Abstract
Chloroplast development in cotyledons differs in a number of ways from that in true leaves, but the cotyledon-specific program of chloroplast biogenesis has not been clarified. The cyo1 mutant in Arabidopsis thaliana has albino cotyledons but normal green true leaves. Chloroplasts develop abnormally in cyo1 mutant plants grown in the light, but etioplasts are normal in mutants grown in the dark. We isolated CYO1 by T-DNA tagging and verified that the mutant allele was responsible for the albino cotyledon phenotype by complementation. CYO1 has a C4-type zinc finger domain similar to that of Escherichia coli DnaJ. CYO1 is expressed mainly in young plants under light conditions, and the CYO1 protein localizes to the thylakoid membrane in chloroplasts. Transcription of nuclear photosynthetic genes is generally unaffected by the cyo1 mutation, but the level of photosynthetic proteins is decreased in cyo1 mutants. Recombinant CYO1 accelerates disulfide bond reduction in the model substrate insulin and renatures RNase A, indicating that CYO1 has protein disulfide isomerase activity. These results suggest that CYO1 has a chaperone-like activity required for thylakoid biogenesis in cotyledons.
INTRODUCTION
In dicotyledonous plants, the development of cotyledons—embryonic leaves formed during embryogenesis—is distinct from that of true leaves, which arise as the result of apical meristem activity. Although both cotyledons and true leaves contain chloroplasts and are photosynthetically active, chloroplast differentiation follows distinct paths in these two organs (Mansfield and Briarty, 1996). In cotyledons, plastids partially develop during embryogenesis, but development stops during seed maturation and dormancy. Upon germination in light, the plastids further develop into functional chloroplasts. By contrast, during true leaf development, proplastids differentiate into mature chloroplasts. Fully differentiated chloroplasts in cotyledons resemble young true leaf chloroplasts, although they usually contain a less extensive thylakoid membrane than mature true leaf chloroplasts (Deng and Gruissem, 1987).
As long as seedlings grow submerged in the soil, they live heterotrophically, using nutrients stored mainly in cotyledon cells. During this initial stage of development, etioplasts develop from proplastids. Once the seedling emerges from the soil and reaches light, it rapidly transforms etioplasts into chloroplasts, thereby enabling photosynthesis and switching from heterotrophic to autotrophic growth. In contrast with cotyledons, true leaf chloroplasts develop directly from proplastids. Mutants with pigment deficiencies that are confined either to cotyledons or true leaves also suggest differences in the regulation of plastid development in these two organs. Arabidopsis thaliana plants with mutations in VAR1 and VAR2 (FtsH proteases) have normal green cotyledons (Sakamoto et al., 2003), whereas a number of other Arabidopsis mutants, including wco (unidentified gene product) (Yamamoto et al., 2000), sco1 (chloroplast elongation factor G) (Albrecht et al., 2006), and sig2 and sig6 (sigma factors) (Privat et al., 2003; Ishizaki et al., 2005), have albino or pale-green cotyledons but normal green leaves. Despite these reports, how cotyledon-specific chloroplasts develop remains largely unknown.
In Escherichia coli, DnaJ, a primary Hsp40 homolog, acts in conjunction with the HSP70 protein DnaK and the nucleotide exchange factor GrpE during many cellular processes, including protein folding, protein transport, degradation of misfolded proteins, and bacteriophage DNA replication (for reviews, see Georgopoulos, 1992; Cyr et al., 1994). DnaJ has chaperone activity, as revealed by its capacity to recognize nonnative proteins and prevent the aggregation of folding intermediates in vitro (Langer et al., 1992; Szabo et al., 1996; Goffin and Georgopoulos, 1998). DnaJ can also catalyze the formation, reduction, and isomerization of disulfide bonds similar to protein disulfide isomerase (PDI) (de Crouy-Chanel et al., 1995). PDI is a molecular chaperone that catalyzes dithiol/disulfide interchange reactions and promotes protein disulfide formation, isomerization, or reduction, depending on the redox potential and nature of the polypeptide substrate (Holmgren, 1979; Freedman, 1989; Gilbert, 1990). Zn2, one of two C4-type zinc finger motifs in DnaJ, is important for this enzymatic activity (Goffin and Georgopoulos, 1998; Tang and Wang, 2001; Shi et al., 2005).
In plants, DnaJ-like proteins are involved in plastid biogenesis. Or is involved in the differentiation of noncolored plastids into chromoplasts for carotenoid accumulation (Lu et al., 2006). BSD2 is required for posttranslational regulation of the L subunit of ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco) (Brutnell et al., 1999). Chloroplast DnaJ homolog2 (CDJ2) is required for the biogenesis and/or maintenance of thylakoid membranes (Liu et al., 2005). RB60 is an atypical PDI that functions as a member of a redox regulatory protein complex controlling translation in Chlamydomonas reinhardtii chloroplasts (Kim and Mayfield, 1997).
We now describe an Arabidopsis mutant, cyo1, with albino cotyledons and cotyledon-specific defects in chloroplast biogenesis. The CYO1 protein has a Zn2 motif similar to that in E. coli DnaJ and has thiol disulfide reduction activity. We also demonstrate that CYO1 is a thylakoid membrane protein and that CYO1 is expressed mainly in cotyledons under illumination. These results suggest a role for CYO1 specifically in cotyledon chloroplast differentiation.
RESULTS
Characterization of the cyo1 Mutant
T-DNA insertional mutants in Arabidopsis thaliana were produced on a large scale by vacuum infiltration to identify mutants with chloroplast development phenotypes (Shirano et al., 2000). Several mutants with an albino cotyledon phenotype were isolated from the ∼3,500 transgenic lines examined. One such mutant, designated cyo1 (shi-yo-u means cotyledon in Japanese), had albino cotyledons but normal green leaves (Figure 1B). On 1/2 MS (for half-concentration of normal Murashige and Skoog) plates containing 1.5% sucrose, germination of cyo1 mutant seeds was normal but expansion of the first leaf was slower than in the wild type. In soil or on 1/2 MS plates containing no sucrose, many mutants could not produce leaves and subsequently died. Mutants with leaves that were transferred from plates to soil grew autotrophically and produced mature seeds by self-pollination. The period between first leaf expansion and flowering was similar in cyo1 mutants and wild-type plants. No chlorophyll could be detected in cotyledons from mutants grown in normal (26 μmol·m−2·s−1) or very dim (0.70 μmol·m−2·s−1) light conditions. These results suggest that the albino cotyledons observed in the mutants were not the result of photobleaching or photoinhibition. The chlorophyll content in mutant rosette leaves was comparable to that in wild-type leaves (see Supplemental Figure 1 online).
Figure 1.
The cyo1 Mutant Plant Has Albino Cotyledons.
A 10-d-old wild-type plant (A), a cyo1 mutant plant (B), and a cyo1 mutant plant complemented with the CYO1 genomic DNA clone (C). All were grown under continuous light at 23°C on 1/2 MS agar plate medium with 1.5% sucrose.
Ultrastructure of Plastids in cyo1 Mutants
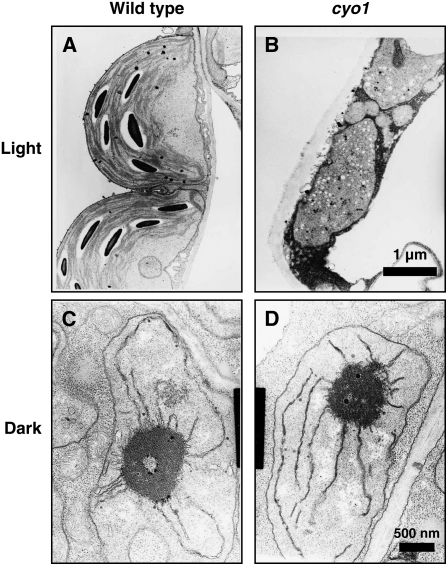
To further characterize the cyo1 mutant, we examined plastid ultrastructure by transmission electron microscopy. In cotyledons from wild-type plants grown under light conditions, chloroplasts were crescent-shaped and contained thylakoid membranes, including stroma thylakoids and grana thylakoids (Figure 2A). In albino cotyledons of cyo1 mutant plants, the plastids were smaller and abnormally shaped (Figure 2B). The plastids did not develop thylakoid membranes but instead contained a large number of electron-dense particles (Figure 2B). Leaf chloroplasts from cyo1 mutant and wild-type plants were similar ultrastructurally (see Supplemental Figure 2 online), as were etioplasts in cotyledons from wild-type and mutant plants grown in the dark (Figures 2C and 2D). These results indicate that the cyo1 mutation affected chloroplast biogenesis under light conditions but did not affect etioplast biogenesis under dark conditions.
Figure 2.
The Chloroplasts in Cotyledons of cyo1 Mutants Are Small and Abnormal in Shape.
Wild-type chloroplast (A) and cyo1 mutant plastid (B) in cotyledons from 7-d-old plants grown under light. The magnification is the same in (A) and (B). Wild-type (C) and cyo1 mutant (D) etioplast in cotyledons from 7-d-old plants grown in the dark. The magnification is the same in (C) and (D).
