Abstract
The timing of the developmental transition to flowering is critical to reproductive success in plants. Here, we show that Arabidopsis thaliana homologs of human Lysine-Specific Demethylase1 (LSD1; a histone H3-Lys 4 demethylase) reduce the levels of histone H3-Lys 4 methylation in chromatin of the floral repressor FLOWERING LOCUS C (FLC) and the sporophytically silenced floral repressor FWA. Two of the homologs, LSD1-LIKE1 (LDL1) and LSD1-LIKE2 (LDL2), act in partial redundancy with FLOWERING LOCUS D (FLD; an additional homolog of LSD1) to repress FLC expression. However, LDL1 and LDL2 appear to act independently of FLD in the silencing of FWA, indicating that there is target gene specialization within this histone demethylase family. Loss of function of LDL1 and LDL2 affects DNA methylation on FWA, whereas FLC repression does not appear to involve DNA methylation; thus, members of the LDL family can participate in a range of silencing mechanisms.
INTRODUCTION
The developmental transition from a vegetative to a reproductive phase (i.e., flowering) is perhaps the most critical event in the plant life cycle. In Arabidopsis thaliana, several pathways form a regulatory network that integrates the endogenous development state of the plant and environmental cues (e.g., daylength and temperature) to control the timing of the initiation of flowering (Mouradov et al., 2002; Putterill et al., 2004; Balasubramanian et al., 2006).
A key component in this regulatory network in Arabidopsis is FLOWERING LOCUS C (FLC), a MADS box transcription factor that blocks the floral transition (Michaels and Amasino, 1999; Sheldon et al., 1999). Therefore, repression of FLC expression results in the acceleration of flowering. The vernalization pathway represses FLC in response to a prolonged cold exposure, whereas the autonomous pathway, which includes FLOWERING LOCUS D (FLD) and FVE, constitutively represses FLC. FRIGIDA (FRI) activates FLC expression such that in the absence of vernalization, flowering is delayed (i.e., FRI establishes a vernalization requirement) (reviewed in Boss et al., 2004; Sung and Amasino, 2005).
Recent studies have revealed that chromatin modification plays an important role in the regulation of FLC expression. Histone H3 trimethylation at Lys-4 (H3K4me3) and histone acetylation are associated with active FLC transcription, whereas histone deacetylation and histone H3 methylation at Lys-9 (H3K9) and Lys-27 (H3K27) are associated with FLC repression (reviewed in He and Amasino, 2005). The autonomous-pathway repressors FLD and FVE are required for the deacetylation of FLC chromatin (He et al., 2003; Ausin et al., 2004; Kim et al., 2004). Vernalization leads to repressive histone modifications of FLC chromatin, including deacetylation, and increased methylation of H3K9 and H3K27 (Bastow et al., 2004; Finnegan et al., 2005; Sung et al., 2006). Activation of FLC expression and the associated increase in H3K4 trimethylation require the PAF1 (for RNA Polymerase II–Associated Factor1)–like complex (He et al., 2004).
FWA, a homeodomain-containing transcription factor first identified based on its ability to delay flowering, is also under epigenetic control. In wild-type Arabidopsis, FWA is silenced in the sporophyte; it is only expressed in female gamete and extraembryonic endosperm tissue in an imprinted (maternal origin–specific) manner (Soppe et al., 2000; Kinoshita et al., 2004). fwa epi-alleles (which do not have a change in the nucleotide sequence of FWA) cause a late-flowering phenotype due to ectopic FWA expression in sporophytic tissues (Soppe et al., 2000); ectopically expressed FWA interacts with FLOWERING LOCUS T and interferes with its function, delaying the floral transition (Ikeda et al., 2007). In the wild-type sporophyte, silent FWA chromatin is marked by repressive histone modifications and cytosine methylation in its 5′ region (Soppe et al., 2000; Lippman et al., 2004; Kinoshita et al., 2007). Epi alleles of fwa frequently arise in mutants defective in DNA methylation, such as met1 (for methyltransferase1) (Saze et al., 2003), and in mutants defective in chromatin remodeling, such as ddm1 (for decreased DNA methylation1) (Soppe et al., 2000).
Histone H3K4 methylation, which is associated with actively transcribed genes, plays an important role in regulating transcription (Martin and Zhang, 2005). The ɛ amino group of H3K4 residues can be monomethylated, dimethylated, or trimethylated. In budding yeast (Saccharomyces cerevisiae), trimethylated H3K4 is associated exclusively with active euchromatic genes (Santos-Rosa et al., 2002), whereas H3K4 dimethylation (H3K4me2) occurs in both inactive and active euchromatic genes (Ng et al., 2003). H3K4me2 is most prevalent in coding regions and the 3′ end of genes, and this pattern is thought to play a role in determining a transcriptionally permissive chromatin environment (Santos-Rosa et al., 2002; Ng et al., 2003). Similar to its association in S. cerevisiae, H3K4 trimethylation is associated with active transcribed genes in multicellular eukaryotes; however, in contrast with yeast, H3K4 dimethylation is also associated with active genes in multicellular eukaryotes (Schneider et al., 2004). For instance, active genes in chicken are marked with elevated levels of H3K4me2 and H3K4me3 at promoters and 5′ transcribed regions (Schneider et al., 2004). It has also been shown that in Arabidopsis, elevated levels of H3K4me2 and H3K4me3 are associated with active genes and that these modifications occur in 5′ promoters and coding regions but are absent from nontranscribed intergenic regions (Alvarez-Venegas and Avramova, 2005).
Histone H3K4 methylation is dynamically regulated by histone methylases and demethylases (Martin and Zhang, 2005). A component of transcriptional corepressor complexes, Lysine-Specific Demethylase1, has been shown to demethylate H3K4 and repress target gene expression in mammalian cells (Shi et al., 2004). Human LSD1 specifically demethylates monomethyl and dimethyl H3K4 (Shi et al., 2004; Forneris et al., 2005; Lee et al., 2005) and, when complexed with an androgen receptor, also destabilizes dimethyl H3K9 (H3K9me2) (Metzger et al., 2005). LSD1 is an integral component of several mammalian histone deacetylase (HDAC) corepressor complexes (Humphrey et al., 2001; Hakimi et al., 2002) in which HDACs and LSD1 may cooperate to remove activating acetyl and methyl histone modifications (Shi et al., 2005; Lee et al., 2006). Consistent with this model, in one such complex (the BRAF-HDAC complex), the enzymatic activities of HDACs and LSD1 are closely linked, as HDAC inhibitors diminish histone demethylation activity and the abrogation of LSD1 activity decreases the deacetylation activity of this complex (Lee et al., 2006).
We previously identified and characterized a plant homolog of human LSD1, FLD, which promotes flowering in Arabidopsis by constitutively repressing FLC expression (He et al., 2003). In this report, we demonstrate that FLD homologs, which we refer to as LSD1-LIKE1 and LSD1-LIKE2 (LDL1 and LDL2), also contribute to the repression of FLC and that LDL1 and LDL2, but not FLD, contribute to the sporophytic silencing of FWA.
RESULTS
Arabidopsis Has Four Relatives of Human LSD1
LSD1 is evolutionarily conserved among multicellular eukaryotes (Shi et al., 2004). We previously reported that FLD is a plant homolog of a repressor complex component that was later designated LSD1 (He et al., 2003). In addition to FLD, Arabidopsis has three other homologs of LSD1: LDL1 (At_1g62830), LDL2 (At_3g13682), and LDL3 (At_4g16310) (Figure 1). Among these LSD1 relatives, FLD, LDL1, and LDL2 show extensive similarity (the similarity between FLD and LDL1 is 74% over a 624–amino acid region, and the similarity between LDL1 and LDL2 is 69% over a 739–amino acid region), whereas LDL3 shows less similarity to the other proteins (Figure 1).
Figure 1.
Amino Acid Sequence Alignment of Arabidopsis thaliana LDL1 (At LDL1), LDL2 (At LDL2), LDL3 (At LDL3), FLD (At FLD), and Homo sapiens LSD1 (Hs LSD1).
Numbers refer to amino acid residues; identical residues are shaded with black, and similar residues are shaded with gray. The SWIRM domain is indicated with a solid line; the conserved histone demethylation domain is indicated with a broken line. The broken triangle indicates the spacer region of Hs LSD1 (Shi et al., 2004) omitted from the alignment; the solid triangle indicates a 129–amino acid region of At LDL3 omitted from the alignment because it does not align with the rest of the proteins.
