Abstract
Background
Vascular plants respond to pathogens by activating a diverse array of defense mechanisms. Studies with these plants have provided a wealth of information on pathogen recognition, signal transduction and the activation of defense responses. However, very little is known about the infection and defense responses of the bryophyte, Physcomitrella patens, to well-studied phytopathogens. The purpose of this study was to determine: i) whether two representative broad host range pathogens, Erwinia carotovora ssp. carotovora (E.c. carotovora) and Botrytis cinerea (B. cinerea), could infect Physcomitrella, and ii) whether B. cinerea, elicitors of a harpin (HrpN) producing E.c. carotovora strain (SCC1) or a HrpN-negative strain (SCC3193), could cause disease symptoms and induce defense responses in Physcomitrella.
Results
B. cinerea and E.c. carotovora were found to readily infect Physcomitrella gametophytic tissues and cause disease symptoms. Treatments with B. cinerea spores or cell-free culture filtrates from E.c. carotovoraSCC1 (CF(SCC1)), resulted in disease development with severe maceration of Physcomitrella tissues, while CF(SCC3193) produced only mild maceration. Although increased cell death was observed with either the CFs or B. cinerea, the occurrence of cytoplasmic shrinkage was only visible in Evans blue stained protonemal cells treated with CF(SCC1) or inoculated with B. cinerea. Most cells showing cytoplasmic shrinkage accumulated autofluorescent compounds and brown chloroplasts were evident in a high proportion of these cells. CF treatments and B. cinerea inoculation induced the expression of the defense-related genes: PR-1, PAL, CHS and LOX.
Conclusion
B. cinerea and E.c. carotovora elicitors induce a defense response in Physcomitrella, as evidenced by enhanced expression of conserved plant defense-related genes. Since cytoplasmic shrinkage is the most common morphological change observed in plant PCD, and that harpins and B. cinerea induce this type of cell death in vascular plants, our results suggest that E.c. carotovora CFSCC1 containing HrpN and B. cinerea could also induce this type of cell death in Physcomitrella. Our studies thus establish Physcomitrella as an experimental host for investigation of plant-pathogen interactions and B. cinerea and elicitors of E.c. carotovora as promising tools for understanding the mechanisms involved in defense responses and in pathogen-mediated cell death in this simple land plant.
Background
Plants are continuously subjected to pathogen attack and respond by activating a range of defense mechanisms. Recognition of the pathogen or elicitors derived either from the pathogen or released from the plant cell wall is accompanied with the production of molecular signals including salicylic acid [1], jasmonic acid [2] and ethylene [3] that lead to the induction of defense gene expression. This in turn results in the accumulation of functionally diverse pathogenesis-related (PR) proteins and metabolites (e.g., phenylpropanoids) [4,5]. Recognition of the pathogen or elicitors is usually accompanied by the rapid death of the infected cells, known as the hypersensitive response (HR), which limits the access of the pathogen to water and nutrients thereby restricting its growth [6,7]. HR can be triggered either by non-specific elicitors recognized by plant receptors, or by specific elicitors (encoded by pathogen avirulence (avr) genes) recognized by corresponding encoded products of plant resistance (R) genes [8,9]. Several studies have suggested that plant cell death resulting from the HR is a type of programmed cell death (PCD). Plant cells undergoing PCD share a number of characteristic morphological and biochemical features in common with animal cell apoptosis [7,10,11]. Moreover, cell death with apoptotic features has also been observed in plants susceptible to virulent pathogens [12,13].
Although bryophytes are non-vascular plants and are considered to be primitive among the embryophyta, mosses have been shown to respond to a variety of environmental stimuli and to several common plant growth factors much like vascular plants. Thus, in spite of having diverged from vascular plants approximately 700 million years ago [14], mosses are well-suited for the study of fundamental processes in plant biology. Furthermore, mosses have a simple developmental program and a life cycle with a predominant haploid phase which greatly facilitates genetic analysis [15].
Physcomitrella patens, a relatively small moss, has recently become a model plant to study plant gene function in that it exhibits high-frequency homologous recombination comparable with that of Saccharomyces cerevisiae, enabling the construction of gene knock-outs [16,17]. The assembled Physcomitrella genome has recently been released and full-length cDNAs in addition to 80,000 ESTs are available in the databases [18-20]. These advantages together with the presence of a great number of Physcomitrella ESTs with high sequence identity to defense-related genes of vascular plants, many of them with unknown functions, makes this plant a very useful model to study plant-pathogen interactions. The susceptibility of distinct tissues to pathogens can also be studied, since Physcomitrella can be maintained as a haploid gametophyte with distinct developmental stages. These consist of the protonema which is a filamentous network of cells, and the radially symmetric gametophore which is a leafy shoot composed of a non-vascular stem with leaves as well as rhizoids [21]. Disease development can be visualized microscopically in that the leaves and protonemal filaments are formed of a monolayer of cells.
There have been very few reports on either pathogen infection or the activation of defense responses in mosses. In silico analysis of the Physcomitrella genome, however, indicates the presence of several encoded proteins with high similarity to R gene products found in flowering plants [22]. Regarding natural infection, the fungus Scleroconidioma sphagnicola (S. sphagnicola) can infect and cause disease symptoms in the moss Sphagnum fuscum (S. fuscum) [23] and viruses were detected in Antarctic mosses [24]. S. sphagnicola hyphae can grow inside the cell wall of S. fuscum, digesting wall components, penetrating into cells of leaves and causing chlorosis of the tissue. In more advanced stages of disease development, necrosis of infected leaf and stem cells, as well as host death can be observed [23].
In this study we aimed to identify plant pathogens capable of infecting and triggering a defense response in Physcomitrella, with the goal of establishing a model system to conduct molecular, cellular and genetic studies on Physcomitrella-pathogen interactions. We used two pathogens with a broad host range, the bacterium Erwinia carotovora ssp carotovora (E.c. carotovora) and the fungus Botrytis cinerea (B. cinerea). E.c. carotovora is a soft-rot Erwinia which causes disease on many vascular plants [25,26]. The main virulence factors of E.c. carotovora are the plant cell wall-degrading enzymes including cellulases, proteases and pectinases [26]. These enzymes cause maceration of the infected tissues and the released cell wall fragments can act as elicitors of the plant defense response [27-31]. Previous studies have shown that cell-free culture filtrate (CF) containing plant cell wall-degrading enzymes from E.c. carotovoraSCC3193 produces similar symptoms and defense gene expression as those caused by E.c. carotovoraSCC3193 infection and enhanced disease resistance in CF-treated plants [28,30-32]. Some E.c. carotovora strains produce harpins, which are small, acidic, glycine-rich, heat-stable proteins, that elicit HR and induction of plant defense responses [33,34]. In the present study we have used two strains of E.c. carotovora; i) E.c. carotovoraSCC1 which is a harpin (HrpN) producing strain [35], and ii) E.c. carotovoraSCC3193 which is a HrpN-negative strain [36]. B. cinerea is a necrotrophic fungal pathogen that attacks over 200 different plant species [37], by producing multiple proteins and metabolites that kill the host cells [38]. The main virulence factors of B. cinerea vary depending on the isolate, and include toxins and cell wall degrading enzymes such as endopolygalacturonases and xylanases [39-41]. In this study, we demonstrate that E.c. carotovora-derived elicitors and B. cinerea cause disease symptoms and induce a defense response in the moss Physcomitrella.
