 (27) | 0.07 (30) | ND |
| Lichkov | 1.6 (24) | ND | 0.31 (23) | 0.03 (41) | ND | 0.37 (34) | 0.08 (30) | 0.03 (64) | 0.04 (43) | ND |
| Červená Voda | 2.1 (14) | ND | 0.12 (57) | ND | ND | 0.07 (22) | 0.02 (33) | ND | 0.01 (48) | ND |
| Perch | ||||||||||
| Hluboká n/V | 0.7 (22) | ND | 0.25 (19) | 0.01 (62) | 0.01 (27) | 0.18 (41) | 0.08 (41) | 0.02 (45) | 0.02 (38) | ND |
| Podolí | 1.0 (27) | ND | 0.29 (49) | 0.02 (62) | 0.03 (21) | 0.31 (23) | 0.09 (42) | 0.02 (31) | 0.06 (47) | 0.42 (6) |
| Klecany | 0.6 (17) | ND | 0.90 (23) | ND | ND | 0.28 (8) | 0.16 (11) | 0.05 (6) | 0.09 (5) | 0.49 |
| Kunětice | 0.8 (13) | ND | 0.35 (39) | 0.02 (50) | ND | 0.31 (32) | 0.12 (28) | 0.02 (4) | 0.03 (42) | 0.91 (21) |
| Srnojedy | 0.9 (20) | ND | 1.82 (59) | 0.04 (44) | 0.03 (62) | 0.43 (75) | 0.38 (45) | 0.16 (61) | 0.17 (65) | 1.59 (21) |
| Hřensko | 0.7 (5) | ND | 0.32 (44) | ND | ND | 0.31 (30) | 0.11 (25) | 0.04 (40) | 0.04 (36) | 0.86 (21) |
| Chub | ||||||||||
| Hluboká n/V | 2.7 (44) | 0.06 (36) | 1.19 (69) | 0.05 (53) | 0.09 (49) | 0.08 (75) | 0.16 (53) | 0.14 (63) | 0.17 (60) | 1.57 (43) |
| Podolí | 2.1 (30) | ND | 0.45 (26) | 0.02 (53) | ND | ND | 0.12 (53) | 0.09 (83) | 0.08 (50) | 1.34 (16) |
| Klecany | 3.2 (28) | 0.58 (113) | 5.76 (61) | ND | 0.80 (53) | 0.47 (98) | 1.73 (103) | 0.82 (97) | 0.56 (50) | 3.68 (24) |
| Kunětice | 1.9 (43) | 0.08 (59) | 1.33 (90) | 0.03 (69) | 0.08 (97) | ND | 0.30 (74) | 0.13 (65) | 0.13 (82) | 4.08 (94) |
| Srnojedy | 2.1 (44) | 0.24 (55) | 3.53 (80) | 0.04 (86) | 0.10 (55) | ND | 0.66 (72) | 0.31 (65) | 0.42 (63) | 3.84 (35) |
| Hřensko | 3.0 (52) | 0.09 (51) | 1.58 (53) | ND | 0.07 (67) | 0.09 (18) | 0.25 (49) | 0.16 (49) | 0.25 (46) | 1.37 (70) |
| Bream | ||||||||||
| Hluboká n/V | 4.1 (74) | ND | 1.56 (90) | 0.06 (75) | ND | ND | 0.18 (89) | 0.11 (34) | 0.13 (91) | ND |
| Podolí | 3.5 (55) | ND | 1.83 (45) | ND | 0.05 (29) | ND | 0.23 (47) | 0.07 (89) | 0.19 (53) | 1.38 |
| Klecany | 4.6 (20) | 0.25 (25) | 13.08 (16) | 0.22 (51) | 0.79 (35) | 0.48 (28) | 2.80 (4) | 0.81 (19) | 1.21 (55) | 7.39 |
| Kunětice | 2.1 (16) | 0.05 (60) | 3.64 (95) | 0.14 | 0.19 (74) | ND | 0.54 (83) | 0.13 (69) | 0.22 (78) | 6.89 (54) |
| Srnojedy | 2.1 (44) | 0.05 (43) | 6.26 (34) | ND | 0.19 (57) | ND | 1.17 (45) | 0.47 (55) | 0.89 (60) | 2.27 (43) |
| Hřensko | 2.3 (27) | 0.04 (83) | 2.76 (36) | ND | 0.09 (27) | ND | 0.39 (38) | 0.07 (48) | 0.37 (37) | 0.79 (42) |
| Barbel | ||||||||||
| Podolí | 3.4 (19) | 0.30 (30) | 5.30 (26) | ND | 0.12 (43) | ND | 0.53 (8) | 0.27 (8) | 0.34 (11) | 2.31 (1) |
