Abstract
Purpose
Although previous neuroimaging efforts clearly indicate visual cortical dysfunction in adults with amblyopia, the extent of abnormalities remains unclear.
Methods
This fMRI study directly compared activity in visual cortex produced by monocular stimulation in 18 adults (6 esotropic strabismics, 6 anisometropes, and 6 controls). Measures were made in three cortical regions of interest, individually defined using standard retinotopic mapping techniques in the nonamblyopic eye, corresponding to extrafoveal V1, extrafoveal V2, and the foveal representation at the occipital pole. Fixation stability was monitored and found not to differ significantly between subject groups.
Results
Overall results showed depressed fMRI signal magnitude for amblyopic eyes compared with sound eyes, although a few subjects did not show this trend. Assessment of the spatial extent of activation using an ocular dominance index did show significantly larger interocular differences for both strabismics and anisometropes compared with control subjects for whom eye dominance was carefully defined. In addition, both amblyopic groups showed less cortical area able to be significantly driven by either eye. The magnitude of these effects was equivalent in V1, V2, and the foveal representation, as well as between amblyopic groups. No difference was detected in the strength of signal from the nasal versus temporal retina in either amblyopic group.
Conclusions
Asymmetries in magnitude of monocular activation do occur in subjects with amblyopia, but these basic measures are limited in terms of sensitivity for mild to moderate amblyopia and for specificity between subtypes.
Amblyopia is defined as visual impairment without ocular lesion, resulting from abnormal neural development.1 The two most prevalent etiologies result in anisometropic and strabismic amblyopia.2 The interruption of normal visual experience during critical developmental periods leads to alterations in subsequent organization and function of visual cortex.3 Psychophysical investigations of human amblyopia have found impaired visual acuity and contrast sensitivity in amblyopic eyes,4–6 particularly for the central visual field.7,8 Furthermore, asymmetric visual field deficits have been reported in strabismics with inward eye deviation; nasal retina input is impaired more than temporal retina.9,10 This asymmetry could possibly result from biased competition between the nasal retina of the deviated eye and the fovea of the sound eye, leading to suppression of amblyopic eye input.9,11
The amblyopic deficit is thought to include primary visual cortex (V1), where monocular inputs are first combined.12–14 Animal studies using models of amblyopia15,16 show shifts in ocular dominance, some binocularity losses, and impaired cortical acuity and contrast sensitivity for the affected eye.17,18 Nevertheless, these V1 losses do not fully account for behavioral deficit, thereby implicating visual areas beyond V1.19,20 The neuroimaging techniques of positron-emission tomography (PET) and functional magnetic resonance imaging (fMRI) have recently expanded the study of human amblyopia. These studies have shown reduced cerebral blood flow and glucose metabolism in V1 and extrastriate cortex for amblyopic eye viewing.21–27 Nevertheless, these studies have not yielded a consistent answer regarding the spatial extent of the effect, and the relative deficit in V1 versus V2 remains unclear. Most studies have not localized deficits to specific visual areas or field locations, with some exceptions.26,28 Finally, very few studies have specifically compared amblyopic subtypes with the same paradigm. Previous work has found less activation at high spatial frequencies for anisometropes,29 while more impaired binocular responses were reported for strabismics.30
The present fMRI study was conducted to specifically compare visual areas V1 and V2. We also quantitatively reexamined previously reported abnormalities by comparing strabismic and anisometropic amblyopes with matched control subjects, and carefully defined eye dominance in control subjects. We included important controls such as eye tracking, and we used standard psychophysical tests to confirm diagnoses. We used monocular hemifield mapping to examine interocular and between-group metabolic signal differences. We hypothesized that (1) amblyopic eyes would produce reduced signal magnitude compared to sound eyes and normal eyes, especially in foveal representations; (2) ocular dominance would shift toward the sound eye, and binocularity would be reduced in amblyopes, in all cortical regions; (3) nasal retina stimulation would produce weaker fMRI signal than temporal retina in strabismic amblyopes, especially in extrafoveal regions. We obtained results that supported hypotheses 1 and 2, but these effects were variable, only of moderate strength, and did not distinguish strabismic and anisometropic subtypes. Hypothesis 3 was not supported by our data.
Materials and Methods
Subjects
We studied 18 adult volunteers aged 18 to 35 years (13 female, 5 male). Six were control subjects (CTL), 6 had previously been diagnosed with strabismic amblyopia (STRAB), and 6 had previously been diagnosed with anisometropic amblyopia (ANISO). Any subject with other neurological conditions was excluded. These subjects were recruited through public advertisement in the surrounding regions of West Virginia, Pennsylvania, and Maryland. Informed consent was obtained from all subjects (IRB protocol #14788), in accordance with the Declaration of Helsinki and HIPAA. Subject groups were matched for mean age (CTL = 25, STRAB = 27, ANISO = 28) and mean years of education (CTL = 15, STRAB = 16, ANISO = 15). All amblyopic subjects had a history of patch occlusion treatment during childhood (Table 1). Of the 6 strabismic subjects, 3 also reported surgical correction of their deviation in childhood.
Table 1.
Subject Characteristics
| CTL (N=6) | Age at Diagnosis (years) | Patch Duration (months) | Age at Surgery (years) | Current Deviation (prism diopters) | WE | WE Dist VA (logMAR) | BE Dist VA (logMAR) | IRD (diopters) | Stereo-acuity (arcsec) |
|---|---|---|---|---|---|---|---|---|---|
 C1 C1
 C2 C2
 C3 C3
 C4 C4
 C5 C5
 C6 C6 |
-
- - - - - |
-
- - - - - |
-
- - - - - |
-
- - - - - |
OS
OD OS OD OS OD |
0.10
0.00 0.00 0.00 0.00 0.20 |
0.10
−0.10 0.00 0.00 −0.10 0.10 |
0
0.25 0.25 0.25 0.25 0 |
40
40 40 40 80 40 |
| Mean | - | - | - | - | - | 0.05 | 0.00 | - | - |
| STRAB (N=6) | Age at Diagnosis (years) | Patch Duration (months) | Age at Surgery (years) | Current Deviation (prism diopters) | AE | AE Dist VA (logMAR) | SE Dist VA (logMAR) | IRD (diopters) | Stereo-acuity (arcsec) |
 S1 S1
 S2 S2
 S3 S3
 S4 S4
 S5 S5
 S6 S6 |
3
4 6 2 10.5 4 |
24
6 2 36 0 24 |
-
15 - 2,13 - 6 |
20
20 6 12 25 8 |
OD
OS OD OS OD OS |
0.40
0.10 0.20 0.40 0.30 0.90 |
0.00
0.00 0.00 0.10 0.00 0.00 |
0.75
0 0.75 2.75* 0 1.0 |
>3000
>3000 400 >3000 >3000 3000 |
| Mean | 4.9 | 15.3 | - | 11.8 | - | 0.38 | 0.02 | 0.50 | - |
| ANISO (N=6) | Age at Diagnosis (years) | Patch Duration (months) | Age at Surgery (years) | Current Deviation (prism diopters) | AE | AE Dist VA (logMAR) | SE Dist VA (logMAR) | IRD (diopters) | Stereo-acuity (arcsec) |
| ■ A1
◆ A2 ▲ A3 ✕ A4  A5 A5
● A6 |
9
5 5.5 10 9 9 |
2
12 24 2 1 12 |
-
- - - - - |
ORT
ORT ORT 4-6 E ORT ORT |
OS
OD OS OD OD OD |
0.60
0.30 0.10 0.20 0.30 1.00 |
0.10
0.10 0.00 0.00 0.00 0.30 |
3.5
1.75 2.0 1.5 2.75 0.5 |
800
400 800 800 100 >3000 |
| Mean | 7.9 | 8.8 | - | - | - | 0.42 | 0.08 | 2.00 | - |
WE –worse control eye, BE – better control eye, AE – amblyopic eye, SE – sound eye.