Results
E.c. carotovora and B. cinerea infect Physcomitrella patens
In order to determine whether E.c. carotovora infects Physcomitrella tissues, a gfp-labelled E.c. carotovoraSCC3193 strain was inoculated onto Physcomitrella leaves as described in methods. Two days after inoculation tissue examined by confocal microscopy indicated that the labelled cells of E.c. carotovora had occupied the apoplast between leaf cells, as well as the cellular space of some plant cells in this same tissue (Figure 1A).
Figure 1.
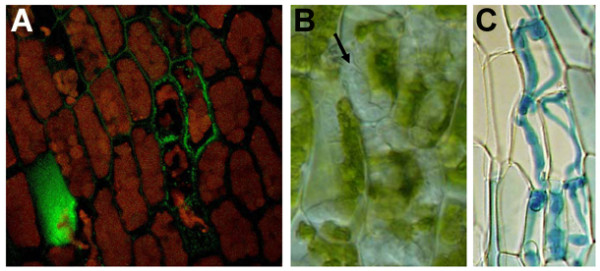
E.c. carotovora and B. cinerea inoculation of Physcomitrella leaves. A. Leaves of Physcomitrella gametophores inoculated with E.c. carotovoraSCC3193 carrying a GFP-expressing plasmid at 2 dpi. B. B. cinerea inoculated leaves at 2 dpi. C. Trypan blue stained B. cinerea hyphae in inoculated leaves at 2 dpi. Arrow indicates hyphae growing in Physcomitrella.
When B. cinerea inoculated leaf tissue was examined, outlines of fungal hyphae were apparent inside the cell cavity displacing the cytoplasmic contents (Figure 1B). Infection of Physcomitrella tissues by B. cinerea was examined in more detail by staining fungal hyphae with trypan blue. Two days post infection (dpi) hyphae appeared to be within the limits of the cell walls in Physcomitrella leaves (Figure 1C). Our observation that B. cinerea hyphae appeared within plant cells is likely in that Physcomitrella leaves are composed of a contiguous monolayer of adjacent cells.
E.c. carotovora, CFs and B. cinerea cause disease symptoms in Physcomitrella
Development of disease symptoms by E.c. carotovora was initiated by inoculating the harpin HrpN-producing E.c. carotovoraSCC1 strain and the HrpN-negative E.c. carotovoraSCC3193 strain onto Physcomitrella leaves. Inoculation with both strains caused visible symptoms around the wounded tissue within 2 days when observed with a magnifying glass, while mock inoculated tissues did not (Figure 2).
Figure 2.
Symptom development in response to E.c. carotovora inoculation. Leaves of Physcomitrella gametophores were wounded and inoculated with 0.9% NaCl (A, D), E.c. carotovoraSCC3193 (B, E) or E.c. carotovoraSCC1 (C, F). Pictures of representative colonies were taken at 2 dpi.
Physcomitrella infection by E.c. carotovora and subsequent development of symptoms required that we first wound the plant mechanically (Figures 1 and 2). Growth or colonization of E.c. carotovora in planta as determined by enumeration of bacteria in a given tissue could not be done due to the difficulty of consistently wounding the tissue sufficiently without causing excessive damage and dessication. Instead of continuing our studies with E.c. carotovora bacterial inoculation, cell-free culture filtrate (CF) was used to elicit a defense response since: i) in vascular plants CF incites the same disease symptoms and induces defense gene expression in the same way as does inoculation with E.c. carotovora [30-32], ii) CF is sprayed onto the colonies allowing for direct and homogeneous contact, and iii) it overcomes the technical difficulty of introducing a sufficient number of small wounds on the moss tissue to allow inoculation by E.c. carotovora for a comprehensive evaluation of the plant defense response.
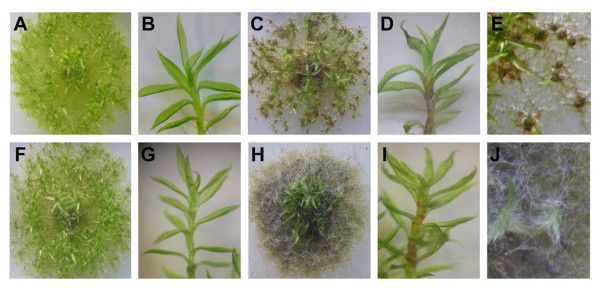
Disease symptom development was observed upon treating Physcomitrella colonies with: (i) CF of E.c. carotovoraSCC1 (CF(SCC1)), (ii) CF of E.c. carotovoraSCC3193 (CF(SCC3193)), or (iii) B. cinerea spores. Disease symptoms such as tissue maceration developed in colonies two days after treatment with either CF(SCC1), CF(SCC3193) or after inoculation with B. cinerea spores, as shown in Figure 3. In control experiments, moss colonies treated with either Luria-Bertani (LB) (Figure 3A), potato dextrose broth (PBD) growth media (not shown) or with spores of a non-pathogen, Aspergillus nidulans (not shown), did not develop disease symptoms. Protonemal filaments treated with either CF(SCC1) or CF(SCC3193) developed maceration symptoms, with CF(SCC3193) causing less tissue damage than CF(SCC1) (Figures 3C and 3F). Additionally, CF(SCC1) -treated colonies acquired a brownish aspect as seen in the protonemal filaments shown in Figures 3C and 3E. CF-treated gametophores, also known as leafy shoots, did not show maceration symptoms, although brownish stems were observed in CF(SCC1)-treated colonies (Figure 3D).
Figure 3.
Symptom development in response to CF treatment and B. cinerea inoculation. Moss colonies and gametophores treated with LB (A, B), CF(SCC1) (C, D), CF(SCC3193) (F, G) or with B. cinerea spores (H, I). A closer view of colonies treated with CF(SCC1) (E), or inoculated with B. cinerea spores (J) is shown. Pictures of representative colonies were taken 2 days after treatment.
Physcomitrella showed a clear susceptibility to B. cinerea, involving a characteristic proliferation of mycelium and the appearance of necrotic protonemal tissue in addition to the browning of stems (Figures 3H, 3J and 3I). Inoculated tissues were soft, macerated and were easily separated from the rest of the moss colony. Four dpi B. cinerea-infected moss tissues were completely macerated (data not shown). Protonemal filaments were more susceptible than leaves to CF treatments and B. cinerea inoculation. Taken together, these results show that CF(SCC1), CF(SCC3193) and B. cinerea are capable of causing disease symptoms in Physcomitrella.
