Abstract
Background:
Chronic pain models are commonly defined as either nerve-injury or inflammation models, but recent work suggests inflammatory processes are important in nerve injury-induced pain.
Methods:
In the rat spinal nerve ligation model, the authors examined effects of systemic corticosteroid triamcinolone acetonide (TA) on the cytokine protein profile and sympathetic sprouting in the axotomized sensory ganglia, excitability of sensory neurons, and mechanical sensitivity.
Results:
By postoperative day 3, marked increases (5- to 16-fold) in monocyte chemoattractant protein-1, growth-related oncogene (GRO/KC or CXCL1), and interleukin (IL)-6 were observed, whereas IL-4 and IL-2 levels fell more than 4-fold. The increased cytokines and number of sympathetic basket formations in the sensory ganglia were reduced toward normal values by TA given starting at the time of injury. IL-4 and IL-2 levels were not restored by TA. Systemic TA also reduced the firing rate and incidence of bursting activity, but not the overall incidence of spontaneous activity, in large- and medium-sized neurons. Mechanical hypersensitivity on postoperative day 3 was reduced by TA, and some effect could still be observed 4 days after cessation of TA. However, starting TA at day 7 was ineffective.
Conclusions:
Several components of the spinal nerve injury model are responsive to corticosteroid, suggesting inflammatory processes are important in the development of neuropathic pain. The observation that TA was effective when given starting at the time of injury suggests that steroid treatment might alter the development of chronic pain after surgical procedures that involve nerve injury, such as amputation or hernia repair.
INTRODUCTION
Our understanding of chronic pain has benefited from a number of different animal models. Such models are often classified as either nerve injury or inflammation models (for review, see 1). In the peripheral nervous system, commonly used nerve injury models include complete or partial transection of the sciatic nerve or its branches,2-4 chronic constriction of the sciatic nerve,5 and spinal nerve ligation (SNL).6 Chronic inflammation models include those in which inflammatory substances such as carrageenan, Freund's adjuvant, or zymosan are injected beneath the skin7 or deposited adjacent to a nerve.8
More recent studies have shown that the distinction between nerve injury and inflammation models is not clear-cut. Nerve injury, as a form of tissue damage, evokes an inflammatory response. This response not only mediates tissue repair and regeneration, but contributes importantly to chronic pain behaviors. Thus, inflammatory cytokines and chemokines, as well as growth factors with similar functions, have been shown to be upregulated in many nerve injury models; and genetic or pharmacological reduction of these molecules can reduce pain behaviors and other pathologies present in these models. In addition, many studies have demonstrated important roles for resident and invading immune cells and for glial cells in nerve injury pain models. Neurons including nociceptors express functional receptors for many of these inflammatory mediators (for review, see 9-12).
In view of the emerging importance of inflammatory effects on neurons, we recently developed a model in which effects of direct localized inflammation of the DRG neurons could be examined in the absence of nerve injury.13 In this model, inflaming the L5 dorsal root ganglion (DRG) by depositing a small drop of the immune stimulus zymosan in the nearby epidural space led to prolonged mechanical allodynia and hyperalgesia, increased spontaneous activity and hyperexcitability of the sensory neurons, and sprouting of sympathetic fibers around sensory neurons, which is implicated in neuropathic pain. We also measured increases in certain pro-inflammatory cytokines within the DRG, along with decreases in some anti-inflammatory cytokines.
We were interested in examining the role of inflammation in the initiation of neuropathic pain by comparing the localized inflammation of the DRG model with a commonly used nerve injury model, the SNL model.6 In the SNL model, robust pain behaviors, sympathetic sprouting into the axotomized DRG, and spontaneous activity and enhanced excitability of the sensory neurons are observed. The model involves ligation of the L5 (or L5 and L6) spinal nerves, allowing separation of the axotomized neurons in the L5/L6 DRG from the intact neurons in L4 that mediate the observed evoked pain behaviors. Although this is commonly used as a neuropathic pain model, like other nerve injury models,14 it has been shown to induce infiltration of macrophages and T cells into the axotomized and adjacent DRG.15 In this study, we report that the cytokine changes in the DRG induced by the SNL model bear many similarities to those observed in the localized inflammation model. This led us to test the response of the SNL model to subcutaneous injections of a commonly used corticosteroid anti-inflammatory drug, triamcinolone acetonide (TA).
MATERIALS AND METHODS
Animals
Sprague-Dawley rats obtained from Harlan (Indianapolis, IN, USA) were housed in groups of 2-4 in 40×60×30 cm plastic cages with soft bedding under a 12/12 h day/night cycle. The rats were kept 7-10 days under these conditions before surgery and up to 2 weeks after surgery. The experimental protocol was approved by the Institutional Animal Care and Use Committees of the University of Cincinnati, Cincinnati, OH, USA.
