Abstract
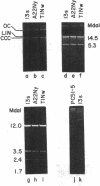
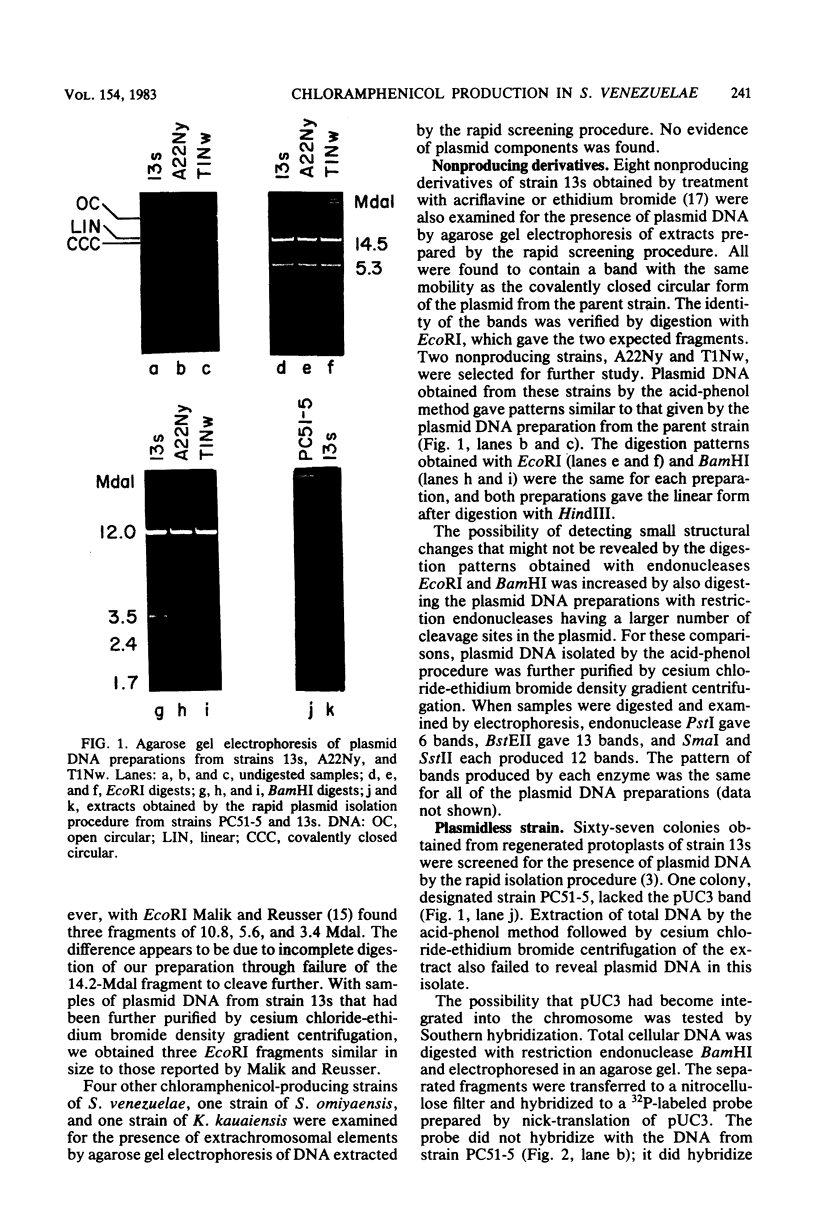
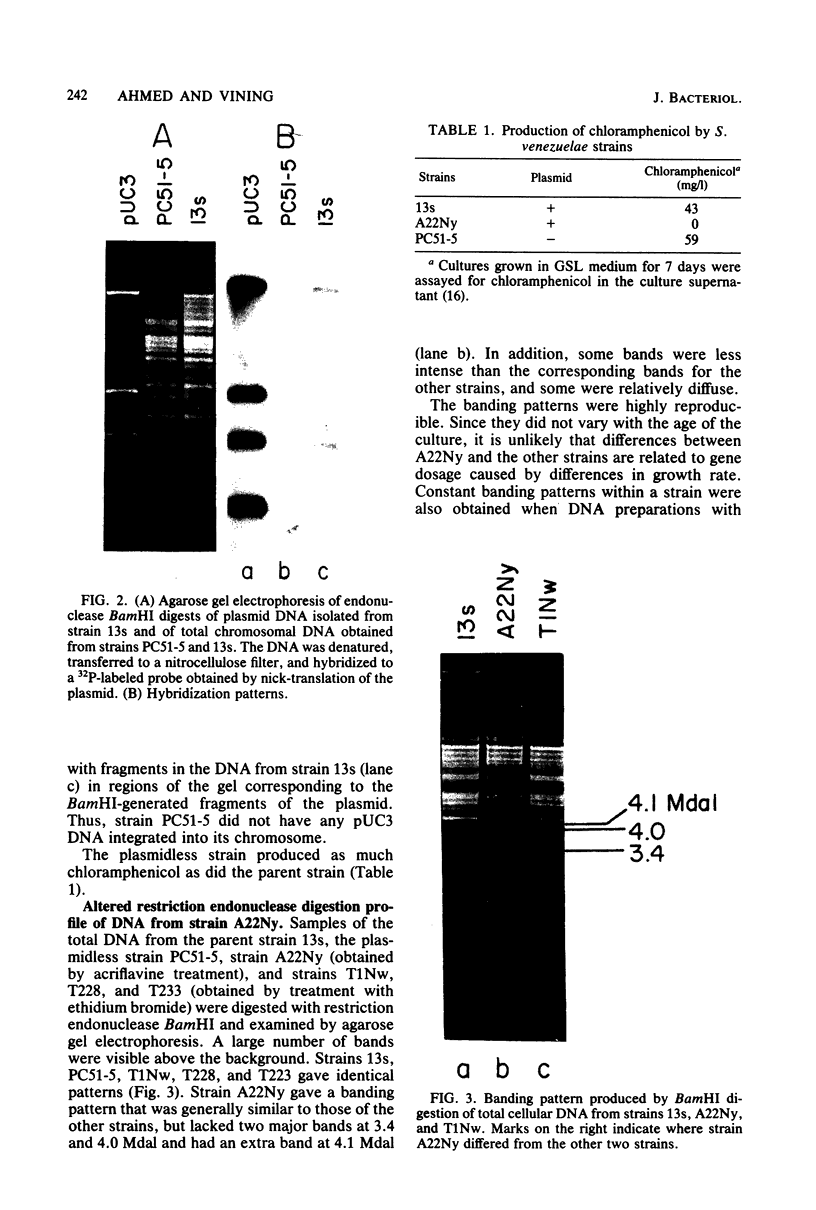
Of seven chloramphenicol-producing actinomycetes examined, only Streptomyces venezuelae strain 13s contained extrachromosomal DNA detectable by agarose gel electrophoresis and cesium chloride-ethidium bromide density gradient centrifugation. The single 17-megadalton plasmid present in this strain was indistinguishable from plasmid pUC3 previously isolated from mutagenized cultures. Strains selected for their inability to produce chloramphenicol after treatment with acriflavine or ethidium bromide still contained a plasmid that had the same electrophoretic mobility as plasmid pUC3 and yielded similar fragments when digested with restriction endonucleases. By regenerating protoplasts of strain 13s and screening for isolates lacking extrachromosomal DNA, strain PC51-5 was obtained. The absence of plasmid pUC3 sequences in this strain was confirmed by Southern hybridization using 32P-labeled plasmid as a probe. Since the plasmidless strain produced as much chloramphenicol as did the parent strain, pUC3 contains neither structural nor regulatory genes for antibiotic production. Evidence from electrophoretic analysis of BamHI digests of total cellular DNA from wild-type and dye-treated nonproducing progeny indicated that acriflavine caused structural changes in the chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagawa H., Okanishi M., Umezawa H. A plasmid involved in chloramphenicol production in Streptomyces venezuelae: evidence from genetic mapping. J Gen Microbiol. 1975 Oct;90(2):336–346. doi: 10.1099/00221287-90-2-336. [DOI] [PubMed] [Google Scholar]
- Akagawa H., Okanishi M., Umezawa H. Genetics and biochemical studies of chloramphenicol-nonproducing mutants of Streptomyces venezuelae carrying plasmid. J Antibiot (Tokyo) 1979 Jun;32(6):610–620. doi: 10.7164/antibiotics.32.610. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. F., Hopwood D. A. Unstable naturally occurring resistance to antibiotics in streptomyces. J Gen Microbiol. 1978 Jun;106(2):377–381. doi: 10.1099/00221287-106-2-377. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Bacterial protoplast fusion: recombination in fused protoplasts of Streptomyces coelicolor. Mol Gen Genet. 1978 Jul 4;162(3):307–317. doi: 10.1007/BF00268856. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M., Bibb M. J., Cohen S. N. Genetic recombination through protoplast fusion in Streptomyces. Nature. 1977 Jul 14;268(5616):171–174. doi: 10.1038/268171a0. [DOI] [PubMed] [Google Scholar]
