Abstract
Plant photosynthesis declines when the temperature exceeds its optimum range. Recent evidence indicates that the reduction in photosynthesis is linked to ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco) deactivation due to the inhibition of Rubisco activase (RCA) under moderately elevated temperatures. To test the hypothesis that thermostable RCA can improve photosynthesis under elevated temperatures, we used gene shuffling technology to generate several Arabidopsis thaliana RCA1 (short isoform) variants exhibiting improved thermostability. Wild-type RCA1 and selected thermostable RCA1 variants were introduced into an Arabidopsis RCA deletion (Δrca) line. In a long-term growth test at either constant 26°C or daily 4-h 30°C exposure, the transgenic lines with the thermostable RCA1 variants exhibited higher photosynthetic rates, improved development patterns, higher biomass, and increased seed yields compared with the lines expressing wild-type RCA1 and a slight improvement compared with untransformed Arabidopsis plants. These results provide clear evidence that RCA is a major limiting factor in plant photosynthesis under moderately elevated temperatures and a potential target for genetic manipulation to improve crop plants productivity under heat stress conditions.
INTRODUCTION
Increasing crop yield to meet worldwide future food demand is one of the major challenges for agricultural research (Cassman, 1999). Although crop yields dramatically increased in the 20th century, the estimated growth in the world's population to ∼8 billion by 2020 and the slowing rate of increase in crop yield suggests a requirement for new strategies to improve food security (Miflin, 2000; Rosegrant and Cline, 2003). Additionally, plant productivity is often challenged by environmental stress, including high temperature and drought. Increased global temperature in the future will have both ecological and agricultural consequences. High temperature negatively impacts plant growth, survival, and yield. Field studies and mathematical modeling revealed that decadal variations in temperature have a significant effect on crop productivity. Over the last 17 years, a negative correlation between regional temperature during the growing season and maize (Zea mays) and soybean (Glycine max) yield has been described (Lobell and Asner, 2003).
Photosynthesis, the process through which plants accumulate biomass by converting inorganic carbon to carbohydrates using light energy, is a major target for improving crop productivity via conventional breeding practices (Richards, 2000) and by crop transgenic approaches (Dunwell, 2000; Sinclair et al., 2004). Genes involved in photosynthetic enhancement, such as sedoheptulose-1,7-bisphosphatase (SBPase) (Miyagawa et al., 2001), sucrose-phosphate synthase (Boxter et al., 2003; Lunn et al., 2003), and the C4 cycle enzymes phosphoenolpyruvate carboxylase and pyruvate phosphate dikinase (reviewed in Matsuoka et al., 2001; Leegood, 2002) were overexpressed in transgenic tobacco (Nicotiana tabacum) and rice (Oryza sativa) plants. Expression of the Escherichia coli glycolate catabolic pathway enzymes (i.e., glucolate dehydrogenase, glyoxylate carboligase, and tartronic semialdehyde reductase) (Kebeish et al., 2007) in C3 plants alleviated the negative effects on net photosynthesis from photorespiration in transgenic Arabidopsis thaliana plants. As one of the most heat-sensitive physiological processes, photosynthesis is also a target for crop improvement under heat stress conditions. At moderate heat stress, inhibition of photosynthesis is reversible, whereas extended recovery period at optimum growth temperature is required after exposure to high temperature (Salvucci and Crafts-Brandner, 2004a). Artificially increasing the intracellular CO2 concentration significantly improves photosynthesis and stimulates photosynthetic electron transport during short exposures to heat stress (Haldimann and Feller, 2004). Although several components of the photosynthetic apparatus and associated metabolic pathways are sensitive to moderate heat stress, numerous studies hypothesize that the loss of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the CO2 fixing enzyme, is the primary limiting factor of net photosynthesis under moderate heat stress (Feller et al., 1998; Salvucci and Crafts-Brandner, 2004a). The activation state of Rubisco, defined as the fraction of active sites that are catalytically competent, is regulated by the chloroplast-localized enzyme Rubisco activase (RCA), a member of the AAA+ family of ATPases associated with diverse cellular activities (Neuwald et al., 1999; Portis, 2003). Through protein–protein interactions and ATP hydrolysis, RCA can remove naturally occurring sugar-phosphate inhibitors from both active (carbamylated) and inactive (decarbamylated) Rubisco sites, a function that is essential for maintaining Rubisco catalytic competency.
Crafts-Brandner and Salvucci (2000) demonstrated that higher-plant Rubisco is a thermostable enzyme that exhibits increased catalytic turnover rates as temperatures are elevated up to 45°C, while RCAs from several crop plants are extremely thermolabile. Under moderately elevated temperatures, the loss of RCA activity, reduction in Rubisco's activation state, and lower plant photosynthetic rates are tightly correlated. Transgenic Arabidopsis plants expressing suboptimal levels of RCA were much more sensitive to inhibition by moderate heat stress than plants expressing normal levels of RCA (Salvucci et al., 2006). Based on these data, the thermolability of RCA was hypothesized as the primary cause for the loss of photosynthetic CO2 fixation activity during moderate heat stress. A corollary was the prediction that a thermostable RCA would be able to maintain Rubisco at a high activation state and increase photosynthetic CO2 fixation rates when the plants are exposed to moderate heat stress, in both C3 (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004a) and C4 plants (Crafts-Brandner and Salvucci, 2002).
Many plants contain two forms of RCA: the 43-kD β (short; RCA1) isoform and the 46-kD α (long; RCA2) isoform that is regulated by the redox state of the chloroplast via oxidation of two Cys residues at the C terminus portion (Zhang et al., 2002). In several plant species, such as Arabidopsis, spinach (Spinacia oleracea), and rice, the short and long isoforms are generated by alternative splicing of a single pre-mRNA (Werneke et al., 1989; To et al., 1999), while in maize, a single pre-mRNA encodes two polypeptides through limited proteolysis of the long isoform at its N-terminal region (Vargas-Suarez et al., 2004). In cotton (Gossypium hirsutum), the short and long isoforms are encoded by separate genes (Salvucci et al., 2003). Expression of the long form in cotton, maize, and wheat (Triticum aestivum) that requires prolonged exposure to heat stress and the significant thermostability properties in vitro of the cotton long form may indicate the importance of a thermostable activase under heat shock conditions.
To test the hypothesis that RCA limits photosynthesis under moderate heat stress, we used gene shuffling (Stemmer, 1994) to generate several variants of the Arabidopsis RCA1 exhibiting improved thermostability and expressed them in a fast-neutron mutagenized Arabidopsis RCA deletion line (Δrca) (Li et al., 2001). The positive effects of shuffled thermostable RCA variants on Rubisco activation state, rates of photosynthesis, and growth under moderate heat stress clearly demonstrate that RCA is a limiting factor in plant productivity under heat stress and provides a new strategy for improving crop yield under such stress conditions.
RESULTS
Evolution of Thermostable RCA Variants
To identify variants with improved thermostability, we developed a high-throughput screening method that directly assays Rubisco activation activity. In the primary screen, RCA1 variants expressed in E. coli were selected for their ability, in crude cell lysates, to activate Arabidopsis Rubisco at 25°C. A second tier screen assayed for the relative thermostability of active clones by incubating the host E. coli soluble fraction at different temperatures prior to the Rubisco activation assay. Selected RCA variants were purified and assayed in a third tier screen to determine specific activity and thermostability. After two rounds of shuffling and screening (3200 clones per round), seven variants exhibiting improved thermostability were identified.