| Klecany | 4.8 (40) | 0.21 (42) | 12.54 (37) | ND | 0.17 (22) | 0.50 (40) | 1.32 (38) | 0.53 (48) | 1.17 (56) | 8.34 (29) |
| Kunětice | 4.0 (32) | 0.22 (103) | 9.16 (67) | 0.19 (40) | 0.14 (78) | ND | 1.20 (65) | 0.50 (70) | 0.70 (55) | 15.55 (41) |
| Srnojedy | 3.6 (19) | 0.06 | 5.47 (20) | ND | 0.02 | 0.14 (48) | 0.67 (11) | 0.45 (51) | 0.62 (49) | 3.62 (62) |
| Hřensko | 2.4 (45) | 0.09 (66) | 4.76 (56) | ND | 0.12 (58) | ND | 0.57 (45) | 0.26 (48) | 0.89 (46) | 1.81 (25) |
ND, not detected.
Table 3 shows that in all examined fish samples, the major PBDE congener was BDE-47. Levels of this 2,2′4,4′-tetrabromo-diphenyl ether were approximately one order of magnitude higher than those of other monitored congeners. This was not surprising, as BDE-47 was a main component in various kinds of technical mixtures (e.g., Bromkal 70-5DE) commonly used in industry. As in our samples, this congener typically makes the major contribution to the total PBDE content in the environmental samples collected in Europe. Pentabromodiphenyl ether congeners BDE-99 and BDE-100, and hexabromo-diphenylether congeners BDE-153 and BDE-154 were also present in most samples. The levels of these congeners exceeded the LOD in 70% of fish, and at least one of these PBDEs was detected. The presence of BDE-49 was confirmed in only about 10% of the samples; BDE-66 and BDE-183 were not detected in any sample. In accordance with similar studies (de Boer et al. 2003; Eljarat et al. 2004, 2005), no detectable decabromodiphenyl ether (congener 209) was present in any examined fish sample. According to several authors (Geyer et al. 1999; Sellström et al. 1998), the superlipophilic nature of this chemical (log Kow ~ 10) might be responsible for the lack of detection. BDE-209 can be strongly bound to sediments, hence its actual dissolved concentration in water is very low, and thus only a negligible fraction of this BFR is expected to be bioavailable to fish. As discussed by Eljarat et al. (2005), the low bioaccumulation potential of this chemical is due to its large molecular size that hinders a passage over membranes (Andersson and Blomkvist 1981). The alternative explanation of minimal occurrence of the deca-BDE congener in aquatic organisms is its rapid excretion and/or biotransformation after entering their body (Eljarrat et al. 2005; Eriksson et al. 2004). Regardless, BDE-209 is a relatively labile substance that easily decomposes under environmental conditions in yielding a large range of lower brominated congeners in addition to other bromine-containing products when illuminated by sunlight (Eriksson at al. 2004; Söderström et al. 2004).
