Abstract
Oligodendrocytes myelinate axons in the vertebrate central nervous system (CNS). They develop from precursor cells (OPCs), some of which persist in the adult CNS. Adult OPCs differ in many of their properties from OPCs in the developing CNS. In this study we have purified OPCs from postnatal rat optic nerve and cultured them in serum-free medium containing platelet-derived growth factor (PDGF), the main mitogen for OPCs, but in the absence of thyroid hormone in order to inhibit their differentiation into oligodendrocytes. We find that many of the cells continue to proliferate for more than a year and progressively acquire a number of the characteristics of OPCs isolated from adult optic nerve. These findings suggest that OPCs have an intrinsic maturation program that progressively changes the cell's phenotype over many months. When we culture the postnatal OPCs in the same conditions but with the addition of basic fibroblast growth factor (bFGF), the cells acquire these mature characteristics much more slowly, suggesting that the combination of bFGF and PDGF, previously shown to inhibit OPC differentiation, also inhibits OPC maturation. The challenge now is to determine the molecular basis of such a protracted maturation program and how the program is restrained by bFGF.
Keywords: oligodendrocyte precursor cells, cell cycle, optic nerve, PDGF, bFGF
Introduction
In many cell lineages the behavior of precursor cells changes as development proceeds, but it is generally not known to what extent the changes reflect changes in the cells' environment, an intracellular program for change built into the cells themselves, or both. We have been addressing this problem in the oligodendrocyte cell lineage.
Oligodendrocytes develop from proliferating precursor cells (OPCs) that initially arise in germinal zones and then migrate throughout the CNS (Small et al. 1987; LeVine and Goldman 1988; Hardy and Reynolds 1991; Pringle and Richardson 1993; Ono et al. 1997). The OPCs divide a limited number of times before they stop and terminally differentiate into postmitotic oligodendrocytes (Temple and Raff 1986; Gard and Pfeiffer 1990). Although OPCs can differentiate into astrocytes (type-2 astrocytes) in culture and have therefore been called oligodendrocyte type-2 astrocyte (O-2A) progenitor cells (Raff et al. 1983), there is still no convincing evidence that they normally do so in vivo (Skoff 1990; Fulton et al. 1992).
Various growth factors can promote OPC proliferation in culture, including PDGF (Noble et al. 1988; Raff et al. 1988; Richardson et al. 1988), bFGF (Bögler et al. 1990; McKinnon et al. 1990), insulin-like growth factor I (IGF-I; McMorris and Dubois-Dalcq 1988), ciliary neurotrophic factor (CNTF) and its relatives (Barres et al. 1996), neurotrophin-3 (NT-3; Barres et al. 1994a), and neuregulins (NRG; Canoll et al. 1996; Vartanian et al. 1999). However, only PDGF can stimulate the proliferation of purified OPCs on its own (Ibarrola et al. 1996), and it is probably the most important mitogen in vivo (Calver et al. 1998; Fruttiger et al. 1999).
OPC differentiation is tightly coupled to cell cycle withdrawal and, in cultures of OPCs isolated from the postnatal rat optic nerve at least, it appears to be regulated by a cell-intrinsic timer (Temple and Raff 1986; Gao et al. 1997). The timer consists of at least two components: a timing component that measures elapsed time and an effector component that stops cell proliferation and initiates differentiation when time is up (Barres et al. 1994b; Bögler and Noble 1994). Although the timer is cell intrinsic, its normal function depends on extracellular signals: PDGF is required for the timing component to operate (Noble et al. 1988; Raff et al. 1988), while thyroid hormone (TH) regulates the effector component (Barres et al. 1994b). In the absence of PDGF, OPCs immediately stop dividing and prematurely differentiate into oligodendrocytes (Noble and Murray 1984; Temple and Raff 1985); in the presence of PDGF and the absence of TH, most OPCs tend to keep dividing and do not differentiate for at least 16 d (Barres et al. 1994b; Ahlgren et al. 1997; Gao et al. 1998). Bögler et al. 1990 used a combination of PDGF and FGF, rather than PDGF in the absence of TH, to inhibit oligodendrocyte differentiation, and showed independently that the timer in OPCs consists of separable timing component and effector components. There is evidence that the cyclin-dependent kinase inhibitor P27kip1 (Durand et al. 1997, Durand et al. 1998) and the β1 thyroid hormone receptor (Gao et al. 1998) are both part of the intrinsic timer.
OPCs are present in the adult CNS (ffrench-Constant and Raff, 1986; Levine and Gard 1987; Levine and Stallcup 1987; Wolswijk and Noble 1989; Fulton et al. 1992; Wren et al. 1992; Shi et al. 1998), although they have different properties from perinatal OPCs: OPCs isolated from adult rat optic nerve, for example, proliferate, differentiate, and migrate more slowly in culture than do OPCs isolated from postnatal optic nerve (ffrench-Constant and Raff, 1986; Wolswijk and Noble 1989; Shi et al. 1998). Noble and his colleagues provided evidence that cells with some of the properties of adult OPCs can arise in culture from neonatal OPCs (Wren et al. 1992). Neonatal OPCs also differ from embryonic OPCs (Gao and Raff; 1997; Gao et al. 1998): embryonic cells have a simpler morphology and lower levels of TRβ1; they proliferate for longer before they differentiate; and they divide and migrate faster. Moreover, when purified embryonic OPCs are cultured in PDGF without TH, they acquire the properties of the neonatal OPCs of the equivalent age, suggesting that progressive maturation is an intrinsic property of developing OPCs (Gao et al. 1998).
In this study, we investigate how purified OPCs from neonatal rat optic nerve behave when cultured continuously for many months in the presence of PDGF, or PDGF and bFGF, but in the absence of TH. We compare these long-term cultured OPCs with OPCs freshly isolated from adult optic nerves of different ages. The results suggest that the long-term cultured OPCs gradually acquire several of the characteristics of adult OPCs, suggesting that OPCs have an intrinsic maturation program that progressively changes the cells' properties over many months.
Materials and Methods
Animals and Reagents
Sprague-Dawley rats were obtained from the Animal Facility at University College London. Recombinant human PDGF-AA and NT-3 were purchased from Peprotech, bFGF from Promega. NRG (glial growth factor 2, or GGF2) was a gift from Cambridge NeuroScience. The following antibodies were used: monoclonal anti-RAN-2 (Bartlett et al. 1981); A2B5 monoclonal (Eisenbarth et al. 1979); monoclonal anti-galactocerebroside (anti-GC; Ranscht et al. 1982); O1 and O4 monoclonals (Sommer and Schachner 1981); monoclonal anti-myelin basic protein (anti-MBP; Boehringer Mannheim); affinity-purified rabbit anti-proteolipid protein (anti-PLP) antibodies (gift from W. Stoffel); rabbit antiserum against glial fibrillary acidic protein (anti-GFAP, Pruss 1979); goat antiserum against vimentin (Santa Cruz Biotechnology); monoclonal anti-rat nestin (rat 401; Hockfield and McKay 1985; PharMingen); monoclonal anti-bromodeoxyuridine (anti-BrdU; Maguad et al. 1988); and rabbit antiserum against the NG2 proteoglycan (PharMingen). Most of these antibodies have been described in previous studies (Barres et al. 1992; Durand et al. 1997). All other chemicals were purchased from Sigma unless indicated otherwise.
Purification and Culture of OPCs
OPCs were prepared from optic nerves of postnatal day zero (P0), P7, P9, or P14 rats and purified by sequential immunopanning as previously described (Barres et al. 1992; Gao et al. 1997). The purified OPCs (generally >98% purity) were plated at either clonal (2,000 cells) or higher (15,000 cells) density into poly-d-lysine (PDL)-coated 25-cm2 flasks (T25, Falcon) in 3 ml culture medium. In some cases they were cultured in PDL-coated slide flasks (Nunc) at a density of 500–1,000 cells/flask in 1.5 ml culture medium. The serum-free culture medium was a modified B-S medium (Bottenstein and Sato 1979), based on DME containing bovine insulin (10 μg/ml), NT-3 (5 ng/ml), human transferrin (100 μg/ml), BSA (100 μg/ml), progesterone (60 ng/ml), sodium selenite (40 ng/ml), N-acetyl-cysteine (60 μg/ml), putrescine (16 μg/ml), forskolin (5 μM), trace minerals (GIBCO BRL), and penicillin-streptomycin (GIBCO BRL). Human PDGF (10 ng/ml) was added to one set of cultures, and bFGF and PDGF (both 10 ng/ml) were added to another set. TH was not added unless indicated otherwise. In some experiments, mixed optic nerve cells were directly plated onto PDL-coated glass coverslips and cultured in the above medium without PDGF for 2–3 d. These cells were used to determine the optimal concentrations of antibodies for immunofluorescence staining.
Cultures were maintained at 37°C in an 8% CO2 incubator, and half of the culture medium was changed every 48 h. For high-density cultures, fresh PDGF was added every 8 h. Cells were passaged using 0.0125% trypsin (GIBCO BRL) when they reached 80–90% confluence, which generally took 15–20 d. About 2,000 cells were plated into T25 flasks at each passage. A total of eight independent sets of P7 cultures were initiated and followed through multiple passages. The morphology of the cells was regularly observed by inverted phase microscopy and photography (Tmax 100 B/W film) and sometimes by time-lapse video recording (see below).
Purification and Culture of Adult OPCs
Adult OPCs were purified from P50, P300, P390, or P510 rat optic nerves, using a collagenase-trypsin protocol initially described by Wolswijk and Noble 1989 and later modified by Shi et al. 1998(the detailed protocol was kindly provided by B. Barres). The dissection of adult optic nerves and subsequent enzymatic dissociation procedures were essentially the same as described by Shi et al. 1998. The OPCs were purified by incubating the cell suspension first on an anti–RAN-2 antibody-coated dish for 30 min to deplete type-1 astrocytes, and meningeal cells, and microglia, and then on an A2B5-coated dish for 1 h to select the OPCs. Negative selection on an anti-GC antibody-coated dish was omitted, as adult OPCs express GC on their surface (Shi et al. 1998; this study). Finally, after extensive washing, adult OPCs were removed from the A2B5 plate with trypsin and plated on PDL-coated coverslips or slide flasks. The P50 OPCs were cultured in PDGF-supplemented B-S medium as described above for perinatal precursor cells. For OPCs purified from older rats, we added NRG (50 ng/ml), as we found this was required for the cells to survive.
