Abstract
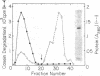
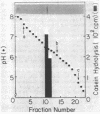
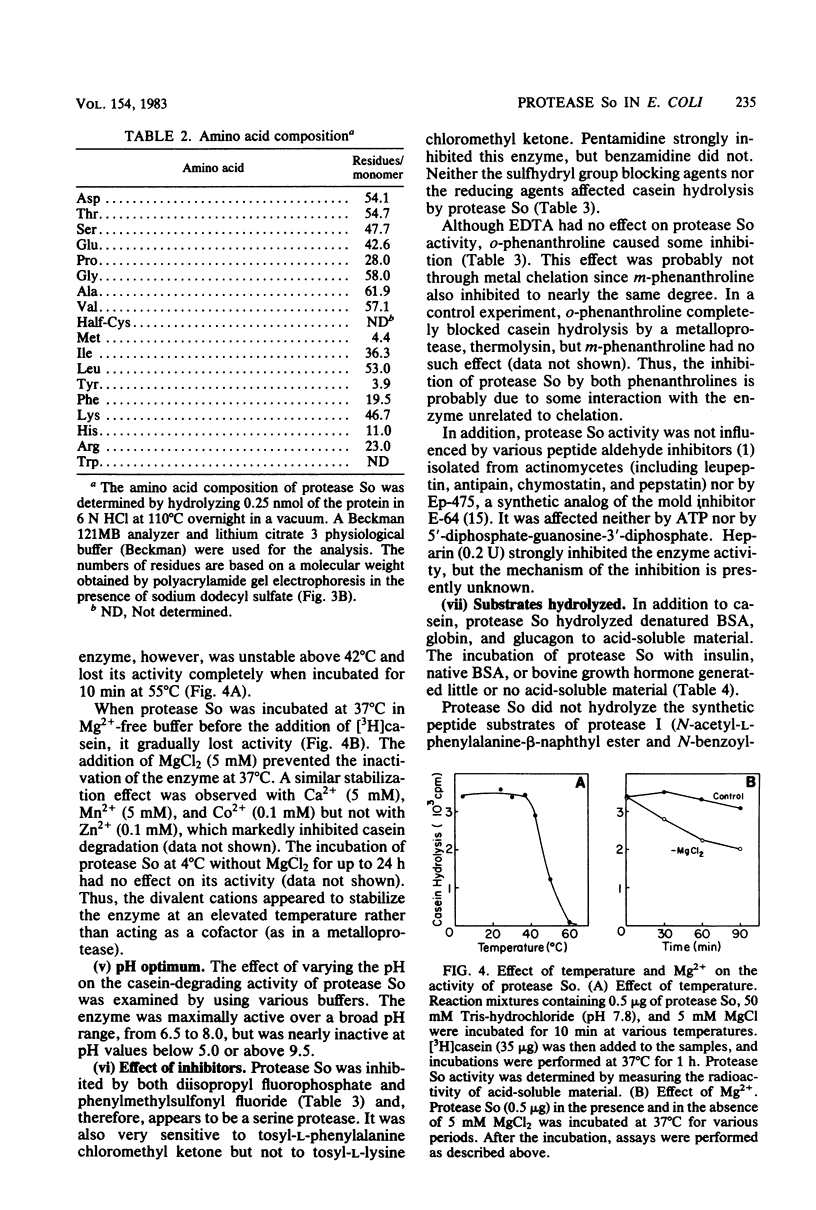
A new cytoplasmic endoprotease, named protease So, was purified to homogeneity from Escherichia coli by conventional procedures with casein as the substrate. Its molecular weight was 140,000 when determined by gel filtration on Sephadex G-200 and 77,000 when estimated by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. Thus, it appears to be composed of two identical subunits. Protease So had an isoelectric point of 6.4 and a Km of 1.4 μM for casein. In addition to casein, it hydrolyzed globin, glucagon, and denatured bovine serum albumin to acid-soluble peptides but did not degrade insulin, native bovine serum albumin, or the “auto α” fragment of β-galactosidase. A variety of commonly used peptide substrates for endoproteases were not hydrolyzed by protease So. It had a broad pH optimum of 6.5 to 8.0. This enzyme is a serine protease, since it was inhibited by diisopropyl fluorophosphate and phenylmethylsulfonyl fluoride. Although it was not inhibited by chelating agents, divalent cations (e.g., Mg2+) stabilized its activity. Protease So was sensitive to inhibition by N-tosyl-l-phenylalanine chloromethyl ketone but not by N-tosyl-l-lysine chloromethyl ketone. Neither ATP nor 5′-diphosphate-guanosine-3′-diphosphate affected the rate of casein hydrolysis. Protease So was distinct from the other soluble endoproteases in E. coli (including proteases Do, Re, Mi, Fa, La, Ci, and Pi) in its physical and chemical properties and also differed from the membrane-associated proteases, protease IV and V, and from two amino acid esterases, originally named protease I and II. The physiological function of protease So is presently unknown.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles L. K., Konisky J. Cleavage of colicin Ia by the Escherichia coli K-12 outer membrane is not mediated by the colicin Ia receptor. J Bacteriol. 1981 Jan;145(1):668–671. doi: 10.1128/jb.145.1.668-671.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lazdunski C. Interaction of colicin E4 with specific receptor sites mediates its cleavage into two fragments inactive towards whole cells. Eur J Biochem. 1979 Jun 1;96(3):525–533. doi: 10.1111/j.1432-1033.1979.tb13066.x. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Zipser D. Purification and characterization of protease III from Escherichia coli. J Biol Chem. 1979 Jun 10;254(11):4698–4706. [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Effects of protease inhibitors on protein breakdown and enzyme induction in starving Escherichia coli. Nat New Biol. 1971 Nov 10;234(45):51–52. doi: 10.1038/newbio234051a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Swamy K. H., Chung C. H., Larimore F. S. Proteases in Escherichia coli. Methods Enzymol. 1981;80(Pt 100):680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Kowit J. D., Choy W. N., Champe S. P., Goldberg A. L. Role and location of "protease I" from Escherichia coli. J Bacteriol. 1976 Dec;128(3):776–784. doi: 10.1128/jb.128.3.776-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Goldberg A. L. Intermediate steps in the degradation of a specific abnormal protein in Escherichia coli. J Biol Chem. 1977 Dec 10;252(23):8350–8357. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larimore F. S., Waxman L., Goldberg A. L. Studies of the ATP-dependent proteolytic enzyme, protease La, from Escherichia coli. J Biol Chem. 1982 Apr 25;257(8):4187–4195. [PubMed] [Google Scholar]