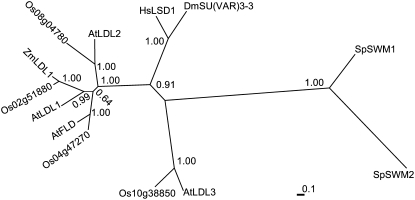
LSD1 is a single-copy gene in the human genome (Shi et al., 2004), and the Drosophila LSD1 homolog SU(VAR)3-3 is also a single-copy gene (Rudolph et al., 2007), whereas both Arabidopsis (a eudicot) and rice (Oryza sativa; a monocot) have four homologs of LSD1 (Figure 2), indicating that these LSD1-like genes were likely to have been duplicated before the monocot–eudicot split. Phylogenetic analysis of LSD1 relatives from different organisms showed that LDL1, LDL2, and FLD form a separate cluster that is related to the cluster of LSD1 and SU(VAR)3-3 (Figure 2). FLD has been shown to repress FLC expression and thus to promote flowering (He et al., 2003); however, the roles of the LDLs are unknown.
Figure 2.
Phylogenetic Tree of LSD1 Relatives in Different Organisms.
The unrooted phylogram was generated using Mrbayes (version 3.1.2); amino acid sequences were aligned with ClustalW. At, Arabidopsis thaliana; Dm, Drosophila melanogaster; Hs, Homo sapiens; Os, Oryza sativa; Sp, Schizosaccharomyces pombe; Zm, Zea mays (Zm LDL1, AZM4_71848; http://maize.tigr.org). Sp SWM1 (SPBC146.09c) and Sp SWM2 (SPAC23E2.02) are distant relatives of Hs LSD1. Clade credibility (posterior probability) values for each branch are shown.
LDL1 and LDL2 Promote the Floral Transition
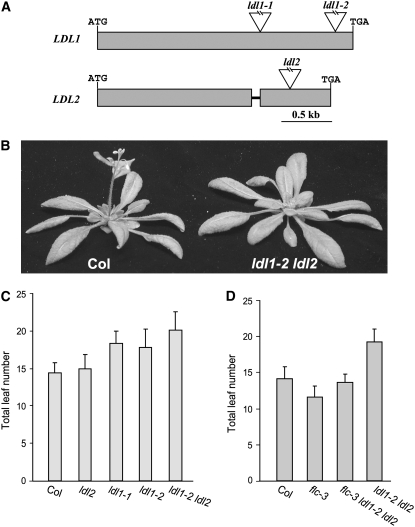
To elucidate the biological roles of these LSD1 relatives, we first identified two loss-of-function mutants of LDL1 and one of LDL2 (Figure 3A). In long days (LD; 16 h of light and 8 h of dark), ldl1 mutants flowered later than the wild-type Columbia (Col), whereas no phenotype was observed in the ldl2 single mutant (Figure 3C; flowering is measured by the developmental criterion of the number of leaves formed, prior to flowering, from the primary apical meristem). The ldl1-2 ldl2 double mutant flowered later than the ldl1 single mutant, but otherwise it appeared normal (Figures 3B and 3C). The differences in flowering behavior in LD between the wild type, the ldl1 single mutant, and the ldl1 ldl2 double mutant were moderate but statistically significant (Table 1). There was no alteration in the rate of leaf initiation in this double mutant relative to Col (data not shown).
Figure 3.
Phenotypes of ldl1, ldl2, and ldl1 ldl2.
(A) Gene structure of LDL1 and LDL2. Exons are represented by closed boxes, and introns are represented by lines. Triangles indicate T-DNA insertions.
(B) Phenotypes of Col and the ldl1 ldl2 mutant grown in LDs.
(C) Flowering times of ldl1, ldl2, and ldl1 ldl2 mutants grown in LDs. The total number of primary rosette and cauline leaves at flowering was counted, and for each line at least 10 plants were scored. The values shown are means ± sd.
(D) Flowering times of flc, ldl1 ldl2, and flc ldl1 ldl2 mutants grown in LDs. Total leaf number at flowering was scored. Fifteen plants were scored each for flc, flc ldl1 ldl2, and ldl1 ldl2; for Col, 10 plants were scored. The values shown are means ± sd.
Table 1.
Student's t Test for the Flowering Times of ldl1, ldl2, and ldl1 ldl2 Mutants in LDs
| Value | Col versus ldl1-1 | Col versus ldl1-2 | Col versus ldl2 | Col versus ldl1-2 ldl2 | ldl1-2 versus ldl1-2 ldl2 | ldl2 versus ldl1-2 ldl2 |
|---|---|---|---|---|---|---|
| t value | 6.42 | 4.75 | 0.75 | 8.38 | 3.03 | 7.43 |
| P value | 0.00 | 0.00 | 0.23 | 0.00 | 0.00 | 0.00 |
The Late-Flowering Phenotype of ldl1 ldl2 Is Partially Dependent on FLC
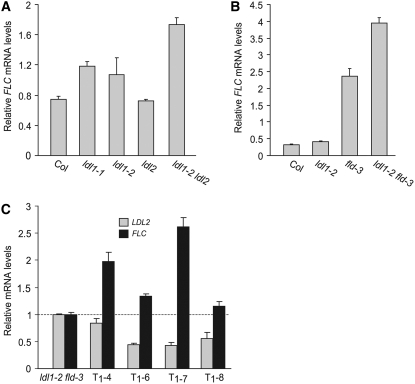
FLD, a homolog of LDL1 and LDL2, constitutively represses FLC expression (He et al., 2003). To examine whether LDL1 and LDL2 also contribute to FLC repression, we examined whether the late-flowering phenotype of ldl1 ldl2 is altered by the introduction of an flc null mutation. flc ldl1 ldl2 triple mutants flowered earlier than ldl1 ldl2 but still slightly later than the flc single mutant in LD (Figure 3D). Thus, the late-flowering phenotype of ldl1 ldl2 is partly dependent on FLC, indicating that LDL1 and LDL2 may also repress the expression of another floral repressor(s). We also quantified FLC transcripts in the ldl single mutants and the ldl1 ldl2 double mutant using real-time quantitative PCR. Consistent with the flowering phenotypes, FLC was upregulated in ldl1 and ldl1 ldl2 mutants and remained unchanged in ldl2 mutants (Figure 4A).
Figure 4.
Repression of FLC by LDLs.
(A) Relative FLC mRNA levels in seedlings of ldl1, ldl2, and ldl1 ldl2 quantified by real-time PCR.
(B) Relative FLC mRNA levels in seedlings of ldl1, fld, and ldl1 fld quantified by real-time PCR.
(C) Relative mRNA levels of LDL2 and FLC in rosette leaves of ldl1 fld transformed with a double-stranded RNA interference construct targeting LDL2. Four independent T1 transgenic plants were examined; gray and black bars represent mRNA levels of LDL2 and FLC, respectively.
The values shown are means ± sd.
LDL1, LDL2, and FLD Are Partially Redundant in Repressing FLC Expression
As noted above, a component of the delayed flowering resulting from the loss of LDL1 and LDL2 is due to FLC. FLD also represses FLC expression; hence, it was of interest to examine the contributions of FLD, LDL1, and LDL2 to the FLC expression level. As shown in Table 2, although the fld single mutant displays a strong late-flowering phenotype, the ldl1 fld double mutants flowered even later than fld, and this further delay in flowering was FLC-dependent (Figure 4B, Table 2). In addition, we quantified FLC transcript levels in seedlings of ldl1-2, fld-3, and ldl1-2 fld-3 by real-time quantitative PCR. Consistent with the flowering phenotypes, FLC mRNA levels were higher in the ldl1 fld double mutant than in the fld single mutant (Figure 4B). Hence, LDL1 and FLD are partially redundant in repressing FLC expression, with FLD playing a major role. FLC expression in the double mutants was still suppressed by vernalization (see Supplemental Figure 1 online), indicating that these genes are not part of the vernalization pathway in Arabidopsis.
Table 2.
Total Leaf Number at Bolting for ldl1, fld, and ldl1 fld Mutants in LDs
| Col | ldl1-2 | fld-3 | ldl1-2 fld-3 | flc-3 | flc-3 ldl1-2 fld-3 |
|---|---|---|---|---|---|
| 14.1 ± 1.7 (12) | 16.1 ± 2.7 (15) | 78.4 ± 10.8 (10) | >90.5 ± 3.6 (6)a | 11.6 ± 1.6 (15) | 14.9 ± 1.9 (15) |
Values shown are means ± sd of total number of rosette and cauline leaves; numbers in parentheses indicate the number of plants scored.
