Abstract
Hyaluronan is a multifunctional glycosaminoglycan that forms the structural basis of the pericellular matrix. Hyaluronan is extruded directly through the plasma membrane by one of three hyaluronan synthases and anchored to the cell surface by the synthase or cell surface receptors such as CD44 or RHAMM. Aggregating proteoglycans and other hyaluronan-binding proteins, contribute to the material and biological properties of the matrix and regulate cell and tissue function. The pericellular matrix plays multiple complex roles in cell adhesion/de-adhesion, and cell shape changes associated with proliferation and locomotion. Time-lapse studies show that pericellular matrix formation facilitates cell detachment and mitotic cell rounding. Hyaluronan crosslinking occurs through various proteins, such as tenascin, TSG-6, inter-alpha-trypsin inhibitor, pentraxin and TSP-1. This creates higher order levels of structured hyaluronan that may regulate inflammation and other biological processes. Microvillous or filopodial membrane protrusions are created by active hyaluronan synthesis, and form the scaffold of hyaluronan coats in certain cells. The importance of the pericellular matrix in cellular mechanotransduction and the response to mechanical strain are also discussed.
Keywords: Proteoglycan, Hyaluronan, Versican, Aggrecan, CD44, RHAMM, Mechanotransduction, Cell Adhesion, Cell Traction
Introduction
Hyaluronan, or hyaluronic acid, is a multifunctional glycosaminoglycan that forms the basis of the pericellular matrix. Hyaluronan is a linear polymer composed of repeating disaccharides of glucuronic acid and N-acetylglucosamine [−β (1,4)-GlcUA-β (1,3)-GlcNAc-]n, and is synthesized by 3 different but related hyaluronan synthases, HAS1, HAS2 and HAS3 [1,2]. These are enzymes with multiple transmembrane domains that synthesize hyaluronan at the inner surface of the plasma membrane. During synthesis, the growing polymer chain is extruded through the membrane into the pericellular space. This is in contrast to the mode of synthesis of other glycosaminoglycans, which are made and covalently linked to core proteins in the Golgi apparatus to make a proteoglycan, and secreted by normal exocytotic mechanisms. Hyaluronan chains can be anchored to the cell surface via the synthase enzyme or through binding to a cell surface receptor such as CD44 or RHAMM (receptor for hyaluronic acid mediated motility). Hyaluronan is cleaved by one of several hyaluronidases. There are six hyaluronidase genes in humans, encoding enzymes with different properties and different cell locations [3]. Under normal physiological conditions, hyaluronan ranges in relative molecular mass from 106–107 (~2000–25,000 disaccharides) with polymer lengths of 2–25 μm. (See review by Toole [4]). Hyaluronan is capable of an amazing variety of conformations when deposited on mica surfaces; from extended chains, to relaxed coils, to condensed rods, and pearl necklaces of helical coils, rods, hairpins, and toroids [5]. Hyaluronan can also self-associate to form fibers, networks, and stacks. When retained at the cell surface, hyaluronan can form a voluminous pericellular matrix or “coat”, which has also been termed “glycocalyx”. The hyaluronan-dependent coat has multiple important roles, from serving structural and mechanochemical functions, to the regulation of cell division and motility, as well as cancer progression and metastasis. This review will discuss various aspects of hyaluronan-dependent pericellular matrix structure and function.
Pericellular Matrix Structure
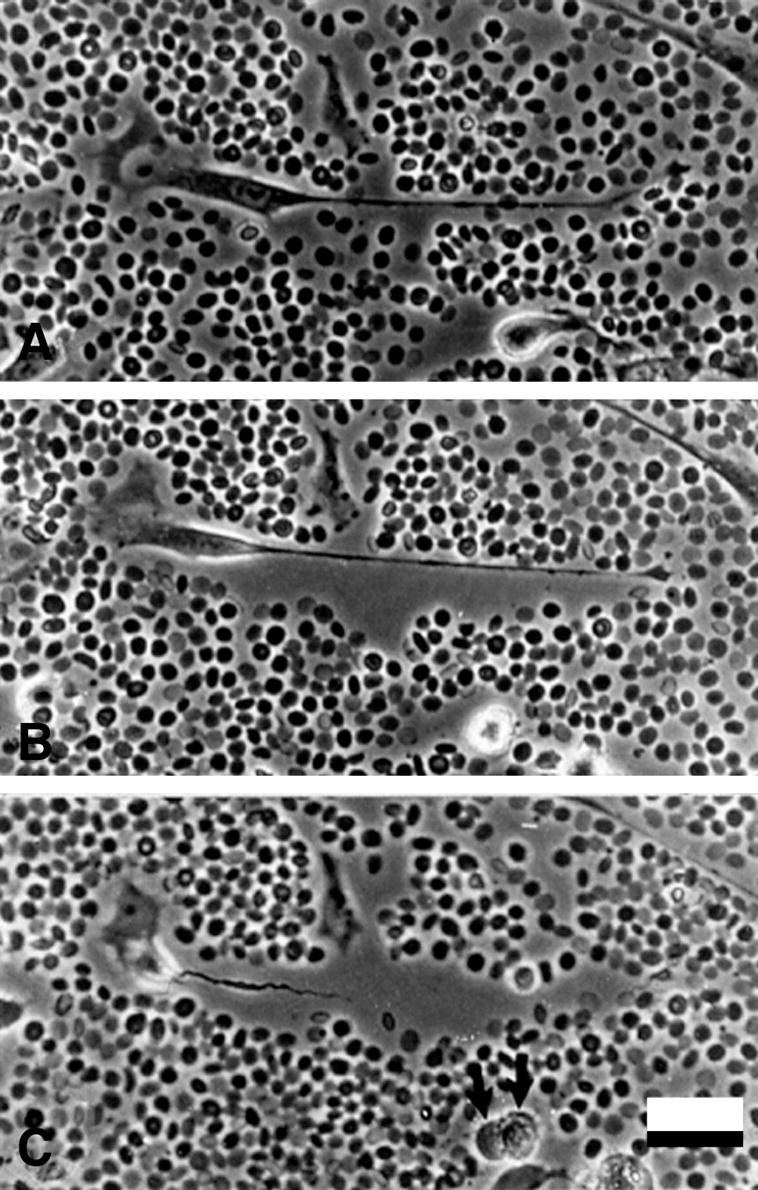
Several studies have investigated the structure and formation of the pericellular matrix. One of the most widely used techniques to view the hyaluronan-dependent pericellular matrix is the particle exclusion assay, which was first utilized nearly forty years ago [6]. In this assay, a suspension of particles, usually fixed erythrocytes, is allowed to settle and a clear zone surrounding the cell is made apparent by virtue of the exclusion of the red blood cells by the gel-like hyaluronan coat (Fig. 1). Treatment of cells with hyaluronan-specific Streptomyces hyaluronidase removes the pericellular coat, indicating that matrix integrity is hyaluronan-dependent.
Figure 1.
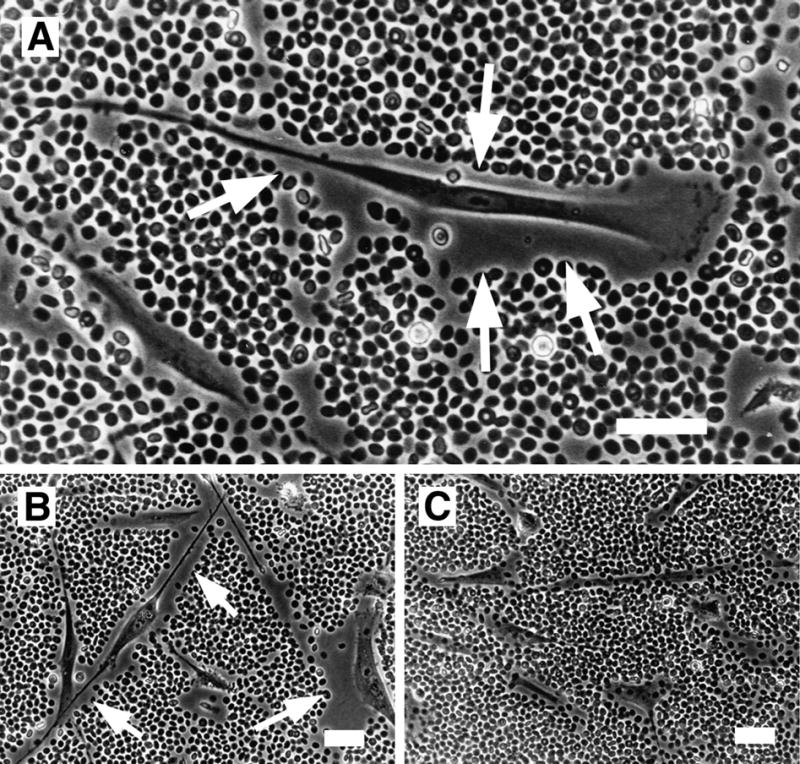
Hyaluronan-dependent pericellular matrix in human smooth muscle cells visualized using the particle exclusion assay. The cell coat excludes the fixed erythrocytes and is seen as a clear zone surrounding the cell (arrows). A. A typical locomoting cell with a small amount of pericellular matrix at the lammellipodium in front and more abundant matrix along the cell flanks and trailing uropod. B, C. Pericellular matrices were visualized before, B, or after, C, digestion with Streptomyces hyaluronidase. Bars equal 50 μm. Panel A originally published in: S. Evanko, J. Angello, T. Wight, Formation of hyaluronan and versican rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol., 1999, 19(4):1004–1013. Used with permission.
Since the thickness of the hyaluronan coat often exceeds 20 μm, which roughly corresponds to the extended length of a single high molecular mass hyaluronan, it is obvious that there must be a way to stretch out the chains, rather than allow their random coil formation close to the cell surface. This notion is in line with the fact that the pericellular coat in many cells requires an aggregating proteoglycan, such as aggrecan or versican, in order to exclude erythrocytes in the particle assay [7]. The repulsion between the highly charged chondroitin sulfates in these proteoglycans apparently forces the perpendicular, extended state of cell surface hyaluronan, and results in the formation of a thick coat. The aggregating proteoglycans interact with hyaluronan via the Link module in the N-terminal globular G1 domain. The Link module is shared by other hyaluronan binding proteins such as link protein and CD44. In chondrocytes, aggrecan is the predominant proteoglycan in the pericellular matrix, while in fibroblasts and smooth muscle cells, versican is the major hyaluronan binding proteoglycan [7–9]. Neurocan, brevican, and phosphacan, as well as versican and aggrecan are all candidates for binding hyaluronan in the perineuronal nets of nervous tissue [10–12].
