Abstract
This study examines the temporal properties of geniculocortical and corticogeniculate (CG) pathways that link the lateral geniculate nucleus (LGN) and primary visual cortex in the ferret. Using electrical stimulation in the LGN to evoke action potentials in geniculocortical and CG axons, results show that conduction latencies are significantly faster in geniculocortical neurons than in CG neurons. Within each pathway, axonal latency and visual physiology support the view of sub-classes of neurons. By examining the timing of visual responses and the latency of CG feedback, estimates indicate that visual information can reach the cortex and return to the LGN as early as 60 msec following the onset of a visual stimulus. These findings place constraints on the functional role of corticogeniculate feedback for visual processing.
Keywords: LGN, V1, corticogeniculate, geniculocortical
INTRODUCTION
Neurons in the lateral geniculate nucleus (LGN) of the thalamus play a pivotal role in visual processing. Not only are they the major source of feedforward input to the primary visual cortex, they also receive direct and robust feedback from the cortex. As a result of this reciprocal feedforward–feedback loop, the LGN and cortex share an intimate relationship whereby the cortex has the opportunity to dynamically influence its own supply of visual information. As a first step towards understanding the functional interactions between the geniculocortical and corticogeniculate pathways, it is important to determine the temporal properties that are associated with each pathway.
In this study, we examine the temporal properties of geniculocortical and corticogeniculate pathways in the ferret. Similar to other carnivores, the LGN in the ferret is a laminar structure with layers A and A1 that project primarily to layer 4 and the C layers that largely bypass layer 4 and project primarily to the superficial layers (LeVay and Gilbert, 1976; Zahs and Stryker, 1988). Although on-center and off-center neurons are segregated within sublamina in layers A and A1, there is no segregation of X and Y cells within these layers (Zahs and Stryker, 1985; McConnel and LeVay, 1986; Zahs and Stryker, 1988; Morgan and Thompson, 1993; Kawasaki et al., 2004). W cells are found exclusively in the C layers (Guillery and Oberdorfer, 1977; Kawasaki et al., 2004). Earlier studies in cats report that axonal conduction velocity of X, Y and W cells differ significantly from each other, with Y cells displaying the fastest conduction velocity and W cells the slowest (Reviewed in Stone, 1983). It remains to be determined whether geniculocortical axons in the ferret differ in their conduction velocity.
Similar to other mammals, corticogeniculate (CG) neurons are located in layer 6 of the ferret primary visual cortex. Based on their location and morphological properties, CG neurons have the opportunity to receive direct input from the LGN via either the collaterals of feedforward geniculocortical axons that provide weak input to layer 6 and/or their own apical dendrites that ramify within layer 4 (Gilbert and Wiesel, 1979; Lund et al., 1979; Katz, 1987; Callaway and Lieber, 1996; Usrey and Fitzpatrick, 1996; Nahmani and Erisir, 2005). Last, in both cats and ferrets, axons of the CG neurons are type 1 as described by Guillery (Guillery, 1969) and tend to target multiple lamina within the LGN (Robson, 1983; Claps and Casagrande, 1990). Whether CG axons in ferrets have a range of conduction velocities is unknown, however, previous results from cats indicate that axonal conduction velocity can be used to distinguish three distinct classes of neurons (Tsumoto and Suda, 1980).
Using electrical stimulation in the LGN to drive action potentials in geniculocortical and CG axons, we find that the two pathways differ significantly in their conduction latency. In addition, we find that both pathways contain a mixture of cell types. Finally, by measuring the temporal properties of visual responses in geniculocortical recipient (GCR) neurons and CG neurons, we estimate that the CG pathway is well suited to influence visual processing in the LGN within 60 msec of the onset of a visual stimulus. These results indicate that the geniculocortical and CG pathways should not be viewed in isolation because the evolution of visual responses in the visual cortex almost certainly includes effects mediated by dynamic interactions between the geniculocortical and CG streams.
OBJECTIVE
The primary goal of this study is to determine the temporal properties of feedforward and feedback pathways that link the LGN and visual cortex in the ferret. To accomplish this, we used electrical stimulation to identify GCR neurons and CG neurons in cortical area 17. We then measured response latencies following electrical and visual stimulation. For a subset of neurons in the dataset, we also measured visual response properties to sine-wave gratings and white-noise stimuli.
METHODS
This study used twelve adult ferrets (Mustela putorius furo), age years. All surgical and experimental procedures conformed to guidelines from the NIH, the American Physiological Society, the Society for Neuroscience and the Animal Care and Use Committee at the University of California, Davis.
Surgery and preparation
Surgical anesthesia was induced either by intramuscular injection of ketamine (40 mg kg−1) and acepromazine (0.04 mg kg−1) or isoflurane (2%). Following tracheotomy and stereotaxic placement, animals were ventilated artificially and anesthesia maintained with isoflurane (1–1.5%) in oxygen and nitrous oxide (2:1). For the duration of the experiment, the animal’s temperature, expired CO2, electrocardiogram (ECG) and electroencephalogram (EEG) were monitored continuously. A midline scalp incision was made and two small craniotomies were made, one over the LGN and the other over area 17. All wound margins were infused with lidocaine. The eyes were dilated with 1% atropine sulfate, fitted with contact lenses and focused on a tangent screen 76 cm in front of the animal. Following completion of all surgical procedures, animals were paralyzed with vecuronium bromide (0.2 mg kg−1 hr−1). Proper depth of anesthesia was ensured throughout the experiment by (1) monitoring the EEG for changes in slow-wave/spindle activity and (2) monitoring the ECG and expired CO2 for changes associated with a decrease in the depth of anesthesia. The concentration of isoflurane was increased immediately if any of these measures indicated a decrease in depth of anesthesia.