- Morrison S. L., Zipser D. Polypeptide products of nonsense mutations. I. Termination fragments from nonsense mutations in the Z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Jun 14;50(2):359–371. doi: 10.1016/0022-2836(70)90198-1. [DOI] [PubMed] [Google Scholar]
- Mount D. W. The genetics of protein degradation in bacteria. Annu Rev Genet. 1980;14:279–319. doi: 10.1146/annurev.ge.14.120180.001431. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Purification and characterization of two novel proteolytic enzymes in membranes of Escherichia coli. Protease IV and protease V. J Biol Chem. 1982 Apr 25;257(8):4333–4339. [PubMed] [Google Scholar]
- Pacaud M., Richaud C. Protease II from Escherichia coli. Purification and characterization. J Biol Chem. 1975 Oct 10;250(19):7771–7779. [PubMed] [Google Scholar]
- Pacaud M., Uriel J. Isolation and some propeties of a proteolytic enzyme from Escherichia coli (protease I). Eur J Biochem. 1971 Dec 10;23(3):435–442. doi: 10.1111/j.1432-1033.1971.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Conklin K., Rosenthal E., Goldberg A. L. Further evidence for the involvement of charged tRNA and guanosine tetraphosphate in the control of protein degradation in Escherichia coli. J Biol Chem. 1978 Jun 10;253(11):3945–3951. [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of starvation for potassium and other inorganic ions on protein degradation and ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1980 Sep;143(3):1223–1233. doi: 10.1128/jb.143.3.1223-1233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Gorodetsky D. I., Stepanov V. M. The study of Escherichia coli proteases. Intracellular serine protease of E. coli-an analogue of bacillus proteases. J Gen Microbiol. 1979 Feb;110(2):443–451. doi: 10.1099/00221287-110-2-443. [DOI] [PubMed] [Google Scholar]
- Swamy K. H., Goldberg A. L. E. coli contains eight soluble proteolytic activities, one being ATP dependent. Nature. 1981 Aug 13;292(5824):652–654. doi: 10.1038/292652a0. [DOI] [PubMed] [Google Scholar]
- Swamy K. H., Goldberg A. L. Subcellular distribution of various proteases in Escherichia coli. J Bacteriol. 1982 Mar;149(3):1027–1033. doi: 10.1128/jb.149.3.1027-1033.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Voellmy R. W., Goldberg A. L. ATP-stimulated endoprotease is associated with the cell membrane of E. coli. Nature. 1981 Apr 2;290(5805):419–421. doi: 10.1038/290419a0. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Goldberg A. L. Guanosine-5'-diphosphate-3'-diphosphate (ppGpp) and the regulation of protein breakdown in Escherichia coli. J Biol Chem. 1980 Feb 10;255(3):1008–1014. [PubMed] [Google Scholar]
- Waxman L., Goldberg A. L. Protease La from Escherichia coli hydrolyzes ATP and proteins in a linked fashion. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4883–4887. doi: 10.1073/pnas.79.16.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]