CF(SCC1) and B. cinerea trigger cytoplasmic shrinkage, accumulation of autofluorescent compounds and chloroplast browning
Pathogen infection or elicitor treatment can induce plant cell death with characteristic changes in cells, including cytoplasmic shrinkage, alteration of chloroplast organization and accumulation of autofluorescent compounds [13,42-44]. In the present study, we examined cellular changes occurring in Physcomitrella tissue showing macroscopic disease symptoms after exposure to B. cinerea spores or CF of the E.c. carotovora strains. CF(SCC1)-treated (Figures 4C and 4D) and B. cinerea-inoculated (Figure 4E) protonemal cells showed cytoplasmic shrinkage after 2 days. In contrast, no cytoplasmic shrinkage was evident in cells treated with LB (control for CF treatments, Figure 4A), CF(SCC3193) (Figure 4B) or PDB (control for B. cinerea inoculation, data not shown). Other morphological changes were also observed in Physcomitrella CF(SCC1)-treated and B. cinerea-inoculated cells. After 2 days, both treatments caused browning of the chloroplasts in a high proportion of cells (Figures 4D and 4E). Chloroplast browning was evident only in cells showing cytoplasmic shrinkage (Figures 4D and 4E). Additionally, CF(SCC1)-treated (Figures 4F and 4G) and B. cinerea-inoculated cells (data not shown) with brownish chloroplasts showed lack of red autofluorescence of chlorophyll. CF(SCC1)-treated protonemal cells having cytoplasmic shrinkage and brown chloroplasts were more abundant after 4 days. Within this treated protonemal tissue, cells with fewer brown chloroplasts were also observed suggesting that they were being brokendown (Figure 4H). In contrast, CF(SCC3193)- or LB-treated protonemal filaments did not show such changes and green chloroplasts were evident at least 6 days after treatment (data not shown).
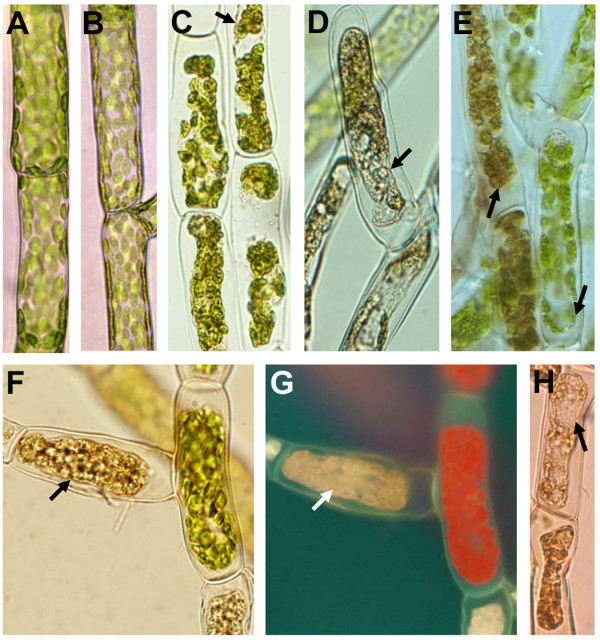
Figure 4.
Analysis of protonemal filament changes in response to treatments with CF and B. cinerea spores. Protonemal filaments examined under transmitted light after 2 days of treatment with LB (A), CF(SCC3193) (B), CF(SCC1) (C, D), and B. cinerea spores (E). Cytoplasmic shrinkage observed with CF(SCC1) and B. cinerea spores are indicated with arrows. CF(SCC1)-treated protonemal cells showing browning of chloroplasts and loss of red chlorophyll autofluorescence after UV-excitation are indicated with arrows (F, G). A cell with collapsed cytoplasm and fewer chloroplasts is shown 4 days after treatment (indicated with an arrow) (H).
After an apparent initial contact of B. cinerea hyphae with individual cells within the leaf, plant cells were observed to respond by accumulating autofluorescent compounds (Figure 5A). Cells in B. cinerea-inoculated leaves developing light blue to yellow autofluorescence (AF) were also observed. This AF appeared confined to the cytoplasm now separated from the cell wall (Figure 5C). No AF, however, was observed in CF(SCC1)- or CF(SCC3193)-treated leaves (data not shown). AF was clearly evident in protonemal filaments of B. cinerea-inoculated and CF(SCC1)-treated colonies (Figures 5E and 5G). In contrast, no AF was seen in PDB-treated leaves (Figure 5B) or PDB- or LB-treated protonemal filaments (Figures 5D and 5F), and only a few CF(SCC3193)-treated cells accumulated autofluorescent compounds (data not shown). Also, accumulation of autofluorescent compounds was generally observed once cytoplasmic shrinkage occurred (Figures 5H and 5I). In summary, our results show that CF(SCC1) and B. cinerea induce cellular changes in Physcomitrella protonemal cells, including cytoplasmic shrinkage, browning of chloroplasts and accumulation of autofluorescent compounds, suggesting a cell death process.
Figure 5.
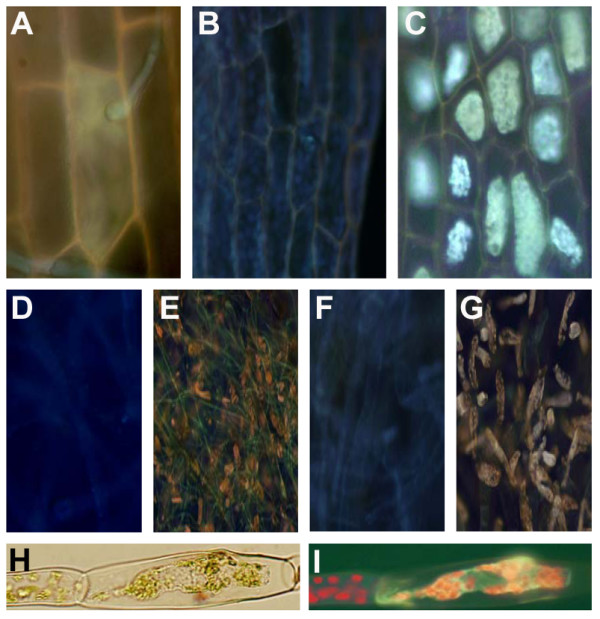
Accumulation of autofluorescent compounds in Physcomitrella after CF treatment and B. cinerea inoculation. Examination of UV-stimulated autofluorescence of B. cinerea-inoculated leaf (A, C), PDB-treated leaf (B), PDB- (D), B. cinerea spores- (E), LB- (F) and CF(SCC1)-treated protonemal filaments (G). A closer view of a CF(SCC1)-treated protonemal cell with cytoplasmic shrinkage and UV-stimulated autofluorescence is shown (H, I). Observations were made 2 days after treatments.
E.c. carotovora elicitors and B. cinerea trigger cell death in Physcomitrella
To assess whether CF and B. cinerea caused cell death of Physcomitrella tissues, we stained moss colonies with Evans blue, a dye that is excluded by membranes of living cells but diffuses into dead cells [45]. Figure 6 shows pictures of representative tissues. Two days after treatments, an increase in stained cells was observed with either CF(SCC3193), CF(SCC1) or B. cinerea spores, compared with control treatments. Although, while in CF(SCC3193)-treated tissue a low proportion of stained protonemal cells was observed (Figure 6B), CF(SCC1)-treated or B. cinerea-inoculated tissues showed a high proportion of stained protonemal cells (Figures 6C and 6E). In control treatments, almost no stained cells were visible (Figures 6A, 6D).