Procedures for spinal nerve ligation and triamcinolone acetonide injections
Male Sprague-Dawley rats weighing 200-250 g at the time of surgery were anesthetized with isoflurane. An incision was made on the back between L2 and S1. The L5 spinal nerve was exposed and tightly ligated with 6-0 silk and cut about 5 mm distal to the ligature. For experiments in which cytokine levels in the DRG were measured, both the L4 and the L5 spinal nerves were cut in order to increase the amount of tissue from DRGs with spinal nerve ligation obtained from each animal. The anti-inflammatory corticosteroid TA was given beginning on the day of surgery, one subcutaneous injection on the back of the neck about 60 min prior to surgery (1.5 mg/kg), followed by one additional injection per day for the first 3 days after surgery, for a total of 4 injections. The control group received 4 injections of normal saline in the same volume. In other experiments, animals received the same series of injections but without the SNL procedure, or received the injections starting on post-operative day (POD) 7, as indicated. For sham surgeries used in cytokine measurement experiments, both of the L4/L5 spinal nerves were exposed but without nerve ligation.
Behavioral testing for mechanical allodynia
The testing procedure has been described in detail in previous publications.16,17 Rats were inspected and tested every other day for 5 days before surgery (3 testing sessions). After surgery, behavioral testing was performed on day 3 and day 7, and on additional days as indicated in the figures. To avoid potential bias, the person performing the behavioral testing was blinded as to the experimental group. Rats were placed in a Plexiglas box with a plastic mesh floor. To obtain percent withdrawal responses, von Frey filaments capable of exerting bending forces of 20, 40, 60, 80, and 120 mN, but each having the same tip diameter of 0.1 mm, were applied, in the order of ascending force, to 6 designated loci distributed over the plantar surface of the foot. Each filament was applied alternately to each foot and to each locus. The withdrawal threshold, defined as the force (filament) associated with 50% response of foot withdrawal, was calculated by fitting the force-response data to the Hill Equation using a program, Microcal Origin 7.0 (Microcal Software, Inc., MA, USA).18 The mean withdrawal thresholds (baseline) of each hind paw before surgery were obtained from an average of 3 testing sessions (as day 0).
Immunohistochemical staining for sympathetic fibers
Rats were anesthetized on POD 8 with pentobarbital sodium (40 mg/kg, i.p.) and fixed by perfusing 200–300 ml of Zamboni's fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH=7.4) through the left ventricle of the heart. The axotomized L5 DRG was removed, post-fixed in the perfusion fixative for 1 hour at 4°C, and embedded in gelatin overnight. The ganglia were horizontally sectioned with a Vibratome (Vibratome Company, St. Louis, MO, USA) at a thickness of 40 μm.
Tissue sections were incubated in antibodies to tyrosine hydroxylase (TH) at a dilution of 1:1000 for 48 hours at 4°C, followed by reaction with biotinylated secondary antibody and, finally, with Vector ABC reagent. The TH antibody is an affinity purified polyclonal rabbit antibody; the antigen is purified denatured rat TH isolated from pheochromocytoma cells. Specificity is demonstrated by ability to stain the noradrenergic and dopamine systems in rat brain with low background (Pel-Freeze, Rogers, AR, USA). Triton-X (0.3%) was used in all reaction solutions to enhance antibody penetration. Immunoreaction products were visualized by the diaminobenzidine method in the presence of H2O2 in 0.1 M phosphate buffer. Tissues were then mounted on gelatin-coated slides, air dried, dehydrated, and coverslipped for light-microscopic observation.
Slides from control and experimental groups were labeled with numbers so that the person performing the image analysis was blinded as to the experimental group. In addition, all images were captured and analyzed by an investigator other than the one who performed immunohistostaining to avoid possible bias. Each DRG was sectioned into 15 to 20 sections that were then mounted on a single slide. Using ImagePro Plus software (Media Cybernetics, Inc., Silver Spring, MD, USA), images from all sections of each DRG were captured under a light microscope (20x) equipped with a SPOT Insight colored digital camera (Diagnostic Instruments, Inc., Burlingame, CA, USA), and stored in a Pentium IV computer for measurement. The number of neuronal somata surrounded by TH-immunoreactive basket-like structures or rings were counted from 4 (randomly chosen) out of 15-20 sections of the TH-immunostained DRG. Only DRG neurons encircled by TH-immunoreactive fibers for at least two-thirds of the circumference of the somata, and which had a clearly visible nucleus, were counted. The average density of the TH-immunoreactive basket/ring within each DRG was obtained by dividing the total number of baskets/rings by the size of the total measured cellular area (area in mm2).
Cytokine measurement
Cytokine expression profiles in the DRG were evaluated on POD 3 and 7 after the SNL (with or without TA treatment) or sham surgery using Bio-Plex System (BioRad, Hercules, CA, USA) combined with Linco 14-Plex Rat Cytokine Detection Kit (Linco Research, Inc., St. Charles, MO, USA). A total of 14 rat cytokines were measured simultaneously from a single well according to the manufacturer's protocols, as previously described.13 Briefly, ipsilateral DRGs (L4 and L5) were isolated from the rats and homogenized in lysis buffer (Bio-Rad, Hercules, San Diego, CA, USA) supplemented with a protease inhibitor cocktail (Sigma, St. Louis, MO, USA) followed by centrifugation (13,000 rpm) at 4°C for 30 min to obtain extracted protein. Protein samples (25 μl) extracted from DRG tissue were thawed and run in duplicate. A broad sensitivity range of standards (Linco Research, Inc., MI, USA) ranging from 4.88 to 20,000 pg/ml was used to allow the quantization of a dynamic wide range of cytokine concentrations and provide the greatest sensitivity. This method was chosen because it has sensitivity and performance similar to ELISA methods, but requires much smaller sample volumes and is suitable for mutiplexing.19
The concentrations of cytokines in these assays were calculated using a standard curve. A regression analysis was performed to derive an equation that was then used to predict the concentration of the unknown samples. The measured cytokines were interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70), lL-18, interferon-γ, tumor necrosis factor-α, granulocyte-macrophage colony stimulating factor, growth related oncogene (GRO/KC) and monocyte chemoattractant protein-1 (MCP-1).