Studies using immunogold labeling and time-lapse microscopy indicate that aggrecan is tethered to the chondrocyte surface, at least in part, by its association with hyaluronan [13]. The data lead to a model that is a bottle brush-like arrangement, similar to isolated hyaluronan-proteoglycan aggregates [13,14]. Figure 2 shows a model of hyaluronan and associated proteoglycans anchored to the cell surface via CD44. Scanning electron microscopy of smooth muscle cells fixed in the presence of ruthenium red to precipitate the proteoglycans revealed numerous proteoglycan granules attached to HA chains [15]. In its simplest form, the pericellular matrix of smooth muscle cells consists of hyaluronan and associated proteoglycans and other proteins extending perpendicularly from the cell surface (Fig. 3A). In other locations in the same cultures or on different parts of the same cell, the hyaluronan chains and proteoglycans are found in a dense tangled network, or with the proteoglycans and other associated molecules in more condensed, and sometimes beadlike, clusters in the matrix and on the cell surface (Fig. 3B). This beading could be an artifact of dehydration, but nevertheless provides important structural information about matrix organization. Digestion of the cells with Streptomyces hyaluronidase, which is specific for hyaluronan, removes the hyaluronan filaments and the proteoglycan granules (Fig. 3C).
Figure 2.
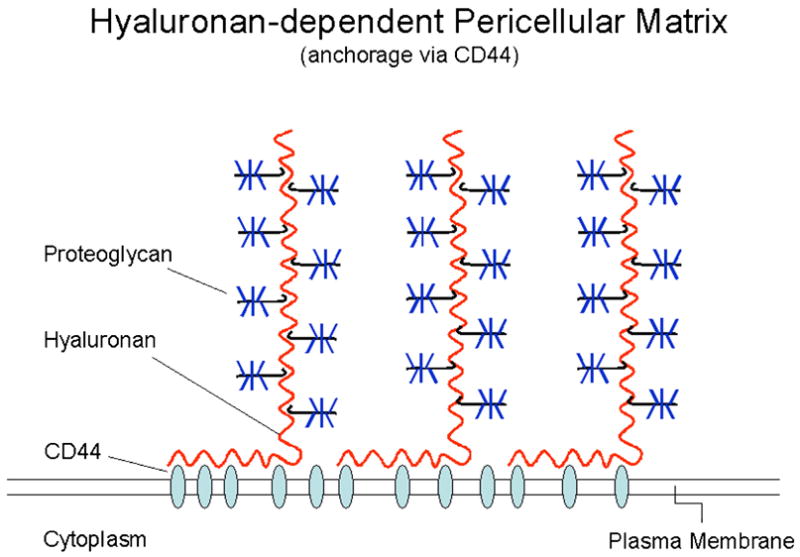
Model depicting the pericellular matrix with the hyaluronan chains anchored to the cell surface via CD44 and the associated aggregating proteoglycans. Adapted by permission from Macmillan Publishers LTD: Nature Reviews Cancer, Toole, B.P., “Hyaluronan: from extracellular glue to pericellular cue,” 4:528 (2004).
Figure 3.
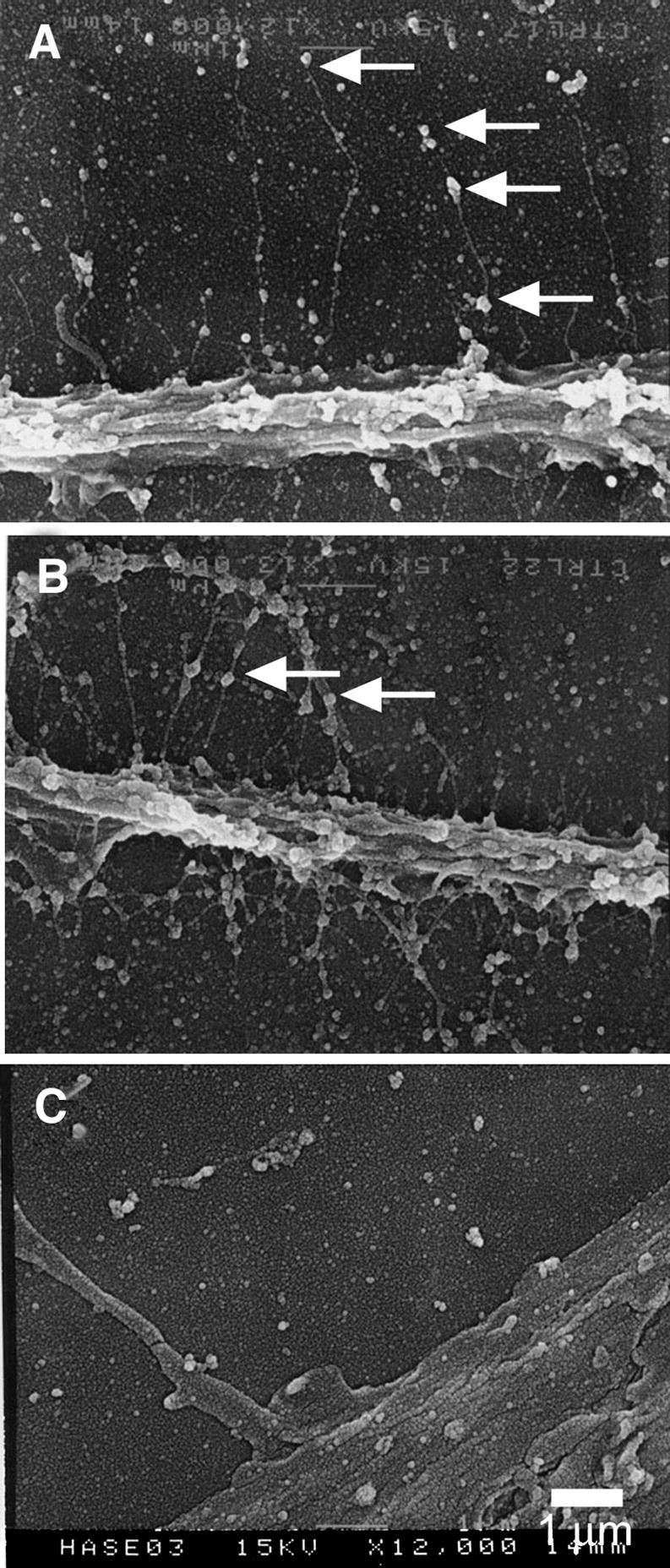
Scanning electron microscopy of the pericellular matrix. Smooth muscle cells were fixed in the presence of ruthenium red, air-dried and coated for electron microscopy. A, Individual hyaluronan chains, several micrometers long extend perpendicularly from the surface of a trailing uropod of a locomoting cell. Proteoglycans are seen as large granules periodically decorating the hyaluronan filaments. B. An example of pericellular matrix that is more tangled with more condensed clusters of proteoglycan granules. C. Hyaluronidase digestion removes the hyaluronan strands and granules from the pericellular matrix and cell surface. Bar equals 1 micrometer. Panels A, B, and C were originally published in: S. Evanko, J. Angello, T. Wight, Formation of hyaluronan and versican rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol., 1999, 19(4):1004–1013. Used with permission.
The presence of aggregating proteoglycans in the pericellular matrix confers a high fixed negative charge density due to the numerous chondroitin sulfate chains, and can have important effects on the material properties and permeability of the matrix. Adding proteoglycans increases the viscosity of hyaluronan solutions [16]. Proteoglycans allow the hyaluronan chain to remain in an extended form due to mutual repulsion of the chondroitin sulfate domains. Thus, the osmotic swelling pressure of the pericellular matrix is increased when more proteoglycan is present. The presence of proteoglycans would also act to stiffen a network of hyaluronan by effectively shortening the hyaluronan chain as it wraps around the globular G1 domain [17]. When excess proteoglycan is added to the coat, the pore size of the pericellular matrix is decreased, with particles 0.3 microns or larger excluded [18]. Link proteins stabilize the interaction between hyaluronic acid and proteoglycans [19–21] and have been shown to have a shortening effect on the length of hyaluronan that is similar to the proteoglycan [17]. Others have shown that adding an excess of link proteins can cause fragmentation or disruption of hyaluronan networks [22].
At the tissue level, the swelling pressure provided by proteoglycan-hyaluronan aggregates has been shown to contribute directly to the compressive stiffness, as well as the shear modulus, of cartilage and fibrocartilage [23–25]. Proteoglycans enmeshed in the collagen matrix inflate the collagen network and induce a tensile pre-stress in the collagen fibrils [25]. This interaction of collagen and proteoglycan aggregates within cartilage matrix provides the complex mechanism that allows the tissue to resist shear deformation. This mechanism undoubtedly occurs in other tissues, albeit to varying degrees, depending on amount and type of aggregating proteoglycan. For example, large amounts of hyaluronan and versican contribute to the swelling in restenotic arterial tissue following balloon angioplasty [26,27]. In addition, the dynamics of the ß1-4 linkage of hyaluronan indicate that it can rapidly exchange between distinct states that make the molecule conformationally restless [28]. It has been proposed that the dynamic properties of hyaluronan make it a perfect space-filling molecule. The nanosecond dynamics of hyaluronan shape change would allow it to fill voids, adjust to surfaces and, when perturbed significantly from its preferred extended states, impose a counteracting or pushing force. Such behavior may be important for cell mobility during wound healing and development.
Hyaluronan Support for Plasma Membrane Protrusions
Epithelial cells can form relatively thick pericellular coats when hyaluronan synthesis is induced by growth factors like EGF [29]. This occurs even though the cells do not express significant amounts of aggregating proteoglycans. The coat formation is even more pronounced in epithelial cells transfected with GFP-Has2 and GFP-Has3 [30,31] (Fig. 4). The GFP-label imaged in live cells reveals that the coat is actually scaffolded by numerous elongated microvilli, on which Has accumulates (Fig. 5) [31]. Similar thin microvilli embedded in HA-coat have been found in untransfected cells such as those of mesothelium, known to synthesize high amounts of HA (unpublished data).The length of the microvilli can exceed 20 μm and they are extremely thin, almost undetectable in ordinary phase contrast microscopy, and easily destroyed during fixation. These new data suggest that the possible contribution of microvillous projections in the coat structure should be studied by live cell microscopy as well as in other cells known to exhibit a hyaluronan-dependent particle exclusion space.
Figure 4.
