Abstract
Background
Studies have implicated the 5-HT7 receptor in physiological and pathophysiological phenomena, including thermoregulation, central control of micturition and locomotion, regulation of circadian rhythm, sleep, and depression. Further, several antidepressant and antipsychotic drugs have high affinity for the 5-HT7 receptor.
Methods
We examined the role of 5-HT7 receptors in a rodent analogue of sensorimotor gating deficits in schizophrenia: phencyclidine (PCP)-induced disruption of prepulse inhibition (PPI) of acoustic startle. We used mice lacking the 5-HT7 receptor due to a targeted inactivation of this receptor gene, and the selective 5-HT7 receptor antagonist SB-269970.
Results
SB-269970 did not affect either baseline PPI or PCP-disrupted PPI. There was no difference between 5-HT7+/+ and 5-HT7−/− mice in startle reactivity or PPI regardless of prepulse intensity (74−82 dB), inter-stimulus interval (25−500 ms), or pulse intensity (90−120 dB). Nevertheless, the disruption of PPI produced by PCP (10 mg/kg) in wild-type mice was reduced in 5-HT7−/− mice, although it was not affected by the 5-HT7 antagonist SB-269970. By contrast, the PPI-disruptive effects of apomorphine (5 mg/kg) and amphetamine (7.5 mg/kg) were comparable in both genotypes.
Conclusions
The results indicate a partial role for the 5-HT7 receptor in the glutamatergic PPI model of sensorimotor gating deficits in schizophrenia that is sensitive to atypical antipsychotics, and no involvement of this receptor in the dopaminergic PPI model that is sensitive to typical antipsychotics. Thus, the 5-HT7−/− mice may provide a useful tool to study the role of 5-HT7 receptor in the action of atypical antipsychotic drugs and schizophrenia.
Keywords: serotonin, schizophrenia, 5-HT7 receptor knockout mice, startle, amphetamine, apomorphine, SB-269970
Dysfunctions of the serotonin (5-HT) system have been suggested to be important in the neurobiology of several psychiatric disorders, including depression and schizophrenia (Geyer et al 2001; Markou et al 1998; Meltzer 1995). Several classes of drugs used to treat psychiatric disorders have relatively high affinity for the 5-HT7 receptor (Roth et al 1994), implicating the 5-HT7 receptor in several neurophysiological and pathophysiological phenomena (Hedlund and Sutcliffe 2004). These drugs included typical and atypical antipsychotics as well as antidepressants (Monsma et al 1993; Shen et al 1993). Further, recent research has suggested the 5-HT7 receptor as a candidate target for the treatment of depression (Guscott et al 2005; Hedlund et al 2005; Wesolowska et al 2006). Using both knockout mice lacking the 5-HT7 receptor and selective 5-HT7 receptor antagonists, it has been demonstrated that blockade or inactivation of this receptor leads to an antidepressant-like profile in behavioral models of antidepressant activity, such as the forced swim test and the tail suspension test (Guscott et al 2005; Hedlund et al 2005; Wesolowska et al 2006).
The role of 5-HT7 receptors in schizophrenia has not been studied extensively. The atypical antipsychotic clozapine has higher affinity for the 5-HT7 receptor than the dopamine D2 receptor generally believed to be the main receptor involved in the mediation of antipsychotic effects (Roth et al 1994). Furthermore, 5-HT7 receptor mRNA is downregulated in the prefrontal cortex of schizophrenia patients (Thomas and Hagan 2004). Interestingly, clozapine may restore neuronal function by upregulating 5-HT7 receptor expression, something that has been shown at least in cell lines (Zhukovskaya and Neumaier 2000). Direct evidence implicating the 5-HT7 receptor in schizophrenia is provided by a study linking single nucleotide polymorphisms in the 5-HT7 receptor gene to schizophrenia in a Japanese population (Ikeda et al 2006). Thus, it is of interest to study the potential role of 5-HT7 receptors in behaviors of relevance to schizophrenia.
Prepulse inhibition (PPI) is the reduction of a startle response elicited by an intense acoustic stimulus (pulse) when it is immediately preceded by a stimulus of lower intensity (prepulse). PPI of startle has been used as a measure of the loss of sensorimotor gating that may contribute to the sensory flooding and cognitive deficits seen in schizophrenia and other psychotic disorders (Braff et al 2001; Geyer et al 2001; Swerdlow and Geyer 1998). Reversals of PPI deficits induced by various manipulations are widely used to assess antipsychotic activity (Geyer et al 2001). PPI deficits have been induced in rodents with drug treatments, including the dopamine receptor agonists apomorphine and amphetamine (Geyer et al 2002; Mansbach et al 1988), and the N-methyl-d-aspartate (NMDA) receptor antagonist phencyclidine (PCP) (Bakshi and Geyer 1995; Geyer and Ellenbroek 2003; Geyer et al 2002; Mansbach and Geyer 1989). It has been suggested that the behavioral effects of dopamine receptor agonists model some aspects of “positive” symptoms of schizophrenia, which respond to treatment with typical antipsychotics, while the behavioral effects of NMDA antagonists model some aspects of “negative” symptoms of schizophrenia, which have been reported to respond preferentially to treatment with atypical antipsychotics (Geyer and Ellenbroek 2003). One published study on the involvement of the 5-HT7 receptor in PPI demonstrated that in rats the selective antagonist SB-258741 dose-dependently reversed PPI-disruption induced by PCP (Pouzet et al 2002). SB-258741 had no effect on amphetamine-induced disruption of PPI, thus suggesting that the 5-HT7 receptor can modulate glutamatergic, but not dopaminergic, effects on PPI. Another study using 5-HT7 receptor knockout mice showed that these mice did not exhibit any alteration in startle amplitude or PPI under baseline conditions (Guscott et al 2005).
In the present study, we further investigated the possible role of 5-HT7 receptors in PPI. We evaluated the capacity of the 5-HT7 receptor antagonist SB-269970 to reduce PCP-induced disruptions of PPI in both rats and mice. Further, we extended previous studies in 5-HT7 receptor knockout mice and explored the role of 5-HT7 receptors in amphetamine-, apomorphine- and PCP-induced disruptions of PPI in these mice.
