Abstract
Spinocerebellar ataxia-1 (SCA1) is caused by the expansion of a polyglutamine repeat within the disease protein, ataxin-1. The overexpression of mutant ataxin-1 in SCA1 transgenic mice results in the formation of cytoplasmic vacuoles in Purkinje neurons (PKN) of the cerebellum. PKN are closely associated with neighboring Bergmann glia. To elucidate the role of Bergmann glia in SCA1 pathogenesis, cerebellar tissue from 7 days to 6 wks old SCA1 transgenic and wildtype mice were used. We observed that Bergmann glial S100B protein is localized to the cytoplasmic vacuoles in SCA1 PKN. These S100B positive cytoplasmic vacuoles began appearing much before the onset of behavioral abnormalities, and were negative for other glial and PKN marker proteins. Electron micrographs revealed that vacuoles have a double membrane. In the vacuoles, S100B colocalized with receptors of advanced glycation end-products (RAGE), and S100B co-immunoprecipated with cerebellar RAGE. In SCA1 PKN cultures, exogenous S100B protein interacted with the PKN membranes and was internalized. These data suggest that glial S100B though extrinsic to PKN is sequestered into cytoplasmic vacuoles in SCA1 mice at early postnatal ages. Further, S100B may be binding to RAGE on Purkinje cell membranes before these membranes are internalized.
Keywords: Purkinje cells, S100B, spinocerebellar ataxia-1, cerebellum, vacuoles, neurodegeneration, glia
INTRODUCTION
Spinocerebellar ataxia 1 (SCA1), an autosomal dominant disease, belongs to a group of trinucleotide repeat disorders of the nervous system. SCA1 is associated with progressive ataxia resulting from the loss of cerebellar Purkinje cells and neurons in the brainstem (21,40). The mutant protein ataxin-1 has an expanded polyglutamine tract (2,11,27). However, the exact function of this protein is not fully understood. Further, understanding the precise pathogenic mechanisms of selective neuronal death in SCA1 is important if effective therapies have to be designed.
SCA1 trangenic mice (line B05) express mutant ataxin-1 with 82 glutamines. Overexpression of mutant ataxin-1 in Purkinje cells of these mice results in a progressive ataxia and Purkinje cell pathology that are very similar to those seen in SCA1 patients (4). In transgenic mice, a hallmark of SCA1 pathology is the presence of intranuclear inclusions and cytoplasmic vacuoles in Purkinje cells. These vacuoles are believed to originate as large invaginations of the outer cell membrane, and later on contain proteins from degenerating Purkinje cell somatodendritic membranes, especially PKC gamma (32). It is not fully understood why increased expression of mutant ataxin-1 results in the formation of cytoplasmic vacuoles. However, they are relevant to disease as they are formed only when mutant ataxin-1 (with expanded repeats) is expressed.
S100 proteins are a multigenic family of non-ubiquitous Ca2+-modulated proteins of the EF-hand type expressed in vertebrates exclusively, and have been involved in the regulation of protein phosphorylation, some enzyme activities, the dynamics of cytoskeleton components, transcription factors, Ca2+ homeostasis, and cell proliferation and differentiation (8,39). Certain S100 members are released into the extracellular space by an unknown mechanism. S100B is known to exist as a non-covalently associated dimer (betabeta) under physiological concentrations and reducing conditions (9), and is capable of functionally crossbridging two homologous or heterologous target proteins in a Ca2+-dependent manner (24). S100B is abundantly expressed in the nervous system (18,29,30,39). It is mainly expressed in astrocytes, oligodendrocytes, and Schwann cells.
Receptor for advanced glycation end products (RAGE) is a multiligand member of the immunoglobulin superfamily of cell surface molecules whose repertoire of ligands includes advanced glycation end products (AGEs), amyloid fibrils, amphoterins and S100/calgranulins (18,31). Several isoforms of RAGE are expressed in human brain (7,13). Schmidt et al. (31) have proposed that in RAGE expressing cells, RAGE ligands have the capacity to upregulate the receptor thus enhancing the initial response. Whereas soluble RAGE (secreted isoform of RAGE), has the ability to prevent RAGE signaling by acting as a decoy (16). S100B has been reported to activate RAGE inducing neurite outgrowth and activation of transcription factor NF-kappaB in vitro (1,18).
Bergmann glia located in the Purkinje cell layer in cerebellar cortex elaborately ensheathe Purkinje cell dendrites, synapses and soma, and are structurally and functionally related with differentiating Purkinje cells (38). Recently, Pakhotin and Verkhratsky (28) have demonstrated electrical coupling between Bergmann glia and Purkinje cells. In addition Bergmann glia are gaining recognition as key players in the clearance of extracellular glutamate, and these cells express high levels of S100B (33). Therefore, the objective of the present study was to determine if Bergmann glia have any role in SCA1 pathogenesis.
METHODS
SCA1 transgenic mice
The SCA1 transgenic mice are generated by Drs. Harry Orr and Huda Zoghbi (4) at the University of Minnesota. We have a colony of SCA1 mice in our animal facility.