To further characterize the properties of the improved variants and to select leads for plant transformation, purified proteins were analyzed using three different assays (Figure 1). Analysis of Rubisco activation by the shuffled leads indicates that the 2nd round variants (301C7 and 382D8) exhibit high thermostability at 40 and 45°C treatments (Figure 1A). The activity of 382D8 after 45°C treatment was 80% higher than RCA1 at the same temperature treatment and only 10% less than RCA1 activity incubated at 25°C. The response of activase activity to elevated temperatures was also evaluated by monitoring its ability to maintain Rubisco in an active state during heat treatment (Rubisco activation under catalytic conditions; Crafts-Brandner and Salvucci, 2000). While wild-type RCA maintained a Rubisco activation state of 0.5 at 40°C, the three leads were able to maintain activation states of 0.62 to 0.72 under the same condition (Figure 1B). Relative to reactions at 25°C, the activation state of Rubisco maintained by the thermostable variants at 40°C was in the range of 78 to 98%, versus 70% for the wild-type enzyme. The protein displaying the highest specific activity at 25°C was the best variant isolated in the first round, 183H12 (Figures 1A and 1B).
Figure 1.
Characterization of Wild-Type RCA (RCA1) and Thermostable Variants (183H12, 301C7, and 382D8).
(A) Rubisco activity after activation by activase after treatment at 25°C (white), 40°C (gray), and 45°C (black). Activase proteins were incubated at the indicated temperatures for 15 min prior to assaying at 25°C.
(B) Activation of Rubisco under catalytic conditions at 25°C (white) and 40°C (gray).
(C) ATPase activity (relative to RCA1 activity at 25°C) of activase proteins incubated at the indicated temperatures for 15 min prior to assaying at 25°C.
RCA is an ATPase (contains the AAA+ domain) that requires ATP to loosen the binding of Rubisco for sugar phosphates (Portis, 2003). We therefore monitored the ATPase activity of the RCA complex independent of its interaction with Rubisco. Results (Figure 1C) showed that the stability of 2nd round variants at 35 and 40°C was improved >10-fold compared with RCA1, whereas 183H12 exhibited 20 and 30% improvement at 25 and 40°C, respectively. These results indicate that the improvement through gene shuffling affects multiple RCA1 properties: the thermostability of the RCA1 molecules to hydrolyze ATP (ATPase activity), the residual activity of the RCA1- RCA1 multimeric complex to activate Rubisco (activation of inactive Rubisco), and the RCA1-Rubisco complex (Rubisco activation under catalytic conditions).
Sequence analysis revealed that one amino acid substitution in 183H12 (T274R) was sufficient to improve activity and thermostability. Three amino acid substitutions in the 2nd round variants 301C7 (F168L, V257I, and K310N) and 382D8 (M131V, V257I, and K310N) resulted in a 10°C increase in stability of Arabidopsis RCA1. The variant 383A12 contains mutations from both 183H12 and 301C7 (F168L, V257I, T274R, and K310N) and exhibited relative low activity at 25°C (82% of the 183H12 activity) but maintained high activity at 40°C (102% of 301C7 activity). Two substitutions shared in variants 301C7 and 382D8 are also present as natural variation in plant species; V257I is present in the cucumber (Cucumis sativus) enzyme, and K310N is conserved in wheat, rice, spinach, and maize.
Characterization of an RCA Deletion Mutant
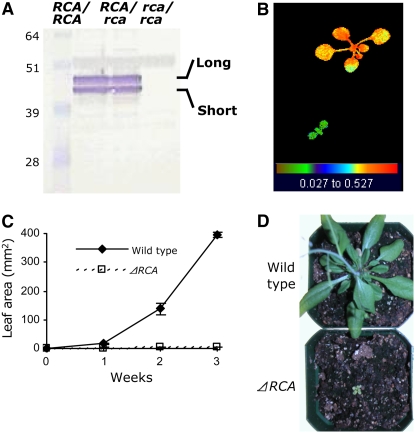
To study the physiological effects associated with the thermostable RCA variants, we screened fast neutron deletion mutagenesis Arabidopsis lines (Li et al., 2001) and isolated an RCA deletion mutant harboring a 3.4-kb deletion (Δrca; see Methods). The Δrca lacks exons 5 to 7 of the rca locus. Our Δrca mutant possesses major advantages as a host system. First, the phenotype and photosynthesis and growth rates of a complemented mutant are affected directly and solely by the properties of the shuffled variants, without the influence of the wild-type enzyme. Secondly, the absence of endogenous RCA1 and RCA2 mimics the screening and selection process for improved shuffled variants, which was performed in the absence of the wild-type genes. Finally, the absence of RCA1 and RCA2 avoids the potential formation of a heterocomplex with the shuffled variants, which could affect their complex properties.
The Δrca mutant was characterized for expression of endogenous RCA, photosynthetic performance, and growth rates to link the genotype to the phenotype. Immunoblot analysis of wild-type, heterozygous, and homozygous plants (genetic backgrounds RCA/RCA, RCA/Δrca, and Δrca/Δrca, respectively) revealed that the gene products (long and short forms) were expressed at similar levels in wild-type and heterozygous plants (Figure 2A). The absence of the short and long isoforms in plants homozygous for the deletion confirmed that the mutation abrogates the expression of both RCA1 and RCA2. Δrca plants grown at ambient CO2 levels exhibited low photosynthetic performance (Fq′/Fm′ values) compared with the wild type (0.185 ± 0.038 and 0.332 ± 0.033, respectively) (Figure 2B) and significantly lower leaf area after 3 weeks on soil (2.93 ± 0.49 and 395.4 ± 8.75 mm2, respectively) (Figure 2C). Two-month-old Δrca homozygotes were severely stunted and chlorotic in comparison with wild-type plants (Figure 2D). We used the deletion line as a host to study the physiological effects of transgenes specifying the expression of RCA1 and thermostable variants.
Figure 2.
Characterization of the Δrca Mutant at Ambient CO2.
(A) Immunoblot analysis from leaves of Arabidopsis wild-type (RCA/RCA), heterozygous (RCA/Δrca), and homozygous (Δrca/Δrca) plants. The blot was immunodecorated with polyclonal antibodies raised against the recombinant Arabidopsis RCA1.
(B) Photosynthetic performance (Fq′/Fm′) of 3-week old wild-type (top) and Δrca (bottom) plants as measured using fluorescence image analysis.
(C) Leaf area (mm2) of the plants described in (B) (50 plants per phenotype) at the indicted age.
(D) Eight-week-old wild-type (top) and Δrca (bottom) plants.