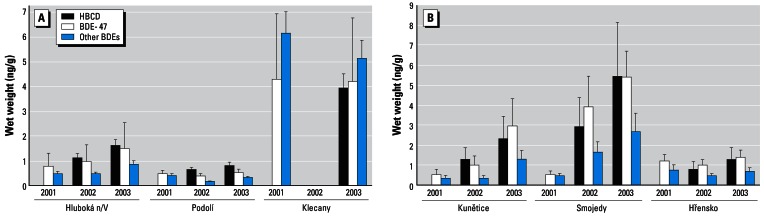
The presence of HBCD in fish collected in 2002 and 2003 was detected in more than 80% of tested samples, with the highest contamination found in fish species from Srnojedy (Elbe River). Figure 2A,B shows examples of concentrations of BDE-47, other ∑PBDEs (congeners 28, 49, 85, 99, 100, 153, and 154), and HBCD in chub from all sampling localities. The average concentration of BFRs in fish from Klecany at Vltava River (aggregated data obtained within the monitoring period) was almost 5 times that of samples obtained in Podolí upstream from Prague. The data obtained by analysis of chub from the Elbe River in 2001–2003 indicated that Srnojedy, located downstream from Pardubice (a large industrial area) was the most polluted locality along the Elbe River.
Figure 2.
Concentration of BDE-47, other ∑PBDEs (BDE-28, −49, −66, −99, −100, −153, and −154), and HBCD in chub samples from sampling sites (ng/g wet weight). Error bars represent mean ± SD. (A) River Vltava and (B) River Elbe.
In Hluboká n/V (Vltava River) and Hřensko (Elbe River), no significant variation among the monitoring years was found, whereas the concentrations of BFRs in the lower part of the Elbe River were largely variable (Figure 2B). In addition to being caused by increasing pollution, this trend might be attributed to differences of seasonal flows in this part of Elbe River for individual years. Extreme floods in 2002 were probably accompanied by the removal of contaminated sediments from monitored localities in the upper part of the Elbe River and the apparent drop of aquatic ecosystem pollution, hence reduction of fish exposure to bioaccumulatively chemicals. On the other hand, the total rainfall in the upper part of the Elbe River basin in 2003 were below the long-term average values (ELbe InformationsSystEm 2006) and the flow was low. Because of the existence of permanent emission sources (industrial wastes) of PBDEs and HBCD along the upper part of the Elbe River, the increases in pollution at monitoring localities occurred again in the following year.
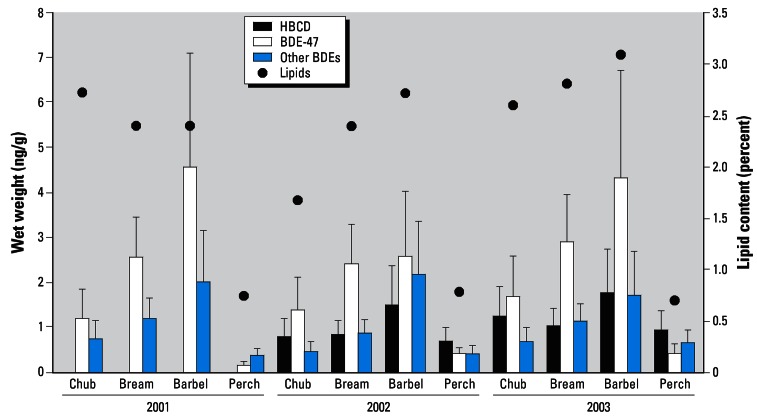
Figure 3 shows large differences in the extent of PBDE and HBCD bioaccumulation among the examined fish species. It is important to note that regardless of the monitoring year and sampling place, the concentrations of these BFRs (based on wet weight) were found in the following order: barbel > bream > chub > perch. de Boer and Brinkman (1994) and Geyer et al. (1999) have shown that the contribution of persistent organohalogen compound buildup in the food chain becomes relevant when log Kow values are > 5–6.5. Because log Kow values of major PBDE congeners monitored in our study range from 5.5 to 7, biomagnification of these chemicals (i.e., their transfer within the trophic levels of examined fish species that leads to a stepwise increase in contamination) might be expected. Similarly, higher levels of PBDEs in lipids were, for example, found in bass, a predator fish (collected in Penobscot River in central Maine), compared with those in white sucker, a benthic feeder, from the same locality (Anderson and MacRae 2006). Conversely, the lowest extent of contamination (regardless of the expression of BFR content on wet weight or lipids) was found in perch, which represent the highest trophic level among the fish examined in our study. Similar trends were also reported in other studies (Table 4). For example, Covaci et al. (2006) showed that the concentration of PBDEs in benthic bream from the Danube delta in Romania was about 50% higher than that in predator perch from the same locality. This controversy could be attributed to differing fat content in these two species, which is in addition to other factors related to differences in their feeding habits. Another reason for the lower concentration of PBDEs in predator species such as perch might be the fish’s higher growth rate (the ratio between weight and age), leading to “dilution” of accumulated pollutants because of a rapid increase of fish muscle tissue. Generally, slow-growing fish species are exposed to polluted environments for a longer time. Moreover, in the case of benthic species (represented in our study by barbel and bream), intensive contact with highly contaminated sediments is also considered a factor in the higher levels of their contamination (Covaci et al. 2006).