VA – visual acuity at distance, reported as logMAR (MAR in arc-minutes).
ORT – orthophoria, E – esophoria.
IRD – interocular refractive difference, reported as diopters of spherical equivalent.
IRD consistent with mixed strabismic/anisometropic diagnosis.
All subjects completed a full ophthalmologic exam at the WVU Eye Institute to confirm their diagnosis based on best-corrected distance acuity. Diagnosis of anisometropic amblyopia was assigned on the basis of: (1) interocular refractive difference of hyperopia ≥ +1.0 diopter (D), astigmatism ≥ +1.0 D, or myopia ≥ −2.5 D; or (2) history of anisometropia but no history of strabismus or strabismus surgery. Diagnosis of strabismus was made on the basis of a history of strabismus or strabismus surgery, but no anisometropia (as defined above). In clinical practice it is common to find that some subjects with amblyopia present with a mixed anisometropic/strabismic diagnosis, although little consensus exists regarding additional subtypes. One of our strabismic subjects (S4) had an interocular difference of hyperopia of 2.75 D, consistent with a potential mixed diagnosis. The direction and magnitude of strabismic deviation in our subjects was determined with cover-uncover, alternate cover, and prism testing. All six of our strabismic subjects showed esotropia. Manifest deviations for our subjects ranged from 0Δ to 25Δ (Table 1).
The ophthalmologic tests also included examination of the fundus with dilation, documentation of ductions and versions, autorefraction, and a sensory exam including Snellen visual acuity chart (Lombart Instrument, Norfolk, VA), and Titmus stereoacuity test (Titmus Optical, Inc., Petersburg, VA) (Table 1). The Titmus stereoacuity test was scored according to highest level of detectable horizontal disparity for the Wirt rings or for the Titmus fly. The crudest stereoacuity measurable with this test is 3000, assigned for patients able to perceive disparity only in the Titmus fly illustration.
Finally, in order to facilitate comparison of amblyopic subjects and controls, control subjects were assigned a better eye and a worse eye based on visual acuity. We used Snellen acuity as the primary criterion, but in three subjects with Snellen equal in both eyes, grating acuity was used. All amblyopic subjects had worse Snellen and grating acuity in their amblyopic eye than their sound eye.
Psychophysical Testing
All psychophysical tests were administered with all subjects’ typical optical correction. Translucent plastic patches covered the nontesting eye. All tests included practice trials to ensure that the stimuli were visible and the task instructions were adequately understood. We used nonparametric statistics for data analysis to avoid dependence on strictly normal distributions. Three tests were administered, grating acuity, contrast sensitivity and contour integration. For the test of grating acuity, standard, computerized, forced-choice methods were used, and are described elsewhere.31 The contrast sensitivity test was also a computerized, 2 alternative forced choice version (Figure 1A).32 For the test of contour integration, stimuli were displayed on a CRT monitor. The task was to determine the orientation (ie, pointing left or right) of a perceived egg-shaped contour made up of 15 small, aligned Gabor patches embedded in a field of randomly oriented patches of identical contrast and spatial frequency (Figure 1B).33–38 This test was administered using the method of constant stimuli and 2-AFC at six levels of increasing difficulty. Task difficulty increased as a function of increasing orientation jitter of the Gabor patches along the contour.
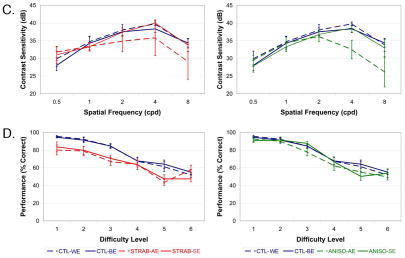
Fig. 1.
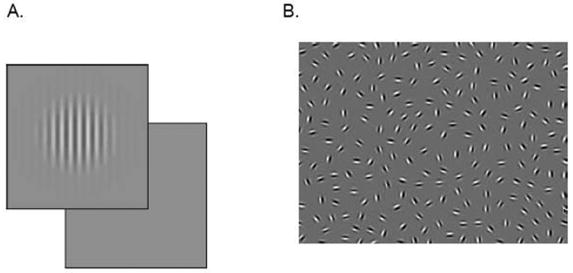
Psychophysical Testing. A. Depiction of stimuli in 2-alternative-forced-choice contrast sensitivity test. B. Depiction of stimuli in contour integration test. C. Contrast sensitivity functions for strabismic and anisometropic subjects show deficits in the higher spatial frequency range. D. Contour integration is impaired at all levels for both amblyopic and sound eyes of strabismic subjects.
Functional Magnetic Resonance Imaging
Data Acquisition
Subjects were scanned in a General Electric 3 Tesla MR scanner (Fairfield, CT), using a visual surface coil (Nova Medical, Inc., Wilmington, MA), as has been previously described.39–40 After a sagittal localizing scan, a T1-weighted inversion recovery sequence (TR = 400 ms) was used to acquire 20 interleaved oblique 4 mm slices with 0.86 × 0.86 mm in-plane resolution, oriented perpendicular to the calcarine sulcus, beginning at the occipital pole. These anatomical scans were used to register functional data to the cortical surface model. Next, multiple functional scans were acquired with 1.72 × 1.72 mm in-plane resolution using a spiral gradient echo sequence [TE = 40 ms, Flip Angle = 65°, TR = 2000 ms].41
Eye movements were monitored using the Sensomotorics iView (Needham, MA) system in order to ensure fixation stability during the functional scans. The iView system was used to measure gaze position in the stimulated eye, and was calibrated using a nine-point display at a screen resolution of 832 × 624 pixels, subtending approximately 30° horizontal × 23° vertical of visual angle. The recording rate of the iView camera was 60 Hz and accuracy < 1°. Calibrated tracking was not achieved in a minority of subjects due to hardware or software failures. For these subjects, fixation during fMRI was monitored manually via the video camera, and was similar to other subjects.
