Abstract
Although immunotherapy based on the adoptive transfer of tumor-specific T lymphocytes has been shown to result in dramatic clinical responses in some patients, the relatively low levels of engraftment and persistence of the adoptively transferred cells may limit these responses in many patients. In an attempt to develop strategies for prolonging the survival of adoptively transferred T cells, we have carried out studies in which T cells obtained from healthy donors as well as tumor-specific T cells were transduced with a retrovirus expressing the human Bcl-2 gene. Our results indicate that these transduced T cells overexpress Bcl-2, are resistant to death, and have a survival advantage following interleukin-2 withdrawal compared with control T cells transduced with a retrovirus expressing green fluorescent protein. Tumor-specific T cells overexpressing Bcl-2 maintained their ability to specifically recognize and respond to target cells. Furthermore, we show that adoptive immunotherapy of an established B16 tumor can be significantly enhanced by overexpressing Bcl-2 in melanoma-specific T-cell receptor transgenic T cells. Our data suggest that adoptive immunotherapy approaches to the treatment of cancer patients may be enhanced using Bcl-2–modified tumor-reactive T cells.
Introduction
Adoptive immunotherapy can be achieved by the transfer of tumor-specific T cells in the form of tumor-infiltrating T lymphocytes (TIL) along with their growth factor interleukin-2 (IL-2; refs. 1, 2). The inefficient engraftment and persistence of adoptively transferred tumor-reactive T cells, however, seems to represent one of the factors that may limit these responses (2–4). The nearly complete regression of multiple metastatic lesions that were observed in some patients that were pretreated with a non-myeloablative chemotherapy regimen seemed to be associated with long-term persistence of adoptively transferred tumor-reactive T cells (4). The lack of sufficient levels of T-cell growth factors such as IL-2 may contribute to the death of the transferred T cells (5, 6), but the administration of high dose IL-2 is limited by the toxicity of this cytokine (1). Several alternative approaches to enhance the survival of transferred T cells are being explored (4, 7, 8).
Recent studies suggest that T-cell death resulting from the withdrawal of the cytokines IL-2 or IL-15 is accompanied by the expression of lower levels of antiapoptotic genes such as Bcl-2 (6, 9–12). Studies have also shown that cell death due to growth factor withdrawal is executed through an intrinsic death pathway that is blocked in cells that overexpress Bcl-2 (5, 9, 10, 13–15). We thus hypothesized that overexpression of an antiapoptotic protein inside T cells could promote the survival of cells suffering from a lack of stimulation by requisite growth factors.
In the current studies, we examined the effects of Bcl-2 overexpression on tumor-reactive T cells. The results showed that overexpression of Bcl-2 enhanced the survival of human tumor-reactive T cells following cytokine withdrawal. In addition, overexpression of Bcl-2 enhanced the in vivo efficacy of murine tumor-reactive T cells in a model adoptive immunotherapy system.
Materials and Methods
Cell Lines and Clones
T-cell lines and clones used in this study are listed in Table 1. TIL lines were grown in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum, L-Glu, β-mercaptoethanol, and antibiotics, all from Gibco, Invitrogen, Carlsbad, CA), containing 3,000 international units (IU) human IL-2/mL (complete medium-2; IL-2 was kindly supplied by Chiron, Emeryville, CA). Cryopreserved peripheral blood mononuclear cells were collected from healthy donor blood donation (Department of Transfusion Medicine, NIH, Bethesda, MD) by centrifugation on Ficoll-Hypaque gradients (Lymphocyte Separation Medium, Organon Teknika, Durham, NC) as per procedure of the manufacturer. Peripheral blood mononuclear cells were stimulated with 10 ng/mL CD3 (ORTHOCLONE OKT3, Ortho Biotech, Bridgewater, NJ) and 600 IU IL-2/mL (16). Three days later, cells were transduced as described below. T-cell clones were grown in complete medium in the presence of 600 IU IL-2/mL. One week before transduction T-cell clones were stimulated and expanded using a rapid expansion protocol, which was carried out by stimulating T cells with OKT3 in the presence of allogeneic feeder cells, as previously described (17).
Table 1.
Specificity, designation, and transduction efficacy of T-cell lines and clones used in this study
| T cells/TIL/Clone* | Specificity † | Restriction ‡ | Transduced with | Designated | Transduction efficacy (%)§ |
|---|---|---|---|---|---|
| PBL-T1 | NA|| | NA | MIG | PT1-G | 55 |
| MIG-Bcl | PT1-B | 40 | |||
| PBL-T2 | NA | NA | MIG | PT2-G | 22 |
| MIG-Bcl | PT2-B | 39 | |||
| TIL (1931-2F4) | MART-1 (27-35) | HLA-A2 | MIG | 1931-G | 15 |
| MIG-Bcl | 1931-B | 25 | |||
| TIL (1749) | MART-1 (27-35) | HLA-A2 | MIG | 1749-G | 24.4 |
| MIG-Bcl | 1749-B | 35 | |||
| Cl-2 1290 (CD4) | KI-67 | DR-16 | MIG | Cl-2-G | 13 |
| MIG-Bcl | Cl-2-B | 19.4 | |||
| Cl-8-1541 (CD4) | Tyrosinase-related protein-2 | DR-15 | MIG | Cl-8-G | 7.2 |
| MIG-Bcl | Cl-8-B | 14.5 | |||
| D4F12 (CD8) | GP100 (209-217) | HLA-A2 | MIG | D4-G | 20 |
| MIG-Bcl | D4-B | 25 | |||
| C22 (CD8) | Aim-1 | HLA-A2 | MIG | C22-G | 14.2 |
| MIG-Bcl | C22-B | 28.5 |
Two different peripheral blood lymphocyte-derived T-cell lines from healthy donors (PBL-T1 and PBL-T2) and two different TIL lines as well as four different tumor-specific T-cell clones (two clones are MHC-I restricted and two clones are MHC-II restricted) were used in this study.