Two plants did not bolt in 3 months, and the mean number is the average of six plants scored.
The ldl2 single mutation does not cause any flowering phenotype, and levels of FLC transcripts in ldl2 remain the same as in Col (Figures 3C and 4A), but it is possible that LDL2 contributes redundantly with LDL1 and FLD to FLC repression. Due to the chromosomal proximity of LDL2 to FLD, it would be difficult to create the fld ldl2 double mutant. Therefore, a double-stranded RNA interference approach using a 223-bp LDL2-specific fragment with no homology with LDL1, LDL3, or FLD was employed to knock down LDL2 expression in ldl1 fld double mutants (in effect, mimicking a triple mutant). We quantified transcripts of LDL2 and FLC in leaves of four independent T1 transgenic plants and found that in all transformants (which developed normally except for delayed flowering), LDL2 expression was reduced and FLC expression was further elevated (Figure 4C). Thus, LDL2 appears to act redundantly with LDL1 and FLD to repress FLC expression.
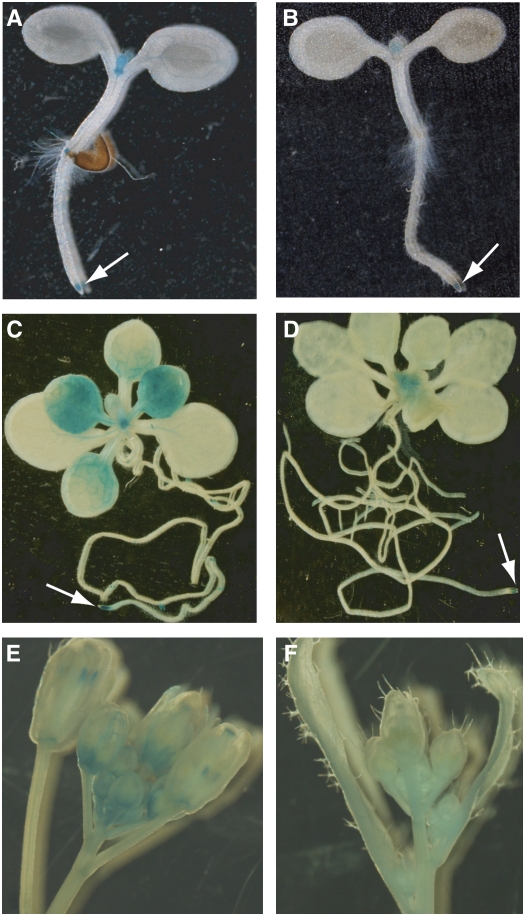
FLC is preferentially expressed in shoot and root apical regions, which are enriched in dividing cells, and is also expressed in leaf vasculature (Bastow et al., 2004; Sung and Amasino, 2004). To examine whether LDL1 and LDL2 display a spatial expression pattern similar to that of FLC, we fused 5′ promoters and part of the coding regions of LDL1 and LDL2 with the reporter gene GUS (for β-GLUCURONIDASE). LDL1 and LDL2 were preferentially expressed in shoot and root apical regions of young seedlings (Figures 5A and 5B); in addition, LDL1:GUS was also expressed in vascular tissues of cotyledon leaves and was readily detectable in leaves of ∼2-week-old plants (Figures 5A and 5C). Furthermore, we found that both LDL1 and LDL2 were expressed in inflorescences (Figures 5E and 5F).
Figure 5.
Histochemical Analysis of the Expression of LDL1 and LDL2.
(A), (C), and (E) Spatial expression patterns of LDL1 in 4-d-old seedlings (T1), ∼2-week-old plants (T2) grown on half-strength Murashige and Skoog medium, and inflorescence revealed by the GUS reporter gene driven by the LDL1 promoter plus the 5′ part of LDL1 CDS.
(B), (D), and (F) Spatial expression patterns of LDL2 in 4-d-old seedlings (T1), ∼2-week-old plants (T2), and inflorescence revealed by GUS driven by the LDL2 promoter plus the 5′ part of LDL2 CDS.
LDL1 and LDL2 Also Repress the Expression of FWA
As noted above, the delay in flowering of ldl1 ldl2 double mutants appears to have an FLC-independent component. There is a close relative of FLC in Arabidopsis, FLOWERING LOCUS M (FLM), which is also a floral repressor (Scortecci et al., 2001). Because we and others previously found that both FLC and FLM are coordinately regulated by chromatin-modifying complexes such as the PAF1-like complex (He et al., 2004; Oh et al., 2004), it was of interest to determine whether LDL1 and LDL2 also repress FLM expression. We quantified FLM transcript levels in Col and ldl1 ldl2 and found that FLM in ldl1 ldl2 was expressed at a similar level to that in Col (see Supplemental Figure 2 online). Hence, unlike the PAF1-like complex, LDL1 and LDL2 repress the expression of FLC but not FLM.
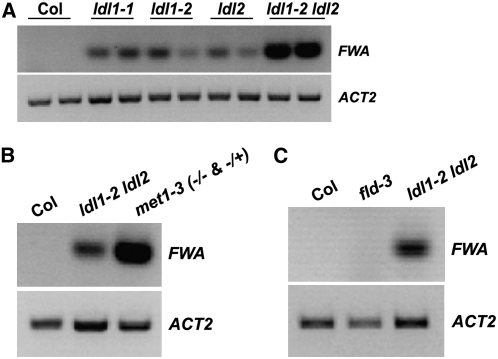
We found, however, that expression of the homeodomain gene FWA was ectopically activated in rosette leaves of plants and seedlings of ldl1 and ldl2 single mutants and was even more elevated in the ldl1 ldl2 double mutant (Figures 6A and 6B). FWA is normally silenced in the sporophyte but is expressed in extraembryonic tissue such as endosperm (Soppe et al., 2000; Kinoshita et al., 2004). Ectopic expression of FWA during sporophyte development (as occurs in fwa epi-alleles) leads to a delay in flowering (Soppe et al., 2000; Saze et al., 2003; Kinoshita et al., 2004). We examined FWA expression in seedlings of ldl1-2 ldl2 and met1-3 (a null allele in which FWA silencing is completely abrogated; Saze et al., 2003) and found that FWA transcript levels in ldl1 ldl2 were lower than those in met1 (Figure 6B), which is consistent with the weaker late-flowering phenotype of flc ldl1 ldl2 relative to met1. We also examined the transcript levels of another heterochromatic locus, Ta2 (a retrotransposon expressed at a low level during vegetative development; Mathieu et al., 2005), in ldl1 ldl2 and found that, unlike the case for FWA, Ta2 transcript levels in ldl1 ldl2 were similar to those in Col (data not shown).
Figure 6.
Derepression of FWA in ldl1, ldl2, and ldl1 ldl2.
(A) Ectopic activation of FWA in rosette leaves of ldl1, ldl2, and ldl1 ldl2. FWA transcripts were examined by RT-PCR, and duplicate lanes for each sample represent duplicate reactions. The constitutively expressed ACTIN2 served as a control.
(B) Analysis of FWA expression in seedlings of ldl1-2 ldl2 and met1-3.
(C) Analysis of FWA expression in rosette leaves of fld.
Because FLD is a homolog of LDL1 and LDL2, we examined whether the loss of FLD also affected FWA expression. As in the wild type, FWA was not expressed in fld during vegetative development (Figure 6C); in addition, when an fld mutation was introduced into ldl1-2 mutants, FWA expression was not enhanced (data not shown), which indicates that FLD is not involved in FWA repression. We also confirmed that the FWA activation in ldl2 was indeed due to the ldl2 lesion by transgenically rescuing the repression of FWA by introducing an LDL2 construct (see Supplemental Figure 3 online). Thus, LDL1 and LDL2, but not FLD, are required to silence FWA in the sporophyte.
We found no evidence for cross-regulation between FLC and FWA. In fwa-1 epi-mutants (in which FWA is highly expressed), FLC mRNA levels were not altered relative to those in wild-type Landsberg erecta (data not shown). Also, as noted above, we did not observe FWA activation in the fld mutant, in which FLC is highly expressed (Figure 6C).
