Abstract
Neuregulin-1 provides an important axonally derived signal for the survival and growth of developing Schwann cells, which is transmitted by the ErbB2/ErbB3 receptor tyrosine kinases. Null mutations of the neuregulin-1, erbB2, or erbB3 mouse genes cause severe deficits in early Schwann cell development. Here, we employ Cre-loxP technology to introduce erbB2 mutations late in Schwann cell development, using a Krox20-cre allele. Cre-mediated erbB2 ablation occurs perinatally in peripheral nerves, but already at E11 within spinal roots. The mutant mice exhibit a widespread peripheral neuropathy characterized by abnormally thin myelin sheaths, containing fewer myelin wraps. In addition, in spinal roots the Schwann cell precursor pool is not correctly established. Thus, the Neuregulin signaling system functions during multiple stages of Schwann cell development and is essential for correct myelination. The thickness of the myelin sheath is determined by the axon diameter, and we suggest that trophic signals provided by the nerve determine the number of times a Schwann cell wraps an axon.
Keywords: cre-loxP, neuregulin, myelin, glia, neuropathy
Introduction
Neural crest cells constitute a migratory and pluripotent cell population that emerges from the dorsal neural tube. Upon reaching their target sites, neural crest cells differentiate into various cell types, including Schwann cells, which is a population of glial cells that ensheathes axons of sensory and motoneurons (Le Douarin 1982). Schwann cell precursors migrate and proliferate along preexisting axon tracts during development, and progress through a series of defined stages that are characterized by the expression of specific genes encoding proteins such as P0, S100, SCIP, or Krox20 (Mirsky and Jessen 1996; Zorick and Lemke 1996). Proliferation, survival, and differentiation of Schwann cell precursors depend critically on signals that are provided by the associated axons. During the first week of postnatal life, the majority of Schwann cells destined to myelinate can already be distinguished morphologically in mice. They establish a 1:1 relationship with the accompanying axon and cease to proliferate, but remain capable of entering the cell cycle, a property important in adult regeneration processes (Zorick and Lemke 1996). The myelination program in Schwann cells is characterized by the expression of specific transcription factor genes like SCIP (also known as Oct-6 or Tst-1) and Krox20 (also known as Egr-2; Chavrier et al. 1988; He et al. 1989; Monuki et al. 1989; Wilkinson et al. 1989; Suzuki et al. 1990). Genetic analysis in mice shows that SCIP determines the correct onset of myelination, whereas Krox20 is required for proper ensheathment of the axon and for the expression of genes encoding myelin proteins (Topilko et al. 1994; Weinstein et al. 1995; Bermingham et al. 1996; Jaegle et al. 1996; Zorick et al. 1999). Myelin proteins are major constituents of the myelin sheath, and the molecular composition of the sheath as well as its appropriate thickness are essential for the normal functioning of a nerve fiber. Mutations in humans and rodents can cause neuropathies accompanied by myelin defects, and are typically associated with a reduced thickness of myelin and a disturbed transmission of the action potential along the affected nerves (Nave 1994; Martini and Schachner 1997; Scherer 1997; Suter 1997).
Early Schwann cell precursors rely on axonal signals for maintenance, proliferation, and differentiation. One neuronal signal that controls survival and proliferation is provided by Neuregulin-1, also named GGF, for glial growth factor (Raff et al. 1978; Lemke and Brockes 1984; Goodearl et al. 1993; Marchionni et al. 1993). Alternative names used for this factor are NDF (Neu differentiation factor), Heregulin, or ARIA (acetylcholine receptor inducing activity; for reviews see Alroy and Yarden 1997; Burden and Yarden 1997). Neuregulin-1 is produced by sensory and motoneurons as a transmembrane molecule inserted into axonal membranes (Ho et al. 1995; Bermingham-McDonogh et al. 1997; Yang et al. 1998). This axon-derived signal is recognized by Schwann cells via a receptor tyrosine kinase composed of a heterodimer of ErbB2 and ErbB3, which signals through tyrosine phosphorylation (Levi et al. 1995; Morrissey et al. 1995; Grinspan et al. 1996; Syroid et al. 1996; Vartanian et al. 1997). This concept is supported by genetic studies in the mouse. Mice with mutations in neuregulin-1, erbB2, or erbB3 all show severe reductions in the numbers of early Schwann cell precursors; at later developmental stages, erbB3 and erbB2 mutants lack Schwann cells (Meyer and Birchmeier 1995; Erickson et al. 1997; Meyer et al. 1997; Riethmacher et al. 1997; Britsch et al. 1998; Woldeyesus et al. 1999; Morris et al. 1999).
The time period during which Schwann cell precursors critically depend on Neuregulin-1 for proliferation and survival ends with the transition from an early precursor to a more mature, differentiating Schwann cell (Dong et al. 1995; Grinspan et al. 1996; Syroid et al. 1996; Murphy et al. 1996). After this transition, the differentiating Schwann cells generate survival factors in an autocrine loop, and become independent of Neuregulin-1, although they are still able to respond to the factor (Rosenbaum et al. 1997; Cheng et al. 1998; Meier et al. 1999; Syroid et al. 1999). Interestingly, neuregulin-1 continues to be expressed in sensory and motoneurons even in adulthood, and both differentiating and mature Schwann cells continue to express the Neuregulin receptor genes erbB2 and erbB3, albeit erbB2 is expressed at reduced levels (Chen et al. 1994; Corfas et al. 1995; Grinspan et al. 1996). We investigate here the functions of the Neuregulin signaling system in myelinating Schwann cells by the use of a Cre-recombinase–induced erbB2 mutation. We observe severe defects in myelination, which results in the formation of abnormally thin myelin sheaths. This correlates with ataxia, tremor and wasting of the animals. Moreover, a postnatal loss of motor axons occurs. Thus, the Neuregulin signaling system not only regulates Schwann cell numbers, but is also necessary for formation of an adequate myelin sheath.
Materials and Methods
Generation of a Targeting Vector and erbB2flox Strain of Mice
The isolation of genomic erbB2 DNA derived from the 129 mouse strain has been described (Britsch et al. 1998). Oligonucleotides encoding the loxP sequence together with an additional EcoRV site were inserted 5′ of exon p. A neomycin cassette flanked by loxP sites was inserted 3′ of exon n (see Fig. 1 A). The erbB2floxneoflox targeting vector was electroporated into E14.1 embryonic stem (ES) cells; homologous recombination events were enriched by selection with G418, and identified by Southern blot hybridization using an external genomic probe located 5′ to exon r (data not shown). As described previously (Torres and Kühn 1997), independent ES cell clones heterozygous for the erbB2floxneoflox allele (see Fig. 1 A) were electroporated with pICcre; colonies were screened by Southern blot hybridization using probe 1 (see Fig. 1 A). Two colonies that contained the erbB2flox allele derived from independent parental erbB2floxneoflox clones were used for a generation of mice that carry this allele as described (Riethmacher et al. 1997). Homozygous erbB2flox animals appeared normal and were fertile. To establish the erbB2 Δ strain, erbB2flox homozygotes were crossed with deleter mice (Schwenk et al. 1995). Cre-mediated deletion of the floxed exons p-n removes 362 nucleotides of coding sequence and, thus, introduces a frameshift mutation. The predicted protein product encoded by the erbB2 Δ allele contains the first 386 amino acids of ErbB2 followed by 51 amino acids from an altered reading frame. Mice heterozygous for the erbB2 Δ allele were bred with erbB2+/− mice; all embryos with the genotype erbB2 Δ/− identified at E11.5 were dead as judged by the absence of heartbeat and signs of resorption. Histological analysis of E10.5 erbB2 Δ/− embryos showed the previously described phenotypes, i.e., lack of trabeculation and abnormal cranial sensory ganglia. All analyses of conditional erbB2 animals were carried out on the mixed C57BL/6/129 background. As controls for Krox20-cre/+; erbB2flox/− animals, littermates with a genotype Krox20-cre/+; erbB2flox/+ were routinely used. Littermates with genotypes Krox20+/+; erbB2flox/−, or Krox20+/+; erbB2flox/+, and also Krox20-cre/+; erbB2+/− animals were analyzed by light microscopy and thin myelin was not apparent.