The heterozygous PS–82 BO5 line of mice identified using a transgene specific PCR assay were backcrossed to the parental FVB/N strain (N=10) to establish congenic line with homogeneous background strain. The line B05 has 30 copies of the transgene PS-82. Transgenic mice of the B05 line develop progressive loss of Purkinje cells and cerebellar function. Heterozygous B05 transgenic mice become visibly ataxic (as assessed by home cage behavior) at 12 wks of age, whereas in homozygotes, the onset of ataxia is at 6 wks postnatally. Though these animals are ataxic, they can reproduce (with normal litter size), eat and drink, and survive for more than one year.
Tissue processing, histology and electron microscopy
SCA1 heterozygous and wildtype mice at different postnatal ages were anesthetized and perfused with 4% buffered paraformaldehyde according to the method described by Neuroscience Associates, Knoxville, TN. The brains were removed and immersed in the perfusion fixative and further fixed overnight, transferred to phosphate-buffered saline (PBS), and were either used for cryostat sectioning or processed for paraffin embedding. Six μm paraffin sections were cut from the midline sagittal plane of cerebella. Some perfused cerebellar tissue pieces, fixed overnight with 4% with buffered paraformaldehyde were transferred to EM fixative. The tissues blocks were then processed for electron microscopic embedding and sectioning.
Immunohistochemistry
The formalin-fixed paraffin embedded or frozen sections of mouse brain (7 days to 6 wks old) were immunohistochemically stained with monoclonal or polyclonal antibodies to S-100B, calbindin D28k, PKC gamma, β-III tubulin, glial fibrillary acidic protein (GFAP) or RAGE as described earlier (35). Briefly, 6 μm thick tissue sections (paraffin embedded) were deparaffinized and were then incubated with 3% H2O2 for 10 min, microwaved for 14 min and then with 5% blocking goat serum for 10 min with thorough intervening washes with PBS. After washing the sections with PBS, the sections were incubated for 1 hr at room temperature or overnight at 4°C with primary antibody (at optimal dilutions), followed by incubation with (30 min) anti-mouse or rabbit second antibody biotinylated (for HRP staining) or conjugated to Alexa 488 and/or 546 fluorescent dyes. The monoclonal antibody against GFAP (Sigma) was directly conjugated with Cy3, therefore, no secondary antibody was used. The sections were then mounted and photomicrographed using an Olympus BAX60 Epi-fluorescence Microscope. Images were processed using Adobe Photoshop CS (version 8.0; San Jose, CA)
Western blots
For Western blot analysis, cerebellar cytosolic or membrane fractions prepared from SCA1 and wildtype mice were subjected to SDS-PAGE (4–20% or 15% acrylamide gels) (35). For each postnatal age equal amounts of proteins from animals in the various groups were used. Protein estimations were carried out in the nuclear and cytosolic fractions using protein assay kit (Bio-Rad). Proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Bio-Rad), blocked for 1 hr with blocking solution (Western Breeze, Invitrogen) and incubated for 1 hr or overnight with appropriate concentration of primary antibodies. Immunoreactive proteins were visualized by incubation (for 1 hr) in the goat anti-mouse alkaline phosphatase-labeled secondary antibody followed by reaction with the luminescent substrates (Western Breeze, Invitrogen). The blots were then exposed to hyperfilm-ECL (Amersham).
Co-immunoprecepitation
Co-immunoprecipitation of S100B was performed using Protein A IP kit (KBL, Inc. Gaithersburg, MD). Briefly, samples containing 400 μg/ml protein from 4wks old wildtype and SCA1 heterozygous mice were rotated overnight at 4 °C with the rabbit anti-RAGE antibody and 25 μl of a 50% slurry of protein A-agarose. Immunoprecipitates were collected by centrifugation, and the protein A-agarose beads were washed four times with 700 μl of lysis buffer. Agarose beads were then mixed with SDS-gel sample buffer, incubated 15 min at room temperature, and spun down briefly. Eluted samples were resolved by SDS-PAGE and transferred for immunoblot analysis as described previously. Electrophoretically separated proteins were transferred to PVDF membranes, blocked 1 h in blocking solution (Western Breeze, Invitrogen), and incubated overnight with monoclonal S100B antibody. Immunoreactive proteins were visualized by incubation (for 1 hr) in the goat anti-mouse alkaline phosphatase-labeled secondary antibody followed by reaction with the luminescent substrates (Western Breeze, Invitrogen). The blots were then exposed to hyperfilm-ECL (Amersham).
Purkinje cell cultures
Cerebellar Purkinje cell mixed or enriched cultures were prepared by the method of Messer(26) as described previously (34) with slight modifications. We used Thy 1.2 (CD90.2) coated Dynabeads to prepare Purkinje cell enriched cultures.