Identification and Characterization of the CYO1 Gene
cyo1 plants were reciprocally backcrossed to wild-type plants, and segregation of the albino cotyledon phenotype was examined in the F1 and F2 progeny. All of the F1 progeny showed wild-type cotyledons (green color) and hygromycin resistance. In F2 progeny, the hygromycin sensitivity segregated ∼3:1 (hygromycin-resistant = 177, hygromycin-sensitive = 54; χ2 = 0.32, P > 0.05) and the cotyledon phenotype segregated ∼2:1 (hygromycin-resistant/green cotyledon = 129, hygromycin-resistant/albino cotyledon = 48; χ2 = 3.08, P > 0.05). These results indicate that the mutant phenotype segregated as a single recessive mutation and cosegregated with the T-DNA, suggesting that the mutation was caused by insertion of the T-DNA into the nuclear genome. DNA gel blot hybridization analysis indicated that the cyo1 mutant genomic DNA contained a single T-DNA (see Supplemental Figure 3 online).
To determine the T-DNA insertion site, we isolated the T-DNA by plasmid rescue and sequenced the flanking DNA. The T-DNA was inserted in exon 1 of At3g19220, which had been annotated previously (accession number AY059925) as an unknown protein-coding gene. Because the T-DNA contained a set of concatenated enhancer fragments derived from the 35S promoter of Cauliflower mosaic virus for activation tagging, it was possible that the cyo1 mutant phenotype could be due either to transcriptional activation of mutant CYO1 or neighboring genes or to disruption of CYO1. However, heterozygous cyo1/CYO1 plants exhibited normal green cotyledons, arguing against the former possibility. In addition, the albino cotyledon phenotype in cyo1 homozygotes was rescued by introduction of a wild-type At3g19220 genomic fragment (Figure 1C). These results confirm that At3g19220 is CYO1.
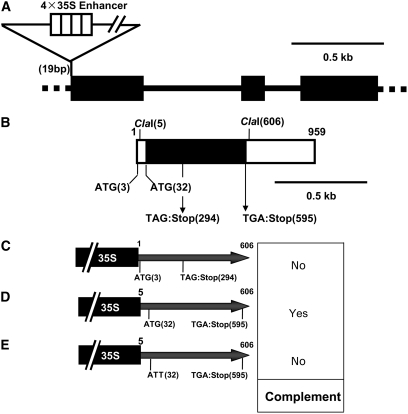
The sequence of a full-length At3g19220 cDNA was reported previously by Seki et al. (2002). We cloned the full-length cDNA by RT-PCR and confirmed the sequence of the amplified fragment. The 959-bp At3g19220 cDNA has three exons (Figure 3A). The T-DNA insertion site was in the first exon, at 19 bp. The cDNA had putative translation initiation codons (ATG) at 3 and 32 bp. To analyze which of the two possible initiation codons is used, cDNA fragments between +1 and +606 bp, or between +5 and +606 bp, were ligated into a vector under the control of the cauliflower mosaic virus 35S promoter, and the vector was introduced into the cyo1 mutant. If the ATG at 3 bp was the initiation codon for translation, the stop codon would have to be at 294 bp (TAG), whereas if the ATG at 32 bp was the initiation codon, the stop codon would have to be at 595 bp (TGA). Only the vector containing the cDNA between +5 and +606 bp could complement the cyo1 phenotype (Figures 3C and 3D). Furthermore, to exclude the possibility that At3g19220 encoded a noncoding RNA, we constructed a vector containing the cDNA between 5 and 606 bp with the ATG at 32 bp mutated to ATT. When this construct was introduced into mutant plants, transformants harboring the vector had albino cotyledons (see Supplemental Figure 4 online). These results indicate that the translation initiation codon of At3g19220 is at 32 bp.
Figure 3.
Molecular Characterization of the CYO1 Gene.
(A) Structure of CYO1. Boxes and bars represent exons and introns, respectively.
(B) Structure of the CYO1 cDNA. The black box and white boxes represent the open reading frame and untranslated region, respectively.
(C) to (E) DNA fragments used for the complementation assay. The DNA fragment in (C) contains the ATG codon at 3 bp, whereas the DNA fragments between 5 and 606 bp, shown in (D) and (E), do not. The ATG at 32 bp was mutated to ATT in the DNA fragment shown in (E). 35S represents the cauliflower mosaic virus 35S promoter.
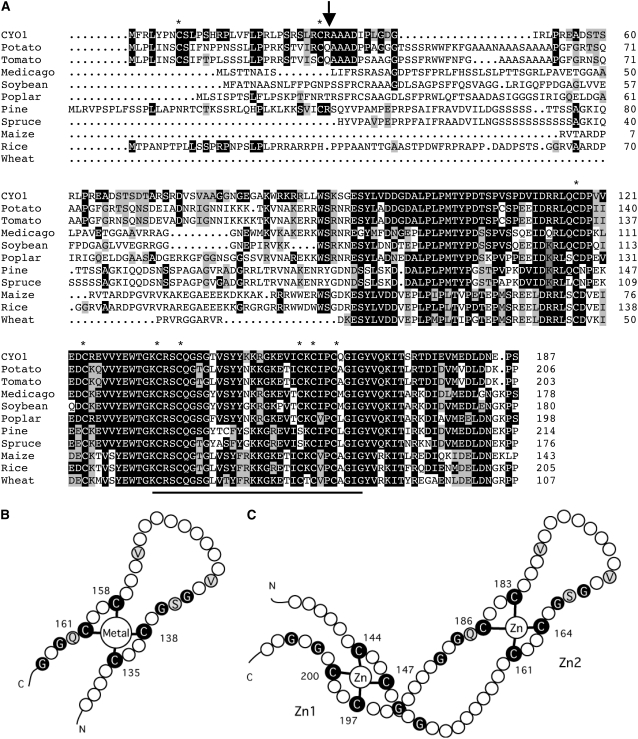
At3g19220/CYO1 encodes a protein of 187 amino acids with a putative molecular weight of 20,842. CYO1 contains nine Cys residues and has a predicted zinc finger–like domain between residues 135 and 164 similar to that found in DnaJ (Figures 4B and 4C). A BLAST search revealed that many plants (e.g., potato [Solanum tuberosum], tomato [Solanum lycopersicum], Medicago truncatula, soybean [Glycine max], poplar [Populus sp], pine [Pinus sp], spruce [Picea abies], maize [Zea mays], rice [Oryza sativa], and wheat [Triticum aestivum]) have a CYO1 homolog (Figure 4A). CYO1 homologs could not be identified in moss or algae.
Figure 4.
Homologs and Putative Topology of CYO1.
(A) Alignment of the amino acid sequence of CYO1 (accession number AY059925) and CYO1 homologs from potato (TC133656), tomato (TC180110), Medicago truncatula (ABE80527), soybean (TC228649), poplar (TC44537), pine (TC76401), spruce (TC24344), maize (TC343209), rice (TC332052), and wheat (TC257683). Sequences of potato, tomato, soybean, poplar, pine, spruce, maize, rice, and wheat were drawn from The Institute for Genomic Research Gene Indices (http://tigrblast.tigr.org/tgi/). The sequences of spruce, maize, and wheat are not full-length amino acid sequences. Conserved residues are shown in black, and similar residues are shown in gray. Asterisks indicate Cys residues, the bar shows the zinc finger motif, and the vertical arrow indicates the putative cleavage site of the transit peptide of Arabidopsis CYO1.
(B) Putative topology of the zinc finger motif in CYO1. Residues conserved between CYO1 and DnaJ are highlighted. Residues conserved in the zinc finger domain are shown in black, and identical residues between CYO1 and DnaJ are shown in gray.
(C) The topology of E. coli DnaJ (Shi et al., 2005).
Expression Analysis of CYO1
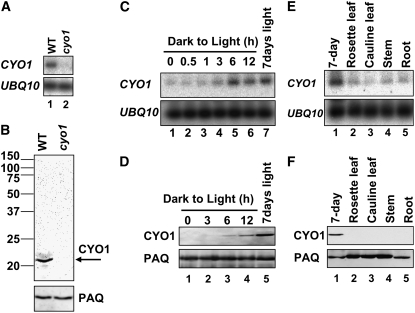
We analyzed CYO1 mRNA levels in 7-d-old wild-type and cyo1 mutant plants. The CYO1 transcript was undetectable by RNA gel blot hybridization; thus, nonsaturating RT-PCR conditions were used for quantification. As shown in Figure 5A, the wild-type transcript but not the cyo1 mutant transcript could be detected. To analyze the amount of CYO1 protein in plants, we prepared a rabbit antibody against recombinant CYO1 and performed protein gel blotting. Total protein from 7-d-old Arabidopsis grown under light on 1/2 MS plates containing 1.5% sucrose was separated by SDS-PAGE. Anti-CYO1 recognized a band with a molecular weight of ∼20,000 in the wild type, consistent with the predicted molecular weight, but not in cyo1 mutant samples (Figure 5B). These results indicate that the abnormal chloroplast phenotype observed in cyo1 mutant plants was caused by a lack of CYO1 protein.