Figure 3.
Comparison of BDE-47, other ∑PBDEs (BDE-28, −49, −66, −99, −100, −153, and −154), and HBCD levels in tested fish species in Hřensko on the Elbe river (ng/g wet weight) from 2001 to 2003. Error bars represent mean ± SD.
Table 4.
Comparison of levels of PBDEs in freshwater fish samples from some similar studies (ng/g wet weight) with results obtained in this study.
| Fish | Area | BDE-47 | BDE-99 | BDE-100 | BDE-153 | ∑PBDEs | HBCD | Reference |
|---|---|---|---|---|---|---|---|---|
| Barbel | Cinca River, Spain, upstream Monzón | 0.8 | NA | n.q. | 0.3 | ND | Eljarrat et al. 2004 | |
| Barbel | Cinca River, Spain, downstream Monzón | 22.1 | NA | 2.1 | 125.5 | 529.7 | Eljarrat et al. 2004 | |
| Barbel | River Ebro, Spaina | 0.63 | NA | Lacorte et al. 2006 | ||||
| Barbel | River Cinca, Spaina | 113 | NA | Lacorte et al. 2006 | ||||
| Bleak fish | River Cinca Spain, upstream Monzón | 5.4 | NA | NA | 0.6 | ND | Eljarrat et al. 2005 | |
| Bleak fish | River Cinca Spain, downstream Monzón | 20.0 | NA | NA | 228 | 1,501 | Eljarrat et al. 2005 | |
| Bream | River Vltava – Klecany, Czech Republic | 13.1 | 0.5 | 2.8 | 0.8 | 7.4 | This study | |
| Bream | River Elbe – Srnojedy, Czech Republic | 6.3 | ND | 1.2 | 0.5 | 1.4 | This study | |
| Bream | River Viskan, Sweden | 500 | 2.4 | 24 | NA | Sellström et al. 1993 | ||
| Bream | River Rhine, the Netherlandsa | 16 | 0.1 | ND | 0.9 | NA | de Boer et al. 2003 | |
| Bream | River Danube Delta, Romania | 0.04 | NA | Covaci et al. 2006 | ||||
| Barp | Zuun, Belgium | 0.45 | 0.62 | NA | Covaci et al. 2005 | |||
| Barp | Canal Willebroek, Belgium | 2.9 | 3.8 | NA | Covaci et al. 2005 | |||
| Perch | River Danube Delta, Romania | 0.03 | NA | Covaci et al. 2006 | ||||
| Bass | Penobscot River, USAb | 6,490 | 5,630 | 1,790 | 544 | NA | Anderson and MacRae 2006 | |
| Pike | River Viskan, Sweden | 2.5 | < 0.3 | 0.5 | 39.2 | Sellström et al. 1998 | ||
| Pike | River Viskan, Swedenb | 2,000 | 78 | 170 | NA | Sellström et al. 1993 | ||
| Pike | Lake Bolmen, Sweden | 0.3 | 0.06 | 0.08 | 0.02 | NA | Kierkegaard et al. 2004 | |
| Pike | River Danube Delta, Romania | 0.02 | NA | Covaci et al. 2006 | ||||