Methods and materials
Animals
Ten-to-twelve week old male 5-HT7−/− mice and their male 5-HT7+/+ sibling controls were used. The generation of the 5-HT7−/− mouse strain has been described previously (Hedlund et al 2003). Briefly, the 5-HT7−/− mice were created by targeted disruption within exon II of the 5-HT7 receptor gene, thus inactivating all known splice variants of the receptor protein. The inactivation was done in embryonic stem cells derived from 129Sv mice and breeding was then performed in C57BL/6J mice. The mice used in this study had been back-crossed on the C57BL/6J background for at least 14 generations.
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) arrived in the laboratory when they were 10−12 weeks of age and were group housed (4 per cage). Male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 250−275 g upon arrival in the laboratory were grouped housed (2 per/cage). For one week after arrival in the vivarium, animals were allowed to habituate to their new environment. Animals were housed in a humidity-(60±10%) and temperature-controlled (21±1°C) animal facility on a reversed light/dark cycle (lights off at 6 am for mice or 7 am for rats) with free access to food and water except during testing. All tests and other manipulations were conducted during the dark phase of the cycle. Animal facilities were AAALAC-approved, and protocols were in accordance with the “Guiding Principles in the Care and Use of Animals” (provided by the American Physiological Society) and the guidelines of the National Institutes of Health.
Drugs
The drugs amphetamine (Sigma, St. Louis, MO; 7.5 mg/kg, intraperitoneal injection in a volume 10 ml/kg given 15 min before a testing session), apomorphine (Sigma, St. Louis, MO; 5 mg/kg, subcutaneous injection in a volume 5 ml/kg given immediately before a testing session), or PCP (Sigma, St. Louis, MO; in mice: 10 mg/kg, intraperitoneal injection in a volume 10 ml/kg; in rats: 1.5 mg/kg, subcutaneous injection in a volume 1 ml/kg given 15 min before a testing session including 5 min habituation to startle box) were used to disrupt PPI. Amphetamine, PCP, and SB-269970 were dissolved in saline (0.9% NaCl); except for the experiment in rats where SB-269970 was dissolved in sterile water; apomorphine was dissolved in 0.1% ascorbic acid. Saline (0.9% NaCl) injections were used as controls for all drugs, except apomorphine for which 0.1% ascorbic acid served as a control.
SB-269970 (Sigma, St. Louis, MO; in mice: 3, 10, 15, 20, or 30 mg/kg, intraperitoneal injection in a volume 10 ml/kg; in rats: 10 mg/kg, subcutaneous injection in a volume 2 ml/kg, given 30 min before a testing session) was used to inhibit 5-HT7 receptors. The doses and time of administration were chosen based on previous studies in which pharmacokinetic analysis has shown that SB-269970 penetrates into the brain, at least in rats, with a maximum concentration after 30 min (Hagan et al 2000). Several studies have shown physiological effects within the dose range used (Hagan et al 2000; Hedlund et al 2005; Wesolowska et al 2006).
Acoustic startle procedure
Testing occurred in startle chambers (San Diego Instruments, San Diego, CA) that consisted of clear non-restrictive Plexiglas cylinders resting on a platform inside a ventilated and illuminated chamber. A high-frequency loudspeaker inside the chamber produced both a continuous background noise of 70 dB (mice) or 65 dB (rats) and the various acoustic stimuli. As described previously (Mansbach et al 1988), the whole-body startle response of the animal caused vibrations of the Plexiglas cylinder, which were then converted into analog signals by a piezoelectric unit attached to the platform. These signals were then digitized and stored by a computer and interface unit. Monthly calibrations were performed on the chambers to ensure the accuracy of the sound levels and measurements. Sound levels were measured as described previously (Mansbach et al 1988) using the dB(A) scale.
Before drug testing, C57BL/6J mice and Wistar rats underwent a brief startle session (5 min in duration) to create treatment groups matched for baseline startle reactivity and PPI response. During this baseline session, the 70-dB (mice) or 65-dB (rats) background noise was presented for 1 min and continued throughout the remainder of the session. A total of 17 trials (mice) or 24 trials (rats) were presented [five (mice) or four (rats) pulse-alone trials, followed in a pseudo-random order by six (mice) or nine (rats) 120-dB pulse alone and six 78 dB prepulse+pulse trials (mice) or six 77 dB prepulse+pulse trials (rats)].
During testing sessions animals were placed in the startle chambers and the background noise was presented for a 5 min acclimatisation period and continued throughout the test session. During a testing session, all trial types were presented several times in a pseudorandom order. In mouse studies, 60 trials were used to assess PPI (12 pulse-alone trials, 12 no stimulus trials, 12 74-dB prepulse+pulse trials, 12 78-dB prepulse+pulse trials, and 12 82-dB prepulse+pulse trials). In rat studies, 84 trials were used to assess PPI (12 pulse-alone trials, 42 no stimulus trials, 10 69-dB prepulse+pulse trials, 10 73-dB prepulse+pulse trials, and 10 77-dB prepulse+pulse trials). In addition, six pulse-alone trials, which were not included in the calculation of prepulse inhibition (PPI) values (because the most rapid habituation of the startle reflex occurs within the first few presentations of the startling stimulus), were presented at the beginning and six more pulse-alone trials at the end of each test session to assess startle habituation throughout the session. The time between trials averaged 15 s (ranging from 12 to 30 s) and the total duration of a test session was approximately 25 min. The pulse-alone trial consisted of a 40 ms 120 dB pulse of broadband noise. The prepulse+pulse trials consisted of a 20 ms noise prepulse, a 100 ms delay, then a 40-ms 120-dB startle pulse (120 ms onset-to-onset interval). The no stimulus trial consisted of background noise only and allowed the assessment by the piezoelectric accelerometer of general activity in the startle chamber when no acoustic stimuli were presented.
For the initial characterization of startle response and PPI in 5-HT7−/− mice, a longer testing session (approximately 60 min in duration) was created where pulse values varied from 70- to 120-dB in steps of 5; prepulse intervals were 20, 40, 60, 80, 100, 200, 500 ms; prepulse intensities were 74, 78, and 82 dB, with a 70 dB background level.