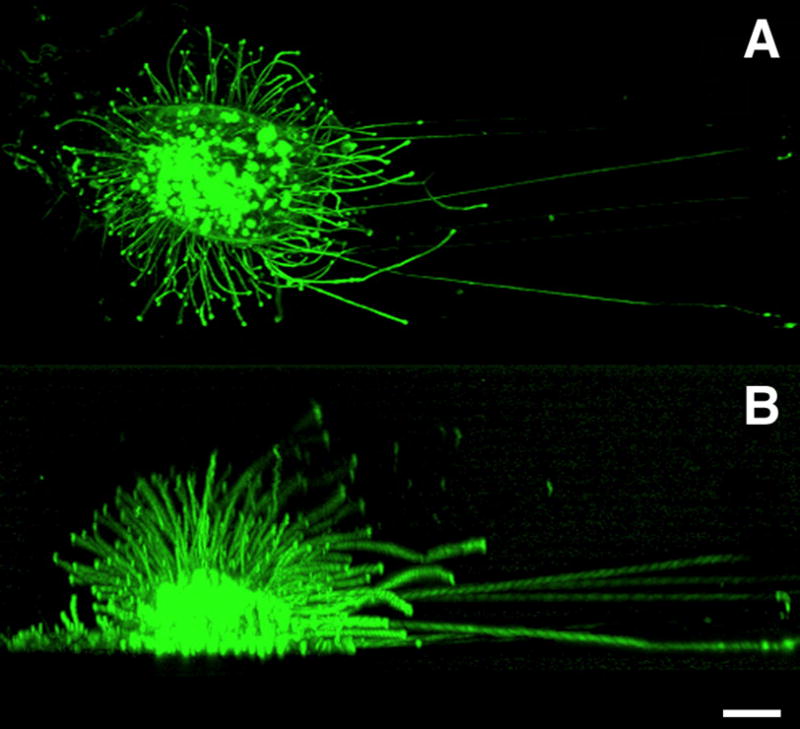
Microvillous plasma membrane protrusions induced by hyaluronan synthesis. GFP- labeled Has3 was transfected into LP-9 cells. The next day, a live cell was studied at multiple horizontal optical sections by confocal microscope. The stack of the optical sections was combined for a downwards (A) and sideways view (B). Bar equals 20 μm.
Figure 5.
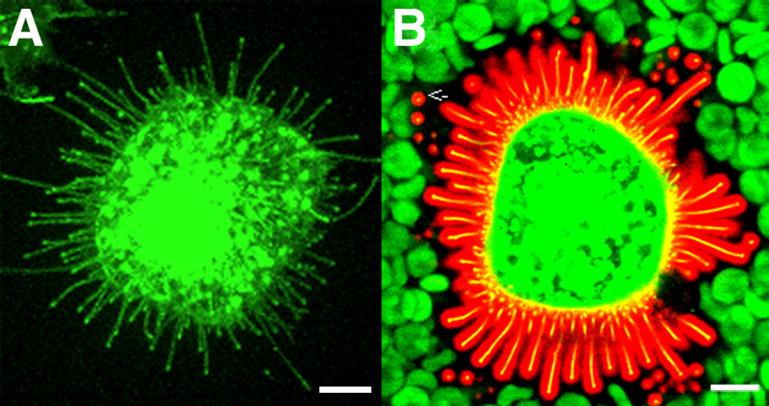
Hyaluronan coat on MCF-7 cells expressing GFP-HAS3. The microvilli (with green GFP-Has3), shown alone in (A), are actually covered by a 0.5–2 μm layer of hyaluronan (B), visualized by a probe made of aggrecan G1 domain and link protein tagged with Alexa Fluor 594® (red). Note that the hyaluronan coat visualized by red blood cells (green) corresponds to the space occupied by the microvilli (yellow) and their hyaluronan cover. The image represents a single confocal optical section; many of the microvilli are shown in cross section (arrow). Bar equals 10 μm.
Hyaluronan Crosslinking
Several different mechanisms of crosslinking hyaluronan have been identified that can influence pericellular matrix assembly and material properties [32]. For example, the C-terminal lectin-like domain of versican has been shown to bind to multimeric tenascin-R and tenascin-C in a calcium-dependent manner, creating the possibility of crosslinking hyaluronan through non-covalent versican-tenascin-versican interactions [33,34]. Other studies have implicated inter-alpha trypsin inhibitor (IαI) as necessary for the formation of cell coats [35,36], for the maintenance of hyaluronan scaffold integrity during cumulus expansion [37], and for potentiating CD44-mediated leukocyte adhesion to hyaluronan-rich matrices [38,39]. IαI heavy chains are transferred from the chondroitin sulfate chain of IαI to the hyaluronan. This process occurs through a transesterification reaction (i.e., a break of the ester bond between a heavy chain and the glycosaminoglycan chain of bikunin) and the formation of an equivalent covalent linkage between the heavy chain and hyaluronan [40]. This would potentially create both intra- and inter-chain crosslinks.
TSG-6 (35 kDa-secreted product of the tumour necrosis factor-stimulated gene-6), is another protein with a Link module that serves in hyaluronan binding and participates with other proteins in the formation of higher-order crosslinked hyaluronan structures [32]. TSG-6 can act as a cofactor in the transfer of the IαI heavy chains to hyaluronan as described above. TSG-6 can also form complexes with pentraxin 3 (PTX3) potentially to link up to 20 hyaluronan chains, thus forming a node in the extracellular matrix [41]. Complexes such as these are likely to be formed in the cumulus matrix and at inflammatory sites [41], and produced by endothelial cells, macrophages, fibroblasts [42], and probably other cells.
Another kind of matrix node may involve thrombospondin-1 (TSP1), a disulfide-linked homotrimer of 150 kDa subunits that has been found to interact with the Link module of TSG-6 probably through its N-terminal ‘N’-module [43]. The binding sites on TSG-6 for HA and TSP1 are non-overlapping. Therefore, it is possible that TSG-6–TSP1 complexes could bring together three hyaluronan chains.
Cells can also generate massive cable-like structures or fibers of hyaluronan in response to various stimuli [e.g. inflammation, viral infection, endoplasmic reticulum (ER) stress and hyperglycemia] and these fibers have specific leukocyte-binding properties [38,44-47]. The hyaluronan chains from one cell often merge with those from other cells to form extremely large cables that can span many cell lengths. In fibroblast cultures, we have observed cables that nearly span an entire 22 mm coverslip (Evanko and Wight, unpublished observations). The leukocyte retention assay suggests that the cables are mechanically more robust than non- or less-crosslinked hyaluronan, and help hold the leukocytes during the assay washing procedures. These cables also contain versican, IαI heavy chains, and TSG-6 [47]. It has been proposed that the presentation of hyaluronan in a crosslinked form leads to receptor clustering on leukocytes (or co-receptor engagement through the presence of accessory molecules on the cables) which would promote adhesion [32,47]. It has been suggested that this may be pro-inflammatory, with the cables acting as distress signals that might perpetuate chronic inflammation [45]. Others have suggested that the cables could be anti-inflammatory and control leukocyte activation by preventing adhesion through ICAM-1 [48], VCAM-1 or VLA-4[49], or by preventing loss of matrix [32,47].
The longest cables appear to emanate from the apical surface of the cell in the perinuclear region. Long cables are not typically seen on the leading lamellipodia of migrating fibroblasts, indicating that the large cable structures are not necessarily required for locomotion, and may actually impede migration ([50] and Evanko and Wight, unpublished observation). The activation of latent hyaluronan synthesis activity from predominantly perinuclear regions, particularly under conditions of ER stress suggests that cable formation may be initiated in the perinuclear and or endoplamic reticulum membranes [45,47]. This can give rise to hyaluronan cable-based nuclear interconnections that can span vast distances, forming novel sort of mechanochemical intercellular network that may serve an important role during inflammation.
Increased hyaluronan synthesis induced by HAS1 overexpression led to increased hyaluronan cable formation and promoted hyaluronan-dependent monocyte binding, while overexpression of HAS1, 2, or 3 all promoted resistance to cell detachment by trypsin/EDTA and decreased migration and proliferation in SMC [50]. In contrast, increased HAS2 expression in proximal tubular kidney epithelial cells led to increased pericellular hyaluronan but inhibited cable formation [51]. However, more work will be needed to define the HAS isoform(s) responsible for cable formation, the role of cables in inflammation, proliferation and migration in other cells, and to better determine whether there are actual structural and biochemical distinctions between hyaluronan coats and hyaluronan cables.
Role of the Pericellular Matrix in ECM Assembly
The hyaluronan dependent pericellular matrix may be involved directly or indirectly in the assembly of other ECM components, either by serving as a scaffold or through interactions of pericellular matrix constituents with other matrix proteins. For example, fibronectin and collagen were found in the pericellular matrix of fibroblasts [52]. Thus the cell coat may serve to retain newly secreted collagen and fibronectin prior to assembly. Elastic fiber formation may also be regulated by the pericellular matrix. For example, retrovirally mediated overexpression of versican splice variant V3 by arterial smooth muscle cells induced tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury [53]. This was proposed to be due to removal of the chondroitin sulfate containing forms of versican, VO and V1, by competition for hyaluronan in the pericellular matrix. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury [54]. Others have shown that hyaluronan oligosaccharides have an elastogenic effect, possibly by removal of the hyaluronan and associated versican chondroitin sulfate from the cell surface by competition with hyaluronan receptors [55].
Role for the Pericellular Matrix in Cell Proliferation and Migration
Hyaluronan and versican have been found to play a role in the maintenance of proliferative and migratory phenotypes in various cells following growth factor treatment or injury [15,56–63]. Hyaluronan is localized in tissues and cell cultures using a highly specific biotinylated hyaluronan binding protein (bHABP) preparation from cartilage [64]. The probe is prepared from trypsin digests of cartilage extracts and is isolated using a hyaluronan affinity column. It consists of a mixture of N-terminal fragments of aggrecan and link proteins. Figure 6 shows the localization of hyaluronan in specimens of non-injured and balloon-injured rat carotid arteries using this probe. Note how the hyaluronan staining is increased in the neointima of the injured artery and surrounds the proliferating (PCNA-positive) smooth muscle cells [65].
Figure 6.
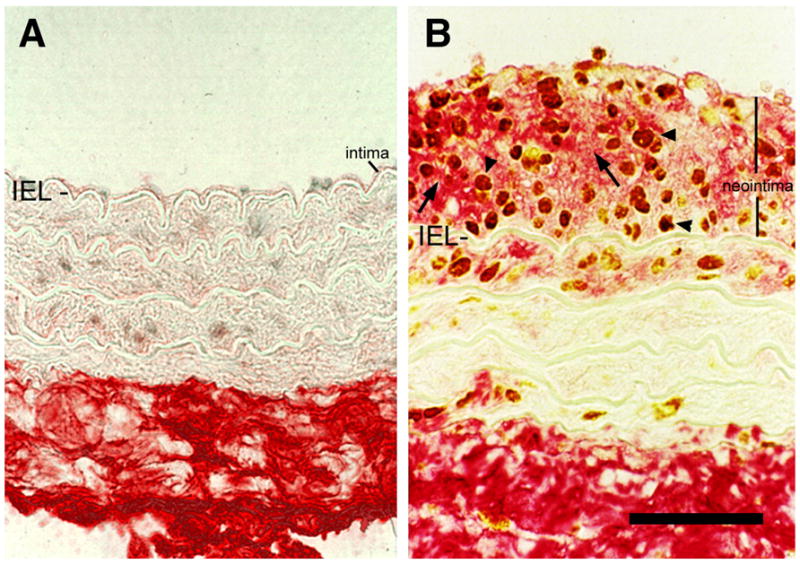
Hyaluronan is increased in arterial neointima following balloon injury. Hyaluronan staining of arterial tissue using biotinylated-HABP (red, arrows) and PCNA staining to localize proliferating cells (brown, arrowheads) in uninjured, A, and injured B, rat carotid arteries. Note the abundance of hyaluronan surrounding the proliferating cells in the neointima of the injured vessel. IEL, internal elastic lamina. Bar equals 50 μm. Panels A and B were originally published in: R. Riessen, T.N. Wight, C. Pastore, C. Henley, J.M. Isner, Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation, 1996, 93(6):1141–1147. Used with permission.