Stimuli
The visual stimuli were generated using the Psychophysics Toolbox42–43 and MATLAB 5 (Natick, MA) for Macintosh OS 9 on a PowerMac G4 computer (Cupertino, CA) with dual SVGA display drivers (output resolution = 832 × 624 pixels, 30° horizontal × 23° vertical). The stimuli were displayed in the scanner using the Avotec SilentVision dichoptic projector (Stuart, FL). Subjects viewed the images with both eyes open by looking straight ahead into the eyepieces, and used the built-in optical correction. For each functional scanning run, one eyepiece displayed the stimulus and fixation target while the other displayed an isoluminant gray screen. Left and right eye stimulation was alternated during each experiment. A central fixation target was present at all times for the tested eye. The target was a small arrowhead (0.5°) pointing in one of four directions (ie, up, down, left, or right), which randomly changed direction every 4 s. Subjects reported the appearance of the fixation point using a fiber-optic button pad. The tested eye was shown 16 s periods of a high-contrast, moving wedge in the left or right visual hemifield, alternating with 16 s of fixation-only. The wedge contained a radial square-wave grating pattern alternately expanding and contracting every 2 s at 7°/s. This wedge spanned either 100° (40° from vertical meridian) or 160° (10° from vertical meridian), and spared the central 0.5° of visual space, where the fixation target was presented (see Figure 2). Eight scans of 256 s were collected, two for each combination of wedge size and eye.
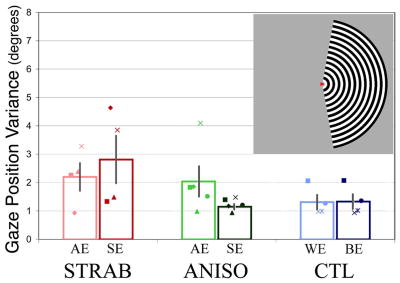
Fig. 2.
fMRI Fixation Stability and BOLD Signal Magnitude. A. Upper right inset shows depiction of hemifield stimulus. The subject maintained fixation on the central “arrowhead” target. Fixation stability is shown for each group. No significant differences of fixation stability are seen for either intergroup or interocular comparisons. Group mean (open bars) and individual data are shown (from left-to-right, strabismic, anisometropic, and control). Saturated colors correspond to the better/sound eye (BE/SE) and lighter shades to the worse/amblyopic eye (WE/AE). Calibration error resulted in lack of eye tracking data in two strabismics (S5, S6), one anisometrope (A5), and two controls (C2, C3). B. Mean BOLD signal magnitude is depressed for every intergroup and interocular comparison, although a few amblyopic patients do not follow this trend (S2, S5, and A4, see text for details). There is also a consistent trend for less signal magnitude in control worse versus better eyes. Asterisks indicate significant intergroup differences.
Statistical Analysis
Surface reconstructions of each subject’s cerebral cortex were generated from high-resolution anatomical images obtained in a General Electric 1.5 Tesla scan session prior to the fMRI experiments, using the freely available (http://www.nmr.mgh.harvard.edu/freesurfer) FreeSurfer software package.44 The functional analysis was completed using the FS-FAST software tools freely available at ftp://ftp.nmr.mgh.harvard.edu/pub/flat/fmri-analysis. Analysis consists of motion-correction and intensity normalization, followed by selective averaging of the blocks corresponding to stimulation of each of the left and right visual hemifields, separately for each eye. A voxel-wise F-test computed the significance of the contrast between BOLD (blood oxygen level dependent) signal for each block and fixation baseline. The functional results were then viewed on an individual’s cortical surface, producing maps of statistical significance (p < 0.05) on inflated or flattened representations.
Region-of-Interest Analysis
Retinotopic mapping experiments were conducted for all subjects in a separate session using standard, previously described phase-encoded eccentricity and polar angle stimuli.40 The maps generated from each subject’s sound/better eye were used to define regions of interest. These regions of interest were defined on each subject’s cortical surface and were thus composed of 2-D elements termed vertices (note the difference from voxels, which are 3-D elements of individual slices). Objectively determined borders were available for V1 and V2 for all subjects. In addition, a “foveal” region of interest (FOV) was defined as the region of occipital pole activated by the sound/better eye in the central 2.5° of visual angle. Regions of interest corresponding to extrafoveal V1 and V2 (V1EF and V2EF) were defined as the remainder of these areas (ie, 2.5–15° eccentricity) after subtraction of FOV.
These regions of interest were used to extract BOLD signal from the hemispheres contralateral to the stimulated hemifield. BOLD signal was then compared across diagnoses using a multifactorial random-effect ANOVA on rank-ordered data. Initially, five factors were included in this analysis: subject group, region of interest, wedge size, stimulated hemiretina (nasal vs temporal), and eye (amblyopic vs sound eye or worse vs better eye). There were no significant effects of wedge size or hemiretina, so these factors were collapsed across conditions. Wilcoxon tests were used to compare amblyopic to sound and control eyes for each subject group, within each region of interest.
Computation of Interocular Indices
The regions of interest defined above were also used to quantify the difference in fMRI response between eyes at the individual subject level. Two separate indices were defined, using the cortical vertices that were significantly activated (p < 0.05).
Ocular Dominance Index
The dominance index (DI) was calculated as the difference between the number of vertices activated by each eye, divided by the sum of the number of vertices activated by each eye, ie, DI = (sound eye − amblyopic eye)/(sound eye + amblyopic eye). A positive DI indicates more vertices activated by the sound eye, while a negative DI indicates more vertices activated by the amblyopic eye. Better and worse eye were used in place of sound and amblyopic eye for control subjects. The mean DI for each subject group was compared with the control group using Wilcoxon tests.
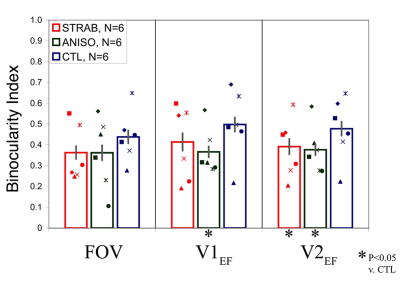
Binocularity Index
A vertex was classified as binocular if monocular stimulation through both eyes resulted in significant MR signal. The binocularity index (BI) was thus calculated as the intersection divided by the union of vertices activated by each monocular stimulation, ie, BI = (OS ∩ OD)/(OS ∪ OD).30 Unlike Lee et al,30 who calculated the binocularity index using voxels in a volumetric region of interest, our index was calculated using vertices intersecting the cortical surface. The mean BI for each diagnosis group was compared with the control group using Wilcoxon tests.