Preparation of dissociated cultures and feeding
The whole cerebellum was removed from 0–1 day old mouse pup. Meninges were carefully removed under the dissection microscope. Tissue was rinsed in cold Ca2+ and Mg2+ free Hanks balanced salt solution(CMF-HESS) and then 3ml of cold CMF-HESS was added to petri dish containing 6 cerebella. The tissue was minced and was then added to the centrifuge tubes. Tubes were left at room temperature for 5 min so that the tissue pieces can settle down with gravity. Supernatant was gently removed and 3 ml of 1% trypsin/0.1% DNase (in CMF-HESS) was added. Pellet (minced tissue pieces) was incubated for 14 min at room temperature followed by washing and then trituration in 3 ml Dulbecco’s modified Eagle’s medium (DMEM)/0.05%. The dissociated cells were centrifuged gently and the top 2.4 ml of media was removed and with the remaining media the cells were titrated and the cell pellet was thoroughly chilled on ice. The pellet was resuspended in DMEM and cells were counted. Cells were diluted with the plating medium (DMEM and 10% horse serum) and the cells were plated at 1–1.5×106 cells/1.5 ml of medium and grown in Lab Tek II Camber Slides with 4 wells/slide. Alternatively, to 1 ml of cell suspension, 25μl of Thy 1.2 coated dynabeads were added. Cells were chilled on ice for 5 min and were incubated at room temperature for 15 min with gentle tilting and rotation. The microfuge tubes were placed on Dynal MPC for 1min and 500 μl of DMEM-1% horse serum was added to the pelleted beads. Beads were washed twice with DMEM-1% horse serum and cells were resuspended in 1 ml plating media. The microfuge tubes or plates were incubated overnight at 35.5° C with 5% CO2 and 100% humidity. Next day cells were flushed (attached to dynabeads) vigorously and released cells were transferred into a flask containing serum free supplement, [50ml DMEM, 50 ml H-12, 1ml (100x) insulin-transferrin-selenium supplement (Gibco BRL) 1ml(100x) penicillin-streptomycin, 100 μM putrescine, and 20nM progesterone]. These cultures initially comprise a very heterogeneous mixture of cell types, but the smallest and most numerous type (granule cell precursors) gradually dies off in the first 6 to 9 days. The remaining cells include (relatively) flat glial cells, bipolar and tripolar cells 8–10 μm in diameter, and about an equal number of Purkinje cells (of similar size with slightly more elaborate asymmetric arbors off to one side of the soma). By 10 – 15 days these arbors were more elaborate.
Immunocytochemistry
The cultured Purkinje cells were immunostained for calbindin D28k or S100B. Briefly, the cultures were fixed for 30 min in 4% paraformaldehyde in PBS. After 3x5 min rinse in PBS the cultures were incubated with 10% blocking goat or horse serum containing 0.05% Triton X-100 for 10 min with thorough intervening washes with PBS. The sections were then incubated overnight at 4 °C with optimal dilution of primary antibody. After washing in PBS, cultures were incubated for 1 hr with anti-mouse or anti-rabbit IgG conjugated with Alexa 546 or Cy3 (diluted 1:100 in 5% goat or horse serum prepared in PBS containing 0.05% Triton X-100). After mounting, the immunostained cells were photomicrographed using an Olympus BAX 60 epi-fluorescence microscope.
S100B protein labeling
Bovine brain purified S100B protein (Sigma) was labeled with fluorescent dye Oregon Green using Oregon Green 514 protein labeling kit (Molecular Probes). 5–14 days old cultures were incubated at different time intervals with labeled S100B protein. Unlabeled S100B protein was detected by indirect immunofluorescence.
RESULTS
To gain insight into a molecular basis of the vacuole formation in SCA1 Purkinje cells we looked at the expression of Bergmann glial and Purkinje cell marker proteins. The immunohistochemical analysis revealed that S100B though expressed exclusively in Bergmann glia was localized to Purkinje cell vacuoles (Fig. 1). We saw S-100B positive cytoplasmic vacuoles in Purkinje cells of heterozygous SCA1 mice as early as 16 days postnatally, and by day 20 a marked number of vacuole were visible (not shown). However, these vacuoles was negative for PKC gamma, β-III tubulin, calbindin D28k and glial GFAP, especially in the central core of the vacuoles (Fig. 1, 2A and 3). No S100B vacuoles were seen in wildtype animals and A02 transgenic line with normal CAG repeats (data not shown).
Figure 1.
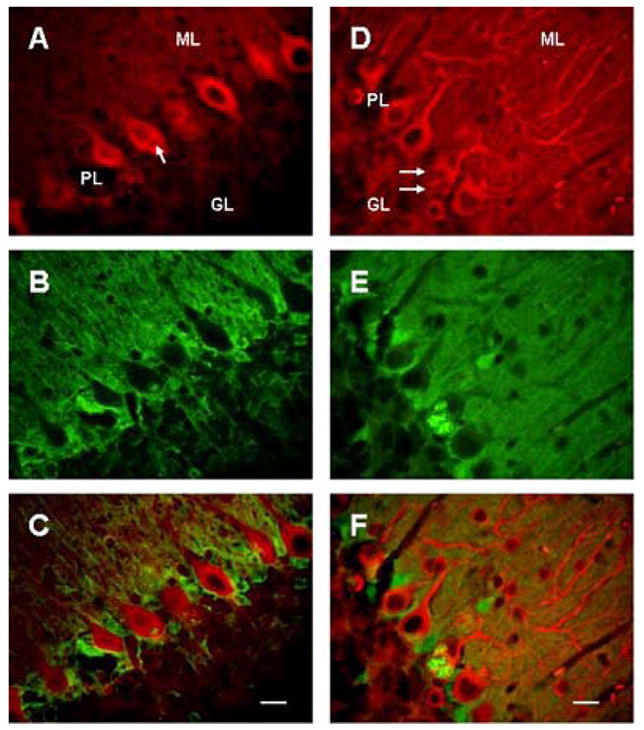
Cytoplasmic vacuoles (arrows) in Purkinje cells in 23 days (A–C) and 5 wks old (D–F) SCA1 heterozygous mice. A–C: Cerebellar section showing double immunostaining of PKC gamma (A) and S100B (B), and staining in merged image (C); bar = 10 μm. D–F: Double immunofluorscence of beta III tubulin (D) and S100B (E), and fluorescence in merged image (F); bar = 10 μm. GL, ML and PL are granule, molecular and Purkinje cell layers respectively. Vacuoles are positive for S100B and appear to be negative for both PKC gamma and beta III tubulin. Most of the vacuoles contain S100B immunoreactive central core.