Determination of Cell Cycle Time by BrdU Incorporation
To label OPCs in S phase, cells that had been in culture for different periods of time were plated at a density of 3,000 cells/13-mm PDL-coated glass coverslip and cultured for 2 d in medium containing either PDGF or PDGF plus bFGF. Then 2.5 μM BrdU (Boehringer Mannheim) was added to the culture medium for 2–144 h, as indicated in Results. Half of the media was changed every 3 d and fresh BrdU was added. Finally, the cells were fixed in 4% paraformaldehyde (5 min, room temperature), permeabilized in 70% ethanol (in PBS, −20°C) for 10 min, incubated in 6N HCl and 1% Triton X-100 (15 min, room temperature) to denature nuclear DNA, and then incubated in 0.1 M sodium borate (in PBS and 1% Triton X-100) for 10 min. The cells were subsequently blocked with 50% normal goat serum and 1% Triton X-100 for 30 min and then incubated with monoclonal anti-BrdU antibody (supernatant; used at 1:5 dilution) for 1 h, followed by a goat anti–mouse IgG-biotin and streptavidin-FITC (diluted 1:100, 30 min each; Amersham). Finally, the nucleus of all cells was labeled with bisbenzamide (Hoechst 33342 dye). In some experiments, cells were first surface-labeled with the A2B5 monoclonal antibody, before ethanol fixation (see below), using Texas red–coupled goat anti-mouse Ig antibodies. To label the proliferating OPCs freshly isolated from adult animals, cells growing on coverslips for 2–3 d were pulsed with 2.5 μM BrdU for 8 h and then processed for immunofluorescence staining as described above. All coverslips were mounted with Citifluor (Chemistry Lab) on glass slides and sealed with nail vanish. Cells were examined in a Zeiss Axioplan-2 fluorescence microscope and photographed on either Tmax-400 black and white film or Kodak Ektochrome 100 color slide film.
A total of 1,000–1,500 cells (identified by bisbenzamide nuclear staining) were counted per coverslip to determine the proportion of BrdU+ cells. Two coverslips were counted for each condition. The labeling index was plotted against the BrdU pulse time to obtain a cumulative labeling curve. The estimate of cell cycle time (Tc) was determined from the cumulative labeling index (Nowakowski et al. 1989), using CricketGraph. Using the formula y = ax + b, where y is the labeling index, a the slope, x the cumulative BrdU labeling time, and b the intercept on the y axis, we determined Tc = 1/a and the rough S-phase time Ts = bTc. The linear region of the curve (generally between 2 and 24 h) was used to deduce Tc. In all cases, the linear regression coefficient γ2 was >0.95. All the experiments were repeated at least twice.
Determination of Population Doubling Time, Cell Death, and Spontaneous Differentiation by Clonal Analysis
Purified OPCs that were in culture for different periods of time were plated at clonal density (2,000 cells/flask) into PDL-coated T25 flasks and cultured in either PDGF or PDGF plus bFGF for 8 d. Duplicate or triplicate flasks were set up for each time point. OPCs and oligodendrocytes were identified by their characteristic morphologies (Temple and Raff 1986). On day 8, the numbers of live, dead, and differentiated cells were counted for each clone; a total of 200–400 clones were randomly counted for each time point. The proportions of cell death and spontaneous differentiation were determined by dividing the number of dead or differentiated cells by the total number of cells (including live, dead, and differentiated) in each clone. The approximate population doubling time was determined by using the formula d = t/log2N, where d is the population doubling time, t is the time of cells in culture, and N is the total number of cells in the clone.
Induced Differentiation
Differentiation of OPCs was induced by either the addition of TH (T3 at 30 ng/ml; Barres et al. 1994b) or PDGF withdrawal (Noble and Murray 1984; Temple and Raff 1985). Several approaches were employed to quantitate the differentiation response. For the majority of experiments, OPCs of different ages were plated at high density (15,000 cells/flask) into PDL-coated T25 flasks and cultured for 2 d in either PDGF or PDGF plus bFGF. Then, either TH was added to the culture medium or cells were switched to medium without PDGF. Half of the culture medium was replaced with fresh medium every 3 d. Differentiated oligodendrocytes were counted, based on their characteristic morphology (Raff et al. 1978; Temple and Raff 1985). Duplicate flasks were set up for each time point and generally ∼1,000 cells were counted for each flask. At the end of the experiment, cells were harvested by scraping and used in RT-PCR assays to determine the levels of mRNAs encoding the myelin proteins PLP and MBP. RT-PCR analysis of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used as a control. mRNA was extracted using a QuickPrep Micro mRNA Purification kit (Pharmacia), according to the manufacturer's instructions. cDNA synthesis was then carried out using 0.5 μg of mRNA, Superscript II MMLV reverse transcriptase, and 3′-PCR primers (for MBP, 5′-GAAGAGACAGCCGCTCTG-3′; for PLP, 5′-GTATAGGCAGTCTCTGCGC-3′; for GAPDH, 5′-TCCACCACCCTGTTGCTGTA-3′) in a 20-μl reaction under the conditions previously described (Tang et al. 1993). PCR was performed using 2 μl of the respective cDNA and corresponding PCR primers (the 3′-primers were described above; the 5′-primers for MBP, PLP, and GAPDH were: 5′-AAGTACTTGGCCACAGCAAG-3′, 5′-CAGTATGTCATCTATGGAAC-3′, and 5′-ACCACAGTCCATGCCATCAC-3′, respectively). All PCR primers were designed to span introns to distinguish PCR products derived from RNA and the products derived from genomic DNA. PCR conditions were as previously described (Tang et al. 1993), and the cycling profile was 94°C × 15 s, 53°C × 30 s, and 72°C × 1 min for 30 cycles.
In some experiments cells were plated at clonal density (1,000 cells/flask) in PDL-coated slide flasks and treated as described above. The number of differentiated oligodendrocytes in each clone was counted every 2 d, and clones were scored as precursor clones or oligodendrocyte clones according to the predominant (i.e., >50%) cell type present in the clone (Barres et al. 1993, Barres et al. 1994b). Duplicate flasks were set up for each time point and about 100 clones were counted for each flask. At the end of the experiment, the slides were stained for MBP. The experiments were carried out twice.
Immunofluorescence Staining
Cells of different ages were plated onto either PDL-coated 13-mm glass coverslips (3,000 cells/coverslip) or slide flasks (10,000 cells/flask) and cultured for 2–3 d before they were immunostained. For cell surface staining with A2B5, O1, O4, or anti-GC antibodies, cells were fixed in 3.7% paraformaldehyde for 5 min at room temperature. For staining for NG2 antigen, MBP, and PLP, cells were fixed and permeabilized for 5 min in acid alcohol (95% ethanol and 5% glacial acetic acid) at room temperature. For intracellular labeling of vimentin, nestin, and GFAP, cells were fixed and permeabilized for 10 min in methanol:acetone (1:1) at –20°C. Following washing, cells were blocked for nonspecific binding sites with either 50% horse serum (for anti-vimentin antibody) or goat serum (for the other antibodies) for 30 min at room temperature. Primary antibodies were used at either 1:10 (for supernatants) or 1:100 (for the others), diluted in 25% blocking serum in PBS. The incubations were generally 1 h at room temperature. After extensive washing, coverslips were incubated (30 min, room temperature) in goat anti-mouse IgG-biotin, goat anti–rabbit IgG-biotin, or donkey anti–goat IgG-biotin, all diluted 1:100 in 25% blocking serum in PBS. Finally, cells were incubated with a mixture of either FITC-streptavidin or Texas red–streptavidin (both at 1:100) and either bisbenzamide (1:200) or propidium iodide (PI, 1 μg/ml) for 30 min at room temperature. Coverslips were mounted and observed as described for BrdU staining. The fluorescent and biotin-coupled reagents were all obtained from Amersham.
Time-Lapse Video Recording
OPCs of different ages were cultured at clonal density (1,000 cells/flask) in PDL-coated slide flasks in medium containing either PDGF or PDGF plus bFGF. After 2–3 d, the flask was placed on the stage of a Zeiss inverted phase-contrast microscope and maintained at 37°C. Time-lapse video recordings were made using a Sony CCD black and white video camera and a Sony video cassette recorder. Cell cycle times were determined by measuring the time between mitotic telophases. Cell motility was measured by determining the distance that the cell body had moved during a specific period of time, and the results were expressed as μm/h. Generally, clones of 2–10 cells were chosen for analysis.
Results
Establishment of Long-Term OPC Cultures and Changes in Morphology
When purified P7 OPCs were cultured in serum-free B-S medium containing either PDGF or PDGF plus bFGF, but in the absence of TH, most of the cells initially had a bipolar or unipolar morphology with unbranched processes (Fig. 1A and Fig. B). When cultured at high density (15,000–20,000 cells/T25 flask) in PDGF alone, the cells divided rapidly and reached near confluence (∼300,000 cells/flask) in 7–8 d; when cultured at clonal density (2,000 cells/T25 flask), they reached near confluence in ∼2 wk. When cultured in PDGF plus bFGF, the cells reached near confluence later (10–12 d at high density and ∼20 d at low density), suggesting that bFGF inhibited PDGF-stimulated OPC proliferation (see below).
Figure 1.
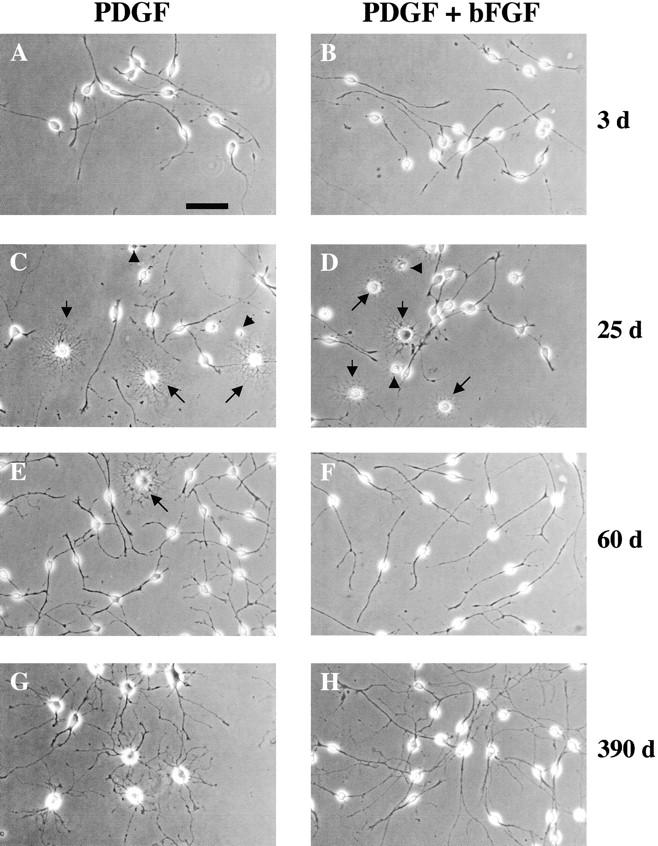
Photomicrographs showing the morphologies of OPCs cultured without TH in either PDGF or PDGF plus bFGF for different periods of time. In C and D, the arrowheads point to dead cells and the arrows point to differentiated oligodendrocytes. In E, the arrow points to a differentiated oligodendrocyte. Bar, 10 μm.