Stimulation of smooth muscle cells with PDGF increased the size of the pericellular coat, coordinately upregulated versican and link protein mRNA expression, and caused an increase in the amount of link protein-stabilized versican-hyaluronan aggregates [58], suggesting that alterations in the organization and material properties of hyaluronan-dependent matrices induced by altered levels of hyaluronan binding proteins underlie some of the biological effects. Figure 7 demonstrates a model of pericellular matrix expansion following PDGF treatment that accompanies the increase in migratory and proliferative activity induced by the growth factor. In actively proliferating cells, dense accumulations of the pericellular matrix that stain positively for hyaluronan and versican can be seen around the mitotic figures, indicating that a more viscous, compacted pericellular matrix, with higher osmotic pressure may be required for the mitotic process (Fig. 8A–D). Disruption of the pericellular matrix of human smooth muscle cells using hyaluronan oligosaccharides inhibited PDGF-induced proliferation (Fig. 8E) and migration, and also caused a dramatic cell shape change to a flattened and more adherent phenotype (Fig. 8F,G) [15]. The effect of hyaluronan fragments of different molecular weights varies with cell type, however. For example, in contrast to their effects on smooth muscle cells, hyaluronan oligosaccharides are pro-proliferative and pro-migratory in endothelial cells and thus promote angiogenesis [66].
Figure 7.
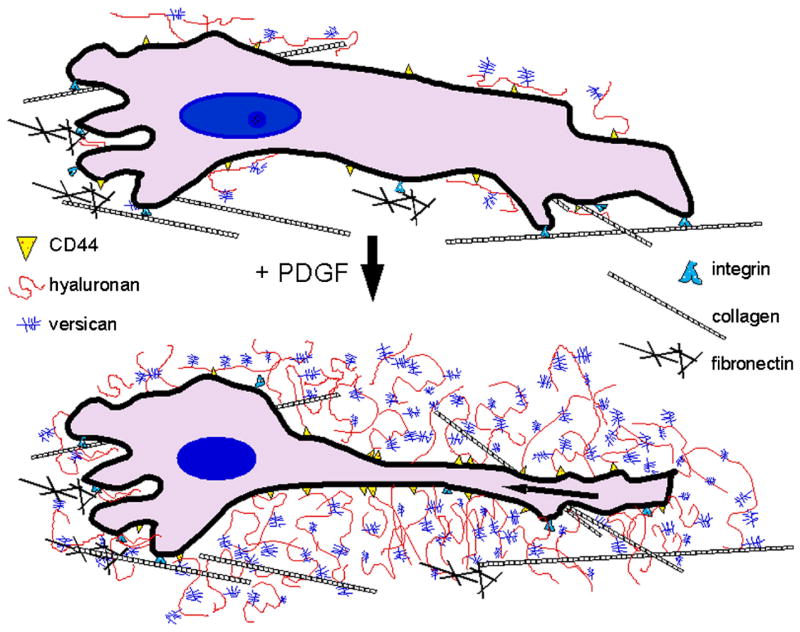
Model of pericellular matrix expansion and cell shape change following stimulation with PDGF.
Figure 8.
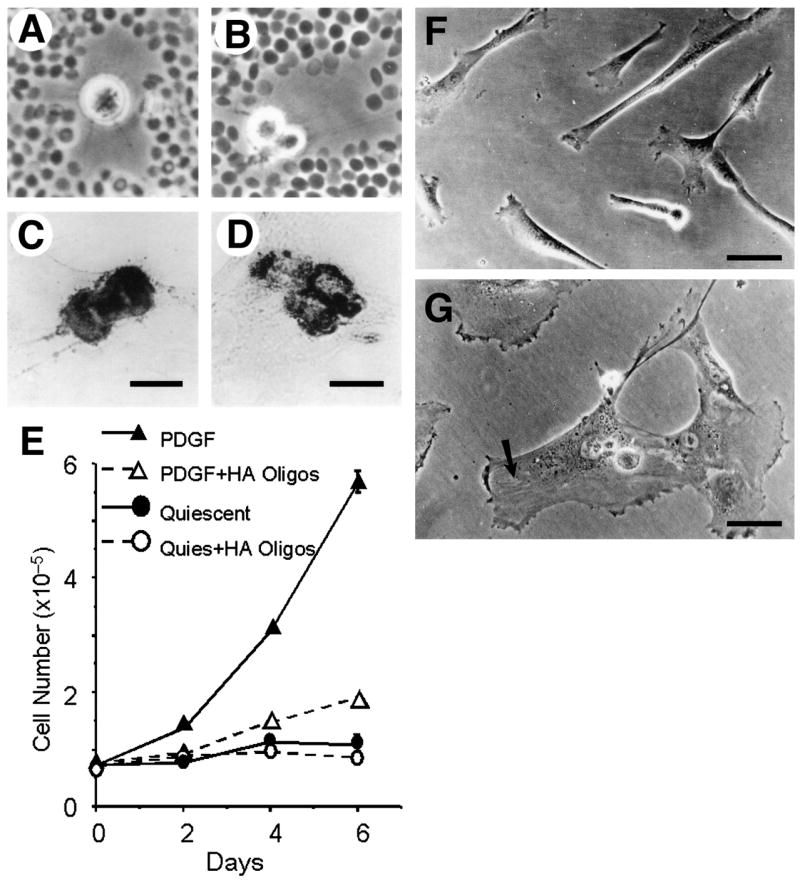
Hyaluronan-dependent pericellular matrix regulates proliferation and cell shape. A, B, Particle exclusion assay showing cell coats surrounding mitotic smooth muscle cells in different stages of division. Mitotic cells stained for hyaluronan, C, and versican D, show concentrated deposits of these components. E, treatment of human smooth muscle cells with oligosaccharides of hyaluronan (20 μg/ml) inhibits PDGF-induced proliferation. Oligosaccharides also stimulated flattening of SMC. F, untreated cells. G, cells treated with oligosaccharides. Bar equals 50 μm. Portions of this figure were originally published in: S. Evanko, J. Angello, T. Wight, Formation of hyaluronan and versican rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol., 1999, 19(4):1004–1013. Used with permission.
In addition to providing large numbers of negative charges, the proteoglycans have other biological activities. For example, the C-terminal domain of versican has been shown to promote tumor growth and angiogenesis [67,68]. Furthermore, different functions can be ascribed to the various splice variants of versican [69–71], adding to the complexity of the roles for proteoglycans in the pericellular matrix.
Pericellular Matrix Regulation of Cell Adhesion
Work from a number of laboratories shows that hyaluronan and/or proteoglycan in the pericellular matrix can have both adhesive and anti-adhesive properties, which are regulated on several levels. Evidence for an anti-adhesive role of the pericellular matrix comes from several studies indicating that hyaluronan promotes cell detachment [72–74]. Surface coatings of purified hyaluronan and chondroitin sulfate proteoglycans are generally anti-adhesive and can form barriers to cell movement and create guidance pathways for neurite outgrowth or neural crest migration [38,75–77]. Avoidance of hyaluronan and versican substrate coatings also occurs in fibroblasts and smooth muscle cells (Evanko and Wight, unpublished observations). Elevated hyaluronan synthesis occurs during the G2/M phase, and promotes mitotic cell detachment and rounding, which was hypothesized to be due, in part, to the steric exclusion properties of the cell coat [60].
Time-lapse microscopy, in conjunction with the particle exclusion assay, showed that a pericellular matrix several micrometers in thickness can form within 15–40 minutes, just before premitotic cells retracted their extended processes, providing visual evidence for a dynamic causal relationship between the formation of the cell coat and subsequent detachment and rounding [15] (Fig. 9). Observations that the coat is enriched along the less adherent portions of the membrane, and that flattened and highly spread smooth muscle cells produce very small or no cell coats, also support an anti-adhesive role for the cell coat. Hyaluronan and versican were localized between focal contacts on the underside of fibroblasts, indicating that the swelling properties of the pericellular matrix may act to counterbalance the tensile forces exerted by cells on solid substrates or fibrillar matrix components [78]. In other words, rapid formation of a concentrated pericellular matrix on the undersurface of cultured cells may provide a countervailing force and a means by which a cell can “push” itself away from a substrate or from other cells. This may be the mechanism by which hyaluronan regulates cell spacing during developmental processes [79], or in cell separation during cytokinesis [56].
Figure 9.
Pericellular matrix formation accompanies cell detachment and mitotic cell rounding. Time-lapse microscopy was begun 24 h after PDGF treatment, when cells are actively moving and dividing. The formation and expansion of the pericellular matrix occurs mainly at the time cells detach from the tissue culture substrate. (A) At time zero (immediately after settling of the red blood cells), an elongated cell with a long, trailing process and relatively little hyaluronan-dependent matrix. (B) 40 min later, a distinct pericellular matrix has formed around the cell and trailing process, pushing the red blood cells away, while the main cell body is beginning to detach from the substrate. (C) At 80 min, the cell is more rounded and is retracting the trailing process through the sleeve of hyaluronan-rich matrix. Note also the rounded cell with a distinct pericellular matrix in the lower part of the field in A and which has completed mitosis in C (arrows). Bar equals 50 μm.
Active degradation and/or traction of the pericellular matrix may be needed to effect cell condensation at other times in development. We have noted the enrichment of pericellular matrix around retraction fibers, left behind as the cell edge withdraws from a substrate, suggesting that cellular tractional forces may be important for concentrating or organizing the hyaluronan-rich matrix into either pro- or anti-adhesive forms. Time-lapse data further show that the cell coat is highly malleable and easily tractioned, as indicated by the movement of red blood cells that are atop the hyaluronan gel and pulled toward the cell as it retracts (data not shown). It has also been noted that hyaluronan that is shared between cells can be concentrated and drawn into aligned fibers and that this involves CD44 [80]. Both CD44 and RHAMM associate with the cytoskeleton [81,82], control tumor cell invasiveness, and transduce signals involved in proliferation, locomotion, focal adhesion turnover [83,84], indicating that both receptors may be responsible for hyaluronan traction. CD44 has been demonstrated to mediate phagocytosis [85,86], bringing up the idea that hyaluronan in the pericellular matrix may by used by cells to draw in other matrix components, cellular debris or other particles, thus facilitating phagocytosis. The full role of cellular traction on hyaluronan organization is not yet clear. However, these observations highlight the idea that hyaluronan may be involved in both pushing and pulling at the cellular level.