Figure 1.
Strategy employed for the generation of the erbB2flox allele. (A) The structure of the wild-type erbB2 gene is shown at the top (i). The erbB2floxneoflox allele (ii) was generated by homologous recombination in ES cells. In this allele, three exons (green) are flanked by loxP sequences (red arrowheads). The 5′-most loxP sequence also contains, at its border, an EcoRV site shown in red. In addition, the neomycin resistance cassette (neo) is present that is also surrounded by loxP sequences. The erbB2flox allele (iii) was generated by transient expression of Cre in ES cells; in this allele, the neo-cassette has been removed, but two loxP sites that flank three exons of erbB2 are retained. The erbB2 Δ allele (iv) is obtained after Cre-induced recombination of erbB2flox. Exons are indicated by boxes and lettered (see Britsch et al. 1998); black hatched and green exons were sequenced directly, whereas the sequences of white exons were inferred from the cDNA. Probes 1 and 2 are indicated and were used for Southern hybridization in Fig. 1 B and Fig. 2 A, respectively. Restriction enzyme sites (RV, EcoRV; and B, BamHI) are indicated, as are the sizes and positions of predicted fragments obtained after digestion of genomic DNA. (B) Southern hybridization of EcoRV-digested genomic DNA from ES cells before and after transient transfection of pICcre. Lane 1, parental ES cell with erbB2floxneoflox/+ genotype; lanes 2–4, erbB2flox/+ cells; and lane 5, wild-type DNA.
Determination of Recombination Specificity and Efficiency
Tissues from 6-wk-old mice double heterozygous for a reporter-lacZ allele and Krox20-cre were stained with 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal) as described (Akagi et al. 1997). Blue staining indicative of Cre-mediated recombination was observed in peripheral nerves, hair follicles, and cartilage in which Krox20 expression has been described (Levi et al. 1996). Bundles of fibers from sciatic nerves were teased, wholemount stained with 4′,6-diamidino-2-phenylindole (DAPI), and cleared in 80% glycerol in PBS. Alternatively, the nerve sheaths were disrupted to enhance fixation and allow access of X-gal to Schwann cells. The nerves were osmicated and embedded in resin (see also below), and 1-μm sections were counterstained with neutral red and eosin. For immunohistochemical analysis, teased nerves from Krox20-cre/+; reporter-lacZ animals were fixed for 20 min at 4°C in 4% paraformaldehyde (PFA), blocked with 10% goat serum in PBS containing 0.5% Triton X-100, and incubated with monoclonal anti-myelin basic protein antibody (Boehringer Mannheim) and rabbit polyclonal anti–β-galactosidase antibody (ICN Cappel). FITC-conjugated anti-rabbit IgG and Cy3-conjugated anti-mouse IgG (Dianova) were used as secondary antibodies and nuclei were counterstained with DAPI. Genomic DNA of tissues from adult Krox20-cre/+; erbB2flox/+ animals was used for Southern blot hybridization; spinal roots were pooled from several animals to allow detection of erbB2 alleles.
Histology, Electron Microscopy, and Axon/Schwann Cell Counts
Nerves were isolated from animals perfused with 2.5% glutaraldehyde in phosphate buffer, postfixed, and contrasted with osmium tetroxide as described (Topilko et al. 1994). For light microscopy, nerves were embedded in Technovit 7100 resin (Kulzer); 1-μm sections were stained with toluidine blue. Preparation for electron microscopy was done as described (Topilko et al. 1994). Numbers of axons in the fifth lumbar segment (L5) ventral roots were determined from composite light micrographs. The axon numbers determined in individual mutant animals were 549, 679, 721, 739, 773, 778, 800, 855, 887, 1,014 and 1,096; in control animals, 956, 968, 974, 1,026, 1,092 and 1,103 axons were counted. Neuron numbers in the dorsal root ganglia were determined as described (Riethmacher et al. 1997). Numbers of Schwann cell nuclei associated with nerves were determined from 1-μm sections.
Determination of Apoptosis and Proliferation Rates in Nerves
Animals between postnatal day 3.5 and 15 (P3.5 and P15, respectively) were injected once intraperitoneally with bromodeoxyuridine (BrdU; 100 μg/g body weight) and killed 80 min after injection. To increase numbers of BrdU-positive cells in nerves from 5-wk-old and adult animals, multiple BrdU injections (1 each day, over 4 d) were used. Animals were perfused with 4% PFA in PBS, nerves were dissected, postfixed for 2–4 h, and embedded in OCT compound (Sakura). Frozen sections (6 μm) were processed for TUNEL staining using the ApopTag kit (Oncor); BrdU-positive nuclei were detected using a monoclonal anti-BrdU antibody (Sigma Chemical Co.). Sections were counterstained with DAPI (Sigma Chemical Co.) to determine overall numbers of Schwann cell nuclei.
Protein Extraction and Western Blot Analysis
Sciatic nerves were dissected, flash-frozen in liquid nitrogen, and stored at –70°C. Total protein lysates of nerves were prepared by sonication in Laemmli sample buffer (Laemmli 1970). Samples were separated in 12.5% acrylamide gels under reducing conditions and transferred to nitrocellulose membranes. Western blotting was performed using rabbit polyclonal antibodies directed against Krox20, SCIP, or PMP22, or a mouse mAb directed against P0; for detection, an ECL kit (Amersham) was used.
Preparation of Digital Images
Southern and Western blots, hybridized with 32P-labeled probes, or incubated with ECL reagents, respectively, were exposed to Kodak Bio-Max film and scanned into Adobe Photoshop® 3.0. PhosphorImage scans of Southern blots were analyzed using the software package TINA 2.08e© 1993 Raytest Isotopenmeßgeräte GmbH. Adobe Photoshop® 3.0 was also used to prepare the optical and electron micrograph images.
Results
Conditional Mutagenesis of erbB2
We generated an erbB2flox allele in which two loxP sites flank three exons encoding part of the extracellular domain of the receptor. We chose these exons since their deletion introduces a frameshift mutation, which results in the production of an mRNA that encodes a truncated receptor. A two-step procedure was employed to generate the floxed allele in ES cells (Fig. 1; see also Gu et al. 1993). The mutant ES cells were used to generate mice that carry the erbB2floxallele. Mice homozygous for the erbB2flox allele appeared normal and were fertile. The erbB2flox allele was crossed into a cre-deleter strain (Schwenk et al. 1995). The resulting erbB2 Δ allele (Fig. 1 A) was combined with the previously described genetical-null allele of erbB2 (Britsch et al. 1998). ErbB2−/erbB2Δ mice die before E11.5 and are phenotypically indistinguishable from erbB2−/− mice, indicating that Cre-induced recombination of the erbB2flox allele generates a nonfunctional erbB2 gene.