Experimental design
5-HT7−/− and 5-HT7+/+ mice
The first cohort of 5-HT7−/− and 5-HT7+/+ mice (n=12) was examined for their initial startle and PPI responses in the long startle session without any drug treatment. Then, over a 6-week period, these mice were used to evaluate the effects of the three different PPI-disruptive drugs in a repeated crossover design. Thus, we examined the effects of PCP (10 mg/kg, crossover design 1), apomorphine (5 mg/kg, crossover design 2), and amphetamine (7.5 mg/kg, crossover design 3). As a result, all mice received both drug and vehicle treatments during the course of the study, but there was a 2-week gap between different treatments. The second cohort of 5-HT7−/− and 5-HT7+/+ mice underwent crossover testing with PCP/vehicle only. There was no baseline session for 5-HT7−/− mice.
C57BL/6J mice
First we examined the effects of SB-269970 alone on PPI and startle. Injections of vehicle, 3, 10, or 30 mg/kg SB-269970 were given intraperitoneally 30 min before testing to 40 mice (n=10 per dose, between-subject design). Then, the interactive effects of PCP (vehicle, 10 mg/kg) and SB-269970 (vehicle, 10 mg/kg) on PPI and startle were examined in the same mice as well as in drug-naïve mice in a second replication of the experiment. Finally, a third cohort of C57BL/6J mice was used to further examine the interactive effects of PCP (vehicle, 10 mg/kg) and higher SB-269970 doses (vehicle, 15, and 20 mg/kg). For statistical analyses, all three cohorts of C57BL/6J mice were combined to evaluate the interactive effects of PCP and SB-269970.
Wistar rats
The interactive effects of PCP (vehicle, 1.5 mg/kg) and SB-269970 (vehicle, 10 mg/kg) on PPI and startle were examined (n=8−10 per dose, between-subject experimental design). The doses were chosen based on previously published findings showing normalization of PCP-disrupted PPI with the different, but structurally related, 5-HT7 receptor antagonist SB-258741 (Pouzet et al 2002).
Data analyses
The acoustic startle data (measures: startle amplitude and prepulse inhibition) were analyzed with a two-way or three-way ANOVA with Drug doses or Genotype as a between subjects factor, and Block (for startle amplitude) and Intensity (for PPI) as the respective within-subject factors. The amount of PPI was calculated as a percentage score for each prepulse trial type: % PPI = 100 – {[(startle response for prepulse+pulse)/(startle response for pulse-alone)] × 100}. To explore potential time-dependent effects of the manipulations made, all PPI data were divided into two blocks before analysis. As described below, this approach revealed several significant effects and for consistency all data were analyzed and presented this way. Startle amplitude was calculated as the average response to all of the pulse-alone trials, excluding the first and last blocks of five pulse-alone trials. For all experiments, statistically significant results were followed by Newman-Keuls post-hoc analyses. The level of significance was set at P < 0.05.
Results
Startle and PPI response in 5-HT7+/+ and 5-HT7−/− mice under baseline conditions
With startle pulse intensity varying from 70 to 120 dB, the startle response was similar in 5-HT7+/+ and 5-HT7−/− mice (Figure 1A) with a greater response at higher intensities. ANOVA analyses revealed a significant effect of Pulse (F(7,154)=41.76, p<0.0001), but no significant effect of Genotype or their interaction. With a fixed pulse intensity of 120 dB, there was no difference in PPI with prepulse intensities of 74, 78, or 82 dB in 5-HT7+/+ and 5-HT7−/− mice (Figure 1B). PPI increased with increasing prepulse intensity in both genotypes as indicated by a significant main effect of Prepulse intensity (F(2,44)=40.79, p<0.0001). There were no significant effects of Genotype or their interaction. There was also no difference in the PPI response between the two genotypes when PPI was assessed at interstimulus intervals ranging from 25 ms to 500 ms (Figure 1C). ANOVA analyses revealed a significant effect of Interstimulus interval (F(6,132)=14.46, p<0.0001), but no effect of Genotype or their interaction.
Figure 1.
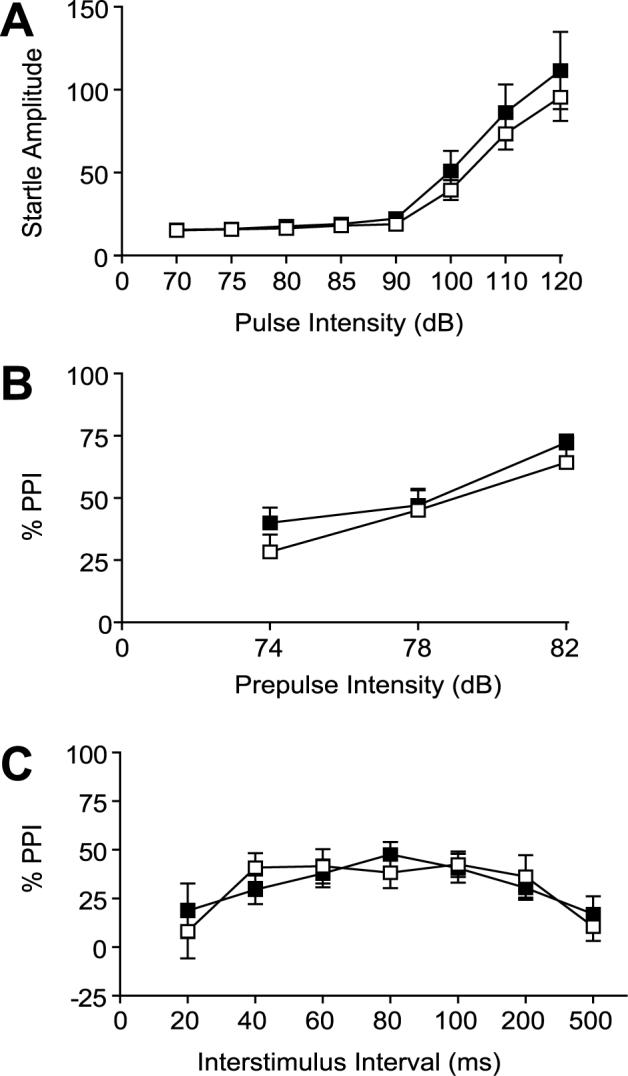
Startle response and PPI was not altered in 5-HT7−/− mice (■) compared to 5-HT7+/+ mice (□). Startle amplitude (A) was measured within a pulse intensity range of 70−120 dB. Values (arbitrary units) represent mean startle amplitude ± SEM. PPI with a fixed pulse intensity of 120 dB (B) was tested at three prepulse intensities (74, 78, and 82 dB). Values are presented as mean % prepulse inhibition ± SEM. PPI response with a fixed pulse intensity of 120 dB and a prepulse intensity of 82 dB (C) was measured with prepulse intervals ranging from 20−500 ms. Values are presented as mean % prepulse inhibition ± SEM. n = 12 per group.