Specificity is tested by the ability of the indicated cell line or clone to respond to target cell line that was either loaded with the indicated peptide(or controls) or transfected with the indicated antigen (or control antigens). Response was detected by measuring the IFN-γ that was secreted into the supernatant by specific ELISA.
Restriction is determined by using allele-specific transfectants and blocking by antibodies.
Transduction efficacy measured by the percentage of GFP-positive T cells 1 week after the transduction with retroviruses.
Not applicable.
Retroviral Constructs and the Isolation of High-Titer Producer Clones
Retroviral constructs based on the modified mouse stem-cell virus system MIG (18, 19) were kindly provided by Dr. Luk Van Parijs (MIT, Cambridge, MA). In the two constructs that were used in this study, recombinant gene expression was driven by the mouse stem-cell virus long terminal repeat. In the first construct, a cDNA encoding green fluorescent protein (GFP) was inserted downstream of an IRES sequence, referred to as pGFP, and in the second construct, the GFP cDNA was followed by an IRES which preceded a cDNA encoding the human Bcl-2 gene product, referred to as pBcl. High-titer vesicular stomatitis virus-G pseudotyped retroviral supernatants concentrated 50× by ultracentrifugation (20) were used to infect the PT-67 cell line (Clontech, Palo Alto, CA). High virus titer PT-67 clones with high GFP expression evaluated using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and supernatants from these clones were used to transduce the human melanoma cell line 888 (21) to provide a relative estimate of retroviral titers (22). One clone that produced the GFP expressing virus (MIG) with a titer of 2 × 108 transduction units and one that produced the GFP and Bcl-2 expressing virus (MIG-Bcl) with a titer of 4 × 108 transduction units were selected for producing viruses for T-cell transduction.
T-Cell Transduction
Two rounds of T-cell transduction were done as previously described (16). T cells were expanded by stimulating with OKT3 in the presence of feeder cells (17), and 1 to 3 weeks later the percentage of the transduced cells was estimated by fluorescence-activated cell sorting analysis based on GFP expression using a FACScan (BD, San Jose, CA). GFP+ cells were then sorted using a FACSvantage (BD) cell sorter. Sorting efficiency was high for peripheral blood lymphocyte-derived T cells and TILs, as 80% to 90% of the sorted cells remained GFP+ when tested during the course of these studies. Lower sorting efficiencies were achieved with the T-cell clones, as only 20% to 40% of the sorted cells were GFP+. Unless otherwise stated, sorted cells were used. The T cells were maintained in complete medium-2, and every 3 to 4 weeks the rapid expansion protocol was used to expand the cells.
Detection of Bcl-2 Protein
Bcl-2 was detected using intracellular staining with phycoerythrin-conjugated monoclonal antibody specific for human Bcl-2 (clone Bcl-2/100, Alexis, San Diego, CA). Cells were fixed and permeabilized using the Cytofix/Cytoperm kit from BD as per procedure of the manufacturer, and flow cytometric analysis carried out using FACScan.
Apoptosis Induction and the Estimation of T-Cell Survival
T-cell susceptibility to mitogen-induced cell death was tested by incubating cells in complete medium for 16 hours in the presence of 1 μg/mL phytohemagglutinin (PHA)-L (PHA-L, Sigma, St. Louis, MO). The susceptibility of T cells to passive cell death as a result of growth factor withdrawal was measured by culturing the cells in complete medium containing decreasing concentrations of IL-2 or in the absence of exogenously added IL-2. T-cell death was estimated by trypan blue exclusion and by staining with phycoerythrin-conjugated Annexin V (Alexis) according to the instructions of the manufacturer, and analyzed by fluorescence-activated cell sorting. All assays were done in duplicate and were repeated two to five times. Live transduced T cells represent those cells that were detected in the GFP-positive and Annexin V-negative quadrant derived from the fluorescence-activated cell sorting analysis. To calculate the number of live cells, both trypan blue-negative and -positive cells were microscopically counted, the total number of cells per well was calculated, and this number was multiplied by the percentage of cells that are both Annexin V-negative and GFP-positive as estimated from the fluorescence-activated cell sorting analysis. The ratio of live cells in cultures without exogenously added IL-2 was calculated using the following formula: Ratio = percentage of live cells among MIG-Bcl-transduced T cells/percentage of live cells among MIG-transduced T cells.
IFN-γ Production Assays
Melanoma-specific T cells (1–2.5 × 104) were cocultured with target cells (1 × 105) for 16 hours in 200 μL complete medium per well of 96-well flat-bottomed plates (Corning, Inc., Acton, MA). Target cells used in this study were either melanoma cell lines or EBV-transformed B-cell lines (EBV-B) that were unpulsed or pulsed with 1 0μg/mL MHC class-I- or 50 μg/mL MHC-class-II-binding peptide. An ELISA specific for human IFN-γ (R&D Systems, Minneapolis, MN) was done according to the protocol of the manufacturer. Intracellular cytokine detection was done as earlier described (23). All assays were done in duplicate and were repeated two to four times.