Complementation of Δrca
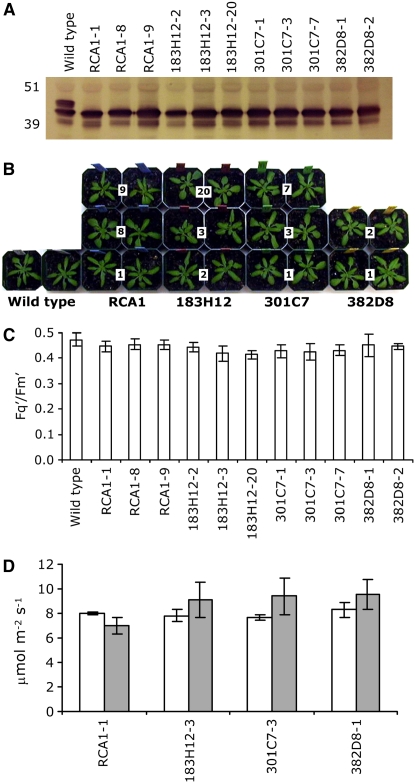
Since Δrca homozygotes cannot flower and produce seeds, we have used the following complementation cascade: (1) selection of heterozygous plants for the deletion by high-throughput PCR using a single-leaf 96-well DNA extraction method (Xin et al., 2003) with specific primers for the wild-type and deleted alleles (see Methods), (2) transformation with the gene of interest, (3) T0 selection for antibiotic resistance and PCR analysis for homozygosity, and (4) self-pollination of the resultant homozygous plants to obtain T1 transgenic lines. As shown in Figure 3A, the wild type (RCA/RCA) expresses short and long isoforms of activase, whereas transgenic Δrca lines complemented by the transgenes express only the 43-kD short isoform. Since RCA is extremely sensitive to proteolytic degradation (Salvucci et al., 1993), the additional 39-kD faint band detected by the RCA antibodies is most likely a degradation product of the transgene. Under 22°C growth conditions (see Methods), the transgenic lines exhibited similar growth rates as the wild-type untransformed plants (Figure 3B). The Fq′/Fm′ values of transgenic deletion lines expressing RCA1 or variants 183H12, 301C7, or 382D8 (ΔrcaRCA1, Δrca183H12, Δrca301C7, and Δrca382D8, respectively) were similar to wild-type untransformed plants, indicating that expression of the short form is sufficient for functional complementation of Δrca under normal growth conditions (Figure 3C). Under these conditions, the photosynthetic activity of ΔrcaRCA1-1 was similar to Δrca183H12-3, Δrca301C7-3, and Δrca382D8-1 (Figure 3D). Temporary exposure to 30°C for 1 h resulted in 12% decreased photosynthesis in ΔrcaRCA1-1. Conversely, lines Δrca183H12-3, Δrca301C7-3, and Δrca382D8-1 exhibited 16, 22, and 16% increased photosynthesis after 1 h at 30°C.
Figure 3.
Functional Complementation of Δrca Mutants Expressing RCA1 and Thermostable Variants 183H12, 301C7, and 382D8 under Normal Growth Conditions (22°C).
(A) Immunoblot analysis of total protein from 3-week old leaves. Numbers indicate the line designations of independent transformation events.
(B) Photographs depicting the similar size of all the plants described above when grown under normal conditions.
(C) Photosynthetic performance (Fq′/Fm′) of the plants (8 to 10 plants/independent line) described above monitored by fluorescence image analysis.
(D) Effect of temporary (1 h) moderate heat stress treatment on photosynthesis rates (μmol m−2 s−1) of Δrca transgenic lines expressing RCA1 and thermostable variants. The net photosynthesis of four independent plants per line was monitored using an infrared gas analyzer at 22°C (white) and 30°C (gray).
Effect of Thermostable Activase on Growth and Development under Moderate Heat Stress Conditions
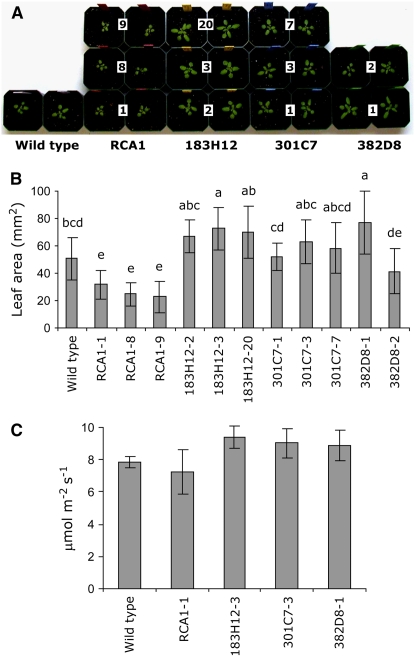
Since exposure of Arabidopsis plants to 30°C causes minor induction of heat shock proteins (typically induced at 32°C and above) and minor effects on stomatal aperture (Feller et al., 1998; Salvucci et al., 2001), we predicted that growth under prolonged heat treatment would be positively affected by a thermostable activase. Four-week-old transgenic lines exposed for 2 weeks to moderate heat stress (see Methods) displayed normal phenotype and leaf color but varied in size (Figure 4A). ΔrcaRCA1 (lines 1, 8, and 9) were stunted in comparison with wild-type untransformed plants and to the Δrca lines that express the shuffled variants (Figure 4B). Transgenic Arabidopsis expressing the 1st round variant (Δrca183H12-3, Δrca183H12-2, and Δrca183H12-20) that possesses the highest in vitro–specific activity were the largest plants. Lines that expressed the thermostable 2nd round variants Δrca301C7 and Δrca382D8 were larger than ΔrcaRCA1 lines but did not reach the leaf area levels of the Δrca183H12 lines. While Δrca lines expressing only the short form of the wild-type gene (RCA1) were smaller than wild-type untransformed lines (expressing both short and long forms), most transgenic lines expressing the shuffled variants exhibited greater leaf area than wild-type untransformed plants (Figure 4B), and all lines expressing the shuffled variants were significantly larger (P = 0.01) than the Δrca transformants expressing RCA1. The best line from each variant was further analyzed for photosynthetic activity during the moderate heat stress cycle (after 2 h at 30°C). Transgenic lines showed a CO2 fixation pattern that correlated with leaf area. Rates of CO2 fixation in lines Δrca183H12-3, Δrca301C7-3, and Δrca382D8-1 were 30, 25, and 23% higher, respectively, than in the ΔrcaRCA1-1 line (Figure 4C). These results demonstrate that RCA is a limiting factor in photosynthesis under the experimental conditions.
Figure 4.
Effect of Moderate Heat Stress (30°C for 4 h per d) on Wild-Type Plants and Δrca Mutants Expressing RCA1 or Thermostable Variants 183H12, 301C7, and 382D8 at the Vegetative Stage.
(A) Photograph of the plants showing differential growth rates mediated by the RCA variant. Numbers indicate the line designations of independent transformation events.
(B) Leaf area (mm2) of 8 to 10 independent plants per line, analyzed using a fluorescence image analysis system. Means followed by common letters are not significantly different at P = 0.05 using a protected least significant difference.
(C) Net photosynthesis (μmol m−2 s−1) of four independent plants from selected lines, monitored by gas exchange analysis after 2 h at 30°C.
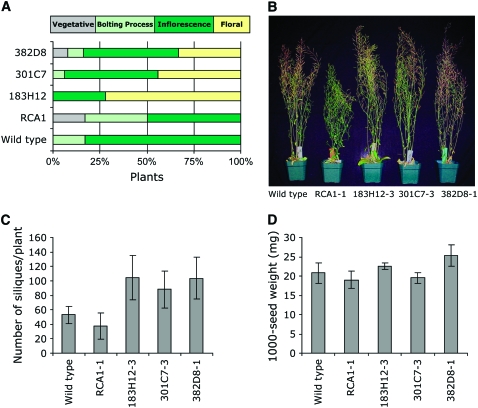
Four-week-old plants exposed to 2 weeks of moderate heat stress also showed differences in rates of plant development (Figure 5A). At the end of the treatment period, 74, 44, and 33% of Δrca183H12, Δrca301C7, and Δrca382D8, respectively, had mature inflorescences with open flowers, while 100% of untransformed wild-type plants and 88% of ΔrcaRCA1 lines had emerging immature inflorescences with no open flowers. Additionally, 12% of the ΔrcaRCA1 lines were in the vegetative stage with no visible inflorescences. Under normal growth conditions, Arabidopsis plants flower after 4 weeks. Therefore, the relatively high percentage of Δrca183H12, Δrca301C7, and Δrca382D8 lines showing normal development is likely due to improved RCA thermostability, which minimized the inhibition of photosynthesis and growth under moderate heat stress conditions.