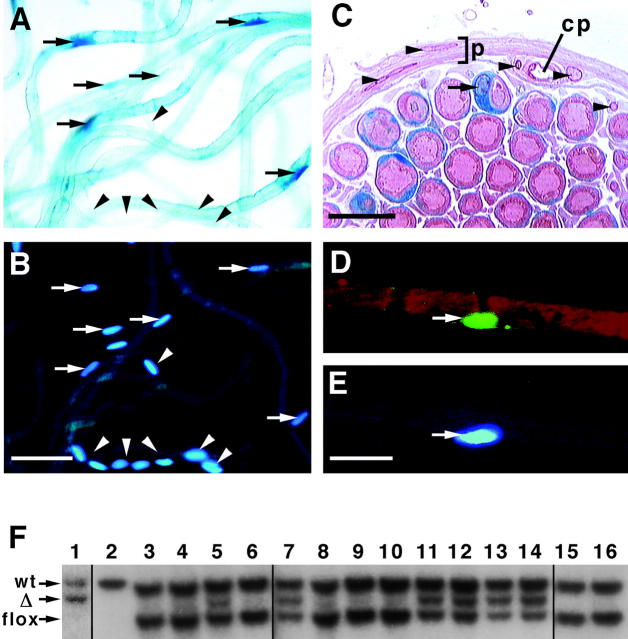
The Krox20 gene encodes a transcription factor expressed in promyelinating and myelinating Schwann cells and, thus, appears from ∼E16 in peripheral nerves (Topilko et al. 1994). Furthermore, Schwann cell precursors that reside in the spinal roots express Krox20 earlier, starting ∼E11. To mutate erbB2, we used a mouse strain in which cre was introduced into the Krox20 locus by homologous recombination. In this strain, cre expression faithfully reproduces the expression pattern of the endogenous Krox20 gene; its detailed characterization is reported elsewhere (Voiculescu et al. 2000). The Krox20-cre allele was combined with a reporter allele, a lacZ gene which is expressed upon Cre-induced recombination (Akagi et al. 1997). In teased preparations of the sciatic and other nerves of such mice, we observed β-galactosidase staining in the majority of cells with elongated nuclei that were spaced at regular intervals along the nerve. In contrast, more closely and irregularly spaced cells were not stained (Fig. 2A and Fig. B). Sectioning of nerves, complemented by immunohistochemical analysis of teased fibers using antibodies directed against β-galactosidase and myelin basic protein confirmed the identity of the stained cells as myelinating Schwann cells (Fig. 2, C–E). Southern hybridization was used to quantify recombination of the erbB2flox allele, and showed that recombination occurred in 40–50% of cells associated with sciatic nerves and spinal roots in animals with the genotype Krox20-cre/+; erbB2flox/+ (Fig. 2 F). It should be noted that these nerves contain significant numbers of nonmyelinating Schwann cells, connective tissue, blood cells, and vessels in which recombination does not occur. Recombination was also observed in the skin and ear: hair follicles express Krox20, accounting for the recombination in these tissues (Levi et al. 1996). No recombination was detected in the spinal cord.
Figure 2.
Tissue specificity of Krox20-cre–induced mutations. (A–E) Nerves from reporter lacZ mice (see Akagi et al. 1997) that also contain a Krox20-cre allele were stained for β-galactosidase or β-galactosidase activity. (A and B) Teased nerves stained with X-gal (A) and DAPI (B). (C) X-gal–stained nerves were also osmicated, sectioned, and counterstained with neutral red and eosin. The myelin and axon of an individual nerve fiber stained dark and light red, respectively. (D and E) Teased nerve fiber triple stained with antibodies against myelin basic protein (D, red), β-galactosidase (D, green), and DAPI (E, blue). Arrows point towards β-gal–positive Schwann cells; arrowheads point towards other cells and nuclei that do not stain; perineurium (p) and capillaries (cp). (F) Southern hybridization analysis of DNA digested with BamHI and EcoRV. Lane 1, erbB2 Δ/+; lane 2, wild type; lanes 9 and 10, erbB2flox/+; all other lanes contain DNA obtained from tissues of mice with a genotype Krox20-cre/+; erbB2flox/+. Lane 3, liver; lane 4, muscle; lane 5, ear; lane 6, lung; lane 7, skin; lane 8, spleen; lanes 11 and 12, sciatic nerves; lanes 13 and 14 spinal roots; lane 15, spinal cord; and lane 16, ovaries. Densitometric analysis showed that recombination occurred in 40–50% of cells associated with peripheral nerves. Bars: (B) 50 μm; (C and E) 20 μm.
ErbB2 Is Required for Correct Myelination of Peripheral Nerves
Conditional mutant animals with the genotype Krox20-cre/+; erbB2flox/− were viable, but displayed various behavioral abnormalities within the first weeks of postnatal life. Alterations included kinked or serpentine tails, gait abnormalities, difficulties in hindlimb movement, and wasting associated with weight loss and premature death (5 out of a group of 37 mutants died within 6 mo). Such behavioral abnormalities were observed in all conditional mutants, but were variable in the time of onset and severity. Control animals, for instance animals with the genotype Krox20-cre/+; erbB2+/−, did not display these behavioral phenotypes.
Examination of the sciatic nerves from Cre-induced mutants showed no gross changes in morphology or histology at P3.5 (Fig. 3 A, compare to B), indicating that the Schwann cell precursor pool in this nerve is established. However, sciatic nerves of mutants at P15 were translucent and thin. Histologically, a strikingly reduced thickness of myelin sheaths was apparent (Fig. 3C and Fig. D). Reduced thickness of the myelin sheaths persisted, and was also observed at 6 or 14 mo (Fig. 3, E–J, Fig. 4, arrowheads, and data not shown). Myelin of normal thickness (Fig. 3 E, arrow) was found only in 1.7 ± 1.0% of all myelinated axons in the sciatic nerve. Thus, the Krox20-cre–induced erbB2 mutation reproducibly affected virtually all myelinating Schwann cells. Thin myelin was not observed in control mice with a genotype Krox20-cre/+; erbB2+/−, or Krox20-cre/+; erbB2flox/+, or Krox20+/+; erbB2flox/−, or Krox20+/+; erbB2flox/+. Electron micrographs were used to quantify myelin thickness (Fig. 4). In 6-mo-old animals, the thickness of myelin was reduced two- to threefold. This is reflected in G ratios (ratios of axon diameters to fiber diameters) of 0.83 ± 0.04 in mutant, and 0.68 ± 0.04 in control sciatic nerves (P < 10−6). Hypomyelination in sciatic, sural, saphenous nerves and nerves innervating lower leg muscles were observed in all mutant animals examined (28 animals). In the spinal roots, the phenotype was more dramatic (see below). The thin myelin was due to the presence of fewer wraps of myelin around the axon.
Figure 3.
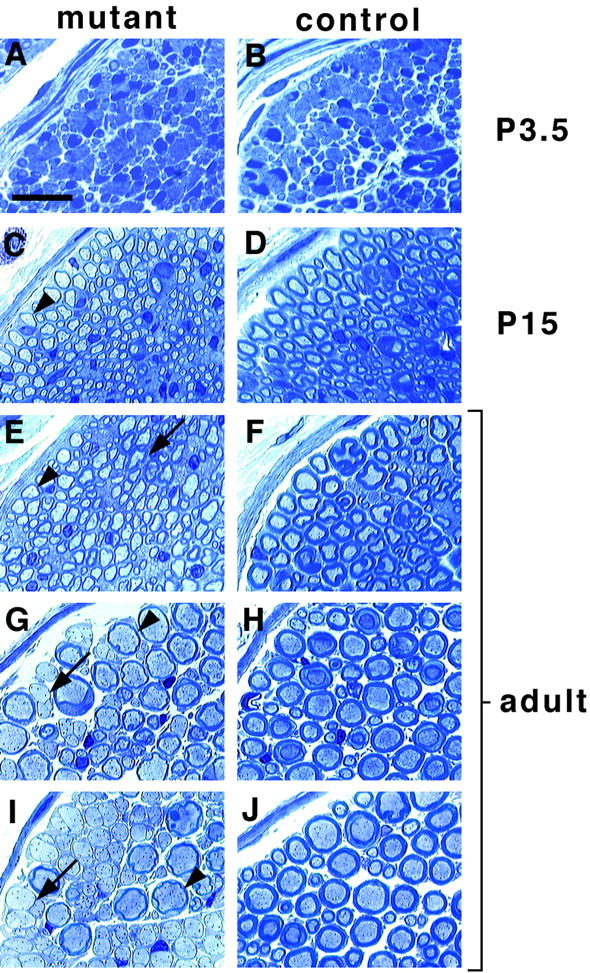
Peripheral nerve histology in mice with conditional erbB2 mutation. Semi-thin sections of peripheral nerves from Krox20-cre/+; erbB2flox/− (A, C, E, G, and I) and control (B, D, F, H, and J) animals. The age of the animals is indicated. Shown are the tibial (A–D) and sural (E and F) branches of the sciatic nerve and nerves innervating lower leg muscles (G–J). Arrowheads (C, E, G, and I) point towards axons with abnormally thin myelin sheaths. The arrow in E points towards rare axons with myelin of normal thickness; arrows in G and I point towards large diameter axons that are not myelinated. Bar, 20 μm.