Mice, Tumor Challenge, and Immunotherapy
C57BL/6 Bcl-2 transgenic mice (The Jackson Laboratory, Bar Harbor, ME) were intercrossed with Pmel mice (24) that express a transgenic T-cell receptor that recognizes gp100+ cells in H-2Db restricted manner, resulting in mice with double transgenic T cells (Pmel-Bcl-2). Similarly, GFP transgenic mice (The Jackson Laboratory) were intercrossed with Pmel transgenic mice resulting in the double transgenic T cells (Pmel-GFP) which served as a control for Pmel-Bcl-2 cells. B16 melanoma cell line was obtained from the National Cancer Institute tumor repository. The mouse adoptive immunotherapy model system has previously been described (24, 25). Briefly, mice with 14 days s.c. B16 tumor were untreated or treated with one or all of the following treatments: 500 cGy radiation, 1 × 106 of 1-week cultured T cells, vaccinated with 2 × 107 plaque-forming units of recombinant fowlpox virus encoding human gp100, or injected with 600,000 IU IL-2. The number of animals used in each group is five. This experiment has been repeated independently two additional times with similar results. All experiments were done with randomization of the mice and were done in a “blinded” fashion (i.e., the person taking the measurement had no knowledge of the group that the mouse was derived from).
Results
T-Cell Transduction by MIG and MIG-Bcl
Healthy donors’ peripheral blood lymphocyte-derived T cells, TILs, and CD4+ or CD8+ tumor-specific T cells were all efficiently transduced with MIG or with MIG-Bcl retroviruses regardless of their origin (Table 1). The average transduction efficiency achieved using the MIG virus was 21.4 ± 13.7, whereas for the MIG-Bcl retrovirus it was 28.3 ± 8.6 (Table 1).
Increased Levels of Bcl-2 Generated by T-Cell Transduction
The relative levels of Bcl-2 expression in the transduced T-cell populations were then examined. T cells that were transduced with the MIG-Bcl retrovirus (Fig. 1C and D) overexpressed Bcl-2 protein when compared with T cells transduced with the MIG retrovirus (Fig. 1A and B). The levels of Bcl-2 protein generated as a result of MIG-Bcl transduction were 1.5- to 2.7-fold higher than those found in control GFP-transduced or nontransduced T cells (Fig. 1B and D and data not shown). Significantly, the expression of GFP seemed to be correlated with the expression of Bcl-2 in the MIG-Bcl-transduced population (Fig. 1D).
Figure 1.

Transduction of Bcl-2 results in protein overexpression and resistance to mitogen-induced cell death. PT1-G (A and B) and PT1-B (C and D) were stained with a phycoerythrin-conjugated anti–human Bcl-2 antibody (B and D) or with an isotype- and flurochrome-matched antibody (A and C). Geometric mean of FL-2 fluorescence [phycoerythrin (PE)] in the upper right quadrant is indicated. PT2-G (E–G) and PT2-B cells (H–J) were cultured in complete medium in the presence of 600 IU IL-2/mL (E and H) or in the absence of exogenously added IL-2 (F and I) or in the absence of IL-2 and the presence of 1 μg/mL PHA (G and J). The percentage of viable transduced GFP+ T cells, which are Annexin V-negative, detected after 16 hours of culture, is indicated in the lower right quadrant.
Bcl-2 Overexpression Protects T Cells from Cell Death Induced by Mitogen or Cytokine Withdrawal
In order to examine the effects of overexpression of Bcl-2 on T-cell survival, control MIG- or MIG-Bcl-transduced T cells derived from healthy donor peripheral blood lymphocytes (PT2-G and PT2-B, respectively) were cultured in medium containing PHA for 16 hours in the absence of IL-2 and compared with T cells maintained in medium with or without IL-2. The PT2-G and PT2-B cells that were maintained in the presence or absence of IL-2 but were not treated with PHA remained highly viable 16 hours following culture initiation (Fig. 1E, F, H, and I). Treatment of T cells with PHA resulted in substantial cell death, as only 27% of the treated PT2-G cells were viable at this time point (Fig. 1G), but Bcl-2 overexpression seemed to partially block apoptosis, as 68% of the PHA-treated PT2-B cells remained viable (Fig. 1J). Similar data were obtained with phorbol 12-myristate 13-acetate after 48 hours of culture under similar conditions (data not shown).
