Abstract
Objective To determine the impact of the European Union’s Clinical Trials Directive on the number of academic drug trials carried out in Denmark.
Design Retrospective review of applications for drug trials to the Danish Medicines Agency, 1993-2006.
Review methods Applications for drug trials for alternate years were classified as academic or commercial trials. A random subset of academic trials was reviewed for number of participants in and intended monitoring of the trials.
Results Academic and commercial drug trials showed an identical steady decline from 1993 to 2006 and no noticeable change after 2004 when good clinical practice became mandatory for academic trials.
Conclusion The Clinical Trials Directive introduced in May 2004 to ensure good clinical practice for academic drug trials was not associated with a decline in research activity in Denmark; presumably because good clinical practice units had already been in place in Danish universities since 1999. With such an infrastructure academic researchers can do drug trials under the same regulations as drug companies.
Introduction
The European Clinical Trials Directive1 came into force on 1 May 2004. The directive made good clinical practice mandatory for all clinical drug trials, including academic trials, and not just commercial trials as previously.
The good clinical practice quality standard includes a large amount of paperwork, with documentation, monitoring, and audits, thereby increasing demands on resources. This demand raised a debate that predicted the decline or even disappearance of academic clinical research.2,3,4 The dissatisfaction among academics was due to a general perception that the good clinical practice quality standard is bureaucratic and time consuming and does not ensure higher quality.
We studied all applications for clinical trials submitted from 1993 to 2006 to the Danish Medicines Agency to test the hypothesis of an immediate and noticeable decline in the number of academic clinical trials after the enforcement of the Clinical Trials Directive.
Methods
We studied paper and electronic files from the Danish Medicines Agency for alternate years from 1993 to 2005. Data from the 2005 European clinical trials database5 agreed with the electronic and paper files therefore we included the data from 2006 to guarantee uniformity. Two researchers (LB and CH) determined the rates of monitoring and publication for a random sample of approved academic clinical trials. Both researchers were trained to ensure good inter-rater agreement. Ratings by the Swedish and Norwegian competent authorities for drug approval were comparable.
We defined clinical applications from academic researchers on the basis of the data as well as the publication rights being the property of publicly employed researchers and the absence of a pharmaceutical company named on the first page of the trial protocol.
Clinical applications from the commercial sector were defined on the basis of a submission by a pharmaceutical company, a pharmaceutical company named on the first page of the trial protocol, or trial data or publication rights seeming to be the property of a pharmaceutical company. We classed those applications that did not fall into either category as non-classifiable—for example, missing files or missing protocols.
We reviewed a random subset of academic clinical trials for intended monitoring and number of participants on alternate years from 1993 to 2003 and all academic clinical trials after 1 May 2004, when the European Clinical Trials Directive for the good clinical practice quality standard in academic trials came into force. The publication rate of approved trials was determined by a Medline search of investigators’ names and drug names (preferably by MeSH term).
Results
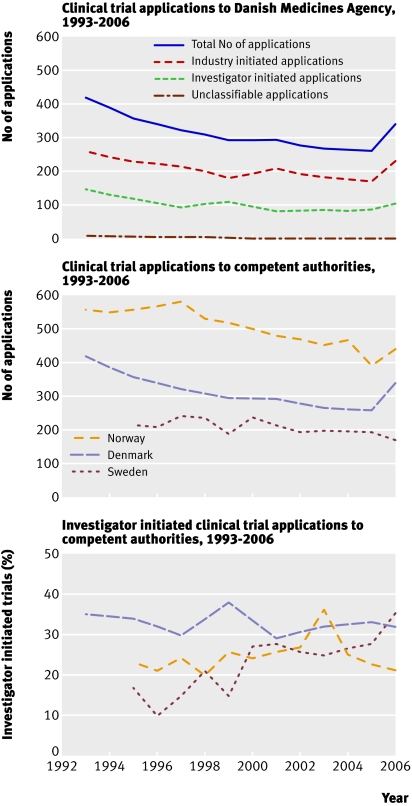
Applications for clinical trials to the Danish Medicines Agency from 1993 to 2006 showed a continual decline, from 417 in 1993 to 260 in 2005 and an increase to 336 in 2006. Applications from academic researchers decreased from 147 in 1993 to 86 in 2005, increasing to 107 in 2006, and those from the commercial sector decreased from 262 in 1993 to 174 in 2005, increasing to 229 in 2006 (figure). These figures were also compared with applications to competent authorities for drug approval in Norway and Sweden (figure).
Number of applications for clinical trials from academic researchers and commercial sector in Denmark compared with applications to Danish, Norwegian, and Swedish competent authorities for drug approval, 1993-2006. Swedish and Norwegian data are used with permission of the respective competent authorities
The median number of participants in academic, national and international (multicentre), trials did not change during the study period. The table also provides 10th and 90th centiles for these trials. The percentage of applications by academic researchers remained at about one third throughout the study (figure).