The final concentrations of the 14 cytokines were obtained from an average of values observed in 3 to 6 samples for each time point and condition, as indicated in Table I. Each individual sample contained 4 DRGs combined from 2 rats, measured 2 or 3 times. Data were normalized to the amount of protein; similar results were obtained using normalization to the tissue weight.
Table I.
Cytokine levels in the axotomized DRGs on POD 3 and 7
| POD 3 |
POD 7 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cytokine | Normal | Sham | SNL | SNL + steroid |
Sham | SNL | SNL + steroid |
Estimated Detection limit |
| GM-CSF | - | - | - | - | - | - | - | - |
| IL-1α | 14.0 ± 2.4 | 9.3 ± 1.7 | 11.2 ± 6.1 | 2.8 ± 2.0& | 7.5 ± 3.6 | 11.3 ± 4.2 | 14.1 ± 3.2 | 0.10 (0.25 pM) |
| MCP-1 | 21.8 ± 2.9 | 30.6 ± 8.5 | 106 ± 52# | 45.4 ± 4.8# | 28.8 ± 12.1 | 63.2 ± 20.1& | 36.4 ± 3.6& | 0.06 (0.19 pM) |
| IL-4 | 1.8 ± 0.5 | 2.2 ± 1.9 | 0.1 ± 0.1# | 0.1 ± 0.1# | 1.8 ± 1.0 | 0.0 ± 0.0$ | 0.4 ± 0.1& | 0.04 (0.12 pM) |
| IL-1β | 13.6 ± 4.0 | 10.4 ± 3.6 | 16.2 ± 1.4 | 8.5 ± 2.4 | 14.0 ± 1.8 | 11.6 ± 3.5 | 16.7 ± 3.5 | 0.04 (0.09 pM) |
| IL-2 | 19.1 ± 3.1 | 11.2 ± 3.7 | 4.4 ± 1.0# | 3.7 ± 0.7$ | 12.9 ± 4.1 | 4.7 ± 1.1# | 7.4 ± 1.5# | 0.10 (0.17 pM) |
| IL-6 | 11.0 ± 1.9 | 25.4 ± 11.1 | 180 ± 37$ | 106 ± 10$ | 11.3 ± 1.8 | 42.2 ± 4.0$ | 59.4 ± 10.0$ | 0.16 (0.32 pM) |
| IL-10 | 3.8 ± 2.6 | 5.0 ± 3.2 | 1.3 ± 1.3 | 0.7 ± 0.6 | 1.3 ± 1.3 | 1.0 ± 0.8 | 4.1 ± 1.9 | 0.06 (0.14 pM) |
| IL-12p70 | 5.3 ± 1.3 | 3.3 ± 0.7 | 1.6 ± 0.6 | 1.2 ± 0.7 | 3.3 ± 1.4 | 1.6 ± 0.3& | 2.8 ± 0.6 | 0.07 (0.04 pM) |
| IL-5 | 1.7 ± 0.8 | 2.4 ± 2.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.6 ± 1.3 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.05 (0.15 pM) |
| IFN | 19.0 ± 4.8 | 24.9 ± 15.5 | 173 ± 162 | 6.3 ± 3.2 | 21.2 ± 6.3 | 198 ± 120 | 17.6 ± 4.0 | 0.08 (0.22 pM) |
| IL-18 | 147 ± 30 | 117 ± 22 | 170 ± 4 | 166 ± 21 | 164.0 ± 44.0 | 128 ± 12 | 263 ± 46 | 0.08 (0.14 pM) |
| GRO/KC | 17.0 ± 3.9 | 23.9 ± 8.1 | 154 ± 74# | 31.8 ± 0.8 | 22.2 ± 5.9 | 28.1 ± 8.4 | 39.1 ± 15.1 | 0.03 (0.19 pM) |
| TNFα | 12.9 ± 3.1 | 13.3 ± 7.5 | 6.0 ± 2.3 | 9.4 ± 1.3 | 14.2 ± 5.1 | 7.5 ± 2.4 | 16.1 ± 4.2 | 0.07 (0.18 pM) |
Cytokine abbreviations: GM-CSF, granulocyte-macrophage colony stimulating factor; GRO/KC, growth related oncogene; IF ,interferon-γ; interleukin (IL); MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-α. Values are given as mean ± S.E.M in units of pg/mg protein. Four steroid or normal saline (vehicle) injections were given daily starting immediately before the surgery. Significance of changes (vs. normal) is indicated by & (p<0.05), # (p<0.01), and $ (p < 0.001), based on ratio t-tests (on the logs of the values, i.e., examining the significance of the fold change). N = 3 – 6 experiments for each experimental group, and 5 for the normal group; each experimental measurement was on samples containing 4 ganglia combined from 2 rats, measured two or three times. The estimated detection limits were obtained from the lowest detectable cytokine concentration (as specified by the kit manufacturer) and the average experimental tissue average experimental protein weight, for each experiment.