The effects of Bcl-2 overexpression on the survival of unstimulated T cells cultured in the absence of exogenous cytokines were then examined. In the presence of 300 or 600 IU/mL IL-2, 60% to 75% of peripheral blood lymphocytes that were transduced with either the retroviral construct encoding GFP or the retroviral construct encoding Bcl-2 remained viable throughout the follow-up period with no significant differences in the percentage of live cells (Fig. 2A and B and data not shown). Culturing the MIG-transduced T cells in lower IL-2 concentrations (6-60 IU/mL IL-2) resulted in an evident decrease of viability in PT2-G, 1931-G TIL, and Cl-8-G (Fig. 2A–C and data not shown). The viability of MIG-Bcl-transduced cells was not affected by the reduction in IL-2 concentrations with the exception of the 6 IU/mL concentration (Fig. 2A–C and data not shown). In the absence of exogenously added IL-2 the viability of the MIG-transduced T cells decreased continuously down to near zero within 1 to 2 weeks (Fig. 2A–E). Under the same conditions, MIG-Bcl-transduced cells exhibited resistance to passive cell death and up to 65% of these cells remained viable (Fig. 2A–E). Following 4 weeks of culture in complete medium without the addition of exogenous IL-2, between 10% and 20% of the MIG-Bcl-transduced T cells remained viable, whereas essentially no viable cells were detected in the GFP transduced or nontransduced populations (data not shown). Among the different T-cell populations that were tested, CD4+ T-cell clones specific for melanoma antigens were most susceptible to passive cell death (Fig. 2D and E and data not shown). Calculated from data presented in Fig. 2, in the absence of exogenously added IL-2, the ratio of live cells in MIG-Bcl-transduced T cells to live cells in MIG-transduced T cells was highest in CD4 clones (ratio of 5 on day 3 and ≥40 on day 14) followed by TIL (ratio of 12 on day 14) and the least in peripheral blood lymphocyte-derived T cells (ratio of 7.5 on day 7 and 6.4 on day 14).
Figure 2.
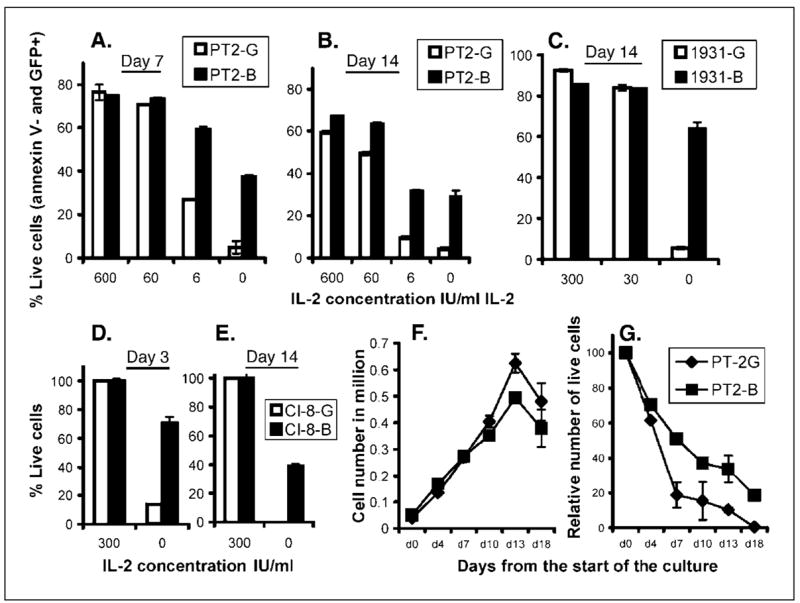
Resistance of Bcl-2-transduced T cells to passive cell death. PT2-G and PT2-B cells (A and B), 1931-G and 1931-B (C), and Cl-8-G and Cl-8-B (D and E) were cultured in complete medium in the presence of 0 to 600 IU/mL exogenously added IL-2 as indicated by the abscissa. Viability was then analyzed after 3 (D), 7 (A), or 14 (B, C, and E) days by Annexin V-phycoerythrin staining and fluorescence-activated cell sorting analysis. Growth (F) and survival (G) curves of PT2-G and PT2-B cells cultured in complete medium in the absence (G) or presence of 300 IU IL-2/mL (F).
In most clinical settings transduced T cells would represent a fraction of the total T-cell population. Thus, we next compared the ability of MIG- and MIG-Bcl-transduced cells to survive in the presence or absence of exogenously added growth factors when MIG-Bcl-transduced T cells represented ~10% of the total T cells. Identified on the basis of GFP expression, the number of live-transduced cells was calculated as described in Materials and Methods. PBL-T2 cells that were transduced with 9% to 10% efficiency were used for these experiments without any prior sorting. In control cultures containing 300 IU/mL of IL-2, the number of PT2-G and PT2-B increased ~10-fold over a period of 2 weeks (Fig. 2F). In the absence of exogenously added IL-2, the number of transduced PT2-G cells declined rapidly within 1 week and essentially no viable cells were present 18 days following IL-2 withdrawal (Fig. 2G). In contrast, ~50% of transduced PT2-B cells were viable 1 week following IL-2 withdrawal, and on day 18 about 20% of these cells survived in the absence of any exogenously added IL-2 (Fig. 2G). Similar data were obtained with TILs and melanoma-specific CD4+ and CD8+ T-cell clones (data not shown).
Correlation between Bcl-2 Expression Level and Protection from Passive Cell Death
Based on the level of GFP expression, which seemed to correlate with the level of BCL-2 expression (Fig. 1D), MIG-Bcl-transduced T cells cultured in the presence of IL-2 could be divided into two approximately equal populations: GFPdim and GFPbright (Fig. 3A and D). When gated on live Annexin V-negative cells, there was a major loss of live cells in the GFPdim population when the amount of exogenously added IL-2 was decreased or eliminated in the culture (Fig. 3A–F). In the absence of exogenously added IL-2, the GFPbright population represented the majority of live T cells remaining in the culture for up to 4 weeks (Fig. 3C and F).
Figure 3.

Correlation of T-cell survival with Bcl-2 expression levels. PT2-B (A–C) and 1749-B (D–F) cells cultured in complete medium in the presence of either 300 or 30 IU/mL of IL-2 or in the absence of exogenous IL-2. Cell viability was estimated as described in Materials and Methods on the indicated days. Numbers above the histograms indicate the percentage of the relative cell number in each marker area.