Identifying CG and GCR neurons
A pair of platinum/iridium electrodes (Frederick Haer and Co.) was inserted into the LGN to electrically stimulate geniculocortical and CG axons. These electrodes were placed in regions of the LGN that contained neurons with receptive fields that were in retinotopic register with those of neurons in the exposed area 17. To electrically excite regions around the electrodes, biphasic pulses (0.2 msec duration, ~10 volts) were delivered at intervals of >3 secs using an AM Systems isolated pulse stimulator.
Single-unit recordings were made from neurons in area 17 using tungsten-in-glass microelectrodes (Alan Ainsworth). Neuronal responses were amplified, filtered and recorded to a computer equipped with a Power 1401 data acquisition system and the Spike2 software package (Cambridge Electronic Design). Spike isolation was based on waveform analysis and the presence of a refractory period, as indicated by the autocorrelogram (Usrey et al., 2000; Usrey et al., 2003). To identify cortical neurons that either projected to the LGN or received input from the LGN, cortical electrodes were advanced slowly across the depth of area 17 while simultaneously stimulating the LGN. Once a unit was encountered that responded to the electrical stimulation, two experimental tests were performed to determine the identity of the unit. In the first test (a ‘non-collision’ test), responses were recorded following a shock delivered every 3–5 secs. This test was used to measure the latency and temporal precision of responses to electrical stimulation, and the reliability of evoked responses. With this test alone, however, it is not certain whether the cortical unit projects to the LGN or receives input from the LGN. This distinction was made clear with the second ‘collision test’. In the collision test, a spontaneous spike in the cortical neuron triggered (within 1 msec) the electrical shock. In the collision test, a lockout was used to ensure that shocks did not occur with intervals <3 secs. If the cortical unit was a CG neuron, then the antidromic spike resulting from the shock would collide with the spontaneous orthodromic spike and the antidromic spike would not reach the cortex. By contrast, if the cortical unit received feedforward input from the LGN, then the spike resulting from the shock would reach the cortex and drive a spike from the cortical neuron. Once the identity of a neuron was determined, the pulse stimulator was turned off and the visual-response properties of the neuron assessed as described below.
Visual stimuli
Visual stimuli were created with a VSG2/5 visual stimulus system (Cambridge Research Systems) and presented with a gamma-calibrated Sony monitor running at 140 Hz. The mean luminance of the monitor was 38 candelas m−2. Neurons were characterized using drifting, sine-wave gratings and white-noise stimuli. The grating stimulus was used to classify neurons as either simple cells or complex cells on the basis of the ratio of the first Fourier coefficient (f1) to mean response (simple cells, f1/mean >1.0; complex cells, f1/mean <1.0) (Skottun et al., 1991). For neurons with ratios >1.0, the white-noise stimulus was used to examine receptive fields in both space and time (Sutter, 1992; Reid et al., 1997). The white-noise stimulus consisted of a 16 × 16 grid of black and white squares. Each square in the grid was modulated independently in time according to an m-sequence of length 215 – 1. The stimulus was updated every 28.5 msec. Receptive-field maps and impulse responses were calculated using reverse-correlation analysis.
RESULTS
Using electrical stimulation to drive orthodromic and antidromic responses from neurons in area 17 of the ferret, we identified individual GCR neurons and CG neurons, respectively (Fig. 1). Fig. 2 shows the electrically evoked responses of two GCR neurons and two CG neurons. In this figure, all traces are aligned to the stimulus artifact (time zero). The blue traces show the average response of each neuron following an electrical shock delivered every 3 sec or 5 sec. Although each neuron faithfully produced a spike in response to the shock, they did so with different latencies, 1.7 msec and 4.7 msec for the two GCR neurons and 2.2 msec and 6.1 msec for the two CG neurons. The red traces in Fig. 2 show each neuron’s response to a collision test in which shocks are delivered immediately following (<1 msec) a spontaneous spike by the cortical neuron. If the cortical neuron was a CG neuron, then the spike that triggered the shock collided with the spike that resulted from the shock and, because of the refractory state of the axon, the cortical neuron did not respond to the shock (Fig. 2C, D, red traces). Alternatively, if the cortical neuron was a GCR neuron, then the two spikes did not collide and the cortical neuron responded to the shock with a spike at the fixed latency (Fig. 2A, B, red traces).
Fig. 1. Orthodromic and antidromic activation.