Figure 2.
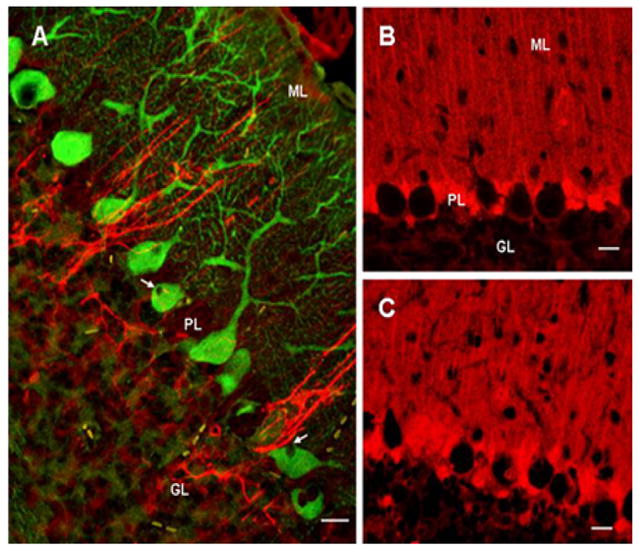
GFAP (red) and calbindin-D 28k (green) double immunostaining in cerebellum in 4-wks old SCA1 heterozygous mouse (A). Purkinje cell vacuoles (arrows) are negative for both GFAP and calbindin; bar = 10 μm. B and C: S100B immunostained Bergmann glia in 4wks old wildtype (B) and SCA1 heterozygous mice (C); bar = 10 μm. The number of immunoreactive Bergmann glia to S100B appears to have increased in SCA1 cerebellum.
Figure 3.
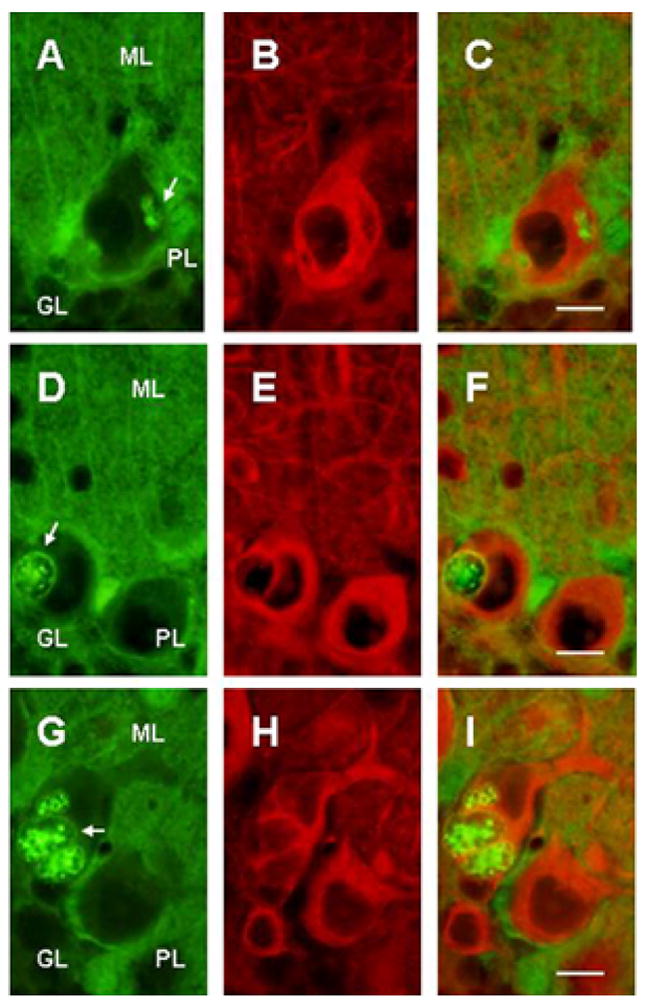
Double immunostained cerebellar sections of 5 wks SCA1 heterozygous mice showing S100B (A,D,G) and beta III tubulin (B,E,H) immunoreactions. C, F and I are merged images. The arrows indicate multiple S100B immunoreactive vacuoles of various shapes and sizes in Purkinje cells. Smaller vacuoles have somewhat homogeneous distribution of S100B protein. However, majority of vacuoles are devoid of beta III tubulin, especially in the central core. Bar = 10 μm.
S100B containing vacuoles were of different shapes and sizes. Most of the vacuoles had S100B immunoreactive outer membrane and central core. Whereas, smaller vacuoles had somewhat homogeneous distribution of S100B protein (Fig. 3). Electron microscopy of the cerebellar sections from 6 wks old heterozygous mice revealed that vacuoles appeared to be originating near Purkinje cell membranes (Fig. 4A), and in close proximity of Bergmann glial cells. Further, the outer membrane of the internalized vacuoles in fact was a double membrane (Fig. 4B, inset) suggesting that vacuoles may be formed outside Purkinje cells,which are then internalized. At early postnatal ages, when the vacuoles had just started appearing, there were no differences between the number of Bergmann glia in SCA1 and wildtype mice, however, around 4 wks in SCA1 heterozygous mice, the number of Bergmann glia began to increase in the Purkinje cell layer as compared to the age-matched wildtype animals (Fig 2B and C).