Bcl-2 Overexpression Does Not Impact on the Function or Specificity of Melanoma-Specific T Cells
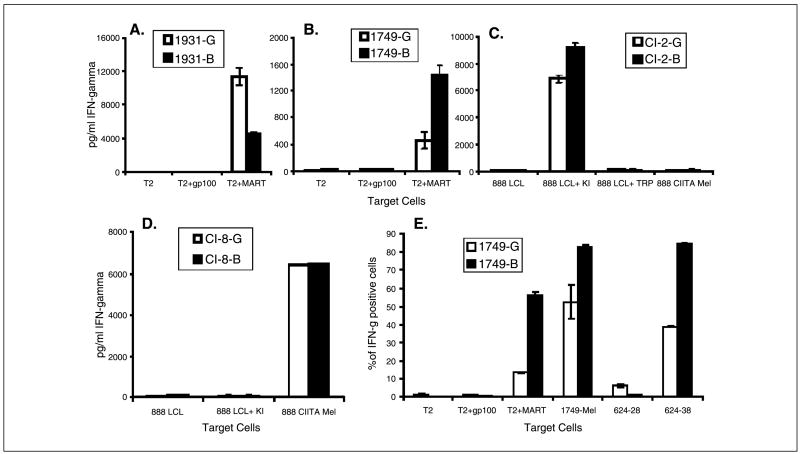
Two predominantly CD8+ TILs that recognized the immunodominant MART-1:27-35 peptide were cocultured with T2 target cells pulsed either with the MART-1 or control gp100:209-217 peptide. Alternatively these TILs and melanoma-specific T-cell clones were cultured with autologous or allogeneic melanoma cell lines. Significant levels of IFN-γ were detected in supernatants from either MIG-or MIG-Bcl-transduced TILs when cultured with the MART-1:27-35 peptide, whereas no significant levels of IFN-γ were detected in supernatants from the control cultures (Fig. 4A and B). The 1931-G TIL line produced more IFN-γ in response to MART-1:27-35 peptide-pulsed T2 stimulation than did the 1931-B TIL line (Fig. 4A). In contrast, the 1749-B TIL line produced more IFN-γ in response to T2 cells pulsed with the MART-1:27-35 peptide than the 1749-G TIL line (Fig. 4B). Similarly, the 1749-B line produced more IFN-γ in response to autologous 1749-mel stimulation than the 1749-G cell line (data not shown). As this response only reflects an MHC class I restricted immune response, the impact of Bcl-2 overexpression was studied in the MHC class II restricted T-cell clones Cl-2 1290 (Cl-2) and Cl-8-1541 (Cl-8). The Cl-2 cells recognize an exogenously (Fig. 4C) but not endogenously presented mutated (L for S at position 3,202) epitope from the Ki-67 antigen corresponding to amino acids 3,197 to 3,213 (SMCLRLRKTKSQPAAST) in the context of HLA-DR16.1 A slight but insignificant increase in IFN-γ production by Cl-2B as compared with Cl-2G T-cell response could be detected (Fig. 4C). The antigen reactivity of a second CD4+ T cell that recognizes an epitope of tyrosinase-related protein-2 (21) was also examined following transduction with MIG and MIG-Bcl. Both Cl-8-G and Cl-8-B cells have the same specificity and produced similar amounts of IFN-γ in response to the autologous 888 mel cells, which were transduced with CIITA to up-regulate HLA class II expression, but did not respond to HLA-DR-matched but tyrosinase-related protein-2 nonexpressing 888 EBV-B cell line unpulsed or pulsed with the control KI epitope (Fig. 4D).
Figure 4.
Bcl-2-overexpressing T cells maintained their functional activity and specificity. IFN-γ release ELISA of 1931-G and 1931-B (A), 1749-G and 1749-B (B), Cl-2-G and Cl-2-B (C), or Cl-8-G and Cl-8-B (D) effector cells. Abscissa, targets were either unpulsed or pulsed with control or relevant peptide. E, quantitative measurement of the effector response of Bcl-2-overexpressing tumor-specific T cells. Percentage of 1749-G and 1749-B cells that recognize the indicated target cells was estimated by carrying out fluorescent cell analysis of permeabilized cells with an anti-IFN-γ antibody.
To further quantify the response of MIG- and MIG-Bcl-transduced T cells, we have monitored the IFN-γ immune response to target cell stimulation using an intracellular cytokine detection assay. The results from this assay correlated with the ELISA results (Fig. 4B and E and data not shown). The 1749-B and 1749-G TIL lines produced IFN-γ in response to MART-1:27-35 peptide-pulsed T2 cell stimulation but did not respond to T2 cells that were unpulsed or were pulsed with a control peptide (Fig. 4E). The 1749-B and 1749-G TIL lines also produced IFN-γ in response to autologous melanoma stimulation and to stimulation by an HLA-A2-matched melanoma 624.38 but not to HLA-A2-loss variant 624.28, both of which were derived from the 624 melanoma (Fig. 4E). The results obtained using the intracellular cytokine detection assay were similar to those obtained when cytokine release assays were used to compare the response of 1931-G with 1931-B or that of Cl-8-G with Cl-8-B cells (data not show).