| Trout | River Tichá Orlice – Králíky, Czech Republic | 1.1 | 0.7 | 0.2 | 0.1 | ND | This study | |
| Trout | Lake Michigan, USA | 23 | 7.9 | 4.8 | 1.5 | NA | Asplund et al. 1999 | |
| Trout | Lake Ontario, Canada | 58 | 14 | 5.7 | 4.9 | NA | Luross et al. 2002 | |
| Trout | Lake Erie, Canada | 16 | 2 | 2.5 | 0.9 | NA | Luross et al. 2002 | |
| Trout | Dalsland Canal, Swedenb | 232 | 227 | 65 | NA | Sellström et al. 1993 | ||
| Trout | Lochnagar Lake, Scotlandb | 0.3 | 0.6 | 0.07 | 0.1 | NA | Vives et al. 2004 | |
| Yellow eel | River Meuse, Eijsden, the Netherlandsb | NA | NA | NA | 32 | Morris et al. 2004 | ||
| White sucker | Penobscot River, USAb | 4,700 | 980 | 910 | 79 | NA | Anderson and MacRae 2006 |
Abbreviations: NA, not analyzed; ND, not detected
ng/g dry weight.
ng/g lipid weight
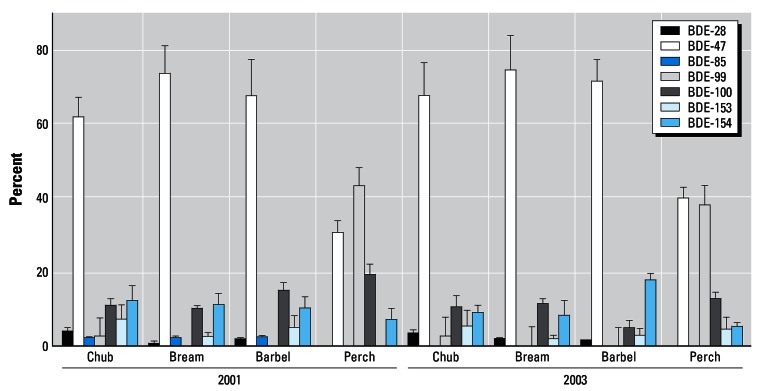
Substantial differences were observed between the contamination pattern of perch and other fish species. Figure 4 shows aggregated data obtained for four experimental biomonitors collected in Hřensko (Elbe River) in two monitoring years, 2001 (before floods) and 2003 (after floods, low rainfalls). As illustrated in the figure, the spectrum of PBDEs (regardless of their total concentrations, see Table 3) was almost identical to that found in omnivorous and benthic fish with distinctly dominating BDE-47 (40–75% of the total PBDE content). In perch the content of BDE-99 was equal to or even higher than that of BDE-47. Their contribution to the total PBDEs ranged between 30 and 40% and 25 and 45%, respectively. In most studies (Covaci et al. 2005; Luross et al. 2002; Sellström et al. 1998; de Boer et al. 2003), the dominanting congeners were also BDE-47, BDE-99, and BDE-100. On the other hand, in Spanish studies by Eljarat et al. (2004 and 2005), hexa-BDEs (BDE-153 and BDE-154) as well as hepta-BDE (183) were the most abundant BFRs occurring in fish (barbel and bream) samples. Probably less common PBDE technical mixtures were released into the aquatic environment.
Figure 4.
Pattern of PBDE congeners in various fish species in Elbe-Hřensko. Error bars represent mean ± SD.