Figure 4.
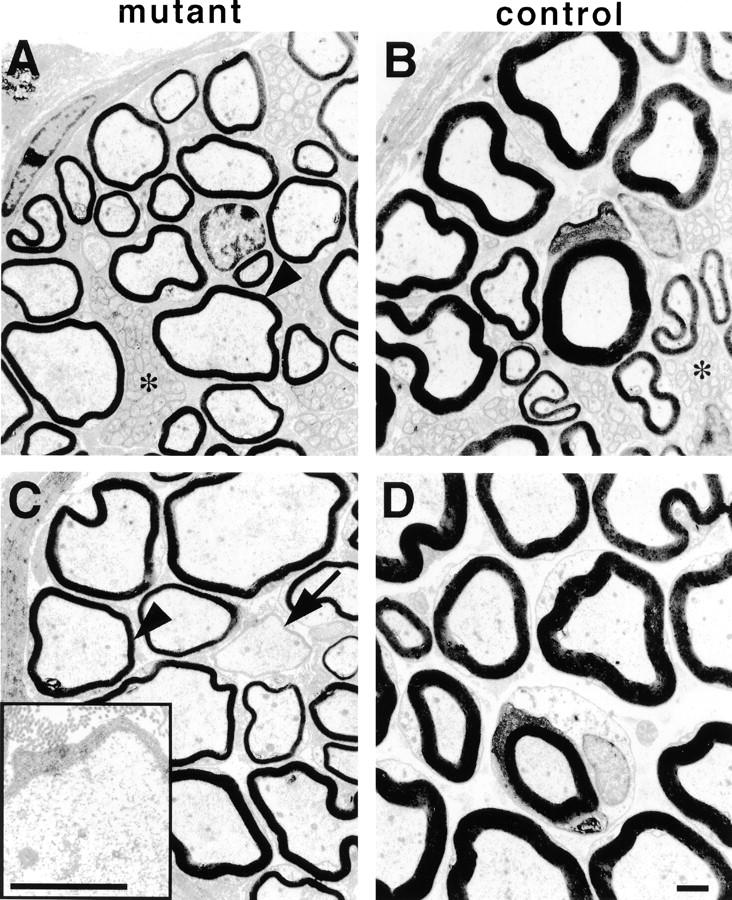
Electron microscopy of sciatic nerve branches from conditional erbB2 mutants. Electron microscopic analysis of peripheral nerves from Krox20-cre/+; erbB2flox/− (A and C) and control (B and D) animals at 6 mo of age. Shown are sural nerves (A and B) and nerves innervating lower leg muscles (C and D). Arrowheads (A and C) point towards abnormally thin myelin sheaths in nerves from mutant mice; the arrow in C indicates a large diameter axon surrounded by a basal lamina, which is also shown enlarged in the inset. Asterisks denote bundles of nonmyelinated small diameter axons. Bars, 1 μm.
In addition, we observed occasionally large-caliber axons lacking myelin that were still surrounded by a Schwann cell basal lamina in the sciatic nerve (Fig. 4 C, arrow and inset). Such Schwann cells frequently ensheathed several axons (Fig. 3G and Fig. I, arrows). These profiles were more frequent in nerves innervating muscle than in cutaneous sensory nerves. Extensive patches of amyelinated axons were observed in some Cre-induced mutants (Fig. 3 I). The histology of nonmyelinating bundles appeared normal (Fig. 4, asterisks).
In early postnatal stages or in the embryo, a more severe phenotype was observed in dorsal and ventral spinal roots. These nerves display a severe lack of Schwann cells in all animals with Krox20-cre–induced erbB2 mutations at P3.5 and at P15 (10 examined; Table , Fig. 5, and data not shown). In accordance, myelin was absent in ventral roots, whereas thin myelin sheaths were surrounding a small proportion of axons in dorsal roots. This coincides with the early onset of Krox20-cre expression in these cells, revealing the early requirement for ErbB2 in establishment of the Schwann cell precursor pool. As animals reached adulthood, Schwann cell numbers increased, but the myelin sheaths formed were thin (Fig. 5). Localized patches of amyelinated axons persisted. Moreover, a single perineurium surrounds the entire spinal root in control animals, whereas perineurial sheaths compartmentalized the roots into six to seven branches in mutants (Fig. 5, arrowheads).
Table 1.
Effect of Krox20-cre–induced Mutation of erbB2 on Schwann Cell Number
| Sciatic | Ventral root | Dorsal root | ||||
|---|---|---|---|---|---|---|
| Mutant | Control | Mutant | Control | Mutant | Control | |
| P3.5 | 564 ± 31 | 588 ± 27 | 5 ± 2 | 51 ± 3 | 6 ± 2 | 33 ± 4 |
| P15 | 395 ± 12 | 409 ± 21 | 18 ± 3 | 74 ± 4 | 96 ± 10 | 179 ± 5 |
| P 6 mo | 209 ± 33 | 204 ± 20 | 40 ± 13 | 41 ± 7 | 101 ± 9 | 112 ± 8 |
Schwann cell nuclei were counted from semi-thin sections of nerves at the indicated ages; values are displayed as numbers of nuclei per section. The number of nuclei within spinal roots at P3.5 was determined from sections of lumbar blocks (L4-L5 level); at later stages, L5 roots were examined. Data are shown as mean ± SD.
Figure 5.
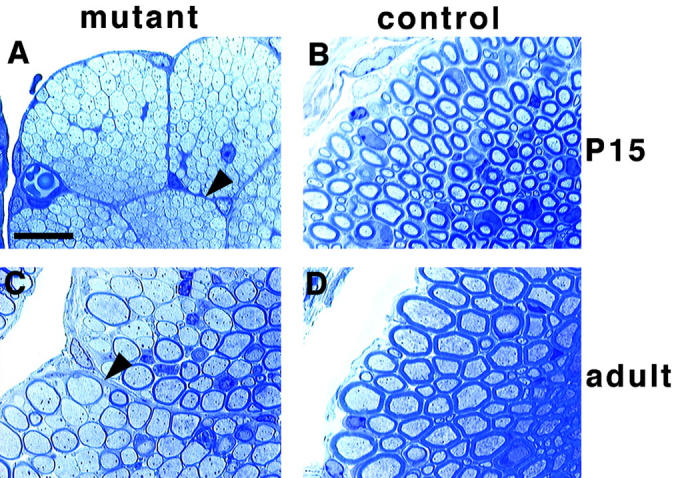
Histology of spinal nerve roots from conditional erbB2 mutants. Semi-thin sections of ventral roots from Krox20-cre/+; erbB2flox/− (A and C) and control (B and D) animals at P15 (A and B) and adult stages (C and D). Arrowheads (A and C) point towards the abnormal perineuria observed in mutant animals. Note that in mutant animals, the extreme lack of Schwann cells observed at P15 is compensated at later stages, although myelin sheaths remain abnormally thin. Bar, 20 μm.