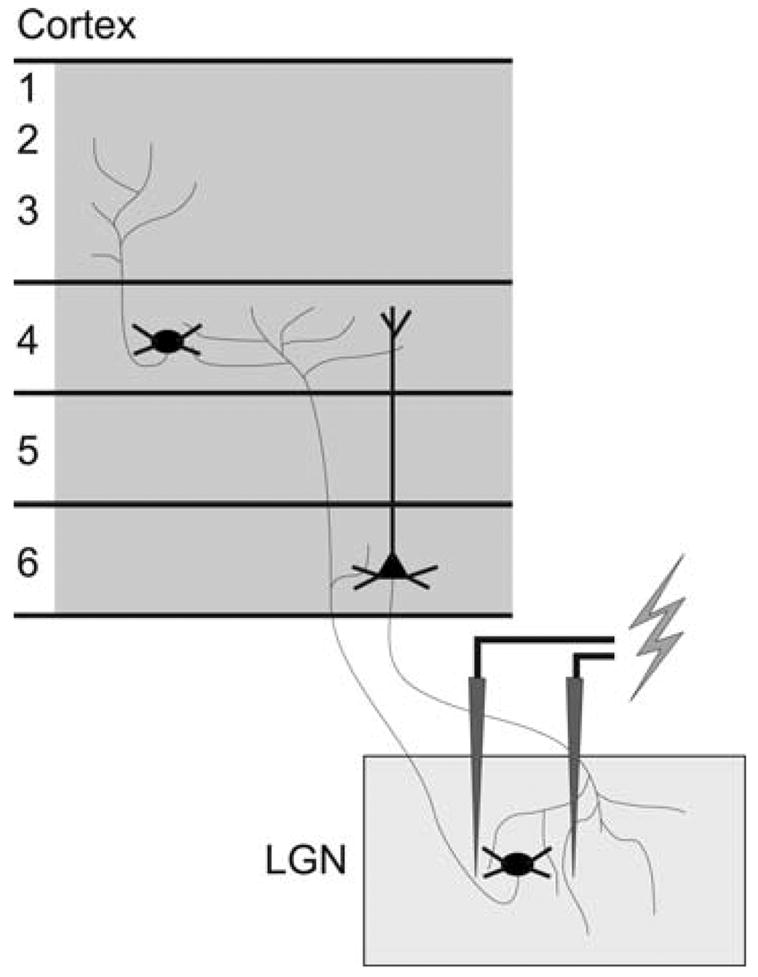
A schematic representation of the stimulation paradigm including a bipolar stimulating electrode in the LGN that orthodromically activates GCR neurons in layers 4 and 6, and antidromically activates CG neurons in layer 6.
Fig. 2. Identifying GCR neurons and CG neurons.
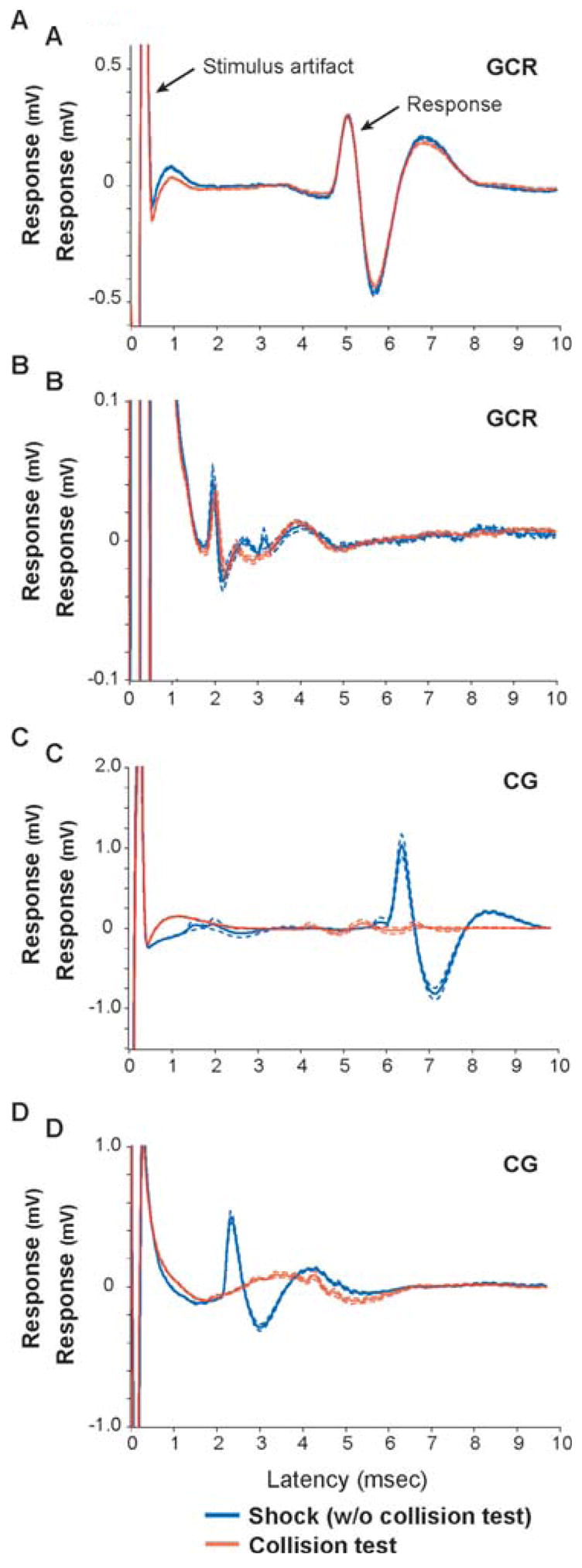
A–D. Averages of waveform traces for four representative neurons, two GCR neurons (A, B) and two CG neurons (C, D). Traces are aligned to the stimulus artifact representing time 0 (left arrow in top trace). Responses to shock trials (without collision test) are shown in blue and responses to collision trials are shown in red. Dashed lines indicate the standard error for each trial type average. The two GCR neurons have latencies of 4.7 msec and 1.7 msec. The two CG neurons have latencies of 6.1 msec and 2.2 msec.
