Abstract
Combining an effective psychological treatment with conventional anxiolytic medication is typically not more effective than unimodal therapy for treating anxiety disorders. However, recent advances in the neuroscience of fear reduction have led to novel approaches for combining psychological therapy and pharmacological agents. Exposure-based treatments in humans partly rely on extinction to reduce the fear response in anxiety disorders. Animal studies have shown that d-cycloserine (DCS), a partial agonist at the glycine recognition site of the glutamatergic N-methyl- d-aspartate receptor facilitates extinction learning. Similarly, recent human trials have shown that DCS enhances fear reduction during exposure therapy of some anxiety disorders. This article discusses the biological and psychological mechanisms of extinction learning and the therapeutic value of DCS as an augmentation strategy for exposure therapy. Areas of future research will be identified.
Keywords: Exposure therapy, Extinction learning, Anxiety disorders, Phobias, d-cycloserine, NMDA, Glycine, Glutamate, Translational research
Introduction
The most effective strategies for treating anxiety disorders include exposure-based interventions and anxiolytic pharmacotherapy (such as benzodiazepines, tricyclic antidepressants, monoamine oxidase inhibitors, and selective serotonin reuptake inhibitors, to name only a few). Exposure-based treatments are effective interventions for a range of anxiety disorders, including social phobia (e.g., Feske & Chambless, 1995), panic disorder and agoraphobia (e.g., Clum, Clum, & Surls, 1993), obsessive–compulsive disorder (e.g., Abramowitz, 1996), post-traumatic stress disorder (e.g., Foa et al., 1999), and specific phobias (e.g., Öst, Svensson, Hellstrom, & Lindwall, 2001). Nevertheless, there is still considerable room for improvement for these interventions. In an attempt to maximize treatment response, it has been generally assumed that combining two effective interventions should result in greater gains than only one of them. Surprisingly, there is no clear evidence to suggest that a combination strategy of exposure-based therapy and pharmacotherapy is more effective than unimodal therapy in the long term (for a review, see Foa, Franklin, & Moser, 2002; Otto, Smits, & Reese, 2005). For example, clinical trials of panic disorder showed that patients who received a combination therapy between cognitive behavioral therapy (CBT) and imipramine (Barlow, Gorman, Shear, & Woods, 2000) or benzodiazepine (Marks et al., 1993) medications showed a loss of efficacy once the medication was discontinued relative to patients who received CBT alone. Moreover, the combination between CBT and the active drug did not outperform the combination between CBT and a pill placebo (e.g., Barlow et al., 2000).
Similarly, in a randomized trial comparing the individual and combined effects of fluoxetine and CBT for social anxiety disorder, Davidson et al. (2004) found a combination treatment response rate of 54.2% as compared with a response rate of 51.7% for CBT alone, and 50.8% for fluoxetine alone at post-acute. Consistent results were reported in a clinical trial comparing unimodal therapy and the combined effects of exposure plus response prevention and clomipramine for obsessive–compulsive disorder (Foa et al., 2005). The findings of this trial revealed no significant difference in the efficacy between CBT and combination treatment. The response rates for CBT were 62% for the intent-to-treat sample relative to 70% for combination treatment. Similar results were reported in other trials with obsessive–compulsive disorder (e.g., Franklin, Abramowitz, Bux, Zoellner, & Feeny, 2002).
This brief review of the clinical trial literature shows no clear advantage of combination therapy relative to exposure-based intervention plus pill placebo at post-acute, and also no advantage as compared with unimodal therapy at follow-up. The reason for the disappointing efficacy of combined therapy is not known. It is possible that the affect-modulating properties of pharmacotherapy and their associated side effects may interfere with CBT by providing conditions for state-dependent learning during the exposure-based therapy sessions (e.g., Otto et al., 2005). In other words, discontinuing the medication may alter the internal state, which may in turn interfere with the learning during exposure-based therapy that happens during an internal state due to the influence of the pharmacological agent. This perspective receives some support from animal studies suggesting that extinction learning from exposure to feared cues is sensitive to context effects (e.g., Bouton, 2002, 2004). It is also possible that the anxiolytic effect of pharmacotherapy inhibits the full activation of the fear structure, leading to a suppression of emotion processing of the feared stimuli (Foa & Kozak, 1986). This account is consistent with studies suggesting that strategies to decrease the perceived threat of exposure lead to less fear reduction (e.g., Powers, Smits, & Telch, 2004; Rodriguez & Craske, 1993). Finally, it is possible that fear reduction during exposure-based therapy is attributed to the pill rather than the exposure practices during combined treatment, which may negatively affect the person’s perception of self-efficacy with regard to the treatment gains.
In sum, the context and the affect-modulating properties of pharmacotherapy, while offering effective treatment of anxiety disorders, may also undermine some of the beneficial effects of CBT, so that combined treatment may not outperform CBT alone and may place patients at relatively greater risk of relapse when the medication is discontinued. We have previously outlined a number of recommendations in order to maximize the efficacy of combination therapy (Otto, Basden, Leyro, McHugh, & Hofmann, 2007). Following these recommendations, exposure-based therapy is more likely to be enhanced if the pharmacological agent (1) produces few side effects and has little affect-modulating effects to minimize state-dependent learning; (2) allows full activation of the fear structure and emotional processing of feared stimuli during the exposures; and (3) can be delivered in isolated dosing to allow patients to attribute a significant degree of the therapeutic effects to the successful exposures. Finally, and perhaps most importantly, the pharmacological agent should support aspects of the therapeutic mechanism exposure-based therapy. These recommendations seem to be overly ambitious and practically unattainable with conventional pharmacotherapy for anxiety disorders. However, basic research in animal extinction learning has recently been translated into clinical applications. This research points to a pharmacological agent that meets all of the above-mentioned recommendations for enhancing exposure-based therapy. The following will summarize the preclinical (animal) studies that led to the discovery of this agent, and that were more recently translated into research with clinical and nonclinical (human) subjects.
Extinction learning in exposure-based therapy
Exposure therapy is rooted in behaviorism that began in the early 1900s (Watson, 1924) and grew out of the Pavlovian fear conditioning paradigm. In this paradigm, an initially innocuous stimulus, such as a light or tone (conditioned stimulus, CS), is presented together with an innately aversive stimulus, such as a foot shock (unconditioned stimulus, US), which leads to an adaptive fear response (unconditioned response, UR). After repeated pairings of the CS and US, the subject (typically a mouse or rat) exhibits a conditioned fear response (conditioned response, CR) to the CS. The strength of the fear response in animals is determined by the number of repetitions of association between the CS and US, and the intensity of the UR. Moreover, the repeated presentation of the CS in the absence of the US leads to a gradual decrease of the CR, which was termed extinction.