Improved Immunotherapy Achieved through Overexpression of Bcl-2 in Melanoma-Specific T Cells
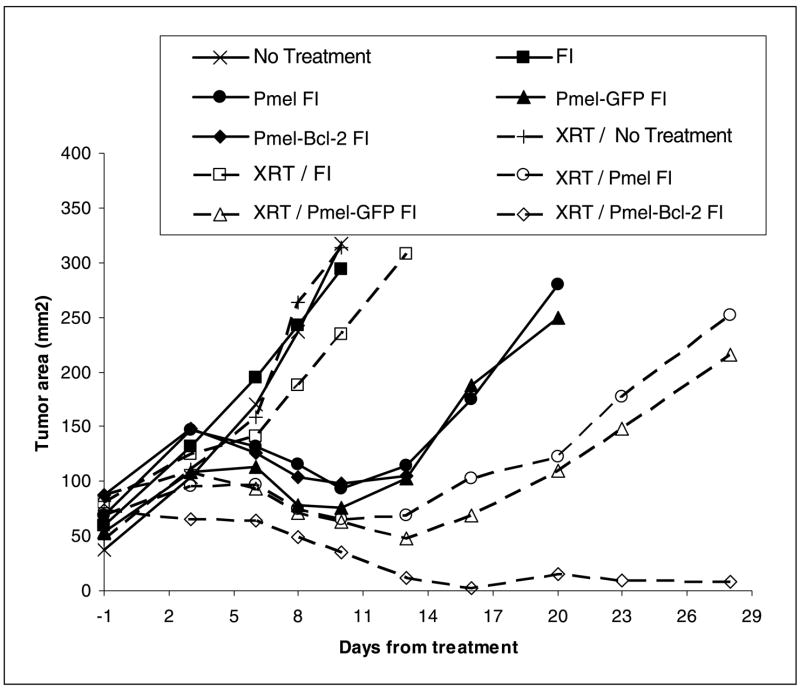
The effects of increased Bcl-2 expression on in vivo immune responses were then examined in a mouse adoptive immunotherapy model employing Pmel cells by comparing Pmel-Bcl-2 with Pmel-GFP or Pmel cells in their ability to treat established tumors (Fig. 5). Mice carrying 14-day established s.c. B16 melanoma received no treatment or any of the following treatments alone or in combination: lymphodepleted by irradiation (X-ray therapy), received adoptive transfer of single or double transgenic T cells from Pmel, Pmel-Bcl-2, or Pmel-GFP mice, vaccinated with fowlpox (F), or injected with IL-2 (I). Among these various treatment protocols, the most efficient one was that in which Pmel-Bcl-2 double transgenic T cells were transferred into a lymphodepleted recipients followed by vaccination and IL-2 administration, which resulted in nearly complete regression 2 weeks following transfer. Similar treatment with adoptively transferred Pmel-GFP double transgenic T cells resulted in a less durable therapeutic effect that was similar to that obtained using Pmel cell adoptive transfer. Other treatment regimens resulted in even less effective therapy. When tumor graphs were compared using the Wilcoxon rank sum test, results indicating that Bcl-2–overexpressing Pmel T-cell receptor transgenic cells were superior to Pmel cells that did not overexpress the Bcl-2 transgene were significant at P <0.05 in the experiment shown as well as in an independent repeat. These data clearly show the positive impact that Bcl-2 has on the therapeutic efficacy of tumor-specific T cells.
Figure 5.
Overexpression of Bcl-2 enhances the therapeutic efficacy of adoptively transferred tumor-reactive T cells. C57BL/6 mice were inoculated s.c. with B16 melanoma and 14 days later received varying combinations of the following treatments: Pmel, Pmel-GFP, or Pmel-Bcl-2 T-cell transfer, recombinant gp-100 fowlpox vaccination (F), IL-2 administration (I), or sublethal irradiation [X-ray therapy (XRT)]. Dashed lines, groups that received 500 cGy of total body irradiation. Caliper measurements were used to determine tumor outgrowth. Representative of two independent experiments.
Taken together, these results indicated that the enforced overexpression of Bcl-2 in tumor-reactive T-cell lines as well as T-cell clones increased their resistance to mitogen-induced as well as passive cell death without altering their functional specificity. In addition, the results of the studies in the mouse model system indicate that Bcl-2-overexpressing tumor-reactive T cells are more effective at mediating tumor regression than control counterpart T cells, and suggest that overexpression of Bcl-2 in human tumor-reactive T cells may lead to improvements in the therapeutic efficacy of these cells.
Discussion
Multiple studies have shown that T cells derived from healthy donors can be retrovirally transduced with relatively high efficiency (26). Only limited data, however, is available on the ability of retroviruses to transduce either TIL or T-cell clones (27–29). In this study we showed that six different tumor-specific TIL and clones can be efficiently transduced with mono- and bi-cistronic retroviral constructs, which may allow the utilization of these transduced cells in a clinical setting without a prior selection.
Lymphocyte survival is controlled in part by the outcome of competing pro- and antiapoptotic members of the Bcl-2 families and other apoptosis-related proteins (9, 10, 15). Moderate levels of Bcl-2 expression in T cells could be detected throughout the different stages of T-cell development with the highest level detected in CD4 and CD8 single positive subpopulations (30, 31). Memory type CD8+ T cells express higher levels of Bcl-2 than naïve cells, suggesting an essential role for Bcl-2 expression in maintaining the survival of T cells (11, 12, 32). Our data suggest that increased levels of Bcl-2 generated by retroviral transduction of activated human T cells can lead to enhanced T-cell survival. This result was uniformly seen in different types of T cells but was more evident in CD4+ T cells. This is in agreement with the recently available data analyzing responses in both humans and mice, indicating that CD8+ T cells that have expanded following antigen stimulation seem to be maintained for longer periods of time than activated CD4+ T cells (6, 11, 12, 32). In one of these studies, antigen-specific CD4+ memory cells seemed to express significantly lower levels of Bcl-2 than antigen-specific CD8+ T cells (32).