Results
Psychophysics
Grating Acuity
Consistent with Snellen acuity (Table 1), grating acuity measures for sound eyes were always better than for amblyopic eyes. For the control subjects, 5/6 eyes classified as better had better grating acuity, because this was the definition. Across groups, amblyopic subjects had decreased grating acuity in their impaired eyes compared with both controls and their sound eyes (CTL= 0.78/0.84 min; STRAB = 0.94/1.49 min; ANISO = 0.84/1.24 min, respectively, for better/worse eye). Consistent with previous work, amblyopic grating acuity exceeded Snellen acuity (Table 1),45,46 which is known to have additional sensitivity to higher-order effects such as crowding.47,48
Contrast Sensitivity
Contrast sensitivity in the amblyopic eyes of both groups was depressed compared with control worse eyes at 2, 4, and 8 cpd, consistent with the commonly reported higher spatial frequency deficit in amblyopia (Figure 1C).49–51 Multifactorial ANOVA on rank-ordered data showed a main effect of spatial frequency (F = 22.9; p < 0.0001). The pair-wise differences reached significance for anisometropic eyes at 4 cpd (CTL = 39.8 dB, ANISO = 32.5 dB; c = 4.05; p < 0.04), but not at other spatial frequencies. No other significant differences were seen between amblyopic vs sound eyes or for sound vs control better eyes for either patient group. Results were unchanged when we calculated the integral of each subject’s sensitivity function.
Contour Integration
The 75% correct threshold level was interpolated for every subject. Both eyes of strabismics were impaired compared to controls (Figure 1D) (weaker eye CTL = 3.5, STRAB = 1.8, c = 5.02; p = 0.02—better eye CTL = 3.6, STRAB = 2.2; c = 4.39; p = 0.03). These results are consistent with reports of impairments at this task in both strabismic and sound eyes, independent of acuity.52 In contrast, no deficit was found for anisometropic eyes compared with control eyes, although an interocular comparison approached significance, as has been shown elsewhere (sound eye = 3.6, amblyopic eye = 3.3; c = 2.6; p = 0.07).35 Previous work has suggested that strabismic and anisometropic amblyopia can be distinguished based on differences in higher-order visual functions such as vernier acuity and contour integration,53,54 so a greater impairment for strabismics was expected. Overall, these results suggest that our strabismic and anisometropic subject groups are comparable to previous studies.
Functional Magnetic Resonance Imaging
Fixation Stability and Head Motion
In order to provide a single index of fixation during each scan, the standard deviation of horizontal gaze position was calculated for each subject. Vertical gaze position was also measured, but was less variable between subjects, consistent with the known pattern of fixational eye movements in amblyopes.55,56 Mean gaze position variance was calculated from the raw eye position data after filtering for blinks. The means did not significantly differ between amblyopic and control better eyes (CTL = 1.3°, STRAB = 2.2°, ANISO = 2.0°), although more variability existed between subjects in the amblyopic groups than in the control group (Figure 2A). Also, both amblyopic groups displayed somewhat reduced mean fixation stability compared to controls. Interestingly, two of the strabismic subjects actually had better fixation stability when viewing with their amblyopic eye (S2 and S4), but all anisometropic subjects had better stability when viewing with their sound eyes. Control subjects had similar fixation stability in both better and worse eyes. Review of fixation task response data confirmed that all subjects provided feedback for > 75% of the trials, with no significant differences in accuracy between the groups. Finally, head motion, estimated as the mean vector magnitude of translational motion within each scan, did not differ between eyes or groups.
fMRI Activation Maps
Qualitatively, for both eyes, stimulation in one hemifield of visual space resulted in an increase in BOLD signal in the contralateral visual cortex in every subject, consistent with the normal retinogeniculocortical projection. In 4/6 strabismics and 5/6 anisometropes, a clear preference for the sound compared with amblyopic eye was observable in both hemispheres in both the extent and the magnitude of the activation pattern. Exceptions S2 and S5 instead exhibited stronger activation from the nasal retina than the temporal retina of each eye. The mild anisometrope A4 had stronger activation in both hemispheres in response to stimulation of amblyopic than sound eye. In comparison, only 3/6 control subjects displayed greater activation for their better eye.
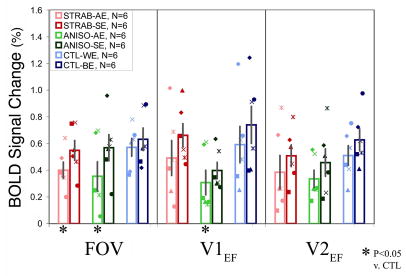
Region of Interest Analysis of Signal Magnitude
Consistently reduced BOLD signal was seen for the amblyopic or worse eye in most individual subjects (Figure 2B). The three exceptions (S2, S5, and A4) were noted above. The multifactorial ANOVA (rank-ordered data) revealed a main effect of diagnosis (CTL = 0.61%, STRAB = 0.50%, ANISO = 0.40%; F = 12.5; p < 0.0001), and a main effect of eye (sound/better eye = 0.57%, amblyopic/worse eye = 0.44%; F = 13.6; p = 0.0003). At a group level, reduced signal for amblyopic eyes compared to sound eyes was present for each condition, although none of these pair-wise Wilcoxon tests were significant (Table 2). There was always reduced signal in amblyopic compared to control worse eyes, and this was significant for strabismic eyes in FOV, and for anisometropic eyes for FOV and V1EF (Table 2).
Table 2.
Comparison of BOLD Magnitude and Indices in Regions-of-lnterest
| BOLD Signal | Indices | |||
|---|---|---|---|---|
| STRAB
(N = 6) |
AE vs SE | AE vs CTL | DI vs CTL | BI vs CTL |
| FOV | + | 0.04 (3.85) | + | + |
| V1EF | + | + | + | + |
| V2EF | + | + | 0.04 (4.09) | 0.04 (4.09) |
|
ANISO
(N = 6) |
AE vs SE | AE vs CTL | DI vs CTL | BI vs CTL |
| FOV | + | 0.03(4.81) | 0.0005(12.3) | + |
| V1EF | + | 0.03(4.81) | 0.01 (6.54) | 0.003 (8.69) |
| V2EF | + | + | 0.02 (5.43) | 0.02 (5.43) |
AE – amblyopic eye, SE – sound eye, CTL – control subjects.
DI – dominance index, BI – binocularity index.
FOV – foveal ROI, V1EF – extrafoveal V1, V2EF – extrafoveal V2.
indicates expected trend for reduced AE signal compared to SE or CTL. (Significant comparisons are given as p(χ))
The comparison of nasal vs temporal hemiretina did not show any significant differences, regardless of region of interest, eye, or subject group. There was not even consistently greater signal magnitude from the nasal or temporal hemiretina for any group. With the exception of the two strabismic subjects described above, there does seem to be any support for a hemiretinal bias in this group of subjects under the current viewing conditions.
We determined if fixation stability correlated with the BOLD signal, even though it did not significantly differ between groups. One significant linear correlation was found, between signal magnitude in FOV and fixation variance for strabismic eyes (R2 = 0.92; p < 0.05), so in this case the reduced magnitude for strabismic eyes should be viewed with caution. However, neither V1EF nor V2EF correlated with fixation for strabismics, nor did any of the regions of interest for anisometropic subjects.
Finally, we tested for any linear correlation between BOLD signal and the subject’s performance on visual acuity, grating acuity, contrast sensitivity, and contour integration. However, none of these correlations were significant.
