Abstract
Atypical diurnal patterns of hypothalamic-pituitary-adrenal (HPA) axis activity have been observed samples of individuals following early life adversity. A characteristic pattern arising from disrupted caregiving is a low early morning cortisol level that changes little from morning to evening. Less well understood is the plasticity of the HPA axis in response to subsequent supportive caregiving environments. Monthly early morning and evening cortisol levels were assessed over 12 months in a sample of 3- to 6-year-old foster children enrolled in a randomized trial of a family-based therapeutic intervention (N = 117; intervention condition n = 57; regular foster care condition n = 60), and a community comparison group of same-aged, nonmaltreated children from low-income families (n = 60). Latent growth analyses revealed stable and typical diurnal (morning-to-evening) cortisol activity among community comparison children. Foster children in the intervention condition exhibited cortisol activity that became comparable to the comparison group children over the course of the study. In contrast, children in regular foster care condition exhibited increasingly flattened morning-to-evening cortisol activity over the course of the study. In sum, improvements in caregiving following early adversity appear to have the potential to reverse or prevent disruptions in HPA axis functioning.
Keywords: foster care, maltreatment, neglect, preschool, intervention, HPA axis, cortisol
Introduction
Alterations in hypothalamic-adrenal-pituitary (HPA) axis activity (as measured by salivary cortisol activity) have been observed in children following a range of adverse early life experiences. For example, atypical patterns of HPA axis activity have been reported in children who experienced the early loss of a caregiver (Meinlschmidt and Heim, 2005), in children who were maltreated in their family of origin (De Bellis et al., 1999; Shea et al., 2004), and in children who were subjected to severe neglect as a result of institutional rearing in developing countries (Carlson and Earls, 1997). The characteristic pattern of alterations noted for these children is a flattening of diurnal (morning-to-evening) cortisol activity, owing largely to low early morning cortisol levels (Gunnar and Vazqeuz, 2001). Similar patterns associated with chronic stress have been noted in adults (Heim et al., 2000), and it is presumed that these flattened or hypoactive patterns reflect downregulation of the HPA axis following periods of heightened activity early in life (Friese et al., 2005).
Animal models of early adverse experiences have provided evidence that manipulations affecting the development and functioning of the HPA axis operate, at least in part, via their impact on maternal care (Smotherman and Bell, 1980). In rodents, manipulations impacting the dam’s licking and grooming of her pup have shown long-term effects on HPA axis reactivity and regulation; similar results were found when dams characteristically provided high or low levels of licking and grooming (Meaney and Szyf, 2005). In nonhuman primates, manipulations that disrupt the mother–infant relations—including maternal deprivation and repeated separation—produce offspring that are behaviorally vulnerable to stressors (Suomi, 1997; Levine, 2005). In several recent studies of rhesus infants reared under conditions of maternal deprivation or repeated, unpredictable maternal separations, researchers have reported atypical diurnal cortisol activity resulting particularly from low early morning cortisol levels (Boyce et al., 1995; Sánchez et al., 2005). These animal data suggest that circumstances that limit a caregiver’s ability to serve as an external source of affective and physiological regulation during critical points in development impact the development of the HPA axis. Moreover, these studies have implications for understanding how disturbances in the caregiving system during early human development might impact the developing child (Heim and Nemeroff, 2001; Gunnar et al., 2006).
The association between disruptions in maternal care and altered HPA axis activity might be particularly relevant for foster children. Several recent studies have shown that, like other maltreated children and children reared in neglectful institutional care, foster children show atypical diurnal cortisol activity. These patterns often involve very low early morning cortisol levels with little change from morning to evening. For example, Dozier et al. (2006) reported smaller morning-to-evening cortisol changes in a sample of 55 foster children (placed in care in infancy) relative to a non–foster care sample. Similarly, Fisher et al. (2006) documented that a significantly greater proportion of foster preschoolers entering new placements had very low early morning cortisol levels compared to a non–foster care sample of comparable socioeconomic class. Consistent with the hypothesis that disruptions in caregiving might mediate these effects, there is emerging evidence (Bruce et al., 2007) that foster children who exhibit extremely low early morning cortisol levels are likely to have experienced more severe neglect and more foster placement disruptions during infancy and toddlerhood compared to those with more typical early morning cortisol levels.
Inasmuch as there are over 500,000 foster children in the United States (U.S. Department of Health and Human Services, 2006), issues surrounding altered HPA axis activity and the associated symptoms of anxiety and affective dysregulation in foster children could have considerable public health implications. Numerous studies have documented exceptionally high risk for poor outcomes in this population. For example, foster children have been found to exhibit very high rates of psychiatric symptoms and mental disorders (Landsverk et al., 2001), substance abuse (Hurlburt et al., 2004), poor academic outcomes (Fanshel, 1978; Wodarski et al., 1990; Stock and Fisher, 2006), and physical growth retardation (Wyatt et al., 1997; Pears and Fisher, 2005). Increased understanding about the associations between specific dimensions of early stress, alterations in HPA axis activity, and negative outcomes has the potential to clarify explanatory models regarding risk and protection in foster children.
In addition, there is a need to better understand the limits of HPA axis plasticity following exposure to early adversity. Although this is a relatively new line of inquiry, converging sources of evidence have suggested that the HPA axis remains mutable over time and could be impacted by therapeutic interventions and other environmental changes. First, studies of rodents exposed to “enriched environments” following early adversity have shown improved neural development (e.g., greater synaptic density; Turner and Greenough, 1985), although the data on whether enriched environments lead to more typical neuroendocrine functioning are equivocal (Moncek et al., 2004). Second, psychotherapeutic treatment studies on adult human populations (with and without psychopathology) have shown similar improvements in HPA axis functioning. For example, Mommersteeg et al. (2006a) examined diurnal cortisol levels in a psychotherapy treatment study of adults with burnout following chronic stress exposure. Compared to the healthy adults from the comparison group, the adults in the treatment group exhibited lower pretreatment early morning cortisol levels that increased significantly following 14 treatment sessions. Similarly, several studies have documented the positive effects of stress management interventions in reducing healthy individuals’ cortisol responses to acute stressors (Gaab et al., 2003; Hammerfald et al., 2005; Gaab et al., 2006). Third, in a series of studies on children adopted in the United States following institutional rearing in developing nations, researchers have documented low early morning cortisol levels at the time of adoption that became more typical (i.e., having a morning peak in cortisol that declines gradually through the day) after time in the adoptive families (Bruce et al., 2000; Gunnar et al., 2001). The evidence from these three sources supports the idea that therapeutic interventions might have the potential to impact HPA axis functioning in children following exposure to early stress.
In the present study, we examined whether foster preschoolers randomly assigned to a therapeutic family-based intervention would develop more typical diurnal cortisol activity over time compared to foster preschoolers randomly assigned to regular foster care. Typical diurnal cortisol activity was defined by comparing children in both foster care groups to a group of same-aged, nonmaltreated, low-income community children. The intervention (described below) has been documented to reduce the risk (particularly when multiple prior foster placements are involved) for failed adoptions or reunifications with birth parents following foster care (Fisher et al., 2005). To examine changes in diurnal cortisol activity, we tracked monthly early morning and evening cortisol levels over 12 months following a new foster placement.
It is important to note that the focus of the current study was on only one component of HPA axis functioning: basal diurnal cortisol activity. There is a vast literature involving human and animal studies focusing on the responsiveness of the HPA axis to laboratory-induced and naturally occurring stressors. We chose to focus on basal activity for several reasons. First, as is noted above, alterations in this area of HPA functioning have been observed among foster children and similar human and animal populations with early disruptions in maternal care. Second, no reports in the literature have shown laboratory-induced social stressors to reliably produce HPA axis activity in preschoolers. Third and finally, there are ethical concerns with using social or physical stressors in this vulnerable population.