We repeatedly passaged the cells when they reached near confluence, replating 2,000 cells per T25 flask at each passage. Many cells died in the initial few passages, and among the surviving cells the rate of spontaneous differentiation and apoptotic cell death, which occurred randomly in most clones (Fig. 1C and Fig. D; see below), increased progressively, as previously described (Gao et al. 1998). Even at its peak, however, <50% of the cells differentiated and/or died (see below). Between 45 and 60 d, the amount of spontaneous differentiation and cell death decreased, and the surviving OPCs could be readily passaged (Fig. 1E and Fig. F). The OPCs in PDGF alone progressively developed a more complex morphology (Fig. 1E and Fig. G), whereas the OPCs in PDGF and bFGF maintained a simpler morphology (Fig. 1F and Fig. H). By 4–6 mo, the majority of OPCs in PDGF had a large cell body and multibranched processes and this morphology was maintained thereafter (Fig. 1 G). In contrast, the majority of OPCs cultured in PDGF plus bFGF maintained a small cell body and a bipolar morphology for more than a year (Fig. 1 H), suggesting that the combination of PDGF and bFGF inhibits the morphological maturation of OPCs, as previously described (Bögler et al. 1990).
When OPCs cultured in PDGF alone for 75 d were switched to PDGF plus for bFGF for 60 d, they had a bipolar morphology. When OPCs cultured in PDGF alone for 300 d were switched to PDGF plus bFGF for 30 d, cell death increased, but most cells maintained a complex morphology (not shown).
Analysis of Cell Cycle Time, Spontaneous Differentiation, and Cell Death
To quantitate the changes that occurred with time in culture, we carried out clonal analyses to determine population doubling times and the amount of spontaneous differentiation and cell death. We did BrdU labeling experiments to estimate Tc. As shown in Table , P7 OPCs cultured in PDGF for 8 d had a population doubling time of ∼27 h, while cells cultured in PDGF plus bFGF for the same time period had a population doubling time of ∼47 h. Using cumulative BrdU labeling to estimate Tc, we found that the inhibitory effect of bFGF on the population doubling time in the first 2–3 wk culture resulted at least partly from an increase in Tc (Fig. 2): at 2 d, for example, OPCs cultured in PDGF had a Tc of ∼25 h, whereas the same preparation of cells cultured in PDGF plus bFGF had a Tc of ∼30 h (Fig. 2 C). bFGF also enhanced the rate of cell death at 8 d: the rate was ∼7% in cells cultured in PDGF alone and ∼12% in cells cultured in PDGF plus bFGF (Table ). At these early times, the rate of spontaneous differentiation was not statistically different in cells cultured in PDGF plus bFGF compared with cells cultured in PDGF alone (Table ).
Table 1.
Population Doubling Times of OPCs in Culture
| Days in culture | Population doubling time | |
|---|---|---|
| PDGF | PDGF + bFGF | |
| h | ||
| 8 | 27 ± 4 (306) | 47 ± 4 (203) |
| 18 | 43 ± 6 (332) | 63 ± 3 (455) |
| 90 | 40 ± 4 (119) | 36 ± 5 (127) |
| 330 | 45 ± 4 (335) | ND |
| 390 | 45 ± 7 (143) | 37 ± 3 (136) |
Purified OPCs that had been in culture for various periods of time were cultured at clonal density (2,000 cells per T25 flask) in B-S media containing either PDGF or PDGF + bFGF for 8 d before the number of cells in each clone was counted. The number of clones analyzed is indicated in parentheses. Days in culture refers to the total time that the cells were in culture before replating at clonal density for the assay. The population doubling time was determined as in Materials and Methods. Data are shown as mean ± SEM.
Figure 2.
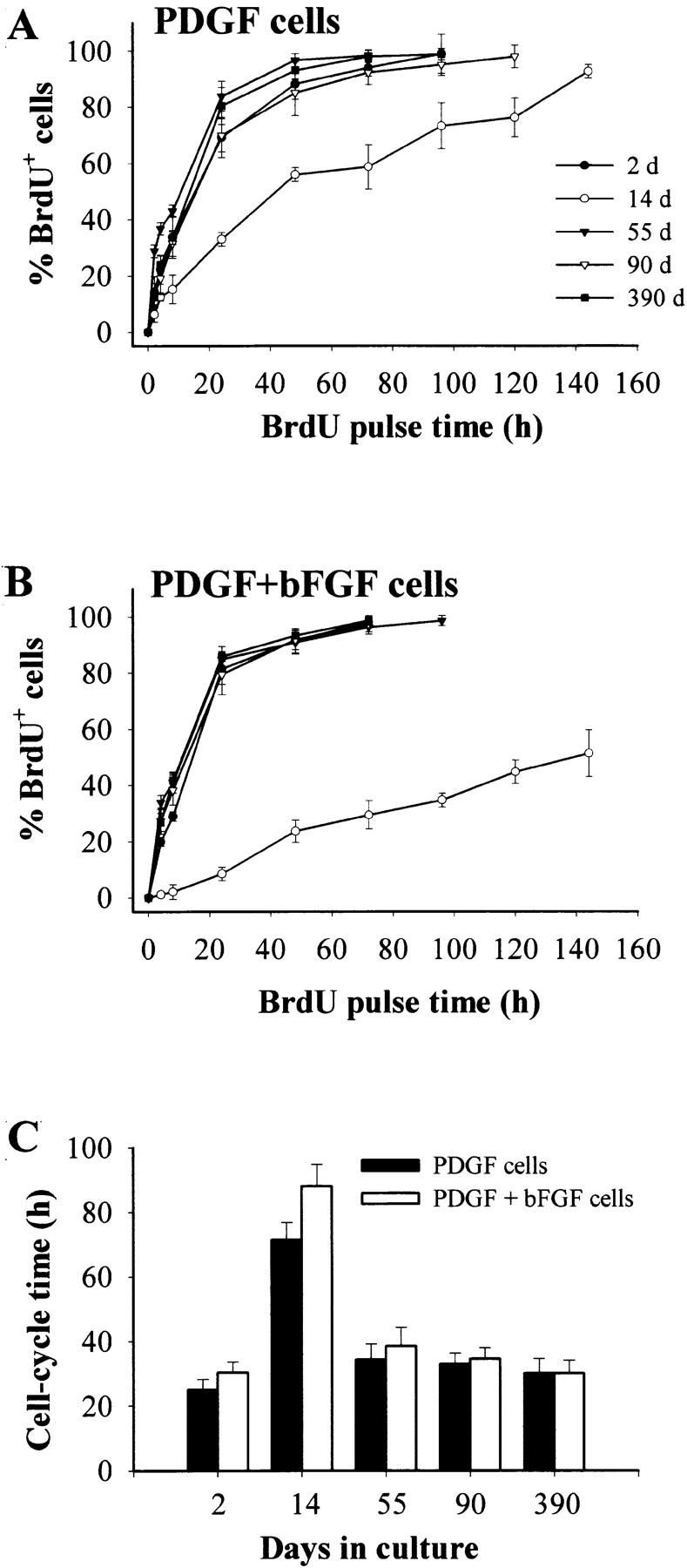
Cell cycle properties of OPCs in culture without TH for various periods of time in either PDGF (A) or PDGF plus bFGF (B). The cells were pulsed with BrdU for the times indicated, and the percentage of BrdU+ cells was determined by immunofluorescence. The results are expressed as mean ± SEM of two independent experiments, each with duplicate cultures for each time point. The calculated Tcs (see Materials and Methods) are shown in C.
Table 2.
Clonal Analysis of Spontaneous Differentiation and Cell Death in OPCs in Culture*
| Days in culture(passage number) | Cell death‡ | Differentiation‡ | ||
|---|---|---|---|---|
| PDGF | PDGF + bFGF | PDGF | PDGF + bFGF | |
| % | ||||
| 8 (0) | 7 ± 2 (203) | 12 ± 4 (203)§ | 10 ± 2 (306) | 9 ± 0.3 (203) |
| 17 (0) | 18 ± 4 (289) | 17 ± 3 (356) | 14 ± 3 (289) | 15 ± 2 (356) |
| 17 (1) | 18 ± 4 (231) | 15 ± 5 (328) | 14 ± 2 (231) | 16 ± 1 (328) |
| 23 (1) | 28 ± 6 (101) | 26 ± 4 (127) | 17 ± 2 (101) | 17 ± 4 (127) |
| 90 (5) | 6 ± 3 (249) | 3 ± 1 (215)§ | 8 ± 1 (249) | <1 (215)§ |
| 374 (29) | 5 ± 2 (335) | ND | 3 ± 0.1 (N 7 335) | ND |
| 390 (30) | 7 ± 2 (143) | 3 ± 2 (205)§ | 4 ± 0.1 (143) | <1 (143)§ |
*Purified OPCs that had been in culture for various periods of time were cultured at clonal density (2,000 cells per T25 flask) in B-S media containing either PDGF or PDGF + bFGF for 8 d before counting. Days in culture refers to the total time that the cells were in culture before replating at clonal density or the assay.
‡The numbers of dead or differentiated (cells with a characteristic oligodendrocyte morphology) cells in each individual clone were counted. The number of clones analyzed is indicated in parentheses. Data are shown as mean ± SD for all the clones analyzed.
§Significant difference (P < 0.05) compared with PDGF only cultures, when analyzed by Student's t test.
By 2–3 wk after initial plating, the population doubling times had increased and were still much higher in PDGF plus bFGF than in PDGF alone (Table ). Cumulative BrdU labeling experiments (Fig. 2A and Fig. B) indicated that part of the increase with time was due to an increase in Tc, while clonal analyses indicated that increased spontaneous differentiation and cell death also contributed (Table ). Nonetheless, the difference between PDGF alone and PDGF plus bFGF was mainly due to the difference in Tcs (Fig. 2A and Fig. B).
The increased rates of spontaneous differentiation and cell death at 2–3 wk were not related to the passaging of the cells, as cells cultured for 17 d with or without passaging had very similar rates of spontaneous differentiation and cell death (Table ). This observation raised the possibility that the increase in Tc, spontaneous differentiation, and cell death may reflect the normal maturation of OPCs. To test this possibility, we purified OPCs from P0, P9, and P14 rat optic nerves and performed cumulative BrdU labeling experiments to estimate Tc and clonal analyses to determine population doubling time, spontaneous differentiation, and cell death. As shown in Fig. 3, P14 OPCs had longer Tcs and produced smaller clones than P9 OPCs, which in turn had longer Tcs and produced smaller clones than P0 OPCs; furthermore, P9 and P14 OPCs had significantly higher rates of spontaneous differentiation and cell death than P0 OPCs. The results were remarkably similar to those obtained with cultured OPCs of equivalent ages (Fig. 2; Table and Table ), supporting the possibility that the changes with time in culture reflect normal OPC cell maturation, rather than a cell culture artefact.
Figure 3.