The concentration of hyaluronan and versican in areas of high focal adhesion turnover, such as ruffling membrane, and especially to the cell flanks during locomotion, further supports the idea that the pericellular coat can be anti-adhesive [15,82]. Time-lapse microscopy shows that a modest pericellular matrix is continuously elaborated along the flanks of locomoting cells, and may provide a kind of lubricative function along the plasma membrane/substrate interface. This also implies that hyaluronan both facilitates and accumulates as a by-product of locomotion and proliferation. As mentioned above, removal of the pericellular coat of human smooth muscle cells by competition with oligosaccharides resulted in a flatter, more adherent phenotype, again consistent with an anti-adhesive property of the coat. Evidence of increased focal adhesion turnover following addition of hyaluronan to migrating cells highlights the dynamic role of the pericellular matrix [87]. Rapid uptake of the hyaluronan and translocation to the nucleus, with signaling mediated through RHAMM, was associated with increased motility. In addition to being concentrated in the pericellular matrix, hyaluronan is also internalized in premitotic cells and co-distributes around the mitotic spindle with microtubule-associated RHAMM [88,89]. Microtubules are compression-bearing elements of the cytoskeleton that target focal adhesions for dissolution [90]. This raises the possibility that hyaluronan internalized from the pericellular matrix may also regulate or facilitate events involving microtubules during cell shape changes.
It is not clear what role the hyaluronidases play during cell detachment, but fragmentation of the hyaluronan is likely to play a role. HYAL 2 has been proposed as a co-receptor for hyaluronan at the cell surface along with CD44 [91], where it begins the degradation of hyaluronan to smaller chains (~20 kDa) [92] as the endosome forms. These chains are then further degraded by HYAL 1 and other glycosidases in the lysosome [29,91]. Recently, a novel mechanism for creating acidic microenvironments for hyaluronan degradation at the cell surface has been described [93]. In this model, hyaluronan degradation is based on the formation of acidic microenvironments under the control of the Na+/H+ exchanger, NHE1, following hyaluronan binding to CD44.
Studies showing that decreased synthesis of hyaluronan in keratinocytes expressing HAS2 antisense RNA resulted in increased vinculin-positive adhesion plaques and decreased migration provide more evidence that the cell coat may antagonize adhesion to other matrix components [94]. It was proposed that it is the dynamic synthesis of hyaluronan that regulates migration [94]. Thus, continuous, active synthesis and/or internalization may be important for the effects of the pericellular matrix on cell movement and internal motor activities related to cell locomotion and mitotic cell rounding.
On the pro-adhesive side, it was recently shown that the extended hyaluronan chains in the pericellular coat of chondrocytes mediate the early, long-range adhesive interactions that precede the formation of firm integrin-mediated adhesion complexes when cells are plated on tissue culture substrates [95,96]. This makes sense because the hyaluronan chains extend several micrometers away from a cell and would be the first thing to contact an artificial substrate in cell culture. However, the pro- or anti-adhesive character of the pericellular matrix may be regulated by the amount, size, cellular location, and malleability of the hyaluronan and would therefore be more complex in three-dimensional conditions. In experiments looking at binding of cells to calcium tartrate crystals, it was shown that hyaluronan was pro-adhesive if only one surface (either the cell or the substrate) was coated with the hyaluronan, whereas, no adhesion took place if both the cells and the substrate were coated with hyaluronan [97]. This implies that hyaluronan can be self-limiting in its adhesive properties, and that a critical concentration may be required for both surfaces to be coated with enough hyaluronan to promote cell detachment. However, matters are complicated by time-lapse sequences that show how the pericellular coat can apparently promote cell detachment and retraction at one moment, and within a few minutes, the same matrix can provide guidance for filopodial re-extension by the same cell (data not shown). It is tempting to speculate that traction-induced alignment and/or concentration of the hyaluronan matrix may later facilitate filopodial re-extension or guidance.
Hyaluronan that is present in the form of cable structures clearly is pro-adhesive. Hyaluronan cables promote the adhesion of monocytes in inflammatory environments in cell culture and within tissues such as inflamed intestinal mucosa and kidney [38,46,50,98]. The high degree of crosslinking of hyaluronan within the cables would create a more stable structure and more ordered presentation of hyaluronan and/or its associated proteins, which can influence receptor clustering and intracellular signaling. In the early stages of inflammation, the adhesive interactions between hyaluronan on the endothelial cell surface and CD44 on leukocytes that help mediate leukocyte rolling on endothelium have been well described [99,100]. This hyaluronan-mediated adhesion sets up the conditions for extravasation that is mediated by other adhesion molecules. Both high molecular weight hyaluronan and hyaluronan fragments are found in the inflammatory environment, but it is the small fragments of hyaluronan that are thought to be proinflammatory [101].
Other data indicating a pro-adhesive role for hyaluronan comes from time-lapse studies on cell locomotion. Promotion of lammellipodial formation was initiated by application of exogenous hyaluronan to an “inactive” cell edge with a micropipette, suggesting that hyaluronan may promote the formation of cell protrusions and has a chemotactic effect [102]. Another study showed that the enzymatic activity of HAS is coupled to plasma membrane residence and the HAS is localized to cell protrusions [30]. In this recent study, HAS overexpression and hyaluronan production was also found to influence the formation of these microvillous protrusions in adenocarcinoma cells and other epithelial cells [31] (Fig. 4). While the functions of the microvilli induced by overexpression of HAS are not known, such protrusions could explore the surrounding matrix and adjacent cells for potential adhesion sites, and/or pathways for migration. Interestingly, the microvilli are rapidly retracted following hyaluronidase digestion (Fig. 10) and they wither following the addition of an inhibitor of hyaluronan synthesis. However, the microvilli were not affected by competition with hyaluronan oligosaccharides and disruption of the CD44 gene, indicating that their formation was independent of hyaluronan receptors. The data point out the novel concept that the glycocalyx created by dense arrays of hyaluronan chains, tethered to the HAS enzyme during biosynthesis, can induce and maintain prominent microvilli.
Figure 10.
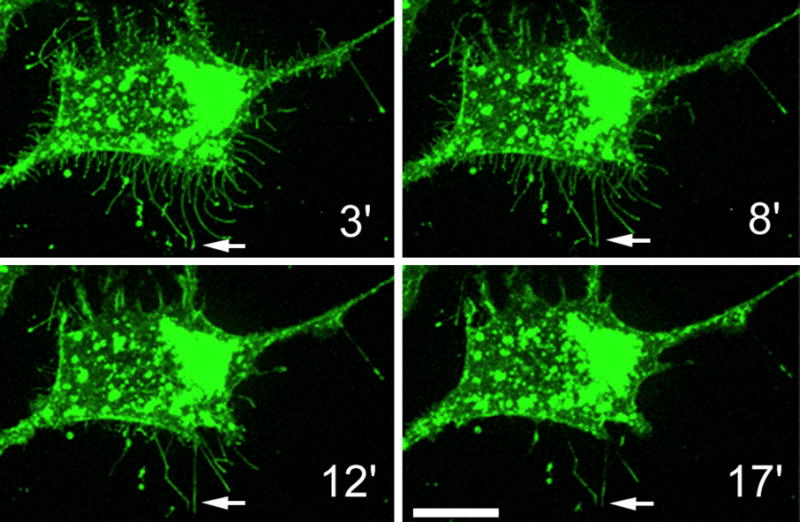
The maintenance of the microvilli is dependent on the hyaluronan coat. Streptomyces hyaluronidase was introduced in a MCF-7 cell culture overexpressing GFP-HAS3 to degrade cell surface hyaluronan. The microvilli gradually shrink and eventually disappear when the external support given by the Has-associated hyaluronan is lost. Note that those microvilli which have adhered to the substratum do not retract (arrows).
Based on other time-lapse observations of coat formation, hyaluronan-mediated adhesion and de-adhesion may occur on different parts of the same cell simultaneously. For example, the formation of pericellular matrix around extending filopodia in spreading smooth muscle cells suggests that hyaluronan at the leading edge facilitates forward extension while the hyaluronan along the flanks and uropod apparently promote detachment. Disruption of the cell coat with oligosaccharides prevented cell spreading, and is at least consistent with this idea [15]. Studies examining localization of GFP-tagged HAS indicate that the initial adhesion during extension of a filopodium may be mediated by the hyaluronan that is emanating from the synthase located at the tip and along the sides of the filopodium [30]. This could promote filopodial growth in that direction. At some later time, or along a different part of the cell, as hyaluronan synthesis continues, a critical concentration is reached where the pericellular matrix coats both the cell and the substrate and becomes anti-adhesive and/or more malleable. The apparent dual role of hyaluronan in adhesion and de-adhesion, therefore, is based on a complex interplay between hyaluronan synthesis, degradation and internalization, local concentrations of hyaluronan and binding and/or crosslinking proteins which would dictate the viscosity and physical form presented to cell surface receptors and the consequent intracellular signaling. The relative amount of pericellular matrix components compared to the fibrillar components like collagen and fibronectin is also an important parameter to consider.
Hyaluronan is the major pericellular/matrix molecule in the epidermis, while fibrous collagens, fibronectin, and other common components of connective tissues matrices are absent. The unique dominant position of HA in this tissue makes a good example of the dual role of hyaluronan in cell adhesion. Hyaluronan can mediate adhesion between keratinocytes in suspension cultures via bridging CD44 on adjacent cells [103], while in the epidermal tissue hyaluronan accumulation between spinous and granular keratinocytes inhibits the formation of desmosomes, the main cell-cell contacts between keratinocytes [104]. Therefore, all stimuli that activate epidermis, like growth factors, trauma, and inflammation, increase hyaluronan synthesis [105] and influence cell adhesion. Hyaluronan accumulation on keratinocytes enhances the turnover of stable cell-cell contacts, stimulates cell migration and proliferation, and delays the terminal differentiation of the keratinocytes.
Collectively, the data also imply that there can be a “merging” of pericellular and intracellular environments under certain conditions, and that the form and location of hyaluronan can directly influence cell structure. For example, hyaluronan cable structures that arise from within a perinuclear location and connect multiple cell nuclei over long distances form the basis for a novel, mechanically continuous network between cells [47]. Other data suggest that there is a pool of latent HAS in these perinuclear locations that can be activated by various stimuli [30,38]. We have previously observed intracellular hyaluronan in close proximity to nuclear clefts and furrows, as well as binding of exogenous hyaluronan to nucleoli [88]. There are also several observations of a close relationship between intracellular hyaluronan, hyaluronan receptors and structural elements such as microtubules and the microtubule-associated HA receptor RHAMM [88,89,106]. Other studies show that RHAMM, possibly through interactions with intracellular hyaluronan or hyaluronan in the cell coat, plays a role in regulating microtubule activity and stabilization of the mitotic spindle [107]. This indicates an important role for pericellular and/or intracellular hyaluronan in the maintenance of the proper milieu for normal cell shape changes, and as part of the cellular architectural framework that regulates gene expression.
