Abstract
MyoD expression is thought to be induced in somites in response to factors released by surrounding tissues; however, reverse transcription-PCR and cell culture analyses indicate that myogenic cells are present in the embryo before somite formation. Fluorescently labeled DNA dendrimers were used to identify MyoD expressing cells in presomitic tissues in vivo. Subpopulations of MyoD positive cells were found in the segmental plate, epiblast, mesoderm, and hypoblast. Directly after laying, the epiblast of the two layered embryo contained ∼20 MyoD positive cells. These results demonstrate that dendrimers are precise and sensitive reagents for localizing low levels of mRNA in tissue sections and whole embryos, and that cells with myogenic potential are present in the embryo before the initiation of gastrulation.
Keywords: myogenesis, epiblast, segmental plate, in situ hybridization, muscle transcription factor
Introduction
Reverse transcription PCR (RT-PCR) can reveal the presence of messenger RNA not detectable by in situ hybridization. This raises the question of whether messages present in low abundance are functionally significant, an issue particularly relevant to the study of the MyoD family of transcription factors that regulate skeletal muscle development (Weintraub et al. 1991; Rudnicki and Jaenisch 1995; Molkentin and Olson 1996). A widely held view states that the expression of MyoD in the somites of avian embryos and Myf5 in the mouse is initiated by factors secreted by the neural tube, notochord, and ectoderm. mRNA for these factors is not detected by in situ hybridization until after somites pinch off from the segmental plate mesoderm and an intact segmental plate will not form muscle in vitro unless it is cocultured with the neural tube and/or notochord (for review see Cossu et al. 1996; George-Weinstein et al. 1998; Buckingham and Tajbakhsh 1999). However, MyoD has been detected by RT-PCR in the segmental plate and the epiblast that gives rise to the mesoderm during gastrulation (George-Weinstein et al. 1996a,George-Weinstein et al. 1996b). Furthermore, both the segmental plate and epiblast give rise to an abundance of skeletal muscle when isolated from surrounding tissues, dissociated into a single cell suspension, and cultured in serum-free medium (George-Weinstein et al. 1994, George-Weinstein et al. 1996a, George-Weinstein et al. 1997). These and other experiments (Krenn et al. 1988; Choi et al. 1989; Holtzer et al. 1990; Chen and Solursh 1991; von Kirschhofer et al. 1994) suggest that myogenic cells are present in early embryos, but are repressed from differentiating in vivo until after somite formation. Inductive factors secreted by the neural tube and notochord may upregulate the expression of MyoD in cells that already have low levels of message. Thus, in this case, low abundance mRNA appears to be an indicator of developmental potential.
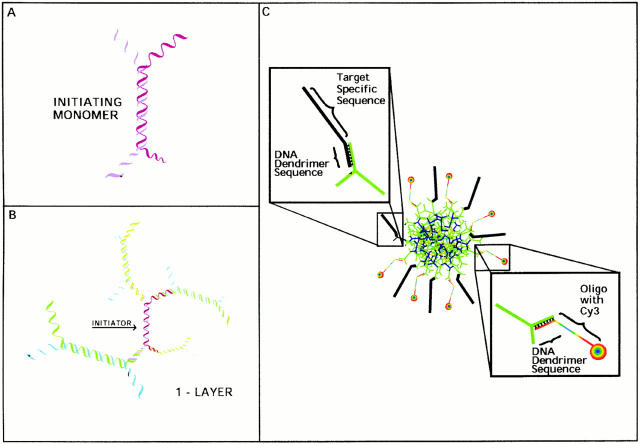
Whereas RT-PCR can detect low levels of mRNA, it does not reveal how many cells contain MyoD or where they are located within the embryo. To identify those cells that express MyoD before somite formation, we have performed in situ hybridizations with increasingly younger tissues using the recently developed and sensitive probes, fluorescently labeled 3DNA dendrimers. Dendrimers are highly branched, multilayered structures synthesized by sequential hybridizations of partially complimentary heteroduplexes called DNA monomers (Fig. 1; Nilsen et al. 1997; Vogelbacker et al. 1997; Wang et al. 1998). Greater than 500 molecules of fluorochrome, 32P, digoxigenin, or biotin can be incorporated into the dendrimer. Many of the single-stranded outer arms are cross-linked with an oligonucleotide sequence specific for a particular mRNA or DNA sequence. Since dendrimers produce a 100- to 1,000-fold increase in signal compared with single-stranded oligonucleotide probes in Northern and Southern blots and can be tagged with a fluorochrome, they were predicted to be precise and sensitive probes for mRNA in single cells in tissue sections. In situ hybridizations with Cy3 dendrimers revealed that subpopulations of MyoD positive cells are present throughout the segmental plate, epiblast, mesoderm, and hypoblast.
Figure 1.
Synthesis of the DNA dendrimer. Core dendrimers were synthesized from initiating monomers consisting of a double-stranded waist and four single-stranded arms (A). Sequential hybridizations produced one-layer (B) and four-layer (C) dendrimers. Antisense oligonucleotides for particular mRNAs (target-specific sequence) and oligonucleotides containing the fluorochrome Cy3 were hybridized to the outer arms of the four-layer dendrimer (C).
Materials and Methods
Synthesis of DNA Dendrimers
Core DNA dendrimers were synthesized as described previously (Nilsen et al. 1997; Vogelbacker et al. 1997; Wang et al. 1998). Assembly proceeds by sequential hybridization of seven single-stranded DNA 116-mers. Each 116-mer is designed to partially hybridize via a central region of 50 nucleotides, yielding a heteroduplex monomer with a double-stranded waist region and four single-stranded arms (Fig. 1 A). These initiator dendrimers are hybridized to monomers to produce the one-layer growing structure (Fig. 1 B). Dendritic assembly was continued by the subsequent addition of monomers to the growing one-layer structure to yield a four-layer dendrimer (Fig. 1 C). After each hybridization, the structure is covalently cross-linked with 4,5′,8 trimethyl psoralen (trioxsalen; 1/15 vol/vol of psoralen-saturated ethanol), followed by a 10-min exposure to UVA light in a Simms Instruments ultraviolet reaction chamber 3000. The four-layer core dendrimer was purified from denaturing sucrose gradients (10–50% sucrose, 50% formamide, 50 mM tris-HCl, 10 mM EDTA, pH 8.0) at 40°C to 3.5Ω11 w^2T.