Methods
Participants
The sample was comprised of 3- to 6-year-old foster preschoolers entering new placements (N = 117) under the care of a public child welfare agency in a moderate-sized, Pacific Northwest city with a population of about 150,000. To be eligible for the study, the placement had to be expected to last for at least 3 months. Recruitment occurred continuously over 3.5 years.
Eligible participants were randomly assigned to the Multidimensional Treatment Foster Care for Preschoolers (MTFC-P) intervention condition or to a regular foster care (RFC) comparison condition. Following randomization, a staff member contacted the child’s caseworker (i.e., the legal guardian while the child is in care) and requested consent for the child to participate in the salivary cortisol collection project, as it was not a part of the original randomized trial data collection. A staff member then contacted the foster parent(s) for recruitment purposes. To be successfully recruited, the caseworker and the foster family had to consent to participate. Consent for participation was obtained for 89% of the MTFC-P children (n = 57) and 82% of the RFC children (n = 60). Acceptance rates did not differ between the groups, χ2 = 1.291, df = 1, p = .26, indicating that randomization prior to recruitment did not introduce sampling bias into the study. All research staff members involved in data collection were blind to the condition of children and caregivers.
The foster placements at study entry included first-time foster placements, moves between foster homes, or reentries into foster care following failed permanent placements. Children in the two foster care groups did not differ on placement type at baseline, χ2(3) = 2.24, p = .52, and had spent comparable numbers of days in care prior to baseline (RFC M = 139.20, SD = 141.03; MTFC-P M = 204.40, SD = 221.19; t(114) = −1.9, p = .06).
A second comparison group of same-aged, low-income, nonmaltreated community children (CC) and their families were also recruited to participate (n = 60). The CC families consented to a screening using computerized child welfare system records to verify that they had no previous reports of maltreatment or involvement in child welfare services. Median income for the CC sample was $15,000–19,999, and median education (assessed via a categorical system) was some community college/vocational school.
There were no differences between groups on mean child age or gender. The mean age in years at baseline was 4.4 (M range = 4.3–4.5; SD range = .79–.86), F(2, 174) = 1.13, p = .33. At baseline, boys made up 49% (n = 28) of the MTFC-P group, 58% (n = 35) of the RFC group, and 53% (n = 32) of the CC group, χ2(2) = .10, p = .61. The ethnicity breakdown did not differ between groups, χ2(8) = 9.07, p = .34. Combined across groups, the sample was 89% European American, 1% African American, 5% Latino, and 5% Native American, which is representative of the community in which the sample was recruited. Across groups, retention was high: 96.6% at baseline and 80% at 12-months postbaseline. Treatment of missing data is discussed below.
MTFC-P Group Procedures
MTFC-P is family-based therapeutic intervention that was designed to addresses the developmental and social-emotional needs of foster preschoolers. It was delivered via a team approach to foster children, their foster parents, and their permanent placement resources (birthparents or adoptive relatives/nonrelatives). Prior to placement, foster parents completed 12 hours of intensive training. After placement, foster parents worked with trained consultants and received support and supervision via daily telephone contacts, weekly foster parent group meetings, and 24-hour staff availability. The foster parent consultants facilitated the maintenance of a warm, responsive, consistent environment in which positive behavior was encouraged and problem behavior was limited. Children received individualized treatment designed to improve behavior in preschool/daycare and home settings. Children attended weekly therapeutic playgroup sessions designed to facilitate school readiness in which behavioral, social, and developmental competencies are addressed. When children were to be permanently placed, family therapists worked with these families to familiarize them with the parenting techniques used by the foster parents. This helped to facilitate consistency between settings. Children typically received services for 6–9 months, including the period of transition to a permanent placement. For children remaining in long-term foster care, the services lasted until their behavior stabilized and the risk of placement disruption appeared to have been mitigated. Treatment fidelity for all MTFC-P components was monitored via progress notes and checklists completed by the clinical staff.
MTFC-P has been shown to impact attachment-related behaviors, having a particularly strong effect on increasing secure attachment behaviors and decreasing avoidant attachment behaviors compared to children in regular foster care (Fisher and Kim, in press). MTFC-P has also been documented to improve permanent placement success rates (Fisher et al., 2005). A more detailed description of the MTFC-P program and its theoretical underpinnings can be found in Fisher et al. (1999) and in Fisher and Chamberlain (2000).
RFC Group Procedures
RFC children received routine services in state foster care, which commonly involved weekly individual psychotherapy. Some RFC children also received developmental screening and, if delays were found, special education services.
Measures
Salivary cortisol collection and assay
Monthly early morning and evening salivary cortisol samples were gathered on 2 consecutive days for 12 months (M1–M12). For foster children, M1 assessments occurred 3–5 weeks postplacement. Saliva collections occurred 30 min after the child awoke and before eating or drinking (AM) and 30 min before bedtime (PM). Following procedures described in Schwartz et al. (1998), caregivers were trained by research staff members to complete saliva collection at home. To stimulate saliva flow, the child chewed a piece of Trident® Original Flavor Gum (Cadbury Adams USA, Plano, TX) for 1 min. The child then spat the gum out, and the caregiver tipped a Salivette® (Sarstedt, Newton, NC) absorbent roll from a protective plastic tube into the child’s mouth without touching the roll. The child kept the role in his/her mouth for 1 min and was instructed not to touch it with his/her fingers. The caregiver then assisted the child in inserting the roll back into the protective tube. The caregiver then filled out the date and time of the collection on the tube label. All samples were kept in the participants’ freezers until collected for assay by a research staff member. Samples were assayed using the High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics, State College, PA). Both samples from each child were included in the same assay batch to minimize within-subject variability. Samples were assayed in duplicate and were averaged. Duplicates varying by more than 15% were reassayed. The intraassay and interassay coefficients of variance were 2.69% and 10.98%, respectively.
Certain medications, general health, food intake, and sleep patterns have been shown to affect cortisol levels (de Kloet, 1991). Thus, children using steroid-based medications (e.g., steroidal asthma inhalers) on a regular basis were excluded from the study. Caregivers were instructed and reminded during daily phone conversations to avoid sampling when their children periodically used steroid-based medications or were ill. Caregivers also completed brief questionnaires regarding the sampling times and their children’s eating and sleeping behaviors on the sampling days. These questionnaires were inspected to ensure compliance with sampling guidelines. Of nearly 8,000 saliva samples, 572 were missing (7%; 315 AM samples and 257 PM samples) because the caregivers did not return the samples or did not collect data at one of the sampling times. In addition, 9 samples were excluded due to out-of-range cortisol values (> 2.0 μg/dl; 2 AM samples and 7 PM samples), and 72 samples were excluded due to incorrect sampling time (i.e., collection time recorded on sample tube and in diary differed by more than 30 min or did not correspond to the sampling window; 36 AM samples and 36 PM samples).
Data Analysis and Statistical Modeling
The primary outcome measure was a difference score computed by subtracting the daily PM cortisol level from the daily AM cortisol level (AM–PM cortisol change). This provided a rough index of the diurnal cortisol activity. Although additional diurnal cortisol collections (e.g., midmorning and afternoon) would have better defined the diurnal pattern, the highly vulnerable nature of the children in this sample and an emphasis on limiting the assessment burden on foster children and their caregivers guided our selection of only two time points.