In total, we identified 15 GCR neurons and 9 CG neurons. Fig. 3 shows the distribution of latencies (between stimulus and response) for both populations of neurons. Although the two populations have partially overlapping distributions, the distribution corresponding to the GCR population is shifted to the left, toward shorter latencies, compared with the CG population. This shift was significant (P < 0.05; t-test), with the mean latency between stimulus and response for GCR and CG neurons 2.9 ± 0.3 msec and 4.0 ± 0.6 msec, respectively (Fig. 3, inset). It should be noted that the GCR latency includes both the time required for spike propagation from LGN to cortex and the time required for synaptic transmission (Fig. 1). By contrast, the CG latency reflects only the time required for spike propagation. As a consequence, the two populations of neurons are even more distinct from each other in terms of their conduction velocities. Earlier studies in cats have shown that conduction velocity can distinguish different classes of LGN and CG neurons (Cleland et al., 1971; Hoffman et al., 1972; Tsumoto et al., 1978; Tsumoto and Suda, 1980). Along these lines, our sample of GCR neurons is not unimodal (P = 0.01, Hartigan’s Dip Test) because it contains neurons with at least two clusters of latency values (Fig. 3).
Fig. 3. Latency distributions for GCR and CG neurons.
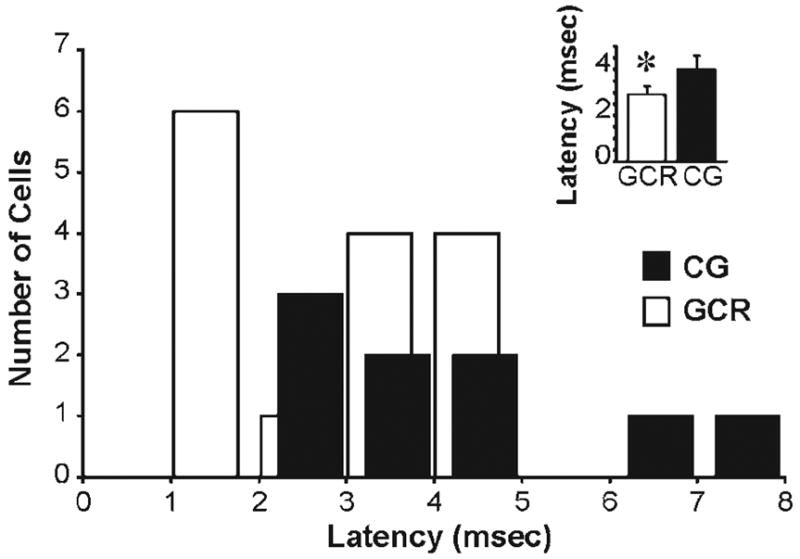
The histogram shows the latencies between shock and spike for 15 GCR neurons (white) and 9 CG neurons (black). Inset, average latencies of each population. GCR neurons had an average latency of 2.9 ± 0.3 and CG neurons had an average of 4.0 ± 0.6. *, latencies for GCR and CG neurons are significantly different (P < 0.05, t-test).
For a subset of neurons in our sample, we measured visual responses to a drifting sine-wave grating. By comparing the first Fourier coefficient (f1) to mean response, this stimulus can quantitatively distinguish simple cells from complex cells, because the f1:mean is >1.0 for simple cells and <1.0 for complex cells (Skottun et al., 1991). As shown in Fig. 4A, both populations of GCR and CG neurons contained a mixture of simple cells and complex cells. Because simple cells are the dominant cell type in layer 4 of ferrets (Usrey et al., 2003), it seems likely the GCR neuron with complex characteristics resides in layer 6 (Fig. 1).
Fig. 4. Visual physiology of GCR and CG neurons.