Region-of-Interest Interocular Indices
Ocular Dominance Index
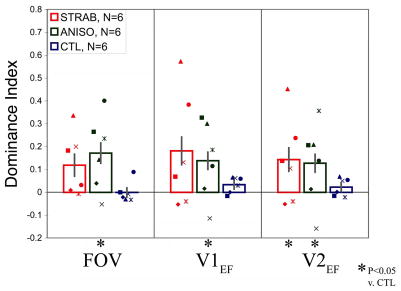
In general, individual strabismic and anisometropic subjects showed greater DI relative to control subjects (Figure 3A). The DIs for the control subjects were distributed tightly around zero, indicating no consistent ocular dominance, at least in number of activated vertices. In contrast, all but three of the amblyopic subjects showed sound eye dominance, with positive DIs. Consistent with the region of interest magnitude results, the exceptions were S2, S5, and A4. However, it is worth noting that all these subjects are mild amblyopes, and S2 and S5 have no interocular refractive difference (Table 1). Despite the variability between subjects, the anisometropic group had mean DIs which were significantly more positive than controls, as did strabismics in V2EF (Table 2). Although DI was calculated from signal extent (number of vertices), it also strongly correlated across all subjects with an analogously calculated signal magnitude measure in FOV (R2 = 0.74; p < 0.00001), V1EF (R2 = 0.73; p < 0.00001), and V2EF (R2 = 0.68; p < 0.0001), thus supporting the known relationship between extent of activation and signal magnitude.
Fig. 3.
Ocular Dominance and Binocularity Index by Group and ROI. A. Group data is shown for each region of interest (from left-to-right, strabismic, anisometropic, and control). Mean DI is shifted toward the sound eye for strabismic and anisometropic groups. B. Mean BI is reduced in all ROIs for strabismic and anisometropic amblyopes. Asterisks indicate significant differences. Labeling conventions are the same as in Fig. 2.
Binocularity Index
A wide range of individual BI values was seen for strabismic and anisometropic subjects, overlapping somewhat with the BI distribution for control subjects (Figure 3B). However, mean BIs both amblyopic groups trended smaller than for controls, reaching significance for V1EF and V2EF of the anisometropic group and V2EF for strabismics (Table 2).
Discussion
We show here that amblyopia and the associated interruption of early visual experience leads to reduction in neural processing of visual stimuli, regardless of clinical etiology. Our study improved upon previous work by using a relatively large number of subjects, monitoring fixation, and individually defining retinotopic regions of interest on the flattened cortical surface. Although our results were consistent with previous PET and fMRI studies that suggested reduced recruitment of cortical neurons by amblyopic eye input, we found impairments in evoked BOLD magnitude in V1 and V2 to be variable and of moderate size when we compared mild-to-moderate amblyopes with carefully matched controls. These fMRI measures did not relate strongly to any of our behavioral measures. These results are discussed in turn below.
Fixation Stability
Given evidence that fixation stability can be impaired in amblyopia,55 it is a possible confounding factor. However, we did not find significantly impaired fixation stability in our amblyopes. Furthermore, fixation task performance was equivalent across groups, further supporting our claim that fixation and attention were maintained throughout the experiment. These measurements suggest that impaired fixation does not necessarily preclude meaningful fMRI experiments in mild-to-moderate amblyopic subjects. In fact, this is the first neuroimaging study of amblyopia to measure fixation. Finally, the lack of correlations between fixation stability and fMRI measures in all but one case (STRAB, FOV) provides further argument against eye movements as a general confounding factor. While we cannot rule out a small effect from fixation, we are confident that it does not generally explain our results.
Signal Magnitude Deficits
Overall, we report here a main effect of eye in fMRI signal magnitude. Given our stringent inclusion of eye dominance in control subjects, it is notable that acuity-based eye dominance was an effective predictor of interocular signal magnitude in controls (Figure 2), a novel result. We do not claim that our method of assigning eye dominance extends to dominance for all visual functions, but this implementation provided a conservative control for amblyopia. The main effect of eye was apparent as group-level trends for both amblyopic subtypes, consistent with previous PET22,23,57 and fMRI.25–27 Furthermore, amblyopic eyes showed group-level trends for less signal than control eyes.
In general, these results did not distinguish between FOV, V1EF or V2EF; amblyopic eyes produced lower signals in all regions-of-interest. This is consistent with the most recent neuroimaging studies that carefully defined visual areas.26 Muckli et al28 found an unexpected increase in activation for areas V1 and V2 for the amblyopic eye. We did not replicate that result here, but there are many differences in the experimental paradigm. This study is the first to define a separate region of interest for the central 2.5° of the visual field. This was done in part because of the difficulty of reliably distinguishing visual area boundaries in the confluent foveal representation, but also because of the selective impairment of central vision in amblyopia. Nevertheless, we did not find a selective reduction in FOV. Future examination of this issue might use stimuli with higher spatial frequencies or other viewing conditions.
We found that signals in V1 generally correlated with signal in V2, suggesting a common source of impairment. Another important issue is the extent of correlation between signal in V1, V2, and psychophysical measures. There is some suggestion that calcarine activation correlates with visual acuity,22 but the majority of studies have not found correlations with either visual acuity or contrast sensitivity.24,25,28,58 We also did not find significant linear correlations between grating acuity, contrast sensitivity, or visual acuity and V1 or V2, with our sample size. However, when we separated the amblyopic subjects according to the severity of amblyopia, we did note that subjects S6 and A6, subjects with large acuity losses, exhibited large BOLD reductions. Recent studies of human amblyopia have increasingly emphasized the dysfunction of higher-level visual cortex in the parietal and temporal cortex.59,32 It may be that many behavioral losses in amblyopia are better predicted by activation of these areas.28
This study focused on subjects with mild to moderate amblyopia, a result of our public recruitment. In this context, we point out that some individual mild amblyopes could not be reliably distinguished from the range of control subjects based on our fMRI measures. This is a conclusion not readily apparent from the existing neuroimaging literature. It is also apparent that our results did not differentiate anisometropic and strabismic subtypes. Future studies could focus on more severe patients, separate patients based on factors other than clinical diagnosis, or employ tasks that relate to other psychophysical deficits.
Nasotemporal Asymmetry
Studies of amblyopia have often shown greater impairments for the nasal retina, that is, visual acuity and luminance detection,9 reaction time for suprathreshold light detection,60 and increment thresholds.61 However, in some cases the opposite bias has been reported. 8,60,62 Nevertheless, a disadvantage for the nasal retina has been clearly postulated to result from constant, active suppression of input from the amblyopic eye’s nasal retina by the foveal region of the sound eye’s temporal retina under binocular viewing conditions in esotropes.9 We thus hypothesized that nasal retina stimulation might result in less fMRI activation. However, we found no evidence for this effect with our current methods. Future studies of this issue could adopt viewing conditions that encourage greater interocular suppression or focus more specifically on the oculomotor system.