Effects of PCP on startle and PPI response in 5-HT7+/+ and 5-HT7−/− mice
There was no difference in the startle response of 5-HT7+/+ or 5-HT7−/− mice after PCP (10 mg/kg) administration (Table 1). ANOVA analyses on the startle reactivity data revealed no significant PCP × Genotype or PCP × Genotype × Block of startle interactions. Nevertheless, there was a significant PCP × Block interaction (F(3,192)=9.92, p<0.0001). There was also a significant effect of PCP treatment (F(1,64)=8.92, p<0.01) on startle reactivity and a significant effect of Genotype (F(1,64)=5.37, p<0.05), but no interaction of PCP × Genotype. PCP induced a slight increase in startle reactivity mainly in block 1 of the startle session and this effect dissipated in blocks 3 and 4 indicating similar habituation of the startle response in both 5-HT7+/+ or 5-HT7−/− mice treated either with PCP or vehicle.
Table 1.
Phencyclidine, apomorphine or amphetamine had no effect on startle response habituation in 5-HT7+/+ and 5-HT7−/− mice.
| Block 1 | Block 2 | Block 3 | Block 4 | |
|---|---|---|---|---|
| Phencyclidine | ||||
| 5-HT7+/+ (vehicle) | 129.57±13.6 | 142.41±16.7 | 108.14±14.4 | 101.75±14.7 |
| 5-HT7+/+ (10 mg/kg) | 221.88±26.88 | 169.72±19.11 | 134.09±16.63 | 146.46±22.74 |
| 5-HT7−/− (vehicle) | 103.07±13.11 | 95.96±10.11 | 102.4±9.14 | 98.78±10.86 |
| 5-HT7−/− (10 mg/kg) | 172.08±15.36 | 145.36±13.25 | 104.62±14.87 | 94.03±10.71 |
| Apomorphine | ||||
| 5-HT7+/+ (vehicle) | 127.74±18.56 | 96.8±14.24 | 92.05±13.92 | 82.59±14.52 |
| 5-HT7+/+ (5 mg/kg) | 70.39±7.77 | 68.28±10.76 | 68.49±9.53 | 54.82±8.35 |
| 5-HT7−/− (vehicle) | 176.92±34.48 | 170.97±24.08 | 136.82±24.29 | 126.9±17.18 |
| 5-HT7−/− (5 mg/kg) | 91.64±13.17 | 90.99±10.48 | 81.97±14.27 | 85.13±13.41 |
| Amphetamine | ||||
| 5-HT7+/+ (vehicle) | 95.10±11.4 | 69.3±5.94 | 62.10±8.76 | 73.3±11.74 |
| 5-HT7+/+ (7.5 mg/kg) | 67.9±10.7 | 71.6±10.9 | 60.7±10.9 | 55.0±10.8 |
| 5-HT7−/− (vehicle) | 145.5±35.6 | 117.7±29.4 | 106.6±28.10 | 84.3±21.9 |
| 5-HT7−/− (7.5 mg/kg) | 105.1±17.02 | 90.4±16.64 | 79.1±13.5 | 61.8±12.3 |
Values (arbitrary units) are presented as mean of startle amplitude ± SEM in blocks 1−4, where each block consisted of 6 trials.
The PPI data were separated into two blocks for analyses, with the greatest effects of PCP treatment being observed in the first block (Figure 2A). ANOVA analyses of PPI data in Block 1 demonstrated a significant effect of Genotype (F(1,64)=4.32, p<0.05), PCP treatment (F(1,64)=13.48, p<0.001) and their interaction (F(1,64)=4.48, p<0.05). There was a significant effect of Prepulse intensity (F(2,128)=69.22, p<0.0001), but no interaction between the factors Genotype, PCP treatment and Prepulse intensities. Visual inspection of the data showed that the disruptive effects of PCP were more pronounced in 5-HT7+/+ mice compared to 5-HT7−/− mice. Additional statistical analyses revealed that PCP significantly (Newman-Keuls test, p<0.01) decreased PPI in 5-HT7+/+ mice at the tested prepulse intensities of 74, 78, and 82 dB. By contrast, the effects of PCP on PPI were not significant in 5-HT7−/− mice, except at prepulse intensity 78 dB where there was a small, but significant, decrease in PPI in 5-HT7−/− mice treated with PCP compared with 5-HT7−/− mice treated with vehicle.
Figure 2.