Figure 6.
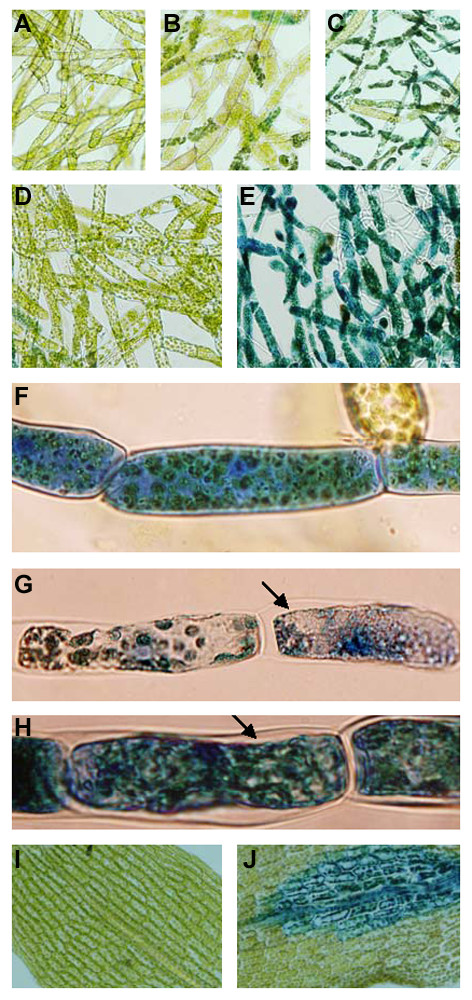
Analysis of cell death in Physcomitrella. Evans blue staining of protonemal tissues after treatments with LB (A), CF(SCC3193) (B), CF(SCC1) (C), PDB (D) and B. cinerea spores (E). A closer view of CF(SCC3193)- (F), CF(SCC1)- (G) and B. cinerea inoculated (H) protonemal cells is shown. Arrows indicate cytoplasmic shrinkage. Leaves treated with CF(SCC1) (I) and B. cinerea spores (J) were also stained with Evans blue. Pictures of representative tissues were taken 2 days after treatment.
Cytoplasmic shrinkage was evident in most Evans blue stained protonemal cells treated with CF(SCC1) or B. cinerea spores (Figures 6G and 6H). Whenever cytoplasmic shrinkage occurred, cells were stained with Evans blue, indicating that these cells were dying or dead. In contrast, most CF(SCC3193)-treated protonemal cells did not show cytoplasmic shrinkage and the dye was distributed homogeneously in the cells (Figure 6F). Three days after treatment, stained CF(SCC3193)-treated protonemal cells did not exhibit cytoplasmic shrinkage suggesting that this response does not develop at a later stage (data not shown). In gametophores, Evans blue stained cells could be detected in leaves inoculated with B. cinerea (Figure 6J), whereas stained cells were not seen after CF(SCC1) (Figure 6I), CF(SCC3193) or control treatments (not shown).
B. cinerea and E.c. carotovora elicitors mediate activation of defense-related genes
To analyze whether CF treatment or B. cinerea inoculation trigger Physcomitrella defense gene expression, we characterized the expression of a number of defense-related gene homologues including; (i) PR-1, (ii) LOX, (iii) PAL, and (iv) CHS. LOX (lipoxygenase) is a key enzyme in the synthesis of defense-related compounds including JA [46], PAL (phenylalanine ammonia-lyase) mediates the biosynthesis of phenylpropanoids and SA [5,47] and CHS (chalcone synthase) is the first enzyme in the synthesis of flavonoids [5]. The results in Figure 7 show that expression of the Physcomitrella homologues increased after CF treatment or B. cinerea inoculation. Clearly, three types of expression patterns were observed. The level of PR-1 expression peaked at 24 h in CF(SCC3193)-treated moss colonies, while in CF(SCC1)-treated and B. cinerea-inoculated tissues expression of PR-1 peaked at 4 h. Expression of PAL and CHS peaked at 4 h in tissues treated with either of the CFs or with B. cinerea spores, although among the treatments, higher expression levels were observed with CF(SCC1). In the case of CHS, two transcripts with an identical expression pattern were detected. LOX expression was moderately induced in CF(SCC1)- and CF(SCC3193)-treated moss colonies at 4 and 24 h, while transcript levels increased significantly in B. cinerea inoculated Physcomitrella tissues, reaching the highest expression level at 24 h. The results obtained in this study show that several conserved defense-related gene homologues of Physcomitrella were induced in response to treatment with E.c. carotovora elicitors or B. cinerea spores.
Figure 7.
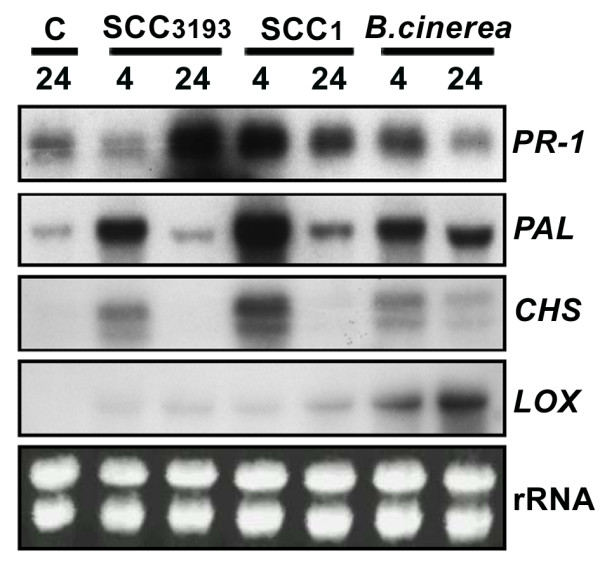
CF and B. cinerea-induced expression of defense-related genes in Physcomitrella. Expression of PR-1, PAL, CHS and LOX genes was characterized by RNA-gel blot hybridization after the following treatments: moss colonies sprayed with LB (control, C), CF(SCC1), CF(SCC3193) or inoculated with B. cinerea spores (2 × 105 spores/ml). Plant samples were harvested at the indicated times (hours) after treatment. 10 μg of RNA was separated on formaldehyde-agarose gels, transferred to nylon membranes and hybridized to 32P-labeled DNA probes. Ethidium bromide staining of rRNA was used to ensure equal loading of RNA samples. Similar results were obtained from two independent experiments.
Discussion
The developmental simplicity, ease of genetic analysis and the evolutionarily relationship between Physcomitrella and other plants has prompted us to study the interaction between this moss and two broad host range pathogens, the bacterium E.c. carotovora and the fungus B. cinerea. Our results indicate that both B. cinerea and E.c. carotovora can infect Physcomitrella tissues and cause disease symptoms. Since Physcomitrella gametophytes do not have stomata, E.c. carotovora entered plant tissues through wounds, while B. cinerea hyphae probably entered by hydrolyzing the plant cell wall using hydrolytic enzymes or by secreting cell wall permeable toxins that kill plant cells. We have observed B. cinerea hyphae within plant cells, including cells in which hyphae were apparently inside the cell cavity displacing the cytoplasm. Hyphae of other necrotrophic fungi, including S. sphagnicola, Tephrocybe palustris and Nectria mnii, are capable of penetrating live cells of moss leaves, resulting in cell death a posteriori [23,48]. In case of Nectria mnii, it was shown that intracellular hyphae could displace the host cell contents [48].