Figure 5.
Effect of Moderate Heat Stress (30°C for 4 h per d) on Mature Wild-Type Plants and Δrca Mutants Expressing RCA1 or Thermostable Variants 183H12, 301C7, and 382D8.
(A) Developmental analysis of plants described in Figure 4. Numbers represent the percentage of plants in each developmental stage after 4 weeks of growth.
(B) Photograph of mature plants (8 weeks old).
(C) Number of siliques per plant. Eight to 10 independent plants per line were analyzed.
(D) Seed weight (mg/1000 seeds) of seeds harvested from the selected lines (five independent plants per line).
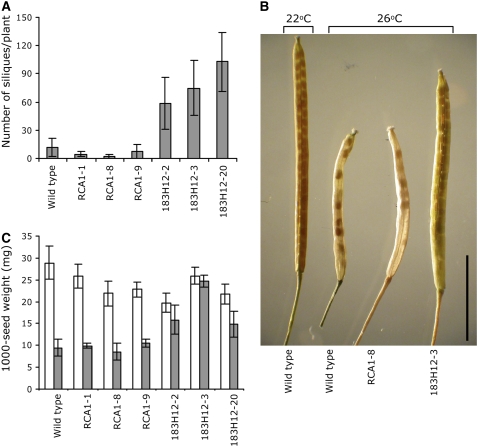
Mature plants (10 weeks old) exposed for 8 weeks to moderate heat stress were similar in appearance. A slight positive effect on plant height was detected in Δrca183H12-3, Δrca301C7-3, and Δrca382D8-1 lines (116, 121, and 119%, respectively) compared with ΔrcaRCA1-1 (Figure 5B). A dramatic difference was observed in the number of siliques per plant, which was 130.8 ± 48.2, 84.3 ± 19.6, and 100.8 ± 26.9 for Δrca183H12-3, Δrca301C7-3, and Δrca382D8-1, respectively, compared with 40.2 ± 16.3 and 47.5 ± 15.8 for ΔrcaRCA1-1 and the wild type, respectively (Figure 5C). To confirm that the relatively enhanced formation of siliques in transformants expressing improved RCA was not at the expense of individual seed size, we compared the weights of lots of 1000 seeds. As shown in Figure 5D, Δrca183H12-3 and Δrca382D8-1 produced slightly larger seeds (18 and 32%, respectively) than ΔrcaRCA1-1, while the seed weight of Δrca301C7-3 and wild-type plants was similar to that of ΔrcaRCA1-1.
Effect of Thermostable Activase on Seed Yield under Continual 26°C Temperature Stress
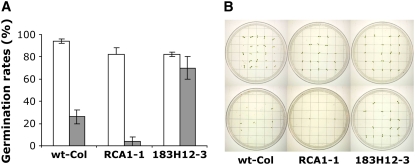
To further analyze the effect of improved RCA on growth under moderate temperature stress, T3 lines expressing the most active clone at 25°C (in vitro), 183H12, were grown continuously at 26°C under higher light intensity and humidity than in the previous experiment (see Methods). Wild-type and ΔrcaRCA1 lines grown at 26°C produced slightly decreased overall biomass and exhibited slow rates of plant development than under normal growth conditions, whereas the biomass and the developmental process of lines of Δrca183H12 was unchanged (data not shown). By contrast, the number of siliques per plant produced by Δrca plants grown at 26°C was dramatically affected by the variant of RCA they expressed (Figure 6A). Δrca183H12 lines possessed 50 to 100 more siliques per plant than ΔrcaRCA1 lines and 40 to 80 more siliques per plant than wild-type plants. In addition, the siliques of Δrca183H12 were larger and produced more seeds than the wild-type plants and the ΔrcaRCA1 lines (Figure 6B). Siliques from Δrca183H12 at 26°C exhibited a similar phenotype to the wild type grown under normal growth conditions but produced fewer seeds. Under normal growth conditions, a minor decrease in seed weight was observed in Δrca lines expressing RCA1 and 183H12 compared with wild-type plants (Figure 6C; white bars). Under continuous exposure to 26°C, however, 50 to 150% greater seed weight was observed in lines of Δrca183H12 than in either wild-type plants or ΔrcaRCA1 lines. In comparing seed weight for each line grown at 26°C to that of the same line grown at 22°C, lines Δrca183H12-2, Δrca183H12-3, and Δrca183H12-20 were strikingly less affected by the higher growth temperature than the ΔrcaRCA1 lines or the wild type. Since exposure to 26°C resulted in small siliques containing few seeds of small seed weight, we further analyzed seed viability using a germination test. Seeds from the wild type, ΔrcaRCA1-1, and Δrca183H12-3 were collected from plants grown at normal growth conditions and 26°C and then germinated at 22°C. Seeds from ΔrcaRCA1-1 and Δrca183H12-3 lines of parents grown at 22°C showed the same germination rate (86%), which was slightly lower than that of wild-type plants (94%) (Figure 7). Complete inhibition of germination (4%) was observed in ΔrcaRCA1-1 seeds collected from parents grown at 26°C and significant inhibition in wild-type seeds (26%). Conversely, Δrca183H12-3 seeds collected from parents grown at 26°C exhibited relatively high germination rates of 70%.
Figure 6.
Effect of 26°C Heat Stress on Development and Yield of Wild-Type Plants and Δrca Mutant Lines Expressing RCA1 and the Thermostable Variant 183H12.
(A) Number of siliques per plant. Ten to 12 independent plants per line were analyzed.
(B) Photograph of siliques from selected lines grown at the indicated temperature showing variation in silique size and seed set. Bar = 0.5 cm.
(C) Seed weight (mg/1000 seeds) of seeds harvested from the plants described in (A).
Figure 7.
Effect of 26°C Heat Stress on the Germination Rates of Seeds from the Wild-Type Plants and the Δrca Mutant Lines Expressing RCA1 and the Thermostable Variant 183H12.
(A) Germination rates (at 22°C) of seeds (250 seeds per line) harvested from Arabidopsis plants that were grown at 22 or 26°C (gray).
(B) Plantlets described in (A) harvested from Arabidopsis plants that were grown at 22°C (top) or 26°C (bottom).
DISCUSSION
Damage to the photosynthetic electron transport chain has been considered the primary cause for inhibition of photosynthesis under heat stress due to the heat sensitivity of photosystem II (Berry and Björkman, 1980). Based on enzyme kinetics and modeling, previous researchers hypothesized that RCA thermolability is a primary cause for the inhibition of plant photosynthesis under moderate temperature stress. Crafts-Brandner and Salvucci (2000) analyzed the thermostability of Rubisco and RCA and demonstrated that RCA is the limiting enzyme in vitro. Similar results were observed across a wide variety of taxa, including C3 plants (spinach and tobacco), C4 plants (maize), plants that grow in hot (cotton and creosote bush [Larrea tridentata]) and cold (Antarctic hairgrass) climatic conditions, and for trees grown under natural conditions (Crafts-Brandner and Salvucci, 2000, 2002; Haldimann and Feller, 2004; Salvucci and Crafts-Brandner, 2004a, 2004b). Based on the CO2, O2, ATP, and ribulose-1,5-biphosphate concentrations, the gas solubilities at elevated temperatures and the activities of the key C4 enzymes, pyruvate phosphate dikinase and PEP carboxylase, and the components of the photosynthetic electron transport chain, Crafts-Brandner and Salvucci (2002) predicted that maize photosynthesis would increase at elevated temperatures (25 to 45°C) if the system included a thermostable activase.