Watson and Rayner (1920) demonstrated the direct application of these Pavlovian principles to human fear conditioning and extinction. In the classic case of Little Albert, Watson and Rayner (1920) first presented an 11-month-old orphan with several objects, including a rat, a rabbit, a fur coat, and a dog. Little Albert showed no initial signs of fear toward these objects. However, the infant exhibited clear distress in response to the US, which was a loud noise made by banging a heavy hammer against a steel bar. During the conditioning trial, the experimenters repeatedly paired the rat (CS) with the noise (US). After seven trials over the course of 1 week, Little Albert was presented with the rat without the noise. As predicted, the boy exhibited signs of distress and fear when only seeing the rat. The experimenters then presented Little Albert with other hairy objects, including a rabbit, a fur coat, and a dog. The boy reacted similarly distressed to each of these objects, suggesting that the response generalized to other objects with hair. A similar reaction was observed 5 days and, to a lesser degree, 31 days after the initial conditioning trial. When the subject was tested in a different context (by moving him to a different room) the fear response was still noticeable but significantly decreased. After repeated presentations of the CS alone, the distress response eventually dissipated.
In sum, Watson and Rayner’s (1920) classic experiment illustrates the Pavlovian principles of fear conditioning, fear generalization, and context effects in humans and also exemplifies the role of extinction learning in exposure-based therapy in humans. As in the case of Little Albert, extinction learning is procedurally very similar to exposure-based therapy. Specifically, exposure-based therapy involves exposure to the feared object or situation in the absence of any danger. This is not to say that extinction learning is the only mechanism through which exposure-based therapy acts, and my intention is not to trivialize psychotherapy. In fact, a host of treatment-relevant variables are likely to contribute to treatment success, ranging from specific maladaptive cognitions over perceived self-efficacy to therapeutic alliance, empathy, and other common factors. However, basic extinction processes are also likely to be involved in exposure-based therapy.
Mechanisms of extinction learning
Associative processes
Although extinction has been described and studied since Pavlov (1927), the process of extinction has remained speculative. Some of the earlier theories assumed that the excitatory association between the CS and the US representation formed during acquisition is weakened and ultimately eliminated (“un-learned”) through extinction training (Mackintosh, 1975; Rescorla & Wagner, 1972). Evidence against the notion that extinction is merely due to an un-learning or erasure of previously acquired fear include the observations that the original fear response returns after the passage of time (spontaneous recovery; e.g., Robbins, 1990), after a change of context from the extinction context (renewal; e.g., Bouton & Bolles, 1979; Rodriguez, Craske, Mineka, & Hladeck, 1999), or after unsignaled presentations of the US that occur within the context of the retention test (reinstatement; e.g., Bouton & Swartzentruber, 1991; Dirikx, Hermans, Vansteenwegen, Baeyens, & Eelen, 2004; Hermans et al., 2005; Rescorla & Heth, 1975).
Modern learning theories of extinction assume that conditioning happens as subjects form representations of the relevant cues (CS and US) and situational contexts, and as they acquire information about the association between these cues and the situations (see Myers & Davis, 2007, for a review). These associations can be either excitatory (i.e., activation of one representation activates another) or inhibitory (i.e., activation of one representation inhibits activation of another). Acquisition of CSs is explained by the formation of an excitatory association between representations of the CS and US. The US representation is activated indirectly through its association with the CS representation, which in turn triggers the CS. Extinction is assumed to proceed through multiple mechanisms (Myers & Davis, 2007) that also include new learning that inhibits the excitatory association between CS and US (e.g., Bouton, 1993; Myers, Ressler, & Davis, 2006). As part of this new form of learning, the subject changes the CS–US contingency in such a way that the CS no longer signals an aversive event and thereby inhibits the expression of the fear response (e.g., Bouton, 1993, 2004; Myers & Davis, 2002).
Cognitive processes
It is typically assumed that animals only learn by using basic associative mechanisms. However, rats can perform causal reasoning, which allows the animal to predict outcomes on the basis of observation without the reliance on any associative processes (Bleisdell, Sawa, Leising, & Waldmann, 2006). Similarly, a number of studies have shown that reductions in the CS–US expectancy are correlated with reductions in CRs during the course of extinction (e.g., Biferno & Dawson, 1977; Lipp & Edwards, 2002). Furthermore, extinction appears to be associated with a reduction in the strength of the CS–US expectancy (Hofmann, in press; Shell, Dawson, & Marinkovic, 1991).
Not surprisingly, the influence of higher cognitive processes in extinction is particularly evident in humans. For example, it has further been shown that experimentally induced autonomic fear responses can be eliminated by simply informing subjects that the US will no longer follow the CS (Grings, 1973). Moreover, extinction can be disrupted by adding a stimulus that serves as a safety signal (Lovibond, Davis, & O’Flaherty, 2000). In sum, the literature on cognitive processes in extinction learning suggests that extinction is accompanied by changes in the CS–US contingency (namely that the CS no longer signals the US). Moreover, verbal instructions, which are part of exposure-based therapies, can directly modify extinction processes by changing this contingency.
Biological processes
Animal research implicates the amygdala during fear extinction, including the medial prefrontal cortex in rats (Milad & Quirk, 2002) and humans (Phelps, Delgado, Nearing, & LeDoux, 2004), and specifically the basolateral nucleus of the amygdale (Quirk, Repa, & LeDoux, 1995; Repa et al., 2001). Although the specific role of the amygdala in memory formation is not completely understood (Cahill, Weinberger, Roozendaal, & McGaugh, 1999), fear learning and extinction appear to involve movement of calcium ions into amygdala neurons, followed by a number of intracellular mechanisms that lead to long-term changes in synaptic function and morphology (Blair, Tinkelman, Moita, & LeDoux, 2003; Boje, Wong, & Skolnick, 1993).