Measurement of spontaneous activity and excitability parameters
At POD 7 (i.e., 4 days after the last injection of steroid or saline), intracellular recording was performed on sensory neurons in whole DRG preparations isolated from saline and steroid treated rats. As described in previous publications, 16,20 the ipsilateral L5 DRG was dissected out of the rat under barbiturate anesthesia, placed in the recording chamber and mounted on the stage of an up-right microscope (BX50-WI, Olympus America Inc., Center Valley, PA, USA). A U-shaped stainless steel rod with 3 pieces of fine nylon filaments crossed from one side to the other was used to gently hold the ganglion in place within the recording chamber. The DRG was continuously perfused at a rate of 2-5 ml/min with oxygenated artificial cerebrospinal fluid containing (in mM): NaCl 130, KCl 3.5, NaH2PO4 1.25, NaHCO3 24, Dextrose 10, MgCl2 1.2, CaCl2 1.2 (pH = 7.3). The temperature was maintained at 36 ± 1 °C by a temperature controller.
DRG cells were visualized under differential interference contrast. Intracellular, electrophysiological recordings were made from each cell with a microelectrode filled with 2M potassium acetate (pH=7.2). Satisfactory recordings were obtained with electrodes of 50-80 MΩ from large- and medium-sized neurons, visually classified by the diameter of the soma (>30μm). The electrophysiological data were collected with the use of single-electrode continuous current-clamp (AxoClamp-2B, Axon Instruments, Inc., Union City, CA, USA) and analyzed with pClamp 9 software (Axon Instruments, Inc., Union City, CA, USA).
In experiments to determine the incidence of spontaneous activity, individual DRG neurons were first impaled with a recording electrode. If spontaneous activity was absent during the first 60 sec of the impaling, incremental currents (up to 4 nA) were then injected to ensure that action potentials could be evoked indicating a healthy cell. If any spontaneous activity was present, then we waited for 3 min to ensure that the activity was not caused by penetrating the somata with the sharp electrode. Next, the following parameters were measured using a series of current pulse injections, as described previously 21: the threshold current (rheobase), action potential threshold, resting membrane potential, input resistance and afterhyperpolarization of the recorded DRG cell. The resting potential was taken 3 min after a stable recording was first obtained.
Data analysis
Significance was ascribed for p<0.05. Behavioral time-course data was analyzed using Two-way repeated measures ANOVA (RM ANOVA), with Bonferroni post test to determine on which days steroid-treated and saline-treated animals were significantly different, if an overall significant drug effect was observed. One-way ANOVA with Tukey's multiple comparison tests was used to compare differences in TH basket density between control, SNL, and SNL + TA DRG sections. Significance of differences in average values of electrophysiological parameters were determined using Student's t-test, unless otherwise indicated (data not normally distributed were tested with the Mann-Whitney rank sum test). Significance of differences in proportions of bursting cells was determined using Fisher's exact test.
RESULTS
SNL increases inflammatory cytokines in the axotomized DRG and corticosteroid treatment partially reverses these increases
Levels of selected cytokines were measured in DRG from normal animals and from the experimental groups on POD 3 and 7. The results are summarized in Table I and Figure 1. Of the 14 cytokines measured, all but one (granulocyte-macrophage colony stimulating factor) were readily detectable in normal DRG. None were significantly elevated from normal levels in sham operated animals on POD 3 or 7. Three cytokines were significantly elevated by SNL on day 3: MCP-1, GRO/KC (CXCL1), and IL-6, all of which are generally classified as pro-inflammatory cytokines. In each case the cytokine level was reduced towards normal levels in animals treated with steroidal anti-inflammatory TA (1.5 mg/kg/day for 4 days beginning just before the surgery), although the values in steroid-treated animals were (except for GRO/KC) still significantly higher than in normal animals. MCP-1 and IL-6 remained elevated in SNL animals on day 7, but the effects of steroid treatment were less apparent at this time. Two cytokines, IL-2 and IL-4 were significantly lower after spinal nerve ligation and remained so through POD 7. Interestingly, the steroid treatment did not appear to elevate these cytokines back towards normal levels. Similar results were obtained if the cytokines were normalized to tissue weight instead of to protein content. It should be noted that some of the average measurements for IL-4 (other than for the normal and day 7 sham groups) include values that were below the detection limit, limiting the quantitative comparisons between the experimental groups.
Figure 1.
Time course of cytokines significantly changed by SNL. The normal value in unoperated animals is indicated by the dotted line. Significance of changes (vs. normal) is indicated by & (p<0.05), # (p<0.01), and $ (p<0.001), based on ratio t-tests (on the logs of the values, i.e., examining the significance of the fold change). See also Table 1.