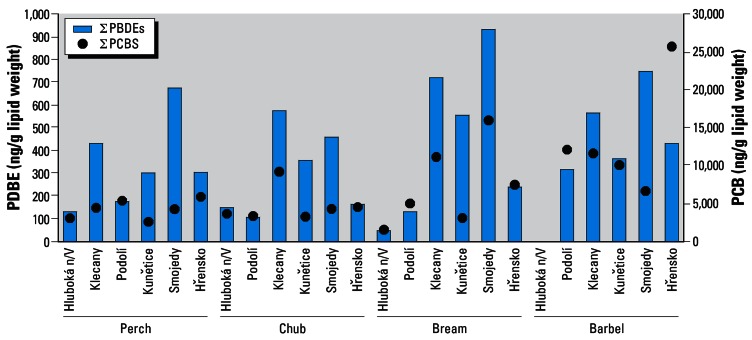
In Figure 5 the mean values of PBDE content (aggregated data) are compared with PCB levels (∑PCB, PCB-28, −52, −101, −118, −138, −153, and −180) determined in the same fish samples in a parallel study concerned with chlorine-containing POPs (Pulkrabová J, Suchan P, Kocourek V, Hajšlová J, Pudil F. Unpublished data 2006). Typically, the content of PCBs in biomonitors was higher by one order of magnitude and generally did not correlate with the extent of contamination in by PBDEs in particular localities. Differences in pollution sources were documented by large variations in the PCB/PBDE ratios calculated for individual fish species. Barbel showed the tendency of fish species to accumulate more PCBs than PBDEs (in Klecany, this phenomenon was not pronounced, with the PCB/PBDE ratio of 59). The correlation coefficient characterizing the relationship between the 10 ∑PBDE congeners and the 7 indicator ∑PCB congeners in all examined fish species was 0.39 (p < 0.05). It is reasonable to believe that such low correlation clearly indicates the independence of PBDEs and organochlorine compounds as sources of pollution.
Figure 5.
Comparison of PBDE and PCB content in fish collected in six sampling localities (aggregated data).
Conclusions
As mentioned previously, this is the first report on the occurrence of PBDEs and HBCD in freshwater fish in Czech rivers. The results of the present study are summarized as follows:
Fish is a suitable species for use as a bioindicator for monitoring BFRs in aquatic ecosystem; the greatest accumulation was measured in fatty benthic species represented by barbel and bream. Conversely, the potential of perch (predator fish) for bioaccumulation of these chemicals was lower.
Contamination patterns and their extent in fish collected in the Elbe and Vltava rivers are comparable with those reported in other European studies conducted in rivers in industrial areas. Technical mixtures based on penta- congeners were probably the source of pollution. When the entire data set generated in our present study was compared with data sets in similar studies conducted abroad, no extremely contaminated locality was found in Czech rivers that we monitored; the extent of pollution was similar to that in other industrial regions, for example, in Canada, Sweden, and Spain.
The levels of PBDEs were about 10–30 times lower than PCB levels determined in the same fish samples. In barbel from Hřensko (Elbe River), the level of PBDEs was 60 times lower compared with that of PCBs. The concentrations of HBCD in fish were of the same order of magnitude as those of the most abundant PBDE-47.
Footnotes
This article is part of the monograph “Endocrine Disruptors—Exposure Assessment, Novel End Points, and Low-Dose and Mixture Effects.”
Funding was provided by European Union project QLRT-2001–00596 FIRE (flame retardants interated risk assessment for endocrine disruption) and a project of the Ministry of Education, Youth and Sports of the Czech Republic (MSM 6046137505).
This financial support is gratefully acknowledged.