Our analytic plan involved examining group differences over time in AM–PM cortisol change and, if significant group differences were obtained, examining AM and PM cortisol levels separately. This strategy would allow us to decompose AM–PM cortisol change into its two component measures to determine whether variations in AM cortisol levels, PM cortisol levels, or both contributed to group differences in this index of diurnal cortisol activity.
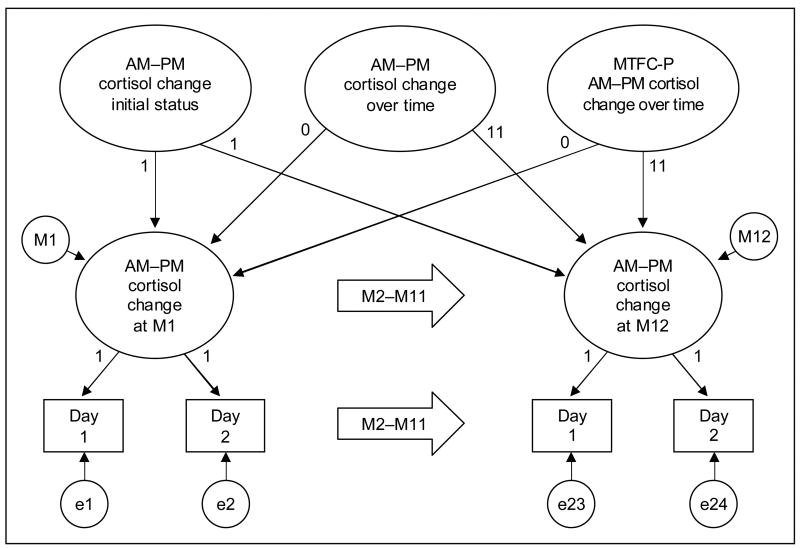
As is illustrated by the path diagram in Figure 1, the AM–PM cortisol change model had three levels, with the two AM–PM cortisol change values on consecutive days (i = 1–2) nested within 12 months (j = 1–12), which were nested within 177 subjects (k = 1–177). The M2–M11 arrows indicate repetition of the cortisol assessments and the monthly latent cortisol variables. In accordance with path diagram conventions, latent variables are shown in circles, measured variables are shown in squares, and regression paths are shown as unidirectional arrows. Residual variances at the month level within subjects (mj,k) and at the day level within months within subjects (ei,j,k) were constrained to be equal.
Figure 1.
Path diagram of the AM–PM cortisol change model.
In our preliminary analyses, the collection time and the difference between time of waking and collection time were entered as time-varying covariates to reduce extraneous day-today variation. The effects were significant but did not alter the group effects or conclusions about their significance, so they were dropped from the model. The subject-level random effects included initial status and linear change over time from M1 to M12 (slope). An intervention effect was included in the MTFC-P group to capture potential variation across participants in response to MTFC-P. The random intervention effect was allowed to correlate with initial status to test the possibility that differential responses to MTFC-P were related to initial AM–PM cortisol change. (For more details about this model and parameterization, see Muthén and Curran [1997]).
Fixed effects included the means of initial status and change over time. Group membership was included by dummy-coded contrasts, with the CC and RFC groups contrasted against the MTFC-P group. Change Over Time × Group interaction effects represent CC and RFC differences from MTFC-P; thus, the Change Over Time × RFC interaction captures the intervention effect, and the Change Over Time × CC interaction indicates whether the MTFC-P group changed at the same linear rate as the CC group. The correlations among the subject-level random effects were freely estimated (although not shown in the diagram). The models for AM and PM cortisol levels were identical to the AM–PM cortisol change model. All models were estimated using the “lme” function (Pinheiro and Bates, 2000), which is a part of both the R (R Development Core Team, 2005) and S-Plus (Insightful Corporation, 2001) statistical packages. Model specifications (i.e., normality of random effects, invariance of month and day level residual variances) were thoroughly checked through residual diagnostic plots, and corrective action was taken as necessary.
Missing data
The models for AM–PM cortisol change, AM cortisol level, and PM cortisol level were estimated using all the available data for all participants at all time points, even when only partial data was available. This approach is the current recommended standard for growth models (Schafer and Graham, 2002) and performs better than the alternative approaches (e.g., use of only participants with complete data or single imputation of missing values) in the sense of minimizing potential bias due to attrition relative to the baseline sample and maximizing power. The model was estimated assuming ignorable missingness; that is, a particular cortisol value on a given day did not interfere with the collecting and recording of that particular cortisol value.
The total amount of missing data was not equally distributed across groups. The amount of missing AM–PM, AM, and PM data was highest for the RFC group, followed by the MTFC-P group and then the CC group. The differences between the MTFC-P and CC groups were not significant, but the two groups had significantly fewer missing data than the RFC group. At no assessment interval were more than 35% of the data missing for any the group. Missing data do not necessarily indicate attrition from the study; rather, among children who remained enrolled in the study, there were instances in which it was not possible to collect cortisol for one or more monthly intervals. Given the robustness of the analytic procedures for dealing with missing data, group differences in missing data, though noteworthy, are unlikely to have affected the results.
Results
The means and standard deviations for monthly AM–PM cortisol change, AM cortisol level, and PM cortisol level by group are shown in Table 1.
Table 1.
Means and Standard Deviations for AM–PM Cortisol Change, AM Cortisol Level, and PM Cortisol Level by Group, Month 1–12
| AM–PM cortisol change
|
AM cortisol level
|
PM cortisol level
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RFC
|
MTFC-P
|
CC
|
RFC
|
MTFC-P
|
CC
|
RFC
|
MTFC-P
|
CC
|
||||||||||
| Month | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| 1 | 0.40 | 0.25 | 0.38 | 0.29 | 0.47 | 0.18 | 0.47 | 0.27 | 0.44 | 0.25 | 0.50 | 0.19 | 0.06 | 0.09 | 0.06 | 0.11 | 0.04 | 0.03 |
| 2 | 0.42 | 0.28 | 0.39 | 0.26 | 0.41 | 0.23 | 0.52 | 0.27 | 0.47 | 0.25 | 0.48 | 0.21 | 0.10 | 0.14 | 0.08 | 0.13 | 0.06 | 0.10 |
| 3 | 0.38 | 0.25 | 0.43 | 0.22 | 0.41 | 0.26 | 0.48 | 0.27 | 0.50 | 0.22 | 0.48 | 0.24 | 0.10 | 0.19 | 0.06 | 0.06 | 0.06 | 0.11 |
| 4 | 0.34 | 0.22 | 0.40 | 0.24 | 0.38 | 0.21 | 0.40 | 0.22 | 0.44 | 0.24 | 0.43 | 0.19 | 0.06 | 0.07 | 0.05 | 0.06 | 0.05 | 0.07 |
| 5 | 0.37 | 0.27 | 0.36 | 0.27 | 0.28 | 0.21 | 0.48 | 0.25 | 0.43 | 0.27 | 0.36 | 0.22 | 0.11 | 0.13 | 0.07 | 0.12 | 0.08 | 0.09 |
| 6 | 0.31 | 0.24 | 0.35 | 0.23 | 0.38 | 0.27 | 0.43 | 0.21 | 0.40 | 0.21 | 0.44 | 0.26 | 0.12 | 0.15 | 0.06 | 0.06 | 0.06 | 0.08 |
| 7 | 0.32 | 0.25 | 0.37 | 0.25 | 0.40 | 0.26 | 0.40 | 0.23 | 0.43 | 0.23 | 0.48 | 0.23 | 0.09 | 0.11 | 0.06 | 0.10 | 0.08 | 0.11 |
| 8 | 0.26 | 0.20 | 0.33 | 0.26 | 0.31 | 0.26 | 0.34 | 0.21 | 0.42 | 0.23 | 0.43 | 0.22 | 0.09 | 0.12 | 0.08 | 0.18 | 0.12 | 0.16 |
| 9 | 0.30 | 0.23 | 0.35 | 0.24 | 0.36 | 0.22 | 0.41 | 0.20 | 0.41 | 0.24 | 0.44 | 0.22 | 0.11 | 0.13 | 0.06 | 0.10 | 0.08 | 0.10 |
| 10 | 0.40 | 0.23 | 0.40 | 0.28 | 0.42 | 0.29 | 0.48 | 0.24 | 0.46 | 0.24 | 0.48 | 0.27 | 0.08 | 0.10 | 0.06 | 0.14 | 0.06 | 0.10 |
| 11 | 0.28 | 0.23 | 0.41 | 0.25 | 0.38 | 0.23 | 0.38 | 0.21 | 0.49 | 0.22 | 0.46 | 0.23 | 0.10 | 0.14 | 0.08 | 0.14 | 0.08 | 0.12 |
| 12 | 0.30 | 0.34 | 0.38 | 0.31 | 0.38 | 0.26 | 0.39 | 0.27 | 0.49 | 0.22 | 0.47 | 0.25 | 0.10 | 0.15 | 0.11 | 0.19 | 0.09 | 0.15 |
AM–PM Cortisol Change
Residual diagnostics on initial fits for AM–PM cortisol change revealed no mean differences across groups at initial status but extremely low and high values for the day-level residuals. To remedy this and to prevent extreme outliers from distorting the results, we trimmed the AM–PM cortisol change distribution at the 1st and 99th percentiles (scores below the 1st percentile and above the 99th percentile were recoded to the 1st and 99th percentiles, respectively). Likelihood ratio tests for initial fits also indicated that the random intervention effect in the MTFC-P group and the associated correlations were not significant, χ2 = 0.76, df = 3, p = .86, so they were dropped from the model.