Shifted Ocular Dominance
Although human post-mortem cases have not found abnormal ocular dominance column periodicity,63,64 two recent neuroimaging studies demonstrated ocular dominance shifts in V1 of human amblyopes. Goodyear et al65 used high resolution (0.5 × 0.5 × 3 mm) fMRI to image ocular dominance columns in six strabismic adults, finding a 60/40 dominance ratio in favor of the sound eye. In another study, Liu et al27 demonstrated dominance for the sound eye in two anisometropic amblyopes with an alternate method that constructs voxel-based ocular dominance histograms using the voxel-wise Student t-statistic for the left eye vs right eye contrast. This is a somewhat remarkable result because the spatial resolution was lower than the size of the normal ocular dominance columns in V1 (ie, 3–4 mm vs 0.5 mm), but subjects with amblyopia may have more monocular segregation at the level of neural populations than subjects with normal vision.66 We used a vertex-counting technique similar to Goodyear et al65 but did not attempt to resolve individual ocular dominance column boundaries. Our method, like that of Liu et al,27 pooled signals across multiple ocular dominance columns to compute mean ocular dominance in larger cortical regions. Our results agree qualitatively with these previous reports. However, these indirect “ocular dominance” measures may have limited sensitivity compared to specific anatomical or electrophysiological measures from single neurons, and may not address more subtle abnormalities in the balance of excitation and inhibition or receptive field location.18,67 For example, in this study, several individuals with amblyopia showed a balanced fMRI DI despite their interocular difference in acuity. Moreover, our results depend upon viewing conditions and might change for conditions favoring more or less interocular suppression.
Impaired Cortical Binocularity
We know from animal models of amblyopia that the number of binocularly driven cells is reduced in V118,68,69 and perhaps reduced even further in extrastriate cortex.70,71 Binocularity has also been extensively studied in humans using psychophysics. In addition to the typical severe loss of stereopsis, studies have demonstrated impaired binocular summation and asymmetries in the strength of binocular inhibition between eyes.72,73 Visual evoked potentials have confirmed this dissociation in both strabismic and anisometropic amblyopes.74,75 A recent pair of fMRI papers has added to these results. Using a binocularity index similar to ours, Lee et al30 reported impaired binocularity for strabismic (N = 6) and anisometropic (N = 5) amblyopes in the calcarine region, while Algaze et al25 found reduced calcarine binocularity for 5 amblyopic subjects of mixed diagnoses.
We improved upon these techniques by using vertices on the cortical surface, in multiple visual areas. We found degraded binocularity in our amblyopes, although not as severe as previously reported. Moreover, we obtained similar results for V1 and V2. Since we did not use a binocular viewing condition, we divided the intersection by the union of monocularly driven vertices. Our index does not account for the possibility of interocular suppression under natural viewing conditions, so we potentially overestimated binocularity. On the other hand, it should be noted that in these experiments the nontested eye viewed a homogeneous gray screen, and this condition may allow more suppression than in experiments that employ occlusion of that eye.
None of our subjects could be classified as having early onset (infantile) strabismus or anisometropia, which might be expected to lead to even greater impairments of binocularity. Nevertheless, we demonstrated that our subject cohort shows significant impairment of stereopsis and have demonstrated other binocular deficits previously.31 Given that the development of binocularity may extend well into childhood,76 impairments are expected even with later onset etiologies. The fact remains, however, that many strabismic subjects showed a severe loss of stereoacuity without a reduced BI, highlighting the limitations of this measure. We know that cortex beyond areas V1 and V2 plays a critical role in binocular perception,77,78 so studies of higher-level areas in subjects with amblyopia represents an important goal for the future.
Acknowledgments
This study was supported by NIH grant EY015219 and COBRE grant P20RR15574, project 2, to JM. We thank Susan Lemieux, James Trimmier, and Ruth Walsh for their contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campos E. Amblyopia. Surv Ophthalmol. 1995 July;40(1):23–39. doi: 10.1016/s0039-6257(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 2.von Noorden GK, Campos EC. Binocular vision and ocular motility: Theory and management of strabismus. 6. St. Louis: Mosby; 2001. [Google Scholar]
- 3.Daw NW. Critical periods and amblyopia. Arch Ophthalmol. 1998 April;116(4):502–5. doi: 10.1001/archopht.116.4.502. [DOI] [PubMed] [Google Scholar]
- 4.Abrahamsson M, Sjostrand J. Contrast sensitivity and acuity relationship in strabismic and anisometropic amblyopia. Br J Ophthalmol. 1988 January;72(1):44–9. doi: 10.1136/bjo.72.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asper L, Crewther D, Crewther SG. Strabismic amblyopia. Part 1. Psychophysics. Clin Exp Optom. 2000 March;83(2):49–58. doi: 10.1111/j.1444-0938.2000.tb04892.x. [DOI] [PubMed] [Google Scholar]
- 6.Ciuffreda KJ, Fisher SK. Impairment of contrast discrimination in amblyopic eyes. Ophthalmic Physiol Opt. 1987;7(4):461–7. [PubMed] [Google Scholar]
- 7.Thomas J. Normal and amblyopic contrast sensitivity function in central and peripheral retinas. Invest Ophthalmol Vis Sci. 1978 August;17(8):746–53. [PubMed] [Google Scholar]
- 8.Sireteanu R, Fronius M. Human amblyopia: structure of the visual field. Exp Brain Res. 1990;79(3):603–14. doi: 10.1007/BF00229328. [DOI] [PubMed] [Google Scholar]
- 9.Sireteanu R, Fronius M. Naso-temporal asymmetries in human amblyopia consequence of long-term interocular suppression. Vision Res. 1981;21(7):1055–63. doi: 10.1016/0042-6989(81)90010-9. [DOI] [PubMed] [Google Scholar]
- 10.Hess RF, Pointer JS. Differences in the neural basis of human amblyopia: The distribution of the anomaly across the visual field. Vision Res. 1985;25(11):1577–94. doi: 10.1016/0042-6989(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 11.Sireteanu R. Human amblyopia: consequence of chronic interocular suppression. Hum Neurobiol. 1982 March;1(1):31–3. [PubMed] [Google Scholar]
- 12.Cynader MS. Competitive neuronal interactions underlying amblyopia. Hum Neurobiol. 1982 March;1(1):35–9. [PubMed] [Google Scholar]