Role of the Pericellular Matrix in Mechanotransduction and Cell Response to Strain
The stiffness of crosslinked hyaluronan gels is important for cellular adhesion and spreading in bioengineering applications [108]. This suggests that local variations in Young’s modulus of the native pericellular matrix may dictate its inherent signal transduction potential, whether it promotes cell adhesion and spreading, or promotes cell detachment and rounding, or contributes to the metastatic potential of cancer cells. Growing evidence that structured hyaluronan is closely associated with cell architecture and that hyaluronan receptors interact with the cytoskeleton point out the need for consideration of the hyaluronan-dependent matrix in cellular mechanical models. According to the tensegrity model, cellular control and cell shape are dependent on a balance of forces between cells and the extracellular matrix [109]. Although most studies in this area have focused on cell surface integrin associations with fibrillar matrix components such as collagen and fibronectin, the mechanical contribution of the hyaluronan-dependent pericellular matrix and its receptors has been largely left out of these models. In addition to contributing to whole tissue material properties (i.e., providing swelling pressure to the local matrix), a few recent observations lead to the hypothesis that hyaluronan and associated proteoglycans may also be important mediators of mechanochemical signaling at the cellular level. The potential of hyaluronan to form cables, together with altering swelling properties, means that the hyaluronan-dependent matrix may itself mediate signals due to tension and shear, as well as tissue compression. Structured hyaluronan therefore, may form the basis of a kind of dynamic and changing web or network through which cells may communicate and respond to external forces.
At present, only a few studies have provided direct evidence that hyaluronan or aggregating proteoglycans are involved in cellular mechanotransduction. One group recently showed that the pericellular matrix was involved in the transduction of shear stress in endothelial cells [110]. Hyaluronidase treatment significantly decreased flow-induced nitric oxide (NO) production, whereas it did not affect acetylcholine-induced NO production. They concluded that hyaluronan within the glycocalyx plays a pivotal role in detecting and amplifying the shear force of flowing blood that triggers endothelium-derived NO production in isolated canine femoral arteries. Similarly, another study found that fluid flow-induced PGE2 release by bone cells is reduced via glycocalyx degradation by hyaluronidase whereas calcium signals are not [111]. In a study examining membrane properties, an atomic force microscopic probe was used to pull “membrane tethers” from the cell surface with and without digestion of the glycocalyx with hyaluronidase [112]. They found that the pericellular matrix contributes significantly to the heterogeneity in membrane properties and its presence increases the number and strength of the membrane tethers. This implies that the number and strength of cellular interconnections in vivo may be influenced by the presence of the pericellular matrix. Along similar lines, another study showed that hyaluronan influences the number of gap-junctional intercellular communications of normal human dermal fibroblasts [113].
Mechanical strain is known to influence hyaluronan and proteoglycan production, and several studies provide evidence that the cells respond to mechanical input by synthesizing a matrix that will allow the cells and/or tissue to adapt to the new mechanical environment. For example, flow-induced shear has been shown to upregulate hyaluronan synthesis in endothelial cells [114]. Cyclic stretch was shown to augment hyaluronan production in human cervical fibroblasts [115]. CD44 levels and expression of mRNA for HAS3 were increased in strained fibrocartilage cells, highlighting the role for movement-induced stimuli in differential extracellular matrix metabolism during joint development, and showing that strain may differentially regulate HA synthase gene expression [116,117]. Cyclic compression of tendon explants resulted in synthesis and accumulation of large aggregating proteoglycans by resident fibroblasts, indicating an adaptive response in the development of fibrocartilage in locations where tendons are under compression [118,119]. Cyclic stretch of smooth muscle cells upregulated versican and TSG-6 and increased the amount of hyaluronan-versican aggregates [120]. Other studies point to a role for the cell coat in the regulation of stress-strain and local fluid flow environment in chondrocytes [121] and invoke fluid flow-induced streaming potentials as a potential mechanism for signal transduction.
Abnormal strains may also create conditions that promote inflammation through effects on the pericellular matrix. For example, low-molecular-weight hyaluronan was induced by cyclic stretch in lung fibroblasts and accumulated in lungs from animals with ventilator-induced lung injury. In hyaluronan synthase-3 knockout mice, these reactions were significantly reduced, except for the capillary leakage that resulted from mechanical injury [122]. Cyclic tensile stretch of chondrocytes caused depolymerization of hyaluronan and induced reactive oxygen species (ROS). Superoxide dismutase inhibited not only ROS induction but also hyaluronan depolymerization caused by the mechanical stress. The authors concluded that ROS play an important role in mechanical stress-induced hyaluronan depolymerization [123]. Oxidant injury plays a critical role in the degenerative changes that are characterized by a decline in cell numbers and viability, and occurs with aging and in the etiology of many diseases. Exogenous hyaluronan and chondroitin-4-sulfate confer protection from oxidative damage in fibroblasts and other cells [124,125]. Other studies showed a direct role for versican expression in protection from oxidation [69]. While hyaluronan, like chondroitin sulfate, acts as a scavenger of ROS, it undergoes degradation during the process [126]. This is consistent with the finding that even the normal turnover of pericellular hyaluronan is regulated by ROS in human epidermis [127]. In histological sections of human breast cancer, staining of cell-associated hyaluronan is inversely related to ROS and NO• synthesis, suggesting that in excess they can form super radicals that induce shedding of pericellular hyaluronan [128]. In general, factors that control the turnover of the pericellular hyaluronan coat, whether enzymatic [129] or mediated by ROS, are poorly known.
Increased cell-associated hyaluronan and increased thickness of the cell coat has been associated with myofibroblast differentiation [130,131]. This may be regulated at the level of hyaluronan degradation [131]. However, more studies will be required to determine the role of structured hyaluronan, specific HAS enzymes, hyaluronan receptors and associated proteins in the maintenance of a contractile phenotype in fibroblasts.
Pericellular hyaluronan expression is tightly associated with, and probably regulates cellular differentiation. In human skin organ cultures, factors like all-trans-retinoic acid which increase hyaluronan synthesis lead to delayed differentiation [132], like in human skin in vivo. Likewise, organotypic epidermal cultures show that keratinocyte differentiation is stimulated and inhibited by factors that decrease and increase hyaluronan synthesis, respectively [133]. Enhancing turnover of cell surface hyaluronan by hyaluronidase promotes terminal differentiation of keratinocytes [134].
Conclusions
The hyaluronan-dependent pericellular matrix is a multifunctional regulator of cell adhesion, cell shape and behavior. Hyaluronan-binding proteoglycans and other associated proteins in the cell coat contribute to a wide variety of structural morphologies and material properties of the pericellular matrix, and add complexity to the biological functions of the matrix. Greater levels of structure and crosslinking of hyaluronan contribute to important processes such as ovulation and inflammation. Hyaluronan synthesis can create and support plasma membrane protrusions. More detailed knowledge of pericellular matrix structure and function under different biological conditions and disease states will aid in formulating therapeutic intervention strategies and bioengineering approaches.
Acknowledgments
The authors would like to thank Dr. Virginia Green for careful reading and editing of the manuscript. Support was provided through NIH grant P01 HL018645-31 (SPE & TNW) and The Academy of Finland and Sigrid Juselius Foundation (RHT & MIT). We also want to thank Dr. Kirsi Rilla and Anne Kultti, MSc for the images of the GFP-Has transfected cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 2.Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277:4581–4584. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- 3.Stern R, Csoka A. Mammalian Hyaluronidases. In: Hascall V, Yanagashita M, editors. Glycoforum/Science of Hyaluronan Today. Seikagaku, Corporation; Tokyo, Japan: 2000. http://www.glycoforum.gr.jp. [Google Scholar]
- 4.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 5.Cowman MK, Spagnoli C, Kudasheva D, Li M, Dyal A, Kanai S, Balazs EA. Extended, relaxed, and condensed conformations of hyaluronan observed by atomic force microscopy. Biophys J. 2005;88:590–602. doi: 10.1529/biophysj.104.049361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarris BJ, Fraser JRE. On the pericellular zone of some mammalian cells in vitro. Exp Cell Res. 1968;49:181–193. doi: 10.1016/0014-4827(68)90530-2. [DOI] [PubMed] [Google Scholar]
- 7.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBaron RG, Zimmerman DR, Rouslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- 9.Wight TN, Potter-Perigo S, Aulinskas T. Proteoglycans and vascular cell proliferation. Am Rev Respir Dis. 1989;140:1132–1135. doi: 10.1164/ajrccm/140.4.1132. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- 12.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 13.Lee GM, Johnstone B, Jacobson K, Caterson B. The dynamic structure of the pericellular matrix on living cells. J Cell Biol. 1993;123:1899–1907. doi: 10.1083/jcb.123.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg RL, Toole BP. Pericellular coat of chick embryo chondrocytes: structural role of hyaluronate. J Cell Biol. 1984;99:2114–2122. doi: 10.1083/jcb.99.6.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evanko S, Angello J, Wight T. Formation of hyaluronan and versican rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 16.Mow VC, Mak AF, Lai WM, Rosenberg LC, Tang LH. Viscoelastic properties of proteoglycan subunits and aggregates in varying solution concentrations. J Biomechanics. 1984;17:325–338. doi: 10.1016/0021-9290(84)90027-7. [DOI] [PubMed] [Google Scholar]
- 17.Morgelin M, Paulsson M, Heinegard D, Aebi U, Engel J. Evidence of a defined spatial arrangement of hyaluronate in the central filament of cartilage proteoglycan aggregates. Biochem J. 1995;307:595–601. doi: 10.1042/bj3070595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldin P, Suzuki M, Teder P, Pertoft H. Chondroitin sulfate proteoglycan modulates the permeability of hyaluronan containing coat around normal human mesothelial cells. J Cell Physiol. 1995;165:54–61. doi: 10.1002/jcp.1041650107. [DOI] [PubMed] [Google Scholar]
- 19.Christner JE, Brown ML, Dziewiatkowski DD. Interactions of cartilage proteoglycans with hyaluronate. Inhibition of the interaction by modified oligomers of hyaluronate. J Biol Chem. 1979;254:4624–4630. [PubMed] [Google Scholar]
- 20.Franzen A, Bjornsson S, Heinegard D. Cartilage proteoglycan aggregate formation. Role of link protein. Biochem J. 1981;197:669–674. doi: 10.1042/bj1970669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardingham TE. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979;177:237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewton RG, Mayne R. Mammalian vitreous humor contains networks of hyaluronan molecules: electron microscopic analysis using the hyaluronan-binding region (G1) of aggrecan and link protein. Exp Cell Res. 1992;198:237–249. doi: 10.1016/0014-4827(92)90376-j. [DOI] [PubMed] [Google Scholar]
- 23.Koob TJ, Vogel KG. Site-related variations in glycosaminoglycan content and swelling properties of bovine flexor tendon. J Orthop Res. 1987;5:414–424. doi: 10.1002/jor.1100050314. [DOI] [PubMed] [Google Scholar]
- 24.Koob TJ. Effects of chondroitinase-ABC on proteoglycans and swelling properties of fibrocartilage in bovine flexor tendon. J Orthop Res. 1989;7:219–227. doi: 10.1002/jor.1100070209. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 26.Wight TN. The vascular extracellular matrix. In: Fuster RRV, Topol EJ, editors. Atherosclerosis and Coronary Artery Disease. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 421–440. [Google Scholar]
- 27.Wight TN, Lara S, Riessen R, LeBaron R, Isner J. Selective deposits of versican in the extracellular matrix of restenotic lesions from human peripheral arteries. Am J Pathol. 1997;151:963–973. [PMC free article] [PubMed] [Google Scholar]
- 28.Sheehan J, Almond A. Hyaluronan: Static, Hydrodynamic and Molecular Dynamic Views Nov. 1, 2001. 2001 Glycoforum: http://www.glycoforum.gr.jp/science/hyaluronan/hyaluronanE.html.