References
- Alaee M, Luross J, Sergeant DB, Muir DCG, Whittle DM, Solomon K. Distribution of polybrominated diphenyl ethers in the Canadian environment. Organohalogen Compounds. 1999;40:347–350. [Google Scholar]
- Allchin CR, Law RJ, Morris S. Polybrominated diphenylethers in sediments and biota downstream of potential sources in the UK. Environ Pollut. 1999;105:197–207. [Google Scholar]
- Anderson T, MacRae JD. Polybrominated diphenyl ethers in fish and wastewater samples from an area of the Penobscot River in Central Maine. Chemosphere. 2006;62:1153–160. doi: 10.1016/j.chemosphere.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Andersson Ö, Blomkvist G. Polybrominated aromatic pollutants found in fish in Sweden. Chemosphere. 1981;10:1051–1060. [Google Scholar]
- Asplund L, Hornung M, Peterson RE, Turesson K, Bergman A. Levels of polybrominated diphenyl ethers (PBDEs) in fish from the Great Lakes and Baltic Sea. Organohalogen Compounds. 1999;40:351–354. [Google Scholar]
- Covaci A, Bervoets L, Hoff P, Voorspoel S, Voets J, Van Campenhout K, et al. Polybrominated diphenyl ethers (PBDEs) in freshwater mussels and fish from Flanders, Belgium. J Environ Monit. 2005;7:132–136. doi: 10.1039/b413574a. [DOI] [PubMed] [Google Scholar]
- Covaci A, Gheorghe A, Hulea O, Schepens P. Levels and distribution of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in sediments and biota from the Danube Delta, Romania. Environ Pollut. 2006;140:136–149. doi: 10.1016/j.envpol.2005.06.008. [DOI] [PubMed] [Google Scholar]
- de Boer J, Brinkman UAT. The use of fish as biomonitor for the determination of contamination of the aquatic environment by persistent organochlorine compounds. Trac Trend Anal Chem. 1994;13:397–404. [Google Scholar]
- de Boer J, de Boer K, Boom JP. Polybrominated biphenyls and diphenyl ethers. In: Paassivirta J, editor. The Handbook of Environmental Chemistry. Vol 3. Part K: New Types of Persistent Halogenated Compounds. Berlin: Springer; 2000a. pp. 62–95. [Google Scholar]
- de Boer J, van der Horst A, Wester PG. PBDEs and PBBs in suspended particulate matter, sediments, sewage treatment plant in- and effluents and biota from the Netherlands. Organohalogen Compounds. 2000b;47:85–88. doi: 10.1016/s0269-7491(02)00280-4. [DOI] [PubMed] [Google Scholar]
- de Boer J, Wester PG, van der Horst A, Leonards PE. Polybrominated diphenyl ethers in influents, suspended particulate matter, sediments, sewage treatment plant and effluents and biota from the Netherlands. Environ Pollut. 2003;122:63–74. doi: 10.1016/s0269-7491(02)00280-4. [DOI] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Eljarrat E, de la Cal A, Raldua D, Duran C, Barcelo D. Occurrence and bioavaiability of polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from the Cinca river, a tributary of the Ebro river (Spain) Environ Sci Technol. 2004;38:2603–2608. doi: 10.1021/es0301424. [DOI] [PubMed] [Google Scholar]
- Eljarrat E, de la Cal A, Raldua D, Duran C, Barcelo D. Brominated flame retardants in Alburnus alburnus from Cinca River Basin (Spain) Environ Pollut. 2005;133:501–508. doi: 10.1016/j.envpol.2004.06.017. [DOI] [PubMed] [Google Scholar]
- ELbe InformationsSystEm. Home Page. 2006. [accessed 15 January 2006]. Available: http://elise.bafg.de/servlet/is/7211/
- Eriksson J, Green N, Marsh G, Bergman A. Photochemical decomposition of 15 polybrominated diphenyl ether congeners in methanol/water. Environ Sci Technol. 2004;38:3119–3125. doi: 10.1021/es049830t. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Rimkus GG, Scheunert I, Kaune A, Kettrup A, Zeeman M, et al. Bioaccumulation and occurrence of endrocrine-disrupting chemicals (ECDs), persistent organic pollutants (POPs), and other organic compounds in fish and other organism including humans. In: Beek B, editor. The Handbook of Environmental Chemistry. Vol. 2. Berlin: Springer; 1999. pp. 1–166. [Google Scholar]