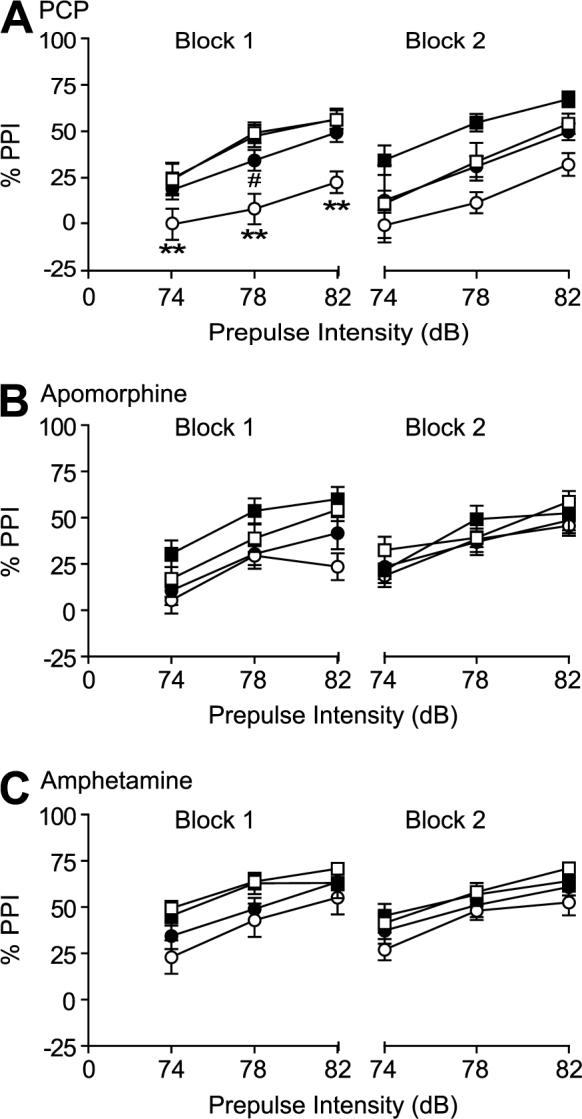
Effects of PCP (10 mg/kg, n=17/group, A), apomorphine (5 mg/kg, n=12/group, B) and amphetamine (7.5 mg/kg, n=12/group, C) on PPI response in 5-HT7+/+ mice (□, vehicle; ○, drug) compared to 5-HT7−/− mice (■, vehicle; •, drug). Values are presented as mean % prepulse inhibition ± SEM. PPI was tested at three prepulse intensities (74, 78, and 82 dB). PCP disrupted PPI (B) in 5-HT7+/+ mice, but failed to do so in 5-HT−/− mice; and the PPI response was significantly lower in 5-HT7+/+ mice after PCP disruption compared to 5-HT7−/− mice at all prepulse intensities tested (** p < 0.01, ANOVA followed by Newman-Keuls test). In 5-HT7−/− mice, PCP significantly disrupted PPI only at the prepulse intensity of 78 dB and only in block 1 (# p<0.05, Newman-Keuls test). The PPI disruptive effect of apomorphine and amphetamine was similar in both 5-HT7+/+ and 5-HT7−/− mice.
ANOVA analyses on PPI data in block 2 demonstrated a significant effect of Genotype (F(1,64)=6.58, p<0.05), PCP treatment (F(1,64)=7.91, p<0.01) and Prepulse intensities (F(2,128)=48.47, p<0.0001). However, there were no interactions between the factors indicating no differences between 5-HT7+/+ and 5-HT7−/− mice in PPI response after vehicle or PCP administration.
Effects of apomorphine on startle and PPI response in 5-HT7+/+ and 5-HT7−/− mice
There was no difference in startle response between 5-HT7+/+ and 5-HT7−/− mice after apomorphine (5 mg/kg) administration (Table 1). ANOVA analyses on the startle reactivity data revealed no Apomorphine × Genotype or Apomorphine × Genotype × Block interactions, but a significant Apomorphine × Block interaction (F(3,129)=3.58, p<0.05). There was a significant effect of Apomorphine treatment (F(1,43)=11.9, p<0.001) and Genotype (F(1,43)=6.73, p<0.01) on startle reactivity. 5-HT7−/− mice treated with vehicle showed a trend for higher startle response compared to 5-HT7+/+ mice treated with vehicle.
Again, for consistency in data presentation, the PPI data were separated into two blocks for analyses (Figure 2B). ANOVA analyses on PPI data from block 1 demonstrated a significant effect of Apomorphine treatment (F(1,43)=9.2, p<0.001), Prepulse intensity (F(2,86)=53.25, p<0.0001), but no significant effect of Genotype, Genotype × Apomorphine or Genotype × Apomorphine × Prepulse intensity interactions.
ANOVA analyses on PPI data from block 2 demonstrated a significant effect of Prepulse intensities (F(2,86)=53.08, p<0.0001), but no effect of Genotype or Apomorphine treatment. There were no interactions between the factors indicating no differences between 5-HT7+/+ and 5-HT7−/− mice in PPI response after vehicle or apomorphine administration.
Effects of amphetamine on startle and PPI response in 5-HT7+/+ and 5-HT7−/− mice
There was no difference in startle response habituation of 5-HT7+/+ or 5-HT7−/− mice after amphetamine (7.5 mg/kg) administration (Table 1). ANOVA analyses on the startle reactivity data revealed no significant Amphetamine × Genotype or Amphetamine × Genotype × Block interactions, but a significant Amphetamine × Block interaction (F(3,132)=3.08, p<0.05) and a significant Genotype × Block interaction (F(3,132)=5.21, p<0.01). However, there was no significant effect of either Amphetamine treatment or Genotype on startle reactivity.
Amphetamine administration disrupted PPI response in both 5-HT7+/+ and 5-HT7−/− mice (Figure 2C). ANOVA analyses on PPI response data from block 1 demonstrated a significant effect of Amphetamine treatment (F(1,44)=6.65, p<0.01) and Prepulse intensity (F(2,88)=55.82, p<0.0001), but no significant effect of Genotype, Genotype × Amphetamine or Genotype × Amphetamine × Prepulse intensities interactions.
ANOVA analyses of PPI data from block 2 demonstrated significant effects of Amphetamine treatment (F(1,44)=4.49, p<0.05) and Prepulse intensities (F(2,88)=53.31, p<0.0001), but no effect of Genotype. There were no interactions between the factors indicating no differences between 5-HT7+/+ and 5-HT7−/− mice in PPI response after vehicle or amphetamine administration.
In view of the crossover design used, additional ANOVAs were performed to explore potential carry-over effects of the drugs. These analyses revealed no interaction between the factors Genotype and Crossover design (data not shown).
Effects of SB-269970 on PPI and startle response in C57BL/6J mice
In C57BL/6J mice, SB-269970 did not alter the startle amplitude at any of the doses tested (Table 2; there was no significant Treatment effect or Treatment × Block interaction). PPI was also not affected by SB-269970 at any prepulse intensity tested (Table 2; there was no significant Treatment effect or Treatment × Prepulse intensities interaction).
Table 2.