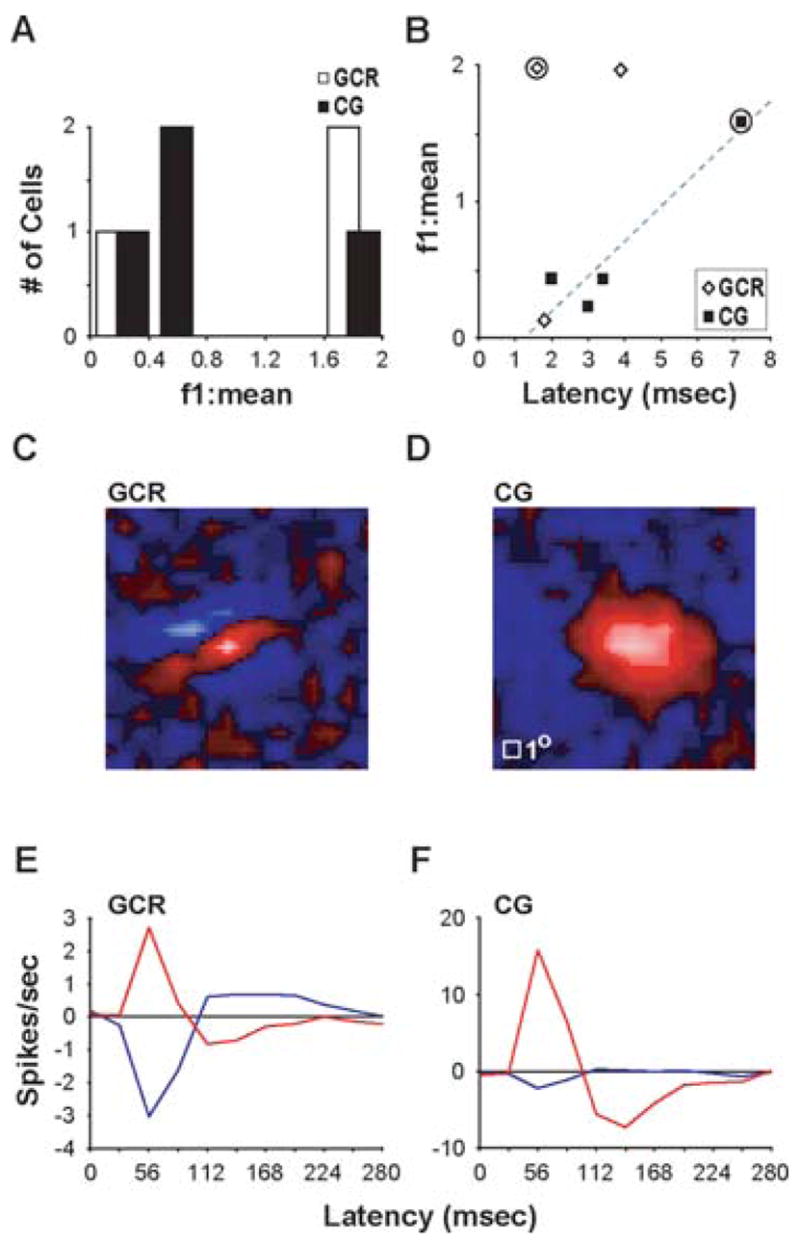
A. The first Fourier component to mean response (f1:mean) distribution for three GCR neurons (white) and four CG neurons (black). B. Relationship between f1:mean and conduction latency. Circles indicate the neurons illustrated in C–F. Gray dashed line indicates the linear fit to the CG neuron data. Regression analysis revealed a linear relationship between f1:mean and conduction latency for the CG neurons (R2 = 0.89), however the data were not significantly fit to the model (P = 0.056). C, D. White-noise receptive field maps for a GCR neuron (C) and a CG neuron (D) with f1:mean >1. Red pixels indicate “On” subregions, blue pixels indicate “Off” subregions. White square at lower left of CG map represents 1 degree of visual angle for both maps. E, F. Impulse responses showing the time course of visual responses for the example neurons in C and D. Red and blue curves represent the timing of the “On” and “Off” subregion of the receptive field, respectively.
In cats, CG neurons with complex receptive fields have shorter conduction latencies than CG neurons with simple receptive fields (Tsumoto and Suda, 1980). Consistent with this, complex cells in our sample all had short latencies (mean, 2.8 ± 0.5 msec) (Fig. 4B). Although most of the neurons with longer latencies were difficult to characterize with visual stimuli, it is interesting to note that the single simple cell in our sample of CG neurons had a longer latency (7.2 msec) (Fig. 4B).
Having determined that the time required for spikes to propagate from LGN to cortex and from cortex to LGN is short (a few milliseconds each way), we asked how soon following the onset of a visual stimulus does the cortex influence LGN activity. To do this we used a white-noise stimulus and reverse-correlation analysis to examine the time course of visual responses in GCR and CG neurons. Fig. 4C–F shows the spatial receptive fields and impulse responses of a GCR neuron and a CG neuron. The peak response of the GCR neuron occurred at a latency of 56 msec following stimulus onset (Fig. 4E). This value is in accordance with previous published values for layer 4 neurons in ferrets (61.2 ± 8.7 msec) (Usrey et al., 2003). Importantly, the peak visual response of the CG neuron also occurred at 56 msec (Fig. 4F). Given that the mean latency for spikes to propagate from CG neurons to the LGN is 4 msec (Fig. 3), cortical influences on LGN activity are likely to occur within 60 msec of stimulus onset.
CONCLUSIONS
GCR neurons have significantly shorter conduction latencies than CG neurons.
Populations of GCR and CG neurons contain both simple cells and complex cells.
CG neurons with complex receptive fields have short conduction latencies.
Based on conduction latency and timing of visual responses, cortical activity is likely to influence LGN activity within 60 msec following the onset of a visual stimulus.