Glutamate is one of the most important excitatory neurotransmitters in the mammalian brain and performs an important role in the brain circuitry underlying fear processing. The N-methyl- d-aspartate (NMDA) receptor is an ionotropic receptor for glutamate. In fact, fear and extinction learning are both blocked by antagonists at the NMDA receptor (see for a review Myers & Davis, 2007). NMDA receptors are heteromeric complexes (Laube, Kuhse, & Betz, 1998) that contain a number of subunits (Lao, Wang, Yasuda, Dunah, & Wolfe, 1997; Laube, Hirai, Sturgess, Betz & Kuhse, 1997). It has been shown that long-term potentiation (LTP) is mediated by NMDA receptors containing NR2A subunits in pyramidal cells of the hippocampus in area CA1 (Liu, Wong, & Pozzza, 2004). Analyses of LTP in cells of this region (Lisman, 1989) suggest that the process is governed by the “Hebb rule” (Hebb, 1949), which states that the induction of LTP involves the NMDA class of glutamate-activated channels in the postsynaptic membrane (e.g., Collinridge, Kehl, & McLennan, 1983) that only open if there are both presynaptic release of glutamate and also substantial depolarization of the postsynaptic membrane (Nowak, Bregestovski, Ascher, Herbert, & Prochiantz, 1984). Variations in the intracellular Ca2+ concentration regulate the induction of long-term synaptic plasticity at the glutamatergic synapses (Bliss & Collingridge, 1993; Malenka, & Nicoll, 1999; Nishiyama, Hong, Mikoshiba, Poo, & Mato, 2000). Activation of NMDA receptors allows flow of Na+ and K+ ions, and influx of a small amount of Ca2+ ions. The influx of Ca2+ ions (Jahr & Stevens, 1993; MacDermott, Mayer, Westbrook, Smith, & Baker, 1986) then triggers an increase in synaptic weight, a cellular mechanism for learning and memory (Lynch, Larson, Kelso, Barrionuevo, & Schottler, 1983). NMDA receptors in the amygdala are also considered essential for LTP, a process that underlies specifically fear learning and extinction (Fanselow & LeDoux, 1999; Lee, Choi, Brown, & Kim, 2000).
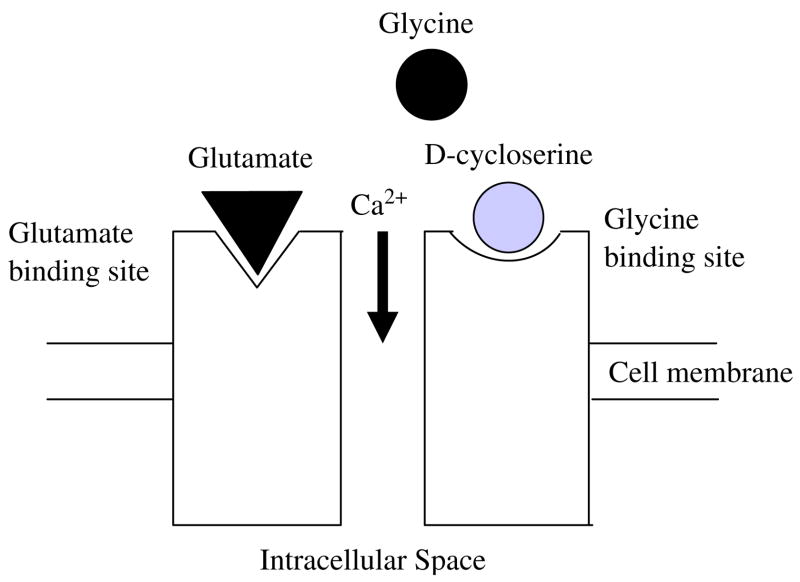
Activation of NDMA receptors requires binding of both glutamate and the co-agonist glycine for the efficient opening of the Ca2+ ion channel. Some of the subunits bind the co-agonist glycine and other subunits bind the neurotransmitter glutamate. It has been shown that d-serine can co-agonize the NMDA receptor with even greater potency than glycine (see for a review, Wolosker, 2006). d-4-amino-3-isoxazolidone (d-cycloserine, DCS) is an analogue of d-alanine and a partial agonist at the glycine recognition site of the glutamatergic NMDA receptor that works very similar to d-serine. Fig. 1 depicts an NMDA receptor that is co-activated by DCS. A more detailed description of NMDA receptor activation can be found in Laube et al. (1998).
Fig. 1.
Schematic depiction of the NMDA receptor that is co-activated by d-cycloserine. NMDA receptors require the binding of two molecules of glutamate or aspartate and two molecules of glycine or glycine agonists.
Studies have shown that DCS facilitates the process of extinction of conditioned fear when administered in individual doses prior to or soon after extinction trials in animals (Davis, Walker, & Myers, 2003; Falls, Miserendino, & Davis, 1992; Parnas, Weber, & Richardson, 2005; Richardson, Ledgerwood, & Cranney, 2004). Some of the most important studies of the recent preclinical literature will be reviewed next.
Augmentation strategies of extinction learning: animal studies
The animal literature consistently reports that NMDA antagonists inhibit extinction. For example, a study by Falls and colleagues showed that intra-amygdala infusions of an NMDA receptor antagonist shortly before extinction training dose-dependently blocked extinction (Falls et al., 1992). This impairment is not due to an effect on NMDA receptors outside the amygdala, damage to or destruction of the amygdala, or an impairment of sensory transmission during extinction training. Furthermore, the inhibitory effect of NMDA antagonists on extinction cannot be explained by state dependency, and blocking NMDA receptors after extinction training also blocks extinction (Santini, Muller, & Quirk, 2001).
The first study to demonstrate that DCS facilitates extinction learning was reported by Walker, Ressler, Lu, and Davis (2002). At the same time, this experiment served as a model study of DCS in animals. In essence, the investigators found that either systemic administration or infusion of DCS into the basolateral nucleus of the amygdala prior to extinction training enhanced the extinction of learned fear in rats, as measured by fear-potentiated startle. Adult rats first received 10 pairings of a light (CS) and a shock (US). Two days later, some rats were given 30 extinction trials by presenting the CS without the US. Thirty minutes prior to these extinction trials, rats were injected with DCS or a saline solution. All rats were then tested the following day to assess the fear response to the CS alone. Specifically, the investigators measured fear-potentiated startle by presenting an acoustic startle stimulus by itself on some of the test trials, and preceded by the CS on other trials. The results showed that rats treated with DCS prior to the extinction trials exhibited less fear response to the CS at test than rats treated with a saline solution. This effect was only observed in rats given the extinction trials and could not be attributed to any nonspecific drug effects. Moreover, the effect of DCS on extinction was dose-dependent because rats systemically injected with either 15 or 30 mg/kg of DCS exhibited facilitated extinction, whereas rats given 3.25 mg/kg of DCS responded similarly to saline-treated controls. Finally, the investigators were able to show that DCS specifically acts on the amygdala.
