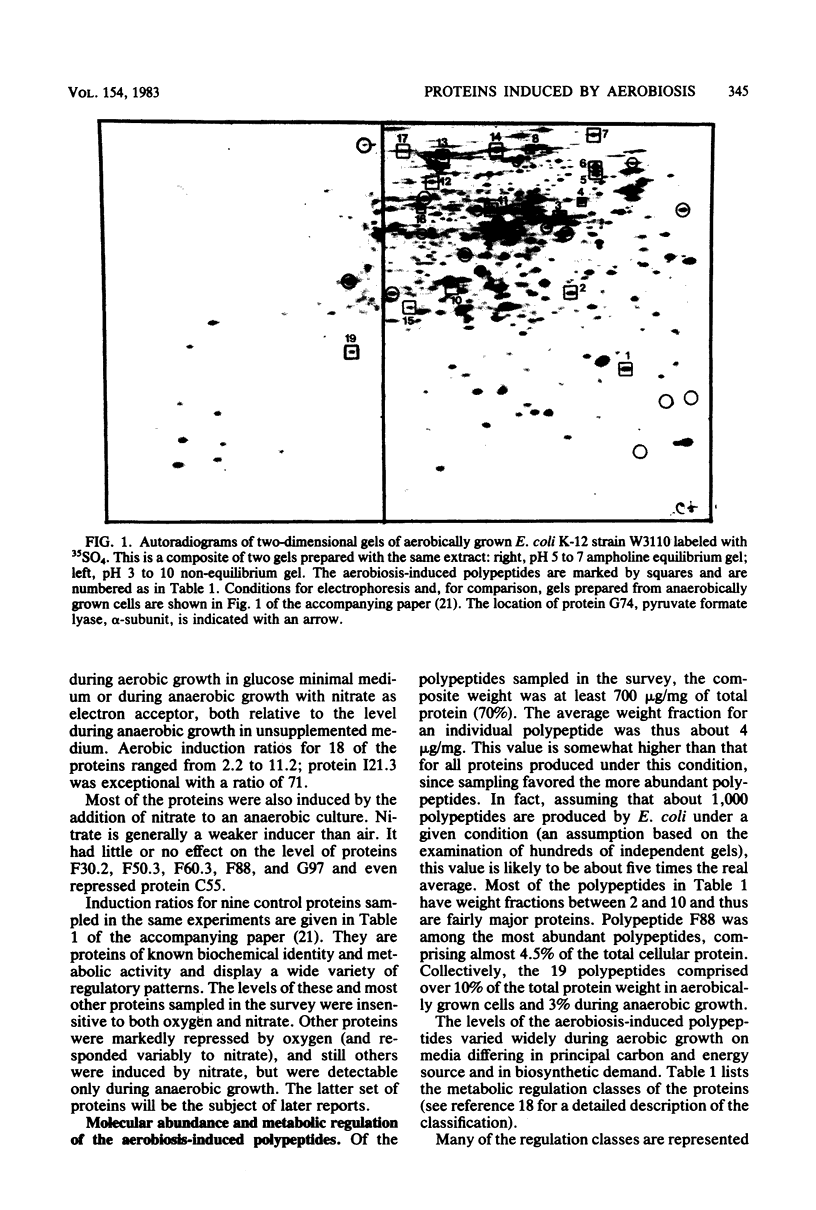
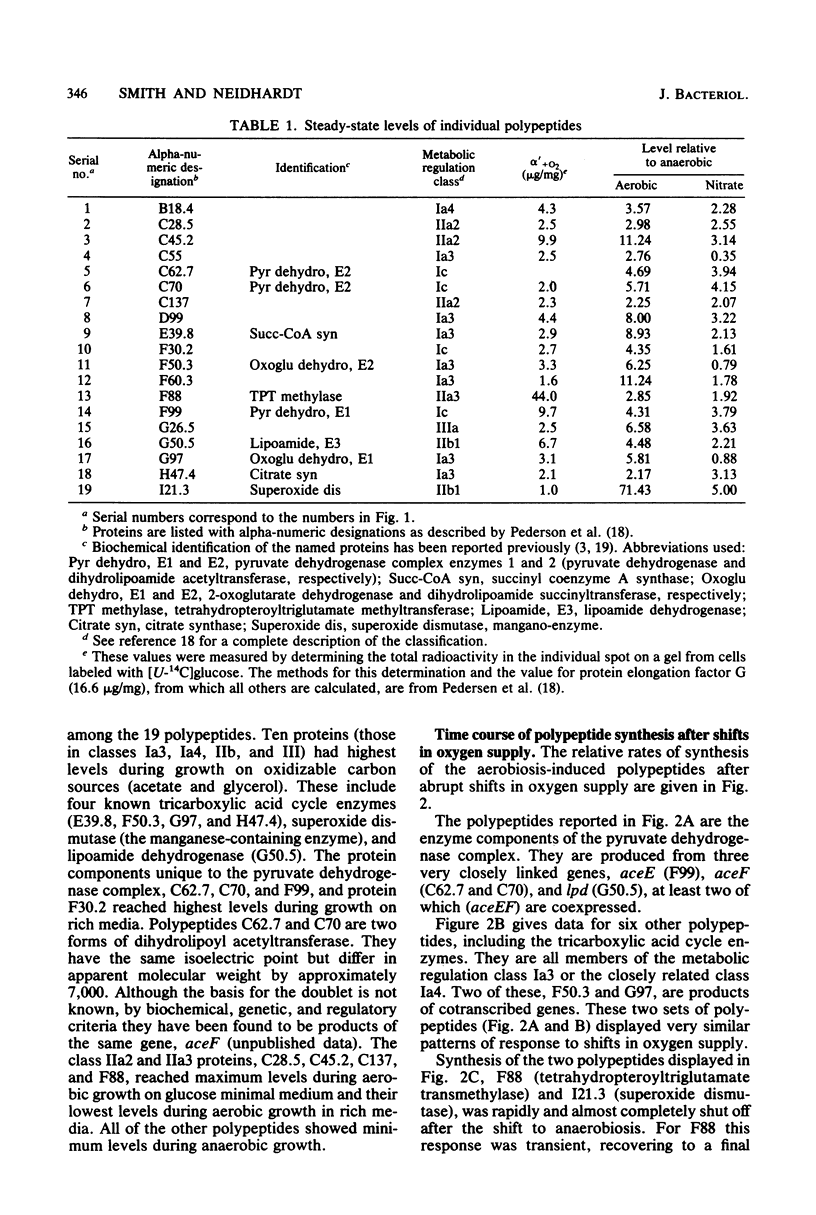
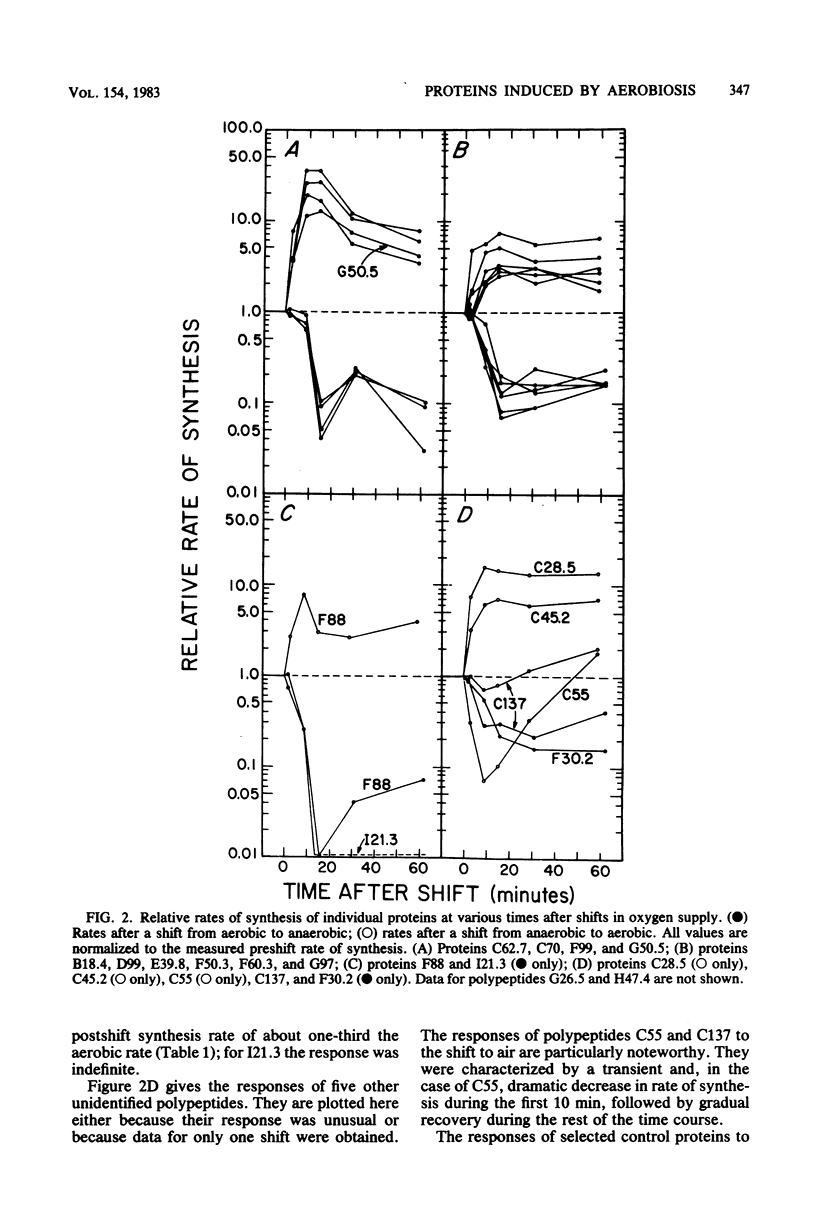
Abstract
The role of protein induction and repression in the adaptation of Escherichia coli to changes in the supply of oxygen and other electron acceptors is only poorly understood. We have studied the changes in cellular protein composition associated with this adaptation by measuring the levels of 170 individual polypeptides produced during aerobic or anaerobic growth of E. coli, with and without nitrate. Nineteen polypeptides had levels highest during aerobic growth. These proteins include the enzymes of the pyruvate dehydrogenase complex, several tricarboxylic acid cycle enzymes, superoxide dismutase, and tetrahydropteroyltriglutamate transmethylase. The other aerobiosis-induced proteins have not been identified. These polypeptides are major cellular proteins during aerobic growth and display several different patterns of regulation in response to medium composition. Induction ratios for oxygen ranged from 2.2 to 11.2, with one exceptional member, superoxide dismutase, increasing 71-fold with aeration. Most of the proteins were also induced by nitrate during anaerobic growth. The time course of induction after shifts in oxygen supply revealed similarities in response among proteins of related function or metabolic regulation class. These