DISCUSSION
In this study, we examined the temporal properties of feed-forward and feedback pathways between the LGN and visual cortex in the ferret. Our results show that spike propagation in both directions can be fast, with the fastest transmission occurring in the feedforward pathway. Given the time course of visual responses in area 17, our results further indicate that cortical feedback can influence LGN activity as early as 60 msec following stimulus onset. In the sections below, we compare our results from the ferret with those of other species and consider their functional implications for sensory processing.
The feedforward pathway from LGN to visual cortex
Across species, distinct classes of relay neurons provide the cortex with information about the visual environment (Reviewed in Stone, 1983; Steriade et al., 1997; Sherman and Guillery, 2005). These classes can be identified using several criteria, including anatomy, visual physiology and axon-conduction velocity. Along these lines, studies in cats and tree shrews have shown that X and Y relay cells differ significantly in their conduction latency, and that Y cells respond with a shorter latency following electrical stimulation of their axons than X cells (Cleland et al., 1971; Hoffman et al., 1972; Sherman et al., 1975). In the present study, we recorded the latency required for cortical neurons to respond to LGN input. Our results reveal a bimodal distribution of neurons with latencies clustered at 1–2 msec and 3–5 msec, which corresponds exactly to the conduction time of Y and X cell afferents, respectively. In cats, studies examining the organization of X and Y cell input into layer 4 suggest that the X and Y streams might be largely segregated (Ferster and LeVay, 1978; Bullier and Henry, 1979; Leventhal 1979; but see Humphrey et al., 1985; Alonso et al., 1996). Although our results are consistent with the idea that distinct populations of cortical neurons receive exclusively X- or Y-cell input, we cannot rule out the possibility of mixed X- and Y-cell input to individual cortical recipient neurons. For example, our sample of GCR neurons might include neurons with both short and longer latency input, with the longer latency input masked by the refractory state of the neuron.
Although we did not make lesions and reconstruct electrode tracts in the current study, almost all of the GCR neurons were recorded from at depths consistent with their location in layer 4. A notable exception, however, came from an electrode penetration where we first recorded from a CG neuron and then, at a slightly deeper depth, recorded from a GCR neuron. Because CG neurons reside exclusively in layer 6 (Gilbert and Kelly, 1975; Lund et al., 1975; Hendrickson et al., 1978; Swadlow and Weyand, 1981; Katz, 1987; Fitzpatrick et al., 1994; Usrey and Fitzpatrick, 1996), it seems likely that at least one of our 15 GCR neurons was a layer 6 neuron. As in the cat and monkey, there is a direct projection from the LGN to layer 6 in the ferret, however, this projection is much more sparse than that directed to layer 4 and less likely to drive responses (LeVay and Gilbert, 1976; Hendrickson et al., 1978; Fitzpatrick et al., 1983; Humphrey et al., 1985; Nahmani and Erisir, 2005). Consistent with this view, none of the CG neurons in our sample were driven by direct LGN input.
Because CG neurons project both to the LGN and to layer 4 of visual cortex, there is the possibility that some of the neurons we identified as GCR neurons might have been synaptically driven by the antidromic spike of a CG neuron traveling along an axon collateral rather than by the orthodromic spike of an LGN afferent. We cannot rule out this possibility, but believe it is unlikely for a number of reasons. First, previous studies have shown that the onset latency and threshold for activation are much greater for CG axon collaterals than for geniculocortical axons (Ferster and Lindstrom, 1985). Second, the response amplitude of layer 4 neurons to layer 6 input is relatively small, potentially sub-threshold, and is, thus, inconsistent with the responses we see (Ferster and Lindstrom, 1985; Stratford et al., 1996). Finally, in our sample many of the GCR neurons have activation latencies that are shorter than the fastest CG neuron, thus, it seems unlikely that these particular neurons are mislabeled.
The feedback pathway from visual cortex to the LGN
Compared with the geniculocortical pathway, much less is known about the anatomy and physiology of the CG pathway. In particular, we lack a firm understanding of the role served by the CG pathway in sensory processing. Given the relative abundance of CG synapses in the LGN (individual LGN neurons receive more synapses from CG neurons than any other single source, including retinal ganglion cells), it seems likely that this pathway has an important role in sensory processing (Guillery, 1969; Erisir et al., 1997a; Erisir et al., 1997b). In addition to sensory processing, several studies indicate that the CG pathway is a crucial component of the circuitry that underlies sleep and wakefulness (Reviewed by Steriade, 2003). Together, it seems likely that the CG pathway has multiple, distinct roles depending on the arousal state of an animal.
The few studies that have examined the anatomy and physiology of the CG pathway report that it is comprised of distinct classes of neurons (Hendrickson et al., 1978; Tsumoto and Suda, 1980; Swadlow and Weyand, 1987; Claps and Casagrande, 1990; Fitzpatrick et al., 1994; Usrey and Fitzpatrick, 1996). Most relevant to the current study are the findings of Tsumota and Suda (Tsumota and Suda, 1980), who distinguish three classes of CG neurons in the cat. These classes differ in their visual response properties and conduction velocities. In general, neurons with fast conduction velocities (13–32 m sec−1) were complex cells, those with intermediate conduction velocities (3.2–11 m sec−1) were simple cells and those with slow conduction velocities (0.3–1.6 m sec−1) were non-responsive to visual stimuli. In the present study, all the CG neurons recorded from in the ferret had conduction latencies within the ranges of the fast and intermediate neurons in the cat. Within this group of CG neurons, both simple cells and complex cells were encountered. Although we did not encounter CG neurons with long conduction latencies (>10 msec), we cannot rule out their existence because they have been documented in the CG pathway of the rabbit and in other sensory and motor pathways linking cortex with thalamus (Swadlow and Weyand, 1987; Swadlow, 1990; Swadlow, 1991; Sirota et al., 1995).