Later studies replicated the facilitation effect of DCS on extinction. For example, Ledgerwood and colleagues similarly reported that DCS facilitates extinction of conditioned freezing when given either systemically or directly into the amygdala (Ledgerwood, Richardson, & Cranney, 2003, 2004). Interestingly, DCS could still facilitate extinction when given up to about 3 h after extinction training, which is consistent with the idea that DCS acts to facilitate memory consolidation of extinction (Richardson et al., 2004). However, DCS temporarily failed to facilitate extinction of learned fear when the animals had been previously exposed to DCS (Parnas et al., 2005). Specifically, Parnas et al. (2005) found that five pre-exposures to DCS over a 10-day period eliminated the enhancing effects of DCS on the extinction of conditioned freezing. Similarly, DCS in animals revealed positive effects on spatial learning only for isolated dosing (Quartermain, Mower, Rafferty, Herting, & Lanthorn, 1994). This is consistent with the demonstration of desensitization of the NMDA receptor complex in cell culture with prolonged exposure to DCS and other glycinergic ligands (Boje et al., 1993). This highlights the importance to consider the number of DCS exposures and the interval separating these exposures, because chronic administration temporarily appears to change the function of the glycine/NMDA receptor complex. In this context, it is also important to note that long-term exposure to all major classes of antidepressants (including selective serotonin reuptake inhibitors, tricyclics, and monoamine reuptake inhibitors) appear to result in neurochemical changes at the glycine-binding site of the NMDA receptor complex (Nowak, Trullas, Layer, Skolnick, & Paul, 1993; Paul, Nowak, Layer, Popik, & Skolnick, 1994). Similar effects have been reported after electroconvulsive shock therapy (Popik, Wrobel, & Nowak, 2000). Therefore, it is possible that previous treatments with conventional pharmacological treatments and electroshock therapy impair the effects of DCS on extinction in humans, at least temporarily.
It has further been shown that DCS not only facilitates extinction but also reduces the occurrence of reinstatement-induced relapse (Ledgerwood et al., 2004). This has led Richardson and colleagues to the hypothesis that DCS not only enhances extinction learning but that DCS-treated patients may also be less likely to relapse following successful exposure-based treatment (Richardson et al., 2004). In fact, as I will review further below, the early clinical data do suggest that the effects of DCS tend to be more pronounced at follow-up.
The research literature is most convincing and robust for the effects of DCS on extinction learning (cf., Richardson et al., 2004). However, there are isolated reports of at least some facilitation of other learning tasks, including spatial learning in a Morris water maze (Lelong, Dauphin, & Boulouard, 2001), a thirst-motivated linear maze learning task (Quartermain et al., 1994), inhibitory avoidance learning (Land & Riccio, 1999), and a visuospatial-guided delayed win-shift performance task (Pussinen, & Sirvio, 1999). These studies provide some support for the potential efficacy of DCS outside extinction learning, but leave open questions as to whether enhancement effects are more optimal around extinction-based or stress-based tasks.
Augmentation strategies of exposure therapy: clinical studies
Early studies
DCS is an established antibiotic medication for the chronic treatment of tuberculosis at high doses (500 mg daily) in humans. It should be noted that the antibiotic effects are unrelated with DCS’s ability to facilitate extinction learning. In addition, DCS has been applied as a chronic-dose strategy to improve negative symptoms in schizophrenia (Goff & Coyle, 2001; Goff et al., 1999; Rowland et al., 2005), social behavior in autistic disorder (Posey et al., 2004), and cognitive functioning in Alzheimer’s disease (Schwartz, Hashtroudi, Herting, Schwartz, & Deutsch, 1996; Tsai, Falk, Gunther, & Coyle, 1999). However, despite some early promise in its application to both schizophrenia and Alzheimer’s disease, the evidence in general has been disappointing. For example, across 13 studies between 1995 and 2005, at least half of the trials reported nonsignificant effects (Otto et al., 2007). Similarly, a review of four studies using DCS for Alzheimer’s disease revealed that the drug was generally ineffective (Laake & Oeksengaard, 2002). As noted earlier, one potential reason for the lack of efficacy for DCS in these applications may be the reliance on chronic rather than isolated dosing strategies. Moreover, it is possible that the DCS effects are limited to enhancing extinction learning in exposure-based therapy. The use of DCS with minimal side effects when given acutely in small doses allows it to be easily tested as a potential adjunctive treatment to exposure-based therapy for anxiety disorders.
Specific phobia
The first study to examine the utility of DCS as a method to enhance exposure therapy in humans was conducted by Ressler et al. (2004). The authors randomized 28 subjects with a DSM-IV diagnosis of specific phobia of heights (acrophobia) to two treatment conditions. In one condition, participants received two sessions of virtual reality exposure therapy preceded in double-blind fashion by administration of single doses of DCS (50 or 500 mg) taken 2–4 h prior to each of the sessions. In the other condition, participants received a placebo pill before the identical intervention. The results showed that exposure-based therapy combined with DCS resulted in significantly greater reductions of acrophobic symptoms at 1 week and 3 months following treatment. No difference in efficacy was observed between the two doses, and no adverse effects from DCS administration were reported. Subjects who received DCS prior to the exposures further showed significantly greater decreases in skin conductance fluctuations during the virtual exposures and significantly greater improvement on general measures of real-world acrophobia symptoms as compared with subjects receiving placebo prior to the exposures. These group differences became evident early in treatment and further improved at the 3-month follow-up.
Social anxiety disorder
My research team conducted the second double-blind placebo-controlled study in a human sample of anxiety disorder patients. This study randomly assigned 27 patients with a principal DSM-IV diagnosis of social anxiety disorder (social phobia) to receive either exposure therapy plus DCS (50 mg) or exposure therapy plus pill placebo.
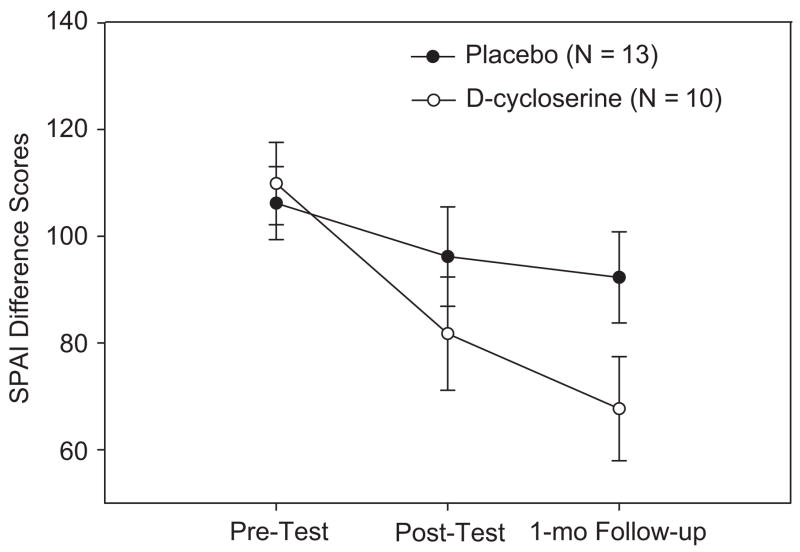
The first treatment session consisted of an introduction of the treatment model and psychoeducation. The following 4 sessions emphasized stepwise social exposures, with administration of the study pill 1 h before each session. The exposure practices consisted of giving speeches of increasing difficulty level about topics chosen by the therapists in front of the other group members or confederates and a video camera. At the end of each exposure session, patients were encouraged to continue to apply home-practice strategies (such as giving speeches in front of a mirror). Although the in-session exposure practices only focused on public speaking, 51.9% of the subjects had a generalized subtype of social anxiety disorder, and 40.7% had at least one additional DSM-IV axis I diagnosis. The level of social anxiety was assessed at baseline, post-treatment, and at the 1-month follow-up. The primary treatment outcome measure consisted of the Social Phobia and Anxiety Inventory (SPAI; Turner, Beidel, Dancu, & Stanley, 1989). Additional measures included the Clinical Global Impression Scale, Severity (Guy, 1976) and the Liebowitz Social Anxiety Scale (Liebowitz, 1987). Participants who received DCS augmentation as compared with placebo augmentation demonstrated significantly greater improvement at post-treatment as assessed by clinician-rated and self-report instruments, with evidence of maintained and extended treatment effects at the follow-up evaluation.