Figure 5.
RNA Expression and Immunoblot Analysis of CYO1.
(A) Analysis of CYO1 transcripts from 7-d-old wild-type (lane 1) and cyo1 mutant (lane 2) plants grown under light. Ubiquitin10 (UBQ10) was analyzed as a loading control.
(B) Immunoblot analysis of CYO1 levels in 7-d-old wild-type (left lane) and cyo1 mutant (right lane) plants grown under light conditions. Fifty micrograms of total protein was loaded in each lane. The arrow indicates CYO1. Plasma Aquaporin (PAQ) was analyzed as a loading control.
(C) Effect of light on CYO1 transcript levels. Seven-day-old wild-type Arabidopsis plants grown in continuous dark were illuminated for the times indicated (0 to 12 h; lanes 1 to 6). Lane 7 shows 7-d-old plants grown under continuous light.
(D) Effect of light on CYO1 protein levels. Seven-day-old wild-type Arabidopsis plants grown in continuous dark were illuminated for the times indicated (0 to 12 h; lanes 1 to 4). Lane 5 shows 7-d-old plants grown under continuous light. Fifty micrograms of total protein was loaded in each lane.
(E) Organ-specific transcripts of CYO1 in continuously illuminated adult plants. Total RNA was isolated from 4-week-old wild-type Arabidopsis for organ-specific quantification of the CYO1 transcript (lanes 2 to 5).
(F) Organ-specific CYO1 protein levels. Fifty micrograms of total protein was loaded in each lane.
The CYO1 transcript level was very low in etiolated seedlings, but upon illumination it gradually increased and reached a maximum at 6 h (Figure 5C). Similarly, CYO1 protein was not detected in etiolated seedlings but increased upon illumination (Figure 5D). Although CYO1 transcript levels in seedlings illuminated for 6 h were almost the same as in 7-d-old seedlings grown under continuous light, CYO1 protein levels in seedlings illuminated for 12 h did not reach those of 7-d-old seedlings grown under continuous light.
Wild-type plants grown for 7 d in the light expressed CYO1, but significantly less CYO1 expression was detected in the organs of 4-week-old Arabidopsis (Figures 5E and 5F). These results indicate that CYO1 is expressed mainly in young plants grown under light conditions.
Subcellular Localization of CYO1
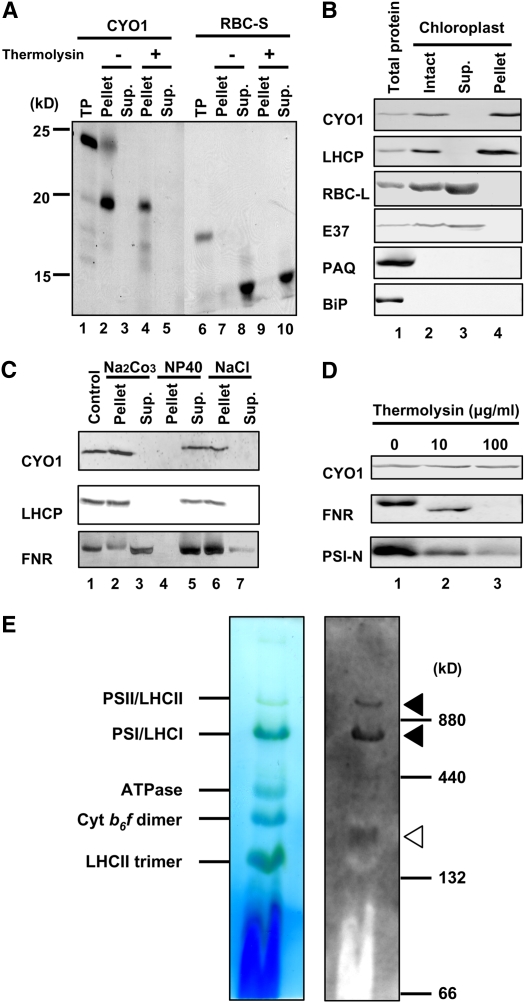
The TargetP program (http://www.cbs.dtu.dk/services/TargetP/) predicted a mitochondrial presequence between residues 1 and 30 in CYO1, but the WoLF PSORT program (http://wolfpsort.seq.cbrc.jp/) (Horton et al., 2007) suggested that CYO1 is a chloroplast protein. The potato, tomato, Medicago truncatula, soybean, poplar, pine, and rice proteins were also predicted to be chloroplastic by TargetP. To investigate CYO1 localization, we performed an in vitro chloroplast import experiment. Full-length 35S-labeled CYO1 was produced by in vitro translation. A control assay was performed with the stroma-localized small subunit of Rubisco (RBC-S). After incubation of the CYO1 translation product with isolated pea (Pisum sativum) chloroplasts, radiolabeled CYO1 was detected in the chloroplast fraction, as was control protein (Figure 6A). When the chloroplasts were treated with thermolysin, a protease that does not penetrate the envelope membrane, mature CYO1 and RBC-S were protected from proteolysis (Figure 6A). These results indicate that CYO1 is targeted to chloroplasts. Although the calculated molecular weight of full-length CYO1 is 20,842, the apparent molecular weight of the full-length CYO1 translation product was ∼25,000 by SDS-PAGE. After incubation of this CYO1 translation product with isolated pea chloroplasts, a protein with a molecular mass of ∼20,000 was detected. These results suggest that CYO1 contains a chloroplast transit peptide.
Figure 6.
Localization of CYO1 Protein.
(A) In vitro chloroplast import and protease protection assay for CYO1. 35S-labeled CYO1 and RBC-S were produced by in vitro translation. The proteins were incubated with isolated pea chloroplasts in the presence of 4.0 mM Mg-ATP for 30 min at room temperature. Chloroplasts were recovered by sedimentation through 40% (v/v) Percoll. The recovered intact chloroplasts were then incubated without (lanes 2, 3, 7, and 8) or with (lanes 4, 5, 9, and 10) thermolysin for 30 min at 4°C. Intact chloroplasts were recovered by centrifugation through 40% (v/v) Percoll and fractionated into total membrane (Pellet; lanes 2, 4, 7, and 9) and soluble (Supernatant [Sup.]; lanes 3, 5, 8, and 10) fractions. CYO1 was detected in the chloroplast fraction (Pellet), as was the control protein. TP represents 10% of in vitro translated products (lanes 1 and 6).
(B) Immunoblot analysis of CYO1. Total protein is whole cell protein (lane 1). Intact chloroplasts (lane 2) were lysed, and soluble (Sup.; lane 3) and insoluble (Pellet; lane 4) proteins (10 μg/lane) were separated by 12.5% SDS-PAGE. Each protein fraction was prepared from 7-d-old plants. RBC-L (stroma protein), E37 (chloroplast envelope protein), PAQ (plasma membrane protein), and BiP (ER protein) were analyzed as controls for each fraction.
(C) Sonicated thylakoids were prepared from 7-d-old plants and were treated with 0.1 M sodium carbonate (lanes 2 and 3), 1.0% Nonidet P-40 (NP40; lanes 4 and 5), or 2.0 M NaCl (lanes 6 and 7) and then soluble (Sup.; lanes 3, 5 and 7) and insoluble (Pellet; lanes 2, 4, and 6) fractions were separated at 12,000g. LHCP and FNR were used as marker proteins for intrinsic and extrinsic thylakoid membrane proteins, respectively.
(D) Sonicated thylakoids were prepared from 7-d-old plants and were treated with thermolysin (0, 10, or 100 μg/mL) for 60 min on ice. FNR and PSI-N were used as stroma- and lumen-exposed peripheral thylakoid protein markers, respectively.
(E) Intact thylakoids of 7-d-old plants were solubilized by treatment with 1% n-dodecyl-β-d-maltoside and separated by 5 to 14% blue native gel electrophoresis. After electrophoresis, the gel was incubated in 0.1% SDS containing transfer buffer (100 mM Tris, 192 mM Gly, and 5% methanol) for 10 min at room temperature and blotted to a membrane (left panel). The membrane was reacted with antibodies against CYO1 (right panel). Without additional staining, protein complexes were detected by bound Coomassie blue dye and chlorophylls. Bands corresponding to various photosynthetic complexes are indicated (Asakura et al., 2004) (see also http://www.hos.ufl.edu/clineweb/BNgel.htm). Ferritin (880 and 440 kD) and BSA (132 and 66 kD) were used as molecular mass marker proteins. Arrowheads show the bands of CYO1. Closed arrowheads show that CYO1 bands comigrate with PSI/LHCI and PSII/LHCII, and the open arrowhead shows the CYO1 band in an unidentified thylakoid protein complex.