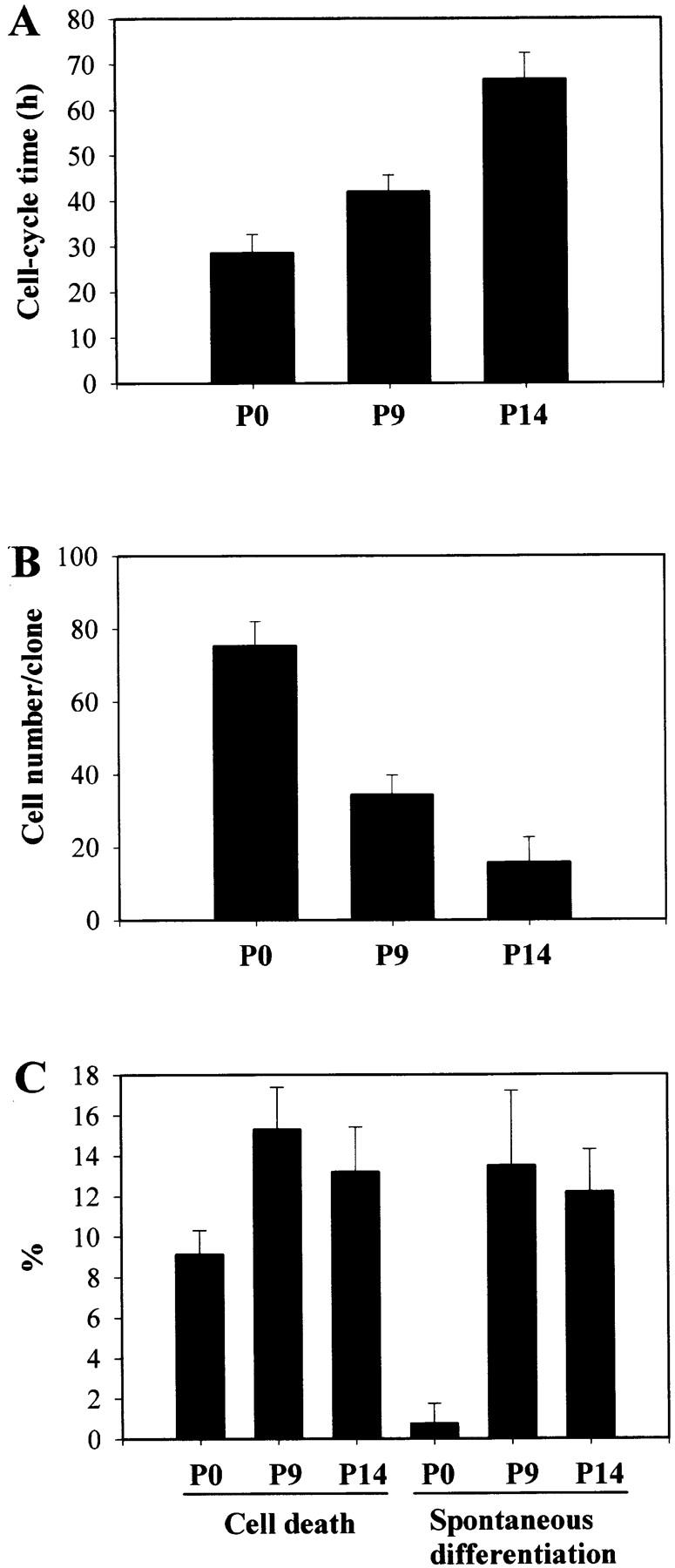
Analyses of Tc, clonal expansion, spontaneous differentiation, and cell death in cultures of OPCs purified from P0, P9, and P14 rat optic nerve. Cells were cultured in PDGF without TH. (A) After 2 d, BrdU was added for 2–96 h before cells were stained for BrdU. The cumulative BrdU labeling indices were used to calculate the cell cycle time, as in Fig. 2. The results are shown as mean ± SEM of two independent experiments, each with triplicate cultures for each time point. The results are significantly different (P < 0.01) between P0 and P9 and between P9 and P14. In B and C, the OPCs were cultured at clonal density for 7 d, and then the number of cells per clone (B) and the proportions of oligodendrocytes and dead cells per clone (C) were determined. The results are shown as mean ± SEM of two independent experiments. In B, the results are significantly different (P < 0.01) between P0 and P9 and between P9 and P14. In C, the results are significantly different (P < 0.01) between P0 and P9 and P14 for cell death, and also (P < 0.001) between P0 and P9 and P14 for differentiation.
After 17 d in culture, spontaneous differentiation and cell death continued to increase (Table ), reaching plateau levels by 4–6 wk (Table and data not shown). By 55 d in culture, the Tcs stabilized at ∼35 h (Fig. 2). Spontaneous differentiation and cell death rates stabilized at <10%, and now cells in PDGF plus bFGF showed lower spontaneous differentiation and death rates than cells in PDGF alone (Table and not shown). Once established after 40–60 d in culture, the OPCs appeared to self renew indefinitely. This was the case for eight of eight separate long-term experiments. In each case, the established OPCs in either PDGF or PDGF plus bFGF had Tcs of ∼35 h (Fig. 2 C). The cells in PDGF alone had longer population doubling times than the same set of cells in PDGF plus bFGF (Table ), mainly because the spontaneous differentiation and death rates were lower in bFGF (Table ).
Induced Differentiation
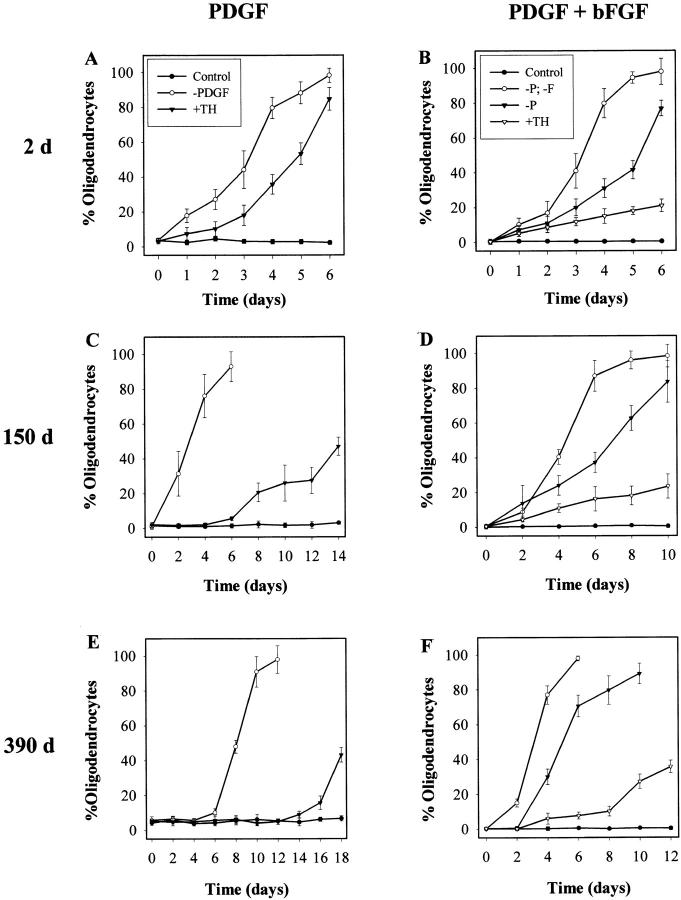
When cells in PDGF were induced to differentiate by the addition of TH after various periods of time in culture, the rate at which oligodendrocytes developed progressively slowed as the time before TH addition increased, although the rate of oligodendrocyte development when PDGF was withdrawn slowed much less. After 2 d in culture, ∼100% of P7 OPCs differentiated into morphologically typical oligodendrocytes within 6 d when PDGF was withdrawn (Fig. 4 A). Although the response of such cells to TH addition was slower than to PDGF withdrawal, >80% differentiated within 7 d (Fig. 4 A). By contrast, only ∼50% of OPCs cultured in PDGF for 75 d differentiated within 1 wk when induced by TH, although their response to PDGF withdrawal was comparable to that of cells cultured in PDGF for 2 d (not shown). After 150 d in PDGF, the response to TH addition was even slower, although the response to PDGF withdrawal was still just as fast as after 2 d in PDGF (Fig. 4 C). After 390 d, it took 18 d for 40% of the cells to differentiate after TH addition and 10 d before almost all of the cells differentiated after PDGF removal (Fig. 4 E). In all cases, differentiation was confirmed by RT-PCR analysis of MBP and PLP mRNAs (Fig. 5 and not shown); for 390-d cultures, differentiation was also confirmed by clonal analysis and MBP staining (not shown). In summary, the response of OPCs to TH decreases with time in culture, whereas the response to PDGF withdrawal decreases much less.
Figure 4.
Speed at which P7 OPCs cultured without TH for various periods of time in PDGF or PDGF plus bFGF differentiate into oligodendrocytes when deprived of either PDGF (−P) or both PDGF and bFGF (−P, −F) or when stimulated by TH (+TH). The number of days that the cells were in culture before they were induced to differentiate is shown on the left. Results are shown as mean ± SEM of triplicate flasks. Similar results were obtained with several sets of cells.
Figure 5.
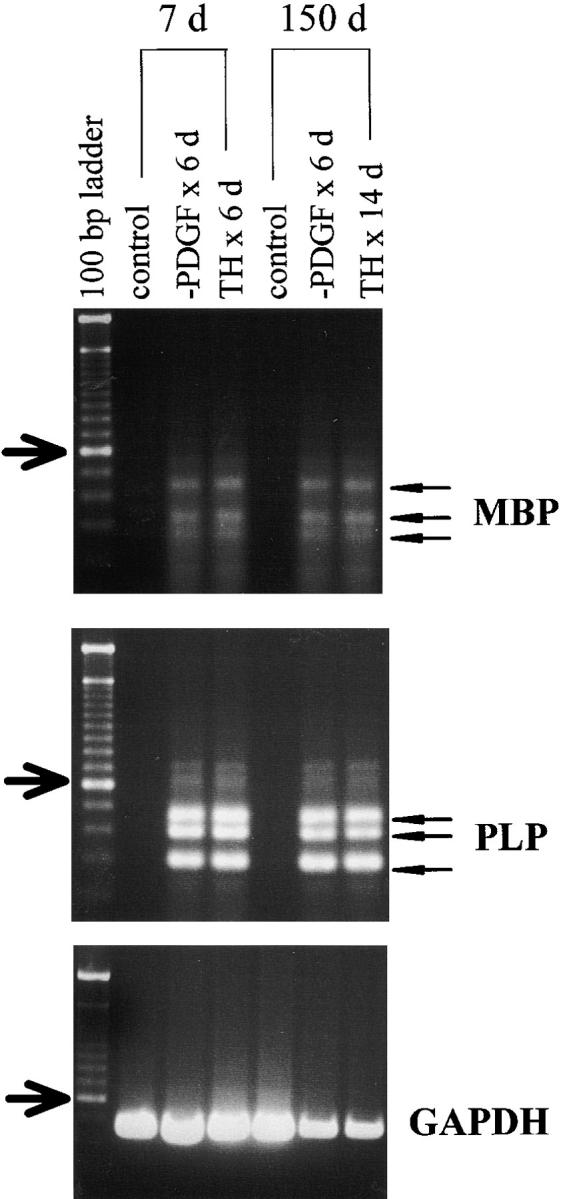
RT-PCR assays for MBP and PLP mRNAs following the induction of differentiation in OPCs that had been cultured in PDGF without TH for 7 or 150 d before induction by either PDGF withdrawal or the addition TH. Note that at least three bands are detected for both MBP and PLP (arrowheads), both of which are known to have multiple mRNA species produced by alternative RNA splicing (Roach et al. 1983; Milner et al. 1985; Malotka and Dornmair 1995). The left lane is a 100-bp ladder of size markers, and the arrows indicate the 600-bp band. The GAPDH mRNA was used as a control.