SB-269970 had no effect on PPI or startle response under baseline conditions in wild-type (C57BL/6J) mice.
| SB-269970 (mg/kg) | ||||
|---|---|---|---|---|
| Vehicle | 3 | 10 | 30 | |
| Prepulse inhibition response | ||||
| 74 dB prepulse | 27.8±6.56 | 27.13±3.93 | 20.83±5.88 | 19.43±6.07 |
| 78 dB prepulse | 33.9±5.81 | 35.13±6.29 | 31.2±6.4 | 28.64±3.65 |
| 82 dB prepulse | 43.4±8.19 | 43.9±6.91 | 45.76±5.11 | 37.16±5.48 |
| Startle response | ||||
| Block 1 | 238.33±33.51 | 217.72±46.01 | 223.5±40.45 | 241.12±37.21 |
| Block 2 | 195.88±20.77 | 194.98±28.95 | 175.15±26.56 | 194.7±31.08 |
| Block 3 | 187.85±26.57 | 153.00±20.31 | 163.38±16.58 | 172.5±21.75 |
| Block 4 | 169.00±23.55 | 149.37±29.84 | 177.95±31.78 | 138.5±29.52 |
Values are presented as mean percent of PPI ± SEM or mean startle amplitude (arbitrary units) ± SEM. Startle response is presented as blocks 1−4, with each block being the mean of 6 trials.
Effects of SB-269970 on PCP-induced disruption of startle and PPI response in C57BL/6J mice
ANOVA analyses on startle amplitude values revealed a significant effect of startle Block (F(3,396)=37.63, p<0.00001), SB-269970 treatment (F(3,132)=5.01, p<0.01) and a strong trend for an effect of PCP treatment (F(1.132)=3.66, p<0.058, ns). However, the SB-269970 × PCP × Block interaction was not significant indicating that startle habituation was similar in all treatment groups (Table 3).
Table 3.
Effects of PCP alone or in combination with SB-269970 on startle response in C57BL/6J mice and Wistar rats.
| Treatment | N | Block 1 | Block 2 | Block 3 | Block 4 |
|---|---|---|---|---|---|
| C57BL/6J mice | |||||
| vehicle + vehicle | 30 | 247.9±18.2 | 208.5±15.1 | 182.2±13.1 | 163.8±15.4 |
| vehicle + PCP (10 mg/kg) | 30 | 292.9±22.8 | 245.2±18.5 | 209.2±16.3 | 208.5±18.2 |
| SB-269970 (10 mg/kg) + vehicle | 20 | 199.62±19.6 | 176.7±16.6 | 154.2±15.1 | 153.2±13.9 |
| SB-269970 (10 mg/kg) + PCP (10 mg/kg) | 20 | 223.5±18.2 | 192.3±17.5 | 170.1±14.9 | 156.2±12.2 |
| SB-269970 (15 mg/kg) + vehicle | 11 | 273.9±28.4 | 261.3±20.2 | 202.8±14.8 | 218.1±17.2 |
| SB-269970 (15 mg/kg) + PCP (10 mg/kg) | 10 | 287.8±38.8 | 273.7±29.4 | 232.9±26.3 | 212.2±25.2 |
| SB-269970 (20 mg/kg) + vehicle | 10 | 224.3±22.3 | 216.1±22.2 | 200.3±23.5 | 204.3±20.1 |
| SB-269970 (20 mg/kg) + PCP (10 mg/kg) | 9 | 278.8±37.8 | 254.4±29.8 | 243.5±39.8 | 224.5±27.0 |
| Wistar rats | |||||
| vehicle + vehicle | 10 | 1673.4±177.8 | 1339.4±225.5 | 969.9±154.2 | 794.4±143.3 |
| vehicle + PCP (1.5 mg/kg) | 8 | 1384.3±187.1 | 1103.0±132.0 | 800.5±128.8 | 799.4±142.9 |
| SB-269970 (10 mg/kg) + vehicle | 10 | 1611.8±167.2 | 1183.3±183.8 | 962.4±134.9 | 868.8±137.0 |
| SB-269970 (10 mg/kg) + PCP (1.5 mg/kg) | 10 | 1589.8±192.9 | 1308.0±164.5 | 982.7±179.2 | 872.3±170.9 |
PCP alone or in combination with SB-269970 had no effect on startle response in either C57BL/6J mice or Wistar rats. Values (arbitrary units) are presented as mean startle amplitude ± SEM. Startle response is presented in blocks 1−4.
As expected, PCP disrupted PPI in C57BL/6J mice (F(1,132)=34.35, p<0.00001). Pretreatment with SB-269970 did not significantly alter the PCP-induced disruption (Table 4, there was no SB-269970 × PCP interaction). However, the effect of treatment with SB-269970 was significant (F(3,132)=2.8, p<0.05). Although there was a significant effect of SB-269970 treatment, there was no significant interaction with the factor PCP, and there were no significant post-hoc effects. Separate ANOVA analyses on PPI values in block 1 and 2 revealed similar results (data not shown).
Table 4.
SB-269970 had no effect on PCP-disrupted PPI response in C57BL/6J mice or Wistar rats.
| Treatment | N | Prepulse intensity (dB) | ||
|---|---|---|---|---|
| 74 | 78 | 82 | ||
| C57BL/6J mice | ||||
| vehicle + vehicle | 30 | 25.01±3.10 | 34.2±3.68 | 45.81±3.57 |
| vehicle + PCP (10 mg/kg) | 30 | 1.31±3.69 | 10.8±3.57 | 21.38±4.65 |
| SB-269970 (10 mg/kg) + vehicle | 20 | 26.56±3.67 | 41.51±4.27 | 58.6±3.25 |
| SB-269970 (10 mg/kg) + PCP (10 mg/kg) | 20 | 4.68±4.33 | 18.64±3.06 | 33.93±4.75 |
| SB-269970 (15 mg/kg) + vehicle | 11 | 17.48±2.57 | 25.19±3.08 | 35.19±2.86 |
| SB-269970 (15 mg/kg) + PCP (10 mg/kg) | 10 | 1.25±4.14 | 17.7±7.81 | 26.08±5.55 |
| SB-269970 (20 mg/kg) + vehicle | 10 | 17.69±3.8 | 26.34±3.7 | 39.48±2.62 |
| SB-269970 (20 mg/kg) + PCP (10 mg/kg) | 9 | 5.12±9.34 | 9.45±10.0 | 27.98±7.30 |
| Treatment | N | Prepulse intensity (dB) | ||
|---|---|---|---|---|
| 69 | 73 | 77 | ||
| Wistar rats | ||||
| vehicle + vehicle | 10 | 29.17±8.67 | 51.85±8.11 | 55.86±8.07 |
| vehicle + PCP (1.5 mg/kg) | 8 | 20.47±6.66 | 35.23±7.25 | 37.41±8.09 |
| SB-269970 (10 mg/kg) + vehicle | 10 | 30.16±5.96 | 46.96±7.08 | 58.67±7.64 |
| SB-269970 (10 mg/kg) + PCP (1.5 mg/kg) | 10 | 7.96±5.27 | 23.66±6.32 | 33.20±7.72 |
Values are presented as mean percent of PPI ± SEM.