Oligonucleotide sequences for specific mRNAs plus 7–30 bases complementary to the dendrimer were either psoralen cross-linked or ligated to at least 20 of the outer surface dendrimer arms. Fluorescent dendrimers were prepared by hybridizing and cross-linking a Cy3-labeled oligonucleotide to at least one half of the arms on the outer surface of the dendrimer. Each dendrimer contained from 250–500 Cy3 molecules.
Dendrimers contained the following cDNA sequences for antisense mRNA: chicken MyoD (Dechesne et al. 1994), 5′-TTC TCA AGA GCA AAT ACT CAC CAT TTG GTG ATT CCG TGT AGT AGC TGC TG-3′; chicken embryonic fast myosin (Freyer and Robbins 1983), 5′-CAG GAG GTG CTG CAG GTC CTT CAC CGT CTG GTC CAG GTT CTT CTT CAT CCT CTC TCC AGG-3′; and chicken glyceraldehyde-3-phosphate dehydrogenase (Dugaiczyk et al. 1983), 5′-ATC AAG TCC ACA ACA CGG TTG CTG TAT CCA AAC TCA TTG TCA TAC CAG GAA-3′. Dendrimers lacking a specific recognition sequence were used as a negative control for background fluorescence.
In Situ Hybridization
The in situ hybridization protocol was modified from that of Sassoon and Rosenthal 1993 and Raap et al. 1994. White Leghorn chick embryos (Truslow Farms) were staged according to the method of Hamburger and Hamilton 1951. Stage 16 (28 pairs of somites), stages 13–14 (17–22 pairs of somites), and stage 4 embryos were fixed in 4% formaldehyde, embedded in paraffin, sectioned transversely at 10 μm, and applied to 3-well teflon printed slides (Electron Microscopy Sciences) coated with 0.2% gelatin. Cells were permeabilized with 0.1% Triton X-100 for 10 min and treated for 5–10 min with 0.1% pepsin (Sigma Chemical Co.) in 0.01 M HCl. 30 μl of hybridization buffer containing 60% deionized formamide, 2× SSC buffer, 50 mM sodium phosphate, 5% dextran sulfate (Sigma Chemical Co.), 15 μg yeast RNA, 15 μg salmon sperm DNA (Boehringer), and 18 ng of Cy3-labeled dendrimers was applied to each section. Sections were incubated at 80°C for 10 min then at 37°C overnight. After rinsing in 60% formamide, nuclei were labeled with bis-benzamide (Sigma Chemical Co.; 1 ng/ml deionized water). Sections were mounted in Gelmount (Fisher Scientific) and observed with a Nikon Eclipse E800 epifluorescence microscope (Optical Apparatus). Photomicrographs of differential interference contrast (DIC) images, bis-benzamide–labeled nuclei, and Cy3 dendrimers were produced with the Optronics DEI 750 video camera and Image-Pro Plus image analysis software (Phase 3 Imagining Systems). Results were consistent in sections from 9 stages 13–14 embryos and 5 stage 4 embryos.
In situ hybridizations also were performed on whole, unsectioned embryos. Hamburger and Hamilton 1951 stage 1 embryos were further divided into stages X–XII by the method of Eyal-Giladi and Kochav 1976. Stages X–XII and stage 2 embryos were fixed and permeabilized with Triton X-100 and pepsin as described above. Each embryo was applied to a one-well teflon printed slide (Electron Microscopy Sciences), incubated with 100 μl of hybridization buffer, and processed as described above. Consistent results for MyoD localization were obtained in 4 stage X, 5 stage XI, 3 stage XII, and 5 stage 2 embryos.
Immunofluorescence Localization
Myosin protein was localized in tissue sections with the MF20 mAb to myosin heavy chain (Bader et al. 1982) obtained from the Developmental Studies Hybridoma Bank. Sections were deparaffinized, rehydrated, permeabilized in 0.5% Triton X-100, and incubated in primary then secondary antibodies diluted in 10% goat serum in PBS. The secondary antibody was affinity-purified, goat anti–mouse IgG F(ab′)2 fragments conjugated with rhodamine (The Jackson Laboratory). Nuclei were counterstained with bis-benzamide.
Reverse Transcription–Polymerase Chain Reaction
RT-PCR was carried out as described previously (George-Weinstein et al. 1996a,George-Weinstein et al. 1996b). RNA was extracted from Eyal-Giladi and Kochav 1976 stages X–XII embryos and Hamburger and Hamilton 1951 stage 2 embryos. Stage 39 (day 13) pectoralis muscle was included as a positive control for MyoD expression. Primer pairs for MyoD were: nucleotides 620–639, 5′-CGT GAG CAG GAG GAT GCA TA-3′; and nucleotides 864–883, 5′-GGG ACA TGT GGA GTT GTC TG-3′ (Lin et al. 1989). Primer pairs for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were: nucleotides 680–699, 5′-AGT CAT CCC TGA GCT GAA TG-3′; and nucleotides 990–1009, 5′-AGG ATC AAG TCC ACA ACA CG-3′ (Dugaiczyk et al. 1983). Reaction products were separated on 6% polyacrylamide gels and 32P-incorporation visualized by autoradiography.
Results
Localization of MyoD and Myosin mRNA in the Somites
Validation of the use of dendrimers as probes for mRNA in tissue sections was carried out, in part, by comparing the expression of myosin mRNA and protein in the stage 16 embryo (28 somites). Myosin dendrimers and the MF20 antibody to myosin heavy chain protein localized to the myotome of the somite (Fig. 2C and Fig. D). A few myosin dendrimers also were found in the sclerotome and dermatome (Fig. 2 C). The expression of MyoD was similar to, but more extensive than, myosin. MyoD dendrimers bound most abundantly to the dorsal–medial portion of the dermatome and mytome closest to the neural tube (Fig. 2 H). Some dendrimers were observed within the chondrogenic sclerotome and neural tube (Fig. 2 H). By contrast, dendrimers with a recognition sequence for the enzyme GAPDH bound to cells throughout the section (Fig. 2 F), whereas only one to three dendrimers lacking a specific recognition sequence were randomly distributed throughout each section (Fig. 2 E).
Figure 2.