To further determine the subcellular distribution of CYO1, Arabidopsis chloroplasts were isolated by two-step Percoll gradient centrifugation (Aronsson and Jarvis, 2002) and lysed by osmotic disruption. Soluble and insoluble fractions separated by a low-speed centrifugation (3000g, 5 min) that sediments the thylakoid but not the envelope membranes were analyzed by protein gel blotting using the anti-CYO1 antibody. CYO1 was detected in the chloroplast pellet fraction (Figure 6B), which contained the thylakoid membrane marker protein LHCP but not the stroma protein large subunit of Rubisco (RBC-L), the envelope protein E37, a major envelope protein (Teyssier et al., 1996), the plasma membrane protein aquaporin PAQ (Ohshima et al., 2001), or the endoplasmic reticulum (ER) protein BiP (Hatano et al., 1997). This result indicates that CYO1 is a thylakoid membrane protein.
To confirm that CYO1 was a thylakoid membrane protein, we treated sonicated thylakoid fractions with 0.1 M sodium carbonate, 1.0% Nonidet P-40, or 2.0 M sodium chloride (Figure 6C). Neither CYO1 nor LHCP was extracted by sodium carbonate or sodium chloride, but both were solubilized by Nonidet P-40. By contrast, FNR (for ferredoxin NADP+ oxidoreductase), which is attached to the thylakoid membrane, was partially extracted by sodium carbonate and sodium chloride (Figure 6C).
To analyze the topology of CYO1 in the thylakoid membrane, we performed a protease protection assay (Motohashi and Hisabori, 2006). Incubation of sonicated thylakoid membrane samples with the protease thermolysin resulted in the degradation of FNR, which is located on the stroma-exposed surface of thylakoid membranes, and PSI-N (for the N subunit of photosystem I), which is located on the lumen-exposed surface of the thylakoid membrane. By contrast, CYO1 was not degraded (Figure 6D). These results suggest that CYO1 is an intrinsic thylakoid membrane protein. Since CYO1 contains no predicted membrane-spanning segments, it may be protected from thermolysin degradation by its association with other thylakoid membrane proteins.
To begin investigating whether CYO1 might be in a complex with other thylakoid proteins, we performed blue native gel electrophoresis (Asakura et al., 2004) (Figure 6E, left) and immunoblotting (Figure 6E, right) with thylakoids isolated from 7-d-old wild-type Arabidopsis plants and solubilized with 1% n-dodecyl-β-d-maltoside. CYO1 comigrated with the PSI/LHCI and PSII/LHCII complexes (closed arrowheads, 1030 and 710 kD). It was also detected in a band of 220 kD that migrated between cytochrome b6f and the LHCII trimer (open arrowhead). These results suggest that CYO1 associates with multiple thylakoid complexes in vivo.
Expression of Photosynthetic Genes and Proteins
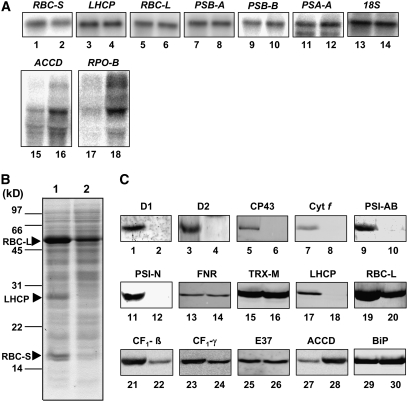
We analyzed transcript levels for several chloroplast and nuclear photosynthetic genes in 7-d-old wild-type and cyo1 mutant plants (Figure 7A). The nuclear transcripts we tested encoded RBC-S and LHCP, and the chloroplast transcripts tested encoded the RBC-L, the A1 protein of PSI (PSA-A), the D1 protein of PSII (PSB-A), the CP47 protein of PSII (PSB-B), acetyl-CoA carboxylase (ACCD), and the β-subunit of plastid-encoded RNA polymerase (RPO-B). RBC-L, PSA-A, PSB-A, and PSB-B are transcribed by plastid-encoded RNA polymerase (PEP), whereas ACCD and RPO-B are transcribed by nucleus-encoded RNA polymerase (NEP) (Hess and Borner, 1999; Kanamaru and Tanaka, 2004). Transcript levels of all genes tested were comparable in wild-types and cyo1 plants, with the exception of ACCD and RPO-B, which were significantly increased in the mutant. These results suggest that NEP-mediated gene transcription was increased by the cyo1 mutation, whereas PEP-mediated transcription was not influenced by the mutation.
Figure 7.
RNA Expression and Immunoblot Analysis of Chloroplast Proteins.
(A) Total RNA was prepared from 7-d-old wild-type (lane 1) and cyo1 mutant (lane 2) plants. One microgram of total RNA was loaded, and transcript levels were measured in wild-type (odd-numbered lanes) and cyo1 mutant (even-numbered lanes) plants for the following nuclear genes: RBC-S, LHCP, and 18S (18S rRNA); the following chloroplast genes transcribed by PEP: RBC-L, PSB-A (D1), PSB-B (CP47), and PSA-A (PsaA); and the following chloroplast genes transcribed by NEP: ACCD and RPO-B.
(B) Twenty micrograms of total protein of 7-d-old wild-type (lane 1) and cyo1 mutant (lane 2) plants was loaded. The SDS-PAGE gel (20 μg of protein per lane) was stained with Coomassie blue. The arrowheads show the major bands of RBC-L, RBC-S, and LHCP.
(C) Immunoblot analysis of photosynthesis and control proteins (20 μg of protein per lane) in 7-d-old wild-type (odd-numbered lanes) and cyo1 mutant (even-numbered lanes) plants. D1, D2, and CP43 are proteins of PSII, Cyt f is a protein of cytochrome b6f, PSI-AB and PSI-N are proteins of PSI, FNR is ferredoxin NADP+ oxidoreductase, TRX-M is thioredoxin m, RBC-L is the large subunit of Rubisco, CF1-β and CF1-γ are subunits of chloroplast ATPase, E37 is a chloroplast envelope protein, ACCD is acetyl-CoA carboxylase, and BiP is an ER protein.
To analyze the relative amounts of chloroplast proteins in the mutant and the wild type, we performed SDS-PAGE and protein gel blotting. Coomassie blue staining revealed that the density of the three major bands, LHCP, RBC-L, and RBC-S, was less in cyo1 mutant plants than in wild-type plants (Figure 7B, lanes 1 and 2). For protein gel blotting, we used antibodies against seven nucleus-encoded proteins—PSI-N, FNR, TRX-M (thioredoxin m), LHCP, CF1-γ (chloroplast ATPase), E37 (envelope protein), and BiP (ER protein)—and eight chloroplast-encoded proteins—D1, D2, and CP43 (PSII), cytochrome f (cytochrome b6f), PSI-AB (PSI protein), RBC-L, CF1-β (chloroplast ATPase), and ACCD. The levels of D1, D2, CP43, cytochrome f, PSI-AB, PSI-N, LHCP, RBC-L, CF1-β, and CF1-γ were significantly lower in cyo1 mutants than in the wild type (Figure 7C). The levels of FNR, TRX-M, E37, and BiP were comparable in wild-type and cyo1 plants, and the level of ACCD was increased significantly in the mutant.
CYO1 Has Protein Zn-Dependent Disulfide Isomerase Activity
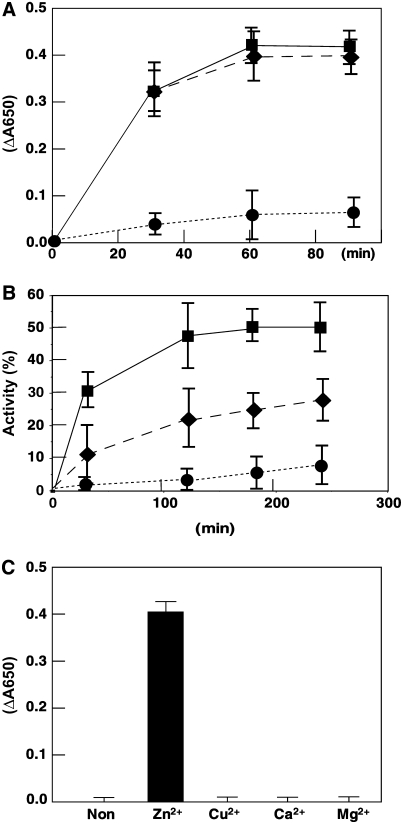
CYO1 residues 135 to 165 are similar to the C4-type zinc finger-2 (Zn2) of E. coli DnaJ (Figures 4B and 4C). Zn2 is important for the enzymatic activity of DnaJ (Tang and Wang, 2001), such as reduction of insulin disulfides and protein disulfide formation (e.g., oxidative renaturation of reduced RNase) (de Crouy-Chanel et al., 1995). To analyze the catalytic properties of CYO1, we tried to express full-length CYO1 in E. coli, but it was insoluble under all E. coli growth/detergent conditions we tried. Therefore, we expressed a truncated CYO1 that included residues 31 to 187 (Δ30CYO1) (data not shown). This Δ30CYO1 was soluble and could be used for rapid spectrophotometric assay of disulfide isomerase activity based on the reduction of insulin (Holmgren, 1979). Mature insulin contains two polypeptide chains, A and B, linked by disulfide bonds. When these bonds are broken, the free B chain is insoluble and precipitates, increasing absorbance at 650 nm. DnaJ in E. coli catalyzes the DTT-dependent reduction of insulin (de Crouy-Chanel et al., 1995; Tang and Wang, 2001; Shi et al., 2005). We measured the reduction of insulin (130 μM) by DTT (0.3 mM) in the presence of 1.0 μM Δ30CYO1 or DnaJ (Figure 8A). Insulin B chain precipitation was observed after 30 min in the presence of Δ30CYO1 or DnaJ and leveled off after 60 min. Significantly less B chain precipitated in the absence of Δ30CYO1 or DnaJ.