Two approaches to validate their hypothesis are (1) transgenic replacement of the native RCA with an enzyme modified to increase thermostability or (2) expression of a thermostable RCA isolated from a heat-resistant species in a heat-sensitive host, such as Arabidopsis. Using site-directed mutagenesis to convert Gln-111 to Glu, Kallis et al. (2000) increased Arabidopsis RCA1 activity by 250% in vitro but observed no improvement in thermostability. Heat-tolerant activase enzymes from plants grown in warm regions, such as creosote bush, jojoba (Simmondsia chinensis), tobacco, and cotton, have been characterized (Salvucci and Crafts-Brandner, 2004b), but the enzymes have not yet been expressed in transgenic heat-sensitive hosts. Such experiments could be complicated by the species dependence of the Rubisco–RCA interaction (Wang et al., 1992). Gene shuffling represents an attractive approach to improve thermostability while maintaining species specificity.
In our efforts to generate Arabidopsis RCA variants with both improved activity and thermostability, we developed a method to screen directly for improved activation of Rubisco. This strategy eliminated the risk that screening for increased ATPase activity or thermostability would not result in corresponding improvement of Rubisco activation. Measuring RCA activity using the ATP hydrolysis assay represents the intrinsic activity of activase, regardless of its interaction with Rubisco, whereas the Rubisco activation assay takes RCA subunit interaction and RCA–Rubisco interaction into account. The improvement of thermostable ATPase activity was more dramatic than the improvement in Rubisco activation activity (cf. Figures 2A and 2C), which is a more biologically significant parameter.
We isolated variants exhibiting higher Rubisco activation activity at 40°C than the activity of the wild-type enzyme at 25°C. After two rounds of shuffling, we increased the ratio of activity after treatment at 40°C versus treatment at 25°C from 54% for the wild-type enzyme to an average of 93%, while maintaining specific activity at 25°C comparable to the wild-type enzyme. Variants 301C7 and 382D8 also possessed greater thermostability than the redox-regulated Arabidopsis RCA long form (RCA2) (Zhang and Portis, 1999; Zhang et al., 2002). Two rounds of DNA shuffling allowed us to identify RCA variants similar to those reported from plants adapted to warm temperatures, such as tobacco, cotton, and creosote bush (Salvucci and Crafts-Brandner, 2004b).
Introducing shuffled RCA variants into an Arabidopsis Δrca line enabled us to directly evaluate the physiological effects of increased RCA thermostability under elevated temperature conditions. Higher photosynthetic rates were observed after 1 h at 30°C in Δrca lines. Under prolonged exposure to moderate heat stress (30°C for 4 h per day), transgenic lines expressing the thermostable variants exhibited increased leaf area and silique number in comparison to Δrca lines expressing RCA1 and a minor increase compared with the wild-type plants (Figures 4 and 5). The comparison of Δrca 183H12 and Δrca RCA1 lines under 26°C showed similar results; namely, Δrca 183H12 exhibited larger leaf area, a higher number of siliques, higher seed weight, and higher germination rates compared with Δrca RCA1 lines and wild-type plants under this treatment (Figure 6).
An additional strategy to improve the Rubisco activation state under heat stress is by overexpression of enzymes that indirectly increase RCA stability. Yang et al. (2005) recently reported an alternate strategy to improve RCA activity under heat stress. They observed that accumulation of Gly betaine in the tobacco chloroplast prevents sequestration of RCA to the thylakoid membranes and maintains higher Rubisco activation state, growth rate, and photosynthetic rate at elevated temperatures (Yang et al., 2005). However, this strategy might not effectively stabilize RCA in all plants since accumulation of Gly betaine requires adequate amounts of choline in the chloroplast. Protection of RCA under heat stress conditions was recently shown by the expression of the enzyme SBPase (Feng et al., 2007). Although the mechanism of RCA thermoprotection of SBPase in vivo is not clear, transgenic rice plants overexpressing SBPase exposed to heat stress exhibited high photosynthetic rates and accumulated more biomass compared with wild-type plants. Protein gel blot analysis of wild-type plants and transgenic rice plants showed that under heat stress, the RCA content in the soluble fractions decreased and the association of RCA with thylakoids increased in wild-type plants. Thus, Feng et al. (2007) suggested that in transgenic rice plants exposed to heat stress, the levels of RCA available to activate Rubisco in the stroma are higher than in wild-type plants.
Kim and Portis (2005), studying Arabidopsis mutants deficient in both short and long form of RCA (rca-), demonstrated that the short-term photosynthetic performance of the mutant expressing the short form had a similar pattern to wild-type plants in response to temporary heat stress. Similar results were recently demonstrated by Salvucci et al. (2006), who analyzed the effect of suboptimal levels of RCA on photosynthesis inhibition under moderate heat stress. Low levels of the short isoform (12% of total levels of short and long forms) expressed in the rca- mutants resulted in lower growth rates at normal growth conditions and greater sensitivity of photosynthesis to inhibition by moderate heat stress compared with wild-type plants. By contrast, we observed a significant heat sensitivity of the ΔrcaRCA1 (expressing short form, but lacking long form) lines compared with wild-type plants (expressing both short and long forms) when exposed to extended heat stress. All the transgenic lines under moderate heat treatment (4 h, 30°C) failed to maintain the leaf area, silique number, and seed weight that were observed under normal growth conditions. It is possible that the long form plays an additional role as a molecular chaperone during prolonged exposure to heat stress that could be partially compensated at 26°C by the thermostable short form (183H12). In the absence of the long form, ΔrcaRCA1 lines express about half as much activase as wild-type plants. This amount could be sufficient to sustain photosynthesis and growth rates under normal conditions while it is insufficient to maintain the wild-type phenotype under moderate heat stress. It is possible that this negative effect can be minimized in lines that express active and thermostable shuffled variants. Another possibility is the effect of heat stress, at 30°C but not 26°C, on photosystem II activity and increased membrane leakiness (Sharkey, 2000). Finally, we cannot rule out that at 26°C the thermostable and active short form is sufficient to maintain the biomass, silique number, and viability of the progenies, whereas under moderate heat stress (30°C, 4 h), additional physiological factors unrelated to photosynthesis are required to produce a normal plant phenotypes.
Transgenic line Δrca183H12-3 grown at 26°C was significantly less affected at leaf area, number of siliques, seed weight, and germination rates compared with Δrca RCA1 lines and wild-type plants (Figures 6 and 7). In developing embryos of Brassica napus under normal growth conditions, Rubisco provides metabolic pathway without the Calvin cycle that increases the efficiency of transforming carbohydrates to oil (Schwender et al., 2004). It is possible that under a moderately elevated temperature (26°C) solely, inactivation of Rubisco due to thermolability of RCA limits seed development and maturation, while under high temperatures, the thermotolerance of mature dry seed and imbibed seed can be acquired by expression of heat shock proteins. Prieto-Dapena et al. (2006) recently demonstrated that expression of the sunflower (Helianthus annuus) seed-specific heat stress transcription factor (Ha HSFA9) in tobacco plants increases the abundance of two classes of cytosol-localized heat shock proteins CI and CII. The transgenic tobacco plants exhibited seed with high germination rates and viability under heat stress conditions.