E.c. carotovoraSCC1, but not E.c. carotovoraSCC3193, was previously shown to harbour the harpin-encoding gene hrpN [35,36]. Harpins are bacterial effector proteins released into the host cells, through a type III secretion system encoded by the hypersensitive reaction and pathogenicity (hrp) gene cluster. When present in plant tissue, harpins cause HR and induction of defense mechanisms [49,50]. In Erwinia spp. hrp genes have been shown to contribute to virulence and to the ability of the pathogen to grow in the plant [35,51]. The higher maceration rate of the protonemal tissues observed with CF(SCC1) compared with CF(SCC3193) is consistent with previous studies showing that polygalacturonase, together with harpin HrpN from E.c. carotovoraSCC1 greatly enhanced lesion formation in Arabidopsis [52].
Cytoplasmic shrinkage is the most common morphological change occurring in plant PCD and has been observed in cells undergoing HR, as well as in tissues of plants susceptible to virulent pathogens [53-55]. Cytoplasmic shrinkage was observed only in Evans blue stained protonemal cells treated with CF(SCC1) but not with CF(SCC3193), probably suggesting that a different mechanism leading to cell death had occurred. This finding is consistent with the induction of HR by harpins and with previous results showing that Pseudomonas syringae pv phaseolicola induced cytoplasmic shrinkage in plant cells, while a hrpD mutant did not [43].
Breakdown of chloroplast membranes and chlorophyll has been observed in cells undergoing PCD, including those treated with elicitors, infected with pathogens or those undergoing senescence [56-59]. Our results showed that treatments with CF(SCC1) and B. cinerea spores induce browning of chloroplasts, which is likely followed by the breakdown of these organelles. This is consistent with previous results showing that S. sphagnicola hyphae are capable of causing degeneration of chloroplasts in the moss S. fuscum [23]. Chloroplasts remained green in protonemal CF(SCC3193)-treated cells, while boiled CF(SCC1), containing the heat-stable HrpN, still induced browning of the chloroplasts in cells also showing cytoplasmic shrinkage (data not shown). These findings suggest that HrpN might trigger cell death associated with cytoplasmic shrinkage and chloroplasts browning. In addition, browning of chloroplasts was associated with chlorophyll breakdown in CF(SCC1)-treated and B. cinerea-inoculated cells, since no red chlorophyll autofluorescence was observed (although quenching by other compounds cannot be excluded). These results are supported by findings showing that E.c. carotovoraSCC1 induced the expression of chlorophyllase 1 in Arabidopsis, which could be involved in the degradation of photoactive chlorophylls to avoid higher levels of reactive oxygen species (ROS) production and cellular damage during pathogen infection [60]. It is also interesting to note that whenever browning of chloroplasts was observed in CF(SCC1)-treated or B. cinerea-inoculated cells, cytoplasmic shrinkage was also present. Since CF(SCC1)-treated protonemal cells with cytoplasmic shrinkage but green chloroplasts were also observed, browning of chloroplasts could be a process occurring later in dying cells after collapse of the cytoplasm. Browning of chloroplasts could be indicative of oxidative processes due to excessive accumulation of ROS in the chloroplasts at late stages of CF(SCC1) and B. cinerea treatments, finally leading to chloroplasts breakdown. To our knowledge, this is the first report in which browning of chloroplasts was observed after pathogen and elicitor treatment. The ability to observe changes in the coloration of the chloroplasts was facilitated in that the leaf tissue, like the protonemal filaments, is composed of a single monolayer of cells.
Accumulation of autofluorescent compounds has been associated with the occurrence of HR in vascular plants [6,44]. CF(SCC1)-treated or B. cinerea-inoculated Physcomitrella tissues developed AF. A previous report demonstrated localized deposition of phenolic compounds at the sites of fungal penetration and also as a second major response that appeared to follow cell death [44]. These findings are consistent with our results, in showing AF confined to the collapsed cytoplasm of dead cells in B. cinerea-inoculated leaves and protonemal filaments.
B. cinerea induce PCD to enable rapid colonization of vascular plants, and Erwinia harpins have been shown to elicit cell death [49,50,61,38]. Cytoplasmic shrinkage, an indicator of plant PCD, correlated with accumulation of autofluorescent compounds and chloroplast browning after inoculation with B. cinerea or treatment with CF of HrpN producing E.c. carotovoraSCC1 suggesting that either treatment results in PCD in Physcomitrella.
Our results also showed that E.c. carotovora elicitors and B. cinerea induced defense-related gene expression in Physcomitrella. Earlier induction of the PR-1-like gene expression and the higher levels of PAL and CHS mRNA accumulation triggered by CF(SCC1) compared with CF(SCC3193), corresponded well with the higher levels of tissue maceration observed with CF(SCC1). CHSs are encoded by multiple genes in vascular plants and Physcomitrella [5,62], and in our study two CHS transcripts with an identical expression pattern were detected. Recently, a new enzymatic activity was described for the same Physcomitrella LOX gene product induced by B. cinerea in this study [63]. Novel oxylipins were generated by this enzyme suggesting a possible involvement in defense responses. In vascular plants PR-1, PAL and LOX are induced by inoculation with E.c. carotovora or by CF treatments [52,64,65] and PR-1 transcript accumulation is increased after B. cinerea infection [66,67]. The results obtained in this study suggest that E.c. carotovora elicitors and B. cinerea similarly induce expression of Physcomitrella defense gene homologues of those studied in vascular plants, and thus validate the use of non-specific plant pathogens or elicitors derived from them to study moss-pathogen interactions.
Conclusion
In the present study, we demonstrate that E.c. carotovora elicitor treatment and B. cinerea inoculation cause disease symptoms and induce defense responses in Physcomitrella. CF(SCC1), CF(SCC3193) and B. cinerea induced the expression of defense-related genes, including PR-1, LOX, PAL and CHS homologues. Compounds produced by LOX, PAL and CHS are involved in the synthesis of JA, phenylpropanoids and SA and flavonoids, respectively, in vascular plants. These compounds could play a role in the defense response of Physcomitrella as has been shown in vascular plants. As such our results further establish E.c. carotovora elicitors, as well as B. cinerea as promising systems to analyze induction of defense responses in Physcomitrella.
Since cytoplasmic shrinkage is the most common morphological change observed in plant PCD, and that harpins and B. cinerea induce this type of cell death in vascular plants, our results suggest that E.c. carotovora CFSCC1 containing HrpN and B. cinerea could also induce this type of cell death in Physcomitrella. Finally, the occurrence of distinct cellular responses leading to cell death by CF(SCC1) and CF(SCC3193) provides a useful system to analyze pathogen-induced cell death and to characterize the key elements involved in its regulation by targeted gene disruption in Physcomitrella.