Figure 8.
CYO1 Has Reductase and Oxidase Activity.
(A) Purified Δ30CYO1 catalyzes the reduction of insulin. The reaction was initiated by adding DTT into 0.1 M potassium phosphate, pH 6.6, containing 0.13 mM bovine insulin in the absence (circles) or presence of 1.0 μM Δ30CYO1 (diamonds) or 1.0 μM DnaJ (squares). The reduction of insulin and the resulting precipitation of the B chain was monitored by following the optical density at 650 nm. Data represent means ± sd of three independent experiments.
(B) Effect of CYO1 on refolding of reduced and denatured RNase A. Refolding of denatured and reduced RNase A (40 μM) was initiated by 10-fold dilution in 50 mM Tris-HCl, pH 8.0, containing 0.1 M NaCl and 0.3 mM DTT at 30°C in the absence (circles) or presence of 1.0 μM Δ30CYO1 (diamonds) or 1.0 μM DnaJ (squares). At the indicated time points, an aliquot containing 40 μmol of RNase A was withdrawn from the reaction to assay RNase A activity. Activity is expressed as a percentage of native RNase A activity and represents the average ± sd of three independent experiments.
(C) Effect of metal on CYO1 activity. Purified Δ30CYO1 was denatured with 6.0 M guanidine hydrochloride, and then the proteins were renatured with dialyzing buffer containing 5.0 mM ZnCl2, CuCl2, CaCl2, MgCl2, or no divalent metal ion. The dialyzed proteins were centrifuged, and the supernatant was used for enzymatic assays. The reduction of insulin and the resulting precipitation of the B chain were monitored by following the optical density at 650 nm. Data represent means ± sd of three independent experiments.
Renaturation of reduced, denatured RNase A, which contains eight sulfhydryl groups, involves the oxidation of its thiol groups followed by rearrangement of the disulfides to the native conformation (with four disulfide bridges) (Anfinsen and Scheraga, 1975). As reported previously (Pigiet and Schuster, 1986), the spontaneous refolding of reduced, denatured RNase A was negligible in air (Figure 8B). Addition of Δ30CYO1 or DnaJ to the reaction stimulated RNase A renaturation. The renaturation activity of Δ30CYO1 was lower than that of DnaJ; however, it may be that the reduced, denatured RNase A is a better substrate for DnaJ than for Δ30CYO1. Nevertheless, Δ30CYO1 catalyzed the reactivation of reduced, denatured RNase A, indicating that CYO1 has PDI activity.
To determine whether CYO1 requires Zn2+ for its enzymatic activity, as does DnaJ, purified Δ30CYO1 was denatured and renatured in a dialyzing buffer containing ZnCl2, CuCl2, CaCl2, MgCl2, or no divalent metal ion. Δ30CYO1 catalyzed the reduction of insulin when renatured in the presence of ZnCl2 but lacked activity when renatured in the presence of any of the other buffers (Figure 8C). This result indicates that Zn2+ is required for the enzymatic activity of CYO1.
DISCUSSION
In this study, we selected a cotyledon-specific albino mutant of Arabidopsis and isolated the gene CYO1, which encodes a thylakoid membrane protein with a DnaJ zinc finger domain. CYO1 copurifies with the PSI/LHCI and PSII/LHCII complexes and has PDI activity.
Many dicotyledonous and monocotyledonous plants have CYO1 homologs. The C-terminal region is highly conserved among CYO1 homologs, suggesting that this region is critical for CYO1 function. Furthermore, Arabidopsis CYO1 has nine Cys residues, all of which are conserved in potato and tomato. Seven of these Cys residues are also conserved in all plants (Figure 4A). Although the phenotype of monocotyledonous plants carrying the cyo1 mutation awaits future investigation, the conservation of Cys residues suggests that they are important for CYO1 function in all angiosperms.
CYO1 transcript and protein accumulate in seedlings and are increased by light irradiation (Figures 5C and 5D). In cyo1 mutants grown under light conditions, the plastids in cotyledons are immature and do not develop any thylakoid membrane (Figure 2B). However, in cyo1 mutants grown under continuous dark, the etioplasts in cotyledons are comparable to those in wild-type plants. Seed maturation in cyo1 mutants is normal; thus, CYO1 likely functions only during chloroplast development in cotyledons.
The cyo1 mutation influences the accumulation of many chloroplast proteins, but it does not affect the accumulation of nuclear transcripts. To analyze the effect of the cyo1 mutation on transcription, we performed a DNA array assay for cyo1 mutant and wild-type plants (see Supplemental Figure 5 online). Scatterplots of mRNA accumulation for 2880 genes differed very little between wild-type and cyo1 plants, suggesting that cyo1 does not influence the transcription of nuclear genes. However, transcript levels for some chloroplast genes (ACCD and RPO-B) transcribed by NEP were increased in the mutant. NEP is largely responsible for the transcription of housekeeping genes during early chloroplast development (Hanaoka et al., 2005); therefore, only chloroplast genes transcribed by NEP are likely to be influenced by the cyo1 mutation.
CYO1 residues 135 to 165 are similar to the Zn2 domain of E. coli DnaJ. DnaJ proteins are molecular chaperones that specifically regulate DnaK-like proteins involved in protein folding. Hsp70s, DnaK homologs in chloroplasts, play an important role in a number of processes (Liu et al., 2005; Lu et al., 2006). However, CYO1 lacks the J domain responsible for stimulating DnaK ATPase activity. Therefore, CYO1 may function without Hsp70s.
DnaJ-like proteins play an important role in plastid biogenesis. The cauliflower (Brassica oleracea) OR gene encodes a DnaJ Cys-rich domain–containing protein that participates in the differentiation of chromoplastids for carotenoid accumulation (Lu et al., 2006); however, the function of OR has yet to be elucidated. CYO1 and OR are present only in higher plants, not in moss and algae. CYO1 (At3g19220) shares only 13% identity with the Arabidopsis OR homolog At5g61670. Maize BSD2 also has a Cys-rich zinc binding domain similar to that in DnaJ and is targeted to chloroplasts (Brutnell et al., 1999). BSD2 is not involved in general photosynthetic complex assembly or protein import but is required for the posttranslational regulation of RBC-L. CDJ2, a chloroplast DnaJ homolog, may function during the biogenesis and/or maintenance of thylakoid membranes (Liu et al., 2005). This protein has the J domain that interacts with Hsp70 but lacks a Cys-rich domain. RB60 is an atypical PDI that functions as a member of a redox regulatory protein complex controlling translation in Chlamydomonas chloroplasts (Kim and Mayfield, 1997). RB47, a member of the eukaryotic poly(A) binding protein family, binds directly to the 5′ untranslated region of psbA mRNA, which encodes the photosynthetic reaction center protein D1 in chloroplasts. RB60 binds to RB47 and modulates its activity via redox and phosphorylation. Unlike CYO1, these proteins are soluble (Yohn et al., 1996), suggesting that CYO1 is a novel PDI in the chloroplast thylakoid membrane that acts as a chaperone-like factor for thylakoid membrane proteins.
Many thylakoid membrane proteins contain Cys residues. Recently, Motohashi and Hisabori (2006) reported that several thylakoid membrane proteins interact with HCF167, a thioredoxin-like protein in thylakoids. All of the proteins they identified, including cytochrome f, Rieske FeS protein, PSI-N, LHCB5, FTSH2, FTSH8, and three CF1 subunits of ATPase, have multiple Cys residues. CYO1 behaves as an intrinsic thylakoid membrane protein and may interact with the PSI/LHCI and PSII/LHCII complexes (Figure 6E). Many hydrophobic subunits of PSI and PSII have Cys residues (Table 1). Because the initial phase of chloroplast biogenesis in the cotyledon is characterized by the rapid synthesis and assembly of the photosynthetic thylakoid membrane system, the PDI activity of CYO1 may be involved directly in disulfide bond formation and/or accelerating the folding of Cys-rich proteins (e.g., hydrophobic subunits of PSI and PSII). This hypothesis requires further investigation.
Table 1.