To determine how early cortical feedback influences LGN neurons following the onset of a visual stimulus we measured the impulse responses of GCR neurons and CG neurons using a white-noise stimulus and reverse-correlation analysis. Results of this analysis indicate that the peak response of both groups of cortical neurons has a latency of ~56 msec. Because the CG pathway contains neurons with short conduction latencies (~4 msec), it is likely that the CG pathway influences visual processing in the LGN within 60 msec of stimulus onset. Furthermore, because the geniculocortical pathway contains neurons with even shorter conduction latencies (~2 msec), the evolution of visual responses in the cortex almost certainly includes effects mediated by the CG pathway. Together, results from this study support the view that the geniculocortical and CG pathways should not be viewed as independent pathways operating in isolation of each other but as members of a complex and dynamic circuit that links the thalamus and cortex for sensory processing.
Acknowledgments
We thank Kelly Henning and Dan Sperka for technical assistance. This work was supported by NIH grants EY13588, EY015580, the McKnight Foundation, and the Esther A. and Joseph Klingenstein Fund.
References
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- Bullier J, Henry GH. Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. Journal of Neurophysiology. 1979;42:1271–1281. doi: 10.1152/jn.1979.42.5.1271. [DOI] [PubMed] [Google Scholar]
- Bullier J, Henry GH. Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. Journal of Neurophysiology. 1979;42:1271–1281. doi: 10.1152/jn.1979.42.5.1271. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Lieber JL. Development of axonal arbors of layer 6 pyramidal neurons in ferret primary visual cortex. Journal of Comparative Neurology. 1996;376:295–305. doi: 10.1002/(SICI)1096-9861(19961209)376:2<295::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Claps A, Casagrande VA. The distribution and morphology of corticogeniculate axons in ferrets. Brain Research. 1990;530:126–129. doi: 10.1016/0006-8993(90)90668-2. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. Journal of Physiology. 1971;217:473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Sherman SM. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proceedings of the National Academy of Sciences of the USA. 1997a;94:1517–1520. doi: 10.1073/pnas.94.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. Journal of Comparative Neurology. 1997b;377:535–549. [PubMed] [Google Scholar]
- Ferster D, LeVay S. The axonal arborizations of lateral geniculate neurons in the striate cortex of the cat. Journal of Comparative Neurology. 1978;182:923–944. doi: 10.1002/cne.901820510. [DOI] [PubMed] [Google Scholar]
- Ferster D, Lindstrom S. Augmenting responses evoked in area 17 of the cat by intracortical axon collaterals of cortico-geniculate cells. Journal of Physiology. 1985;367:217–232. doi: 10.1113/jphysiol.1985.sp015821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Itoh K, Diamond IT. The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey (Saimiri sciureus) Journal of Neuroscience. 1983;3:673–702. doi: 10.1523/JNEUROSCI.03-04-00673.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Usrey WM, Schofield BR, Einstein G. The sublaminar organization of corticogeniculate neurons in layer 6 of macaque striate cortex. Visual Neuroscience. 1994;11:307–315. doi: 10.1017/s0952523800001656. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Kelly JP. The projections of cells in different layers of the cat’s visual cortex. Journal of Comparative Neurology. 1975;163:81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterized neurons in the cat visual cortex. Nature. 1979;280:120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Guillery RW. A quantitative study of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Z Zellforsch. 1969;96:39–48. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Oberdorfer MD. A study of fine and coarse retinofugal axons terminating in the geniculate C-laminae and in the medial interlaminar nucleus of the mink. Journal of Comparative Neurology. 1977;176:515–526. doi: 10.1002/cne.901760404. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. Journal of Comparative Neurology. 1978;182:123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hoffman K-P, Stone J, Sherman SM. Relay of receptive field properties in the dorsal lateral geniculate nucleus of the cat. Journal of Neurophysiology. 1972;35:518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Uhlrich DJ, Sherman SM. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. Journal of Comparative Neurology. 1985;233:159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Katz LC. Local circuitry of identified projection neurons in cat visual cortex brain slices. Journal of Neuroscience. 1987;7:1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Crowley JC, Livesey FJ, Katz LC. Molecular organization of the ferret visual thalamus. Journal of Neuroscience. 2004;24:9962–9970. doi: 10.1523/JNEUROSCI.2165-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Gilbert CD. Laminar patterns of geniculocortical projections in the cat. Brain Research. 1976;113:1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- Leventhal AG. Evidence that the different classes of relay cells of the cat’s lateral geniculate nucleus terminate in different layers of the striate cortex. Experimental Brain Research. 1979;37:349–372. doi: 10.1007/BF00237719. [DOI] [PubMed] [Google Scholar]