Methods
Plant material and growth conditions
Physcomitrella patens Gransden WT isolate [68] was grown on cellophane overlaid BCDAT agar medium consisting of 1.6 g l -1 Hoagland's, 1 mM MgSO4, 1.8 mM KH2PO4 pH 6.5, 10 mM KNO3, 45 μM FeSO4, 1 mM CaCl2, 5 mM ammonium tartrate and 10 g l-1 agar [69]. Protonemal cultures and moss colonies were grown as described previously [70]. Plants were grown at 22°C under a photoperiod of 16 h light and three-week-old colonies were used for all the experiments.
Pathogen inoculation and culture filtrate treatments
Erwinia carotovora ssp carotovora strains SCC3193 [71] and SCC1 [72] were propagated on LB medium [73] at 28°C. Cell-free culture filtrates were prepared by growing bacteria in LB broth overnight, removing bacterial cells by centrifugation (10 min at 4000 g) and filter sterilizing the supernatant (0.2 μm pore size). This filter-sterilized supernatant (CF) was applied by spraying the moss colonies (3 ml per Petri dish containing 16 moss colonies). E.c. carotovoraSCC3193 and E.c. carotovoraSCC1 were grown on LB, and E.c. carotovoraSCC3193 transformed with plasmid pUC18 containing the GFP sequence as reporter gene under control of the lac promoter was grown on LB containing 100 μg/ml ampicillin. After 16 h bacterial cells were centrifuged and suspended in 0.9% NaCl to a final concentration of 5 × 108 cfu/ml. These suspensions were used for inoculation of Physcomitrella leaves previously wounded with a needle to create small lesions. An isolate of B. cinerea from a lemon plant was cultivated on 39 g/L potato dextrose agar (DIFCO) at room temperature. B. cinerea was inoculated by spraying a 2 × 105 spores/ml suspension in half-strength PDB (DIFCO). Symptom development of CF-treated and B. cinerea-inoculated Physcomitrella colonies was analyzed in three independent experiments using two Petri dishes containing16 colonies each. The experiments involving leaves inoculated with E.c. coratovora strains SCC1, SCC3193 and SCC3193 carrying the GFP marker were performed at least three times.
Evans blue and trypan blue staining, autofluorescence detection and microscopy
For detection of cell death, moss colonies were incubated for 2 hours with 0.05% Evans blue and washed 4 times with deionized water to remove excess and unbound dye. Growth and development of B. cinerea mycelium inside leaf tissues was monitored by staining with lactophenol-trypan blue and destaining in saturated chloral hydrate as described previously [74]. For autofluorescent compound detection, leaves were boiled in alcoholic lactophenol and rinsed in ethanol and water [75]. Material was then mounted on a slide in 50% glycerol and examined for Evans blue or trypan blue staining or using ultraviolet epifluorescence for detection of autofluorescent compounds (Microscope Olympus BX61). The infection of Physcomitrella leaves by GFP-tagged E.c. carotovora was visualized with a laser scanning confocal microscope FV 300 (Olympus).
RNA gel blot analysis
Total RNA was isolated from control and treated plant tissue corresponding to 64 moss colonies, using standard procedures based on phenol/chloroform extraction followed by LiCl precipitation. Ten micrograms of total RNA separated by denaturing agarose-formaldehyde gels was transferred to a nylon membrane (Hybond N) following standard procedures [76]. Membranes were prehybridized at 65°C in 6 × SCC, 0.5% SDS, 0.125 mg milk powder and 0.5 mg ml-1 denatured salmon sperm DNA. Hybridizations were performed at 65°C overnight. The DNA fragments to be used as probes were obtained by PCR using the plasmid harbouring the corresponding cDNA as template and the primers M13 forward and reverse. The cDNA clones used were: [DDBJ:BJ182301 (PR-1), DDBJ:BJ201257 (PAL), DDBJ:BJ192161 (CHS) and DDBJ:BJ159508 (LOX)]. PCR fragments were purified using Qiaquick columns (Qiagen), and were labelled with [α32P]-dCTP using Rediprime II Random Prime labelling system (Amersham Biosciences). After hybridization, membranes were washed twice for 30 min at 65°C with 5 × SCC, 0.1% SDS and twice 30 min with 2 × SCC, 0.1% SDS. Subsequently, membranes were exposed on autoradiography film. The amount of RNA loaded was verified by addition of ethidium bromide to the samples and photography under UV light after electrophoresis.
Authors' contributions
IPDL participated in the Northern blot analysis and the microscopic studies, designed this study, drafted and edited the manuscript. JPO carried out the analysis of symptom development and all the microscopic studies. AC and MB participated in the cell death analysis by Evans blue staining. CG participated in the Northern blot analysis. SV helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We gratefully acknowledge E. Tapio Palva, Tarja Kariola and Anne Tuikkala for their generous gift of GFP-tagged E.c. carotovora strain. We thank E. Tapio Palva for the E.c. carotovora strains and Luiz Diaz for the B. cinerea isolate. We would also thank Tomas Cascón for excellent technical assistance, José Roberto Sotelo-Silveira and Anabel Fernández for confocal microscopy assistance. We are grateful to Marcos Montesano and Paul Gill for critical reading of the manuscript and to Carmen Castresana for helpful discussions. This work was supported by Fondo Clemente Estable (Project 9008) DINACYT. The Physcomitrella ESTs were obtained from the RIKEN Biological Research Center.
Contributor Information
Inés Ponce de León, Email: iponce@iibce.edu.uy.
Juan Pablo Oliver, Email: oliver@iibce.edu.uy.
Alexandra Castro, Email: acastro@iibce.edu.uy.
Carina Gaggero, Email: carina@iibce.edu.uy.
Marcel Bentancor, Email: marcelb@fcien.edu.uy.
Sabina Vidal, Email: svidal@fcien.edu.uy.
