Abstract
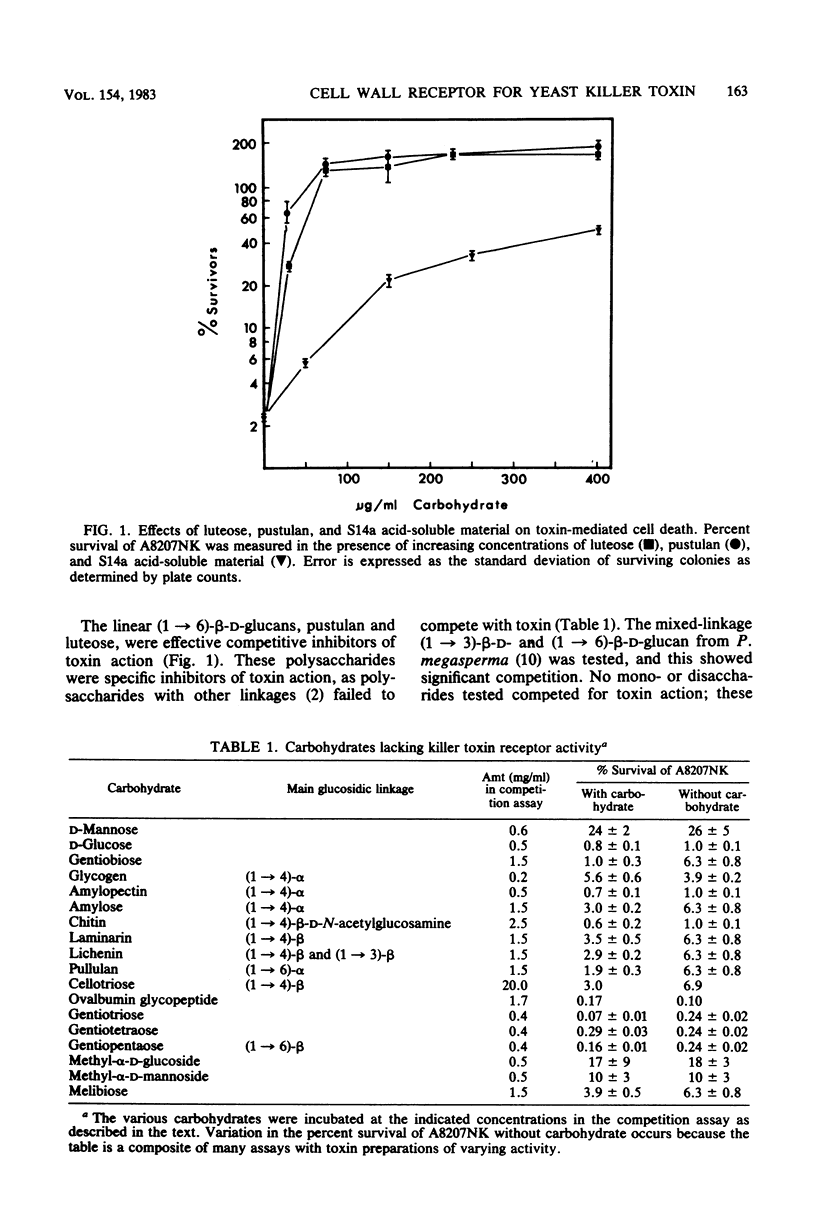
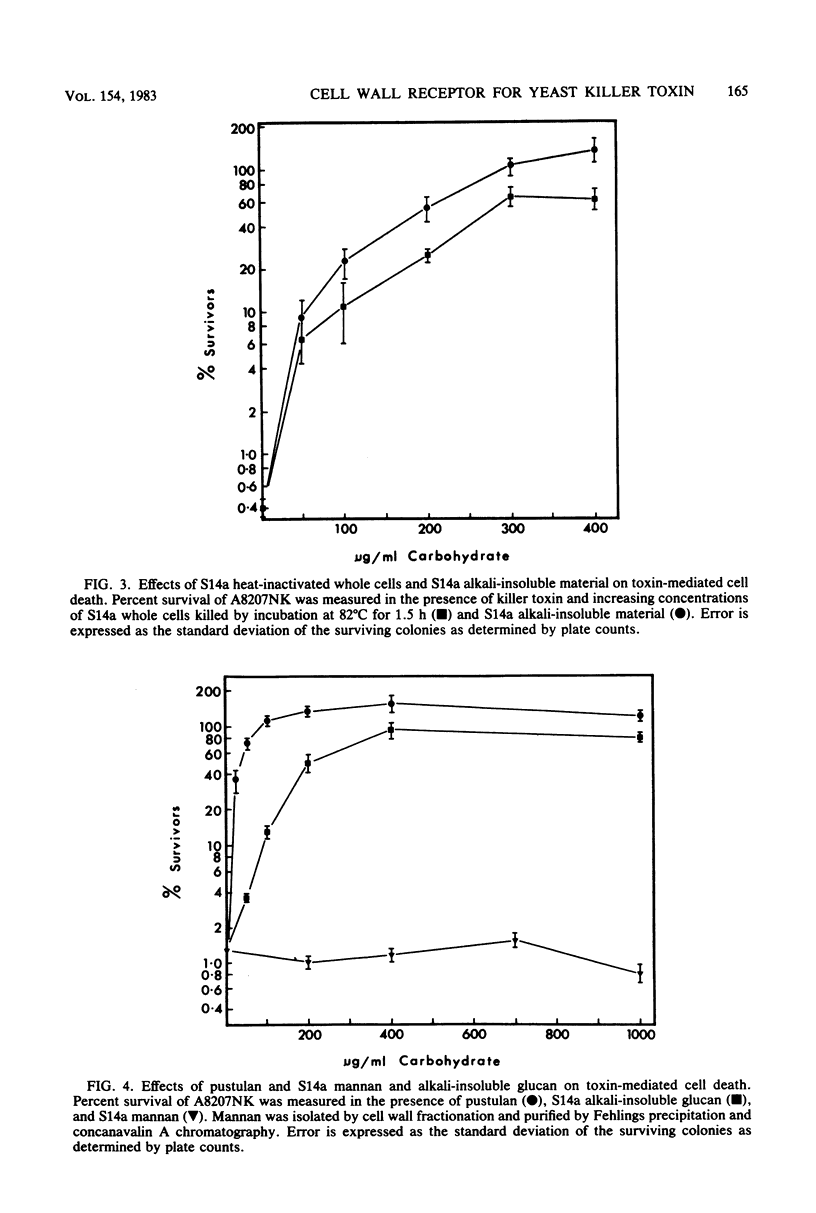
The linear (1 → 6)-β-d-glucans pustulan and luteose were effective competitive inhibitors of killer toxin action. Affinity chromatography of killer toxin on a pustulan-Sepharose column showed that toxin bound directly to a (1 → 6)-β-linked polysaccharide. Other polysaccharides found in yeast cell walls, including (1 → 3)-β-d-glucan, mannan, chitin, and glycogen, were not effective as inhibitors of toxin. Fractionation of yeast cell walls was attempted to identify the toxin receptor in sensitive Saccharomyces cerevisiae. The receptor activity was retained among the insoluble glucans in alkali-washed cells; yeast mannan and alkali-soluble glucan had little receptor activity. A minor fraction of receptor activity was removed from alkali-washed cells by hot acetic acid extraction, a procedure which solubilized some (1 → 6)-β-d-glucan and glycogen. The major fraction (>70%) of receptor activity remained with the acid-insoluble (1 → 6)-β-and (1 → 3)-β-glucans. Zymolyase, an endo-(1 → 3)-β-d-glucanase, solubilized a substantial fraction of the receptor activity in the acid-insoluble glucans. The receptor activity in yeast cell walls was periodate and (1 → 6)-β-d-glucanase sensitive, but was resistant to (1 → 3)-β-d-glucanase and α-amylase. The acid-soluble glucan fractions of a sensitive strain and a krel-l receptor-defective toxin-resistant mutant were examined. The krel-l strain had a reduced amount (ca. 50%) of (1 → 6)-β-d-glucan compared with the sensitive parent strain. A sensitive revertant of the krel-l strain regained the parental level of glucan. These results implicate (1 → 6)-β-d-glucan as a component of the yeast cell wall receptor for killer toxin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Aidroos K., Bussey H. Chromosomal mutants of Saccharomyces cerevisiae affecting the cell wall binding site for killer factor. Can J Microbiol. 1978 Mar;24(3):228–237. doi: 10.1139/m78-041. [DOI] [PubMed] [Google Scholar]
- BADIN J., JACKSON C., SCHUBERT M. Improved method for determination of plasma polysaccharides with tryptophan. Proc Soc Exp Biol Med. 1953 Nov;84(2):289–291. [PubMed] [Google Scholar]