OPCs cultured in PDGF plus bFGF showed much less slowing in their differentiation response with time in culture. Almost all of these cells differentiated within 1 wk when deprived of both PDGF and bFGF, even after they had been in culture for 390 d (Fig. 4B, Fig. D, and Fig. F) or 510 d (not shown). The cells responded somewhat more slowly when deprived only of PDGF (Fig. 4B, Fig. D, and Fig. F), suggesting that bFGF weakly suppresses PDGF withdrawal–induced differentiation. bFGF had a much stronger inhibitory effect on TH-induced differentiation: in the presence of bFGF and PDGF, only ∼16% of OPCs that had been in culture for 2 d differentiated after 6 d of TH treatment (Fig. 4 B), but a similar rate of differentiation was seen when cells cultured in PDGF plus bFGF for 390 d were treated with TH.
Expression of Antigenic Markers and Migration
We next used immunofluorescence to examine the expression of a number of antigenic markers in OPCs after various times in culture. We stained cells for A2B5, O1, O4, GC, NG2, MBP, PLP, vimentin, nestin, and GFAP, most of which are expressed by oligodendrocyte lineage cells at various developmental stages (Gard and Pfeiffer 1990; Goldman and Vaysse 1991; Hardy and Reynolds 1991; Pfeiffer et al. 1993; Grinspan and Franceschini 1995). Some of the results are shown in Fig. 6 and are summarized in Table . Strong A2B5 staining was observed in all the cultures (Fig. 6, A–D), whereas MBP, PLP, or GFAP staining was never observed (not shown). Strong, filamentous vimentin staining was observed in OPCs after 3 d in culture, whereas no such staining was seen after >50 d in culture in either PDGF or PDGF plus bFGF (not shown).
Figure 6.
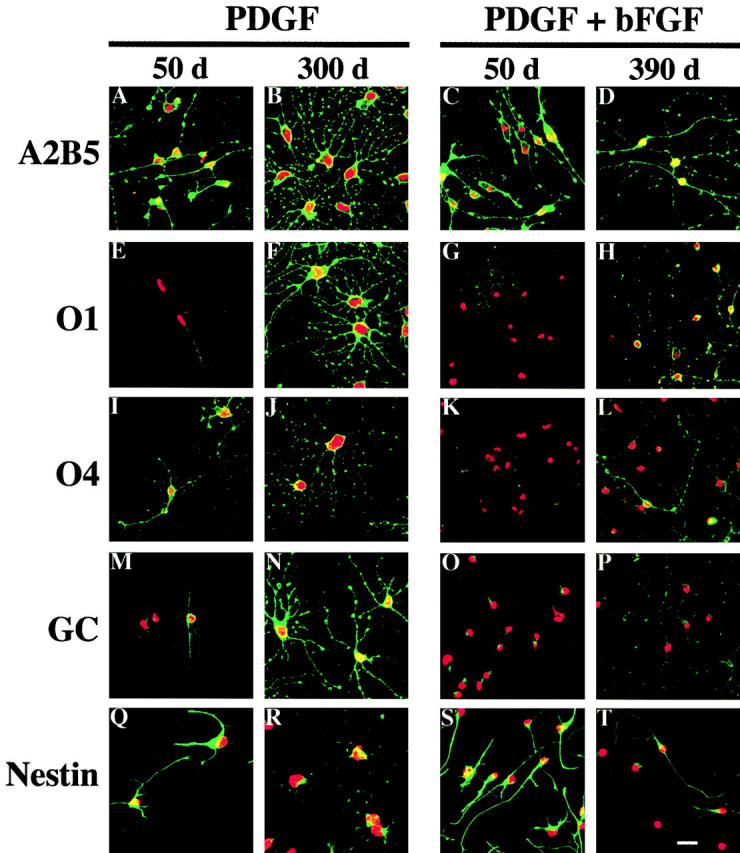
Immunofluores- cence staining with A2B5, O1, O4, Ranscht anti-GC, or anti-nestin antibodies. P7 OPCs were cultured without TH in either PDGF or PDGF plus bFGF for 50 or 390 d. Antibody staining is shown in green. Nuclei were stained with propidium iodide and are shown in red. Bar, 10 μm.
Table 3.
Antigen Expression in OPCs*
| Antigen | PDGF OPCs‡ | PDGF+bFGF OPCs‡ | Fresh adult OPCs§ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 50 d | 120 d | 300 d | 390 d | 50 d | 330 d | 390 d | 50 d | 300 d | |
| A2B5 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| O4 | ± | ++ | 49‖ (+++) | 97 (+++) | 100 (+++) | + | 58 (++) | 77 (++) | + | +++ |
| O1 | ± | + | 54 (+++) | 100 (+++) | 100 (+++) | − | 49 (+) | 62 (++) | + | ++ |
| GC | ± | + | 28 (++) | 100 (+++) | 100 (+++) | ± | 63 (+) | 65 (++) | + | +++ |
| Vimentin | +++ | ± | ± | ± | ± | ND | ND | ± | ± | ± |
| Nestin | +++ | +++ | ND | ± | ± | +++ | ND | 20 (++) | +++ | +++ |
| NG2 | +++ | +++ | ND | ND | +++ | +++ | ND | +++ | +++ | +++ |
*The relative intensity of staining is indicated by an arbitrary scale; ± indicates either that staining was so weak that it was not clear if it was significant or that, in the case of vimentin and nestin, the staining was weak and nonfilamentous.
‡Purified P7 OPCs were cultured in PDGF or PDGF + bFGF for the time indicated. Cells in culture for ≥50 d were passaged multiple times.
§OPCs were purified from P50 or P300 rats. They were then cultured on PDL-coated coverslips for 2 d before immunostaining.
‖The numbers indicate the percentage of cells labeled. An average of 1,000 cells were counted on each coverslip, and each sample was run in duplicate. The numbers represent the mean value from duplicate slides.
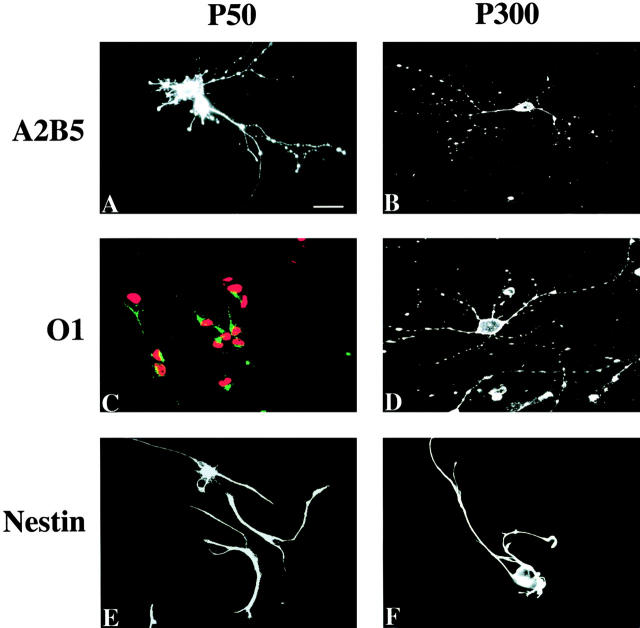
Most interestingly, OPCs cultured in PDGF alone progressively acquired staining by O4, O1, and anti-GC antibodies: whereas cells in culture for 3 d did not stain with any of these antibodies (Table ), those cultured for 50 d showed some staining with all three antibodies (Fig. 6E, Fig. I, and Fig. M). Cells acquired O4 staining first: ∼30% of the OPCs in PDGF for 50 d were stained strongly by O4 antibody (Fig. 6 E, and Table ), whereas most cells of this age showed only weak staining with O1 and anti-GC antibodies (Fig. 6I and Fig. M; Table ). The staining of these antibodies increased with time, and by 300 d, most of the cells showed strong staining with all three antibodies, comparable to that observed for fully differentiated oligodendrocytes (Fig. 6F, Fig. J, and Fig. N).
OPCs cultured in PDGF and bFGF also acquired these antigens but with a much slower time course. After 50 d the cells did not stain with the O1 antibody (Fig. 6 K) and stained only very weakly with the O4 and anti-GC antibodies (Fig. 6G and Fig. O; Table ). The staining with the anti-GC antibody (Ranscht et al. 1982; Fig. 6 O) resembled the polarized patch staining seen with this antibody reported previously on OPCs treated briefly with TH (Tokumoto et al. 1999). Even after 390 d, OPCs cultured in PDGF plus bFGF showed only weak staining with the anti-GC and O1 antibodies and moderate staining with the O4 antibody, and some cells still did not stain at all (Fig. 6H, Fig. L; Table ).
Nestin is an intermediate filament protein characteristic of neural stem cells (Lendahl et al. 1990). OPCs cultured in PDGF alone showed strong, filamentous staining for nestin for up to 50 d (Fig. 6 Q), but by 300 d they showed only weak, nonfilamentous nestin staining (Fig. 6 R). In contrast, ∼25% of OPCs cultured for up to 390 d in PDGF plus bFGF still showed strong filamentous nestin staining (Fig. 6 T).
Although the long-term OPCs cultured in PDGF alone strongly stained with O4, O1, and anti-GC antibodies (Fig. 6F, Fig. J, and Fig. N), the cells were still clearly precursors rather than oligodendrocytes as they also expressed NG2 (Fig. 7 A), an antigen expressed on OPCs that is lost when OPCs differentiate into oligodendrocytes (Levine and Stallcup 1987; not shown), and they were still actively proliferating as evidenced by BrdU incorporation (Fig. 7B and Fig. C). Furthermore, OPCs that had been cultured in PDGF for 16 mo still differentiated into type-2 astrocytes when switched to medium containing 10% FCS (Raff et al. 1983; data not shown).
Figure 7.