References
- Lee HI, Leon J, Raskin I. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA. 1995;92:4076–4079. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I. Signal molecules in systemic plant resistance to pathogens and pests. Cell. 1992;70:879–886. doi: 10.1016/0092-8674(92)90239-9. [DOI] [PubMed] [Google Scholar]
- Linthorst HJM. Pathogenesis-related proteins of plants. Crit Rev Plant Sci. 1991;10:123–150. [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RN, Novacky AJ. The Hypersensitive Reaction in Plants to Pathogens: A Resistance Phenomenon. St. Paul: American Phytopathological Society Press; 1994. [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- Nimchuk Z, Eulgem T, Holt IB, Dangl JL. Recognition and response in the plant immune system. Annu Rev Genet. 2003;37:579–609. doi: 10.1146/annurev.genet.37.110801.142628. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:525–545. doi: 10.1146/annurev.arplant.48.1.525. [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG. Apoptosis: A functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell. 1996;8:375–391. doi: 10.1105/tpc.8.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Yao N. The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol. 2004;6:201–211. doi: 10.1111/j.1462-5822.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Heckman DS, Geiser DM, Eidell BR, Stauffer RL, Kardos NL, Hedges SB. Molecular evidence for the early colonization of land by fungi and plants. Science. 2001;293:1129–1133. doi: 10.1126/science.1061457. [DOI] [PubMed] [Google Scholar]
- Cove D, Benzanilla M, Harries P, Quatrano R. Mosses as model systems for the study of metabolism and development. Annu Rev Plant Biol. 2006;57:497–520. doi: 10.1146/annurev.arplant.57.032905.105338. [DOI] [PubMed] [Google Scholar]
- Schaefer DG, Zrÿd JP. The moss Physcomitrella patens, now and then. Physcomitrella patens. 2001;127:1430–1438. [PMC free article] [PubMed] [Google Scholar]
- Schaefer DG. A new moss genetics: targeted mutagenesis in Physcomitrella patens. Annu Rev Plant Biol. 2002;53:477–501. doi: 10.1146/annurev.arplant.53.100301.135202. [DOI] [PubMed] [Google Scholar]
- Quatrano RS, McDaniel SF, Khandelwal A, Perroud PF, Cove DJ. Physcomitrella patens: mosses enter the genomic age. Current Opinion in Plant Biology. 2007;10:182–189. doi: 10.1016/j.pbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Fujita T, Shin-I T, Seki M, Nishide H, Uchiyama I, Kamiya A, Carninci P, Hayashizaki Y, Shinozaki K, Kohara Y, Hasebe M. Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA. 2003;100:8007–8012. doi: 10.1073/pnas.0932694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Eisinger J, Reski R, Rensing SA. Representation and high-quality annotation of the Physcomitrella patens transcriptome demonstrates a high proportion of proteins involved in metabolism in mosses. Plant Biology. 2005;7:238–250. doi: 10.1055/s-2005-837578. [DOI] [PubMed] [Google Scholar]
- Reski R. Physcomitrella and Arabidopsis: the David and Goliath of reverse genetics. Trends Plant Sci. 1998;3:209–210. doi: 10.1016/S1360-1385(98)01257-6. [DOI] [Google Scholar]
- Akita M, Valkonen JP. A novel gene family in moss (Physcomitrella patens) shows sequence homology and a phylogenetic relationship with the TIR-NBS class of plant disease resistance genes. J Mol Evol. 2002;55:595–605. doi: 10.1007/s00239-002-2355-8. [DOI] [PubMed] [Google Scholar]
- Tsuneda A, Chen MH, Currah RS. Characteristics of a disease of Spagnum fuscum caused by Scleroconidioma sphagnicola. Can J Bot. 2001;79:1217–1224. doi: 10.1139/cjb-79-10-1217. [DOI] [Google Scholar]
- Polischuk V, Budzanivska I, Shevchenko T, Oliynik S. Evidence for plant viruses in the region of Argentina islands, Antartica. FEMS Microbiol Ecol. 2007;59:409–417. doi: 10.1111/j.1574-6941.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- Pérombelon MCM, Kelman A. Ecology of the soft-rot Erwinia. Annu Rev Phytopathol. 1980;12:361–387. doi: 10.1146/annurev.py.18.090180.002045. [DOI] [Google Scholar]
- Toth IK, Bell KS, Holeva MC, Birch PRJ. Soft rot erwiniae: From genes to genomes. Mol Plant Pathol. 2003;4:17–30. doi: 10.1046/j.1364-3703.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Collmer A, Keen NT. The role of pectic enzymes in plant pathogenesis. Annu Rev Phytopathol. 1986;24:383–409. doi: 10.1146/annurev.py.24.090186.002123. [DOI] [Google Scholar]
- Palva TK, Holmström KO, Heino P, Palva ET. Induction of plant defense response by exoenzymes of Erwinia carotovora ssp. carotovora. Mol Plant-Microbe Interact. 1993;6:190–196. [Google Scholar]
- Vidal S, Ponce de León I, Denecke J, Palva ET. Salicylic acid and the plant pathogen Erwinia carotovora induce defense genes via antagonistic pathways. Plant J. 1997;11:115–123. doi: 10.1046/j.1365-313X.1997.11010115.x. [DOI] [Google Scholar]
- Vidal S, Eriksson ARB, Montesano M, Denecke J, Palva ET. Cell wall-degrading enzymes from Erwinia carotovora cooperate in the salicylic acid-independent induction of a plant defense response. Mol Plant-Microbe Interact. 1998;11:23–32. doi: 10.1094/MPMI.1998.11.1.23. [DOI] [Google Scholar]
- Montesano M, Brader G, Ponce de Leon I, Palva ET. Multiple defense signals induced by Erwinia carotovora ssp. carotovora in potato. Molecular Plant Pathol. 2005;6:541–549. doi: 10.1111/j.1364-3703.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- Norman C, Vidal S, Palva ET. Interacting signal pathways control defense gene expression in Arabidopsis in response to the plant pathogen Erwinia carotovora. Mol Plant-Microbe Interact. 2000;13:430–438. doi: 10.1094/MPMI.2000.13.4.430. [DOI] [PubMed] [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defense gene expression in Arabidopsis suspension cultures. Biochem J. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Durner J. Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol Plant-Microbe Interact. 2004;17:131–139. doi: 10.1094/MPMI.2004.17.2.131. [DOI] [PubMed] [Google Scholar]
- Rantakari A, Virtaharju O, Vähämiko S, Taira S, Palva ET, Saarilahti HT, Romantschuk M. Type III secretion contributes to the pathogenesis of the soft-rot pathogen Erwinia carotovora Partial characterization of the hrp gene cluster. Mol Plant-Microbe Interact. 2001;14:962–968. doi: 10.1094/MPMI.2001.14.8.962. [DOI] [PubMed] [Google Scholar]
- Mattinen L, Tshuikina M, Mäe A, Pirhonen M. Identification and characterization of Nip, necrosis-inducing virulence protein of Erwinia carotovora subsp. carotovora. Mol Plant-Microbe Interact. 2004;17:1366–1375. doi: 10.1094/MPMI.2004.17.12.1366. [DOI] [PubMed] [Google Scholar]
- Elad Y, Williamson B, Tudzynski P, Delen N. Botrytis: Biology, Pathology and Control. Dordrecht Kluwer Academic Publishers; 2004. [Google Scholar]
- van Kan JAL. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends in Plant Sci. 2006;11:247–253. doi: 10.1016/j.tplants.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Reino JL, Hernández-Galán R, Durán-Patrón R, Collado IG. Virulence-toxin production relationship in isolates of the plant pathogenic fungus Botrytis cinerea. J Phytopathol. 2004;152:563–566. doi: 10.1111/j.1439-0434.2004.00896.x. [DOI] [Google Scholar]