Figure 4.
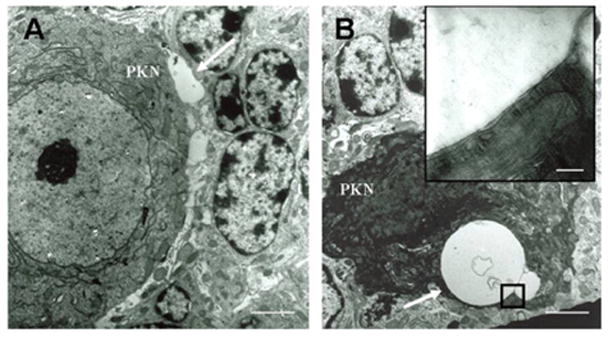
Electronmicrographs of 6wks old SCA1 heterozygous mouse cerebellum (A and B). A: A vacuole like structure, which is not yet internalized is present (arrow) near Purkinje cell’s (PKN) soma; bar = 3 μm. B: A dying Purkinje cell with two cytoplasmic vacuoles (arrow); bar = 3 μm. The inset shows that the vacuole has a double membrane; bar = 0.2 μm.
Now the question was whether S100B just diffused into the vacuoles or Purkinje cells possessed certain binding sites for S100B. We found that Purkinje cells from both wildtype and SCA1 animals expressed RAGE, which are known to bind S100 proteins. In SCA1, RAGE co-localized with S100B, and the outer membrane of the vacuoles showed positive immunoreactivity (Fig. 5A–C). This was supported by co-immunoprecipitation experiments (Fig. 5D).
Figure 5.
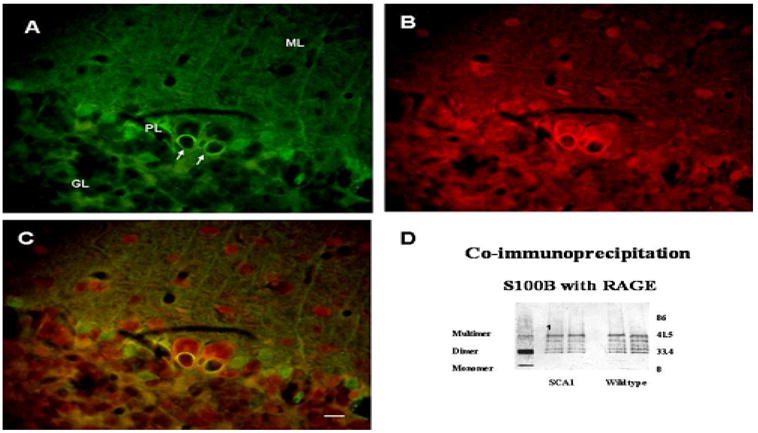
Double immunofluorescence representing S100B (A) and RAGE (B) localization to the cytoplasmic vacuoles (arrows) in Purkinje cells of 6 wks old SCA1 heterozygous mouse. C: Merged image. In SCA1 and wildtype (not shown) mice, RAGE is expressed in Purkinje cells and interneurons in the molecular layer. Bar = 10 μm. D: Western blots showing S100B expression in the cytosolic fraction prepared from the cerebellum of 4 wks old wildtype mouse (lane 1). Mouse cerebellar S100B under reducing conditions exists as a monomer (beta), a dimer (betabeta) and as a multimer. Lanes labeled ‘SCA1’ and ‘Wildtype’ represent S100B co-immunoprecipitated with RAGE using cytosolic fractions of 4ks old SCA1 and wildtype mice. Co-immunoprecipitated S100B appears as multiple bands probably due to different oligomerization states of S100B, and some bands could be other S100 proteins, which bind to RAGE and contain S100B antibody specific beta subunit.
Western blot analysis indicated that RAGE (not shown) and S100B were expressed in the mouse cerebellar tissue fractions (Fig. 5D). Mouse cerebellar S100B under reducing conditions existed as a monomer (beta), a dimer (betabeta) and as a multimer (Fig. 5D). Interestingly, different oligomerization states of S100B co-immunoprecipitated with cerebellar RAGE. Some additional immunoreactive bands seen could be due to the detection of other S100 proteins, which bind to RAGE and contain antibody-specific beta subunit (Fig. 5D).
Localization of S100B protein to the cytoplasmic vacuoles prompted us to study if exogenous S100B protein interacts with SCA1 Purkinje cells in vitro. Purkinje cell enriched cultures were prepared from the cerebella of 0–1 day old wildtype or SCA1 homozygous mouse pups. Purkinje cells were identified by size, asymmetric arbors, immunoreactivity to calbindin-D28k (Fig. 6A) and failure to express GFAP (not shown). Purified S100B protein was labeled with fluorescent dye Oregon Green. 5 days old cultures were incubated at different time intervals with either tagged S100B protein or free dye. Figure 6B shows calbindin positive Purkinje cell with internalized S100B (Fig. 6C). S100B fluorescence was detected in Purkinje cells as early as 2 hr post incubation. Free dye was not internalized (not shown). Cultures without exogenously added S100B did not show any S100B immunoreactivity (not shown).