Number of Cys Residues in Subunits of PSI and PSII in Arabidopsis
| Protein | Number of Cys Residues | Protein | Number of Cys Residues |
|---|---|---|---|
| PSI | PSII | ||
| Hydrophobic subunits | Hydrophobic subunits | ||
| PsaA (A1) | 4 | D1 | 2 |
| PsaB (A2) | 2 | D2 | 4 |
| PsaG | 0 | CP47 | 3 |
| PsaI | 0 | CP43 | 4 |
| PsaJ | 0 | Cytochrome b559 | |
| PsaK | 2 | α Subunit | 0 |
| Hydrophilic subunits | β Subunit | 0 | |
| Stromal orientation | PsbH | 0 | |
| PsaC | 9 | PsbN | 0 |
| PsaD | 1 | 22 kD | 0 |
| PsaE | 0 | Hydrophilic subunits | |
| PsaH | 0 | 33 kD | 4 |
| Lumenal orientation | 23 kD | 1 | |
| PsaF | 3 | 17 kD | 2 |
| PsaN | 5 | 10 kD | 0 |
Cotyledons switch from sink to source status during development in order to support chloroplast biogenesis in the leaf, which is essential for survival in the field. Consequently, chloroplast biogenesis in cotyledons occurs when energy is limited. Therefore, rapid synthesis and assembly of the photosynthetic thylakoid membrane system is critical. CYO1 may be involved directly in this process. In cyo1 mutants, the period between germination and development of the first leaf is longer and the growth rate is slower than in wild-type plants. Consequently, plants that harbor CYO1 may have a selective advantage compared with those that lack this gene.
METHODS
Plant Growth and Isolation of the Mutant
Arabidopsis thaliana (ecotype Wassilewskija) was grown at 23°C under continuous light on 1/2 MS plates or on soil. Transgenic plants were produced on a large scale by vacuum infiltration (Bechtold and Pelletier, 1998), with Agrobacterium tumefaciens strain GV310 (pmp90rk) harboring the activation-tagging vector pPCVICEn4HPT (Hayashi et al., 1992; Shirano et al., 2000). We isolated several mutants with white cotyledons from among ∼3500 transgenic lines. The mutant line designated cyo1 was chosen for further study.
Microscopy
Plant tissues were fixed with glutaraldehyde and paraformaldehyde and embedded in Quetol 812 (Nisshin EM) according to standard procedures. Ultrathin sections were stained with uranyl acetate, then with lead citrate, and observed with a JEM 1200EX transmission electron microscope.
Isolation of the T-DNA–Flanking Sequence
The T-DNA fragment used for Arabidopsis transformation contained the Col E1 origin and the ampicillin resistance gene (Hayashi et al., 1992) and was isolated by plasmid rescue in Escherichia coli. Genomic DNA was extracted from cyo1 mutant plants as described by Saghai-Maroof et al. (1984), digested with ClaI, and then ligated and transformed into E. coli XL2-Blue. The rescued plasmid containing the T-DNA–flanking fragment was sequenced.
RNA Expression Analyzed by RT-PCR
CYO1 gene expression levels were assessed by a kinetic RT-PCR approach. 5′-ATGTTCCGATTATACCCTAATTGCTCTCTG-3′ and 5′-TATCCAAATCTTCCATCACTTCAATGTCCG-3′ were used as CYO1-specific primers, and 5′-CTTCGTCAAGACTTTGACCG-3′ and 5′-GCCCCAAAACACAAACCACC-3′ were used as a control for constitutive expression (UBQ10) (Awai et al., 2001). All genes tested are unique in the Arabidopsis genome and show the same linear amplification rate from 13 to 21 cycles (data not shown). Single-stranded DNA was synthesized from total RNA (0.25 μg) using avian myeloblastosis virus RT (TaKaRa) in the presence of oligo(dT) primer at 55°C for 30 min. PCR aliquots (19th cycle) were analyzed on agarose gels, blotted, and radiolabeled with specific probes corresponding to the amplified fragments.
Immunoblot Analysis
Rabbit antiserum to recombinant full-length CYO1 protein was prepared by a conventional method (Suzuki et al., 2002). Intact chloroplasts of 7-d-old Arabidopsis grown under continuous light were isolated according to the method of Aronsson and Jarvis (2002). Briefly, plants were homogenized and the homogenate was filtered through a double layer of Miracloth (Calbiochem). The filtrate was used as total protein (Figure 6B). The filtrate was centrifuged at 1000g for 5 min, and the pellet was resuspended. The resuspended chloroplasts were loaded onto a two-step Percoll gradient (40 and 85% Percoll) and centrifuged at 2500g for 10 min. The intact chloroplasts that appeared between the phases were washed and used as intact chloroplasts (Figure 6B). The intact chloroplasts were lysed osmotically by suspending them with 50 mM Tris-HCl, pH 7.5, vortexed vigorously, and then centrifuged at 3000g for 5 min. The pellet was washed twice and used as the pellet sample (Figure 6B). The supernatant was centrifuged at 10,000g for 30 min and used as the supernatant sample (Figure 6B). The proteins were electrophoresed on a 12.5% SDS-PAGE gel and electroblotted onto nitrocellulose. The membrane was immunoreacted with each antibody. Immunoreactive proteins were detected using the ECL Advance Western Blotting Detection Kit (GE Healthcare) and Kodak Image Station 2000R for CYO1 or the Alkaline Phosphatase Substrate Kit II (Vector Laboratories) for other proteins.
Intact thylakoids from Arabidopsis were prepared as described (Casazza et al., 2001). Sonicated thylakoids were prepared by sonicating thylakoid membranes for 3 min at 0°C using an ultrasonic disruptor (TOMY, UD-201, output 2, duty cycle 30). For alkaline, detergent, and high sodium chloride treatments, the sonicated thylakoids were resuspended with 100 mM sodium carbonate, pH 11.5, 1.0% Nonidet P-40, or 2.0 M sodium chloride in the extraction buffer. After incubation for 1 h on ice, samples were centrifuged at 10,000g for 10 min at 0°C to separate soluble and insoluble fractions. A protease protection assay was performed as described (Motohashi and Hisabori, 2006).
Blue native gel electrophoresis was performed as described (Asakura et al., 2004) (see also http://www.hos.ufl.edu/clineweb/BNgel.htm) with the following modifications. Intact thylakoids (0.8 mg chlorophyll/mL) were solubilized with 12.5 mM BisTris-HCl, pH 7.0, 10% (w/v) glycerol, and 1% (w/v) n-dodecyl-β-d-maltoside (Dojindo) and incubated for 30 min at 4°C. Samples were centrifuged at 15,000g for 30 min at 4°C. One hundred microliters of the supernatant was combined with 10 μL of blue native sample buffer (5% Coomassie Brilliant Blue G 250 [CBB G 250], 100 mM BisTris-HCl, and 0.5 M 6-amino-capronic acid, pH 7.0). Fifteen microliters of sample (corresponding to 5.5 μg of chlorophyll) was loaded onto a blue native 5 to 14% polyacrylamide gradient gel. Electrophoresis was performed at 100 V at 4°C. The cathode buffer initially contained 0.01% CBB G 250 and was replaced by buffer lacking CBB G 250 after approximately half of the electrophoresis run. After electrophoresis, the gel was incubated with blotting buffer (100 mM Tris, 192 mM Gly, and 5% methanol) containing 0.1% SDS for 10 min at room temperature. The proteins in the gel were electroblotted onto a nitrocellulose membrane filter using blotting buffer.
In Vitro Chloroplast Import Assay
CYO1 protein and RBC-S (Olsen and Keegstra, 1992) were translated and radiolabeled using [35S]Met and the TNT coupled wheat germ extraction system (Promega) according to the manufacturer's protocol. Intact chloroplasts were isolated from 8- to 12-d-old pea (Pisum sativum) seedlings and purified over a Percoll gradient as described previously (Bruce et al., 1994). Intact pea chloroplasts were resuspended in the import buffer (330 mM sorbitol and 50 mM HEPES/KOH, pH 8.0) at a concentration of 1 mg chlorophyll/mL. Import reactions were performed as described by Bruce et al. (1994). Import and extraction experiments were performed as described previously by Tranel et al. (1995).
RNA Gel Blot Analysis
Total RNA was prepared using the RNeasy mini kit (Qiagen) according to the directions supplied. Total RNA (1.0 μg) was electrophoresed on a 1.2% agarose/formaldehyde gel and blotted onto nylon membranes. The membranes were hybridized to random-primed [32P]cDNA probes. After hybridization, the membranes were washed with 0.1× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate)/0.1% SDS at 65°C for 60 min and autoradiographed.