- Lund JS, Lund RD, Hendrickson AE, Bunt AH, Fuchs AF. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. Journal of Comparative Neurology. 1975;164:287–303. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- Lund JS, Henry GH, MacQueen CL, Harvey AR. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. Journal of Comparative Neurology. 1979;184:599–617. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- McConnell SK, LeVay S. Anatomical organization of the visual system of the mink, Mustela vison. Journal of Comparative Neurology. 1986;250:109–132. doi: 10.1002/cne.902500110. [DOI] [PubMed] [Google Scholar]
- Morgan J, Thompson ID. The segregation of ON- and OFF-center responses in the lateral geniculate nucleus of normal and monocularly enucleated ferrets. Visual Neuroscience. 1993;10:303–311. doi: 10.1017/s0952523800003709. [DOI] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. Journal of Comparative Neurology. 2005;484:458–473. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Reid RC, Victor JD, Shapley RM. The use of m-sequences in the analysis of visual neurons: Linear receptive field properties. Visual Neuroscience. 1997;16:1015–1027. doi: 10.1017/s0952523800011743. [DOI] [PubMed] [Google Scholar]
- Robson JA. The morphology of corticofugal axons to the dorsal lateral geniculate nucleus in the cat. Journal of Comparative Neurology. 1983;216:89–103. doi: 10.1002/cne.902160108. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. 2. MIT Press; 2005. [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. X- and Y-cells in the dorsal lateral geniculate nucleus of the tree shrew (Tupaia glis) Brain Research. 1975;93:152–157. doi: 10.1016/0006-8993(75)90294-2. [DOI] [PubMed] [Google Scholar]
- Sirota MG, Swadlow HA, Beloozerova IN. Three channels of corticothalamic communication during locomotion. Journal of Neuroscience. 1995;25:5915–5925. doi: 10.1523/JNEUROSCI.0489-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottun BC, De Valois RL, Grosof DH, Movshon JA, Albrecht DG, Bonds AB. Classifying simple and complex cells on the basis of response modulation. Vision Research. 1991;31:1079–1086. doi: 10.1016/0042-6989(91)90033-2. [DOI] [PubMed] [Google Scholar]
- Steriade M. The corticothalamic system in sleep. Frontiers in Bioscience. 2003;8:d878–899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus. Elsevier Science Publishing; 1997. [Google Scholar]
- Stone J. Parallel Processing in the Visual System. Plenum Press; 1983. [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KA, Bannister NJ, Jack JJ. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382:258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Sutter EE. A deterministic approach to nonlinear systems analysis. In: Pinter R, Nabet B, editors. Nonlinear Vision: Determination of Neural Receptive Fields, Function, and Networks. CRC Press; 1992. pp. 171–220. [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in S-1 forelimb representation of the awake rabbit: receptive fields and axonal properties. Journal of Neurophysiology. 1990;63:1477–1498. doi: 10.1152/jn.1990.63.6.1477. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in second somatosensory cortex of the awake rabbit: receptive fields and axonal properties. Journal of Neurophysiology. 1991;66:1392–1409. doi: 10.1152/jn.1991.66.4.1392. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Weyand TG. Efferent systems of the rabbit visual cortex: laminar distribution of the cells of origin, axonal conduction velocities, and identification of axonal branches. Journal of Comparative Neurology. 1981;203:799–822. doi: 10.1002/cne.902030415. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Weyand TG. Corticogeniculate neurons, corticotectal neurons, and suspected interneurons in visual cortex of awake rabbits: receptive-field properties, axonal properties, and effects of EEG arousal. Journal of Neurophysiology. 1987;57:977–1001. doi: 10.1152/jn.1987.57.4.977. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Creutzfeldt OD, Legendy CR. Functional organization of the corticofugal system from visual cortex to lateral geniculate nucleus in the cat. Experimental Brain Research. 1978;32:345–364. doi: 10.1007/BF00238707. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Suda K. Three groups of cortico-geniculate neurons and their distribution in binocular and monocular segments of cat striate cortex. Journal of Comparative Neurology. 1980;193:223–236. doi: 10.1002/cne.901930115. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Fitzpatrick D. Specificity in the axonal connections of layer VI neurons in tree shrew striate cortex: evidence for distinct granular and supragranular systems. Journal of Neuroscience. 1996;16:1203–1218. doi: 10.1523/JNEUROSCI.16-03-01203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Alonso J-M, Reid RC. Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. Journal of Neuroscience. 2000;20:5461–5467. doi: 10.1523/JNEUROSCI.20-14-05461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Sceniak MP, Chapman B. Receptive fields and response properties of neurons in layer 4 of ferret visual cortex. Journal of Neurophysiology. 2003;89:1003–1015. doi: 10.1152/jn.00749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs KR, Stryker MP. The projection of the visual field onto the lateral geniculate nucleus of the ferret. Journal of Comparative Neurology. 1985;241:210–24. doi: 10.1002/cne.902410208. [DOI] [PubMed] [Google Scholar]
- Zahs KR, Stryker MP. Segregation of ON and OFF afferents to ferret visual cortex. Journal of Neurophysiology. 1988;59:1410–29. doi: 10.1152/jn.1988.59.5.1410. [DOI] [PubMed] [Google Scholar]