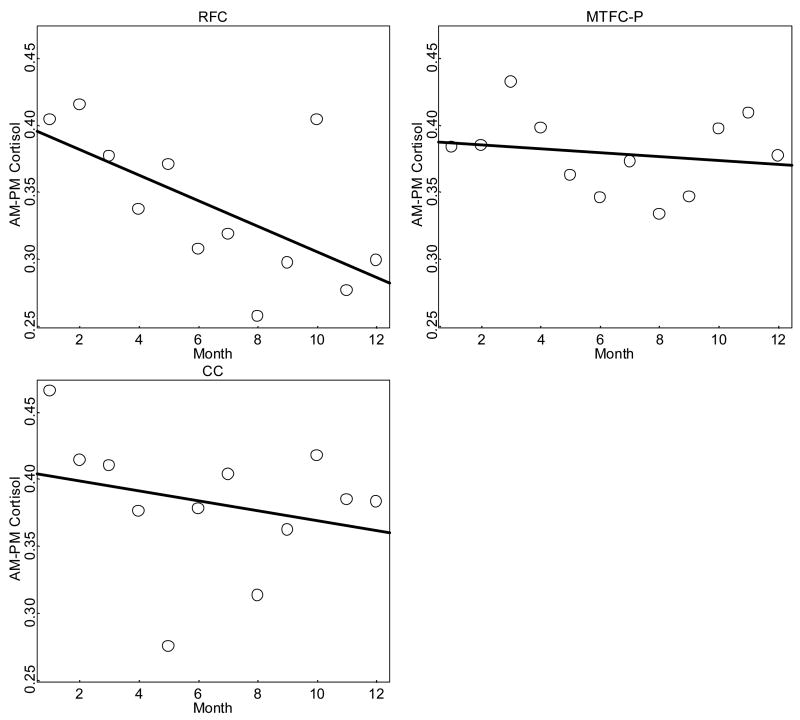
Changes in mean AM–PM cortisol change by group are shown in Figure 2. The RFC group started at a fitted AM–PM cortisol change of about .37 μg/dl; the MTFC-P group started at a fitted AM–PM cortisol change of about .36 μg/dl; and the CC group started at a fitted AM–PM cortisol change of about .39 μg/dl. Over time, however, the groups diverged. The RFC children showed a drop of .09 μg/dl to a fitted mean level of .28 μg/dl by T12. In contrast, the MTFC-P remained unchanged at .36μg/dl, and the CC group showed a marginal decrease of .04 μg/dl to a fitted level of .35 μg/dl at T12.
Figure 2.
Mean AM–PM cortisol change over time by group (μg/dl).
As is shown in Table 2, the Change Over Time × RFC interaction was negative and significant, indicating that the RFC group developed more flattened diurnal cortisol activity over time relative to the MTFC-P group. Follow-up tests (not shown) indicated that the change over time for the RFC group was significantly different from zero. The Change Over Time × CC interaction was not significant, indicating that the CC group’s diurnal cortisol activity did not differ from that in the MTFC-P group. Follow-up tests (not shown) indicated that the change over time was not significantly different from zero for either group. The MTFC-P versus RFC effect size for change over time (when taken as the mean difference in change over time divided by the within-group standard deviation in change over time) was −.65, a medium effect.
Table 2.
Parameter Estimates for Trimmed AM–PM Cortisol Change
| Effect | Value | SE | z | p |
|---|---|---|---|---|
| Fixed effects | ||||
| MTFC-P initial status | 0.358 | 0.025 | 14.580 | 0.000 |
| MTFC-P change over time | 0.000 | 0.003 | 0.116 | 0.907 |
| CC versus MTFC-P | 0.036 | 0.034 | 1.058 | 0.291 |
| RFC versus MTFC-P | 0.011 | 0.035 | 0.329 | 0.743 |
| Change Over Time × CC | −0.006 | 0.004 | −1.259 | 0.208 |
| Change Over Time × RFC | −0.010 | 0.005 | −2.061 | 0.040 |
| Random effectsa | ||||
| SD day residual | 0.197 | 0.010 | 20.652 | 0.000 |
| SD month residual | 0.087 | 0.011 | 8.210 | 0.000 |
| SD subject initial status | 0.138 | 0.013 | 10.387 | 0.000 |
| SD subject change over time | 0.015 | 0.002 | 6.694 | 0.000 |
| Cor initial status and change over time | −0.435 | 0.154 | −2.822 | 0.005 |
| Variance parameter (shrinkage) | 0.905 | 0.026 | 3.670 | 0.000 |
| Variance parameter (exponent) | 0.861 | 0.124 | 6.941 | 0.000 |
Note. Estimated random effects are standard deviations (SD) and correlations (Cor); Variance shrinkage parameter is tested against null value of 1.