Expression and Purification of CYO1 and DnaJ Proteins
The full-length CYO1 cDNA was amplified by RT-PCR using primers that contained sites for NdeI (CYO1-5-Nde, 5′-CCCCATATGTTCCGATTATACCCTAATTGCTCTCTG-3′) and HindIII (CYO1-3-Hin, 5′-GGGAAGCTTAGATGGTTCATTATCCAAATCTTCCATCAC-3′). Similarly, a primer (CYO1-31aa, 5′-CCCCATATGGCCGCCGCTGATATTCCCCTTGGTGACGGA-3′) was constructed, and the primer set CYO1-31aa and CYO1-3-Hin was used for PCR. The amplified DNA fragments encoded the Δ30CYO1 protein containing amino acids 31 to 187. The DnaJ gene in E. coli was amplified by PCR using primers containing sites for NdeI (EdnaJ-5Nde, 5′-GGGCATATGGCTAAGCAAGATTATTACGAGATTTTA-3′) and XhoI (EdnaJ-3-Xho, 5′-GGGCTCGAGGCGGGTCAGGTCGTCAAAAAACTTCTTCAC-3′). The amplified DNA fragments were digested with NdeI and HindIII or XhoI and ligated into the expression vector pET-24a(+) (Novagen). The sequences of the amplified fragments were confirmed. Expression and purification were performed as described previously (Shimada et al., 2004). E. coli strain BL21(DE3) was used for the expression of recombinant fusion proteins bearing a 6xHis tag. Overnight cultured E. coli BL21(DE3) harboring the full-length cDNA of the CYO1 gene was diluted 1:10 with fresh culture medium and further grown at 37°C for 1 h. Isopropyl β-d-thiogalactoside was then added to a final concentration of 1.0 mM, and the cells were further cultured at 37°C overnight. The cells were centrifuged for 10 min at 5000g and 4°C. The cell pellets were suspended in suspension buffer (15.0 mM Tris-HCl, pH 7.0, 50.0 mM NaCl, 0.1 mM EDTA, 0.1 mM DTT, and 1.0 mM phenylmethylsulfonyl fluoride). The crude extracts were sonicated and centrifuged for 10 min at 12,000g and 4°C. The pellets were suspended in suspension buffer supplemented with 6.0 M guanidine hydrochloride and sonicated. The samples were incubated overnight with nickel-nitrilotriacetic acid agarose (Qiagen) at 4°C. The agarose was washed with suspension buffer supplemented with 6.0 M guanidine hydrochloride and then washed with suspension buffer supplemented with 8.0 M urea. Proteins bound to the agarose were eluted with suspension buffer containing 8.0 M urea and 50.0 mM imidazole. The eluted proteins were dialyzed with renaturation buffer (15.0 mM Tris-HCl, pH 6.3, and 50.0 mM NaCl). The dialyzed samples were centrifuged for 10 min at 12,000g and 4°C. The pellet was suspended with renaturation buffer, and the proteins were injected into rabbits to stimulate antibody production.
E. coli strain BL21(DE3) was used for the expression of recombinant Δ30CYO1 and DnaJ fusion proteins with 6xHis tags. Overnight cultured BL21(DE3) harboring the Δ30CYO1 or DnaJ gene was diluted 1:20 with fresh culture medium and further grown at 37°C for 1 h. Isopropyl β-d-thiogalactoside was then added to a final concentration of 1.0 mM, and the cells were further cultured at 30°C (Δ30CYO1) or 20°C (DnaJ) overnight. The cells were centrifuged for 10 min at 5000g and 4°C. The cell pellets were suspended in suspension buffer. The crude extracts were sonicated and centrifuged for 10 min at 12,000g and 4°C. The pellets were suspended in suspension buffer supplemented with 6.0 M guanidine hydrochloride and sonicated. Then, the samples were dialyzed with dialyzing buffer (15 mM Tris-HCl, pH 7.0, and 50 mM NaCl). The samples were centrifuged for 10 min at 12,000g and 4°C, and the supernatant was incubated for 3 h with nickel-nitrilotriacetic acid agarose (Qiagen) at 4°C. The agarose was washed with dialyzing buffer containing 50 mM imidazole. Proteins bound to the agarose were eluted with dialyzing buffer containing 150 mM imidazole. The eluted proteins were denatured with 6.0 M guanidine hydrochloride, and then the proteins were renatured with dialyzing buffer containing 5.0 mM ZnCl2. To analyze the requirement of Zn for the catalytic properties of CYO1, the proteins were renatured with dialyzing buffer containing 5.0 mM ZnCl2, CuCl2, CaCl2, MgCl2, or no divalent metal ion. The samples were centrifuged for 10 min at 12,000g and 4°C. The supernatants were used for enzymatic assays.
Assay of Insulin Disulfide Reduction
The reduction of insulin (Sigma-Aldrich) was assayed by measuring the increase in absorbance at 650 nm (Holmgren, 1979).
Assay of PDI Activity
Reduced RNase A (Sigma-Aldrich) was prepared as described previously (Pigiet and Schuster, 1986). PDI activity was determined by measurement of the reactivation of reduced RNase A (Hasegawa et al., 2003). Yeast RNA (Wako) was dissolved in 50 mM Tris-HCl, pH 7.5, and was precipitated with ethanol to remove the unprecipitable fraction. Precipitated RNA was suspended in the same buffer, mixed with the reduced RNase A, and incubated for 10 min at 37°C. The final concentrations of RNase and RNA were 36 nM and 1 mg/mL, respectively. After the reaction, RNA was precipitated with ethanol by leaving the reaction mixture at −20°C overnight, and the absorption of the supernatant, which contained RNA molecules that had been degraded and become unprecipitable with ethanol, was measured at 260 nm.
Accession Number
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under accession number At3g19220 (CYO1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Levels of Chlorophylls a and b.
Supplemental Figure 2. Ultrastracture of Chloroplasts of cyo1 in Rosette Leaves.
Supplemental Figure 3. Genomic DNA Gel Blot Analysis of T-DNA in cyo1.
Supplemental Figure 4. A Ten-Day-Old cyo1 Mutant Plant Harboring a Vector Containing the cDNA between 5 and 606 bp with the ATG at 32 bp Mutated to ATT.
Supplemental Figure 5. Comparison of mRNA Levels for 2880 ESTs in CYO1 Mutant and Wild-Type Plants.
Supplementary Material
Acknowledgments
We dedicate this article to Ken-ichiro Takamiya, who passed away in an unfortunate traffic accident while this study was being conducted. We thank Toru Hisabori (Tokyo Institute of Technology) for the gift of antibodies against PSI-N, FNR, TRX-M, CF1-β, and CF1-γ; Amane Makino (Tohoku University) for the gift of antibodies against cytochrome f; Maryse A. Block (Centre National de la Recherche Scientifique/Institut Recherche et Developpement/Universite de Perpignan, France) for the gift of antibodies against E37; Yukiko Sasaki and Kan Tanaka (University of Tokyo) for the gift of antibodies against ACCD; Masayoshi Maeshima (Nagoya University) for the gift of antibodies against PAQ; and Ikuko Hara-Nishimura (Kyoto University) for the gift of antibodies against BiP. We are also grateful to Yuji Saito (Tokyo Institute of Technology) for technical advice on the assay for PDI activity and to Toru Hisabori, Ken Motohashi, Hiroyuki Ohta (Tokyo Institute of Technology), Tatsuru Masuda (University of Tokyo), and Hirofumi Kuroda (RIKEN Plant Science Center) for valuable discussions. This study was partially supported by the Ministry of Education, Science, Sports, and Culture (Grant-in-Aid for Scientific Research C, 16770030, to H.S.) and by a grant from the U.S. National Science Foundation (to K.W.O).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Hiroshi Shimada (hshimada@bio.titech.ac.jp).
Online version contains Web-only data.
References
- Albrecht, V., Ingenfeld, A., and Apel, K. (2006). Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: The impact of chloroplast elongation factor G on chloroplast development and plant vitality. Plant Mol. Biol. 60 507–518. [DOI] [PubMed] [Google Scholar]
- Anfinsen, C.B., and Scheraga, H.A. (1975). Experimental and theoretical aspects of protein folding. Adv. Protein Chem. 29 205–300. [DOI] [PubMed] [Google Scholar]
- Aronsson, H., and Jarvis, P. (2002). A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett. 529 215–220. [DOI] [PubMed] [Google Scholar]
- Asakura, Y., Hirohashi, T., Kikuchi, S., Belcher, S., Osborne, E., Yano, S., Terashima, I., Barkan, A., and Nakai, M. (2004). Maize mutants lacking chloroplast FtsY exhibit pleiotropic defects in the biogenesis of thylakoid membranes. Plant Cell 16 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awai, K., Marechal, E., Block, M.A., Brun, D., Masuda, T., Shimada, H., Takamiya, K., Ohta, H., and Joyard, J. (2001). Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82 259–266. [DOI] [PubMed] [Google Scholar]
- Bruce, B.D., Perry, S., Froehlich, J., and Keegstra, K. (1994). In vitro import of protein into chloroplasts. In Plant Molecular Biology Manual, S.B. Gelvin and R.B. Schilperoort, eds (Boston: Kluwer Academic Publishers), p. 1–15.