Corticosteroid treatment reduces mechanical hyperalgesia after SNL
As previously shown, SNL caused a marked mechanical hyperalgesia ipsilateral to the injury site which was evident by POD3.6 Treatment of animals with TA just prior to the surgery and once daily for the next three days caused a significant decrease in the mechanical pain behaviors evoked by SNL (overall p value for drug effect = 0.02; n=11 TA and 12 saline-treated SNL rats). This effect of the steroid was still evident on POD 7, i.e., 4 days after the last steroid injection (Fig. 2). In contrast, injecting the TA for a similar period of time, but starting on POD 7, after mechanical pain behaviors were well established, caused only a transient reduction of pain behavior which did not outlast the period of steroid injection (n=6 TA and 4 saline-treated SNL rats, Fig. 3A). TA injection had no effect on pain behavior in normal, uninjured rats (n=4, Fig. 3B).
Figure 2.
Effect of systemic TA injection on mechanical pain behavior induced by spinal nerve ligation. Withdrawal threshold to mechanical stimulation of the hind paws was measured in the ipsilateral paw on the indicated postoperative day (POD). The POD 0 value is the average of three measurements taken over 5 days prior to the surgery. The shaded area indicates the time of TA injections. *, days on which there was a significant difference between steroid and saline treated animals (Two-way RM ANOVA).
Figure 3.
TA injection has only a transient effect if initiated after pain behaviors are established, and does not affect pain behavior in uninjured animals. A, effect of TA injection beginning on POD7, as indicated by the shaded area. *, only one measurement showed significant differences in individual t tests (p = 0.004 without correction for multiple testing). B. Injection of steroid (shaded area) has no effect on cutaneous sensitivity in unoperated rats.
Corticosteroid treatment reduces sympathetic sprouting after SNL
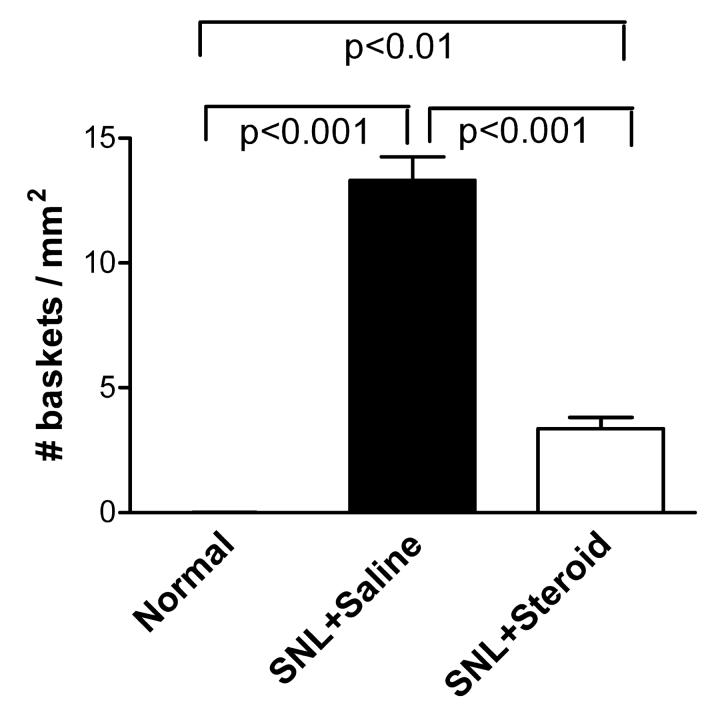
After SNL, sprouting of sympathetic fibers particularly around large- and medium-diameter cells is revealed by immunostaining for TH. The fibers often form distinctive basket-like structures around the neurons. Examples of the basket formations in a saline-treated animal on POD8 are shown in Fig. 4A, and from a TA-treated animal in Fig. 4B. We quantified the degree of sympathetic sprouting by measuring the density of these basket structures in fixed sections of DRG on POD 8. In animals treated with TA (n=11) just before and for the first 3 days after the SNL surgery, the density of such basket formations was reduced almost four-fold, though it was still significantly higher than in control animals (n=12, Fig. 5). As previously reported, basket formations were quite rare in normal, uninjured animals (n=8).
Figure. 4.
Examples of sympathetic fiber basket formations in DRG tissue sections stained for TH. DRG were isolated on POD 8. Arrows indicate examples of neurons surrounded by basket formations. A, example from a saline-injected SNL animal. B, example from a TA injected SNL animal. Scale bar=50 μm.
Figure 5.
TA treatment for the first three days after SNL markedly reduces the density of sympathetic fiber basket formations. DRG sections were stained for TH on POD 8, and basket formations counted as described in Methods. Indicated p values comparing each pair of experimental groups are from one-way ANOVA with Tukey's post test.