The estimated residual standard deviations of all the random effects were significant. The day-level residual was clearly the largest source of variation in AM–PM cortisol change, followed sequentially by group-level initial status and the month residual. The correlation of change over time with initial status was significant and negative. Residual diagnostics on initial fits suggested that the day-level residual variance was smaller in the CC group than in the RFC group and dropped in the MTFC-P group after M5 compared to the RFC group to a level comparable to the CC group. Residual diagnostics also suggested higher day-level variance at higher fitted daily values for all groups. Variance parameters to allow for such shifts are presented in Table 2; as can be seen, the multiplicative shrinkage factor (.92) was significantly smaller than the null hypothesis value of 1, z = 3.04, p < .01, indicating lower day-level variability for the CC group for M1–M12 and comparable lower day-level variability for MTFC-P after M5. Models with a similar shrinkage parameter for the RFC group after M5 (not shown) did not produce significant results. The shrinkage factor for MTFC-P may have resulted from shrinkage in the day-level variance of the PM cortisol levels after M5 (discussed below). The variance parameter to allow for increasing day-level variance at higher fitted daily levels was highly significant, z = 6.94, p < .001. This pattern was not an uncommon departure from standard assumptions especially for positively skewed outcome measures, although, the substantive significance of the pattern was not immediately obvious in this case. Variance stabilizing transformations of the AM and PM cortisol scores eliminated the need for similar variance parameters in those models (presented below).
AM Cortisol Level
Residual diagnostics on initial fits for AM cortisol level revealed that the variance of the day-level residuals was modestly dependent on the fitted level of the outcome, with more variability at higher fitted values. A root transformation (in which y was raised to the power of 1/1.75) of AM cortisol level was used to stabilize the variance of the day-level residuals. Likelihood ratio tests for the initial fits indicated that the random intervention effect in the MTFC-P group and the associated correlations were not significant, χ2 = 2.33, df = 3, p = 0.51, so they were dropped from the model.
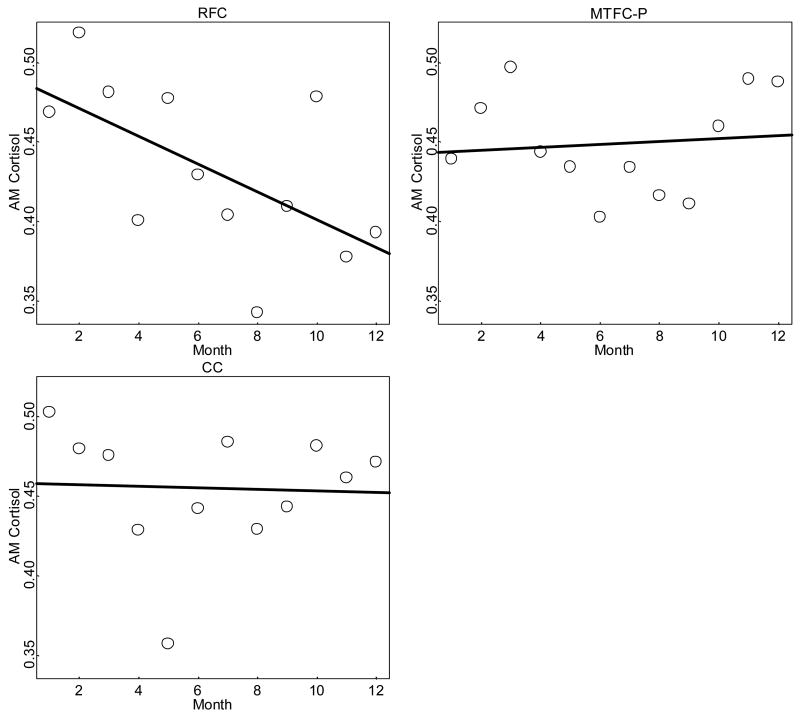
Changes in mean AM cortisol level are shown in Table 3 and Figure 3. As with the results for AM–PM cortisol change, the RFC group showed a large decrease in AM cortisol level over time, starting at .43 μg/dl and dropping to .34 μg/dl by M12. In contrast, the MTFC and CC groups showed very little change over time. The MTFC-P group started at .40 μg/dl and increased to .41 μg/dl by M12. The CC group started at .42 μg/dl and dropped to .40 μg/dl by M12. Indeed, the Change Over Time × RFC interaction was negative and significant, indicating that change over time in the RFC group was significantly more negative (declining) than change over time in the MTFC-P group. Follow-up tests indicated that it was also significantly different from zero.
Table 3.
Parameter Estimates for Transformed AM Cortisol Level
| Effect | Value | SE | z | p |
|---|---|---|---|---|
| Fixed effects | ||||
| MTFC-P initial status | 0.588 | 0.021 | 27.810 | 0.000 |
| MTFC-P change over time | 0.001 | 0.003 | 0.485 | 0.627 |
| CC versus MTFC-P | 0.023 | 0.029 | 0.768 | 0.444 |
| RFC versus MTFC-P | 0.027 | 0.030 | 0.906 | 0.366 |
| Change Over Time × CC | −0.003 | 0.004 | −0.823 | 0.411 |
| Change Over Time × RFC | −0.009 | 0.004 | −2.217 | 0.027 |
| Random effectsa | ||||
| SD day residual | 0.189 | 0.003 | 59.082 | 0.000 |
| SD month residual | 0.097 | 0.006 | 15.873 | 0.000 |
| SD subject initial status | 0.126 | 0.011 | 11.392 | 0.000 |
| SD subject change over time | 0.013 | 0.002 | 7.377 | 0.000 |
| Cor initial status and change over time | −0.578 | 0.131 | −4.400 | 0.000 |
Note. Estimated random effects are standard deviations (SD) and correlations (Cor).
Figure 3.
Mean AM cortisol level change over time by group (μg/dl).
The Change Over Time × CC interaction was not significant, indicating that change over time in the CC group was not significantly different from change over time in the MTFC-P group. Follow-up tests indicated that it was not significantly different from zero in either group. The intervention main effect indicated that change over time in the MTFC-P group was not significantly different from zero. The change over time main effect and Change Over Time × Group interactions indicated that trends for the CC and MTFC-P groups were essentially flat but the RFC group tended to decline over time. The MTFC-P versus RFC effect size for change over time (when taken as the ratio of the mean difference in change over time divided by the within-group standard deviation) was −.66, a medium effect.
The estimated standard deviations of all the random effects were significant. The day-level residual was clearly the largest source of variation in AM cortisol level, followed sequentially by the group-level initial status and the month residual. The correlation of change over time with initial status was significant and negative.
PM Cortisol Level
The results for PM cortisol level were much more skewed than those for AM cortisol level, and it took a much stronger transformation, the reciprocal of a 2.5 root transformation (reverse scaled), to stabilize day-level residual variance. In addition, after the transformation, 28 values (.5% of the total) that were still extremely low were trimmed to prevent undue influence on model parameters. Plots of the estimated month- and individual-level random effects were reasonably normal within each group after transformation and trimming. Likelihood ratio tests for initial fits indicated that the random intervention effect in the MTFC-P group and the associated correlations were not significant, χ2 = 0.05, df = 2, p = 0.97, so they were dropped from the model.