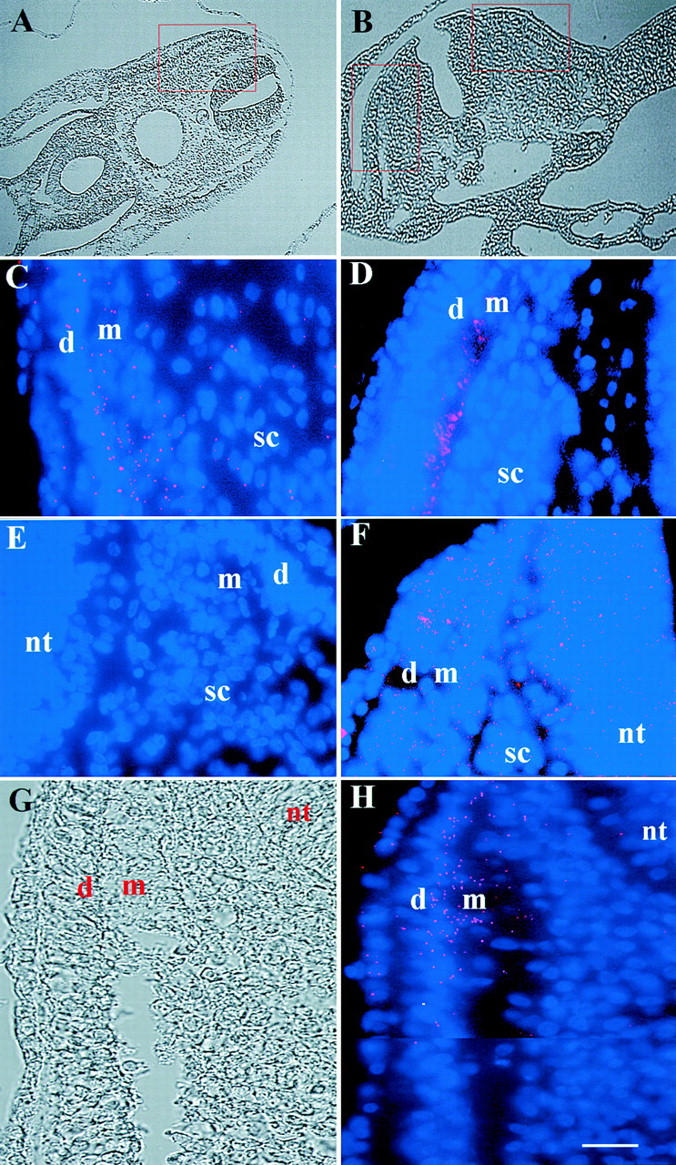
Localization of MyoD and myosin mRNAs in the somite of the stage 16 embryo. A and B, Low magnification DIC images of rostral and caudal sections, respectively. The area outlined in A is shown at higher magnification in C and D. E and F, High magnification images of the areas outlined in B. The fluorescence photomicrographs are merged images of bis-benzamide-labeled nuclei in blue and Cy3-labeled dendrimers in red. The dendrimers bound within the cytoplasm. Rostral somites with fully developed dermatomes (d), myotomes (m), and sclerotomes (s) were hybridized with dendrimers to myosin mRNA (C) and the MF20 antibody to myosin protein (D). Both probes localized to the myotome. Only 1–3 dendrimers lacking a specific recognition sequence bound to each section (E), whereas dendrimers to GAPDH produced fluorescence throughout the somite and neural tube (nt; F). The length of the dermatome and myotome is shown as a composite in G and H. MyoD dendrimers were concentrated in the dorsal–medial portion of these tissues (H). A few dendrimers were found in the sclerotome and neural tube. Bar: (A and B) 54 μm; (C–H) 9 μm.
The labeling pattern of MyoD dendrimers in the less mature, wedge-shaped somites of the stage 14 embryo was similar to that seen in the older embryo. Fluorescence was most abundant in the dorsal–medial portion of the dermomyotome (Fig. 3 B). These results are consistent with previous in situ hybridizations using conventional oligonucleotide probes (Sassoon et al. 1989; Charles de la Brousse and Emerson 1990; Ott et al. 1991; Pownall and Emerson 1992). A few cells of the sclerotome also were fluorescent (Fig. 3 B), supporting the hypothesis that muscle precursors are present in myogenic and chondrogenic regions of the somite (George-Weinstein et al. 1998). MyoD dendrimers also were found in low abundance in the neural tube (Fig. 3 B). This is consistent with the finding that transgenic mice containing lacZ targeted into the Myf5 locus express Myf5 in the neural tube (Tajbakhsh and Buckingham 1995). Furthermore, some cells of the murine neural tube can differentiate into muscle in vitro (Tajbakhsh et al. 1994), and glial-like cells from chick neural tube explants contain MyoD protein (our unpublished observation).
Figure 3.