Biochemical Characteristics and Growth Properties of Schwann Cells in Conditional erbB2 Mutants
We determined the absolute numbers of Schwann cells in sciatic nerves and spinal roots. In the sciatic nerves of animals at all stages tested (P3.5, P15, and 6 mo), Schwann cell numbers were comparable in the control and Cre-induced mutant mice (Table ). In contrast, in dorsal and ventral roots, the numbers of cells were severely reduced. At P3.5, a 10-fold reduction was observed in the ventral, and a 5-fold reduction in the dorsal roots of the mutant mice. This reduction was still apparent at P15, and again more pronounced in ventral than in dorsal roots (Table ). At 6 mo, numbers were similar in the dorsal and ventral roots of control and mutant mice. Thus, compensatory mechanisms exist that allow repopulation of spinal roots with Schwann cells; this repopulation occurs earlier in dorsal than in ventral roots.
Schwann cell proliferation and apoptosis determine the absolute numbers of Schwann cells, and were analyzed using BrdU incorporation and TUNEL labeling, respectively. Compared with the controls, the numbers of BrdU-positive cells were increased in the sciatic nerves of mutants at P3.5 and 5 wk (Fig. 6 A). The numbers of apoptotic nuclei were marginally increased at P3.5 (Fig. 6 B). In adults (age 6 mo), proliferation as well as apoptosis frequencies were below detection limits. Increased cell proliferation was also observed in ventral roots of 5-wk-old animals.
Figure 6.
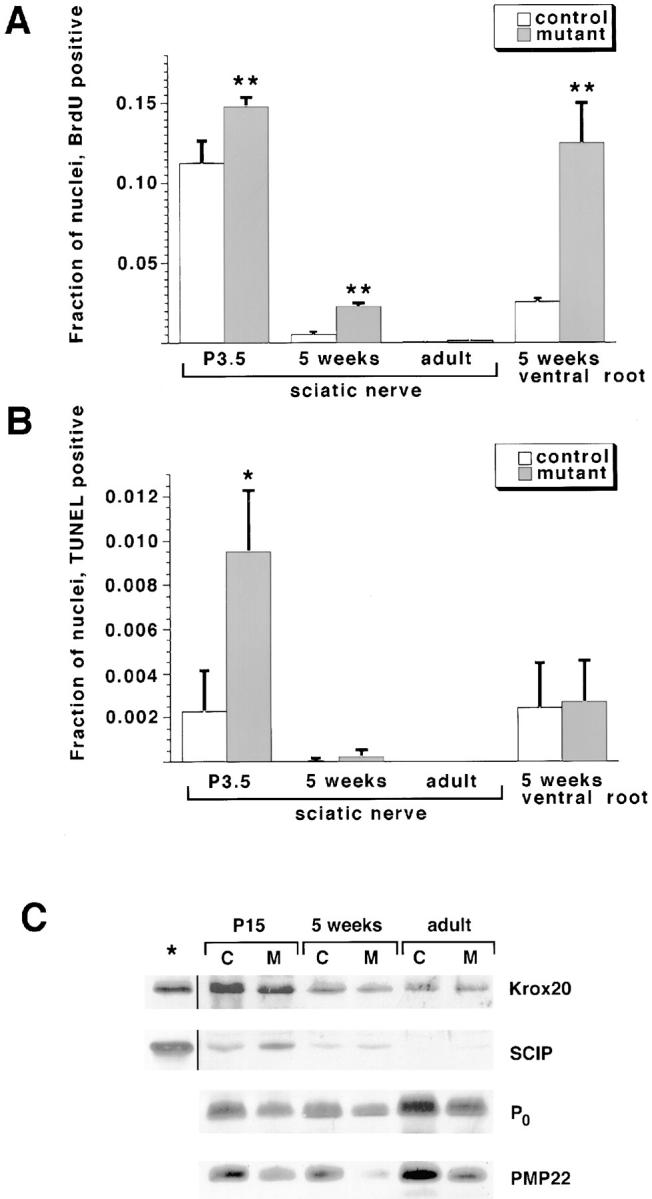
Growth and biochemical properties of Schwann cells in conditional erbB2 mutants. Fractions of nuclei, which were positive for BrdU (A) or TUNEL (B) in sciatic nerves or in the ventral roots, were determined from control or Krox20-cre/+; erbB2flox/− mice at the indicated ages. Data are shown as mean ± SD; *P < 0.17; **P < 0.01. (C) Western analysis of extracts from sciatic nerves using antibodies directed against Krox20, SCIP, P0, and PMP22. Ages of control (C) and Krox20-cre–induced mutant (M) animals are indicated. Asterisk denotes lanes loaded with extracts from COS cells transfected with Krox20 or SCIP expression plasmids.
To assess the effect of the conditional erbB2 mutation induced by Krox20-cre on Krox20 and SCIP protein levels, sciatic nerve extracts were analyzed by Western blotting (Fig. 6 C). The level of Krox20 protein was similar in the nerves of control and Krox20-cre–induced erbB2 mutants. The level of SCIP protein was slightly increased in mutant nerves at P15, but levels attained baseline in control and mutant nerves at later stages. Levels of myelin protein were reduced in conditional mutants, in accordance with the histology.
Neuropathology in Krox20-cre–induced erbB2 Mutants
To assess whether neuron numbers were altered in Krox20-cre–induced erbB2 mutant mice, we counted axons in the L5 ventral root, which contains mainly motor axons. The numbers of such axons were frequently reduced in mutant animals, amounting to 20% loss on average. From 11 mutant animals, and 6 control animals examined, the number of axons in the mutant was 808 ± 152, compared with 1,020 ± 65 in control nerves (P < 0.002). The numbers of neurons in L5 dorsal root ganglia were similar in the control and mutant animals (not shown).
Discussion
The role of Neuregulin-1 as a growth and survival factor during Schwann cell development is well established (Zorick and Lemke 1996; Jessen and Mirsky 1999). Neuregulin-1 provides an axonally derived signal important in establishing the Schwann cell precursor pool, which is received in precursor cells by the Neuregulin-1 receptors ErbB2 and ErbB3 (for review see Adlkofer and Lai 2000). We show here that in addition to its function in the establishment of the precursor pool, the Neuregulin signaling system also plays a role in myelination: abnormally thin myelin sheaths are formed in mice with conditional mutations of erbB2 in myelinating Schwann cells. To introduce the conditional erbB2 mutation, we used a Krox20-cre allele, but it should be noted that thin myelin is also observed when the mutation is introduced by a P0-cre transgene (Garratt, A.N., unpublished observations). The conditional erbB2 mutations also result in an altered behavior of the affected animals, which display movement abnormalities and loss of motoneurons.
The Role of ErbB2 in Myelination
Mutations that cause an abnormal myelin thickness and neuropathy have been extensively characterized (for reviews see Nave 1994; Martini and Schachner 1997; Scherer 1997; Suter 1997; Warner et al. 1999). Peripheral hypo- or hypermyelination are observed in patients with Charcot Marie Tooth disease, and can be caused by mutations in genes encoding the myelin proteins PMP22 and P0, the gap junction protein connexin-32 or the transcription factor Krox20. Even incorrect PMP22 dosage can cause changes in myelination and neuropathy. Mice with targeted or spontaneous mutations in these genes display similar phenotypes. Thus, the thickness of the myelin sheath is strictly controlled, and molecular mechanisms that cause pathological changes are manifold. We show here that hypomyelination and peripheral neuropathy can arise by a novel molecular mechanism, the ablation of the ErbB2 receptor tyrosine kinase. Mutations in signaling molecules have not been observed in patients with hereditary neuropathy. However, afflicted individuals frequently do not carry mutations in known disease causing genes.