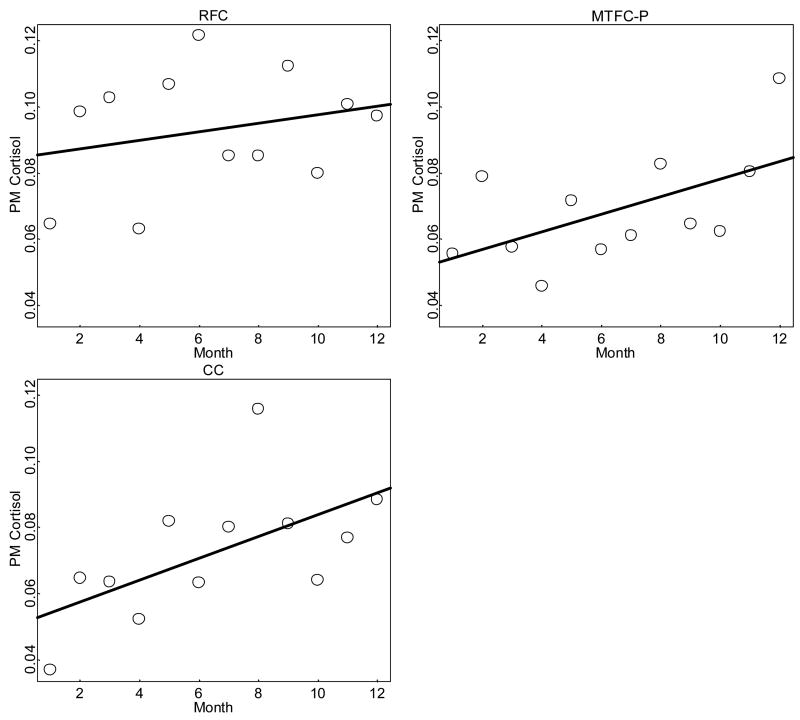
Results for PM cortisol level change over time are shown in Table 4 and Figure 4. The RFC group showed very little change, increasing from .08 μg/dl at M1 to .09 μg/dl at M12. The MTFC-P and CC groups both showed increases from .05 μg/dl at M1 to .08 μg/dl by M12. These changes should be considered in terms of the scale on which they occurred and their clinical significance (discussed below). An additional group difference in PM cortisol level emerged for a shrinkage in day-level residual variation in the MTFC-P group after M5. Inspection of the raw data suggested that these unusually high PM cortisol levels occurred rarely but equally across groups for the first 5 months but became less frequent in the MTFC-P group thereafter.
Table 4.
Parameter Estimates for Transformed PM Cortisol Level
| Effect | Value | SE | z | p |
|---|---|---|---|---|
| Fixed effects | ||||
| MTFC-P initial status | 4.641 | 0.128 | 36.300 | 0.000 |
| MTFC-P change over time | 0.059 | 0.014 | 4.107 | 0.000 |
| CC versus MTFC-P | 0.166 | 0.179 | 0.928 | 0.355 |
| RFC versus MTFC-P | 0.637 | 0.180 | 3.533 | 0.001 |
| Change Over Time × CC | −0.023 | 0.020 | −1.143 | 0.253 |
| Change Over Time × RFC | −0.049 | 0.021 | −2.339 | 0.019 |
| Random effectsa | ||||
| SD day residual | 1.021 | 0.031 | 32.811 | 0.000 |
| SD month residual | 0.622 | 0.028 | 22.514 | 0.000 |
| SD group initial status | 0.814 | 0.062 | 13.121 | 0.000 |
| SD group change over time | −0.864 | 0.053 | −16.382 | 0.000 |
| Cor initial status and change over time | 0.228 | 0.060 | 3.822 | 0.000 |
| Variance parameter (shrinkage—day 2 MTFC-P months 1–5 and non-MTFC-P groups) | 0.894 | 0.036 | 24.493 | 0.000 |
| Variance parameter (shrinkage—day 1 MTFC-P after month 5) | 0.822 | 0.050 | 16.552 | 0.000 |
| Variance parameter (shrinkage— day 2 MTFC-P after month 5) | 0.738 | 0.049 | 14.943 | 0.000 |
Note. Estimated random effects are standard deviations (SD) and correlations (Cor); Variance parameter is tested a against null value of 1.
Figure 4.
Mean PM cortisol level change over time by group (μg/dl).
For the fixed effects in Table 4, the main effect contrast for the RFC group was positive and significant, indicating that the RFC group was initially higher than the MTFC-P group; the main effect for the CC group was not significant, indicating nonsignificant initial differences. Change over time in the RFC group was significantly lower than in the MTFC-P group, and follow-up tests (not shown) indicated that change over time in the RFC group was positive but not significantly different from zero. Change over time in the CC group was not significantly different from change over time in the MTFC-P group, and follow-up tests (not shown) indicated change over time in the CC group was positive and significantly different from zero. The change over time main effect indicated that change over time in the MTFC-P group was positive and significantly different from zero. Notably, however, follow-up tests (not shown) indicated that the three groups were not significantly different at M12. The MTFC-P versus RFC effect size for change over time (when taken as the ratio of the mean difference in change over time divided by the within-group standard deviation in change over time) was −.68, a medium effect.
The estimated standard deviations of all the random effects were strongly significant. The day-level residual was the largest source of PM cortisol level variation, followed sequentially by the group-level initial status and the month residual. The correlation of change over time with initial status was significant and negative. Residual diagnostic plots on initial fits indicated shrinkage in the day-level residual variance on day 2 versus day 1 and suggested that the day-level residual variance dropped in the MTFC-P group after M5. Parameters to allow for such shrinkage are shown in Table 4; as can be seen in the table, all three of the multiplicative shrinkage factors were significantly smaller than the null value of 1. Models with similar parameters for potential shrinkage after M5 for the other two groups (not shown) did not produce significant results. The shrinkage in the PM cortisol day level variability for MTFC-P after M5 is probably responsible for the similar shrinkage in the AM–PM cortisol model. The substantive significance of the shrinkage of the day 2 variability is unclear.
Discussion
This study addressed two interrelated questions regarding the effects of a therapeutic parenting intervention on diurnal cortisol levels in foster preschoolers: whether it could impact diurnal cortisol activity following exposure to early adversity (including neglect, maltreatment, and relationship disruption) and, if so, whether it could improve diurnal cortisol activity by increasing AM cortisol levels. The growth modeling analyses provided fairly unequivocal support for the hypothesis that diurnal HPA function remains mutable in foster preschoolers following early adversity; however, it appears that the primary impact of the therapeutic parenting intervention was not to reverse atypical diurnal cortisol activity but rather to prevent the development of the patterns found in the regular foster care group. In addition, in the MTFC-P group, we observed increased stability in AM and PM cortisol levels over time, a finding that we did not anticipate but that might provide further evidence for the importance of supportive and consistent care in regulating young children’s HPA axis activity. We elaborate on these results and discuss related issues below.
CC Group
Most of the research on children’s diurnal cortisol levels has been conducted with middle- to upper-middle class children (Davis et al., 1999; Watamura et al., 2004; Rosmalen et al., 2005). These studies have indicated that typical diurnal cortisol change ranges from 0.40 to .50 ug/dl. In the present study, we observed AM–PM cortisol change of about 0.39 μg/dl for the CC group. This finding is quite comparable to findings for children from more economically advantaged outcomes. Furthermore, we observed no positive or negative trend for AM–PM cortisol change over time. The findings were comparable to the AM cortisol level analyses. AM cortisol level for the CC group averaged 0.42 μg/dl at M1 and did not change significantly over time. PM cortisol level for the CC group, however, averaged 0.05 μg/dl at M1 and increased to .08 μg/dl by M12, but it is unclear whether this relatively small increase has any biological importance (see below).