- Kars I, Geja H, Krooshof GH, Wagemakers L, Joosten R, Benen JAE, van Kan JAL. Necrotising activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J. 2005;43:213–225. doi: 10.1111/j.1365-313X.2005.02436.x. [DOI] [PubMed] [Google Scholar]
- Brito N, Espino JJ, Gonzalez C. The endo-beta-1,4-xylanase xyn11A is required for virulence in Botrytis cinerea. Mol Plant-Microbe Interact. 2006;19:25–32. doi: 10.1094/MPMI-19-0025. [DOI] [PubMed] [Google Scholar]
- Mittler R, Simon L, Lam E. Pathogen-induced programmed cell death in tobacco. J Cell Science. 1997;110:1333–1344. doi: 10.1242/jcs.110.11.1333. [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Bennett MH, Mansfield JW. Hrp mutant of Pseudomonas syringae pv phaseolicola induces cell wall alterations but not membrane damage leading to the hypersensitive reaction in lettuce (Lactuca sativa) Plant Physiol. 1995;108:503–516. doi: 10.1104/pp.108.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M, Gallagher M, Fagg J, Bestwick C, Paule T, Beale M, Mansfield J. The hypersensitive reaction, membrane damage, and accumulation of autofluorescent phenolics in lettuce cells challenged by Bremia lactucae. Plant J. 1996;9:851–865. doi: 10.1046/j.1365-313X.1996.9060851.x. [DOI] [Google Scholar]
- Gaff DF, Okong'o-Ogola O. The use of nonpermeating pigments for testing the survival of cells. J Exp Bot. 1971;22:756–758. doi: 10.1093/jxb/22.3.756. [DOI] [Google Scholar]
- Feussner I, Wasternack C. The lipoxygenase pathway. Annu Rev Plant Biol. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropenoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. doi: 10.1146/annurev.pp.40.060189.002023. [DOI] [Google Scholar]
- Davey ML, Currah RS. Interactions between mosses (Bryophyta) and fungi. Can J Bot. 2006;84:1509–1519. doi: 10.1139/B06-120. [DOI] [Google Scholar]
- He SY, Huang HC, Collmer A. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell. 1993;2:1255–66. doi: 10.1016/0092-8674(93)90354-S. [DOI] [PubMed] [Google Scholar]
- Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- Yang CH, Gavilanes-Ruiz M, Okinaka Y, Vedel R, Berthuy L, Boccara M, Chen JW, Perna NT, Keen NT. hrp genes of erwinia chrysanthemi 3937 are important virulence factors. Mol Plant-Microbe Interact. 2002;15:472–480. doi: 10.1094/MPMI.2002.15.5.472. [DOI] [PubMed] [Google Scholar]
- Kariola T, Palomäki TA, Brader G, Palva ET. Erwinia carotovora subsp. carotovora and Erwinia -derived elicitors HrpN and PehA trigger distinct but interacting defense responses and cell death in Arabidopsis. Mol Plant-Microbe Interact. 2003;16:179–187. doi: 10.1094/MPMI.2003.16.3.179. [DOI] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/S0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Morel J.-B, Dangl JL. The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 1997;4:671–683. doi: 10.1038/sj.cdd.4400309. [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Paradiso A, Leonetti P, De Gara L. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 2006;48:784–95. doi: 10.1111/j.1365-313X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Arimura SI, Hirata A, Niwa Y, Yun DJ, Tsutsumi N, Uchimiya H, Kawai-Yamada M. Mammalian Bax initiates plant cell death through organelle destruction. Plant Cell Rep. 2005;24:408–17. doi: 10.1007/s00299-005-0948-6. [DOI] [PubMed] [Google Scholar]
- Yao N, Tada Y, Park P, Nakayashiki H, Tosa Y, Mayama S. Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J. 2001;28:13–26. doi: 10.1046/j.1365-313X.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- Navarre DA, Wolpert TJ. Victorin induction of an apoptotic, senescence-like response in oats. Plant Cell. 1999;11:237–250. doi: 10.1105/tpc.11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keates SE, Kostman TA, Anderson JD, Bailey BA. Altered gene expression in three plant species in response to treatment with Nep1, a fungal protein that cause necrosis. Plant Physiol. 2003;132:1610–1622. doi: 10.1104/pp.102.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariola T, Brader G, Li J, Palva ET. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell. 2005;17:282–294. doi: 10.1105/tpc.104.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea . Curr Biol. 2000;10:751–757. doi: 10.1016/S0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- Jiang C, Schommer CK, Kim SY, Suh D-Y. Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens . Phytochem. 2000;67:2531–2540. doi: 10.1016/j.phytochem.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Senger T, Wichard T, Kunze S, Gobel C, Lerchl J, Pohnert G, Feussner I. A multifunctional lipoxygenase with fatty acid hydroperoxide cleaving activity from the moss Physcomitrella patens. J Biol Chem. 2005;280:7588–7596. doi: 10.1074/jbc.M411738200. [DOI] [PubMed] [Google Scholar]
- Aguilar I, Poza-Carrin C, Gui A, Rodrguez-Palenzuela P. Erwinia chrysanthemi genes specifically induced during infection in chicory leaves. Mol Plant Pathol. 2002;3:271–275. doi: 10.1046/j.1364-3703.2002.00118.x. [DOI] [PubMed] [Google Scholar]
- Vidal S. PhD thesis. Swedish University of Agricultural Sciences, Department of Plant Biology; 1998. Molecular Defense responses against the plant pathogen Erwinia carotovora. Signal pathways in the regulation of pathogen-induced gene expression in plants. [Google Scholar]
- Govrin EM, Levine A. Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR) Plant Mol Biol. 2002;48:267–276. doi: 10.1023/A:1013323222095. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003;35:193–205. doi: 10.1046/j.1365-313X.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Schaefer D, Zryd JP, Knight CD, Cove DJ. Stable transformation of the moss Physcomitrella patens . Mol Gen Genet. 1991;226:418–424. doi: 10.1007/BF00260654. [DOI] [PubMed] [Google Scholar]
- Ashton NW, Cove DJ. The isolation and preliminary characterization of auxotrophic and analogue resistant mutants in the moss Physcomitrella patens . Mol Gen Genet. 1977;154:87–95. doi: 10.1007/BF00265581. [DOI] [Google Scholar]
- Saavedra L, Svensson J, Carballo V, Izmendi D, Welin B, Vidal S. A dehydrin gene in Physcomitrella patens is required for salt and osmotic stress tolerance. Plant J. 2006;45:237–249. doi: 10.1111/j.1365-313X.2005.02603.x. [DOI] [PubMed] [Google Scholar]
- Pirhonen M, Heino P, Helander I, Harju P, Palva ET. Bacteriophage T4-resistant mutants of the plant pathogen Erwinia carotovora . Microbe Pathog. 1988;4:359–367. doi: 10.1016/0882-4010(88)90063-0. [DOI] [PubMed] [Google Scholar]
- Saarilahti HT, Palva ET. Major outer membrane proteins in the phytopathogenic bacteria Erwinia carotovora subsp. carotovora and subsp. atroseptica . FEMS Microbiol Lett. 1986;35:267–270. doi: 10.1111/j.1574-6968.1986.tb01540.x. [DOI] [Google Scholar]
- Miller JH. Experiments in Molecular genetics. Cold Spring Harbor, Cold Spring Harbor Press; 1972. [Google Scholar]
- Koch E, Slusarenko A. Arabidopsis is susceptible to infection by downy mildew fungus. Plant Cell. 1990;2:437–455. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, et al. Arabidopsis mutants simulating disease resistance response. Cell. 1994;77:565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fitsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, Cold Spring Harbor Press; 1989. [Google Scholar]