Figure 6.
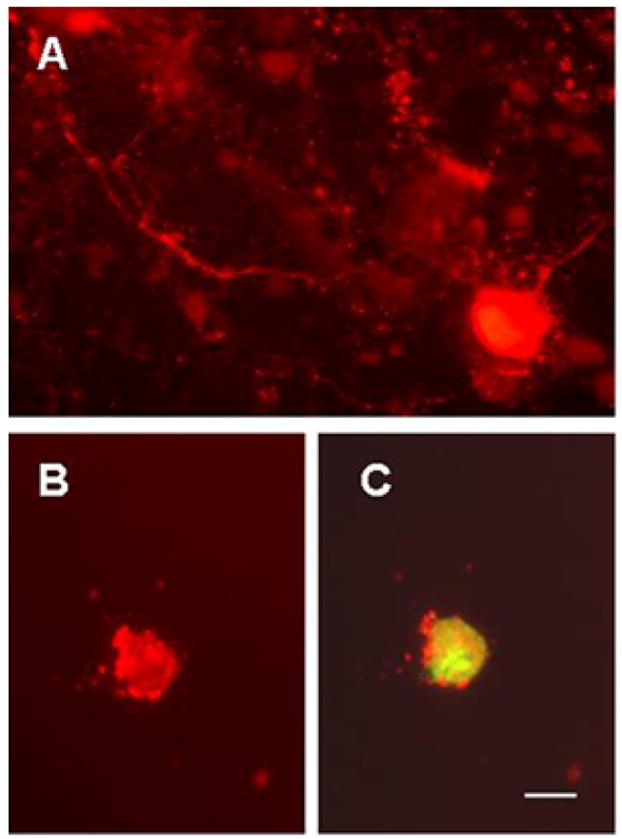
Cultured Purkinje cells showing calbindin D28k immunoreactivity (A and B) and S100B fluorescence (C) in 5 (B and C) and 12 days old (A) cultures. Purkinje cell enriched cultures were prepared from the cerebella of 0–1 day old wildtype (A) and SCA1 homozygous mouse pups (B and C). Purkinje cells were identified by size, asymmetric arbors, immunoreactivity to calbindin-D28k and failure to express glial fibrillary acidic protein (not shown). In this preparation, cultures were incubated with Oregon Green tagged S100B protein (5μg/ml) for 2 hr. Calbindin positive Purkinje cell shows internalized S100B; bar = 10 μm.
DISCUSSION
In SCA1 transgenic mice, two prominent pathological changes that occur early in the disease progression are the formation of cytoplasmic vacuoles and nuclear aggregation of ataxin-1. However, our present study indicates that S100B positive vacuoles appear much earlier, even before ataxin-1 aggregation and alterations in the behavior in SCA1 mice. In addition, we found that the appearance of S100B positive vacuoles does not coincide with the loss of dendritic membranes as reported earlier (32). We also observed that almost all vacuoles had S100B immunoreactive outer membrane and central core. Smaller vacuoles on the other hand had somewhat homogeneous distribution of S100B protein (Fig. 3). Colocalization of S100B and RAGE (S100B receptor) suggests that S100B receptors are expressed in Purkinje cells, and may be associated with normal Purkinje cell- Bergmann glial crosstalk.
Brain S100B functions as a cytokine with both neurotrophic and neurotoxic effects(18,29,30,39). S100B has been shown to be released from astrocytes to promote neuronal survival(3,37) and development(36). However, similar to our studies, increased immunoreactivity to S100B has also been reported in Alzheimer’s disease and Down syndrome(15,19). Increased immunostaining to S100B in SCA1 mice may influence behavior(4) as in other animal studies, changes in the cerebral concentration of S100B cause behavioral disturbances and cognitive deficits(30). Further, S100B binds to RAGE(1,18), and activation of RAGE by S100B promotes cell survival. On the other hand, analysis of RAGE expression in non-demented and Alzheimer’s disease (AD) brains indicated that increases in RAGE protein and percentage of RAGE-expressing microglia paralleled the severity of disease suggesting a role of RAGE in the pathogenesis of AD(23). Therefore, it’s possible that RAGE is a signal-transducing receptor for both trophic and toxic effects of S100B in SCA1 Purkinje cells.
In the present study, the specificity of S100B and lack of localization of other glial and Purkinje cell marker proteins to vacuoles indicate that the vacuoles are not formed due to bulk internalization of extra or intracellular material(32). It could be argued, however, that S100B is released by degenerating Bergmann glia(15,19,30). The Bergmann glial cells display a tight association with Purkinje cells during development and in the adult cerebellum and elaborately ensheathe Purkinje cell dendrites, synapses and soma. Further, in SCA7 mice Purkinje cell abnormalities develop due to the expression of polyglutamine expanded protein in the Bergmann glial cells suggesting that dysfunctional Bergmann glia could cause Purkinje cell degeneration(14). Furthermore, our preliminary electron microscopic observations of young SCA1 mouse cerebellum show signs of pathological changes in Bergmann glia (unpublished observations).