As shown in Fig. 2, the difference between the DCS and placebo group increased linearly with time, with the greatest effects of DCS being evident at the 1-month follow-up. Similar results were observed for the other measures. The between-group effect sizes at both post-treatment and follow-up indicated a medium to very large effect size for the advantage of DCS over placebo, with larger effects at follow-up than immediately following treatment.
Fig. 2.
Means and standard errors of the Social Phobia and Anxiety Inventory (SPAI) at pre-test, post-test and 1-month follow-up assessment of treatment completers. From Hofmann, Meuret et al. (2006). Reprint permission has been requested.
Obsessive– compulsive disorder
Another recent study examined DCS as an augmentation strategy for exposure and prevention for obsessive-compulsive disorder (Kushner et al., in press). In this study, 32 patients with obsessive disorder (Yale-Brown Obsessive–Compulsive Disorder score ≥18; Goodman, Price, Rasmussen, & Mazure, 1989) were randomly assigned to twice-weekly exposure and response prevention plus 10 doses of 125 mg of DCS or exposure-based treatment plus 10 administrations of pill placebo. At pre-test, a hierarchy of the 10 most distressing disorder-related situations was established. Exposure exercises proceeded from the least to most distressing situations. Once a person’s distress rating was reduced by at least 50%, a more difficult exercise was introduced until subjective distress ratings were reduced by 50% as compared with baseline or until the 10th session was reached. The results showed that participants who received DCS were less likely to discontinue the therapy and required fewer exposure sessions to achieve the 50% reduction in distress from baseline. After four exposure sessions, patients in the DCS group reported significantly greater decreases in obsession-related distress as compared with the placebo group. The effect size was medium to large. However, consistent with the animal literature, the difference between the two groups became less pronounced with repeated administrations of the drug. Similar results were reported by Wilhelm et al. (2006). Preliminary analyses of this not yet completed trial suggest that acute administration of DCS facilitates the effects of exposure and response prevention for OCD.
Panic disorder with mild or moderate agoraphobia
Lastly, a recently completed study on the augmentation strategy of DCS for panic disorder recruited 25 outpatients with a primary diagnosis of panic disorder mild to moderate agoraphobia (Tolin et al., 2006). Participants were randomly assigned to either receive 5 weekly sessions of individual exposure therapy for panic disorder plus 50 mg of DCS or the same psychological therapy plus pill placebo. The treatment included standard cognitive restructuring techniques to modify maladaptive thoughts associated with anxiety and panic. During the exposure practices, patients were repeatedly exposed to fearful somatic sensations of anxiety (e.g., hyperventilation to induce dizziness, parasthesias, flushes, etc.). These sessions were delivered in a 90-min format and preceded by administration of the blinded medication 1 h before the session. Home practice assignments were assigned after each session.
The results revealed that, as compared with the placebo condition, the DCS group showed a significantly greater reduction from pre-test to post-test, and a trend toward greater reduction from pre-test to follow-up in symptom severity on dimensional measures (Panic Disorder Severity Scale; Shear et al., 1997). Clinical interview data by blind raters using the Clinical Global Impression Scale, Improvement (CGI, Guy, 1976) further showed that at post-treatment, 62% of patients receiving DCS were considered to be in remission, as compared with 21% of patients in the placebo group. At follow-up, 75% of DCS patients, as compared with 31% of patients in the placebo condition, were in remission. The between-group effect sizes (Cohen’s d) on change scores (from pre-treatment) for the PDSS and CGI at post-treatment and at the 1-month follow-up were large for both the PDSS and CGI, favoring greater change in the DCS group than in the PBO group. At follow-up, the effects remained moderate to large, again showing greater change in the DCS group.
In sum, a number of clinical studies provide strong support for the use of DCS as an augmentation strategy of exposure-based therapy in patients with anxiety disorders, including specific phobia, social anxiety disorder, obsessive–compulsive disorder, and panic disorder with low or moderate agoraphobia. Although the effects found in these studies are surprisingly large, these trials are limited by the relatively small sample size. Future studies are needed to replicate these promising results in larger samples.
Non-clinical studies
In contrast to the above-mentioned clinical studies, research with nonclinical adults has yet to document an advantage for DCS in reducing experimentally acquired fear. Only one research group so far has examined the effect of DCS in subclinical samples (Guastella, Dadds, Lovibond, Mitchell, & Richardson, 2007; Guastella, Lovibon, Dadds, & Mitchell, 2007). In one study, the authors compared the effects of DCS with pill placebo in enhancing extinction in a de novo fear conditioning paradigm (Guastella, Lovibond et al., 2007). The results revealed no effect for DCS when fear acquisition and extinction were conducted on the same day. Likewise, no effect for DCS was found in a revised design using fear-relevant stimuli (i.e., pictures of snakes for nonclinical snake-anxious students) and the separation of acquisition and extinction on separate days. In addition, Guastella, Dadds et al. (2007) used DCS in two studies of nonclinical spider-fearful participants treated in a single session with psycho-education, cognitive therapy, and up to 2 h of intensive exposure practices. All participants tended to respond well (e.g., in one study all participants were able to complete a post-treatment exposure test), and no difference between DCS and placebo augmentation was evident.
These disappointing results may very well be explained due to a ceiling effect because in de novo fear conditioning, little extinction is commonly required to return healthy participants to pre-conditioning levels of arousal. In fact, in the treatment of nonclinical as well as clinical fears of spiders, single-session interventions lead to significant and long-lasting changes (Orr et al., 2000). By way of contrast, the clinical studies demonstrating a treatment facilitation effect of DCS (Hofmann, Meuret et al., 2006; Hofmann, Pollack, & Otto, 2006; Ressler et al., 2004) only administered one-third to one-half the standard number of exposure sessions. In reducing the strength of exposure interventions, these studies were fully in line with the animal research, where only half the standard number of extinction trials is typically used to allow sufficient levels of residual fear to detect the DCS enhancement effect (e.g., Walker & Davis, 2002). Accordingly, in the studies by Guastella, Dadds et al. (2007) and Guastella, Lovibond et al. (2007), the combination of weak levels of fear (i.e., de novo and nonclinical fears), combined with a relatively high dose of exposure intervention, may have created extinction conditions where there was little room to show DCS enhancement due to ceiling effects. Nonetheless, the preliminary studies of DCS as an augmentation strategy for exposure in subclinical fears provide a challenge to the field to better identify the setting conditions where strong DCS effects can be observed.