Effects of SB-269970 on PCP-induced disruption of startle and PPI response in Wistar rats
ANOVA analyses on startle amplitude values revealed a significant effect of Block indicating a habituation effect (F(3,102)=52.48, p<0.00001), but no effects of either SB-269970 or PCP. The SB-269970 × PCP × Block interaction was not significant indicating that startle habituation was similar in all treatment groups (Table 3).
As expected, PCP disrupted PPI in rats (F(1,34)=8.95, p<0.01). Pretreatment with SB-269970 did not significantly alter the PCP-induced disruption (Table 4, effect of treatment with SB-269970 or SB-269970 × PCP interaction were not significant). Separate ANOVA analyses on PPI values from blocks 1 and 2 revealed similar results (data not shown).
Discussion
The main finding of the present study was that inactivation of the 5-HT7 receptor partially prevented PPI disruption by PCP without affecting the ability of amphetamine or apomorphine to disrupt PPI.
Under baseline conditions, there were no differences between 5-HT7+/+ or 5-HT7−/− mice in startle response or PPI regardless of prepulse intensity (74−82 dB), inter-stimulus interval (25−500 ms), or pulse intensity (90−120 dB). These findings are in agreement with the results of a previous study where a different strain of 5-HT7 receptor knockout mice was used (Guscott et al 2005). The present data expand on these previously published results by also testing different interstimulus intervals and a wider range of pulse intensities. In general, both genotypes showed similar habituation responses over blocks of trials and a similar increase in PPI with increasing prepulse intensity.
There was no difference in startle response between 5-HT7+/+ and 5-HT7−/− mice following PCP treatment (10 mg/kg) during all four blocks of startle trials. As expected, PCP (10 mg/kg) disrupted PPI in 5-HT7+/+ mice. Nevertheless, PCP had a significantly lower ability to do so in 5-HT7−/− mice. The genotype difference was more pronounced early in the trial sequence (Block 1), when the effect of PCP on PPI was most pronounced (Figure 2). This timecourse of PCP action is consistent with its reported half-life (28 min) after administration of 10 mg/kg PCP, i.p. in ICR mice (Nabeshima et al 1982). Thus, it is not surprising that PCP-induced PPI disruption was less pronounced, although still significant, in Block 2 compared to Block 1.
In contrast, apomorphine (5 mg/kg) and amphetamine (7.5 mg/kg) continued to have significant PPI-disruptive effects in both 5-HT7+/+ and 5-HT7−/− mice. Thus, taken together these findings indicate a partial alteration in the glutamatergic regulation of the PPI model in the 5-HT7−/− mice. This conclusion is based on previous findings that the glutamatergic component of PPI is sensitive to atypical antipsychotics (Bakshi and Geyer 1995; Geyer and Ellenbroek 2003; Geyer et al 2001). By contrast, the present results suggest that there is no involvement of the 5-HT7 receptor in the dopaminergic disruption of the PPI response.
We also investigated the effects of the relatively selective 5-HT7 receptor antagonist SB-269970 (Hagan et al 2000) on mice and rats. SB-269970 did not affect startle amplitude or PPI at any dose or prepulse intensity tested. SB-269970 also did not have any effect on PCP-induced disruptions of PPI. This lack of modulatory actions of SB-269970 on the effects of PCP on PPI raises several issues. First, it has been shown that it is possible to block PCP-induced PPI disruption using a different selective 5-HT7 receptor antagonist, SB-258741, in Wistar rats (Pouzet et al 2002). To address the hypothesis that these differences in results may be attributable to species differences, we studied the effects of SB-269970 on PCP-induced disruption of PPI in this same strain of rats as in (Pouzet et al 2002). As in the C57BL/6J mice, we did not observe any effect of SB-269970 at the dose of 10 mg/kg that was effective when using SB-258741. An effect seen only in knockout animals may be due to compensatory mechanisms and thus of less physiological relevance. In all, however, the apparent discrepancies may also be due to drug effect differences. It seems most likely that SB-269970 behaves differently than SB-258741, a notion supported by the recent observation that SB-269970 seems to have a very narrow dose range in which it is effective (Wesolowska et al 2006) with no clear dose-response curves, suggesting differences in receptor binding profiles of these two drugs.
Second, in several previous studies it has been possible to mimic effects seen in 5-HT7−/− mice with selective 5-HT7 receptor antagonists. These actions include effects on thermoregulation (Hedlund et al 2003; Hedlund et al 2004), in animal models of depression and antidepressant activity (Guscott et al 2005; Hedlund et al 2005; Wesolowska et al 2006), and in sleep parameters (Hagan et al 2000; Hedlund et al 2005; Thomas et al 2003). These studies provide a solid foundation for the hypothesis that the effects of both blockade and inactivation of the 5-HT7 receptor should be similar. Third, it has been demonstrated that SB-269970 is cleared rapidly from the blood (Hagan et al 2000) and thus that the short half-life could explain the lack of effect. However, the same study found the highest brain concentration in rats 30 min after administration. As noted above, several studies have demonstrated physiological effects attributed to brain action of SB-269970 using similar doses and time intervals between administration and testing as in the present study making it unlikely that the short half-life explains the lack of effect (Hagan et al 2000; Hedlund et al 2005; Wesolowska et al 2006).