- 13.Hess RF. Amblyopia: Sight unseen. Clin Exp Optom. 2001 November;84(6):321–36. doi: 10.1111/j.1444-0938.2001.tb06604.x. [DOI] [PubMed] [Google Scholar]
- 14.Asper L, Crewther D, Crewther SG. Strabismic amblyopia. Part 2. Neural processing. Clin Exp Optom. 2000 July;83(4):200–11. doi: 10.1111/j.1444-0938.2000.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 15.Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque’s visual system. III. Physiological observations. J Neurosci. 1987 May;7(5):1340–51. doi: 10.1523/JNEUROSCI.07-05-01340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiorpes L, Boothe RG. Naturally occurring strabismus in monkeys (Macaca nemestrina) Invest Ophthalmol Vis Sci. 1981 February;20(2):257–63. [PubMed] [Google Scholar]
- 17.Crawford ML, Harwerth RS. Ocular dominance column width and contrast sensitivity in monkeys reared with strabismus or anisometropia. Invest Ophthalmol Vis Sci. 2004 September;45(9):3036–42. doi: 10.1167/iovs.04-0029. [DOI] [PubMed] [Google Scholar]
- 18.Smith EL, III, Chino YM, Ni J, Cheng H, Crawford ML, Harwerth RS. Residual binocular interactions in the striate cortex of monkeys reared with abnormal binocular vision. J Neurophysiol. 1997 September;78(3):1353–62. doi: 10.1152/jn.1997.78.3.1353. [DOI] [PubMed] [Google Scholar]
- 19.Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998 August 15;18(16):6411–24. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Curr Opin Neurobiol. 1999 August;9(4):480–6. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi S, Suzuki Y, Kiyosawa M, Mochizuki M, Ishii K. Differential activation of cerebral blood flow by stimulating amblyopic and fellow eye. Graefes Arch Clin Exp Ophthalmol. 2005 January 14; doi: 10.1007/s00417-004-1009-5. [DOI] [PubMed] [Google Scholar]
- 22.Demer JL, Grafton S, Marg E, Mazziotta JC, Nuwer M. Positron-emission tomographic study of human amblyopia with use of defined visual stimuli. J AAPOS. 1997 September;1(3):158–71. doi: 10.1016/s1091-8531(97)90059-8. [DOI] [PubMed] [Google Scholar]
- 23.Imamura K, Richter H, Fischer H, et al. Reduced activity in the extrastriate visual cortex of individuals with strabismic amblyopia. Neurosci Lett. 1997 April 11;225(3):173–6. doi: 10.1016/s0304-3940(97)00211-5. [DOI] [PubMed] [Google Scholar]
- 24.Goodyear BG, Nicolle DA, Humphrey GK, Menon RS. BOLD fMRI response of early visual areas to perceived contrast in human amblyopia. J Neurophysiol. 2000 October;84(4):1907–13. doi: 10.1152/jn.2000.84.4.1907. [DOI] [PubMed] [Google Scholar]
- 25.Algaze A, Roberts C, Leguire L, Schmalbrock P, Rogers G. Functional magnetic resonance imaging as a tool for investigating amblyopia in the human visual cortex: A pilot study. J AAPOS. 2002 October;6(5):300–8. doi: 10.1067/mpa.2002.124902. [DOI] [PubMed] [Google Scholar]
- 26.Barnes GR, Hess RF, Dumoulin SO, Achtman RL, Pike GB. The cortical deficit in humans with strabismic amblyopia. J Physiol. 2001 May 15;533(Pt 1):281–97. doi: 10.1111/j.1469-7793.2001.0281b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu GT, Miki A, Francis E, et al. Eye dominance in visual cortex in amblyopia using functional magnetic resonance imaging. J AAPOS. 2004 April;8(2):184–6. doi: 10.1016/j.jaapos.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Muckli L, Kiess S, Tonhausen N, Singer W, Goebel R, Sireteanu R. Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Res. 2006 February;46(4):506–26. doi: 10.1016/j.visres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Choi MY, Lee KM, Hwang JM, et al. Comparison between anisometropic and strabismic amblyopia using functional magnetic resonance imaging. Br J Ophthalmol. 2001 September;85(9):1052–6. doi: 10.1136/bjo.85.9.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KM, Lee SH, Kim NY, et al. Binocularity and spatial frequency dependence of calcarine activation in two types of amblyopia. Neurosci Res. 2001 June;40(2):147–53. doi: 10.1016/s0168-0102(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal R, Conner IP, Odom JV, Schwartz TS, Mendola JD. Relating binocular and monocular vision in strabismic and anisometropic amblyopia. Archives of Ophthalmology. 2006;124:844–50. doi: 10.1001/archopht.124.6.844. [DOI] [PubMed] [Google Scholar]
- 32.Mendola JD, Conner IP, Roy A, et al. Voxel-based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp. 2005 April 21;25(2):222–36. doi: 10.1002/hbm.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun J. On the detection of salient contours. Spat Vis. 1999;12(2):211–25. doi: 10.1163/156856899x00120. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs I, Julesz B. A closed curve is much more than an incomplete one: Effect of closure in figure-ground segmentation. Proc Natl Acad Sci U S A. 1993 August 15;90(16):7495–7. doi: 10.1073/pnas.90.16.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandna A, Pennefather PM, Kovacs I, Norcia AM. Contour integration deficits in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 2001 March;42(3):875–8. [PubMed] [Google Scholar]
- 36.Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: Evidence for a local “association field”. Vision Res. 1993 January;33(2):173–93. doi: 10.1016/0042-6989(93)90156-q. [DOI] [PubMed] [Google Scholar]
- 37.Pennefather PM, Chandna A, Kovacs I, Polat U, Norcia AM. Contour detection threshold: Repeatability and learning with ‘contour cards’. Spat Vis. 1999;12(3):257–66. doi: 10.1163/156856899x00157. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs I, Kozma P, Feher A, Benedek G. Late maturation of visual spatial integration in humans. Proc Natl Acad Sci U S A. 1999 October 12;96(21):12204–9. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendola JD, Dale AM, Fischl B, Liu AK, Tootell RB. The representation of illusory and real contours in human cortical visual areas revealed by functional magnetic resonance imaging. J Neurosci. 1999 October 1;19(19):8560–72. doi: 10.1523/JNEUROSCI.19-19-08560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conner IP, Sharma S, Lemieux SK, Mendola JD. Retinotopic organization in children measured with fMRI. J Vis. 2004 June 18;4(6):509–23. doi: 10.1167/4.6.10. [DOI] [PubMed] [Google Scholar]
- 41.Glover G. Simple analytic spiral K-space algorithm. Magn Reson Med. 1999;42:412–5. doi: 10.1002/(sici)1522-2594(199908)42:2<412::aid-mrm25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–6. [PubMed] [Google Scholar]
- 43.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–42. [PubMed] [Google Scholar]
- 44.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001 January;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 45.Mayer DL, Fulton AB, Rodier D. Grating and recognition acuities of pediatric patients. Ophthalmology. 1984 August;91(8):947–53. doi: 10.1016/s0161-6420(84)34209-9. [DOI] [PubMed] [Google Scholar]