results are discussed in relation to previously reported information on the identified aerobiosis-induced proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch P. L., Phillips T. A., Neidhardt F. C. Protein identifications of O'Farrell two-dimensional gels: locations of 81 Escherichia coli proteins. J Bacteriol. 1980 Mar;141(3):1409–1420. doi: 10.1128/jb.141.3.1409-1420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Henning U. Regulation of pyruvate dehydrogenase complex synthesis in Escherichia coli K 12. Identification of the inducing metabolite. Eur J Biochem. 1970 Jun;14(2):258–269. doi: 10.1111/j.1432-1033.1970.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Dills S. E., Dobrogosz W. J. Cyclic adenosine 3',5'-monophosphate regulation of membrane energetics in Escherichia coli. J Bacteriol. 1977 Sep;131(3):854–865. doi: 10.1128/jb.131.3.854-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Stephens P. E. Molecular cloning of the pyruvate dehydrogenase complex genes of Escherichia coli. J Gen Microbiol. 1980 Dec;121(2):277–292. doi: 10.1099/00221287-121-2-277. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hespell R. B. Glycolytic and tricarboxylic acid cycle enzyme activities during intraperiplasmic growth of Bdellovibrio bacteriovorus on Escherichia coli. J Bacteriol. 1976 Nov;128(2):677–680. doi: 10.1128/jb.128.2.677-680.