Interestingly, the survival time of control MIG-transduced T cells in vitro in the absence of exogenously added IL-2 seemed to reflect their survival in vivo following adoptive transfer, as recently reported. When cloned CD8+ T cells were infused in patients, significant levels of cells were not detected in the peripheral blood of recipients 1 week from the infusion date (3, 33), which is the date by which most MIG-transduced cells died in vitro unless Bcl-2 overexpression occurred as a result of transduction or supplementation with IL-2.
Results presented in a recent study suggest that Bcl-XL transduction of human peripheral blood lymphocytes could not protect T cells from death due to IL-2 withdrawal but provided a survival advantage in conditions where T cells were triggered by adding an agonistic anti–T-cell receptor antibody (34). Our studies suggest that Bcl-2 may be more efficient than Bcl-XL in protecting T cells from apoptotic death in spite of the highly homologous functions of these two proteins. Alternatively, the difference could be related to the level of expression of either of these two antiapoptotic proteins achieved in the two different studies.
The ability of transduced T-cell lines and clones to maintain antigenic specificity represents a critical aspect of any T-cell modification protocol. Anti- and proapoptosis genes are often involved in other cellular functions such as activation and proliferation (35). Hence, the ability of Bcl-2-transduced T cells to maintain their functional affinity and fine specificity indicates that the suitability of these cells for adoptive immunotherapy has not been impaired. Differences were observed in the levels of IFN-γ produced by the MIG- and MIG-Bcl-transduced TILs in response to antigenic stimulation; these differences, however, may have been due to changes in growth rates of different T-cell clones comprising these lines. This is supported by the data obtained from MIG- or MIG-Bcl-transduced CD4 and CD8 clones (Fig. 4C and D; J. Charo, preliminary data).
The various protocols used for immunotherapy in our study, apart from those involving double transgenic T cells, have been discussed thoroughly earlier (24, 25, 36). The ability of Bcl-2 transgene overexpression to significantly enhance the efficacy of the adoptive immunotherapy of an established tumor is likely due to its pro-survival properties, which is supported by our in vitro data. This effect of Bcl-2 could has been manifested through various outcomes including allowing the effector T cells to exert their function and outliving tumor cells, resisting the unaccommodating tumor milieu, and the ability of these T cells to act independently of additional help that might be otherwise provided by costimulation or other immune activation mechanisms. Less evident factors might contribute to this effect of Bcl-2 such as the ability of these cells to resist suppression by regulatory T cells or immunosuppressive cytokines.
The development of leukemias in a recent clinical trial involving retrovirally transduced CD34+ cells highlights one of the risks posed by this cell modification approach (37). The introduction of a gene such a sBcl-2 that has been associated with the development of lymphoma indicates that this might in fact represent a risk. Nevertheless, since the advent of gene therapy trials in humans 15 years ago, no such complications have been observed when using mature T cells (29). This is corroborated by the data on the development of T-cell lymphomas, which occur readily early during hematopoiesis (38, 39), suggesting that T-cell modification by retrovirus transduction followed by the adoptive transfer of these cells is unlikely to lead to the generation of additional cancers. In addition, our preliminary results indicate that Bcl-2 transduction does not lead to T-cell immortalization. Whereas Bcl-2 has been implicated in the development of B-cell leukemias, it does not, however, seem to play a significant role in the development of T-cell leukemias (38, 39). The overexpression of Bcl-2 as a transgene, even in the early stages of T-cell development where leukemogenesis is more likely to occur, does not cause the accumulation of pro-T cells and no T-cell leukemia was reported to arise in Bcl-2 transgenic mice (39–41). Some reports even indicate that overexpression of Bcl-2 as a transgene may actually reduce the incidence of T-cell lympho-magenesis (42).
Alternative methods for decreasing apoptosis of T cells may be possible, such as the use of small inhibitory RNAs to target proapoptotic gene members of the Bcl-2 family in T cells (43, 44). The data on the phenotype of bim knockout mice make bim an attractive candidate for these studies (45). The unique features of the pol III promoters used in small inhibitory RNA-encoding vectors may also help to prevent insertional mutagenesis, as these promoters do not transcribe downstream of their termination signal (46).
In conclusion, our data indicate that transduction of T cells with Bcl-2 can enhance their resistance to mitogen-induced apoptosis, as well as apoptosis induced by cytokine withdrawal, without significantly altering their specificity or function. These observations also suggest that adoptive immunotherapy for the treatment of patients with cancer may be improved by carrying out treatments with T cells that have been genetically modified to overexpress Bcl-2.
Acknowledgments
We thank Dr. John R. Wunderlich and the TIL laboratory; Dr. Mark E. Dudley for providing the T cells and melanoma cell lines used for these studies; Arnold Mixon and Shawn Farid for providing assistance with fluorescence-activated cell sorting analysis; and Mona El-Gamil, Linda L. Parker, Jennifer A. Westwood, and Yong F. Li for providing technical assistance.