- 46.Mayer DL, Fulton AB. Preferential looking grating acuities of infants at risk of amblyopia. Trans Ophthalmol Soc U K. 1985;104 ( Pt 8):903–11. [PubMed] [Google Scholar]
- 47.Stuart JA, Burian HM. A study of separation difficulty. Its relationship to visual acuity in normal and amblyopic eyes. Am J Ophthalmol. 1962 53 March;:471–7. [PubMed] [Google Scholar]
- 48.Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory spatial interactions in amblyopic vision. Vision Res. 2002 May;42(11):1379–94. doi: 10.1016/s0042-6989(02)00061-5. [DOI] [PubMed] [Google Scholar]
- 49.Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 1977;17(9):1049–55. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- 50.Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1981 September;21(3):467–76. [PubMed] [Google Scholar]
- 51.McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3(5):380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- 52.Kovacs I, Polat U, Pennefather PM, Chandna A, Norcia AM. A new test of contour integration deficits in patients with a history of disrupted binocular experience during visual development. Vision Res. 2000;40(13):1775–83. doi: 10.1016/s0042-6989(00)00008-0. [DOI] [PubMed] [Google Scholar]
- 53.Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982 July 15;298(5871):268–70. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- 54.Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Res. 1985;25(7):979–91. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- 55.Westall CA, Aslin RN. Fixational eye movements and autokinesis in amblyopes. Ophthalmic Physiol Opt. 1984;4(4):333–7. [PubMed] [Google Scholar]
- 56.Ciuffreda KJ, Kenyon RV, Stark L. Increased drift in amblyopic eyes. Br J Ophthalmol. 1980 January;64(1):7–14. doi: 10.1136/bjo.64.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demer JL, von Noorden GK, Volkow ND, Gould KL. Imaging of cerebral blood flow and metabolism in amblyopia by positron emission tomography. Am J Ophthalmol. 1988 April 15;105(4):337–47. doi: 10.1016/0002-9394(88)90294-2. [DOI] [PubMed] [Google Scholar]
- 58.Anderson SJ, Holliday IE, Harding GF. Assessment of cortical dysfunction in human strabismic amblyopia using magnetoencephalography (MEG) Vision Res. 1999 May;39(9):1723–38. doi: 10.1016/s0042-6989(98)00259-4. [DOI] [PubMed] [Google Scholar]
- 59.Lerner Y, Pianka P, Azmon B, et al. Area-specific amblyopic effects in human occipitotemporal object representations. Neuron. 2003 December 4;40(5):1023–9. doi: 10.1016/s0896-6273(03)00720-7. [DOI] [PubMed] [Google Scholar]
- 60.Chelazzi L, Marzi CA, Panozzo G, Pasqualini N, Tassinari G, Tomazzoli L. Hemiretinal differences in speed of light detection in esotropic amblyopes. Vision Res. 1988;28(1):95–104. [PubMed] [Google Scholar]
- 61.Jacobson SG, Sandberg MA. Nasal-temporal asymmetry of visual thresholds from known retinal areas in strabismic amblyopia. Invest Ophthalmol Vis Sci. 1980;21:271. [Google Scholar]
- 62.Bedell HE. Symmetry of acuity profiles in esotropic amblyopic eyes. Hum Neurobiol. 1982;1:221–224. [PubMed] [Google Scholar]
- 63.Horton JC, Stryker MP. Amblyopia induced by anisometropia without shrinkage of ocular dominance columns in human striate cortex. Proc Natl Acad Sci U S A. 1993 June 15;90(12):5494–8. doi: 10.1073/pnas.90.12.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horton JC, Hocking DR. Pattern of ocular dominance columns in human striate cortex in strabismic amblyopia. Vis Neurosci. 1996 July;13(4):787–95. doi: 10.1017/s0952523800008658. [DOI] [PubMed] [Google Scholar]
- 65.Goodyear BG, Nicolle DA, Menon RS. High resolution fMRI of ocular dominance columns within the visual cortex of human amblyopes. Strabismus. 2002 June;10(2):129–36. doi: 10.1076/stra.10.2.129.8140. [DOI] [PubMed] [Google Scholar]
- 66.Lowel S, Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992 January 10;255(5041):209–12. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- 67.Sengpiel F, Blakemore C. The neural basis of suppression and amblyopia in strabismus. Eye. 1996;10 ( Pt 2):250–8. doi: 10.1038/eye.1996.54. [DOI] [PubMed] [Google Scholar]
- 68.Kumagami T, Zhang B, Smith EL, III, Chino YM. Effect of onset age of strabismus on the binocular responses of neurons in the monkey visual cortex. Invest Ophthalmol Vis Sci. 2000 March;41(3):948–54. [PubMed] [Google Scholar]
- 69.Mori T, Matsuura K, Zhang B, Smith EL, III, Chino YM. Effects of the duration of early strabismus on the binocular responses of neurons in the monkey visual cortex (V1) Invest Ophthalmol Vis Sci. 2002 April;43(4):1262–9. [PubMed] [Google Scholar]
- 70.Schroder JH, Fries P, Roelfsema PR, Singer W, Engel AK. Ocular dominance in extrastriate cortex of strabismic amblyopic cats. Vision Res. 2002 January;42(1):29–39. doi: 10.1016/s0042-6989(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 71.Sireteanu R, Best J. Squint-induced modification of visual receptive fields in the lateral suprasylvian cortex of the cat: Binocular interaction, vertical effect and anomalous correspondence. Eur J Neurosci. 1992;4(3):235–42. doi: 10.1111/j.1460-9568.1992.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 72.Levi DM, Harwerth RS, Smith EL. Binocular interactions in normal and anomalous binocular vision. Doc Ophthalmol. 1980 October 15;49(2):303–24. doi: 10.1007/BF01886623. [DOI] [PubMed] [Google Scholar]
- 73.Harrad RA, Hess RF. Binocular integration of contrast information in amblyopia. Vision Res. 1992 November;32(11):2135–50. doi: 10.1016/0042-6989(92)90075-t. [DOI] [PubMed] [Google Scholar]
- 74.Tsutsui J, Fukai S. Studies on visual evoked cortical response by liquid crystal phase difference haploscope: Amblyopia and/or strabismus. J Pediatr Ophthalmol Strabismus. 1980 May;17(3):185–90. doi: 10.3928/0191-3913-19800501-14. [DOI] [PubMed] [Google Scholar]
- 75.Baitch LW, Levi DM. Evidence for nonlinear binocular interactions in human visual cortex. Vision Res. 1988;28(10):1139–43. doi: 10.1016/0042-6989(88)90140-x. [DOI] [PubMed] [Google Scholar]
- 76.Tychsen L. Binocular Vision. In: Hart W, editor. Adler’s Physiology of the Eye: Clinical Applications. St. Louis: Mosby Year Book; 1992. pp. 773–853. [Google Scholar]
- 77.Backus BT. Stereoscopic vision: What’s the first step? Curr Biol. 2000 October 5;10(19):R701–R703. doi: 10.1016/s0960-9822(00)00707-7. [DOI] [PubMed] [Google Scholar]
- 78.Tsao DY, Vanduffel W, Sasaki Y, et al. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron. 2003 July 31;39(3):555–68. doi: 10.1016/s0896-6273(03)00459-8. [DOI] [PubMed] [Google Scholar]