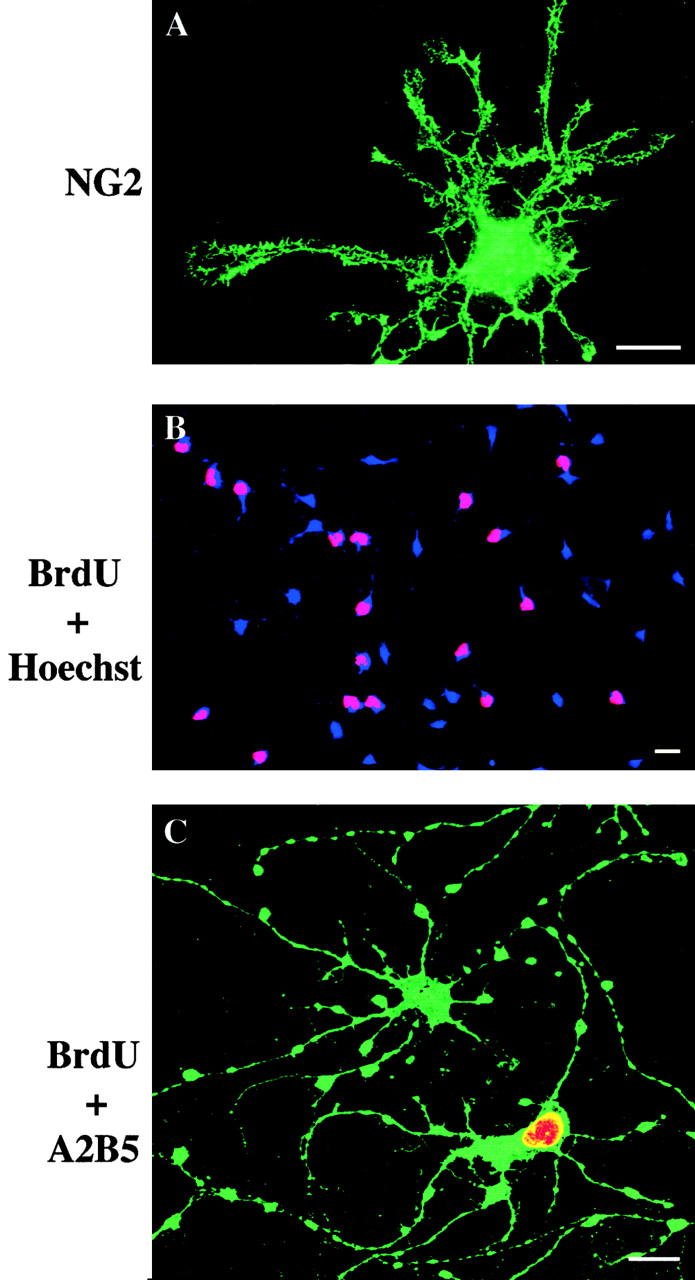
NG2 expression and BrdU incorporation in OPCs cultured without TH in PDGF for 450 d. (A) Cell stained for NG2. (B and C) OPCs were pulsed with BrdU for 4 h before staining for A2B5 (green, in C) and BrdU (red in B and C). Nuclei were stained with Hoechst dye (blue in B). Bars, 10 μm.
We also examined the migration of OPCs after various times in culture. As shown in Table , the migration rate of OPCs cultured in PDGF alone progressively slowed, while that of OPCs cultured in PDGF plus bFGF slowed much less, further suggesting that the presence of bFGF slows down OPC maturation in culture.
Table 4.
Migration Rates of OPCs in Culture
| Time | Migration rate | |
|---|---|---|
| PDGF | PDGF + bFGF | |
| d* | μm/h‡ | |
| 5 | 23 ± 3 | 24 ± 2 |
| 60 | 21 ± 3 | 22 ± 2 |
| 150 | 13 ± 2 | 19 ± 2 |
| 300 | 6 ± 3 | 16 ± 1 |
| 420 | 5 ± 1 | 15 ± 3 |
*Purified P7 OPCs that had been in culture for various periods of time were cultured in B-S medium containing either PDGF or PDGF + bFGF for the days indicated.
‡Cell migration was determined by measuring the distance that the cell body moved during a randomly chosen period of time. Time-lapse video recordings were performed for 48–96 h. Clones of 2–10 cells were chosen, and 15–20 cells from 3–5 different clones were analyzed for each time point. The results are shown as mean ± SEM.
Freshly Purified Adult OPCs
Taken together, our findings suggested that OPCs cultured in PDGF for an extended period of time came to resemble adult OPCs in a number of their properties (ffrench-Constant and Raff 1986; Levine and Gard 1987; Wolswijk and Noble 1989; Fulton et al. 1992; Wren et al. 1992; Shi et al. 1998). To compare our long-term OPCs with adult OPCs, we purified OPCs from adult rat optic nerves of different ages. With advancing age, it became more and more difficult to culture the adult OPCs. Whereas we could easily culture purified OPCs from P50 rats, for example, we were unable to use the same medium to culture purified OPCs from ≥P150 rats. The addition of neuregulin greatly enhanced the survival of the older cells, as we report elsewhere (Fernandez, P.-A., D.G. Tang, L. Cheng, A.W. Mudge, A. Prochiantz, and M.C. Raff, manuscript submitted for publication).
We first compared the ability of purified adult OPCs and age-matched long-term cultured OPCs to incorporate BrdU after an 8-h pulse of BrdU. As shown in Fig. 8 A, ∼45% of OPCs maintained in PDGF for 50 d and ∼35% maintained for 300 d or 390 d incorporated BrdU, whereas <10% of the adult OPCs did so under the same conditions, and the proportion decreased with increasing age of the adult OPCs. The OPCs from P300 and P390 rats were maintained in PDGF and NRG, but NRG did not enhance BrdU incorporation, either by itself or with PDGF (not shown).
Figure 8.
Comparison of BrdU labeling (A) and oligodendrocyte differentiation (B) in P7 OPCs cultured without TH for various periods of time in PDGF alone (open bars and symbols) and freshly isolated OPCs from adult optic nerve of equivalent age (filled bars and symbols). In all cases, OPCs were plated into PDL-coated slide flasks and cultured without TH in PDGF for 2 d. In A, BrdU was added for the last 8 h of the culture, and the cells were then fixed and stained for BrdU. An average of 1,000 cells were counted in duplicate flasks, and the results are shown as mean ± SEM. In B, either the cells were switched after the 2 d to culture medium without PDGF (−PDGF) or TH (+TH) was added for the time indicated. The proportion of differentiated oligodendrocytes was determined using morphological criteria. The results are shown as mean ± SEM of two experiments with duplicate flasks for each condition.
Although adult OPCs proliferated much more slowly than long-term cultured OPCs, the two types of OPCs shared a number of other characteristics. Both populations differentiated equally slowly in response to TH addition, although P50 OPCs also differentiated slowly in response to PDGF withdrawal, whereas P7 OPCs that had been in culture for 50 d differentiated quickly when PDGF was withdrawn (Fig. 8 B). The two types of OPCs were also antigenically similar. Adult OPCs of all ages examined, just like long-term cultured OPCs, stained strongly for A5B5 (Fig. 9A and Fig. B), but they did not stain for MBP, PLP GFAP, or filamentous vimentin (not shown). Moreover, P50 adult OPCs stained only weakly with O1, O4, and anti-GC antibodies, whereas P300 OPCs stained strongly with all these antibodies (Fig. 9C and Fig. D, and not shown). A notable difference, however, was that adult OPCs of all ages stained strongly for filamentous nestin (Fig. 9E and Fig. F), whereas the longterm cultured OPCs in PDGF progressively lost such staining (see Fig. 6 R).
Figure 9.
Immunofluores- cence staining with A2B5, O1, and anti-nestin antibodies of adult OPCs freshly isolated from P50 and P300 rat optic nerve. The cells were cultured without TH in slide flasks in PDGF (P50 cells) or PDGF plus NRG (P300 cells) for 2 d before they were stained. In C, cell nuclei were stained with propidium iodide (red). Bar, 10 μm.
Discussion
Most OPCs in the developing optic nerve divide a limited number of times before they stop and terminally differentiate into postmitotic oligodendrocytes (reviewed in Barres and Raff 1994). Some of these cells, however, seem to follow a different developmental pathway (Wren et al. 1992). Instead of differentiating into oligodendrocytes, they develop into slowly dividing adult OPCs with a distinctive character, which, in culture at least, can still differentiate into either oligodendrocytes or type-2 astrocytes (ffrench-Constant and Raff, 1986; Wolswijk and Noble 1989; Shi et al. 1998). The adult OPCs in the optic nerve have a complex morphology and appear to extend processes exclusively to nodes of Ranvier (Miller et al. 1989; Fulton et al. 1992; Butt et al. 1999). Similar cells are widely distributed in the adult CNS (Levine and Gard 1987). The function(s) of these cells in the adult CNS remains uncertain. It is also uncertain why these cells do not differentiate in vivo, although it has been suggested that they are inhibited from differentiating by Notch signaling, which blocks differentiation of neonatal OPCs in culture (Wang et al. 1998; Shi et al. 1998).
By manipulating the culture medium appropriately one can keep most perinatal OPCs dividing in culture beyond the time when they would normally withdraw from the cell cycle and terminally differentiate. One way is to omit TH from the culture medium (Barres et al., 1994; Ibarrola et al. 1996; Ahlgren et al. 1997; Gao et al. 1998), another is to use a combination of PDGF and bFGF to stimulate proliferation (Bögler et al. 1990; McKinnon et al. 1990). The question we address in this study is what happens if one maintains purified neonatal OPCs in culture in the absence of TH and in the presence of either PDGF or PDGF and bFGF. In particular, do OPCs acquire the properties to OPCs in the adult CNS?
Noble and his colleagues (Wren et al. 1992) showed that adult-like OPCs develop in cultures of neonatal optic nerve cells that are passaged repeatedly on monolayers of astrocytes. They also provided evidence that adult-like OPCs can develop directly from neonatal OPCs: in time-lapse microcinematographic studies, several cells with a neonatal phenotype were seen to give rise to cells with an adult-like phenotype, although the transition sometimes occurred over two or more divisions. Here we provide evidence that the acquisition of some adult OPC properties can occur progressively over months in cultures of purified neonatal OPCs stimulated to divide by PDGF in the absence of TH, suggesting that the changes reflect the operation of an intrinsic maturation program in the OPCs that plays out over many months.
Why Do So Many P7 OPCs Differentiate and/or Die in Our Cultures?
We showed previously that when purified P7 OPCs were cultured for weeks in PDGF without TH their proliferation slowed, their rate of spontaneous differentiation into oligodendrocytes gradually increased, and by 30 d many cells had differentiated and/or died (Gao et al. 1998). We attributed these changes to two intrinsic timing mechanisms operating in the OPCs. One mechanism was proposed to regulate the timing of normal oligodendrocyte differentiation by progressively increasing the probability that an OPC will stop dividing and terminally differentiate; whereas this probability is increased by TH, it is clear that, both in vitro and in vivo, OPCs can terminally differentiate in the absence of TH, although the differentiation is delayed (Ibarrola et al. 1996; Ahlgren et al. 1997; Gao et al. 1998). The second timing mechanism was proposed to be a timer that controls the onset of replicative cell senescence (Gao et al. 1998), which is thought to operate in many kinds of dividing cells in culture (Hayflick 1965; Smith and Pereira-Smith 1996). An alternative explanation for why many OPCs spontaneously differentiate and/or die in our cultures after several weeks is that the extracellular signals that the cells need to survive and/or divide may change over time as the cells proliferate in culture, so that the conditions, at least transiently, become suboptimal for proliferation and survival.