- 29.Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- 30.Rilla K, Siiskonen H, Spicer AP, Hyttinen JM, Tammi MI, Tammi RH. Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J Biol Chem. 2005;280:31890–31897. doi: 10.1074/jbc.M504736200. [DOI] [PubMed] [Google Scholar]
- 31.Kultti A, Rilla K, Tiihonen R, Spicer AP, Tammi RH, Tammi MI. Hyaluronan synthesis induces microvillus-like cell surface protrusions. J Biol Chem. 2006;281:15821–15828. doi: 10.1074/jbc.M512840200. [DOI] [PubMed] [Google Scholar]
- 32.Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Aspberg A, Binkert C, Ruoslahti E. The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc Natl Acad Sci U S A. 1995;92:10590–10594. doi: 10.1073/pnas.92.23.10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundell A, Olin AI, Morgelin M, al-Karadaghi S, Aspberg A, Logan DT. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure. 2004;12:1495–1506. doi: 10.1016/j.str.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004;279:38079–38082. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 36.Blom A, Pertoft H, Fries E. Inter-α-inhibitor is required for the formation of the hyaluronan-containing coat on fibroblasts and mesothelial cells. J Biol Chem. 1995;270:9698–9701. doi: 10.1074/jbc.270.17.9698. [DOI] [PubMed] [Google Scholar]
- 37.Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24:217–227. doi: 10.1055/s-2006-948551. [DOI] [PubMed] [Google Scholar]
- 38.de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuo L, Kanamori A, Kannagi R, Itano N, Wu J, Hamaguchi M, Ishiguro N, Kimata K. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J Biol Chem. 2006;281:20303–20314. doi: 10.1074/jbc.M506703200. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo L, Salustri A, Kimata K. A physiological function of serum proteoglycan bikunin: the chondroitin sulfate moiety plays a central role. Glycoconj J. 2002;19:241–247. doi: 10.1023/A:1025331929373. [DOI] [PubMed] [Google Scholar]
- 41.Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, Mahoney DJ, Day AJ, Siracusa G, Romani L, Mantovani A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 42.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- 43.Kuznetsova SA, Day AJ, Mahoney DJ, Rugg MS, Mosher DF, Roberts DD. The N-terminal module of thrombospondin-1 interacts with the link domain of TSG-6 and enhances its covalent association with the heavy chains of inter-alpha-trypsin inhibitor. J Biol Chem. 2005;280:30899–30908. doi: 10.1074/jbc.M500701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lesley J, Gal I, Mahoney DJ, Cordell MR, Rugg MS, Hyman R, Day AJ, Mikecz K. TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J Biol Chem. 2004;279:25745–25754. doi: 10.1074/jbc.M313319200. [DOI] [PubMed] [Google Scholar]
- 45.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem. 2003;278:47223–47231. doi: 10.1074/jbc.M304871200. [DOI] [PubMed] [Google Scholar]
- 46.Selbi W, de la Motte CA, Hascall VC, Day AJ, Bowen T, Phillips AO. Characterization of hyaluronan cable structure and function in renal proximal tubular epithelial cells. Kidney Int. 2006;70:1287–1295. doi: 10.1038/sj.ki.5001760. [DOI] [PubMed] [Google Scholar]
- 47.Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Zhang XL, Selbi W, de la Motte C, Hascall V, Phillips A. Renal proximal tubular epithelial cell transforming growth factor-beta1 generation and monocyte binding. Am J Pathol. 2004;165:763–773. doi: 10.1016/s0002-9440(10)63339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) J Biol Chem. 1999;274:30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- 50.Wilkinson TS, Bressler SL, Evanko SP, Braun KR, Wight TN. Overexpression of hyaluronan synthases alters vascular smooth muscle cell phenotype and promotes monocyte adhesion. J Cell Physiol. 2006;206:378–385. doi: 10.1002/jcp.20468. [DOI] [PubMed] [Google Scholar]
- 51.Selbi W, Day AJ, Rugg MS, Fulop C, de la Motte CA, Bowen T, Hascall VC, Phillips AO. Overexpression of hyaluronan synthase 2 alters hyaluronan distribution and function in proximal tubular epithelial cells. J Am Soc Nephrol. 2006;17:1553–1567. doi: 10.1681/ASN.2005080879. [DOI] [PubMed] [Google Scholar]
- 52.Hedman K, Kurkinen M, Alitalo K, Vaheri A, Johansson S, Hook M. Isolation of the pericellular matrix of human fibroblast cultures. J Cell Biol. 1979;81:83–91. doi: 10.1083/jcb.81.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- 54.Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- 55.Joddar B, Ramamurthi A. Elastogenic effects of exogenous hyaluronan oligosaccharides on vascular smooth muscle cells. Biomaterials. 2006;27:5698–5707. doi: 10.1016/j.biomaterials.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 56.Tammi R, Tammi M. Correlations between hyaluronan and epidermal proliferation as studied by [3H]glucosamine and [3H]thymidine incorporations and staining of hyaluronan on mitotic keratinocytes. Exp Cell Res. 1991;195:524–527. doi: 10.1016/0014-4827(91)90405-j. [DOI] [PubMed] [Google Scholar]
- 57.Schonherr E, Jarvelainen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-β1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- 58.Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- 59.Deudon E, Berrou E, Breton M, Picard J. Growth-related production of proteoglycans and hyaluronic acid in synchronous arterial smooth muscle cells. Int J Biochem. 1992;24:465–470. doi: 10.1016/0020-711x(92)90040-8. [DOI] [PubMed] [Google Scholar]
- 60.Brecht M, Mayer U, Schlosser E, Prehm P. Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem J. 1986;239:445–450. doi: 10.1042/bj2390445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papakonstantinou E, Karakiulakis G, Roth M, Block LH. Platelet-derived growth factor stimulates the secretion of hyaluronic acid by proliferating human vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1995;92:9881–9885. doi: 10.1073/pnas.92.21.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savani RC, Wang C, Yang B, Zhang S, Kinsella MG, Wight TN, Stern R, Nance DM, Turley EA. Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J Clin Invest. 1995;95:1158–1168. doi: 10.1172/JCI117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savani RC, Turley EA. The role of hyaluronan and its receptors in restenosis after balloon angioplasty: development of a potential therapy. Int J Tissue React. 1995;17:141–151. [PubMed] [Google Scholar]
- 64.Underhill CB, Nguyen H, Shizari M, Culty M. CD44 positive macrophages take up hyaluronan during lung development. Dev Biol. 1993;155:324–336. doi: 10.1006/dbio.1993.1032. [DOI] [PubMed] [Google Scholar]
- 65.Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93:1141–1147. doi: 10.1161/01.cir.93.6.1141. [DOI] [PubMed] [Google Scholar]
- 66.West D, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989;183:179–196. doi: 10.1016/0014-4827(89)90428-x. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y, Chen L, Cao L, Sheng W, Yang BB. Overexpression of the C-terminal PG-M/versican domain impairs growth of tumor cells by intervening in the interaction between epidermal growth factor receptor and beta1-integrin. J Cell Sci. 2004;117:2227–2237. doi: 10.1242/jcs.01057. [DOI] [PubMed] [Google Scholar]
- 68.Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, Wu Y, Kerbel RS, Yang BB. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004;18:754–756. doi: 10.1096/fj.03-0545fje. [DOI] [PubMed] [Google Scholar]
- 69.Wu Y, Wu J, Lee DY, Yee A, Cao L, Zhang Y, Kiani C, Yang BB. Versican protects cells from oxidative stress-induced apoptosis. Matrix Biol. 2005;24:3–13. doi: 10.1016/j.matbio.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 71.Sheng W, Dong H, Lee DY, Lu WY, Yang BB. Versican modulates gap junction intercellular communication. J Cell Physiol. 2007;211:213–219. doi: 10.1002/jcp.20921. [DOI] [PubMed] [Google Scholar]
- 72.Barnhart BJ, Cox SH, Kraemer PM. Detachment Variants of chinese hamster cells. Hyaluronic acid as a modulator of cell detachment. Exp Cell Res. 1979;119:327–332. doi: 10.1016/0014-4827(79)90360-4. [DOI] [PubMed] [Google Scholar]
- 73.Abatangelo G, Cortivo R, Martelli M, Vecchia P. Cell detachment mediated by hyaluronic acid. Exp Cell Res. 1982;137:73–78. doi: 10.1016/0014-4827(82)90009-x. [DOI] [PubMed] [Google Scholar]
- 74.Koochekpour S, Pilkington GJ, Merzak A. Hyaluronic acid/CD44H interaction induces cell detachment and stimulates migration and invasion of human glioma cells in vitro. Int J Cancer. 1995;63:450–454. doi: 10.1002/ijc.2910630325. [DOI] [PubMed] [Google Scholar]
- 75.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 76.Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development. 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- 77.Snow DM, Smith JD, Cunningham AT, McFarlin J, Goshorn EC. Neurite elongation on chondroitin sulfate proteoglycans is characterized by axonal fasciculation. Exp Neurol. 2003;182:310–321. doi: 10.1016/s0014-4886(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 78.Yamagata M, Saga S, Kato M, Bernfield M, Kimata K. Selective distributions of proteoglycans and their ligands in pericellular matrix of cultured fibroblasts. Implications for their roles in cell-substratum adhesion. J Cell Sci. 1993;106:55–65. doi: 10.1242/jcs.106.1.55. [DOI] [PubMed] [Google Scholar]
- 79.Toole BP. Developmental role of hyaluronate. Conn Tiss Res. 1982;10:93–100. doi: 10.3109/03008208209034409. [DOI] [PubMed] [Google Scholar]
- 80.Evanko S, Wight T. Intracellular Hyaluronan. In: Hascall V, Yanagishita M, editors. Glycoforum/Science of Hyaluronan Today. Seikagaku, Corporation; Tokyo, Japan: 2001. http://www.glycoforum.gr.jp. [Google Scholar]
- 81.Underhill C, Toole B. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979;82:475–484. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turley EA, Torrance J. Localization of hyaluronate and hyaluronate-binding protein on motile and non-motile fibroblasts. Exp Cell Res. 1984;161:17–28. doi: 10.1016/0014-4827(85)90486-0. [DOI] [PubMed] [Google Scholar]
- 83.Toole BP. Hyaluronan promotes the malignant phenotype. Glycobiology. 2002;12:37R–42R. doi: 10.1093/glycob/12.3.37r. [DOI] [PubMed] [Google Scholar]
- 84.Lesley J, Hyman R. CD44 structure and function. Front Biosci. 1998;3:d616–630. doi: 10.2741/a306. [DOI] [PubMed] [Google Scholar]