Corticosteroid treatment reduces incidence of bursting in spontaneously active neurons
SNL is well-known to cause an increase in excitability and spontaneous activity in the axotomized cells (see Discussion). We examined spontaneous activity and several measures of excitability in large and medium diameter cells using intracellular recording from isolated whole DRG at 37°C on POD 7 (n = 161 cells from 5 steroid-treated animals and 137 cells from 4 saline-treated animals). Most of the excitability parameters measured were not significantly altered by steroid injection on POD 0 – 4, including resting membrane potential (−60.0 ± 0.48 mV in cells from steroid treated animals vs. −59.4 ± 0.62 mV in cells from saline treated animals, p = 0.42); rheobase (0.85 ± 0.04 vs. 0.95 ± 0.05 nA, p =0.13); action potential threshold (−41.6 ± 0.67 vs. −42.4 ± 0.83 mV, p = 0.45); action potential amplitude (31.2 ± 0.60 vs. 32.2 ± 0.63 mV, p = 0.26); action potential duration (1.8 ± 0.05 msec in both groups, p = 0.54). However, the input resistance was significantly higher after steroid treatment (33.4 ± 1.41 vs. 29.1 ± 1.54 MΩ, p = 0.001, Mann-Whitney test), and the duration of the after-hyperpolarization was significantly longer (3.5 ± 0.18 vs. 2.9 ± 0.16 msec, p = 0.03, Mann-Whitney test). As previously reported, 21 we observed a relatively high incidence of spontaneous activity after SNL which could be readily classified as tonic, bursting, or irregular. The overall incidence of spontaneous activity did not differ between the steroid and saline treated animals (Fig. 6A). However, a lower percentage of the spontaneously active cells showed a bursting pattern of activity after steroid treatment, when measured either as the overall incidence (Fig. 6B), or as the percentage of spontaneously active cells that were of the bursting type (Fig 6C). In addition, the average firing frequency of those cells that were spontaneously active was significantly lower after steroid treatment (Fig 6D).
Figure 6.
Effect of steroid on spontaneous activity (SA). A, incidence of spontaneous activity in large and medium diameter cells was not significantly altered by steroid treatment (p = 0.85, Fisher's exact test). B. The overall incidence of a bursting pattern of spontaneous activity was significantly lower after steroid treatment (p = 0.048). C. Incidence of bursting activity normalized to total number of spontaneously active cells was also significantly lower after steroid treatment (p = 0.03). D. The average action potential frequency in spontaneously active cells was significantly lower after steroid treatment (p = 0.006, Student's t-test).
DISCUSSION
The primary findings of this study are that SNL causes robust increases in several pro-inflammatory cytokines within the DRG, and that a systemic anti-inflammatory steroid, TA, given for 4 days beginning just before spinal nerve ligation, can significantly reduce behavioral pain for at least 7 days, reduce sympathetic sprouting, and mitigate increases in pro-inflammatory cytokines. These findings support the idea that the SNL nerve injury model has important inflammatory contributions. In addition, the observation that TA was effective when given starting at the time of injury suggests that steroid treatment might alter the development of chronic pain resulting from certain surgical procedures that involve nerve injury, such as amputation or hernia repair.
A number of studies have shown that glucocorticoids (applied systemically, locally, or intrathecally) can reduce pain behaviors in various rodent nerve injury models (e.g., 22-24). These studies are generally similar to ours in demonstrating only a partial reduction of pain behavior. However, there are discrepancies in the literature about the ability of steroids to reduce pain behaviors if given after pain is well established. This procedure was ineffective in our study, but in some studies delayed application is effective (e.g. 25,26). In addition, some studies found glucocorticoids to be effective in reducing thermal hyperalgesia (not examined in the present study), but not mechanical hyperalgesia 26, or found that continuous drug application reduced hyperalgesia when daily injections did not 27. An even more striking discrepancy comes from several studies showing that glucocorticoid receptor antagonists given intrathecally can reduce pain behaviors while glucocorticoids enhance them 28,29. These studies were initiated based on the finding that the glucocorticoid receptor in spinal cord neurons is upregulated in the chronic constriction injury model. Glucocorticoids act through three primary mechanisms: direct regulation of transcription, inhibition of transcription effects mediated by nuclear factor-κB (an important transcription factor upregulated in inflammation), and extra-genomic effects. These mechanisms are thought to have different dose-response curves 30. Hence it is likely that understanding the conflicting results about effects of glucocorticoids in nerve injury models will require careful consideration of both dose-response and anatomical site of action of the drugs.