- Hotta K., Okami Y., Umezawa H. Elimination of the ability of a kanamycin-producing strain to biosynthesize deoxystreptamine moiety by acriflavine. J Antibiot (Tokyo) 1977 Dec;30(12):1146–1149. doi: 10.7164/antibiotics.30.1146. [DOI] [PubMed] [Google Scholar]
- Hotta K., Okami Y., Umezawa H. Studies on new aminoglycoside antibiotics, istamycins, from an actinomycete isolated from a marine environment. II. Possible involvement of plasmid in istamycin production. J Antibiot (Tokyo) 1980 Dec;33(12):1510–1514. doi: 10.7164/antibiotics.33.1510. [DOI] [PubMed] [Google Scholar]
- Krell P. J., Stoltz D. B. Unusual Baculovirus of the Parasitoid Wasp Apanteles melanoscelus: Isolation and Preliminary Characterization. J Virol. 1979 Mar;29(3):1118–1130. doi: 10.1128/jvi.29.3.1118-1130.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähler R., Noack D. Action of acridine orange and ethidium bromide on growth and antibiotic activity of Streptomyces hygroscopicus JA 6599. Z Allg Mikrobiol. 1974;14(6):529–533. doi: 10.1002/jobm.3630140610. [DOI] [PubMed] [Google Scholar]
- Malik V. S. Preparative method for the isolation of super-coiled DNA from a chloramphenicol-producing streptomycete. J Antibiot (Tokyo) 1977 Oct;30(10):897–899. doi: 10.7164/antibiotics.30.897. [DOI] [PubMed] [Google Scholar]
- Malik V. S., Reusser F. Restriction enzyme map for streptomycete plasmid pUC3. Plasmid. 1979 Oct;2(4):627–631. doi: 10.1016/0147-619x(79)90060-x. [DOI] [PubMed] [Google Scholar]
- Malik V. S., Vining L. C. Metabolism of chloramphenicol by the producing organism. Can J Microbiol. 1970 Mar;16(3):173–179. doi: 10.1139/m70-030. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Vining L. C. Loss of chloramphenicol production in strains of Streptomyces species 3022alpha treated with acriflavine and ethidium bromide. Can J Microbiol. 1978 Jun;24(6):662–669. doi: 10.1139/m78-111. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Ogawara H. Multiple effects induced by unstable mutation in Streptomyces lavendulae. J Antibiot (Tokyo) 1980 Apr;33(4):420–425. doi: 10.7164/antibiotics.33.420. [DOI] [PubMed] [Google Scholar]
- Nojiri C., Watabe H., Katsumata K., Yamada Y., Murakami T., Kumata Y. Isolation and characterization of plasmids from parent and variant strains of Streptomyces ribosidificus. J Antibiot (Tokyo) 1980 Jan;33(1):118–121. doi: 10.7164/antibiotics.33.118. [DOI] [PubMed] [Google Scholar]