LDL1 and LDL2 Contribute to the de Novo Silencing of the FWA Transgene
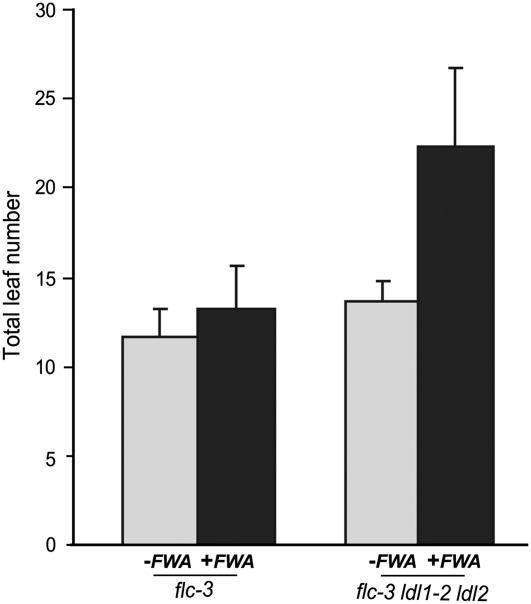
When genomic clones of FWA are introduced into wild-type plants through Agrobacterium tumefaciens–mediated transformation, the FWA transgene is normally silenced and flowering in the transformed plants is not delayed (Chan et al., 2004, 2006a). To evaluate the role of LDLs in de novo silencing of the FWA transgene, we transformed flc and flc ldl1 ldl2 mutants with the FWA transgene (Chan et al., 2004). As predicted from the results reported by Chan et al. (2004, 2006b), T1 transformants of flc mutants (predicted to behave like the wild type) flowered at a similar time as flc (Figure 7). By contrast, T1 transformants of flc ldl1 ldl2 mutants were late-flowering compared with nontransformed controls (Figure 7). Interestingly, the majority of T2 transgenic populations derived from self-pollination of the late-flowering T1 transformants flowered only slightly later than flc ldl1 ldl2 (data not shown), which indicates that over a generation the FWA transgenes became partially silenced. Together, these results suggest that LDL1 and LDL2 play a role in the de novo silencing of FWA.
Figure 7.
Flowering Times of flc and flc ldl1 ldl2 Mutants Transformed with the FWA Transgene.
Plants were grown in LDs. Total leaf number at flowering was scored. Eighteen T1 transformants were scored for each transgenic population (flc and flc ldl1 ldl2 as described in Figure 3D). The values shown are means ± sd.
Around the start site of FWA transcription, there are two sets of tandem direct repeats derived from a retrotransposon that are part of a heterochromatin domain (Soppe et al., 2000; Lippman et al., 2004). These repeats produce short interfering RNAs (siRNAs) through the 24-nucleotide siRNA pathway, which work along with the chromatin-remodeling protein DRD1 (for DEFECTIVE IN RNA-DIRECTED DNA METHYLATION1) to de novo methylate FWA newly introduced into the Arabidopsis genome (Chan et al., 2004, 2006a). Therefore, we sought to examine whether the accumulation of FWA 24-nucleotide siRNAs was disrupted in ldl1 ldl2 mutants. Previously, very low levels of FWA 24-nucleotide siRNAs were detected in wild-type plants (Lippman et al., 2004; Chan et al., 2006b; Kinoshita et al., 2007). Using radioactive probes derived from FWA tandem repeats, we detected low and similar levels of 24-nucleotide siRNAs in both Col and ldl1 ldl2 seedlings (see Supplemental Figure 4 online), which indicates that LDL1 and LDL2 are not involved in the accumulation of FWA 24-nucleotide siRNAs.
LDL1, LDL2, and FLD Are Involved in H3K4 Methylation in Target Gene Chromatin
As noted above, LDL1, LDL2, and FLD are plant homologs of human LSD1 and Drosophila SU(VAR)3-3, which specifically demethylate monomethyl and dimethyl H3K4 (Shi et al., 2004; Forneris et al., 2005; Lee et al., 2005; Rudolph et al., 2007). H3K4me2 and H3K4me3 (presumably converted from H3K4me2) are often linked to gene transcription (Martin and Zhang, 2005). Hence, we evaluated the effect of the loss of LDL activities on the state of H3K4 methylation in the chromatin of FWA and FLC by chromatin immunoprecipitation (ChIP).
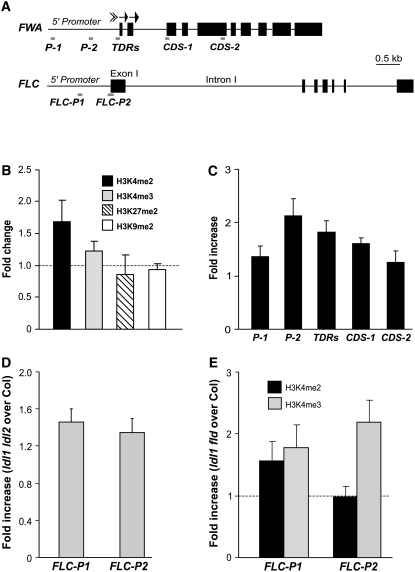
Previously, it was shown that the heterochromatin domain around the start site of FWA transcription, which is marked with repressive histone modifications and cytosine methylation, is involved directly in the silencing FWA in the sporophyte (Soppe et al., 2000; Lippman et al., 2004; Chan et al., 2006b). We first examined the chromatin state of the FWA heterochromatic domain in ldl1 ldl2 using real-time quantitative PCR to quantify genomic fragments from seedlings after ChIP. Consistent with the activation of FWA in ldl1 ldl2 seedlings (Figure 6B), the levels of H3K4me2 in FWA chromatin were increased and the levels of H3K4me3 were also moderately increased, whereas no obvious changes in the levels of H3K9me2 and H3K27me2 were observed in seedlings of ldl1 ldl2 relative to Col (Figure 8B). We further assessed the H3K4me2 state of various FWA regions upstream and downstream of the heterochromatin domain. Immediately upstream, H3K4me2 was enriched in the 5′ promoter region in ldl1 ldl2 relative to Col, whereas in regions 1.3 kb upstream and 1.7 kb downstream, H3K4me2 levels were similar in ldl1 ldl2 compared with Col (Figure 8C). Hence, LDL1 and LDL2 regulate H3K4 methylation only around and within the heterochromatin domain in FWA. Therefore, LDL1 and LDL2 are involved in controlling the state of H3K4 methylation in FWA chromatin, consistent with their putative functions as H3K4 demethylases.
Figure 8.
The Chromatin State of FLC and FWA in Col and ldl Mutants.
(A) Schematic structure of genomic FWA and FLC and the regions analyzed by real-time quantitative PCR after ChIP. The arrows represent two sets of tandem direct repeats in FWA.
(B) Histone methylation state of the heterochromatin domain in FWA chromatin in ldl1 ldl2 and Col seedlings analyzed by ChIP. Each of the immunoprecipitations was performed at least three times. The immunoprecipitated DNA (corresponding to region TDRs) was quantified by real-time PCR and subsequently normalized to an internal control (ACTIN2). The fold changes of ldl1 ldl2 over Col (i.e., the ratio of ldl1 ldl2 to Col) are shown, and the values shown are means ± sd.
(C) H3K4me2 state of various regions in genomic FWA in ldl1 ldl2 and Col. The fold enrichments of ldl1 ldl2 over Col are shown, and the values shown are means ± sd.
(D) H3K4me3 state in FLC chromatin in ldl1 ldl2 and Col seedlings. The fold enrichments of FLC in ldl1 ldl2 over Col are shown, and the values shown are means ± sd.
(E) H3K4 methylation state in FLC chromatin in ldl1 fld and Col. Black and gray bars represent enrichments of H3K4me2 and H3K4me3, respectively. The fold enrichments of ldl1 fld over Col are shown, and the values shown are means ± sd.