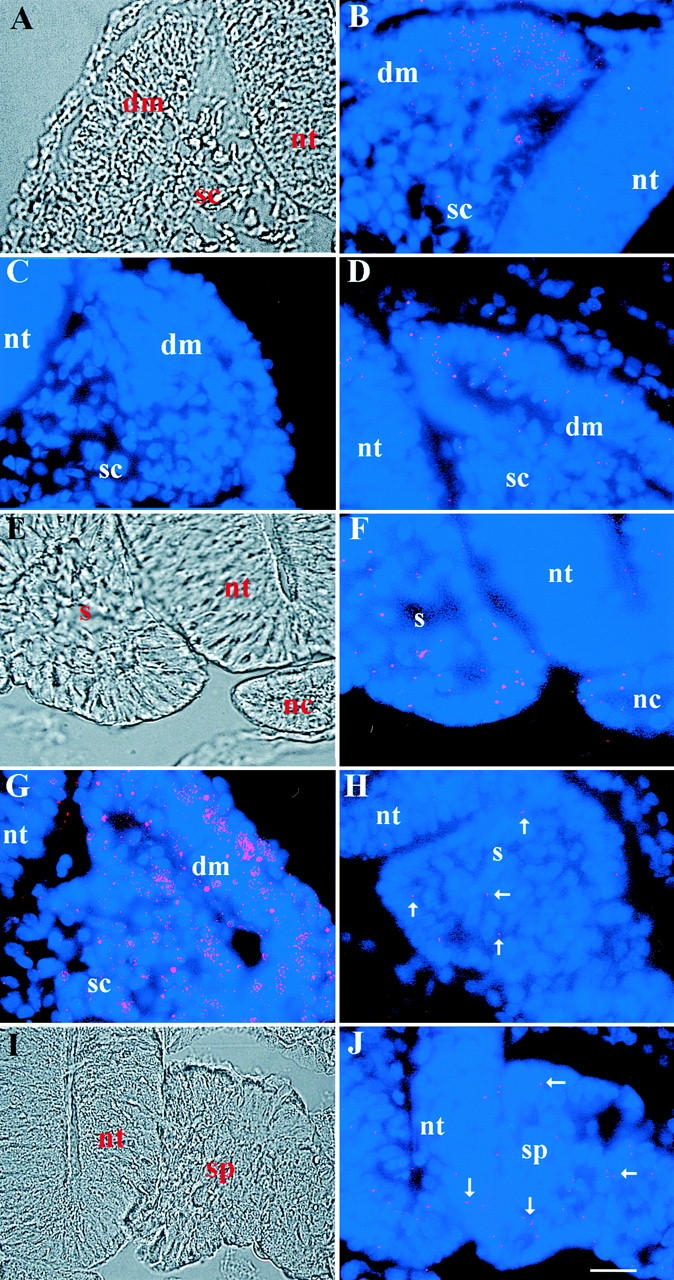
Localization of MyoD and myosin mRNAs in the somites and segmental plate mesoderm of the stage 14 embryo. Photomicrographs in A, E, and I are DIC images of the merged images of bis-benzamide-labeled nuclei and Cy3-labeled dendrimers in B, F, and J, respectively. MyoD dendrimers were concentrated in the dorsal–medial portion of the dermomyotome (dm; B). A few cells of the sclerotome (sc) and neural tube (nt) also were labeled. The pattern of labeling with myosin dendrimers was similar to, but less abundant than, MyoD (D). GAPDH dendrimers produced intense fluorescence throughout the dermomyotome and sclerotome (G), whereas dendrimers lacking a specific recognition sequence did not bind to the somite (C). A subpopulation of cells in the epithelial somite (s; F) and segmental plate (sp; J) contained MyoD dendrimers. A few myosin dendrimers were found in the segmental plate (H). Bar, 9 μm.
The less mature, epithelial somites of the stage 14 embryo contained MyoD positive cells (Fig. 3 F). Dendrimers were concentrated in the ventral region of these somites and those that had just pinched off from the segmental plate (not shown). This could reflect an inductive effect of the notochord on adjacent mesoderm cells via the secretion of Sonic Hedgehog (shh), although mRNA for patched, shh's receptor, was not detected in the segmental plate by conventional in situ hybridization (Borycki et al. 1998).
Dendrimers to embryonic fast myosin produced a similar pattern to MyoD in the wedge-shaped somite, but were less abundant (Fig. 3 D). Fluorescence was most intense in the dorsal–medial portion of the dermomyotome. A few cells in the sclerotome and neural tube also were positive (Fig. 3 D). Since the expression of myosin is downstream of MyoD (Weintraub 1993; Rudnicki and Jaenisch 1995; Molkentin and Olson 1996), MyoD mRNA may be translated into protein in these relatively immature somites. Dendrimers to GAPDH produced intense fluorescence throughout the somite (Fig. 3 G). Only one to three dendrimers lacking a specific recognition sequence were randomly distributed throughout each section (Fig. 3 C).
Localization of MyoD mRNA in the Segmental Plate Mesoderm
Since dendrimers correctly detected MyoD mRNA in the dermomyotome, they were tested for their ability to bind to tissues that give rise to skeletal muscle in vitro, and that contain MyoD mRNA detectable by RT-PCR, but not by conventional in situ hybridization.
The pattern of MyoD expression in the segmental plate was similar to that seen with immature somites. MyoD positive cells were present throughout the segmental plate; however, fluorescence was slightly more abundant in the ventral portion of this tissue (Fig. 3 J). MyoD dendrimers also were found in the neural tube (Fig. 3 J). Only a few myosin dendrimers were present in the segmental plate (Fig. 3 H). One to three dendrimers lacking a specific recognition sequence bound to the entire section (not shown).
Localization of MyoD mRNA in Gastrulating Embryos
The same low level of background seen in older embryos was observed in sections through the stage 4 embryo (Fig. 4C and Fig. I). During this stage of development, cells from the dorsal epiblast layer ingress into the primitive streak to form the mesoderm and endoderm (Rosenquist 1971; Fontaine and Le Douarin 1977; Bellairs 1986; Stern and Canning 1990). MyoD positive cells were a mixture of intensely (>6 dendrimers) and weakly (one to two dendrimers) labeled cells. Consistent with the results obtained with RT-PCR (George-Weinstein et al. 1996a), MyoD dendrimers were found in cells throughout the epiblast, mesoderm, and hypoblast (Fig. 4D, Fig. G, and Fig. H). Cells with a strong signal within the epiblast or hypoblast were adjacent to fluorescent cells in the mesoderm (Fig. 4G and Fig. H).
Figure 4.