Based on the neurotrophic properties of S100B as reported by others, and observed co-localization of S100B and RAGE to both wildtype and SCA1 Purkinje cells in the present study, we believe that S100B protein has a role in Purkinje cell development and function. Since our data shows that S100B binds to RAGE on Purkinje cell membranes, and since RAGE stimulation leads to activation of NF-kappaB pathway(18) we speculate that in SCA1 mice the internalization of S100B-RAGE complexes(10,22) in the form of vacuoles could cause sustained activation of RAGE-dependent pathways. In Huntington’s disease, mutant huntingtin has been shown to elevate NF-kappaB-dependent gene expression(20). NF-kappaB, a heterodimer is present in the cytoplasm in inactive form bound to its inhibitor IkappaB(12). Subsequent to cell stimulation, IkappaB undergoes phosphorylation, ubiquitination and degradation by a proteosome-dependent pathway, allowing nuclear translocation of the active dimeric NF-kappaB transcriptional factor(17,20). Furthermore, NF-kappaB translocation to the nucleus could elevate protein kinase B/Akt expression(25). Interestingly, Akt is a key enzyme that modulates neurotoxicity of ataxin-1(5).
In summary, these data suggest that calcium binding protein S100B though extrinsic to Purkinje cells is sequestered into cytoplasmic vacuoles in SCA1 mice at an early postnatal age. It is possible that S100B has receptors or is a constituent of Purkinje cell membrane. Further, localization of RAGE to the vacuoles suggests that S100B may be binding to RAGE on Purkinje cell membranes before these membranes are internalized. Furthermore, the in vitro data indicates that S100B protein may be internalized into SCA1 Purkinje cells. Whether these cultured cells also contain S100B positive vacuoles, and how these observations compare with Purkinje cells from wildtype mice is currently being pursued.
Acknowledgments
This work was partly supported by grants from the National Institute of Neurological Disorders and Stroke and Lucky Day Foundation, USA.
Footnotes
The Online Journal of Neurological Sciences (Turkish) 1984–2005
This e-journal is run by Ege University Faculty of Medicine,
Dept. of Neurological Surgery, Bornova, Izmir-35100TR
as part of the Ege Neurological Surgery World Wide Web service.
Comments and feedback:
E-mail: editor@jns.dergisi.org
URL: http://www.jns.dergisi.org
Journal of Neurological Sciences (Turkish) ISSNe 1302-1664
References
- 1.Arumugam T, Simeone DM, Schmidt AM, Logsdon CD. S100B stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE) J Biol Chem. 2004;279:5059–5065. doi: 10.1074/jbc.M310124200. [DOI] [PubMed] [Google Scholar]
- 2.Banfi S, Servadio A, Chung MY, Kwiatkowski TJ, Jr, McCall AE, Duvick LA, Shen Y, Roth EJ, Orr HT, Zoghbi HY. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet. 1994;7:513–520. doi: 10.1038/ng0894-513. [DOI] [PubMed] [Google Scholar]
- 3.Barger SW, VanEldik LJ, Mattson MP. S100β protects hippocampal neurons from damage induced by glucose deprivation. Brain Res. 1995;677:167–170. doi: 10.1016/0006-8993(95)00160-r. [DOI] [PubMed] [Google Scholar]
- 4.Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, Duvick LA, Zoghbi HY, Orr HT. SCA-1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–948. doi: 10.1016/0092-8674(95)90273-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen HK, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH, Aitken A, Skoulakism EM, Orr HT, Botas J, Zoghbi HY. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–468. doi: 10.1016/s0092-8674(03)00349-0. [DOI] [PubMed] [Google Scholar]
- 6.Clark HB, Burright EN, Yunis WS, Larson S, Wilcox C, Hartman B, Matilla A, Zoghbi HY, Orr HT. Purkinje cell expression of a mutant allele of SCA-1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17:7385–7395. doi: 10.1523/JNEUROSCI.17-19-07385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Q, Keller JN. Splice variants of the receptor for advanced glycosylation end products (RAGE) in human brain. Neurosci Lett. 2005;373:67–72. doi: 10.1016/j.neulet.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 8.Donato R. Perspectives in S-100 protein biology. Cell Calcium. 1991;12:713–726. doi: 10.1016/0143-4160(91)90040-l. [DOI] [PubMed] [Google Scholar]
- 9.Drohat AC, Nenortas E, Beckett D, Weber DJ. Oligomerization state of S100B at nanomolar concentration determined by large-zone analytical gel filtration chromatography. Protein Sci. 1997;6:1577–1582. doi: 10.1002/pro.5560060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulle S, Mariggio MA, Belia S, Petrelli C, Ballarini P, Guarnieri S, Fano G. Rapid desensitization of PC12 cells stimulated with high concentrations of extracellular S100. Neuroscience. 1999;89:991–997. doi: 10.1016/s0306-4522(98)00386-8. [DOI] [PubMed] [Google Scholar]
- 11.Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet. 2005;6:743–755. doi: 10.1038/nrg1691. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 13.Giron MD, Vargas AM, Suarez MD, Salto R. Sequencing of two alternatively spliced mRNAs corresponding to the extracellular domain of the rat receptor for advanced glycosylation end products (RAGE) Biochem Biophys Res Commun. 1998;251:230–234. doi: 10.1006/bbrc.1998.9446. [DOI] [PubMed] [Google Scholar]