- 85.Moffat FL, Jr, Han T, Li ZM, Peck MD, Falk RE, Spalding PB, Jy W, Ahn YS, Chu AJ, Bourguignon LY. Involvement of CD44 and the cytoskeletal linker protein ankyrin in human neutrophil bacterial phagocytosis. J Cell Physiol. 1996;168:638–647. doi: 10.1002/(SICI)1097-4652(199609)168:3<638::AID-JCP16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 86.Vachon E, Martin R, Plumb J, Kwok V, Vandivier RW, Glogauer M, Kapus A, Wang X, Chow CW, Grinstein S, Downey GP. CD44 is a phagocytic receptor. Blood. 2006;107:4149–4158. doi: 10.1182/blood-2005-09-3808. [DOI] [PubMed] [Google Scholar]
- 87.Hall C, Wang C, Lange L, Turley E. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994;126:575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Evanko S, Wight T. Intracellular localization of hyaluronan in proliferating cells. J Histochem Cytochem. 1999;47:1331–1341. doi: 10.1177/002215549904701013. [DOI] [PubMed] [Google Scholar]
- 89.Evanko SP, Parks WT, Wight TN. Intracellular hyaluronan in arterial smooth muscle cells: association with microtubules, RHAMM, and the mitotic spindle. J Histochem Cytochem. 2004;52:1525–1535. doi: 10.1369/jhc.4A6356.2004. [DOI] [PubMed] [Google Scholar]
- 90.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13:105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- 92.Lepperdinger G, Mullegger J, Kreil G. Hyal2--less active, but more versatile? Matrix Biol. 2001;20:509–514. doi: 10.1016/s0945-053x(01)00170-6. [DOI] [PubMed] [Google Scholar]
- 93.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 94.Rilla K, Lammi MJ, Sironen R, Torronen K, Luukkonen M, Hascall VC, Midura RJ, Hyttinen M, Pelkonen J, Tammi M, Tammi R. Changed lamellipodial extension, adhesion plaques and migration in epidermal keratinocytes containing constitutively expressed sense and antisense hyaluronan synthase 2 (Has2) genes. J Cell Sci. 2002;115:3633–3643. doi: 10.1242/jcs.00042. [DOI] [PubMed] [Google Scholar]
- 95.Cohen M, Joester D, Geiger B, Addadi L. Spatial and temporal sequence of events in cell adhesion: from molecular recognition to focal adhesion assembly. Chembiochem. 2004;5:1393–1399. doi: 10.1002/cbic.200400162. [DOI] [PubMed] [Google Scholar]
- 96.Cohen M, Kam Z, Addadi L, Geiger B. Dynamic study of the transition from hyaluronan- to integrin-mediated adhesion in chondrocytes. Embo J. 2006;25:302–311. doi: 10.1038/sj.emboj.7600960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zimmerman E, Geiger B, Addadi L. Initial stages of cell-matrix adhesion can be mediated and modulated by cell-surface hyaluronan. Biophys J. 2002;82:1848–1857. doi: 10.1016/S0006-3495(02)75535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–10285. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- 99.Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- 100.Nandi A, Estess P, Siegelman MH. Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J Biol Chem. 2000;275:14939–14948. doi: 10.1074/jbc.275.20.14939. [DOI] [PubMed] [Google Scholar]
- 101.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 102.Oliferenko S, Kaverina I, Small JV, Huber LA. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J Cell Biol. 2000;148:1159–1164. doi: 10.1083/jcb.148.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milstone LM, Hough-Monroe L, Kugelman LC, Bender JR, Haggerty JG. Epican, a heparan/chondroitin sulfate proteoglycan form of CD44, mediates cell-cell adhesion. J Cell Sci. 1994;107(Pt 11):3183–3190. doi: 10.1242/jcs.107.11.3183. [DOI] [PubMed] [Google Scholar]
- 104.Tammi R, Tammi M. Influence of retinoic acid on the ultrastructure and hyaluronic acid synthesis of adult human epidermis in whole skin organ culture. J Cell Physiol. 1986;126:389–398. doi: 10.1002/jcp.1041260309. [DOI] [PubMed] [Google Scholar]
- 105.Tammi R, Pasonen-Seppanen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005;124:898–905. doi: 10.1111/j.0022-202X.2005.23697.x. [DOI] [PubMed] [Google Scholar]
- 106.Assmann V, Jenkinson D, Marshall J, Hart I. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. J Cell Sci. 1999;112:3943–3954. doi: 10.1242/jcs.112.22.3943. [DOI] [PubMed] [Google Scholar]
- 107.Maxwell CA, Keats JJ, Crainie M, Sun X, Yen T, Shibuya E, Hendzel M, Chan G, Pilarski LM. RHAMM is a centrosomal protein that interacts with dynein and maintains spindle pole stability. Mol Biol Cell. 2003;14:2262–2276. doi: 10.1091/mbc.E02-07-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schneider A, Francius G, Obeid R, Schwinte P, Hemmerle J, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C. Polyelectrolyte multilayers with a tunable Young's modulus: influence of film stiffness on cell adhesion. Langmuir. 2006;22:1193–1200. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- 109.Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- 110.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003;285:H722–726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 111.Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology. 2003;40:591–603. [PubMed] [Google Scholar]
- 112.Sun M, Graham JS, Hegedus B, Marga F, Zhang Y, Forgacs G, Grandbois M. Multiple membrane tethers probed by atomic force microscopy. Biophys J. 2005;89:4320–4329. doi: 10.1529/biophysj.104.058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park JU, Tsuchiya T. Increase in gap-junctional intercellular communications (GJIC) of normal human dermal fibroblasts (NHDF) on surfaces coated with high-molecular-weight hyaluronic acid (HMW HA) J Biomed Mater Res. 2002;60:541–547. doi: 10.1002/jbm.10171. [DOI] [PubMed] [Google Scholar]
- 114.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into the endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 115.Takemura M, Itoh H, Sagawa N, Yura S, Korita D, Kakui K, Kawamura M, Hirota N, Maeda H, Fujii S. Cyclic mechanical stretch augments hyaluronan production in cultured human uterine cervical fibroblast cells. Mol Hum Reprod. 2005;11:659–665. doi: 10.1093/molehr/gah229. [DOI] [PubMed] [Google Scholar]
- 116.Dowthwaite GP, Ward AC, Flannely J, Suswillo RF, Flannery CR, Archer CW, Pitsillides AA. The effect of mechanical strain on hyaluronan metabolism in embryonic fibrocartilage cells. Matrix Biol. 1999;18:523–532. doi: 10.1016/s0945-053x(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 117.Dowthwaite GP, Flannery CR, Flannelly J, Lewthwaite JC, Archer CW, Pitsillides AA. A mechanism underlying the movement requirement for synovial joint cavitation. Matrix Biol. 2003;22:311–322. doi: 10.1016/s0945-053x(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 118.Koob TJ, Clark PE, Hernandez DJ, Thurmond FA, Vogel KG. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 1992;298:303–312. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- 119.Evanko SP, Vogel KG. Proteoglycan synthesis in fetal tendon is differentially regulated by cyclic compression in vitro. Arch Biochem Biophys. 1993;307:153–164. doi: 10.1006/abbi.1993.1574. [DOI] [PubMed] [Google Scholar]
- 120.Lee RT, Yamamoto C, Feng Y, Potter-Perigo S, Briggs WH, Landschulz KT, Turi TG, Thompson JF, Libby P, Wight TN. Mechanical strain induces specific changes in the synthesis and organization of proteoglycans by vascular smooth muscle cells. J Biol Chem. 2001;276:13847–13851. doi: 10.1074/jbc.M010556200. [DOI] [PubMed] [Google Scholar]
- 121.Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1:317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 122.Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, Quinn DA. The role of hyaluronan synthase 3 in ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:92–98. doi: 10.1164/rccm.200405-652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamazaki K, Fukuda K, Matsukawa M, Hara F, Matsushita T, Yamamoto N, Yoshida K, Munakata H, Hamanishi C. Cyclic tensile stretch loaded on bovine chondrocytes causes depolymerization of hyaluronan: involvement of reactive oxygen species. Arthritis Rheum. 2003;48:3151–3158. doi: 10.1002/art.11305. [DOI] [PubMed] [Google Scholar]
- 124.Campo GM, Avenoso A, Campo S, D'Ascola A, Ferlazzo AM, Calatroni A. Reduction of DNA fragmentation and hydroxyl radical production by hyaluronic acid and chondroitin-4-sulphate in iron plus ascorbate-induced oxidative stress in fibroblast cultures. Free Radic Res. 2004;38:601–611. doi: 10.1080/10715760410001694017. [DOI] [PubMed] [Google Scholar]
- 125.Campo GM, D'Ascola A, Avenoso A, Campo S, Ferlazzo AM, Micali C, Zanghi L, Calatroni A. Glycosaminoglycans reduce oxidative damage induced by copper (Cu+2), iron (Fe+2) and hydrogen peroxide (H2O2) in human fibroblast cultures. Glycoconj J. 2004;20:133–141. doi: 10.1023/B:GLYC.0000018587.67742.4b. [DOI] [PubMed] [Google Scholar]
- 126.Šoltés L, Mendichi R, Kogan G, Schiller J, Stankovská M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 127.Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med. 1997;23:996–1001. doi: 10.1016/s0891-5849(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 128.Karihtala P, Soini Y, Auvinen P, Tammi R, Tammi M, Kosma V-M. Hyaluronan in breast cancer--correlations with nitric oxide synthases and tyrosine nitrosylation. doi: 10.1369/jhc.7A7270.2007. Unpublished manuscript. [DOI] [PubMed] [Google Scholar]
- 129.Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem. 2007;282:5597–5607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- 130.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jenkins RH, Thomas GJ, Williams JD, Steadman R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J Biol Chem. 2004;279:41453–41460. doi: 10.1074/jbc.M401678200. [DOI] [PubMed] [Google Scholar]
- 132.Tammi R, Ripellino JA, Margolis RU, Maibach HI, Tammi M. Hyaluronate accumulation in human epidermis treated with retinoic acid in skin organ culture. J Invest Dermatol. 1989;92:326–332. doi: 10.1111/1523-1747.ep12277125. [DOI] [PubMed] [Google Scholar]
- 133.Pasonen-Seppänen S, Karvinen S, Törrönen K, Hyttinen JM, Jokela T, Lammi MJ, Tammi MI, Tammi R. EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J Invest Dermatol. 2003;120:1038–1044. doi: 10.1046/j.1523-1747.2003.12249.x. [DOI] [PubMed] [Google Scholar]
- 134.Passi A, Sadeghi P, Kawamura H, Anand S, Sato N, White LE, Hascall VC, Maytin EV. Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp Cell Res. 2004;296:123–134. doi: 10.1016/j.yexcr.2004.01.031. [DOI] [PubMed] [Google Scholar]