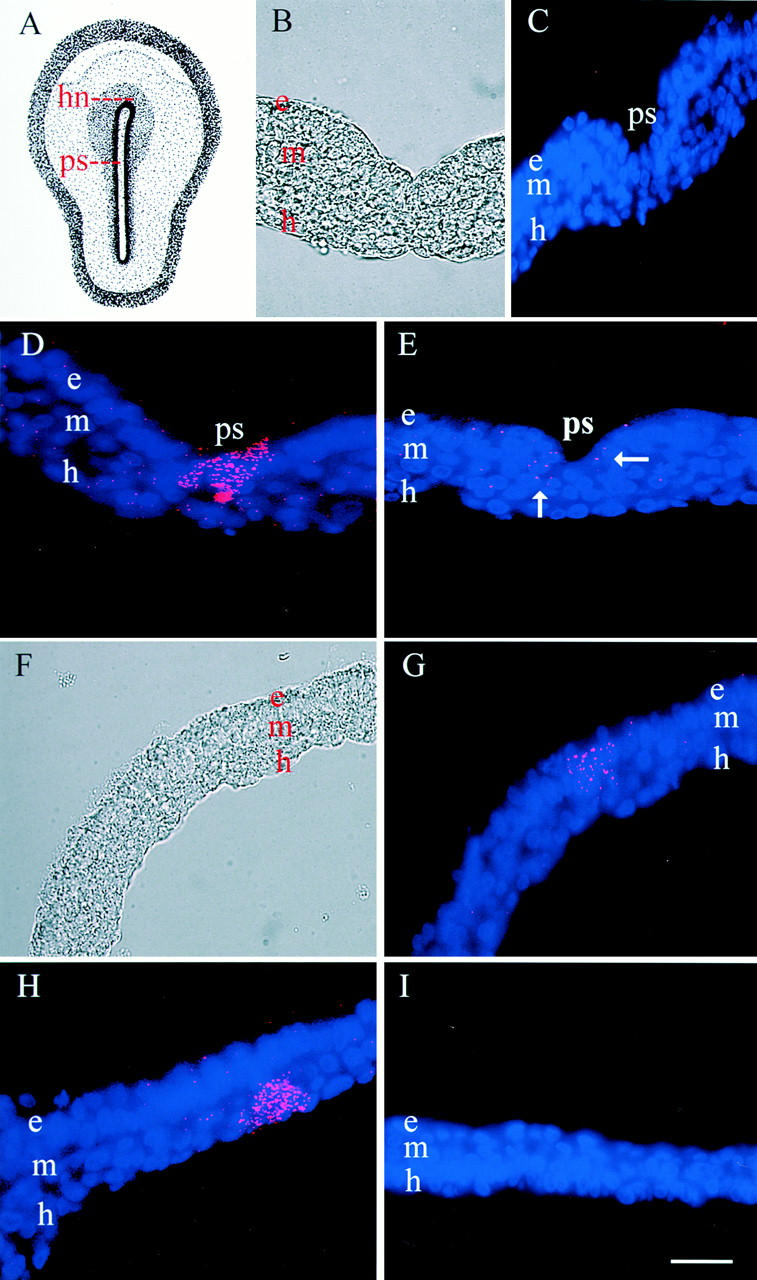
Localization of MyoD in the stage 4 embryo. A drawing of the stage 4 embryo is shown in A. A DIC image of the primitive streak near Hensen's node is shown in B and the lateral region of the embryo in F. Cells ingressing into the primitive streak (ps) in the region of Hensen's node (hn) were intensely labeled with MyoD dendrimers (D). Less fluorescence was observed in more posterior regions of the streak (E). Groups of adjacent epiblast (e) and mesoderm cells (m; G) and mesoderm and hypoblast cells (h; H) also were labeled. Myosin dendrimers were not observed in the primitive streak near Hensen's node (C) or in more lateral regions of the embryo (I). Bar, 9 μm.
The number of labeled cells varied in different regions of the embryo. Staining was strongest in the rostral end of the streak near Hensen's node (Fig. 4 D), a structure that produces a variety of cytokines (Mitrani et al. 1990a,Mitrani et al. 1990b; Cooke and Wong 1991; Kisbert et al. 1995; Stern et al. 1995). Some fluorescence also was observed in more posterior regions of the streak (Fig. 4 E). Cells from the epiblast become mesenchymal and switch from E- to N-cadherin as they ingress into the streak to form the mesoderm (Edelman et al. 1983; Hatta and Takeichi 1986). Since the epiblast epithelium needs to be dissociated and its cells must downregulate E-cadherin and upregulate N-cadherin in order to form muscle in vitro (George-Weinstein et al. 1997), it is not surprising to see expression of MyoD in the primitive streak. Finding MyoD positive cells throughout the entire epiblast is consistent with the fact that cells from all regions of this tissue can form muscle in culture (George-Weinstein et al. 1996a).
Localization of MyoD mRNA in Pregastrulating Embryos
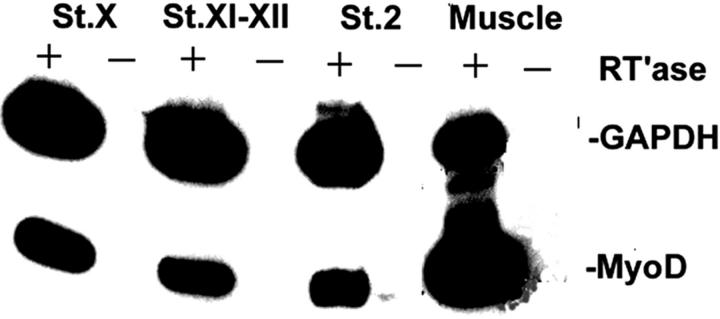
Stages X–XII embryos consist of an epiblast and an incompletely formed hypoblast (Eyal-Giladi and Kochav 1976). MyoD mRNA was detected by RT-PCR in these embryos, as well as in the stage 2 embryo (Fig. 5). Dendrimers were used to localize MyoD in single cells of whole, unsectioned stage X embryos. Approximately 20 MyoD positive cells were located in the posterior epiblast (Fig. 6 C). Most cells were intensely labeled with >10 dendrimers. The number of MyoD positive cells increased in stages XI–XII embryos, extending more laterally in the posterior epiblast (Fig. 6 F). By stage 2, fluorescence also was observed in the anterior–lateral epiblast (Fig. 6 H). The central region of the epiblast was negative. Background from myosin dendrimers (Fig. 6D and Fig. G) and dendrimers lacking a recognition sequence (not shown) was as low as in the older embryos (one to three dendrimers per section).
Figure 5.
Analysis of MyoD expression in pregastrulating embryos by RT-PCR. RNA from Eyal-Giladi and Kochav 1976 stages X and XI–XII embryos and Hamburger and Hamilton 1951 stage 2 embryos were incubated in the presence (+) or absence (−) of reverse transcriptase. Material from stage 39 pectoralis muscle was included as a positive control for MyoD. GAPDH was included as an internal control for the amount of RNA tested. Reaction products were 263 bp for MyoD and 330 bp for GAPDH. MyoD mRNA was detected in stages X–2 embryos.
Figure 6.