In our study, the primary effect of TA on spontaneous electrical activity after SNL was to reduce the number of sensory neurons firing with a bursting pattern, and to reduce the average firing frequency. Previous studies have documented the increase in spontaneous activity in large and medium diameter DRG neurons after SNL (e.g., 31-34). The incidence of spontaneous activity observed in this study after SNL (11.1%) was not significantly different from that observed in our recent study using identical recording procedures (11.5% in large diameter cells, 19.5% in medium diameter cells). 21 In contrast, the incidence of spontaneous activity in large and medium diameter cells is much lower in control rats, e.g. 5% in our previous study, with similarly low values observed in other studies using a variety of recording methods. 17,35-38 In various models of chronic pain, spontaneous activity is one of the earliest abnormalities observed, and blocking this spontaneous activity is a very effective way to block subsequent development of pain behaviors.39. In the present study, we found that although TA was very effective at reducing later-occurring events such mechanical pain, sympathetic sprouting and inflammatory cytokines, it had little effect on the overall incidence of spontaneous activity. This suggests that spontaneous activity may be “upstream” of these other, steroid-responsive effects, consistent with the fact that spontaneous activity is one of the earliest observed consequences of nerve injury. Another possibility is that the bursting form of spontaneous activity, which was the only type of spontaneous activity significantly reduced by TA, is much more effective than other forms of activity at inducing pain behaviors, sprouting and/or cytokine production. Work in other systems indicates that the bursting form of activity may be particularly effective at releasing transmitter, and may specifically increase release of peptidergic transmitters in neurons that have peptidergic and nonpeptidergic co-transmitters.40,41
Although we do not know why systemic steroid treatment reduces fiber sprouting, it may be explained by decreased expression of certain inflammatory cytokines such as IL-6, which has been reported to be able to cause sympathetic growth.42,43 Other cytokines such as GRO/KC may be involved, too, because GRO/KC is known to promote angiogenesis in various tissues, 44,45 and sympathetic fibers are always associated with vascular processes in the DRG.46,47
We recently examined the cytokine profile in the newly developed DRG localized inflammation model, 13 which was designed to examine the effects of inflammation on neurons in the absence of injury (see Introduction). It is interesting that the cytokine profile bears many similarities to that reported in this study in the SNL model, a model designed to examine the effects of nerve injury. All the cytokines observed to increase on POD 3 in the SNL model were also observed to increase in the DRG inflammation model, with roughly comparable fold-increases. Two additional cytokines, IL-18 and IL-1β, were significantly elevated in the localized inflammation model but not the SNL model. However, these increases were much more modest than for other upregulated cytokines in the localized inflammation of the dorsal root ganglion model (∼1.7 fold), so the nonsignificant (∼1.2-fold) increases we observed in SNL may reflect a similar trend, though not quite reaching significance. In both models most cytokines elevated at POD 3 decline by POD 7. However, the two models diverge more at later times. Although both models still have significantly elevated MCP-1 levels on day 7, in the DRG inflammation model, the lL-18 level does not show the early peak typical of other elevated cytokines, being higher on day 7 than on day 3 and showing sustained elevation through at least day 14, and the IL-1β increase, though modest, is also more sustained. As noted above, these cytokines are not significantly elevated in the SNL model and the measured values showed a tendency to decrease, not increase, by day 7. In the SNL model, it is IL-6 that shows a more sustained increase, declining much more slowly than in the localized inflammation model.
The significant elevation of GRO/KC in both models suggests an important role for neutrophils, although it should be noted that cytokines may have roles in the nervous system that differ significantly from their immunological roles in other tissues.48. Neutrophil invasion of peripheral nerve has been demonstrated in several other models of nerve injury, where depletion of neutrophils attenuates pain behaviors 9. The SNL and DRG inflammation models also both show similar, sustained declines in IL-4, IL-2, and IL-12, although the IL-12 decrease was not significant in the SNL model until day 7. The decline in IL-4 is expected in an inflammatory setting, since this is generally considered an anti-inflammatory cytokine. IL-2 and IL-12 are generally considered to be pro-inflammatory cytokines whose primary source is T cells; the observation that they decline rather than increasing suggests that T cell responses are not dominant in either model. An interesting finding in the present SNL study was that TA treatment was very effective at reducing SNL-induced cytokine increases but had no ability to restore levels of the 3 cytokines that declined. This is in contrast to many examples from the immunology literature, in which IL-4 is increased by corticosteroid treatment, consistent with a general view that corticosteroids shift the immune system from a TH1 cell response towards a TH2 cell response.49,50 The failure of TA to increase IL-4 in this study also suggests T cell responses are not dominant in this model.
The finding that pro-inflammatory cytokines that increase on PDO3 have declined again by day 7, while the pain behaviors last much longer, suggests that these cytokines are more likely to play a role in initiation of the pain behaviors than in their maintenance. We also observed that TA treatment was much less effective in reducing pain behavior if initiated on POD 7, after pain behavior was well established. In this case the TA effects did not outlast the application of the drug. This finding is in general agreement with previous work in that it implies that initiation and maintenance of chronic pain behaviors are distinct processes. For example, blockade of spontaneous activity at a nerve injury site is very effective at blocking development of pain behaviors if applied during the first week after the injury, but has only a temporary effect if initiated at a later time point, after pain behaviors are well established.39
The overall gross similarity of the cytokine profiles in the SNL and localized inflammation models is perhaps not surprising, given that the SNL model involves a injury quite close to the DRG and has been previously shown to evoke immune cell infiltration and activation, which is more prolonged than the infiltration observed after the sciatic nerve is transected more distally, at the level of its trifurcation.15 The resulting pain behaviors are unlikely to be due entirely to immunogenic properties of the sutures, rather than to nerve injury, as it has previously been shown that that placing silk sutures adjacent to the DRG without ligating nerves will not evoke pain behaviors.51 This control was not included in the present study, however. Supporting the idea that the SNL model has a relatively large inflammatory component at the DRG level, due to the proximity of the nerve injury, we find that the CCI and axotomy models, in which the nerve injury is more distal, show much more modest and slower increases in some of the same cytokines.39 Our cytokine results highlight the difficulty in drawing a clean distinction between nerve injury and inflammatory models of pain, a point which has been made previously based on other lines of evidence (see Introduction). It seems likely that, at the level of the DRG, either type of model may expose the sensory neurons to increased levels of certain pro-inflammatory cytokines. Understanding the effects of these cytokines on the sensory neurons should contribute to understanding both nerve injury and inflammatory models of chronic pain.