- 14.Garden GA, Libby RT, Fu Y-H, Kinoshita Y, Huatag J, Possin DE, Smith AC, Martinez RA, Fine GC, Grote SK, Ware CB, Einum DD, Morrison RS, Ptack LJ, Sopher BL, La Spada AR. Polyglutamine-expanded ataxin- promotes non-cell-autonomous Purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci. 2002;22:4897–4905. doi: 10.1523/JNEUROSCI.22-12-04897.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2004;279:50019–50024. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- 18.Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275:40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 19.Kato K, Suzuki F, Kurobe N, Okajima K, Ogasawara N, Nagaya M, Yamanaka T. Enhancement of S-100β protein in blood of patients with Down’s syndrome. J Mol Neurosci. 1990;2:109–113. doi: 10.1007/BF02876918. [DOI] [PubMed] [Google Scholar]
- 20.Khoshnan A, Ko J, Watkin EE, Paige LA, Reinhart PH, Patterson PH. Activation of the IkappaB kinase complex and nuclear factor-kappaB contributes to mutant huntingtin neurotoxicity. J Neurosci. 2004;24:7999–8008. doi: 10.1523/JNEUROSCI.2675-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4:62–73. doi: 10.1080/14734220510007950. [DOI] [PubMed] [Google Scholar]
- 22.Kubista H, Donato R, Hermann A. S100 calcium binding protein affects neuronal electrical discharge activity by modulation of potassium currents. Neuroscience. 1999;90:493–508. doi: 10.1016/s0306-4522(98)00422-9. [DOI] [PubMed] [Google Scholar]
- 23.Lue LF, Yan SD, Stern DM, Walker DG. Preventing activation of receptor for advanced glycation endproducts in Alzheimer’s disease. Curr Drug Targets CNS Neurol Disord. 2005;4:249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- 24.McClintock KA, Shaw GS. A logical sequence search for S100B target proteins. Protein Sci. 2000;10:2043–2046. doi: 10.1110/ps.9.10.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng F, D’Mello SR. NF-kappaB stimulates Akt phosphorylation and gene expression by distinct signaling mechanisms. Biochim Biophys Acta. 2003;1630:35–40. doi: 10.1016/j.bbaexp.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Messer A. Primary cultures of cerebellar Purkinje cells. In: Shahar A, DeVllis J, Vernadakis A, Haber B, editors. A Dissection and Tissue Culture Manual of the Nervous System. Alan R Liss Inc; New York: 1989. pp. 198–199. [Google Scholar]
- 27.Orr HT, Chung M-Y, Banfi S, Kwiatkowski TJ, Jr, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LPW, Zoghbi HY. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nature Genet. 1993;4:221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- 28.Pakhotin P, Verkhratsky A. Electrical synapses between Bergmann glial cells and Purkinje neurones in rat cerebellar slices. Mol Cell Neurosci. 2005;28:79–84. doi: 10.1016/j.mcn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Reeves RH, Yao J, Crowley MR, Buck S, Zhang X, Yarowsky P, Gearhart JD, Hilt DC. Astrocytosis and axonal proliferation in the hippocampus of S100b transgenic mice. Proc Natl Acad Sci U S A. 1994;91:5359–5363. doi: 10.1073/pnas.91.12.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003;60:614–632. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 32.Skinner PJ, Vierra-Green CA, Clark HB, Zoghbi HY, Orr HT. Altered Trafficking of membrane proteins in Purkinje cells of SCA1 transgenic mice. Am J Pathol. 2001;159:905–913. doi: 10.1016/S0002-9440(10)61766-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slemmer JE, De Zeeuw CI, Weber JT. Don’t get too excited: mechanisms of glutamate-mediated Purkinje cell death. Prog Brain Res. 2005;148:367–390. doi: 10.1016/S0079-6123(04)48029-7. [DOI] [PubMed] [Google Scholar]
- 34.Vig PJS, McDaniel DO, Subramony SH, Qin Z. The effects of calbindin D-28k and parvalbumin antisense oligonucleotides on the survival of cultured Purkinje cells. Res Commun Mol Pathol Pharmacol. 1999;103:249–259. [PubMed] [Google Scholar]
- 35.Vig PJS, Subramony SH, Burright EN, Fratkin JD, McDaniel DO, Desaiah D, Qin Z. Reduced immunoreactivity to calcium-binding proteins in Purkinje cells precedes onset of ataxia in spinocerebellar ataxia-1. Neurology. 1998;50:106–113. doi: 10.1212/wnl.50.1.106. [DOI] [PubMed] [Google Scholar]
- 36.Whitaker-Azmitia PM, Vingate M, Borella A, Gerlai R, Roder J, Azmitia EC. Transgenic mice overexpressing the neurotrophic factor S-100β show neuronal cytoskeletal and behavioral signs of altered aging processes: implications for Alzheimer’s disease and Down’s syndrome. Brain Res. 1997;776:51–60. doi: 10.1016/s0006-8993(97)01002-0. [DOI] [PubMed] [Google Scholar]
- 37.Winningham-Major F, Staecker JL, Barges SW, Coats S, VanElkik J. Neurite extension and neuronal survival activities of recombinant S100β proteins that differ in the content and position of cysteine residues. J Cell Biol. 1989;109:3064–3071. doi: 10.1083/jcb.109.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cell. Anat Sci Int. 2002;2:94–108. doi: 10.1046/j.0022-7722.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 39.Zimmer DB, Chaplin J, Baldwin A, Rast M. S100-mediated signal transduction in the nervous system and neurological diseases. Cell Mol Biol. 2005;51:201–214. [PubMed] [Google Scholar]
- 40.Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]