We also examined the state of H3K4 methylation in FLC chromatin in ldl1 ldl2 mutants. The levels of H3K4me3 in both FLC-P1 and FLC-P2 were increased in seedlings of ldl1 ldl2 relative to Col (Figure 8D), which is consistent with the moderate derepression of FLC in ldl1 ldl2 (Figure 4A). We further examined the state of H3K4 methylation in FLC chromatin in ldl1 fld mutants and found that H3K4me3 was highly enriched in FLC-P2 (around the transcription start site) and was also increased in FLC-P1 in ldl1 fld relative to Col (Figure 8E). The relative levels of H3K4me3 in FLC in ldl1 fld compared with Col were higher than those in ldl1 ldl2 compared with Col, indicating that FLD plays a more major role than LDL1 and LDL2 in regulating H3K4 methylation in FLC chromatin, consistent with the effects of lesions in these genes on flowering time and FLC expression. Furthermore, we found that the levels of H3K4me2 were also increased in a region in the 5′ promoter of FLC (FLC-P1), and surprisingly, we did not observe any change in the levels of H3K4me2 in FLC-P2 in ld11 fld relative to Col (Figure 8E). It is possible that most of the H3K4me2 in FLC-P2 may have been converted into H3K4me3, which is enriched in this region in ldl1 fld, as it was shown recently that a mammalian H3K4 methyltransferase specifically converts H3K4me2 into H3K4me3 in target gene chromatin (Hayashi et al., 2005). Together, these data indicate that LDL1, LDL2, and FLD are involved in controlling the H3K4 methylation levels of FLC chromatin.
LDL1 and LDL2 Are Required for Full-Level DNA Methylation on FWA
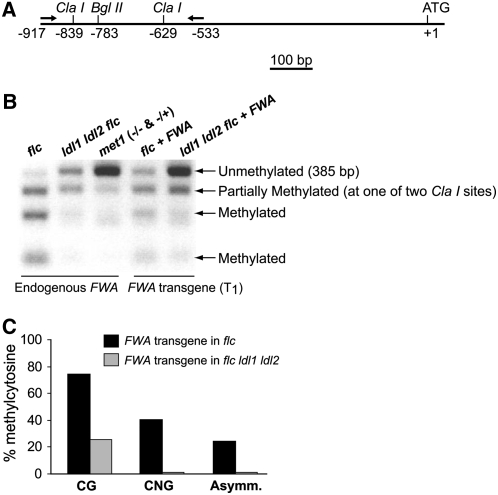
DNA methylation on the heterochromatic region consisting of tandem direct repeats in the FWA locus is involved in its silencing (Soppe et al., 2000; Lippman et al., 2004; Chan et al., 2006b). Hence, it was of interest to examine the state of cytosine methylation of the endogenous FWA and the FWA transgenes introduced into ldl1 ldl2 double mutants. Using a bisulfite PCR/restriction enzyme assay as described by Chan et al. (2006b), regions of the tandem repeats were amplified by PCR, followed by ClaI restriction digestion. CpG methylation protects ClaI restriction sites from bisulfite conversion, thus allowing digestion by ClaI after bisulfite treatment. In the control (flc mutant) background, endogenous FWA was methylated, whereas FWA had reduced methylation in ldl1 ldl2 flc mutants (Figure 9B). As predicted from the results reported by Chan et al. (2004, 2006b), cytosines in the context of CpG in FWA transgenes (in T1 transformants) were effectively de novo methylated in the flc background; by contrast, the majority of CpGs in FWA transgenes were not methylated in ldl1 ldl2 flc mutants (Figures 9B and 9C). Previously, it was shown that the establishment of CpG methylation on FWA transgenes is blocked in mutants in which DRM2 (for DOMAIN-REARRANGED METHYLASE2, a de novo DNA methyltransferase) is mutated (Cao and Jacobsen, 2002). It is noteworthy that in ldl1 ldl2 double mutants, de novo CpG methylation is not affected as strongly as in drm2. Histone modifications often affect cytosine methylation at CpNG and at asymmetric sites (CpHH, where H is A, C, or T) (Aufsatz et al., 2002; Chan et al., 2005; Ebbs and Bender, 2006); therefore, we also determined the levels of methylated cytosines at these two sites in FWA transgenes using bisulfite genomic sequencing. Consistent with the previous findings of Chan et al. (2004, 2006b), ∼40% of CpNG and 25% of CpHH in FWA transgenes were methylated in the control background, whereas both CpNG and CpHH methylation were nearly completely eliminated in ldl1 ldl2 flc (Figure 9C). Hence, LDL1 and LDL2 are essential for non-CpG methylation on FWA trangenes. Therefore, LDL1 and LDL2 not only are required for full-level DNA methylation on endogenous FWA but also are essential for de novo non-CpG methylation on FWA newly introduced into the Arabidopsis genome.
Figure 9.
Methylation Patterns of Endogenous FWA and the FWA Transgene.
(A) Schematic drawing of 5′ FWA. Arrows depict the forward and reverse primers used to amplify the 5′ region of FWA.
(B) Methylation patterns of endogenous FWA and the FWA transgene. Genomic DNA was treated with bisulfite; subsequently, part of the tandem repeats in the 5′ region of FWA was amplified by PCR and followed by ClaI restriction digestion. CpG methylation prevents ClaI restriction sites from bisulfite conversion, thus allowing digestion by ClaI after bisulfite treatment. To analyze the FWA transgene, genomic DNA was first digested with BglII to destroy the endogenous FWA.
(C) FWA transgene (T1) cytosine methylation in CpG, CpNG, and asymmetric sites in flc and flc ldl1 ldl2.
DISCUSSION
Our studies reveal that Arabidopsis relatives of the human histone demethylase LSD1 (LDL1, LDL2, and FLD) reduce the levels of H3K4 methylation in FWA and FLC chromatin and act to repress the expression of these two genes. FLD, LDL1, and LDL2 act in partial redundancy to repress FLC expression, whereas LDL1 and LDL2 act independently of FLD to repress FWA. That different members of this Arabidopsis histone demethylase family have different target preferences may represent specialization related to the different regulatory strategies governing FLC and FWA expression.
FLC is preferentially expressed in shoot and root apical regions throughout vegetative development, and the levels of FLC must be precisely controlled for specific flowering behaviors to be achieved. FLC is repressed by a pathway that monitors seasonal change (vernalization), and the vernalization-mediated repressed state of FLC is reset each generation (Boss et al., 2004; Sung and Amasino, 2005). Autonomous pathway regulators (e.g., FLD and FVE) constitutively repress, but do not silence, FLC expression (i.e., in the presence of these repressors, FLC is still expressed at a low level) (Michaels and Amasino, 2001). Furthermore, DNA methylation does not appear to play a direct role in FLC regulation (Finnegan et al., 2005). Our studies show that FLC is repressed by FLD and its close homologs LDL1 and LDL2. These putative H3K4 demethylases may be part of a corepressor complex involved in FLC repression.
While this article was in preparation, Krichevsky et al. (2007) reported that SWP1 (for SWIRM domain PAO protein1), the gene referred to herein as LDL1, represses FLC expression. They reported that SWP1/LDL1 interacts with the putative H3K9 methyltransferase SUVR5/CZS [for Su(var)3-9–Related5/C2H2 zinc finger-SET domain HMT] and that lower levels of H3K9me2 around the transcription start site of FLC are detected in the wild-type Col but not in swp1/ldl1 and suvr5/czs mutants. Other studies did not reproducibly detect H3K9me2 above the ChIP background levels in the 5′ region of FLC (upstream of the start codon) in Arabidopsis accessions such as Landsberg erecta and FRI-Col without vernalization treatment (Bastow et al., 2004; Liu et al., 2004; Sung and Amasino, 2004). We also examined the levels of H3K9me2 in the 5′ region of FLC in Col and ldl1 ldl2 seedlings by ChIP-PCR and did not observe any difference in the levels of H3K9me2 between Col and ldl1 ldl2 (see Supplemental Figure 5 online). The basis for these different results is not known, and it is intriguing that a SWP1/LDL-containing corepressor complex may work along with SUVR5 to generate a repressive chromatin environment at FLC.
As described above, FLC is repressed, but not silenced, in Arabidopsis lacking FRI. In contrast with FLC, FWA is effectively silenced in the sporophyte (as noted in Figure 6A, we could not detect FWA transcripts in sporophyte tissues after 40 cycles of PCR). Sporophytic silencing of FWA requires cytosine methylation (catalyzed by the MET1 methyltransferase at CpG sites) of the heterochromatin domain consisting of tandem direct repeats in its 5′ transcribed region (Saze et al., 2003). In addition, DDM1, a SWI2/SNF2-like chromatin-remodeling enzyme, is also required for full-level cytosine methylation on FWA (Jeddeloh et al., 1999; Soppe et al., 2000). FWA is ectopically activated in both met1 and ddm1 mutants because of the disruption of the maintenance of cytosine methylation and heterochromatin at the FWA locus (Soppe et al., 2000; Saze et al., 2003).