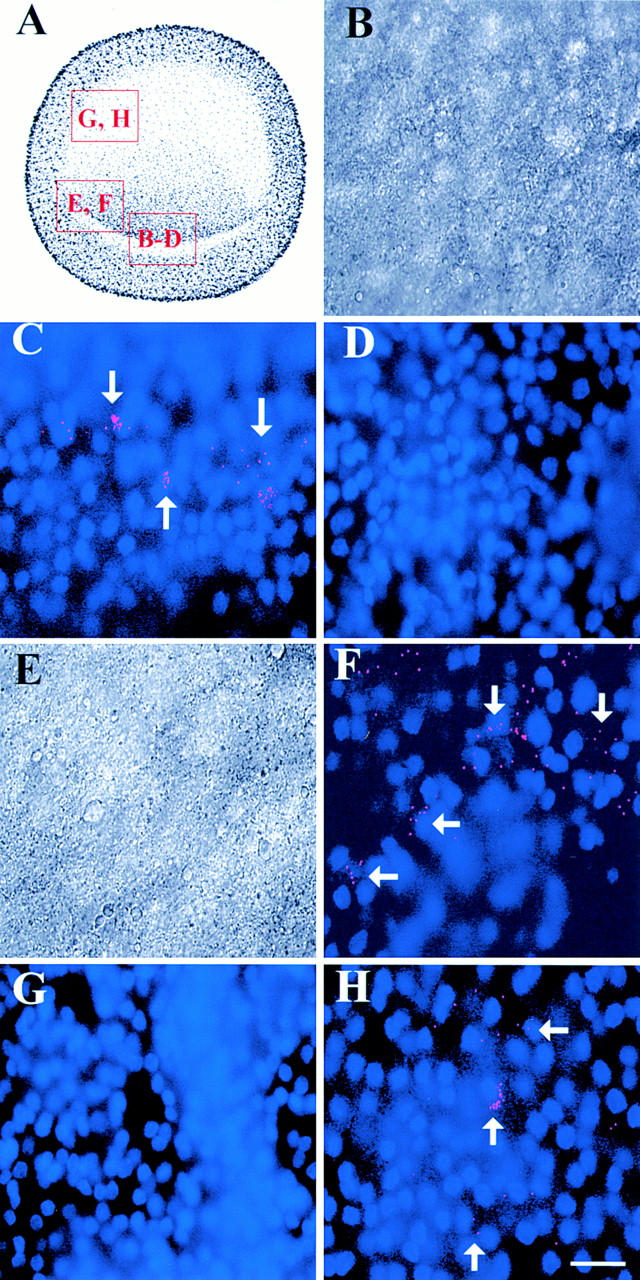
Localization of MyoD in pregastrulating embryos. The areas outlined in the drawing of the pregastrulating embryo (A) are shown in B–H. Photomicrographs in B and E are the DIC images of the merged images of bis-benzamide-labeled nuclei and Cy3-labeled dendrimers in C and F, respectively. The stage X embryo contained ∼20 MyoD positive cells in the posterior epiblast (C). MyoD fluorescence extended more laterally in the posterior epiblast in the stage XII embryo (F). By stage 2, MyoD positive cells also were present in the lateral epiblast (H). Myosin dendrimers did not label stages X (D) or XII (G) embryos. Bar, 15 μm.
Discussion
This study demonstrates, first, that dendrimers are sensitive and precise reagents for detecting low abundance mRNA in tissue sections and whole embryos, and second, that the early chick embryo contains small numbers of cells with MyoD mRNA. Intensely labeled MyoD positive cells were detected in the epiblast at the time the egg is laid. As hypoblast formation progressed, more cells expressed MyoD, although they were less intensely fluorescent than the original population of MyoD positive cells. Whether the increase in labeled cells resulted from proliferation of the original population of positive cells, or the onset of MyoD expression in other cells, remains to be determined. From this stage on, the number of weakly labeled cells exceeded that of intensely labeled ones until the dermomyotome formed in the somite, the time when MyoD or Myf5 can be detected in the somite by in situ hybridization using conventional oligonucleotide probes (Sassoon et al. 1989; Ott et al. 1991; Pownall and Emerson 1992).
We propose that the small number of intensely labeled MyoD positive cells in presomitic tissues are stably committed to the myogenic lineage, whereas the weakly fluorescent population may be programmed to follow other fates, depending on their location within the embryo. The evidence for a committed population of muscle precursors is that the number of epiblast cells from stages X–XII embryos that differentiate into muscle in culture (≤1%) is similar to the number of cells with a relatively high amount of MyoD within the embryo (George-Weinstein et al. 1996a, George-Weinstein et al. 1997; DeLuca et al. 1999). Over the next 24 h, a change occurs within the epiblast that enables >90% of cells to form muscle in culture (George-Weinstein et al. 1996a). This may reflect the increase in the weakly labeled MyoD positive cells in vivo, a release from inhibitory signals when the epiblast is isolated from the mesoderm (George-Weinstein et al. 1996a), the ability of these older epiblast cells to switch from E- to N-cadherin, and cadherin-mediated cell–cell communication in vitro (George-Weinstein et al. 1997). Interestingly, even though >95% of stage 4 epiblast cells synthesize MyoD protein in vitro and most differentiate, a few neurons, chondroblasts, and notochord cells develop among the multitude of muscle cells (George-Weinstein et al. 1996a). This suggests that small numbers of cells are stably committed to a variety of lineages at early stages of development. However, the majority of epiblast cells appear to be uncommitted because, even though most will form muscle in culture, the epiblast does give rise to all tissues of the embryo (Rosenquist 1971; Fontaine and Le Douarin 1977; Bellairs 1986; Stern and Canning 1990).
Cells with MyoD were located throughout the epiblast, mesoderm, and hypoblast. This resembles the ubiquitous expression of MyoD in the Xenopus embryo at the midblastula transition as determined by RT-PCR (Rupp and Weintraub 1991). In theory, stably committed myogenic cells that are randomly distributed throughout the epiblast would eventually become incorporated into nonmuscle tissues as well as the somites. This would explain the presence of cells with myogenic potential in the central nervous system (Fig. 2 and Fig. 3; Tajbakhsh et al. 1994), bone marrow (Wakitani et al. 1995; Ferrari et al. 1998), and dorsal aorta (De Angelis et al. 1999). Although committed to the myogenic lineage, they may remain undifferentiated in an environment that is not permissive for myogenesis.
In the embryo, committed precursors may be responsible for influencing surrounding uncommitted cells to follow the same pathway of differentiation as themselves (Gurdon 1992; Horvitz and Herskowitz 1992; Schnabel 1995). Both committed and uncommitted stem cells are present in the adult (Bjornson et al. 1999; Pittenger et al. 1999). The adult bone marrow contains myogenic cells that can be recruited to regenerate skeletal muscle in vivo (Wakitani et al. 1995; Ferrari et al. 1998). It is not known whether these cells are pluripotent, stably committed myogenic precursors, or both. Given the sensitivity and precision of fluorescently labeled dendrimers, these reagents will be useful in determining the extent of heterogeneity in stem cell populations. Once identified and isolated, stably programmed cells might be used to seed populations of pluripotent cells before implantation into diseased tissues.