Summary and future direction for clinical research
A large number of patients fail to improve after exposure-based therapy and pharmacotherapy. In an attempt to develop more efficacious intervention strategies, a number of large treatment trials have been conducted in the hope of demonstrating greater efficacy of a combination between pharmacotherapy and exposure-based treatments as compared with unimodal therapy. However, these attempts have led to consistently disappointing results. I discussed a number of possible reasons, ranging from state-dependent learning to attribution effects.
Recent translational research from preclinical to clinical work identified d-cycloserine as a pharmacological agent that facilitates extinction learning in rats and probably also exposure-based therapy in humans (see also Davis, Ressler, Rothaum, & Richardson, 2006). These clinical studies suggest that participants who received DCS augmentation as compared with placebo augmentation to exposure-based therapy demonstrated significantly greater improvement at post-treatment as assessed by clinician-rated and self-report instruments, with evidence of maintained and extended treatment effects at the follow-up evaluation in some of the studies.
Despite the highly promising findings, a number of remaining questions need to be addressed in order to move this field of research further ahead. These questions include the following:
Which process in extinction is targeted by DCS?
Extinction is likely to proceed through multiple mechanisms that include some forgetting, associative processes, and higher-level cognitive processes that alter the previously learned CS–US expectancy. It remains to be seen what the primary processes in extinction are, and whether DCS targets all or only some of these processes.
What are the long-term consequences of DCS?
DCS may not only enhance extinction learning or other performance tasks, but it may also prevent relapse following successful exposure-based therapy (Richardson et al., 2004). Some of the clinical studies support this idea because the effects of DCS were maintained or even stronger at follow-up than immediately following the treatment. Clinical trials on DCS with long-term follow-up data are necessary to answer this important question.
Are the effects of DCS limited to fear extinction?
There is some evidence to suggest that DCS not only enhances extinction of previously learned fear, but also enhances other learning tasks, including spatial learning and other performance tasks in animals. However, no human trial thus far has been published on the effects of DCS on performance tasks.
Are the effects on fear extinction limited to DCS?
DCS is only one of many possible agonists at the glycine recognition site of the glutamatergic NMDA receptor. It is possible that other agonists at the glycine recognition site are even more potent than DCS. Moreover, it is possible that other receptor sites, or even other glutamate receptor subtypes, are also involved in extinction learning. For example, the AMPA receptor is another major subtype of glutamate receptors. A recent report has shown that PEPA, 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluorophenoxyacetamide, a potentiator of the AMPA receptor, facilitates extinction learning for contextual fear memory (Zushida, Sakurai, Wada, & Sekiguchi, 2007). Future studies will need to explore these new leads and pursue a similar translational research program that has begun with the early work in DCS.
What is the optimal administration of DCS?
There is strong evidence to suggest that chronic administration of DCS (as well as conventional antidepressants) at least temporarily changes the function of the glycine/NMDA receptor complex. Chronic administration of DCS in previous trials to improve cognitive function in some disorders might have been the reason why the results have been inconsistent and disappointing overall. The animal literature and the recent clinical trials in humans suggest that only acute administration of DCS results in the facilitation effect during extinction and exposure-based therapy. However, it remains unclear what the optimal interval of drug administration is.
What is the optimal timing of DCS administration with relation to exposure therapy?
DCS shows excellent central bioavailability (Nair, Epstein, Baron, & Mulinos, 1956) and it is primarily excreted renally with a half-life of 10 h. A single oral administration of 50 mg leads to peak cerebrospinal fluid DCS levels of approximately 2.9±0.96 mg/dl (D’Souza, Gil, Cassello, & Morrissey, 2000) within 1–2 h, although high-fat meals may delay absorption (Zhu, Nix, Adam, & Peloquin, 2001). Therefore, administering DCS 1 h before a session is likely to result in peak CSF DCS levels during the actual exposure practices and consolidation phase. However, it remains uncertain what the optimal timing of DCS is.
What is the optimal DCS dosage?
Ressler et al.’s (2004) study, which is the only study so far that examined dose dependency, found no difference between 250 and 50 mg. Future studies are necessary to determine the most effective range of dosage.
What is the optimal dosage of exposure-based therapy?
The nonclinical literature seems to suggest that DCS does not show a facilitation effect, which is probably due to a ceiling effect of exposure-based therapy. Similar effects are likely present in clinical samples, which will need to be investigated.
What are the disorder-specific effects of DCS?
So far, there is evidence for the utility of DCS for the treatment of specific phobia, social anxiety disorder, obsessive–compulsive disorder, and panic disorder. Even based on this preliminary literature, it appears that some disorders may be more responsive to DCS-augmented exposure-based therapy than others. A systematic comparison on this issue will be important.
Is DCS effective in all subpopulations?
It is not known whether the effect of DCS can be generalized to all clinical and nonclinical subpopulations, or whether there are differences in diagnostic subtypes, sex, ethnicity, age, and other relevant variables.
What are the drug interactions between DCS and other drugs and interventions?
Animal studies showed that prolonged treatment with antidepressants affects the functional activity of the glycine-activated NMDA receptors such that DCS loses its therapeutic effect. Similar effects have been reported after electroconvulsive shock therapy. This raises the possibility that concomitant or previous treatments with anti-anxiety medication or electroshock therapy impair the effects of DCS on extinction in humans. It is further important to know whether these effects, if they exist, are temporary or permanent.
Concluding comments
It is possible that we are at the beginning stage of a genuine paradigm shift of treatment research, because basic knowledge from the field of neuroscience is directly translated into novel clinical applications. These new applications are unlike the traditional “horse race comparisons” of clinical trials that typically lack a sound theoretical rational for combining pharmacotherapy and psychotherapy. For the first time, a pharmacological agent that does not have any anxiolytic properties was used to enhance some of our most effective psychological treatments for anxiety disorders based on a known mechanism. Future research in this exciting field may not only maximize the efficacy and cost effectiveness of the treatments, but may also answer some of the remaining and important theoretical questions concerning the mechanism of treatment change of exposure-based therapy for anxiety disorders.