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- Langley D., Guest J. R. Biochemical genetics of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: isolation and biochemical properties of deletion mutants. J Gen Microbiol. 1977 Apr;99(2):263–276. doi: 10.1099/00221287-99-2-263. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- Mulligan J. T., Margolin W., Krueger J. H., Walker G. C. Mutations affecting regulation of methionine biosynthetic genes isolated by use of met-lac fusions. J Bacteriol. 1982 Aug;151(2):609–619. doi: 10.1128/jb.151.2.609-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y. Effect of glucose and cyclic adenosine 3',5'-monophosphate on the synthesis of succinate dehydrogenase and isocitrate lyase in Escherichia coli. J Biochem. 1975 Nov;78(5):1097–1100. doi: 10.1093/oxfordjournals.jbchem.a130987. [DOI] [PubMed] [Google Scholar]
- Thomas A. D., Doelle H. W., Westwood A. W., Gordon G. L. Effect of oxygen on several enzymes involved in the aerobic and anaerobic utilization of glucose in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1099–1105. doi: 10.1128/jb.112.3.1099-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Ishimoto M. Effect of nitrate reduction on the enzyme levels in carbon metabolism in Escherichia coli. J Biochem. 1975 Aug;78(2):307–315. doi: 10.1093/oxfordjournals.jbchem.a130909. [DOI] [PubMed] [Google Scholar]