Our studies show that LDL1 and LDL2 are also required to maintain the silencing of endogenous FWA. Recently, it was shown that the Drosophila LSD1 homolog not only represses the expression of euchromatic genes but also functions in heterochromatin formation during embryonic development by blocking the expansion of H3K4 methylation from euchromatin into heterochromatin (Rudolph et al., 2007). Drosophila heterochromatin is defined by repressive histone modifications (Ebert et al., 2006), and CpG methylation is absent in Drosophila (only low amounts of CpT/A methylation have been detected in young embryos) (Lyko et al., 2000). Arabidopsis heterochromatic regions are often marked with cytosine methylation (mainly CpG methylation) in addition to repressive histone modifications (Chan et al., 2005). Although heterochromatic regions in these two organisms bear different features, our observations that H3K4me2 is enriched in and around the FWA heterochromatin domain in ldl1 ldl2 relative to the wild type (Figure 8C) and that cytosine methylation in this domain is also partially lost in ldl1 ldl2 (Figure 9B) lead us to speculate that LDL1 and LDL2 may also function in blocking the spread of H3K4 methylation from euchromatin to the heterochromatic region of endogenous FWA chromatin, and thus maintain this domain in a state of heterochromatin. It is intriguing that these putative histone demethylases may also be involved in maintaining DNA methylation on FWA. Recently, it was shown that the Polycomb group protein Enhancer of Zeste homolog2, a H3K27 methyltransferase, directly controls DNA methylation on target genes in mammalian cells (Vire et al., 2006). We speculate that removal of the activating histone methylations at H3K4 is perhaps required for DNA methyltransferases (e.g., MET1) to methylate cytosines in the heterochromatic FWA region.
De novo silencing of FWA newly introduced into the Arabidopsis genome through Agrobacterium-mediated transformation involves DNA methylation and heterochromatin formation directed by 24-nucleotide siRNAs and the chromatin-remodeling protein DRD1 (Chan et al., 2004, 2006a, 2006b). In this study, we have shown that LDL1 and LDL2 also play an important role in the de novo silencing of FWA newly introduced into Arabidopsis. Indeed, recent work in mammalian systems provides a framework for the role of LDLs in this process. DNMT3L (a mammalian DNA methyltransferase–like protein) binds specifically to histone H3 tails with unmethylated K4 and activates the DNMT3A DNA methyltransferase, resulting in de novo DNA methylation (Jia et al., 2007; Ooi et al., 2007). It is likely that the FWA transgene, newly introduced through Agrobacterium-mediated transformation, may acquire a chromatin state with active H3K4 methylation and that this H3K4 methylation must be removed for siRNA-directed de novo DNA methylation and silencing.
Human LSD1 is a component of several distinct corepressor complexes, such as the BRAF-HDAC complex (Hakimi et al., 2002) and the C-terminal binding protein complex (Shi et al., 2003). It is possible that different complexes with H3K4-demethylating activity are responsible for FLC and FWA repression and that different members of the plant H3K4 demethylase family are preferentially incorporated into different complexes.
That LDL1, LDL2, and FLD are close relatives of LSD1 (Figures 1 and 2) and that H3K4me2 is enriched in FWA chromatin in ldl1 ldl2 relative to the wild type indicate that these LDLs are likely to be H3K4 demethylases. Using recombinant FLD proteins purified from bacteria, we did not detect apparent demethylation activities of H3K4me2 and H3K9me2 with these proteins alone (data not shown). These in vitro enzymatic results are not surprising in light of the recent characterization of human LSD1 (Lee et al., 2005; Shi et al., 2005). Although recombinant LSD1 alone can demethylate H3K4 on free histones, the activities are much lower compared with those of LSD1-containing complexes (Lee et al., 2005); moreover, recombinant LSD1 is unable to demethylate H3K4 on nucleosomes, whereas the LSD1 complexes readily demethylate nucleosomes, indicating that cofactors associated with LSD1 stimulate its demethylation activities (Lee et al., 2005; Shi et al., 2005). In vivo LDL/FLD-containing complexes may possess the H3K4 demethylation activities; purifying these complexes from Arabidopsis will be essential to address the enzymatic characteristics of FLD/LDLs.
METHODS
Plant Materials
Arabidopsis thaliana ldl1-1, ldl1-2, and ldl2 were isolated from the SALK collection (Alonso et al., 2003). fld-3 (He et al., 2003), flc-3 (Michaels and Amasino, 1999), fwa-1 (Soppe et al., 2000), and met1-3 (Saze et al., 2003) were described previously; pooled plants of met1-3/+ and met1-3/met1-3 (selfed progeny of a met1-3/+ heterozygote) were used in this study, as met1-3 homozygotes are genetically unstable.
RNA Isolation
Total RNAs from 10-d-old seedlings and expanded rosette leaves of adult plants were extracted with the RNeasy plant mini kit (Qiagen) or Tri reagent (Sigma-Aldrich) and were treated with RNase-free DNase according to the manufacturer's instructions (Qiagen).
Quantitative RT-PCR Assays
Real-time quantitative PCR was performed on an ABI Prism 7900HT sequence detection system using SYBR Green PCR master mix (Applied Biosystems). PCR proceeded as follows: 50°C (2 min), 95°C (10 min), and 40 cycles of 95°C (15 s) and 60°C (60 s); subsequently, a melting curve was generated to verify the specificity of the amplified fragment. Each sample was quantified at least in triplicate and normalized using TUB2 (At_5g62690) as the control. Primers used are specified in Supplemental Table 1 online.
Analysis of FWA Transcripts in ldl by RT-PCR
Poly(A)+ RNAs were purified from total RNAs extracted from rosette leaves of adult plants with the Oligotex kit according to the manufacturer's instructions (Qiagen). cDNAs were reverse-transcribed from poly(A)+ RNAs with Moloney murine leukemia virus reverse transcriptase (Promega), and cDNAs of FWA were amplified in a 20-μL volume with 40 cycles of 94°C (30 s), 60°C (30 s), and 72°C (30 s). The primer pair used is specified in Supplemental Table 1 online.
siRNA Analysis by RNA Gel Blot
Using the Qiagen Midi kit, low-molecular-weight RNAs were isolated from total RNAs extracted with Tri reagent (Sigma-Aldrich). RNA gel blot analysis was performed as described previously (Kinoshita et al., 2007). Briefly, ∼30 μg of RNAs was fractionated on a 17% polyacrylamide gel containing 7 M urea, transferred to a Hybond N+ nylon membrane (Amersham) via electroblotting, and hybridized to 32P-labeled probes covering the FWA repeat sequences (labeled via random priming) in PerfectHyb Plus buffer (Sigma-Aldrich). The 24-nucleotide RNA marker was described previously (Xie et al., 2004).
Plasmid Construction
To construct LDL1-GUS, a 1.3-kb LDL1 genomic fragment including a 0.8-kb 5′ promoter plus a 0.5-kb coding region (CDS) was inserted into the pBGWFS7 vector (Karimi et al., 2005) via Gateway technology (Invitrogen); LDL1 CDS was in-frame with the downstream GUS reporter gene, although they were not fused directly. To construct LDL2-GUS, a 3.5-kb LDL2 genomic fragment including a 3.1-kb 5′ promoter plus a 0.4-kb CDS was inserted into the pBGWFS7 vector via Gateway technology; LDL2 CDS was in-frame with the downstream GUS reporter gene.
Knockdown of LDL2 via Double-Stranded RNA Interference
A 223-bp LDL2-specific fragment (from +1979 to +2201 of LDL2 cDNA; the transcription start point was +1) was used to create a hairpin RNA by the AGRICOLA consortium (Hilson et al., 2004); the resulting binary plasmid was introduced into Agrobacterium tumefaciens strain GV3101 carrying pMP90 and pSOUP helper plasmids through electroporation and subsequently was introduced into ldl1-2 fld-3 mutants by the floral dip method (Clough and Bent, 1998).