References
- Abramowitz JS. The psychological treatment of obsessive–compulsive disorder. Canadian Journal of Psychiatry. 1996;51:407–416. doi: 10.1177/070674370605100702. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive–behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. Journal of the American Medical Association. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- Biferno MA, Dawson ME. The onset of contingency awareness and electrodermal classical conditioning: An analysis of temporal relationships during acquisition and extinction. Psychophysiology. 1977;14:164–171. doi: 10.1111/j.1469-8986.1977.tb03370.x. [DOI] [PubMed] [Google Scholar]
- Blair HT, Tinkelman A, Moita MA, LeDoux JE. Associative plasticity in neurons of the lateral amygdala during auditory fear conditioning. Annals of the New York Academy of Science. 2003;985:485–487. doi: 10.1111/j.1749-6632.2003.tb07106.x. [DOI] [PubMed] [Google Scholar]
- Bleisdell AP, Sawa K, Leising KJ, Waldmann MR. Causal reasoning in rats. Science. 2006;311:1020–2022. doi: 10.1126/science.1121872. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boje KM, Wong G, Skolnick P. Desensitization of the NMDA receptor complex by glycinergic ligands in cerebellar granule cell cultures. Brain Research. 1993;603:207–214. doi: 10.1016/0006-8993(93)91239-o. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clinical Psychology Review. 1991;11:123–140. [Google Scholar]
- Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdale a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Clum GA, Clum G, Surls R. A meta-analysis of treatments for panic disorder. Journal of Consulting and Clinical Psychology. 1993;61:317–326. doi: 10.1037//0022-006x.61.2.317. [DOI] [PubMed] [Google Scholar]
- Collinridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral–commissural pathway of the rat hippocampus. Journal of Physiology. 1983;224:22–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JRT, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Archives of General Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothaum BO, Richardson R. Effects of d-cycloserine on extinction: Translation from preclinical and clinical work. Biological Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM. Role of the amygdale in fear extinction measured with potentiated startle. Annals of the New York Academy of Science. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- Dirikx T, Hermans D, Vansteenwegen D, Baeyens F, Eelen P. Reinstatement of extinguished conditioned responses and negative stimulus valence as a pathway to return of fear in humans. Learning and Memory. 2004;11:549–554. doi: 10.1101/lm.78004. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Gil R, Cassello K, Morrissey K, et al. IV glycine and oral d-cycloserine effects on plasma and CSF amino acids in healthy humans. Biological Psychiatry. 2000;47:450–462. doi: 10.1016/s0006-3223(99)00133-x. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MD, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. Journal of Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think placsticity underlying Pavlovian fear conditioning occurs in the basolateral amygdale. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Feske U, Chambless DL. Cognitive behavioral versus exposure only treatment for social phobia: A meta-analysis. Behavior Therapy. 1995;26:695–720. [Google Scholar]
- Foa EB, Franklin ME, Moser J. Context in the clinic: How well do cognitive–behavioral therapies and medications work in combination? Biological Psychiatry. 2002;10:987–997. doi: 10.1016/s0006-3223(02)01552-4. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing post-traumatic stress disorder in female assault victim. Journal of Consulting and Clinical Psychology. 1999;67:194–200. doi: 10.1037//0022-006x.67.2.194. [DOI] [PubMed] [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive–compulsive disorder. American Journal of Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- Franklin ME, Abramowitz JS, Bux DA, Zoellner LA, Feeny NC. Cognitive–behavioral therapy with and without medication in the treatment of obsessive–compulsive disorder. Professional Psychology: Research and Practice. 2002;33:162–168. [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. American Journal of Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Levitt J, Amicao E, Mannoach D, Schoenfeld DA, et al. A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Archives of General Psychiatry. 1999;56:21–27. doi: 10.1001/archpsyc.56.1.21. [DOI] [PubMed] [Google Scholar]
- Goodman W, Price L, Rasmussen S, Mazure C. The Yale–Brown Obsessive-Compulsive Scale I: Development, use and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grings WW. Cognitive factors in electrodermal conditioning. Psychological Bulletin. 1973;79:200–210. doi: 10.1037/h0033883. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson GA. A randomized controlled trial of the effect of d-cycloserine on exposure therapy for spider fear. Journal of Psychiatric Research. 2007;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibon P, Dadds MR, Mitchell P. A randomized controlled trial of the effect of d-cycloserine on extinction and fear-conditioning in humans. Behaviour Research and Therapy. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology: Publication ADM 76–338. Washington, DC: US Department of Health, Education and Welfare; 1976. pp. 217–222. [Google Scholar]
- Hebb DO. The organization of behavior. New York, NY: Wiley; 1949. [Google Scholar]
- Hermans D, Dirikx T, Vansteenwegenin D, Baeyens F, Van den Bergh O, Eelen P. Reinstatement of fear responses in human aversive conditioning. Behaviour Research and Therapy. 2005;43:533–551. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy for social anxiety disorder with d-cycloserine. Archives of General Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Pollack MH, Otto MO. Augmentation treatment of psychotherapy for anxiety disorders with d-cycloserine. CNS Drug Reviews. 2006;12:208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clinical Psychology Review. doi: 10.1016/j.cpr.2007.04.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Calcium permeability of the N-methyl- d-aspartate receptor channel in hippocampal neurons in culture. Proceedings of the National Academy of Science USA. 1993;90:11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner M, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. d-cycloserine augmented exposure therapy for obsessive compulsive disorder. Biological Psychiatry. doi: 10.1016/j.biopsych.2006.12.020. in press. [DOI] [PubMed] [Google Scholar]
- Laake K, Oeksengaard AR. d-cycloserine for Alzheimer’s disease. Cochrane Database Systematic Review. 2002;2:CD003153. doi: 10.1002/14651858.CD003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land C, Riccio DC. d-cycloserine: Effects on long-term retention of a conditioned response and on memory for contextual attributes. Neurobiology of Learning and Memory. 1999;72:158–168. doi: 10.1006/nlme.1998.3897. [DOI] [PubMed] [Google Scholar]