We favor the second explanation for several reasons. At the time that our cells become difficult to maintain and passage, they show few of the changes that are characteristic of replicative senescence: they do not stain (unpublished observations), at low pH, with senescence-associated β-galactosidase (Dimri et al. 1995), for example, and the cells differentiate and/or die rather than arrest and survive. The survival signals required by OPCs change after the cells differentiate into oligodendrocytes: PDGF, for instance, promotes the survival of OPCs and newly formed oligodendrocytes (Barres et al. 1992), but oligodendrocytes lose their PDGF receptors within days (Hart et al. 1989; McKinnon et al. 1990) and become insensitive to PDGF. It is perhaps not surprising therefore that the oligodendrocytes that spontaneously differentiate in our cultures soon die. Moreover, the survival signals required by OPCs themselves seem to change as they mature. We show here that the rates of spontaneous differentiation and death are greater in clones of P9 or P14 OPCs than in clones of P0 OPCs in the same culture medium. We also find that NRG is required for the survival of OPCs isolated from ≥P150 rats but not from P50 rats; finally, bFGF increases the death of neonatal OPCs, whereas it significantly inhibits the spontaneous differentiation and death of long-term cultured OPCs. If we are correct that many of the P7 OPCs die during the first few weeks in our culture because the culture conditions are suboptimal for oligodendrocytes and more mature OPCs, we should be able to find conditions where most neonatal OPCs survive through this critical period in culture without differentiation. If this proves possible, we should then be able to determine whether most P7 OPCs can develop into adult OPCs or whether only a subpopulation of P7 OPCs have this potential.
What Distinguishes the OPCs That Survive?
Despite the significant amount of cell death, many OPCs always seem to survive and continue to proliferate indefinitely, or at least for 18 mo, which is as long as we have followed them. We do not know what is special about these cells that enables them to live while many others differentiate and/or die. One possibility is that they preexist as a subpopulation of stem-like cells with exceptional capacity for survival and self renewal. Another is that they arise as immortalized mutants. Although we cannot exclude this latter possibility, we think it unlikely for four reasons: (a) OPCs with the same phenotype developed in eight out of eight experiments. (b) The cells that survive seem to mature over months and acquire at least some of the properties of adult OPCs on much the same schedule as their normal counterparts in vivo, as we discuss below. (c) Even after 15 mo, the cells growing in either PDGF or PDGF plus bFGF show normal cell cycle checkpoint and apoptotic responses (Tang et al., manuscript in preparation). (d) The cells that survive during the critical period are found in most clones.
Yet another possible explanation for what distinguishes the OPCs that survive in our cultures is that they represent the statistical tail of the OPC population with the best survival capabilities. Whatever their origins, it is clear that these cells have the ability for long-term self renewal in culture, in the absence of other cell types.
The Long-Term Survivors in PDGF Progressively Acquire Some of the Properties of Adult OPCs
The most important finding in this study is that in all eight experiments where we established long-term cultures of purified P7 OPCs in PDGF, we find that the cells progressively acquire, over months, some of the properties that are characteristic of OPCs in the adult optic nerve. The cells become larger and their morphology becomes more complex, so that by 5–6 mo they have multiple branching processes. One has to be cautious, however, in interpreting changes in morphology, as cell morphology is very sensitive to environmental influence. Although OPCs in the adult optic nerve have a similar complex morphology (Miller et al. 1989; Fulton et al. 1992; Butt et al. 1999), for example, they can acquire a relatively simple morphology when cultured in TH on astrocyte monolayers (Wolswijk and Noble 1989, Wolswijk and Noble 1992; Wren et al. 1992).
More importantly, the OPCs cultured in PDGF progressively change their antigenic phenotype, mimicking many of the antigenic changes that normally occur in vivo. Most remarkably, the cells gradually acquire the glycolipids, including GC, that are recognized by the O1 (Sommer and Schachner 1981) and Ranscht monoclonal (Ranscht et al. 1982) anti-GC antibodies. Although the cells do not stain, or stain only very weakly, with these antibodies after 50 d in culture, most do stain after 300 d, and the staining is even stronger at 390 d, when it is almost as intense as on differentiated oligodendrocytes. As reported previously by Shi et al. 1998 and confirmed here, although OPCs isolated from developing optic nerve do not stain with these antibodies, OPCs freshly isolated from adult optic nerve do. We find weak staining in P50 cells and strong staining in P300 cells. Thus, the acquisition of GC seems to occur on a similar schedule in vitro and in vivo. Even after 13–16 mo in vitro, however, the cultured P7 OPCs are still proliferating, are intensely stained by the A2B5 and NG2 antibodies, and do not express MBP or PLP mRNA detectable by RT-PCR, indicating that cells have not differentiated into oligodendrocytes. These findings make it clear that one cannot use anti-GC antibodies to identify differentiated oligodendrocytes in the adult CNS and may explain the previous finding that GC+ cells can be a source of remyelinating cells in the injured adult CNS (Wood and Bunge 1991). The expression of nestin, by contrast, differs in the long-term cultured cells and the freshly isolated adult OPCs; whereas cultured cells lose expression, the adult cells retain it.
Noble and colleagues (Wolswijk and Noble 1989, Wolswijk and Noble 1992; Wren et al. 1992) showed previously that the migration rate of OPCs in neonatal optic nerve cell cultures becomes slower with time in vitro. Gao and Raff 1997 provided evidence that this slowing of migration is an intrinsic property of the developing OPCs. We show here that the slowing can continue for many months, compared with the migration rate after 60 d in vitro, the rate is reduced twofold at 150 d and fourfold at 300 d. By 300 d in culture, the migration rate is ∼5 μm/h, which is very similar to the rate (4 μm/h) reported by Wolswijk and Noble 1989 for OPCs in optic nerve cultures prepared from adult rats that were at least 240 d old.
It was also shown previously that adult OPCs differentiate into oligodendrocytes in culture more slowly than do perinatal OPCs (ffrench-Constant and Raff 1986; Wolswijk and Noble 1989; Shi et al. 1998), and we show here that this is also the case for our long-term cultured cells. In response to TH treatment, 150 d cells differentiate more slowly than 7 d cells, and 390 d cells differentiate even more slowly than 150 d cells. Surprisingly, 150 d cells differentiate as fast in response to PDGF withdrawal as do 7 d cells, although 390 d cells differentiate more slowly. This contrasts with freshly isolated adult OPCs, which even at P50 differentiate only slowly in response to PDGF withdrawal.
Taken together, these findings strongly suggest that the surviving OPCs in our cultures continue to mature in vitro for many months, even though they are growing in isolation from other cell types. Gao and Raff 1997 showed previously that purified OPCs from the embryonic rat optic nerve differed in many of their properties from OPCs purified from P7 optic nerve. Embryonic cells were simpler in morphology, proliferated longer before they differentiated when cultured in PDGF and TH, and divided and migrated more rapidly. Remarkably, the embryonic cells acquired the properties of the P7 cells when they were purified and cultured on their own for 10 d in PDGF in the absence of TH, suggesting that OPCs have an intrinsic program that is responsible for their progressive maturation (Gao and Raff 1997). Our present findings suggest that the intrinsic maturation program continues to change cells for many months, raising the intriguing question of what kind of molecular mechanism underlies such a prolonged maturation program.
P7 OPCs cultured for many months in PDGF without TH do not acquire all of the characteristics of adult OPCs. They do not slow their proliferation rate to nearly the same extent, for example. Whereas the cultured cells have a Tc of ∼35 h from 50 d onward, freshly isolated adult OPCs cultured in the same medium divide much more slowly, at a rate that is inversely correlated with the age of the animal from which they are obtained. It is interesting that adult OPCs can be induced to divide rapidly if they are stimulated in culture with the right combination of factors (Wolswijk and Noble 1992; Shi et al. 1998), indicating that the slowing of the cell cycle with maturation is reversible. It will be important to determine how the intracellular cell cycle control system is altered with maturation.
Basic FGF Inhibits the Maturation of OPCs in Culture
Whereas the OPCs growing in PDGF progressively mature and acquire a number of the properties of adult OPCs, those maintained in PDGF plus bFGF mature much more slowly and maintain many of their youthful characteristics. They retain their simple bipolar morphology, their rapid migration rate, a relatively fast differentiation response, and acquire differentiation markers more slowly. Thus, the combination of bFGF and PDGF clearly inhibits OPC maturation in culture, just as it inhibits OPC differentiation into oligodendrocytes (Bögler et al. 1990; McKinnon et al. 1990). It remains to be determined which intracellular signaling pathways activated by FGF receptors, which, like PDGF receptors, are receptor tyrosine kinases, are responsible for these inhibitory effects. It is of interest that bFGF has different effects on P7 OPCs that have been in culture for a short time compared with P7 OPCs that have been in culture for months. Whereas it increases the death and slows down the cell cycle of the young OPCs, it inhibits the spontaneous differentiation and/or death and enhances the cell cycle progression of long-term cultured OPCs. This is further evidence that, as OPCs mature, they change their responsiveness to growth factors.
In summary, we have shown that when purified P7 OPCs are cultured in PDGF (or PDGF plus bFGF) without TH, many of the cells differentiate and/or die after several weeks, but many others continue to proliferate for up to 16 months or more. In PDGF, the surviving cells progressively acquire a number of properties that are characteristic of adult OPCs, while in PDGF and bFGF they retain many of their neonatal properties. These findings suggest that OPCs have an intrinsic maturation program that normally plays out over many months but can be restrained by bFGF. The main challenge now is to determine the molecular basis of such a prolonged intracellular program.
Acknowledgments
The authors are grateful to Mark Marcionni for NRG; Fen-Biao Gao for his initial help in the project; Bill Richardson, Ben Barres, and Anne Mudge for advice; Ben Barres for providing detailed immunopurification protocols for adult precursor cells; Paul Van Heyningen for help with cell cycle analysis; and Lili Cheng and the members of the Raff lab for discussion and support.
D.G. Tang is a recipient of a Hitchings-Elion Award from Burroughs-Wellcome Fund. Y.M. Tokumoto and M.C. Raff are supported by grants from the British Medical Research Council.
Footnotes
Abbreviations used in this paper: BrdU, bromodeoxyuridine; CNS, central nervous system; CNTF, ciliary neurotrophic factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NRG, neuregulins; NT-3, neurotrophin 3; O-2A, oligodendrocyte type-2 astrocyte; OPCs, oligodendrocyte precursor cells; PDL, poly-d-lysine; Tc, cell cycle time; TH, thyroid hormone.