- Bussey H., Saville D., Hutchins K., Palfree R. G. Binding of yeast killer toxin to a cell wall receptor on sensitive Saccharomyces cerevisiae. J Bacteriol. 1979 Dec;140(3):888–892. doi: 10.1128/jb.140.3.888-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Sherman D., Somers J. M. Action of yeast killer factor: a resistant mutant with sensitive spheroplasts. J Bacteriol. 1973 Mar;113(3):1193–1197. doi: 10.1128/jb.113.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Manners D. J. Isolation and composition of an alkali-soluble glucan from the cell walls of Saccharomyces cerevisiae. J Gen Microbiol. 1976 May;94(1):180–192. doi: 10.1099/00221287-94-1-180. [DOI] [PubMed] [Google Scholar]
- HALVORSON H. Studies on protein and nucleic acid turnover in growing cultures of yeast. Biochim Biophys Acta. 1958 Feb;27(2):267–276. doi: 10.1016/0006-3002(58)90333-0. [DOI] [PubMed] [Google Scholar]
- Hahn M. G., Albersheim P. Host-Pathogen Interactions: XIV. Isolation and Partial Characterization of an Elicitor from Yeast Extract. Plant Physiol. 1978 Jul;62(1):107–111. doi: 10.1104/pp.62.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K., Kurokawa M. Separation of fucose and acetylhexosamines by two-dimensional thin-layer chromatography. Anal Biochem. 1968 Dec;26(3):472–474. doi: 10.1016/0003-2697(68)90216-9. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. The complex carbohydrates of mammalian cell surfaces and their biological roles. Essays Biochem. 1975;11:1–36. [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C., Björndal H., Lindberg B. The structure of a beta-(1--6)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):31–36. doi: 10.1042/bj1350031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C. The structure of a beta-(1 leads to 3)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem. 1974 Dec 10;249(23):7685–7694. [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Kern K. A., Antalis C., Ballou C. E. Genetic control of yeast mannan structure. Isolation and characterization of mannan mutants. J Biol Chem. 1973 Jul 10;248(13):4660–4666. [PubMed] [Google Scholar]