Acknowledgments
We thank Drs. Joanna Capparella, James Kadushin, and Pei-Feng Cheng for advice and assistance; Drs. Karen Knudsen, Stephen Kaufman, and Scott Gilbert for critically reading the manuscript; and Dr. Camile DiLullo for providing the graphic images in 1.
This work was supported by the National Institutes of Health (HD36650-01) to M. George-Weinstein.
Footnotes
Harold Weintraub died on March 28, 1995.
Michael Baytion's current address is University of Ohio School of Medicine, Cleveland, OH 44113.
Christian Lopez's current address is Temple University School of Medicine, Philadelphia, PA 19140.
Abbreviations used in this paper: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcription PCR.
References
- Bader D., Masakki T., Fischman D.A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs R. The primitive streak. Anat. Embryol. 1986;174:1–14. doi: 10.1007/BF00318331. [DOI] [PubMed] [Google Scholar]
- Bjornson C.R., Rietz R.L., Reynolds B.A., Magli M.C., Vescovi A.L. Turning brain into blooda hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Borycki A.-G., Mendham L., Emerson C.P. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 1998;125:777–790. doi: 10.1242/dev.125.4.777. [DOI] [PubMed] [Google Scholar]
- Buckingham M., Tajbakhsh S. Myogenic cell specification during somitogenesis. In: Moody S.A., editor. Cell Lineage and Fate Determination. Academic Press; New York: 1999. pp. 617–633. [Google Scholar]
- Charles de la Brousse F., Emerson C.P. Localized expression of a myogenic regulatory gene, qmf1, in the somite dermatome of avian embryos. Genes Dev. 1990;4:567–581. doi: 10.1101/gad.4.4.567. [DOI] [PubMed] [Google Scholar]
- Chen Y.P., Solursh M. The determination of myogenic and cartilage cells in the early chick embryo and the modifying effect of retinoic acid. Roux's Arch. Dev. Biol. 1991;200:162–171. doi: 10.1007/BF00190236. [DOI] [PubMed] [Google Scholar]
- Choi J., Schultheis T., Lu M., Wachtler F., Kurac N., Franke W.W., Bader D., Fischman D.A., Holtzer H. Founder cells for the cardiac and skeletal myogenic lineages. In: Kes L.H., Stockdale F.E., editors. Cellular and Molecular Biology of Muscle Development. A.R. Liss; New York: 1989. pp. 27–36. [Google Scholar]
- Cooke J., Wong A. Growth factor-related proteins that are inducers in early amphibian development may mediate similar steps in amniote (bird) embryogenesis. Development. 1991;111:197–212. doi: 10.1242/dev.111.1.197. [DOI] [PubMed] [Google Scholar]
- Cossu G., Tajbakhsh S., Buckingham M. How is myogenesis initiated in the embryo? Trends Genetics. 1996;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- De Angelis L., Berghella L., Coletta M., Lattanzi L., Zanchi M., Cusella-De Angelis M.G., Ponzetto C., Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J. Cell Biol. 1999;147:869–877. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechesne C.A., Wei Q., Eldridge J., Gannoun-Zaki L., Millasseau P., Bougueleret L., Caterina D., Paterson B.M. E-box- and MEF-2-independent muscle-specific expression, positive autoregulation, and cross-activation of the chicken MyoD (CMD1) promoter reveal an indirect regulatory pathway. Mol. Cell. Biol. 1994;14:5474–5486. doi: 10.1128/mcb.14.8.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca S.M., Gerhart J., Cochran E., Simak E., Blitz J., Mattiacci-Paessler M., Knudsen K., George-Weinstein M. Hepatocyte growth factor/scatter factor promotes a switch from E- to N-cadherin in chick embryo epiblast cells. Exp. Cell Res. 1999;251:3–15. doi: 10.1006/excr.1999.4577. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J.A., Stone E.M., Dennison O.E., Rothblum K.N., Schwartz R.J. Cloning and sequencing of a deoxyribonucleic acid copy of a glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983;22:1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Edelman G.M., Gallin W., Delouvee A., Cunningham B.A., Thiery J.P. Early epochal maps of two different cell adhesion molecules. Proc. Natl. Acad. Sci. USA. 1983;80:4384–4388. doi: 10.1073/pnas.80.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal-Giladi H., Kochav S. From cleavage to primitive streak formationa complementary normal table and a new look at the first stages of the development of the chick. Dev. Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Paolucci E., Cossu G., Mavilio F. Muscle regneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Fontaine J., Le Douarin N. Analyses of endoderm formation into avian blastoderm by the use of quail–chick chimeras. The problem of the neural ectodermal origin of the cells of the APUD series. J. Embryol. Exp. Morphol. 1977;41:209–222. [PubMed] [Google Scholar]
- Freyer G.C., Robbins J. The analysis of a chicken myosin heavy chain cDNA clone. J. Biol. Chem. 1983;258:7149–7154. [PubMed] [Google Scholar]
- George-Weinstein M., Gerhart J., Foti G., Lash J.W. Maturation of myogenic and chondrogenic cells in the presomitic mesoderm of the chick embryo. Exp. Cell Res. 1994;211:263–274. doi: 10.1006/excr.1994.1086. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M., Gerhart J., Reed R., Flynn J., Callihan B., Mattiacci M., Miehle C., Foti G., Lash J.W., Weintraub H. Skeletal myogenesisthe preferred pathway of chick embryo epiblast cells in vitro Dev. Biol. 173 1996. 279 291a [DOI] [PubMed] [Google Scholar]
- George-Weinstein M., Gerhart J., Reed R., Steinberg A., Mattiacci M., Weintraub H., Knudsen K. Intrinsic and extrinsic regulation of the development of myogenic precursors in the chick embryo Basic Applied Myology. 6 1996. 417 430b [Google Scholar]