- Lao J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl- d-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B) Molecular Pharmacology. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: Analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse H, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. Journal of Neuroscience. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. d-cycloserine facilitates extinction of conditioned fear as assessed by freezing in rats. Behavioral Neuroscience. 2003;117:341–505. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. d-cycloserine and the facilitation of conditioned fear: Consequences for reinstatement. Behavioral Neuroscience. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. Journal of Neuroscience. 2000;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelong V, Dauphin F, Boulouard M. RS 67333 and d-cycloserine accelerates learning acquisition in the rat. Neuropharmacology. 2001;41:517–522. doi: 10.1016/s0028-3908(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Lipp OV, Edwards MS. Effect of instructed extinction on verbal and autonomic indices of Pavlovian learning with fear-relevant conditional stimuli. Journal of Psychophysiology. 2002;16:176–186. [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proceedings of the National Academy of Science USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozzza MF, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic placsticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Davis NR, O’Flaherty AS. Protection from extinction in human fear conditioning. Behaviour Research and Therapy. 2000;38:967–983. doi: 10.1016/s0005-7967(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections in EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Baker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–278. [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—A decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Marks IM, Swinson RP, Basaglu M, Kuch K, Nasirvani H, O’Sullivan G, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia: A controlled study in London and Toronto. British Journal of Psychiatry. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction: A review. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learning and Memory. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KGS, Epstein IG, Baron H, Mulinos MG. Absorption, distribution and excretion of cycloserine in man. Antibiotic Annals. 1956:136–140. [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Mato K. Ca2+ stores regulate the polarity and input specificity of synaptic medication. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Nowak G, Trullas R, Layer R, Skolnick P, Paul IA. Adaptive changes in the N-methyl- d-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1380–1386. [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbert A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Öst LG, Svensson L, Hellstrom K, Lindwall R. One-session treatment of specific phobias in youths: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2001;69:814–824. [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Otto MW, Basden S, Leyro TM, McHugh K, Hofmann SG. Clinical perspectives on the combination of d-cycloserine and CBT for the treatment of anxiety disorders. CNS Spectrums. 2007;12:51–61. doi: 10.1017/s1092852900020526. [DOI] [PubMed] [Google Scholar]
- Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: Review and analysis. Clinical Psychology: Science and Practice. 2005;12:72–86. [Google Scholar]
- Parnas SA, Weber M, Richardson R. Effects of multiple exposures to d-cycloserine in extinction of conditioned fear in rats. Neurobiology of Learning and Memory. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Paul IA, Nowak G, Layer R, Popik P, Skolnick P. Adaptation of the N-methyl- d-aspartate receptor complex following chronic antidepressant treatments. Journal of Pharmacology and Experimental Therapeutics. 1994;269:95–102. [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. London, UK: Oxford University Press; 1927. [Google Scholar]
- Phelps EA, Delgado MR, Nearing KJ, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Nowak G. Chronic treatment with antidepressants affect glycine/NMDA receptor function: Behavioral evidence. Neuropharmacology. 2000;39:2278–2287. doi: 10.1016/s0028-3908(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Posey DJ, Kem DL, Swiezy NB, Sweeten TL, Wiegand RE, McDougle CJ. A pilot study of d-cycloserine in subjects with autistic disorder. American Journal of Psychiatry. 2004;161:2115–2117. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JA, Telch MJ. Disentangling the effects of safety-behavior utilization and safety-behavior availability during exposure-based treatment: A placebo-controlled trial. Journal of Consulting and Clinical Psychology. 2004;72:448–454. doi: 10.1037/0022-006X.72.3.448. [DOI] [PubMed] [Google Scholar]
- Pussinen R, Sirvio J. Effects of d-cycloserine, a positive modulator of N-methyl- d-aspartate receptors, and ST 587, a putative alpha-1 adrenergic agonist, individually and in combination, on the non-delayed and delayed foraging behaviour of rats assessed in the radial arm maze. Journal of Psychopharmacology. 1999;13:171–179. doi: 10.1177/026988119901300210. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with d-cycloserine facilitates learning and retention. European Journal of Pharmacology. 1994;157:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux IE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1995;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neuroscience. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. New York, NY: Appleton-Century-Crofts; 1972. [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: Use of d-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by d-cycloserine: Theoretical and clinical implications. Learning and Memory. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Robbins SJ. Mechanisms underlying spontaneous recovery in auto-shaping. Journal of Experimental Psychology: Animal Behavioral Processes. 1990;16:235–249. [Google Scholar]
- Rodriguez BI, Craske MG. The effects of distraction during exposure to phobic stimuli. Behaviour Research and Therapy. 1993;31:549–558. doi: 10.1016/0005-7967(93)90106-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez BI, Craske MG, Mineka S, Hladeck D. Context-specificity of relapse: Effects of therapist and environmental context on return of fear. Behaviour Research and Therapy. 1999;39:845–862. doi: 10.1016/s0005-7967(98)00106-5. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30:633–639. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. Journal of Neuroscience. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BL, Hashtroudi S, Herting RL, Schwartz P, Deutsch SI. d-cycloserine enhances implicit memory in Alzheimer patients. Neurology. 1996;46:420–424. doi: 10.1212/wnl.46.2.420. [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, et al. Multicenter Collaborative Panic Disorder Severity Scale. American Journal of Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Shell AM, Dawson ME, Marinkovic K. Effects of potentially phobic conditioned stimuli on retention, reconditioning, and extinction of the conditioned skin conductance response. Psychophysiology. 1991;28:140–153. doi: 10.1111/j.1469-8986.1991.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Falk W, Gunther J, Coyle J. Improved cognition in Alzheimer’s disease with short-term d-cycloserine treatment. American Journal of Psychiatry. 1999;156:467–469. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Pearlson GD, Krystal JH, Davies M, Brady RE, Simon NM, et al. A controlled trial of d-cycloserine with brief CBT for panic disorder. Paper presented at the 40th annual convention of the Association for Behavioral and Cognitive Therapies; Chicago, IL. 2006. [Google Scholar]
- Turner SM, Beidel DC, Dancu CV, Stanley MA. An empirically derived inventory to measure social fears and anxiety: The Social Phobia and Anxiety Inventory. Psychological Assessment. 1989;1:35–40. [Google Scholar]
- Walker DL, Davis M. The role of amygdale glutamate receptors in fear learning, potentiated startle, and extinction. Pharmacology, Biochemistry, and Behavior. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of d-cycloserine assessed with fear-potentiated startle. Journal of Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB. Behaviorism. New York, NY: Norton; 1924. [Google Scholar]
- Watson JB, Rayner R. Conditioned emotional reactions. Journal of Experimental Psychology. 1920;3:1–34. [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. A randomized, double-blind, placebo-controlled medication trial with d-cycloserine for individuals with OCS currently receiving behavior therapy: A work in progress. Paper presented at the 40th annual convention of the Association for Behavioral and Cognitive Therapies; Chicago, IL. 2006. [Google Scholar]
- Wolosker H. d-Serine regulation of NMDA receptor activity. Science STKE. 2006:pe41. doi: 10.1126/stke.3562006pe41. [DOI] [PubMed] [Google Scholar]
- Zhu M, Nix DE, Adam RDM, Peloquin CA. Pharmacokinetics of cycloserine under fasting conditions and with high-fat meal, orange juice, and antacids. Pharmacotherapy. 2001;21:891–897. doi: 10.1592/phco.21.11.891.34524. [DOI] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: A potentiator of AMPA receptors. Journal of Neuroscience. 2007;271:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]