References
- Ahlgren S.C., Wallace H., Bishop J., Neophytou C., Raff M.C. Effects of thyroid hormone on embryonic oligodendrocyte precursor cell development in vivo and in vitro . Mol. Cell. Neurosci. 1997;9:420–432. doi: 10.1006/mcne.1997.0631. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Raff M.C. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Hart I.K., Coles H.S.R., Burne J.F., Voyvodic J.T., Richardson W.D., Raff M.C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Schmid R., Sendtner M., Raff M.C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Raff M.C., Gaese F., Bartke I., Dechant G., Barde Y.A. A crucial role for neurotrophin-3 in oligodendrocyte development Nature. 367 1994. 371 375a [DOI] [PubMed] [Google Scholar]
- Barres B.A., Lazar M.A., Raff M.C. A novel role for thyroid hormone, glucocorticoids, and retinoic acid in timing oligodendrocyte development Development. 120 1994. 1097 1108b [DOI] [PubMed] [Google Scholar]
- Barres B.A., Burne J.F., Holtmann B., Thoenen H., Sendtner M., Raff M.C. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol. Cell. Neurosci. 1996;8:146–156. doi: 10.1006/mcne.1996.0053. [DOI] [PubMed] [Google Scholar]
- Bartlett P.F., Noble M.D., Pruss R.M., Raff M.C., Rattray S., Williams C.A. Rat neural antigen-2 (RAN-2)a cell surface antigen on astrocytes, ependymal cells, Muller cells and leptomeninges defined by a monoclonal antibody. Brain Res. 1981;204:339–351. doi: 10.1016/0006-8993(81)90593-x. [DOI] [PubMed] [Google Scholar]
- Bögler O., Wren D., Barnett S.C., Land H., Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc. Natl. Acad. Sci. USA. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögler O., Noble M. Measurement of time in oligodendrocyte-type-2 astrocyte (O-2A) progenitors is a cellular process distinct from differentiation or division. Dev. Biol. 1994;162:525–538. doi: 10.1006/dbio.1994.1106. [DOI] [PubMed] [Google Scholar]
- Bottenstein J.E., Sato G.H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A.M., Duncan A., Hornby M.F., Kirvell S.I., Hunter A., Levine J.M., Berry M. Cells expressing the NG2 antigen contact Nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Calver A.R., Hall A.C., Yu W.-P., Walsh F.S., Heath J.K., Betsholtz C., Richardson W.D. Oligodendrocyte population dynamics and the role of PDGF in vivo . Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Canoll P.D., Musacchio M.A., Hardy R., Reynolds R., Marchionni M.A., Salzer J.L. GGF/neuregulin is a neronal signal that promotes the proliferation and survival and inhibits differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O., Peacocke M., Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B., Gao F.-B., Raff M. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:306–317. doi: 10.1093/emboj/16.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B., Fero M.L., Roberts J.M., Raff M. P27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr. Biol. 1998;8:431–440. doi: 10.1016/s0960-9822(98)70177-0. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G.S., Walsh F.S., Nirenburg M. Monoclonal antibodies to a plasma membrane antigen of neurons. Proc. Natl. Acad. Sci. USA. 1979;76:4913–4916. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant C., Raff M.C. Proliferating bipotential glial progenitor cells in adult optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Fruttiger M., Karisson L., Hall A.C., Abramsson A., Calver A.R., Bostrom H., Willetts K., Bertold C.-H., Heath J.K., Betsholtz C., Richardson W.D. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Fulton B.P., Burne J.F., Raff M.C. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J. Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F.-B., Durand B., Raff M.C. Oligodendrocyte precursor cells count time but not cell divisions before differentiation. Curr. Biol. 1997;7:152–155. doi: 10.1016/s0960-9822(06)00060-1. [DOI] [PubMed] [Google Scholar]
- Gao F.-B., Raff M. Cell size control and a cell-intrinsic maturation program in proliferating oligodendrocyte precursor cells. J. Cell Biol. 1997;138:1367–1377. doi: 10.1083/jcb.138.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F.-B., Apperly J., Raff M. Cell-intrinsic timers and thyroid hormone regulate the probability of cell-cycle withdrawal and differentiation of oligodendrocyte precursor cells. Dev. Biol. 1998;197:54–66. doi: 10.1006/dbio.1998.8877. [DOI] [PubMed] [Google Scholar]
- Gard A.L., Pfeiffer S.E. Two proliferative stages of the oligodendrocyte lineage (A2B5+O4− and O4+GalC−) under different mitogenic control. Neuron. 1990;5:615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Goldman J.E., Vaysse P.J.-J. Tracing glial cell lineages in the mammalian forebrain. Glia. 1991;4:149–156. doi: 10.1002/glia.440040206. [DOI] [PubMed] [Google Scholar]
- Grinspan J.B., Franceschini B. Platelet-derived growth factor is a survival factor for PSA-NCAM+ oligodendrocyte pre-progenitors. J. Neurosci. Res. 1995;41:540–551. doi: 10.1002/jnr.490410414. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hardy R., Reynolds R. Proliferation and differentiation potential of rat forebrain oligodendroglial progenitors both in vitro and in vivo . Development. 1991;111:1061–1080. doi: 10.1242/dev.111.4.1061. [DOI] [PubMed] [Google Scholar]
- Hart I.K., Richardson W.D., Heldin C.H., Westermark B., Raff M.C. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development. 1989;105:595–603. doi: 10.1242/dev.105.3.595. [DOI] [PubMed] [Google Scholar]
- Hockfield S., McKay R.D.G. Identification of major cell classes in the developing mammalian nervous system. J. Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrola N., Mayer-Proschel M., Rodriguez-Pena A., Noble M. Evidence for the existence of at least two timing mechanisms that contribute to oligodendrocyte generation in vivo . Dev. Biol. 1996;180:1–21. doi: 10.1006/dbio.1996.0280. [DOI] [PubMed] [Google Scholar]
- Lendahl U., Zimmerman L.B., McKay R.D.G. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Levine J.M., Gard J.P. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellumassociation with smooth protoplasmic astrocytes. J. Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.M., Stallcup W.B. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J. Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine S.L., Goldman J.E. Embryonic divergence of oligodendrocyte and astrocyte lineages in developing rat cerebrum. J. Neurosci. 1988;8:3992–4006. doi: 10.1523/JNEUROSCI.08-11-03992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguad J.P., Sargent I., Mason D.Y. Detection of human white cell proliferative responses by immunoenzymatic measurement of bromodeoxyuridine uptake. J. Immunol. Methods. 1988;106:95–100. doi: 10.1016/0022-1759(88)90276-1. [DOI] [PubMed] [Google Scholar]
- Malotka J., Dornmair K. Alternative splicing and cDNA sequencing of myelin basic protein gene of the Lewis rat. Autoimmunity. 1995;20:67–68. doi: 10.3109/08916939508993341. [DOI] [PubMed] [Google Scholar]
- McKinnon R.D., Matsui T., Dubois-Dalcq M., Aaronson S.A. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- McMorris F.A., Dubois-Dalcq M. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J. Neurosci. Res. 1988;21:199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- Miller R.H., Fulton B.P., Raff M.C. A novel type of glial cell associated with Nodes of Ranvier in rat optic nerve. Eur. J. Neurosci. 1989;1:172–181. doi: 10.1111/j.1460-9568.1989.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Milner R.J., Lai C., Nave K.-A., Lenoir D., Ogata J., Sutcliffe J.G. Nulceotide sequence of two mRNAs for rat myelin proteolipid protein. Cell. 1985;42:931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Noble M., Murray K. Purified astrocytes promote the in vitro divisions of a bipotential glial progenitor cell. EMBO (Eur. Mol. Biol. Organ.) J. 1984;3:2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M., Murray K., Stroobant P., Waterfield M.D., Riddle P. Platelet-derived growth factor promotes the division and motility and inhibits premature differentiation of the oligodendrocyte/type 2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Nowakowski R.S., Lewin S.B., Miller M.W. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J. Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Ono K., Yasui Y., Rutishauser U., Miller R.H. Focal ventricular origin and migration of oligodendrocyte precurors into the chick optic nerve. Neuron. 1997;19:283–292. doi: 10.1016/s0896-6273(00)80939-3. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S.E., Warrington A.E., Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Pringle N.P., Richardson W.D. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Pruss R.M. Thy-1 antigen on astrocytes in long-term cultures of rat central nervous system. Nature. 1979;280:688–690. doi: 10.1038/280688a0. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Mirsky R., Fields K.L., Lisak R.P., Dorfman S.H., Silberberg D.H., Gregson N.A., Leiboweitz S., Kennedy M.C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978;274:813–816. [PubMed] [Google Scholar]
- Raff M.C., Miller R.H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Lillien L.E., Richardson W.D., Burne J.F., Noble M. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P.A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc. Natl. Acad. Sci. USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W.D., Pringle N., Mosley M.J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Roach A., Boylan K.B., Horvath S., Prusiner S.B., Hood L.E. Characterization of cloned cDNA representing rat myelin basic proteinabsence of expression in brain of shiverer mutant mice. Cell. 1983;34:799–806. doi: 10.1016/0092-8674(83)90536-6. [DOI] [PubMed] [Google Scholar]
- Shi J., Marinovich A., Barres B. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J. Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff R.P. Gliogenesis in rat optic nerveastrocytes are generated in a single wave before oligodendrocytes. Dev. Biol. 1990;139:149–168. doi: 10.1016/0012-1606(90)90285-q. [DOI] [PubMed] [Google Scholar]
- Small R.K., Riddle P., Noble M. Evidence for migration of oligodendrocyte–type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Smith J.R., Pereira-Smith O.M. Replicative senescenceimplications for in vivo aging and tumor suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfacesan immunocytochemical study in the central nervous system. Dev. Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Tang D.G., Chen Y., Diglio C.A., Honn K.V. PKC-dependent effects of 12(S)-HETE on endothelial cell vitronectin and fibronectin receptor. J. Cell Biol. 1993;121:689–704. doi: 10.1083/jcb.121.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S., Raff M.C. Differentiation of a bipotential glial progenitor cell in single cell microculture. Nature. 1985;313:223–225. doi: 10.1038/313223a0. [DOI] [PubMed] [Google Scholar]
- Temple S., Raff M.C. Clonal analysis of oligodendrocyte development in cultureevidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- Tokumoto Y.M., Durand B., Raff M.C. An analysis of the early events when oligodendrocyte precursor cells are triggered to differentiate by thyroid hormone, retinoic acid, or PDGF withdrawal. Dev. Biol. 1999;213:327–339. doi: 10.1006/dbio.1999.9397. [DOI] [PubMed] [Google Scholar]
- Vartanian T., Fischbach G., Miller R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc. Natl. Acad. Sci. USA. 1999;96:731–735. doi: 10.1073/pnas.96.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Sdrulla A.D., diSibio G., Bush G., Nofziger D., Hicks C., Weinmaster G., Barres B.A. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wolswijk G., Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Wolswijk G., Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2A adult progenitor cells to rapidly dividing cells with the characteristics of perinatal O-2A progenitor cells. J. Cell. Biol. 1992;118:889–897. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.M., Bunge R.P. The origin of remyelinating cells in the adult central nervous systemthe role of the mature oligodendrocyte. Glia. 1991;4:225–232. doi: 10.1002/glia.440040214. [DOI] [PubMed] [Google Scholar]
- Wren D., Wolswijk G., Noble M. In vitro analysis of the origin and maintenance of O-2A adult progenitor cells. J. Cell. Biol. 1992;116:167–176. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]