- Haglund PL, Zook DR, Buser HR, Hu J. Identification and quantification of polybrominated diphenyl ethers and methoxy-polybrominated diphenyl ethers in Baltic biota. Environ Sci Technol. 1997;31:3281–3287. [Google Scholar]
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int. 2003;29:771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Kierkegaard A, Bignert A, Sellström U, Olsson M, Asplund L, Jansson B, et al. Polybrominated diphenyl ethers (PBDEs) and their methoxylated derivates in pike from Swedish waters with emphasis on temporal trends, 1967–2000. Environ Pollut. 2004;130:187–198. doi: 10.1016/j.envpol.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Lacorte S, Raldúa D, Martínez E, Navarro A, Diez S, Bayona JM, et al. Pilot survey of a broad range of priority pollutants in sediment and fish from the Ebro river basin (NE Spain) Environ Pollut. 2006;140:471–482. doi: 10.1016/j.envpol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Luross JM, Alaee M, Sergeant DB, Cannon CM, Whittle DM, Solomon KR, et al. Spatial distribution of polybrominated diphenyl ethers and polybrominated biphenyls in lake trout from the Laurentian Great Lakes. Chemosphere. 2002;46:665–672. doi: 10.1016/s0045-6535(01)00230-2. [DOI] [PubMed] [Google Scholar]
- Morris S, Allchin CR, Zegers BN, Haftka JJH, Boon JP, Belpaire C, et al. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food web. Environ Sci Technol. 2004;38:5497–5504. doi: 10.1021/es049640i. [DOI] [PubMed] [Google Scholar]
- Ohta S, Ishizuka D, Nishimura H, Nakao T, Aozasa O, Shimidzu Y, et al. Comparison of polybrominated diphenyl ethers in fish, vegetables and meats and levels in human milk of nursing woman in Japan. Chemosphere. 2002;46:689–696. doi: 10.1016/s0045-6535(01)00233-8. [DOI] [PubMed] [Google Scholar]
- Petterson A, Karlsson H. Analysis and Toxicology of Brominated Flame Retardants with Emphasis on PBDEs PhD Dissertation] Sweden: Örebro University; 2001. [Google Scholar]
- Rahman F, Langford KH, Scrimshaw MD, Lester JN. Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ. 2001;275:1–17. doi: 10.1016/s0048-9697(01)00852-x. [DOI] [PubMed] [Google Scholar]
- Sellström U, Jansson B, Kierkegaard A, de Witt C, Odsjö T, Olsson M. Polybrominated diphenyl ethers (PBDE) in biological samples from the Swedish environment. Chemosphere. 1993;26:1703–1718. [Google Scholar]
- Sellström U, Kierkegaard A, de Wit C, Jansson B. Polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from a Swedish river. Environ Toxicol Chem. 1998;17:1065–1072. [Google Scholar]
- Söderström G, Sellström U, de Wit CA, Tysklind M. Photolytic debromination of decabromodiphenyl ether (BDE 209) Environ Sci Technol. 2004;38:127–132. doi: 10.1021/es034682c. [DOI] [PubMed] [Google Scholar]
- van Leeuwen SPJ, Van Cleuvenbergen R, Abalos M, Pasini A-L, Eriksson U, Cleemann M, et al. New certified and candidate certified reference materials for the analysis of PCBs, PCDD/Fs, OCPs and BFRs in the environment and food. Trac Trend Anal Chem. 2006;25:397–409. [Google Scholar]
- Vives I, Grimalt JO, Lacorte S, Guillamón M, Barceló D. Polybrominated diphenyl ether flame retardants in fish from lakes in European high mountains and Greenland. Environ Sci Technol. 2004;38:2338–2344. doi: 10.1021/es030107x. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Kashimoto T, Tatsukawa R. Polybrominated diphenyl ethers in marine fish, shellfish and river and marine sediments in Japan. Chemosphere. 1987;16:2389–2396. [Google Scholar]
- WHO. Environmental Health Criteria 162. Geneva: World Health Organization; 1994. Brominated Diphenyl Ethers. [Google Scholar]
- WHO. Environmental Health Criteria 192: Flame Retardants–General Introduction. Geneva: World Health Organization; 1997. [Google Scholar]
- Zweidinger RA, Cooper SD, Erickson MD, Michael LC, Pellizzari ED. Sampling and analysis for semivolatile brominated organics in ambient air. ACS Symp Ser. 1979;94:217–231. [Google Scholar]