Footnotes
Note: J. Charo is currently in the Max-Delbruck-Center for Molecular Medicine, Robert-Rossle-Strasse 10, 13092, Box 26, Berlin, Germany. Phone: 49-30-94062687; Fax: 49-30-94062453; E-mail: j.charo@mdc-berlin.de.
P.F. Robbins, unpublished data.
References
- 1.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–84. doi: 10.1097/00000658-198910000-00008. discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plas DR, Rathmell JC, Thompson CB. Homeostatic control of lymphocyte survival: potential origins and implications. Nat Immunol. 2002;3:515–21. doi: 10.1038/ni0602-515. [DOI] [PubMed] [Google Scholar]
- 6.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–60. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 7.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167:6356–65. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: Engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–7. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 9.Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–5. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 10.Pinkoski MJ, Green DR. Lymphocyte apoptosis: refining the paths to perdition. Curr Opin Hematol. 2002;9:43–9. doi: 10.1097/00062752-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Whitmire JK, Ahmed R. The economy of T-cell memory: CD4+ recession in times of CD8+ stability? Nat Med. 2001;7:892–3. doi: 10.1038/90923. [DOI] [PubMed] [Google Scholar]
- 12.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–4. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 13.Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common γ chain-deficient mice. Immunity. 1997;7:155–62. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima H, Leonard WJ. Role of Bcl-2 in αβT cell development in mice deficient in the common cytokine receptor γ-chain: the requirement for Bcl-2 differs depending on the TCR/MHC affinity. J Immunol. 1999;162:782–90. [PubMed] [Google Scholar]
- 15.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 16.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–13. [PubMed] [Google Scholar]
- 17.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 18.Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–8. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 19.Lois C, Refaeli Y, Qin XF, Van Parijs L. Retroviruses as tools to study the immune system. Curr Opin Immunol. 2001;13:496–504. doi: 10.1016/s0952-7915(00)00247-8. [DOI] [PubMed] [Google Scholar]
- 20.Yee JK, Friedmann T, Burns JC. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43(Pt A):99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- 21.Robbins PF, El-Gamil M, Li YF, Zeng G, Dudley M, Rosenberg SA. Multiple HLA class II-restricted melanocyte differentiation antigens are recognized by tumor-infiltrating lymphocytes from a patient with melanoma. J Immunol. 2002;169:6036–47. doi: 10.4049/jimmunol.169.10.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mautino MR, Morgan RA. Inhibition of HIV-1 replication by novel lentiviral vectors expressing trans-dominant Rev and HIV-1 env antisense. Gene Ther. 2002;9:421–31. doi: 10.1038/sj.gt.3301674. [DOI] [PubMed] [Google Scholar]
- 23.Fonteneau JF, Larsson M, Somersan S, et al. Generation of high quantities of viral and tumor specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. J Immunol Methods. 2001;258:111–26. doi: 10.1016/s0022-1759(01)00477-x. [DOI] [PubMed] [Google Scholar]
- 24.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–74. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 27.Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–95. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan RA, Cornetta K, Anderson WF. Applications of the polymerase chain reaction in retroviral-mediated gene transfer and the analysis of gene-marked human TIL cells. Hum Gene Ther. 1990;1:135–49. doi: 10.1089/hum.1990.1.2-135. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–8. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 30.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–54. [PubMed] [Google Scholar]
- 31.Gratiot-Deans J, Ding L, Turka LA, Nunez G. bcl-2 proto-oncogene expression during human T cell development. Evidence for biphasic regulation. J Immunol. 1993;151:83–91. [PubMed] [Google Scholar]
- 32.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–9. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 33.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eaton D, Gilham DE, O’Neill A, Hawkins RE. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9:527–35. doi: 10.1038/sj.gt.3301685. [DOI] [PubMed] [Google Scholar]
- 35.Los M, Stroh C, Janicke RU, Engels IH, Schulze-Osthoff K. Caspases: more than just killers? Trends Immunol. 2001;22:31–4. doi: 10.1016/s1471-4906(00)01814-7. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 38.Kitada S, Pedersen IM, Schimmer AD, Reed JC. Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene. 2002;21:3459–74. doi: 10.1038/sj.onc.1205327. [DOI] [PubMed] [Google Scholar]
- 39.Baliga BC, Kumar S. Role of Bcl-2 family of proteins in malignancy. Hematol Oncol. 2002;20:63–74. doi: 10.1002/hon.685. [DOI] [PubMed] [Google Scholar]
- 40.Katsumata M, Siegel RM, Louie DC, et al. Differential effects of Bcl-2 on T and B cells in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:11376–80. doi: 10.1073/pnas.89.23.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strasser A, Harris AW, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci U S A. 1994;91:1376–80. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acton D, Jacobs H, Domen J, Berns A. Bcl-2 reduces lymphomagenesis in δV-TCRβ transgenic mice. Oncogene. 1997;14:2497–501. doi: 10.1038/sj.onc.1201089. [DOI] [PubMed] [Google Scholar]
- 43.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–47. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 44.Choudhury A, Charo J, Parapuram SK, et al. Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, up-regulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer. 2004;108:71–7. doi: 10.1002/ijc.11497. [DOI] [PubMed] [Google Scholar]
- 45.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 46.Tuschl T. Expanding small RNA interference. Nat Biotechnol. 2002;20:446–8. doi: 10.1038/nbt0502-446. [DOI] [PubMed] [Google Scholar]