A high density of 5-HT7 receptors is found in thalamic, cortical, and limbic regions (Hedlund and Sutcliffe 2004; Thomas and Hagan 2004). These regions are also involved in the regulation of behavioral gating (for review, see (Braff et al 2001)) suggesting a possible role for 5-HT7 receptors in the pathophysiology of schizophrenia (e.g., PPI deficits). Further, atypical antipsychotics, that have affinity for 5-HT7 receptors (Roth et al 1994), have been shown to be effective in reversing PPI deficits induced by PCP or apomorphine (Bakshi et al 1994; Swerdlow et al 1994) supporting the hypothesis that 5-HT7 receptors are implicated in PPI. The neural mechanism of 5-HT7 receptor function has not been studied extensively. Recent findings suggest that the 5-HT7 receptors are probably not located on serotonergic neurons and may serve as heteroreceptors in regulation of 5-HT release in the raphe nuclei (Harsing et al 2004). It was recently reported that the selective 5-HT7 receptor antagonist SB-269970 inhibited 5-HT efflux in the dorsal raphe nucleus indirectly by activation of GABAA receptors (Roberts et al 2004). Further, SB-258719, also a selective 5-HT7 receptor antagonist, reversed inhibition of glutamate release induced by the nonselective 5-HT receptor agonist 5-carboxamido-tryptamine, suggesting that the axon terminals of the glutamatergic cortico-raphe neurons may possess 5-HT7 receptors that may modulate glutamate release (Harsing et al 2004). Increases in glutamatergic or serotonergic neurotransmission after administration of PCP (or other NMDA receptor antagonists) or serotonergic compounds (i.e., different 5-HT releasers, 5-HT2 and 5-HT1A receptor agonists) have been shown to disrupt PPI (for review, see (Geyer et al 2001)). Thus, it may be hypothesized that inactivation of the 5-HT7 receptor partially prevented disruption of the PPI response by, at least partially, preventing PCP from inducing serotonin and/or glutamate release. To our knowledge, possible interactions between 5-HT7 receptors and the dopaminergic system have not been studied prior to the present study. However, the lack of effect of 5-HT7 receptor blockade on amphetamine- or apomorphine-induced disruptions of PPI in the present data, together with our finding that SB-269970 and the finding that SB-258741 (Pouzet et al 2002) mediates reversal of PCP-induced PPI disruption suggest that 5-HT7 receptors play a partial role in modulating the glutamatergic, but not the dopaminergic, system.
In conclusion, the results indicate a partial role for the 5-HT7 receptor in the glutamatergic PPI model of schizophrenia that is sensitive to atypical antipsychotics and no involvement in the dopaminergic PPI model that is sensitive to typical antipsychotic drugs. In combination with selective antagonists, the 5-HT7 receptor knockout mice may provide a useful tool to study the role of 5-HT7 receptor function in the action of atypical antipsychotic drugs and schizophrenia. Furthermore, it may be hypothesized that atypical antipsychotics exert at least part of their effect through the 5-HT7 receptor. This effect may be through direct actions at the receptor, a notion supported by the finding that clozapine has relatively high affinity for the 5-HT7 receptor (Roth et al 1994). The effect may also be indirect through other receptor systems, presumably the dopamine D2 receptor. Further studies are required to evaluate the possible benefits of a 5-HT7 receptor antagonist in the treatment of schizophrenia.
Acknowledgments
This work was supported by NIH grants GM32355 (JGS), MH62527 (AM), DA02925 (MAG), and MH73923 (PBH) and by NARSAD (PBH). M.A. Geyer holds an equity interest in San Diego Instruments. We thank Jessica Benedict, Randy Ares, Chelsea Onifer, and Mahálah Buell for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakshi VP, Geyer MA. Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology (Berl) 1995;122:198–201. doi: 10.1007/BF02246096. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J. Pharmacol. Exp. Ther. 1994;271:787–94. [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–9. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol. Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, et al. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, et al. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG, Prauda I, Barkoczy J, Matyus P, Juranyi Z. A 5-HT7 heteroreceptor-mediated inhibition of [3H]serotonin release in raphe nuclei slices of the rat: evidence for a serotonergic-glutamatergic interaction. Neurochem. Res. 2004;29:1487–1497. doi: 10.1023/b:nere.0000029560.14262.39. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur. J. Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 2004;25:481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Kitajima T, et al. Positive association of the serotonin 5-HT7 receptor gene with schizophrenia in a Japanese population. Neuropsychopharmacology. 2006;31:866–71. doi: 10.1038/sj.npp.1300901. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology. 1989;2:299–308. doi: 10.1016/0893-133x(89)90035-3. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94:507–14. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–74. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Role of serotonin in the action of atypical antipsychotic drugs. Clin Neurosci. 1995;3:64–75. [PubMed] [Google Scholar]
- Monsma FJ, Jr., Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993;43:320–7. [PubMed] [Google Scholar]
- Nabeshima T, Sivam SP, Tai CY, Ho IK. Development of dispositional tolerance to phencyclidine by osmotic minipump in the mouse. J. Pharmacol. Methods. 1982;7:239–253. doi: 10.1016/0160-5402(82)90040-7. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Didriksen M, Arnt J. Effects of the 5-HT(7) receptor antagonist SB-258741 in animal models for schizophrenia. Pharmacol. Biochem. Behav. 2002;71:655–665. doi: 10.1016/s0091-3057(01)00744-4. [DOI] [PubMed] [Google Scholar]
- Roberts C, Thomas DR, Bate ST, Kew JN. GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacology. 2004;46:935–941. doi: 10.1016/j.neuropharm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, et al. Binding of typical and atypical antipsychotic agents to 5- hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Jr., Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch. Gen. Psychiatry. 1994;51:139–54. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr. Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Hagan JJ. 5-HT7 receptors. Curr. Drug Target CNS Neurol. Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, et al. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br. J. Pharmacol. 2003;139:705–714. doi: 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT(7) receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Zhukovskaya NL, Neumaier JF. Clozapine downregulates 5-hydroxytryptamine6 (5-HT6) and upregulates 5-HT7 receptors in HeLa cells. Neurosci. Lett. 2000;288:236–240. doi: 10.1016/s0304-3940(00)01225-8. [DOI] [PubMed] [Google Scholar]