- Brutnell, T.P., Sawers, R.J., Mant, A., and Langdale, J.A. (1999). BUNDLE SHEATH DEFECTIVE2, a novel protein required for post-translational regulation of the rbcL gene of maize. Plant Cell 11 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza, A.P., Tarantino, D., and Soave, C. (2001). Preparation and functional characterization of thylakoids from Arabidopsis thaliana. Photosynth. Res. 68 175–180. [DOI] [PubMed] [Google Scholar]
- Cyr, D.M., Langer, T., and Douglas, M.G. (1994). DnaJ-like proteins: Molecular chaperones and specific regulators of Hsp70. Trends Biochem. Sci. 19 176–181. [DOI] [PubMed] [Google Scholar]
- de Crouy-Chanel, A., Kohiyama, M., and Richarme, G. (1995). A novel function of Escherichia coli chaperone DnaJ. Protein-disulfide isomerase. J. Biol. Chem. 270 22669–22672. [DOI] [PubMed] [Google Scholar]
- Deng, X.W., and Gruissem, W. (1987). Control of plastid gene expression during development: The limited role of transcriptional regulation. Cell 49 379–387. [DOI] [PubMed] [Google Scholar]
- Freedman, R.B. (1989). Protein disulfide isomerase: Multiple roles in the modification of nascent secretory proteins. Cell 57 1069–1072. [DOI] [PubMed] [Google Scholar]
- Georgopoulos, C. (1992). The emergence of the chaperone machines. Trends Biochem. Sci. 17 295–299. [DOI] [PubMed] [Google Scholar]
- Gilbert, H.F. (1990). Molecular and cellular aspects of thiol-disulfide exchange. Adv. Enzymol. Relat. Areas Mol. Biol. 63 69–172. [DOI] [PubMed] [Google Scholar]
- Goffin, L., and Georgopoulos, C. (1998). Genetic and biochemical characterization of mutations affecting the carboxy-terminal domain of the Escherichia coli molecular chaperone DnaJ. Mol. Microbiol. 30 329–340. [DOI] [PubMed] [Google Scholar]
- Hanaoka, M., Kanamaru, K., Fujiwara, M., Takahashi, H., and Tanaka, K. (2005). Glutamyl-tRNA mediates a switch in RNA polymerase use during chloroplast biogenesis. EMBO Rep. 6 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, G., Suwa, M., Ichikawa, Y., Ohtsuka, T., Kumagai, S., Kikuchi, M., Sato, Y., and Saito, Y. (2003). A novel function of tissue-type transglutaminase: Protein disulphide isomerase. Biochem. J. 373 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano, K., Shimada, T., Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1997). A rapid increase in the level of binding protein (BiP) is accompanied by synthesis and degradation of storage proteins in pumpkin cotyledons. Plant Cell Physiol. 38 344–351. [DOI] [PubMed] [Google Scholar]
- Hayashi, H., Czaja, I., Lubenow, H., Schell, J., and Walden, R. (1992). Activation of a plant gene by T-DNA tagging: Auxin-independent growth in vitro. Science 258 1350–1353. [DOI] [PubMed] [Google Scholar]
- Hess, W.R., and Borner, T. (1999). Organellar RNA polymerases of higher plants. Int. Rev. Cytol. 190 1–59. [DOI] [PubMed] [Google Scholar]
- Holmgren, A. (1979). Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 254 9627–9632. [PubMed] [Google Scholar]
- Horton, P., Park, K.J., Obayashi, T., Fujita, N., Harada, H., Adams-Collier, C.J., and Nakai, K. (2007). WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 35 W585–W587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki, Y., Tsunoyama, Y., Hatano, K., Ando, K., Kato, K., Shinmyo, A., Kobori, M., Takeba, G., Nakahira, Y., and Shiina, T. (2005). A nuclear-encoded sigma factor, Arabidopsis SIG6, recognizes sigma-70 type chloroplast promoters and regulates early chloroplast development in cotyledons. Plant J. 42 133–144. [DOI] [PubMed] [Google Scholar]
- Kanamaru, K., and Tanaka, K. (2004). Roles of chloroplast RNA polymerase sigma factors in chloroplast development and stress response in higher plants. Biosci. Biotechnol. Biochem. 68 2215–2223. [DOI] [PubMed] [Google Scholar]
- Kim, J., and Mayfield, S.P. (1997). Protein disulfide isomerase as a regulator of chloroplast translational activation. Science 278 1954–1957. [DOI] [PubMed] [Google Scholar]
- Langer, T., Lu, C., Echols, H., Flanagan, J., Hayer, M.K., and Hartl, F.U. (1992). Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature 356 683–689. [DOI] [PubMed] [Google Scholar]
- Liu, C., Willmund, F., Whitelegge, J.P., Hawat, S., Knapp, B., Lodha, M., and Schroda, M. (2005). J-domain protein CDJ2 and HSP70B are a plastidic chaperone pair that interacts with vesicle-inducing protein in plastids 1. Mol. Biol. Cell 16 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S., et al. (2006). The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 18 3594–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1996). The dynamics of seedling and cotyledon cell development in Arabidopsis thaliana during reserve mobilization. Int. J. Plant Sci. 157 280–295. [Google Scholar]
- Motohashi, K., and Hisabori, T. (2006). HCF164 receives reducing equivalents from stromal thioredoxin across the thylakoid membrane and mediates reduction of target proteins in the thylakoid lumen. J. Biol. Chem. 281 35039–35047. [DOI] [PubMed] [Google Scholar]
- Ohshima, Y., Iwasaki, I., Suga, S., Murakami, M., Inoue, K., and Maeshima, M. (2001). Low aquaporin content and low osmotic water permeability of the plasma and vacuolar membranes of a CAM plant Graptopetalum paraguayense: Comparison with radish. Plant Cell Physiol. 42 1119–1129. [DOI] [PubMed] [Google Scholar]
- Olsen, L.J., and Keegstra, K. (1992). The binding of precursor proteins to chloroplasts requires nucleoside triphosphates in the intermembrane space. J. Biol. Chem. 267 433–439. [PubMed] [Google Scholar]
- Pigiet, V.P., and Schuster, B.J. (1986). Thioredoxin-catalyzed refolding of disulfide-containing proteins. Proc. Natl. Acad. Sci. USA 83 7643–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privat, I., Hakimi, M.A., Buhot, L., Favory, J.J., and Mache-Lerbs, S. (2003). Characterization of Arabidopsis plastid sigma-like transcription factors SIG1, SIG2 and SIG3. Plant Mol. Biol. 51 385–399. [DOI] [PubMed] [Google Scholar]
- Saghai-Maroof, M.A., Soliman, K.M., Jorgensen, R.A., and Allard, R.W. (1984). Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto, W., Zaltsman, A., Adam, Z., and Takahashi, Y. (2003). Coordinated regulation and complex formation of yellow variegated1 and yellow variegated2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes. Plant Cell 15 2843–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, M., et al. (2002). Functional annotation of a full-length Arabidopsis cDNA collection. Science 296 141–145. [DOI] [PubMed] [Google Scholar]
- Shi, Y.Y., Tang, W., Hao, S.F., and Wang, C.C. (2005). Contributions of cysteine residues in Zn2 to zinc fingers and thiol-disulfide oxidoreductase activities of chaperone DnaJ. Biochemistry 44 1683–1689. [DOI] [PubMed] [Google Scholar]
- Shimada, H., Koizumi, M., Kuroki, K., Mochizuki, M., Fujimoto, H., Ohta, H., Masuda, T., and Takamiya, K. (2004). ARC3, a chloroplast division factor, is a chimera of prokaryotic FtsZ and part of eukaryotic phosphatidylinositol-4-phosphate 5-kinase. Plant Cell Physiol. 45 960–967. [DOI] [PubMed] [Google Scholar]
- Shirano, Y., et al. (2000). Chloroplast development in Arabidopsis thaliana requires the nuclear-encoded transcription factor sigma B. FEBS Lett. 485 178–182. [DOI] [PubMed] [Google Scholar]
- Suzuki, T., Masuda, T., Singh, D.P., Tan, F.C., Tsuchiya, T., Shimada, H., Ohta, H., Smith, A.G., and Takamiya, K. (2002). Two types of ferrochelatase in photosynthetic and nonphotosynthetic tissues of cucumber: Their difference in phylogeny, gene expression, and localization. J. Biol. Chem. 277 4731–4737. [DOI] [PubMed] [Google Scholar]
- Szabo, A., Korszun, R., Hartl, F.U., and Flanagan, J. (1996). A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. EMBO J. 15 408–417. [PMC free article] [PubMed] [Google Scholar]
- Tang, W., and Wang, C.C. (2001). Zinc fingers and thiol-disulfide oxidoreductase activities of chaperone DnaJ. Biochemistry 40 14985–14994. [DOI] [PubMed] [Google Scholar]
- Teyssier, E., Block, M.A., Douce, R., and Joyard, J. (1996). Is E37, a major polypeptide of the inner membrane from plastid envelope, an S-adenosyl methionine-dependent methyltransferase? Plant J. 10 903–912. [DOI] [PubMed] [Google Scholar]
- Tranel, P.J., Froehlich, J., Goyal, A., and Keegstra, K. (1995). A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO J. 14 2436–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, Y.Y., Puente, P., and Deng, X.W. (2000). An Arabidopsis cotyledon-specific albino locus: A possible role in 16S rRNA maturation. Plant Cell Physiol. 41 68–76. [DOI] [PubMed] [Google Scholar]
- Yohn, C.B., Cohen, A., Danon, A., and Mayfield, S.P. (1996). Altered mRNA binding activity and decreased translational initiation in a nuclear mutant lacking translation of the chloroplast psbA mRNA. Mol. Cell. Biol. 16 3560–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.