Although our examination of the CC group’s AM–PM cortisol change and AM cortisol level showed no evidence of change over time (see Table 1 and Figures 2 and 3), there was marked month-to-month variability in the group mean values. Indeed, the highest and lowest AM–PM cortisol change scores differed by .15 μg/dl, the highest value being at M1 and the lowest value being at M5. It is difficult to determine the source of this variability from the present data. Although seasonal variations in children’s cortisol levels have been reported (Rosmallen et al., 2005), this cannot explain our findings, because children entered the protocol during different months of the year. It is possible that this variation reflects something about the nature of the CC sample; however, it is not likely to result from individual or family factors such as instability in daily routines, as we would expect such factors to be random across families in the CC group and thus unlikely to account for large variations in group-level means at different sampling periods. Interassay error could conceivably account for these variations; however, participants were recruited across several years, and data contributing to a single assessment interval involved samples assayed in multiple assay runs. Moreover, as is reported above, our interassay coefficients of variance were too low to explain the variation. For the moment, the best explanation would seem to be a chance finding, although we are probing other data on these children to determine whether a systematic factor will emerge. In addition to the month-to-month mean variations in the CC group, we noted considerable individual variation from day to day and from month to month. This has been reported in other studies of cortisol sampling for young children, although there is evidence that somewhat greater day-to-day stability occurs among older children (Bartels et al., 2003; Shirtcliff et al., 2005).
MTFC-P and RFC Groups
Counter to our expectations about the effects of intervention on foster preschoolers’ diurnal cortisol activity, we did not observe increased AM–PM cortisol change in the MTFC-P group from M1 to M12. In addition, we did not observe differences in M1 cortisol levels between the foster care groups and the CC group. As is noted above, the CC group’s AM and PM cortisol levels and AM–PM cortisol change were comparable to those reported in other samples of young children; thus, there is little reason to argue that the lack of difference from the foster preschoolers at M1 resulted from atypical diurnal cortisol activity in the CC group. Rather, and in contrast to previous studies (Bruce et al., 2007; Dozier et al., 2006), we did not find mean AM cortisol levels (and thus the mean AM–PM cortisol change) to be unusually low for the foster preschoolers. The difference among these studies might reflect the heterogeneity of foster children. Indeed, Bruce et al. (2007) specified that foster children who had experienced the most severe neglect (i.e., failure to provide), had entered their first foster placement in infancy, and had experienced more than four foster placements prior to their cortisol being assessed were the most likely to exhibit atypically low AM cortisol levels. Few foster preschoolers in the present study (regardless of group) had experienced such severe neglect or instability, which might account for the lack of group-level differences in diurnal cortisol activity relative to the CC group.
The analyses the three groups’ AM–PM cortisol change over time, however, revealed a strong prevention effect in the MTFC-P group. AM–PM cortisol change in the MTFC-P group remained unchanged over time and very similar to the values in the CC group. In contrast, the RFC group showed significantly lower AM–PM cortisol change over time, which is consistent with flattened diurnal cortisol activity. The present research did not address the underlying mechanisms involved in this outcome. However, one possible explanation might lie in prior research showing that chronic stress can lead to the loss of typical diurnal cortisol activity (Pruessner et al., 1999; Mommersteeg et al., 2006b). The foster preschoolers in the present study entered the protocol following a new foster placement, which is widely recognized as a highly stressful event (Eagle, 1994). It may be that, if this stress is confined to a relatively short time following placement and if the child subsequently settles into the foster home, there is less of an impact on diurnal HPA activity. However, if this stress develops chronicity, flattened AM–PM cortisol change might occur. The MTFC-P intervention might help to limit the duration of stress associated with a foster placement, whereas such stress might persist in regular foster care. Consistent with this interpretation are studies documenting that children who received the MTFC-P intervention exhibited more secure and fewer insecure attachment-related behaviors towards caregivers over time compared with children in regular foster care (Fisher and Kim, in press) and are less likely to reenter care following permanent placement (Fisher et al., 2005). However, it is important to recognize the speculative nature of this interpretation and the need for additional research to understand the trends observed in the two foster care groups’ AM–PM cortisol change.
As is noted previously, examining AM–PM cortisol change reveals only one component of HPA axis activity. Thus, although the CC and MTFC-P groups exhibited similar and unchanging AM–PM cortisol change, the AM and PM cortisol levels (at baseline and over time) might have been quite different. Similarly, the flattening observed in the RFC group’s AM–PM cortisol change over time might have resulted from lower morning levels, higher evening levels, or both. Separately analyzing AM and PM cortisol levels over time allowed us to deconstruct the results for AM–PM cortisol change to better understand the nature of the effects observed.
Interestingly, the AM cortisol level analyses were remarkably similar to the AM–PM cortisol change analyses. First, as is discussed above, the growth models revealed no change in the CC group’s AM cortisol level over time. Second, similar to the CC group, the MTFC-P group showed virtually no change in AM cortisol level over time, though AM cortisol level in the RFC group declined significantly. This suggests that the RFC group’s AM–PM cortisol change from M1 to M12 resulted, at least in part, from lowering AM cortisol levels. The change in AM cortisol level in the RFC group is consistent with evidence that hypocortisolism in response to chronic stress is often associated with exceptionally low early morning cortisol levels (Gunnar and Vazquez, 2001; Friese et al., 2005)
The results of the PM cortisol level analyses showed a somewhat different pattern compared to the two other cortisol measures. The CC and MTFC-P groups showed increased PM cortisol levels over time, whereas no change was noted in the RFC group. As is noted above, this increase was quite small and might not be biologically significant. Perhaps more interestingly, however, the MTFC-P group showed reduced day-to-day variability in PM cortisol level, which was not observed in the CC or RFC groups. We can only speculate that this resulted from the MTFC-P intervention’s emphasis on maintaining stable, consistent routines for foster preschoolers. Specifically, MTFC-P treatment staff members encouraged parents to set regular bedtimes, to develop bedtime routines to help settle children in the evenings, and to limit novel or highly stimulating activities between dinner and bedtime. This result is consistent with some of the recent work by Kertes and Gunnar (2004) showing small but notable elevations in evening cortisol among children who participated in structured evening activities. Future research is needed to determine if this intervention, by limiting evening activities, exerts a causal effect on PM cortisol level variability.
There are a number of limitations of the present study. First, as is noted above, the vulnerability of the foster care population and an emphasis on reducing participant burden led to less saliva sampling than might have been optimal. In particular, it would have been helpful to obtain additional saliva samples throughout the day (e.g., midmorning and afternoon), which could have yielded additional information about the nature of the observed AM–PM cortisol change effects. A related issue is that the present study did not gather information about 24-hour cortisol levels. Altered diurnal levels might reflect disturbances to nighttime HPA axis function as well. Low morning cortisol levels, for instance, might indicate a decrease in peak daily cortisol production or might indicate a phase shift in when the peak occurs (e.g., it could be occurring earlier). Future work should explore these issues and should examine whether other physiological variables that typically show circadian rhythmicity (e.g., sleep and body temperature) affect alterations in cortisol activity. Perhaps the cortisol level alterations that we observed were associated with changes in other domains rather than being specific to the HPA axis. Finally, it is important to recognize that the sample was very homogenous with regard to ethnicity. Future research should include more ethnically diverse samples.
Despite these limitations, the current study provides evidence that a therapeutic, family-based intervention for foster preschoolers has the potential to prevent the flattened diurnal cortisol activity (and hence, HPA axis activity) that developed in the RFC group. The implications of these results for foster preschoolers will depend on the mechanisms underlying the prevention effects (an area that is currently under investigation), on whether atypical diurnal cortisol activity relates to increased risk for the cognitive and emotional problems commonly observed in foster preschoolers, and on whether preventing or ameliorating such patterns is associated with reductions in these problems.
Given the limited amount of prior research on preschool-aged children’s HPA axis activity and the absence of extensive longitudinal research on cortisol levels in children in general, these results must be considered preliminary. The monthly variation in cortisol levels for the CC group requires further attention to determine if it resulted from socioeconomic effects or is present in more affluent samples as well. Better understanding typical HPA axis activity over time for preschoolers will make it easier to understand issues of stability and change in special populations such as foster preschoolers. These limitations aside, the current study provides evidence that targeted environmental interventions may affect the HPA axis following exposure to early stress.