ErbB receptor tyrosine kinases modulate the activity of various signaling pathways and are known to affect expression and activity of transcription factors (for reviews see Dougall et al. 1994; Schweitzer and Shilo 1997; Alroy and Yarden 1997, Fromm and Burden 1998; Moghal and Sternberg 1999). We tested whether the conditional erbB2 mutation affects the levels of transcription factors known to regulate myelination. Schwann cells arrest wrapping of axons in the absence of Krox20 (Topilko et al. 1994; Zorick et al. 1999). In vitro, Neuregulin-1 does not affect Krox20 expression directly, but influences the transition from a precursor to a committed Schwann cell that expresses Krox20 (Murphy et al. 1996). In accordance, levels of Krox20 protein are not altered in sciatic nerves of mice with Krox20-cre–induced loss of erbB2. It should be noted that the conditional erbB2 ablation occurs after the transition from a precursor to a committed Schwann cell. In SCIP mutant mice, myelination is delayed. SCIP is expressed in precursors and in Schwann cells during the myelination process, but is downregulated when myelination is completed (Monuki et al. 1989; Jaegle et al. 1996; Zorick et al. 1999). Downregulation of SCIP is delayed in the sciatic nerves of conditional erbB2 mutant mice. This correlates with the prolonged growth phase of Schwann cells, and might not reflect a direct effect of ErbB2 on SCIP expression.
The number of myelin wraps a Schwann cell forms is determined by the diameter of the accompanying axon. Thus, axons not only provide signals that control growth or differentiation, but also instructive signals that determine the size of the membrane surface of Schwann cells and, thus, the number of myelin wraps. In general, the number of myelin wraps might be controlled by trophic signals provided by the axon. Neuregulin provides such a signal, as wrap numbers are reduced in conditional erbB2 mutants. Large diameter axons, because of the enlarged surface area, can present greater amounts of trophic factors and might, therefore, instruct the Schwann cell to form more wraps. Other trophic signals, in addition to the one provided by the Neuregulin signaling system, might be operative, and the sum of these signals would determine myelin thickness and the number of wraps. It is interesting to note that the Drosophila gene DPTEN that encodes a protein and PIP3 lipid phosphatase controls both cell and body size (Goberdhan et al. 1999).
Neuregulin Signaling System in Myelinating Schwann Cells
The balance of cell death and growth in peripheral nerves during the early postnatal phase reflects an adjustment of the numbers of Schwann cell precursors (Grinspan et al. 1996; Syroid et al. 1996; Zorick et al. 1999). The correct 1:1 relationship between axons and myelinating Schwann cells is thus attained, supernumerary cells are removed and areas deficient in Schwann cells are replenished. In early postnatal nerves, Schwann cell survival is regulated in part by access to axonally derived Neuregulin-1, and axotomy-induced death of pre- and perisynaptic Schwann cells can be rescued by exogenous Neuregulin-1 (Grinspan et al. 1996; Syroid et al. 1996; Trachtenberg and Thompson 1996). In our conditional mutants, the importance of ErbB2/Neuregulin-1 signaling in Schwann cell survival and growth is particularly apparent in spinal roots. There, Krox20 is expressed earlier (∼E11), and the Krox20-cre driven mutations are induced earlier than in other peripheral nerves (Topilko et al. 1994; Voiculescu et al. 2000). In the spinal roots, Schwann cells are almost completely absent late in development and into the perinatal period. However, this deficiency is compensated at later stages.
The apoptotic response of Schwann cells to denervation is pronounced in early postnatal life but declines with increasing age (Grinspan et al. 1996; Syroid et al. 1996; Trachtenberg and Thompson 1996). In culture, Schwann cells from the postnatal nerve survive without Neuregulin-1, but secrete survival-promoting growth factors like PDGF, neurotrophin-3, and insulin-like growth factor in an autocrine manner (Porter et al. 1987; Meier et al. 1999; Syroid et al. 1999). Autocrine expression of particular isoforms of neuregulin-1 also has been reported (Rosenbaum et al. 1997; Carroll et al. 1997). In adult mice with Krox20-cre–induced erbB2 mutations, mature myelinating Schwann cells survive, and we do not detect apoptotic nuclei in the nerves. Thus, neither paracrine nor autocrine activation of ErbB2 is essential for survival of mature myelinated Schwann cells.
Regeneration of Schwann Cells
The ability of Schwann cells to regenerate is enormous. In experimentally induced allergic neuritis, myelinating Schwann cells are destroyed, but can be effectively regenerated (Saida et al. 1980). This regenerative capacity is particularly evident in mice that express diphtheria toxin A-chain in myelinating Schwann cells. In such animals, myelinating Schwann cells die and are continuously regenerated through proliferation of the nonmyelinating compartment (Messing et al. 1992). In conditional erbB2 mutant mice, we can still detect single Schwann cells that wrap several large-caliber axons in the adult. Such lesions are not repaired.
We observe a pronounced reduction of Schwann cell numbers in spinal roots of mice with Krox20-cre-induced erbB2 mutations. This severe lack of Schwann cells is compensated during postnatal life, although local patches devoid of Schwann cells remain unrepaired. Interestingly, Krox20 is not expressed in satellite cells of dorsal root ganglia (Murphy et al. 1996); nevertheless, proliferation is increased in dorsal root ganglia of conditional erbB2 mutants (Garratt, A.N., unpublished observations). Thus, satellite cells might replenish Schwann cells in the mutants, particularly in the projections of dorsal root ganglia neurons (dorsal roots) where regeneration is faster than in ventral roots.
Neuropathology in Conditional erbB2 Mutant Mice
The conditional mutation of erbB2 in myelinating Schwann cells results in a moderate loss of motoneurons. Since erbB2 is not mutated in cells of the spinal cord, this is caused by indirect mechanisms. Axonal retractions were previously noted in mice with defective myelination (Giese et al. 1992; Adlkofer et al. 1995; Griffiths et al. 1998; Frei et al. 1999). In mice with Krox20-cre–induced mutations, motoneurons are lost, but sensory neuron numbers are not altered. We observe thin myelin in all those animals and in all nerves. A lack of myelin surrounding large-caliber axons is, however, mainly found in nerves innervating muscle, and is variable in extent in different animals, as is the extent of motoneuron loss. Thus, not thin myelin, but a local lack of myelin in parts of the nerve might cause this damage.
Multiple Functions of Neuregulin in the Schwann Cell Lineage
Neuregulin-1 and its receptors serve several functions in the Schwann cell lineage. The first stage in which this signaling system is required occurs early during development of the lineage. In neuregulin-1, erbB2, or erbB3 mutant mice, the population of early Schwann cell precursors is already diminished as they start to populate the spinal nerves (Meyer and Birchmeier 1995; Erickson et al. 1997; Riethmacher et al. 1997; Britsch et al. 1998; Morris et al. 1999; Woldeyesus et al. 1999). Defective migration of the precursors along the axon might be responsible. It is interesting to note that neural crest cells that form sympathetic ganglia require neuregulin-1, erbB2, and erbB3 for migration (Britsch et al. 1998). However, increased apoptosis and/or a lack of proliferation contribute to the severe Schwann cell phenotype apparent at late stages in null mutants, since precursors that form do not expand. The severe reduction of Schwann cells in spinal roots of conditional erbB2 mutants appears to be caused by cell death, since Krox20 expression and, thus, the Krox20-cre–induced mutation occurs after precursors reach the roots.
Importantly, we demonstrate here that erbB2 is also required for correct myelination in Schwann cells. The thin myelin observed in conditional erbB2 mutant mice is stable, and not repaired. Similarly, local deficits in Schwann cell numbers in peripheral motor nerves are not repaired. Thus, this and other studies provide evidence for a role of erbB2 during repair of dysmyelinated lesions and regeneration of damaged Schwann cells (Carroll et al. 1997; Kwon et al. 1997; Li et al. 1997; Chen et al. 1998). Therefore, Neuregulin/ErbB signaling functions during the entire life span of a myelinating Schwann cell.