Taken collectively, our data support models proposing that the thermolability of RCA plays a significant role in decreased photosynthetic rate, growth rate, and seed yield associated with moderate heat stress. For the past 50 years, Rubisco has been a prime target enzyme for improving crop photosynthetic performance (Parry et al., 2003; Sinclair et al., 2004). To date, it has not proven possible to engineer higher-plant Rubisco with improved carboxylation activity or improved CO2/O2 specificity. Improving RCA represents a promising strategy to enhance Rubisco activity and photosynthetic performance under moderate temperature stress. It is known that the RCA enzymes from several crop plants including maize (Crafts-Brandner and Salvucci, 2002), cotton (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner 2004a), and wheat (Law and Crafts-Brandner, 1999) are thermolabile. Although it is unknown whether or not the thermolability of RCA plays any protective roles by slowing/shutting down photosynthesis/photorespiration under certain temperature conditions, our results demonstrate that under moderate heat stress conditions, increased thermostability of RCA does exert positive impacts on photosynthesis and growth. Use of the methodology developed in this work to improve thermostability of crop species' own RCA is highly feasible. This approach would avoid incompatibility between Rubisco and a foreign RCA and would very likely improve photosynthesis, growth, and yield under moderate heat stress. Nuclear transformation of crop plants has become routine, and RNA interference technology may be used to eliminate or reduce wild-type RCA for maximal performance of the engineered RCA. Our future research will explore expressing improved RCA variants in crop plants with the goal of improving yield under normal and moderate heat stress conditions and expanding cultivated acreage to warmer locations.
METHODS
Arabidopsis thaliana RCA Cloning and Shuffling
Arabidopsis RNA was isolated from green leaves using TrizolR reagent according to the manufacturer's protocol (Invitrogen). RCA cDNA was PCR cloned into TOPOR vector (Invitrogen) using the TITANIUM one-step RT-PCR kit (BD Biosciences-Clontech). For single gene shuffling in the first round, the mature RCA short form (coding region Val-59 to Lys-438) was PCR amplified (Qiagen Taq DNA polymerase or Stratagene Mutazyme DNA polymerase), fragmented, and reassembled in a primerless PCR reaction as previously described (Stemmer, 1994). The shuffled genes were then rescued with flanking primers 5′ (5′-GGCCATGGTGAAAGAAGACAAACAAAC-3′) and 3′ (5′-CGGATCCTTAATGATGATGATGATGATGGCTGCTGCCCTCGAGCTT-3′) that contain an NcoI and BamHI site, respectively. The library of variants was cloned into an Escherichia coli expression vector (pET16b; Novagen) digested with NcoI and BamHI. To increase the pool of genetic diversity in the first round, synthetic shuffling (Ness et al., 2002) was performed using sequences from wheat, rice, cotton, spinach, and cucumber and cloned into pET16b as described above. A second round of gene shuffling using first-round variants as parents was performed as previously described (Crameri et al., 1998).
High-Throughput Screening for Rubisco Activation
E. coli BL21/DE3 cells (Stratagene) expressing shuffled RCA were grown in 200 μL 2xYT media in 96-well plate format, induced (0.4 mM isopropylthio-β-galactoside) at 28°C for 18 h, and harvested by centrifugation at 4000 rpm for 20 min. Cell pellets were resuspended and incubated for 30 min at 4°C in 100 μL sonication buffer that contained 100 mM Tricine- KOH, pH 8, 20 mM ascorbic acid, 3 mM Mg-ATP, 10 mM MgCl2, 10% (v/v) glycerol, 10 mM 2-mercaptoethanol, Protease inhibitor cocktail Set V (EMD-Calbiochem), 10 units mL−1 Benzonase (Novagen), and 1 mg mL−1 Lysozyme (Sigma-Aldrich). Plates were sonicated at 4°C in a 96-well Microplate Sonicator (Misonix) for 5 min. Cell debris was removed by centrifugation at 4000 rpm for 20 min at 4°C. Twenty-two microliters of the soluble fraction of the E. coli cell lysate was incubated with 10 μg of purified noncarbamylated Arabidopsis Rubisco (Salvucci and Crafts-Brandner, 2004b) for 9 min in a 50-μL (final volume) reaction containing an ATP-regenerating system, ribulose-1,5-biphosphate, and [14C]NaHCO3 as previously described (Shen et al., 1991). The activation of Rubisco by cell lysate expressing shuffled variants was terminated by addition of 1 n HCl, and the incorporation of 14CO2 into 3-phosphoglyceric acid was determined by liquid scintillation spectroscopy. All assays were corrected for the background rates of noncarbamylated Arabidopsis Rubisco incubated with E. coli cell lysate expressing empty vector.
Protein Purification and Enzymatic Assays
Recombinant RCA was affinity purified on an Ni-NTA Superflow (Qiagen) column according to the instructions of the manufacturer at 4°C with the following modification: all buffers contained 20 mM ascorbic acid, 3 mM Mg-ATP, 10 mM MgCl2, 10% (v/v) glycerol, and 10 mM 2-mercaptoethanol. Purified proteins were transferred into buffer containing 100 mM Tricine-KOH, pH 8, 3 mM Mg-ATP, 10 mM MgCl2, 10% (v/v) glycerol, and 10 mM 2-mercaptoethanol in a NAP-5 SephadexR G-25 column (Amersham Biosciences). The purity of affinity-purified RCA proteins was 90 to 95% as determined by 10% SDS-PAGE followed by staining with SimplyBlue SafeStain (Invitrogen). Protein concentrations were determined using Coomassie Plus protein assay reagent (Pierce) with BSA as standard.
Recombinant purified RCA was assayed for activation of deactivated Rubisco as described above for high-throughput assay in a 100-μL reaction for 6 min. For heat treatment, purified protein was incubated at the indicated temperature for 15 min prior to assaying. Rubisco activation under catalytic conditions was performed as previously described (Crafts-Brandner and Salvucci, 2000). To increase the heat sensitivity of RCA during the assays, 5% (w/v) polyethylene glycol-3500 was replaced with 10% (v/v) glycerol (Salvucci, 1992). ATPase activity of pretreated RCA at the indicated temperature was determined using the spectrophotometric assay (Shen et al., 1991).
Isolation of the Arabidopsis RCA Deletion Mutant
To select an Arabidopsis RCA deletion mutant (Δrca), 40 mega DNA pools (each mega pool contained DNA representing 2592 lines) were screened by PCR. Using the primers 16057R (5′-GTGGCAACTTGGCCAATCCGCATCGTGTGGAND-3′) and 10314F (5′-TCTCGGAAGTCTCTCATCACCGAGTCTACC-3′) that are located 179 bp from the first exon and 3170 bp from the last exon and flank 5.8 kb in the wild-type genome, we detected a 2.4-kb PCR product. PCR analysis was subsequently performed on the constituent super pools and pools that contained DNA representing 288 and 18 lines, respectively. Seeds from the 18 lines of the selected pool were planted, and the DNA was extracted and analyzed by PCR. Sequence analysis indicated that the deleted fragment contained exons 5 to 7 of the RCA locus and an additional putative open reading frame encoding for 90 amino acids with no homology to any known gene in GenBank.