- George-Weinstein M., Gerhart J., Blitz J., Simak E., Knudsen K. N-cadherin promotes the commitment and differentiation of skeletal muscle precursor cells. Dev. Biol. 1997;185:14–24. doi: 10.1006/dbio.1997.8542. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M., Gerhart J., Mattiacci-Paessler M., Simak E., Blitz J., Reed R., Knudsen K. The roles of stably committed and uncommitted cells in establishing tissues of the somite. Ann. N.Y. Acad. Sci. 1998;842:16–27. doi: 10.1111/j.1749-6632.1998.tb09627.x. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B. The generation of diversity and pattern in animal development. Cell. 1992;68:185–199. doi: 10.1016/0092-8674(92)90465-o. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hatta K., Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Schultheiss T., DiLullo C., Choi J., Costa M., Lu M., Holtzer S. Autonomous expression of the differentiation programs of cells in the cardiac and skeletal myogenic lineages. Ann. N.Y. Acad. Sci. 1990;599:158–169. doi: 10.1111/j.1749-6632.1990.tb42374.x. [DOI] [PubMed] [Google Scholar]
- Horvitz H.R., Herskowitz I. Mechanisms of asymmetric cell divisiontwo Bs or not two Bs that is the question. Cell. 1992;68:237–255. doi: 10.1016/0092-8674(92)90468-r. [DOI] [PubMed] [Google Scholar]
- Kisbert A., Ortner H., Cooke J., Herrmann B.G. The chick Brachyury genedevelopmental expression pattern and response to axial induction by localized activin. Dev. Biol. 1995;168:406–415. doi: 10.1006/dbio.1995.1090. [DOI] [PubMed] [Google Scholar]
- Krenn V., Gorka P., Wachtler F., Christ B., Jacob H.J. On the origin of cells determined to form skeletal muscle in avian embryos. Anat. Embryol. 1988;179:49–54. doi: 10.1007/BF00305099. [DOI] [PubMed] [Google Scholar]
- Lin Z.Y., Dechesne C.A., Eldridge J., Paterson B.M. An avian muscle factor related to MyoD1 activates muscle-specific promotes in nonmuscle cells of different germ-layer origin and in BrdU-treated myoblasts. Genes Devel. 1989;3:986–996. doi: 10.1101/gad.3.7.986. [DOI] [PubMed] [Google Scholar]
- Mitrani E., Gruenbaum Y., Shohat H., Ziv T. Fibroblast growth factor during mesoderm induction in the early chick embryo Development. 109 1990. 387 393a [DOI] [PubMed] [Google Scholar]
- Mitrani E., Ziv T., Shimoni Y., Melton D.A., Bril A. Activin can induce the formation of axial structures and is expressed in the hypoblast of the chick Cell. 63 1990. 495 501b [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Olson E.N. Defining the regulatory networks for muscle development. Curr. Opinion Gen. Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Nilsen T.W., Grayzel J., Prensky W. Dendritic nucleic acid structures. J. Theoretical Biol. 1997;187:273–284. doi: 10.1006/jtbi.1997.0446. [DOI] [PubMed] [Google Scholar]
- Ott M.O., Bober E., Lyons G., Arnold H., Buckingham M. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 1991;111:1097–1107. doi: 10.1242/dev.111.4.1097. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pownall M.E., Emerson C.P. Sequential activation of three myogenic regulatory genes during somite morphogenesis in quail embryos. Dev. Biol. 1992;151:67–79. doi: 10.1016/0012-1606(92)90214-2. [DOI] [PubMed] [Google Scholar]
- Raap K.A., van de Rijke F.M., Dirks R.W. mRNA in situ hybridization to in vitro cultured cells. In: Choo K.H.A., editor. Methods in Molecular Biology. Vol. 33. Humana Press; Totowa, N.J: 1994. pp. 293–300. [DOI] [PubMed] [Google Scholar]
- Rosenquist G.C. The location of pregut endoderm in the chick embryo at the primitive streak stage as determined by radioautographic mapping. Dev. Biol. 1971;26:323–335. doi: 10.1016/0012-1606(71)90131-x. [DOI] [PubMed] [Google Scholar]
- Rudnicki M.A., Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. BioEssays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- Rupp R.A.W., Weintraub H. Ubiquitous MyoD transcription at the midblastula transition preceeds induction-dependent MyoD expression in presumptive mesoderm of X. laevis . Cell. 1991;65:927–937. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- Sassoon D., Rosenthal N. Detection of messenger RNA by in situ hybridization. Methods Enzymol. 1993;225:384–404. doi: 10.1016/0076-6879(93)25027-y. [DOI] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W.E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989;341:303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Schnabel R. Duels without obvious sensecounteracting inductions involved in body wall muscle development in the Caenorhabditis elegans embryo. Development. 1995;121:2219–2232. doi: 10.1242/dev.121.7.2219. [DOI] [PubMed] [Google Scholar]
- Stern C., Canning D.R. Origin of cells giving rise to mesoderm and endoderm in chick embryo. Nature. 1990;343:273–275. doi: 10.1038/343273a0. [DOI] [PubMed] [Google Scholar]
- Stern C.D., Yu R.T., Kakizuka A., Kintner C.R., Mathews L.S., Vale W.W., Evans R.M., Umesono K. Activin and its receptors during gastrulation and the later phases of mesoderm development in the chick embryo. Dev. Biol. 1995;172:192–205. doi: 10.1006/dbio.1995.0015. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Buckingham M.E. Lineage restriction of the myogenic conversion factor myf-5 in the brain. Development. 1995;121:4077–4083. doi: 10.1242/dev.121.12.4077. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Vivarelli E., Cusella-De Angelis G., Rocancourt D., Buckingham M., Cossu G. A population of myogenic cells derived from the mouse neural tube. Neuron. 1994;13:813–821. doi: 10.1016/0896-6273(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Vogelbacker H.H., Getts R.C., Tian N., Labaczewski R., Nilsen T.W. DNA dendrimersassembly and signal amplification. Proc. Am. Chem. Soc. 1997;76:458–460. [Google Scholar]
- von Kirschhofer K., Grim M., Christ B., Wachtler F. Emergence of myogenic and endothelial cell lineages in avian embryos. Dev. Biol. 1994;163:270–278. doi: 10.1006/dbio.1994.1142. [DOI] [PubMed] [Google Scholar]
- Wakitani S., Saito T., Caplan A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Wang J., Jiang M., Nilson T.W., Getts R.C. Dendritic nucleic acid probes for DNA biosensors. J. Am. Chem. Soc. 1998;120:8281–8282. [Google Scholar]
- Weintraub H. The MyoD family and myogenesisredundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T.K., Turner D., Rupp R., Hollenberg S., Zhuang Y., Lassar A. The myoD gene familynodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]