Acknowledgments
We want to thank Ralf Grossfeld and Dieter Riethmacher for help in the initial phase of this project, Gary Lewin for advice, Sven Buchert and Andrea Rehaus for expert technical assistance, as well as Klaus-Armin Nave, Rhona Mirsky and Kristjan Jessen for helpful discussions. We are also indebted to Sandra Göbbels and Klaus-Armin Nave (ZMBH, Heidelberg, Germany) for providing us with a P0-cre transgenic strain. The following provided antibodies: Greg Lemke (anti-SCIP; Salk Institute, San Diego, CA), Pascale Gilardi (anti-Krox20; École Normale Supérieure, Paris, France), Ueli Suter (anti-PMP22; Swiss Federal Institute of Technology, Zürich, Switzerland), Juan Archelos (anti-P0; Graz University Clinic, Graz, Austria), and Michael Wegner (anti-SCIP antibodies and also COS cell transfectants of Krox20 and SCIP expression plasmids; ZMNH, Hamburg, Germany). We also thank Volker Brinkmann for preliminary analysis of mutant nerves by electron microscopy, and Walter Birchmeier for critical reading of the manuscript.
This work was funded by a grant from the European Commission T.M.R. Programme (to A.N. Garratt), and by The Volkswagen Stiftung (to C. Birchmeier).
Footnotes
Abbreviations used in this paper: BrdU, bromodeoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; ES, embryonic stem; L4, L5, fourth and fifth lumbar segments, respectively; P3.5, P15, postnatal day 3.5 and 15, respectively; PFA, paraformaldehyde; X-gal, 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside.
References
- Adlkofer K., Martini R., Aguzzi A., Zielasek J., Toyka K.V., Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat. Genet. 1995;11:274–280. doi: 10.1038/ng1195-274. [DOI] [PubMed] [Google Scholar]
- Adlkofer K., Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Akagi K., Sandig V., Vooijs M., Van der Valk M., Giovannini M., Strauss M., Berns A. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25:1766–1773. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy I., Yarden Y. The ErbB signaling network in embryogenesis and oncogenesissignal diversification through combinatorial ligand-receptor interactions. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Bermingham J.R., Jr., Scherer S.S., O'Connell S., Arroyo E., Kalla K.A., Powell F.L., Rosenfeld M.G. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes. Dev. 1996;10:1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Xu Y.T., Marchionni M.A., Scherer S.S. Neuregulin expression in PNS neuronsisoforms and regulation by target interactions. Mol. Cell. Neurosci. 1997;10:184–195. doi: 10.1006/mcne.1997.0654. [DOI] [PubMed] [Google Scholar]
- Britsch S., Li L., Kirchhoff S., Theuring F., Brinkmann V., Birchmeier C., Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S., Yarden Y. Neuregulins and their receptorsa versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Carroll S.L., Miller M.L., Frohnert P.W., Kim S.S., Corbett J.A. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J. Neurosci. 1997;17:1642–1659. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Zerial M., Lemaire P., Almendral J., Bravo R., Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO (Eur. Mol. Biol. Organ.) J. 1988;7:29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.E., Liu K., Seaber A.V., Katragadda S., Kirk C., Urbaniak J.R. Recombinant human glial growth factor 2 (rhGGF2) improves functional recovery of crushed peripheral nerve (a double-blind study) Neurochem. Int. 1998;33:341–351. doi: 10.1016/s0197-0186(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Chen M.S., Bermingham-McDonogh O., Danehy F.T., Jr., Nolan C., Scherer S.S., Lucas J., Gwynne D., Marchionni M.A. Expression of multiple neuregulin transcripts in postnatal rat brains. J. Comp. Neurol. 1994;349:389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- Cheng L., Esch F.S., Marchionni M.A., Mudge A.W. Control of Schwann cell survival and proliferationautocrine factors and neuregulins. Mol. Cell. Neurosci. 1998;12:141–156. doi: 10.1006/mcne.1998.0706. [DOI] [PubMed] [Google Scholar]
- Corfas G., Rosen K.M., Aratake H., Krauss R., Fischbach G.D. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- Dong Z., Brennan A., Liu N., Yarden Y., Lefkowitz G., Mirsky R., Jessen K.R. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Dougall W.C., Qian X., Peterson N.C., Miller M.J., Samanta A., Greene M.I. The neu-oncogenesignal transduction pathways, transformation mechanisms and evolving therapies. Oncogene. 1994;9:2109–2123. [PubMed] [Google Scholar]
- Erickson S.L., O'Shea K.S., Ghaboosin N., Loverro L., Frantz G., Bauer M., Lu L.H., Moore M.W. ErbB3 is required for normal cerebellar and cardiac developmenta comparison with erbB2 and heregulin deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Frei R., Motzing S., Kinkelin I., Schachner M., Koltzenburg M., Martini R. Loss of distal axons and sensory Merkel cells and features indicative of muscle denervation in hindlimbs of P0-deficient mice. J. Neurosci. 1999;19:6058–6067. doi: 10.1523/JNEUROSCI.19-14-06058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm L., Burden S.J. Transcriptional pathways for synapse-specific, neuregulin-induced and electrical activity-dependent transcription. J. Physiol. 1998;92:173–176. doi: 10.1016/s0928-4257(98)80005-3. [DOI] [PubMed] [Google Scholar]
- Giese K., Martini R., Lemke G., Soriano P., Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–576. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Goberdhan D.C., Paricio N., Goodman E.C., Mlodzik M., Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodearl A.D., Davis J.B., Mistry K., Minghetti L., Otsu M., Waterfield M.D., Stroobant P. Purification of multiple forms of glial growth factor. J. Biol. Chem. 1993;268:18095–18102. [PubMed] [Google Scholar]
- Griffiths I., Klugmann M., Anderson T., Yool D., Thomson C., Schwab M.H., Schneider A., Zimmermann F., McCulloch M., Nadon N., Nave K.A. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Grinspan J.B., Marchionni M.A., Reeves M., Coulaloglou M., Scherer S.S. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerveneuregulin receptors and the role of neuregulins. J. Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Zou Y.R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- He X., Treacy M.N., Simmons D.M., Ingraham H.A., Swanson L.W., Rosenfeld M.G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989;340:35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Ho W.H., Armanini M.P., Nuijens A., Phillips H.S., Osheroff P.L. Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J. Biol. Chem. 1995;270:14523–14532. doi: 10.1074/jbc.270.24.14523. [DOI] [PubMed] [Google Scholar]
- Jaegle M., Mandemakers W., Broos L., Zwart R., Karis A., Visser P., Grosveld F., Meijer D. The POU factor Oct-6 and Schwann cell differentiation. Science. 1996;273:507–510. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- Jessen K.R., Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci. 1999;22:402–410. doi: 10.1016/s0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- Kwon Y.K., Bhattacharyya A., Alberta J.A., Giannobile W.V., Cheon K., Stiles C.D., Pomeroy S.L. Activation of ErbB2 during Wallerian degeneration of sciatic nerve. J. Neurosci. 1997;17:8293–8299. doi: 10.1523/JNEUROSCI.17-21-08293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M. The Neural Crest. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- Lemke G.E., Brockes J.P. Identification and purification of glial growth factor. J. Neurosci. 1984;4:75–83. doi: 10.1523/JNEUROSCI.04-01-00075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A.D., Bunge R.P., Lofgren J.A., Meima L., Hefti F., Nikolics K., Sliwkowski M.X. The influence of heregulins on human Schwann cell proliferation. J. Neurosci. 1995;15:1329–1340. doi: 10.1523/JNEUROSCI.15-02-01329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Topilko P., Schneider Maunoury S., Lasagna M., Mantero S., Cancedda R., Charnay P. Defective bone formation in Krox-20 mutant mice. Development. 1996;122:113–120. doi: 10.1242/dev.122.1.113. [DOI] [PubMed] [Google Scholar]