Plant Transformation and Selection, Growth Conditions, and Photosynthesis Analysis
To express RCA1 and the shuffled variants in their native form in transgenic Arabidopsis plants (Δrca), the transgenes encoding the chloroplast transit peptide and the coding region of rca1 or the shuffled gene variants were cloned into pMAXY4384, which contains the Mirabilis Mosaic Caulimovirus promoter with a double enhancer domain (Day and Maiti, 1999), the UBQ3 terminator, and the kanamycin resistance gene nptII. Heterozygous Deleteagene RCA mutants were transformed by Agrobacterium tumefaciens strain GV3101 using the floral dipping method (Clough and Bent, 1998). To identify Δrca homozygotes expressing shuffled variants, two sets of primers were designed based on the sequence analysis and mapping of the deleted fragment: RCA primers (forward 5′-CAGACAATGTTGGCCTC-3′ and reverse 5′-ACGAGTAACGATGGTAGG-3′) specific for the wild-type allele that produce a 1.5-kb product and rca primers (forward 5′-GTCTATACCTTGAGC-3′and reverse 5′-TCAGTCATACTCGG-3′) that produce a 1.5-kb product in the deleted allele and 4.9 kb in the wild-type allele. To amplify the 1.5-kb product with the rca primers but not the 4.9 kb, we set the PCR amplification cycle to 1.5 min. Those two sets of primers were used to characterize the genetic background of the T1 plants.
Protein was extracted from plant tissue (2 to 3 g fresh weight) in liquid N2 and 1 mL of extraction buffer (100 mM Tricine-KOH, pH 8, 1 mM EDTA, pH 8, 10 mM 2-mercaptoethanol, and Protease inhibitor cocktail Set V). The crude extract was clarified by successive centrifugation for 5 min at 3000g and 20 min at 12,000g. Ten micrograms of soluble protein extract was separated on 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane (according to the instructions supplied by Invitrogen). The blot was immunodecorated with the polyclonal antibodies raised against the recombinant Arabidopsis RCA1, and the proteins were detected using the AP-conjugated substrate kit (Bio-Rad).
Normal growth conditions are defined as 22°C in a 16-h-light (225 μmol photons m−2 s−1)/8-h-dark regime. For moderate heat stress, plants were grown under the same light intensity and regime, but during the light cycle, the temperature was 22°C for 6 h, rapidly increased to 30°C (2°C per min) for 4 h, then returned to 22°C for additional 6 h to complete the day cycle. The temperature during the 8 h dark cycle remained at 22°C. A third temperature treatment was continuous growth at 26°C, with a 16-h-light (300 μmol photons m−2 s−1) and 8-h-dark cycle with 85% humidity.
Steady state net photosynthesis was analyzed during normal and gradual heat treatment (indicated in the text) using the portable infrared gas analyzer (Li-Cor6400; Li-Cor) under 150 μmol photons m−2 s−1 and 350 μbar CO2 supplied by the built-in CO2 injection system. Photosynthetic performance (photosystem II operating efficiency: Fq′/Fm′) and growth rates were analyzed using the chlorophyll a fluorescence imaging system (FluorImager; Qubit Systems) as previously described (Baker et al., 2001). Characterization of growth, biomass, and yield was performed as previously described (Barth et al., 2003).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM179990, AY206372, AF480497, DQ233255, JO3610, X67674, and AY063838.
Acknowledgments
This work was supported by a grant from the National Institute of Standards and Technology-Advanced Technology Program. We thank Eva Lin, Lik Hsueh, and Matthew J. Heckert for technical assistance; Shelly A. Straight for isolating the Arabidopsis activase deletion mutant; John A. Kiser for statistic analysis; Daniel L. Siehl for critically reading the manuscript; and Michael E. Salvucci from the USDA–Agricultural Research Service, Western Cotton Research Laboratory (Phoenix, AZ) for helpful review, discussion, and comments on this work.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Genhai Zhu (genhai.zhu@pioneer.com).
Open Access articles can be viewed online without a subscription.
References
- Baker, N.R., Oxborough, K., Lawson, T., and Morison, J.I. (2001). High resolution imaging of photosynthetic activities of tissues, cells and chloroplasts in leaves. J. Exp. Bot. 52 615–621. [PubMed] [Google Scholar]
- Barth, S., Busimi, A.K., Friedrich, U.H., and Melchinger, A.E. (2003). Heterosis for biomass yield and related traits in five hybrids of Arabidopsis thaliana L. Heynh. Heredity 91 36–42. [DOI] [PubMed] [Google Scholar]
- Berry, J.A., and Björkman, O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31 491–543. [Google Scholar]
- Boxter, C.J., Foyer, C.H., Turner, J., Rolfe, S.A., and Quick, W.P. (2003). Elevated sucrose-phosphate synthase activity in transgenic tobacco sustains photosynthesis in older leaves and alters development. J. Exp. Bot. 54 1813–1820. [DOI] [PubMed] [Google Scholar]
- Cassman, K.G. (1999). Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 96 5952–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner, S.J., and Salvucci, M.E. (2000). Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc. Natl. Acad. Sci. USA 97 13430–13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner, S.J., and Salvucci, M.E. (2002). Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 129 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri, A., Raillard, S.A., Bermudez, E., and Stemmer, W.P. (1998). DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 15 288–291. [DOI] [PubMed] [Google Scholar]
- Day, N., and Maiti, I.B. (1999). Further characterization and expression analysis of mirabilis mosaic caulimovirus (MMV) full-length transcript promoter with single and double enhancer domains is transgenic plants. Transgenics 3 61–70. [Google Scholar]
- Dunwell, J.M. (2000). Transgenic approaches to crop improvement. J. Exp. Bot. 51 487–496. [DOI] [PubMed] [Google Scholar]
- Feller, U., Crafts-Brandner, S.J., and Salvucci, M.E. (1998). Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 116 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, L., Wang, K., Li, Y., Tan, Y., Kong, J., Li, H., Li, Y., and Zhu, Y. (2007). Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep. 26 1635–1646. [DOI] [PubMed] [Google Scholar]
- Haldimann, P., and Feller, U. (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 27 1169–1183. [Google Scholar]
- Kallis, R.P., Ewy, R.G., and Portis, A.R. (2000). Alteration of the adenine nucleotide response and increased Rubisco activation activity of Arabidopsis rubisco activase by site-directed mutagenesis. Plant Physiol. 123 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebeish, R., Niessen, M., Thiruveedhi, K., Bari, R., Hirsch, H.-J., Rosenkranz, R., Stäbler, N., Schönfeld, B., Kreuzaler, F., and Peterhänsel, C. (2007). Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 25 593–599. [DOI] [PubMed] [Google Scholar]
- Kim, K., and Portis, A.R., Jr. (2005). Temperature dependence of photosynthesis in Arabidopsis plants with modifications in rubisco activase and membrane fluidity. Plant Cell Physiol. 46 522–530. [DOI] [PubMed] [Google Scholar]