ChIP and Real-Time Quantitative PCR Analysis
The ChIP experiments were performed as described previously (Johnson et al., 2002) using 10-d-old seedlings. Anti-dimethyl-histone H3 (Lys 4), anti-trimethyl-histone H3 (Lys 4), anti-dimethyl-histone H3 (Lys 27), and anti-dimethyl-histone H3 (Lys 9) were purchased from Upstate Biotechnology. The amounts of genomic DNA immunoprecipitated were determined by real-time quantitative PCR. Quantitative measurements of enrichments from FWA genomic regions and ACTIN2 (At_3g18780) were performed on an ABI Prism 7900HT sequence detection system using TaqMan MGB probes (FAM dye–labeled) made by Applied Biosystems (the TaqMan gene expression assay identifier for ACTIN2 was At02329915_s1) according to the manufacturer's instructions. Relative enrichments of various FWA regions in ldl1 ldl2 over Col were calculated after normalization to ACTIN2; each of the immunoprecipitations was replicated at least three times (ChIP experiments with anti-dimethyl H3K4 were performed twice). Quantitative measurements of enrichments from regions of FLC and TUB2 (At_5g62690) were performed using SYBR Green PCR master mix (Applied Biosystems); relative enrichments of FLC in ldl1 fld or ldl1 ldl2 over Col were calculated after normalization to TUB2; each of the immunoprecipitations was repeated once, and each sample was quantified at least in triplicate.
Bisulfite PCR/Restriction Enzyme Assay and Bisulfite Genomic Sequencing
Approximately 2 μg of genomic DNA was treated with bisulfite as described previously (Soppe et al., 2000; Grunau et al., 2001); subsequently, part of the tandem repeats in the 5′ region of FWA (the bottom strand) was amplified by PCR, followed by ClaI restriction digestion as described by Chan et al. (2006b). Briefly, two nested pairs of primers (5′-CACCATTAATCCAAATACTATTTAATTATT-3′ and 5′-GGGATATTTATTGTAGAGTTAATATAATATTTTT-3′; 5′-CAAATACTATTTAATTATTTAAAATTACTTTTA-3′ and 5′-GGGAATTAAAATTATTTTTTAAATAAAATGTAAA-3′) were used to amplify FWA. PCR products were separated on an agarose gel, and the fragments with the expected size were recovered from the gel and followed by ClaI digestion. To analyze the FWA transgene in T1 transformants of flc and flc ldl1 ldl2 by FWA, genomic DNA was first digested with BglII to destroy the endogenous FWA; the BglII restriction site in the 5′ region of the FWA transgene was eliminated (Chan et al., 2006b). PCR fragments amplified from FWA transgenes (T1) were cloned into pGEM-T Easy vector (Promega) and sequenced further.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At_1g62830 (LDL1), At_3g13682 (LDL2), and At_4g16310 (LDL3).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Repression of FLC in ldl1 fld by Vernalization
Supplemental Figure 2. Relative FLM mRNA Levels in Col and ldl1 ldl2 Seedlings Quantified by Real-Time PCR.
Supplemental Figure 3. Complementation of the ldl2 Mutation.
Supplemental Figure 4. FWA siRNAs in Col and ldl1 ldl2 Analyzed by RNA Gel Blotting.
Supplemental Figure 5. Levels of H3K9me2 in Col and ldl1 ldl2 Seedlings Examined by ChIP-PCR.
Supplemental Table 1. Sequences of Primers Used in RT-PCR and ChIP-PCR Experiments.
Supplementary Material
Acknowledgments
We thank Simon Chan for critical comments and insightful suggestions, Frederic Berger for insightful discussions, Simon Chan and Steve Jacobsen for providing the FWA transgene, Tran Duc Long for the phylogenetic analysis, anonymous reviewers for their insightful comments, and the AGRICOLA consortium for providing the double-stranded RNA interference plasmid targeting LDL2. This work was supported by Academic Research Fund (AcRF) grants from the National University of Singapore and the Singapore Ministry of Education (AcRF Tier 2) and by the Temasek Life Sciences Laboratory to Y.H. Work in R.M.A.'s laboratory was supported by the College of Agricultural and Life Sciences and the Graduate School of the University of Wisconsin and by National Science Foundation Grant 0209786.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Yuehui He (dbshy@nus.edu.sg or yuehui@tll.org.sg).
Online version contains Web-only data.
References
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Alvarez-Venegas, R., and Avramova, Z. (2005). Methylation patterns of histone H3 Lys 4, Lys 9 and Lys 27 in transcriptionally active and inactive Arabidopsis genes and in atx1 mutants. Nucleic Acids Res. 33 5199–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufsatz, W., Mette, M.F., van der Winden, J., Matzke, M., and Matzke, A.J. (2002). HDA6, a putative histone deacetylase needed to enhance DNA methylation induced by double-stranded RNA. EMBO J. 21 6832–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausin, I., Alonso-Blanco, C., Jarillo, J.A., Ruiz-Garcia, L., and Martinez-Zapater, J.M. (2004). Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat. Genet. 36 162–166. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, S., Sureshkumar, S., Lempe, J., and Weigel, D. (2006). Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2 e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow, R., Mylne, J.S., Lister, C., Lippman, Z., Martienssen, R.A., and Dean, C. (2004). Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427 164–167. [DOI] [PubMed] [Google Scholar]
- Boss, P.K., Bastow, R.M., Mylne, J.S., and Dean, C. (2004). Multiple pathways in the decision to flower: Enabling, promoting, and resetting. Plant Cell 16 (suppl.): S18–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X.F., and Jacobsen, S.E. (2002). Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr. Biol. 12 1138–1144. [DOI] [PubMed] [Google Scholar]
- Chan, S.W., Henderson, I.R., and Jacobsen, S.E. (2005). Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 6 351–360. [DOI] [PubMed] [Google Scholar]
- Chan, S.W., Henderson, I.R., Zhang, X., Shah, G., Chien, J.S., and Jacobsen, S.E. (2006. a). RNAi, DRD1, and histone methylation actively target developmentally important non-CG DNA methylation in Arabidopsis. PLoS Genet. 2 e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S.W., Zhang, X., Bernatavichute, Y.V., and Jacobsen, S.E. (2006. b). Two-step recruitment of RNA-directed DNA methylation to tandem repeats. PLoS Biol. 4 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S.W., Zilberman, D., Xie, Z., Johansen, L.K., Carrington, J.C., and Jacobsen, S.E. (2004). RNA silencing genes control de novo DNA methylation. Science 303 1336. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Ebbs, M.L., and Bender, J. (2006). Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18 1166–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert, A., Lein, S., Schotta, G., and Reuter, G. (2006). Histone modification and the control of heterochromatic gene silencing in Drosophila. Chromosome Res. 14 377–392. [DOI] [PubMed] [Google Scholar]
- Finnegan, E.J., Kovac, K.A., Jaligot, E., Sheldon, C.C., Peacock, W. J., and Dennis, E.S. (2005). The downregulation of FLOWERING LOCUS C (FLC) expression in plants with low levels of DNA methylation and by vernalization occurs by distinct mechanisms. Plant J. 44 420–432. [DOI] [PubMed] [Google Scholar]
- Forneris, F., Binda, C., Vanoni, M.A., Mattevi, A., and Battaglioli, E. (2005). Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 579 2203–2207. [DOI] [PubMed] [Google Scholar]
- Grunau, C., Clark, S.J., and Rosenthal, A. (2001). Bisulfite genomic sequencing: Systematic investigation of critical experimental parameters. Nucleic Acids Res. 29 E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi, M.A., Bochar, D.A., Chenoweth, J., Lane, W.S., Mandel, G., and Shiekhattar, R. (2002). A core-BRAF35 complex containing histone deacetylase mediates repression of neuronal-specific genes. Proc. Natl. Acad. Sci. USA 99 7420–7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, K., Yoshida, K., and Matsui, Y. (2005). A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438 374–378. [DOI] [PubMed] [Google Scholar]
- He, Y., and Amasino, R.M. (2005). Role of chromatin modification in flowering-time control. Trends Plant Sci. 10 30–35. [DOI] [PubMed] [Google Scholar]
- He, Y., Doyle, M.R., and Amasino, R.M. (2004). PAF1-complex-mediated histone methylation of FLOWERING LOCUS C chromatin is required for the vernalization-responsive, winter-annual habit in Arabidopsis. Genes Dev. 18 2774–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y., Michaels, S.D., and Amasino, R. (2003). Regulation of flowering time by histone acetylation in Arabidopsis. Science 302 1751–1754. [DOI] [PubMed] [Google Scholar]