- Li H., Terenghi G., Hall S.M. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia. 1997;20:333–347. doi: 10.1002/(sici)1098-1136(199708)20:4<333::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Marchionni M.A., Goodearl A.D.J., Chen M.S., Bermingham-McDonogh O., Kirk C., Hendricks M., Danehy F., Misumi D., Sudhalter J., Kobayashi K. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Martini R., Schachner M. Molecular bases of myelin formation as revealed by investigations on mice deficient in glial cell surface molecules. Glia. 1997;19:298–310. [PubMed] [Google Scholar]
- Meier C., Parmantier E., Brennan A., Mirsky R., Jessen K.R. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J. Neurosci. 1999;19:3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A., Behringer R.R., Hammang J.P., Palmiter R.D., Brinster R.L., Lemke G. P0 promoter directs expression of reporter and toxin genes to Schwann cells of transgenic mice. Neuron. 1992;8:507–520. doi: 10.1016/0896-6273(92)90279-m. [DOI] [PubMed] [Google Scholar]
- Meyer D., Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Meyer D., Yamaai T., Garratt A.N., Riethmacher-Sonnenberg E., Kane D., Theill L.E., Birchmeier C. Isoform specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Jessen K.R. Schwann cell development, differentiation and myelination. Curr. Opin. Neurobiol. 1996;6:89–96. doi: 10.1016/s0959-4388(96)80013-4. [DOI] [PubMed] [Google Scholar]
- Moghal N., Sternberg P.W. Multiple positive and negative regulators of signaling by the EGF-receptor. Curr. Opin. Cell Biol. 1999;11:190–196. doi: 10.1016/s0955-0674(99)80025-8. [DOI] [PubMed] [Google Scholar]
- Monuki E.S., Weinmaster G., Kuhn R., Lemke G. SCIPa glial POU domain gene regulated by cyclic AMP. Neuron. 1989;3:783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Morris J.K., Lin W., Hauser C., Marchuk Y., Getman D., Lee K.F. Rescue of the cardiac defect in erbB2 mutant mice reveals essential roles of erbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Morrissey T.K., Levi A.D., Nuijens A., Sliwkowski M.X., Bunge R.P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl. Acad. Sci. USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P., Topilko P., Schneider M.S., Seitanidou T., Baron V.E.A., Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122:2847–2857. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- Porter S., Glaser L., Bunge R.P. Release of autocrine growth factor by primary and immortalized Schwann cells. Proc. Natl. Acad. Sci. USA. 1987;84:7768–7772. doi: 10.1073/pnas.84.21.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M.C., Abney E., Brockes J.P., Hornby Smith A. Schwann cell growth factors. Cell. 1978;15:813–822. doi: 10.1016/0092-8674(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Riethmacher D., Sonnenberg-Riethmacher E., Brinkmann V., Yamaai T., Lewin G.R., Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Rosenbaum C., Karyala S., Marchionni M.A., Kim H.A., Krasnoselsky A.L., Happel B., Isaacs I., Brackenbury R., Ratner N. Schwann cells express NDF and SMDF/n-ARIA mRNAs, secrete neuregulin, and show constitutive activation of erbB3 receptorsevidence for a neuregulin autocrine loop. Exp. Neurol. 1997;148:604–615. doi: 10.1006/exnr.1997.6696. [DOI] [PubMed] [Google Scholar]
- Saida K., Sumner A.J., Saida T., Brown M.J., Silberberg D.H. Antiserum-mediated demyelinationrelationship between remyelination and functional recovery. Annu. Neurol. 1980;8:12–24. doi: 10.1002/ana.410080103. [DOI] [PubMed] [Google Scholar]
- Scherer S.S. Molecular genetics of demyelinationnew wrinkles on an old membrane. Neuron. 1997;18:13–16. doi: 10.1016/s0896-6273(01)80042-8. [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Shilo B.Z. A thousand and one roles for the Drosophila EGF receptor. Trends Genet. 1997;13:191–196. doi: 10.1016/s0168-9525(97)01091-3. [DOI] [PubMed] [Google Scholar]
- Schwenk F., Baron U., Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U. Myelinkeeping nerves well wrapped up. Curr. Biol. 1997;7:R21–R23. doi: 10.1016/s0960-9822(06)00011-x. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Rohdewohld H., Neuman T., Gruss P., Scholer H.R. Oct-6a POU transcription factor expressed in embryonal stem cells and in the developing brain. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:3723–3732. doi: 10.1002/j.1460-2075.1990.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syroid D.E., Maycox P.R., Burrola P.G., Liu N., Wen D., Lee K.F., Lemke G., Kilpatrick T.J. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc. Natl. Acad. Sci. USA. 1996;93:9229–9234. doi: 10.1073/pnas.93.17.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syroid D.E., Zorick T.S., Arbet Engels C., Kilpatrick T.J., Eckhart W., Lemke G. A role for insulin-like growth factor-I in the regulation of Schwann cell survival. J. Neurosci. 1999;19:2059–2068. doi: 10.1523/JNEUROSCI.19-06-02059.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P., Schneider Maunoury S., Levi G., Baron Van Evercooren A., Chennoufi A.B., Seitanidou T., Babinet C., Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Torres R., Kühn R. Laboratory Protocols for Conditional Gene Targeting in Mice 1997. Oxford University Press; Oxford: pp. 33–35 [Google Scholar]
- Trachtenberg J.T., Thompson W.J. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379:174–177. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- Vartanian T., Goodearl A., Viehover A., Fischbach G. Axonal neuregulin signals cells of the oligodendrocyte lineage through activation of HER4 and Schwann cells through HER2 and HER3. J. Cell Biol. 1997;137:211–220. doi: 10.1083/jcb.137.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiculescu O., Charnay P., Schneider-Maunoury S. Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones and peripheral nervous system. Genesis. 2000;In press doi: 10.1002/(sici)1526-968x(200002)26:2<123::aid-gene7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Warner L.E., Garcia C.A., Lupski J.R. Hereditary peripheral neuropathiesclinical forms, genetics, and molecular mechanisms. Annu. Rev. Med. 1999;50:263–275. doi: 10.1146/annurev.med.50.1.263. [DOI] [PubMed] [Google Scholar]
- Weinstein D.E., Burrola P.G., Lemke G. Premature Schwann cell differentiation and hypermyelination in mice expressing a targeted antagonist of the POU transcription factor SCIP. Mol. Cell. Neurosci. 1995;6:212–229. doi: 10.1006/mcne.1995.1018. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.G., Bhatt S., Chavrier P., Bravo R., Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989;337:461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Woldeyesus M.T., Britsch S., Riethmacher D., Xu L., Sonnenberg-Riethmacher E., Abou-Rebyeh F., Harvey R., Caroni P., Birchmeier C. Genetic rescue of cardiac morphogenesis in erbB2 mutant mice reveals functions of the ErbB2 receptor in development of the peripheral nervous system. Genes. Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Kuo Y., Devay P., Yu C., Role L. A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- Zorick T.S., Lemke G. Schwann cell differentiation. Curr. Opin. Cell Biol. 1996;8:870–876. doi: 10.1016/s0955-0674(96)80090-1. [DOI] [PubMed] [Google Scholar]
- Zorick T.S., Syroid D.E., Brown A., Gridley T., Lemke G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development. 1999;126:1397–1406. doi: 10.1242/dev.126.7.1397. [DOI] [PubMed] [Google Scholar]