Acknowledgments
Support for this research was provided by the following grants: R01 MH059780 and R01 MH065046, NIMH, U.S. PHS; R01 HD045894, NICHD, U.S. PHS; P20 DA017592, NIDA, U.S. PHS; and P30 MH046690, NIMH and ORMH, U.S. PHS. The authors wish to express appreciation to Seymour Levine and Hyoun Kim for their contributions to this manuscript; to members of the Early Experience, Stress Neurobiology, and Prevention Science Network for support and mentorship; to Kristen Greenley, Karla Antoine, Kim Bronz, and the other staff and families of the Multidimensional Treatment Foster Care for Preschoolers project; and to Matthew Rabel for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of diurnal cortisol levels in children. Beh Genetics. 2003;33:421–422. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: reactivity to peer interactions and altered circadian activity. Dev Psychobiol. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- Bruce J, Kroupina M, Parker S, Gunnar M. The relationships between cortisol patterns, growth retardation, and developmental delays in postinstitutionalized children; Paper presented at the International Conference on Infant Studies; Brighton, UK. 2000. Jul, [Google Scholar]
- Bruce J, Pears KC, Levine S, Fisher PA. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. 2007. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann Neurosci. 1997:12. doi: 10.1111/j.1749-6632.1997.tb51936.x. Abstracts 218. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: individual differences in salivary cortisol response in relation to child temperament. Dev Psychobiol. 1999;35:188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. An E. Bennett Research Award: developmental traumatology: part I: biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Frontiers Neuroendocrinol. 1991;12:95–164. [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, et al. Foster children’s diurnal production of cortisol: an exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Eagle R. The separation experience of children in long-term care: theory, resources, and implications for practice. Am J Orthopsychiatry. 1994;64:421–434. doi: 10.1037/h0079546. [DOI] [PubMed] [Google Scholar]
- Fanshel D. Historic themes and landmarks in social-welfare research. Child Welfare. 1978;57:455–456. [Google Scholar]
- Fisher PA, Burraston B, Pears KC. The Early Intervention Foster Care Program: permanent placement outcomes from a randomized trial. Child Maltreatment. 2005;10:61–71. doi: 10.1177/1077559504271561. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Chamberlain P. Multidimensional Treatment Foster Care: a program for intensive parent training, family support, and skill building. J Emotional Behav Disord. 2000;8:155–164. [Google Scholar]
- Fisher PA, Ellis BH, Chamberlain P. Early intervention foster care: a model for preventing risk in young children who have been maltreated. Child Serv: Soc Policy Res Pract. 1999;2:159–182. [Google Scholar]
- Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of a therapeutic intervention for foster children on behavior problems, caregiver attachment, and stress regulatory neural systems. Ann New York Acad Sci. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Kim HK. Intervention effects on foster preschoolers’ attachment-related behaviors from a randomized trial. Prev Sci. doi: 10.1007/s11121-007-0066-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese E, Hesse J, Hellhammer J, Hellhammer D. A new view on hypocortisolism. Psychoneuoendocrinol. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gaab J, Sonderegger L, Scherrer S, Ehlert U. Psychoneuroendocrine effects of cognitive-behavioral stress management in a naturalistic setting—a randomized controlled trial. Psychoneuroendocrinol. 2006;31:428–438. doi: 10.1016/j.psyneuen.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gaab J, Blättler N, Menzi T, Pabst B, Stoyer S, Ehlert U. Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinol. 2003;28:767–779. doi: 10.1016/s0306-4530(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA Early Experience Stress Prevention Science Network. Bringing basic research on early experience and stress neurobiology to bear on preventive intervention research on neglected and maltreated children. Dev Psychopathol. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–627. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected diurnal rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hammerfald K, Eberle C, Grau M, Kinsperger A, Zimmermann A, Ehlert U, et al. Persistent effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects—a randomized controlled trial. Psychoneuroendocrinol. 2005;31:333–339. doi: 10.1016/j.psyneuen.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinol. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hurlburt MS, Leslie LK, Landsverk J, Barth RP, Burns BJ, Gibbons RD, et al. Contextual predictors of mental health service use among children open to child welfare. Arch Gen Psychiatry. 2004;61:1217–1224. doi: 10.1001/archpsyc.61.12.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insightful Corporation. S-PLUS 6 for Windows User’s Guide. Author: Seattle, WA; 2001. [Google Scholar]
- Kertes DA, Gunnar MR. Evening activities as a potential confound in research on the adrenocortical system in children. Child Dev. 2004;75:193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- Landsverk JA, Garland AF, Leslie LK. Mental health services for children reported to child protective services. In: Myers JEB, Hendrix CT, Berliner L, Jenny C, Briere J, Reid T, editors. APSAC Handbook on Child Maltreatment. 2. Sage: Thousand Oaks, CA; 2001. pp. 487–507. [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuoendocrinol. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Meaney M, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinol. 2005;30:568–576. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PMC, Keijsers GPJ, Heijnen CJ, Verbraak MJPM, van Doornen LJP. Cortisol deviations in people with burnout before and after psychotherapy: a pilot study. Health Psychol. 2006a;25:243–248. doi: 10.1037/0278-6133.25.2.243. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PMC, Heijnen CJ, Verbraak MJPM, van Doornen LJP. A longitudinal study on cortisol and complaint reduction in burnout. Psychoneuroendocrinol. 2006b;31:793–804. doi: 10.1016/j.psyneuen.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Moncek R, Dunko BB, Johansson D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- Muthén B, Curran P. General longitudinal modeling of individual differences in experimental designs: a latent variable framework for analysis and power estimation. Psychol Methods. 1997;2:371–402. [Google Scholar]
- Pears KC, Fisher PA. Developmental, cognitive, and neuropsychological functioning in preschool-aged foster children: associations with prior maltreatment and placement history. J Dev Beh Pediatr. 2005;26:112–122. doi: 10.1097/00004703-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS (Statistics and Computing) Springer; New York: 2000. [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC, et al. Determinants of salivary cortisol levels in 10–12 year old children: a population-based study of individual differences. Psychoneuoendocrinol. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Bio Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schwartz EP, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Dev. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Shea A, Walsh C, MacMillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinol. 2004;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson DR. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW. Maternal mediation of early experience. In: Bell RW, Smotherman WP, editors. Maternal Influences and Early Behavior. Spectrum; New York: 1980. pp. 201–210. [Google Scholar]
- Stock CD, Fisher PA. Language delays among foster children: implications for policy and practice. Child Welfare J. 2006;85:445–461. [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. Br Med Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. [Accessed April 19, 2007];The AFCARS Report: Preliminary FY 2005 Estimates as of September 2006. 2006 http://www.acf.hhs.gov/programs/cb/stats_research/afcars/tar/report13.htm.
- Watamura S, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Wodarski JS, Kurtz PD, Gaudin JM, Howing PT. Maltreatment and the school-age child: major academic, socioemotional, and adaptive outcomes. Soc Work. 1990;35:506–513. doi: 10.1093/sw/35.6.506. [DOI] [PubMed] [Google Scholar]
- Wyatt DT, Simms MD, Horwitz SM. Widespread growth retardation and variable growth recovery in foster children in the first year after initial placement. Arch Pediatr Adolesc Med. 1997;151:813–816. doi: 10.1001/archpedi.1997.02170450063010. [DOI] [PubMed] [Google Scholar]