- Hilson, P., et al. (2004). Versatile gene-specific sequence tags for Arabidopsis functional genomics: Transcript profiling and reverse genetics applications. Genome Res. 14 2176–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey, G.W., Wang, Y., Russanova, V.R., Hirai, T., Qin, J., Nakatani, Y., and Howard, B.H. (2001). Stable histone deacetylase complexes distinguished by the presence of SANT domain proteins CoREST/kiaa0071 and Mta-L1. J. Biol. Chem. 276 6817–6824. [DOI] [PubMed] [Google Scholar]
- Ikeda, Y., Kobayashi, Y., Yamaguchi, A., Abe, M., and Araki, T. (2007). Molecular basis of late-flowering phenotype caused by dominant epi-alleles of the FWA locus in Arabidopsis. Plant Cell Physiol. 48 205–220. [DOI] [PubMed] [Google Scholar]
- Jeddeloh, J.A., Stokes, T.L., and Richards, E.J. (1999). Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat. Genet. 22 94–97. [DOI] [PubMed] [Google Scholar]
- Jia, D., Jurkowska, R.Z., Zhang, X., Jeltsch, A., and Cheng, X. (2007). Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature 449 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L., Cao, X., and Jacobsen, S. (2002). Interplay between two epigenetic marks. DNA methylation and histone H3 lysine 9 methylation. Curr. Biol. 12 1360–1367. [DOI] [PubMed] [Google Scholar]
- Karimi, M., De Meyer, B., and Hilson, P. (2005). Modular cloning in plant cells. Trends Plant Sci. 10 103–105. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., Hyun, Y., Park, J.Y., Park, M.J., Park, M.K., Kim, M.D., Lee, M.H., Moon, J., Lee, I., and Kim, J. (2004). A genetic link between cold responses and flowering time through FVE in Arabidopsis thaliana. Nat. Genet. 36 167–171. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., Miura, A., Choi, Y., Kinoshita, Y., Cao, X., Jacobsen, S.E., Fischer, R.L., and Kakutani, T. (2004). One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303 521–523. [DOI] [PubMed] [Google Scholar]
- Kinoshita, Y., Saze, H., Kinoshita, T., Miura, A., Soppe, W.J., Koornneef, M., and Kakutani, T. (2007). Control of FWA gene silencing in Arabidopsis thaliana by SINE-related direct repeats. Plant J. 49 38–45. [DOI] [PubMed] [Google Scholar]
- Krichevsky, A., Gutgarts, H., Kozlovsky, S.V., Tzfira, T., Sutton, A., Sternglanz, R., Mandel, G., and Citovsky, V. (2007). C2H2 zinc finger-SET histone methyltransferase is a plant-specific chromatin modifier. Dev. Biol. 303 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.G., Wynder, C., Bochar, D.A., Hakimi, M.A., Cooch, N., and Shiekhattar, R. (2006). Functional interplay between histone demethylase and deacetylase enzymes. Mol. Cell. Biol. 26 6395–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.G., Wynder, C., Cooch, N., and Shiekhattar, R. (2005). An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 437 432–435. [DOI] [PubMed] [Google Scholar]
- Lippman, Z., et al. (2004). Role of transposable elements in heterochromatin and epigenetic control. Nature 430 471–476. [DOI] [PubMed] [Google Scholar]
- Liu, J., He, Y., Amasino, R., and Chen, X. (2004). siRNAs targeting an intronic transposon in the regulation of natural flowering behavior in Arabidopsis. Genes Dev. 18 2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyko, F., Ramsahoye, B.H., and Jaenisch, R. (2000). DNA methylation in Drosophila melanogaster. Nature 408 538–540. [DOI] [PubMed] [Google Scholar]
- Martin, C., and Zhang, Y. (2005). The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6 838–849. [DOI] [PubMed] [Google Scholar]
- Mathieu, O., Probst, A.V., and Paszkowski, J. (2005). Distinct regulation of histone H3 methylation at lysines 27 and 9 by CpG methylation in Arabidopsis. EMBO J. 24 2783–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger, E., Wissmann, M., Yin, N., Muller, J.M., Schneider, R., Peters, A.H., Gunther, T., Buettner, R., and Schule, R. (2005). LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437 436–439. [DOI] [PubMed] [Google Scholar]
- Michaels, S., and Amasino, R. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R. (2001). Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradov, A., Cremer, F., and Coupland, G. (2002). Control of flowering time: Interacting pathways as a basis for diversity. Plant Cell 14 (suppl.): S111–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, H.H., Robert, F., Young, R.A., and Struhl, K. (2003). Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11 709–719. [DOI] [PubMed] [Google Scholar]
- Oh, S., Zhang, H., Ludwig, P., and van Nocker, S. (2004). A mechanism related to the yeast transcriptional regulator Paf1c is required for expression of the Arabidopsis FLC/MAF MADS box gene family. Plant Cell 16 2940–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi, S.K., Qiu, C., Bernstein, E., Li, K., Jia, D., Yang, Z., Erdjument-Bromage, H., Tempst, P., Lin, S.P., Allis, C.D., Cheng, X., and Bestor, T.H. (2007). DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448 714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill, J., Laurie, R., and Macknight, R. (2004). It's time to flower: The genetic control of flowering time. Bioessays 26 363–373. [DOI] [PubMed] [Google Scholar]
- Rudolph, T., Yonezawa, M., Lein, S., Heidrich, K., Kubicek, S., Schafer, C., Phalke, S., Walther, M., Schmidt, A., Jenuwein, T., and Reuter, G. (2007). Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol. Cell 26 103–115. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa, H., Schneider, R., Bannister, A.J., Sherriff, J., Bernstein, B.E., Emre, N.C., Schreiber, S.L., Mellor, J., and Kouzarides, T. (2002). Active genes are tri-methylated at K4 of histone H3. Nature 419 407–411. [DOI] [PubMed] [Google Scholar]
- Saze, H., Mittelsten Scheid, O., and Paszkowski, J. (2003). Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat. Genet. 34 65–69. [DOI] [PubMed] [Google Scholar]
- Schneider, R., Bannister, A.J., Myers, F.A., Thorne, A.W., Crane-Robinson, C., and Kouzarides, T. (2004). Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat. Cell Biol. 6 73–77. [DOI] [PubMed] [Google Scholar]
- Scortecci, K.C., Michaels, S.D., and Amasino, R.M. (2001). Identification of a MADS-box gene, FLOWERING LOCUS M, that represses flowering. Plant J. 26 229–236. [DOI] [PubMed] [Google Scholar]
- Sheldon, C.C., Burn, J.E., Perez, P.P., Metzger, J., Edwards, J.A., Peacock, W.J., and Dennis, E.S. (1999). The FLF MADS box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., Lan, F., Matson, C., Mulligan, P., Whetstine, J.R., Cole, P.A., Casero, R.A., and Shi, Y. (2004). Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119 941–953. [DOI] [PubMed] [Google Scholar]
- Shi, Y., Sawada, J., Sui, G., El Affar, B., Whetstine, J.R., Lan, F., Ogawa, H., Luke, M.P., Nakatani, Y., and Shi, Y. (2003). Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422 735–738. [DOI] [PubMed] [Google Scholar]
- Shi, Y.J., Matson, C., Lan, F., Iwase, S., Baba, T., and Shi, Y. (2005). Regulation of LSD1 histone demethylase activity by its associated factors. Mol. Cell 19 857–864. [DOI] [PubMed] [Google Scholar]
- Soppe, W.J., Jacobsen, S.E., Alonso-Blanco, C., Jackson, J.P., Kakutani, T., Koornneef, M., and Peeters, A.J. (2000). The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol. Cell 6 791–802. [DOI] [PubMed] [Google Scholar]
- Sung, S., and Amasino, R.M. (2004). Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427 159–164. [DOI] [PubMed] [Google Scholar]
- Sung, S., and Amasino, R.M. (2005). Remembering winter: Toward a molecular understanding of vernalization. Annu. Rev. Plant Biol. 56 491–508. [DOI] [PubMed] [Google Scholar]
- Sung, S., Schmitz, R.J., and Amasino, R. (2006). A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev. 20 3244–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vire, E., et al. (2006). The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439 871–874. [DOI] [PubMed] [Google Scholar]
- Xie, Z., Johansen, L.K., Gustafson, A.M., Kasschau, K.D., Lellis, A.D., Zilberman, D., Jacobsen, S.E., and Carrington, J.C. (2004). Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2 e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.