Number of participants in Danish and international academic trials
| Trials | 1993 | 1995 | 1997 | 1999 | 2001 | 2003 | 2004 | 2005 |
|---|---|---|---|---|---|---|---|---|
| Danish trials*: | ||||||||
| Median No | 30 | 30 | 30 | 31 | 24 | 30 | 40 | 50 |
| 10th-90th centile | 10-193 | 10-112 | 10-120 | 10-101 | 10-174 | 10-126 | 15-73 | 15-154 |
| International trials†: | ||||||||
| Median No | 200 | 30 | 340 | 115 | 300 | 300 | 70 | 300 |
| 10th-90th centile | 60-500 | 30-30 | 40-700 | 30-660 | 45-1500 | 44-9000 | 18-10000 | 58-2640 |
*21-66 trials.
†1-18 trials.
The rates of intended monitoring and publication were estimated for 386 approved academic clinical trials selected at random. The percentage of trials intended to be monitored increased significantly from 4% in 1993 to 33% in 2003 (P<0.05). From 1 May to 31 December 2004 89% of protocols stated that monitoring was intended, in 2005 this value was 98%. The publication rate of approved clinical trials by academic researchers was about one third until 2001, and no publications were found for trials approved after 2004.
Discussion
No decline was shown in the number of Danish drug trials by academic researchers or the commercial sector after 1 May 2004 when the European Clinical Trials Directive for good clinical practice came into force. Since 1993, however, a steady decreasing trend has been observed in numbers of clinical trials. The increase in clinical trials in 2006 is unexplained and needs to be followed up to determine whether this is the start of a trend or a coincidence.
Adherence to the directive was evaluated from the number of trials intended to be monitored; 89% were monitored in 2004 compared with 98% in 2005. Therefore non-adherence to the directive can be dismissed as a cause for lack of effect. The number of participants in academic trials did not noticeably increase despite the inclusion of a few international trials with substantial numbers. The decline therefore seems not to be a drift from many small trials to a few large ones. International academic trials were few but included more participants; it was not possible for us to identify how many of these participants were Danish, but they do not run into thousands.
A survey of investigator initiated trials at a major university hospital in Austria found a 66% decrease in academic research after the introduction of the Clinical Trials Directive whereas the sponsored trials remained constant.6 Intermediary trends were observed in Norway and Sweden. This agrees with differences in the creation of an academic system for the good clinical practice quality standard.
A major difference between Denmark and Austria is that from about 2000 good clinical practice units were already established in Denmark, whereas such a system does not yet exist in Austria. In Denmark the universities and university hospitals fund good clinical practice units that provide free assistance to academic clinical researchers. The manpower invested is about five people per million population. We believe that such units and the focus on available expertise for the good clinical practice quality standard to academic researchers can explain the difference in trend of academic clinical research between Austria and Denmark.
We found no trials published earlier than four years after application; even during the observation period 1993-2006 no more than about 30% were published. The reasons for this are unknown and need to be investigated by the relevant competent authorities.
We conclude that academic researchers can match the demands for the good clinical practice quality standard that industry have adhered to for many years, but only if universities and hospitals allocate resources to good clinical practice units. Allocation or redistribution of such relatively few resources is needed to prevent the decline in drug research and should be made before meeting the demands of more resources to academic drug research.
What is already known on this topic
The good clinical practice quality standard introduced by the Clinical Trials Directive in 2004 increases the demands on resources
Academics perceive the quality standard as bureaucratic and time consuming
What this study adds
The Clinical Trials Directive for good clinical practice in academic trials did not result in a decline in research activity
Contributors: LB helped retrieve and analyse the data. CH helped analyse the data. KFB, KD, and PBA helped plan the study. LGP helped retrieve the data and plan the study. EA supervised and coordinated the study and helped retrieve the data. HEP had the original idea for the study and helped plan the study. LB, CH, and HEP drafted the paper. All authors contributed to the final writing process and data interpretation.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official J Eur Commun 2001;L121:34-44. [PubMed]
- 2.Hemminki A, Kellokumpu-Lehtinen PL. Harmful impact of EU clinical trials directive. BMJ 2006;332:501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayor S. Squeezing academic research into a commercial straitjacket. BMJ 2004;328:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morice AH. The death of academic clinical trials. Lancet 2003;361:1568. [DOI] [PubMed] [Google Scholar]
- 5.European Commission. European clinical trials database. http://eudract.emea.europa.eu
- 6.Singer E. Future of investigator initiated trials in EU academia. [Abstract.] Basic Clin Pharmacol Toxicol 2007;101(suppl 1):11 [Google Scholar]