- Law, R.D., and Crafts-Brandner, S.J. (1999). Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5- bisphosphate carboxylase/oxygenase. Plant Physiol. 120 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood, R.C. (2002). C4 photosynthesis: principles of CO2 concentration and prospects for its introdution into C3 plants. J. Exp. Bot. 53 581–590. [DOI] [PubMed] [Google Scholar]
- Li, X., Song, Y., Century, K., Straight, S., Ronald, P., Dong, X., Lassner, M., and Zhang, Y. (2001). A fast neutron deletion mutagenesis-based reverse genetics system for plants. Plant J. 27 235–242. [DOI] [PubMed] [Google Scholar]
- Lobell, D.B., and Asner, G.P. (2003). Climate and management contributions to recent trends in U.S. agricultural yields. Science 299 1032. [DOI] [PubMed] [Google Scholar]
- Lunn, J.E., Gillespie, V.J., and Furbank, R.T. (2003). Expression of a cyanobacterial sucrose-phosphate synthase from Synechocystis sp. PCC 6803 in transgenic plants. J. Exp. Bot. 54 223–237. [DOI] [PubMed] [Google Scholar]
- Matsuoka, M., Furbank, R.T., Fukayama, H., and Miyao, M. (2001). Molecular engineering of C4 photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 297–314. [DOI] [PubMed] [Google Scholar]
- Miflin, B. (2000). Crop improvement in the 21st century. J. Exp. Bot. 51 1–8. [PubMed] [Google Scholar]
- Miyagawa, Y., Tamoi, M., and Shigeoka, S. (2001). Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosymthesis and growth. Nat. Biotechnol. 19 965–969. [DOI] [PubMed] [Google Scholar]
- Ness, J.E., Kim, S., Gottman, A., Pak, R., Krebber, A., Borchert, T.V., Govindarajan, S., Mundorff, E.C., and Minshull, J. (2002). Synthetic shuffling expands functional protein diversity by allowing amino acids to recombine independently. Nat. Biotechnol. 20 1251–1255. [DOI] [PubMed] [Google Scholar]
- Neuwald, A.F., Aravind, L., Spouge, J.L., and Koonin, E.V. (1999). AAA: A class of chaperone-like ATPases associated with assembly, operation, and disassembly of protein complexes. Genome Res. 9 27–43. [PubMed] [Google Scholar]
- Parry, M.A., Andralojc, P.J., Mitchell, R.A., Madgwick, P.J., and Keys, A.J. (2003). Manipulation of Rubisco: The amount, activity, function and regulation. J. Exp. Bot. 54 1321–1333. [DOI] [PubMed] [Google Scholar]
- Portis, A.R., Jr. (2003). Rubisco activase - Rubisco's catalytic chaperone. Photosynth. Res. 75 11–27. [DOI] [PubMed] [Google Scholar]
- Prieto-Dapena, P., Castano, R., Almoguera, C., and Jordano, J. (2006). Improved resistance to controlled deterioration in transgenic seeds. Plant Physiol. 142 1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, R.A. (2000). Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 51 447–458. [DOI] [PubMed] [Google Scholar]
- Rosegrant, M.W., and Cline, S.A. (2003). Global food security: Challenges and policies. Science 302 1917–1919. [DOI] [PubMed] [Google Scholar]
- Salvucci, M.E. (1992). Subunit interactions of Rubisco activase: Polyethylene glycol promotes self-association, stimulates ATPase and activation activities, and enhances interactions with Rubisco. Arch. Biochem. Biophys. 298 688–696. [DOI] [PubMed] [Google Scholar]
- Salvucci, M.E., and Crafts-Brandner, S.J. (2004. a). Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 120 179–186. [DOI] [PubMed] [Google Scholar]
- Salvucci, M.E., and Crafts-Brandner, S.J. (2004. b). Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol. 134 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci, M.E., DeRidder, B.P., and Portis, A.R., Jr. (2006). Effect of activase level and isoform on the thermotolerance of photosynthesis in Arabidopsis. J. Exp. Bot. 57 3793–3799. [DOI] [PubMed] [Google Scholar]
- Salvucci, M.E., Osteryoung, K.W., Crafts-Brandner, S.J., and Vierling, E. (2001). Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol. 127 1053–1064. [PMC free article] [PubMed] [Google Scholar]
- Salvucci, M.E., Rajagopalan, K., Sievert, G., Haley, B.E., and Watt, D.S. (1993). Photoaffinity labeling of ribulose-1,5-bisphosphate carboxylase/oxigenase activase with ATP γ-benzophenone. J. Biol. Chem. 268 14239–14244. [PubMed] [Google Scholar]
- Salvucci, M.E., van de Loo, F.J., and Stecher, D. (2003). Two isoforms of Rubisco activase in cotton, the products of separate genes not alternative splicing. Planta 216 736–744. [DOI] [PubMed] [Google Scholar]
- Schwender, J., Goffman, F., Ohlrogge, J.B., and Shachar-Hill, Y. (2004). Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature 432 779–782. [DOI] [PubMed] [Google Scholar]
- Sharkey, T.D. (2000). Some like it hot. Science 287 435–436. [DOI] [PubMed] [Google Scholar]
- Shen, J.B., Orozco, E.M., Jr., and Ogren, W.L. (1991). Expression of the two isoforms of spinach ribulose 1,5-bisphosphate carboxylase activase and essentiality of the conserved lysine in the consensus nucleotide-binding domain. J. Biol. Chem. 266 8963–8968. [PubMed] [Google Scholar]
- Sinclair, T.R., Purcell, L.C., and Sneller, C.H. (2004). Crop transformation and the challenge to increase yield potential. Trends Plant Sci. 9 70–75. [DOI] [PubMed] [Google Scholar]
- Stemmer, W.P. (1994). DNA shuffling by random fragmentation and reassembly: In vitro recombination for molecular evolution. Proc. Natl. Acad. Sci. USA 91 10747–10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K.Y., Suen, D.F., and Chen, S.C.G. (1999). Molecular characterization of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in rice leaves. Planta 209 66–76. [DOI] [PubMed] [Google Scholar]
- Vargas-Suarez, M., Ayala-Ochoa, A., Lozano-Franco, J., Garcia-Torres, I., Diaz-Quinonez, A., Ortiz-Navarrete, V.F., and Sanchez-de-Jimenez, E. (2004). Rubisco activase chaperone activity is regulated by a post-translational mechanism in maize leaves. J. Exp. Bot. 55 2533–2539. [DOI] [PubMed] [Google Scholar]
- Wang, Z.-Y., Snyder, G.W., Esau, B.D., Portis, A.R., Jr., and Ogren, W.L. (1992). Species-dependent variation in the interaction of substrate-bound ribulose-1,5-biphosphate carboxylase/oxigenase (Rubisco) and Rubisco activase. Plant Physiol. 100 1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke, J.M., Chatfield, J.M., and Ogren, W.L. (1989). Alternative mRNA splicing generates the two ribulosebisphosphate carboxylase/oxygenase activase polypeptides in spinach and Arabidopsis. Plant Cell 1 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, Z., Velten, J.P., Oliver, M.J., and Burke, J.J. (2003). High-throughput DNA extraction method suitable for PCR. Biotechniques 34 820–826. [DOI] [PubMed] [Google Scholar]
- Yang, X., Liang, Z., and Lu, C. (2005). Genetic engineering of the biosynthesis of glycinebetaine enhanced photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol. 138 2299–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., Kallis, R.P., Ewy, R.G., and Portis, A.R., Jr. (2002). Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc. Natl. Acad. Sci. USA 99 3330–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., and Portis, A.R., Jr. (1999). Mechanism of light regulation of Rubisco: A specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proc. Natl. Acad. Sci. USA 96 9438–9443. [DOI] [PMC free article] [PubMed] [Google Scholar]