- Ochi K., Katz E. The possible involvement of a plasmid(s) in actinomycin synthesis by Streptomyces parvulus and Streptomyces antibioticus. J Antibiot (Tokyo) 1978 Nov;31(11):1143–1148. doi: 10.7164/antibiotics.31.1143. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Ita T., Umezawa H. Possible control of formation of aerial mycelium and antibiotic production in Streptomyces by episomic factors. J Antibiot (Tokyo) 1970 Jan;23(1):45–47. doi: 10.7164/antibiotics.23.45. [DOI] [PubMed] [Google Scholar]
- Omura S., Ikeda H., Tanaka H. Extraction and characterization of plasmids from macrolide antibiotic-producing streptomycetes. J Antibiot (Tokyo) 1981 Apr;34(4):478–482. doi: 10.7164/antibiotics.34.478. [DOI] [PubMed] [Google Scholar]
- Redshaw P. A., McCann P. A., Pentella M. A., Pogell B. M. Simultaneous loss of multiple differentiated functions in aerial mycelium-negative isolates of streptomycetes. J Bacteriol. 1979 Feb;137(2):891–899. doi: 10.1128/jb.137.2.891-899.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson M., Lewis E., Napier E. Occurrence of reiterated DNA sequences in strains of Streptomyces produced by an interspecific protoplast fusion. Mol Gen Genet. 1981;182(2):336–340. doi: 10.1007/BF00269680. [DOI] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721–732. doi: 10.1128/aac.8.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf H. Plasmid loss and changes within the chromosomal DNA of Streptomyces reticuli. J Bacteriol. 1982 Aug;151(2):701–707. doi: 10.1128/jb.151.2.701-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermonti G., Petris A., Micheli M., Lanfaloni L. Chloramphenicol resistance in Streptomyces coelicolor A3(2): possible involvement of a transposable element. Mol Gen Genet. 1978 Aug 4;164(1):99–103. doi: 10.1007/BF00267604. [DOI] [PubMed] [Google Scholar]
- Shaw P. D., Piwowarski J. Effects of ethidium bromide and acriflavine on streptomycin production by Streptomyces bikiniensis. J Antibiot (Tokyo) 1977 May;30(5):404–408. doi: 10.7164/antibiotics.30.404. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yagisawa M., Huang T. S., Davies J. E. Possible involvement of plasmids in biosynthesis of neomycin. J Antibiot (Tokyo) 1978 Aug;31(8):809–813. doi: 10.7164/antibiotics.31.809. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]