Acknowledgements
This work was supported in part by National Institutes of Health (Bethesda, MD, USA) grants NS39568 and NS45594 (Jun-Ming Zhang), and University of Cincinnati Millennium Fund, Cincinnati, OH, USA.
Footnotes
Summary Statement: Systemic injections of corticosteroid (triamcinolone acetonide) mitigate proinflammatory cytokine increases, mechanical pain behaviors and abnormal sympathetic sprouting in dorsal root ganglia after spinal nerve ligation, suggesting this nerve injury model has important inflammatory contributions.
References
- 1.Hogan Q. Animal pain models. Reg Anesth Pain Med. 2002;27:385–401. doi: 10.1053/rapm.2002.33630. [DOI] [PubMed] [Google Scholar]
- 2.Wall PD, Gutnick M. Properties of afferent nerve impulses originating from a neuroma. Nature. 1974;248:740–743. doi: 10.1038/248740a0. [DOI] [PubMed] [Google Scholar]
- 3.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 4.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 5.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 7.Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- 8.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 9.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 12.White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu X, Richardson PM. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. Journal of Neurocytology. 1993;22:334–341. doi: 10.1007/BF01195557. [DOI] [PubMed] [Google Scholar]
- 15.Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112:23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J-M, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- 17.Song XJ, Hu SJ, Greenquist KW, Zhang J-M, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J-M, Li H, Brull SJ. Perfusion of the mechanically compressed lumbar ganglion with lidocaine reduces mechanical hyperalgesia and allodynia in the rat. J Neurophysiol. 2000;84:798–805. doi: 10.1152/jn.2000.84.2.798. [DOI] [PubMed] [Google Scholar]
- 19.duPont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–191. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B, Li HQ, Brull SJ, Zhang JM. Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol. 2002;88:1393–1399. doi: 10.1152/jn.2002.88.3.1393. [DOI] [PubMed] [Google Scholar]
- 21.Xie W, Strong JA, Li H, Zhang JM. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol. 2007;97:492–502. doi: 10.1152/jn.00899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie W, Liu X, Xuan H, Luo S, Zhao X, Zhou Z, Xu J. Effect of betamethasone on neuropathic pain and cerebral expression of NF-kappaB and cytokines. Neurosci Lett. 2006;393:255–259. doi: 10.1016/j.neulet.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 23.Hashizume H, Rutkowski MD, Weinstein JN, DeLeo JA. Central administration of methotrexate reduces mechanical allodynia in an animal model of radiculopathy/sciatica. Pain. 2000;87:159–169. doi: 10.1016/S0304-3959(00)00281-5. [DOI] [PubMed] [Google Scholar]
- 24.Clatworthy AL, Illich PA, Castro GA, Walters ET. Role of peri-axonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci Lett. 1995;184:5–8. doi: 10.1016/0304-3940(94)11154-b. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Sawamura S, Sekiyama H, Tamai H, Hanaoka K. Effect of methylprednisolone on neuropathic pain and spinal glial activation in rats. Anesthesiology. 2004;100:1249–1257. doi: 10.1097/00000542-200405000-00029. [DOI] [PubMed] [Google Scholar]
- 26.Johansson A, Bennett GJ. Effect of local methylprednisolone on pain in a nerve injury model. A pilot study. Reg Anesth. 1997;22:59–65. doi: 10.1016/s1098-7339(06)80057-x. [DOI] [PubMed] [Google Scholar]
- 27.Kingery WS, Agashe GS, Sawamura S, Davies MF, Clark JD, Maze M. Glucocorticoid inhibition of neuropathic hyperalgesia and spinal fos expression. Anesth Analg. 2001;92:476–482. doi: 10.1097/00000539-200102000-00037. [DOI] [PubMed] [Google Scholar]
- 28.Takasaki I, Kurihara T, Saegusa H, Zong S, Tanabe T. Effects of glucocorticoid receptor antagonists on allodynia and hyperalgesia in mouse model of neuropathic pain. Eur J Pharmacol. 2005;524:80–83. doi: 10.1016/j.ejphar.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci. 2004;24:8595–8605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 31.Liu CN, Michaelis M, Amir R, Devor M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic pain. J Neurophysiol. 2000;84:205–215. doi: 10.1152/jn.2000.84.1.205. [DOI] [PubMed] [Google Scholar]
- 32.Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman V, Suzuki R, Dickenson AH. Electrophysiological characterization of spinal neuronal response properties in anaesthetized rats after ligation of spinal nerves L5-L6. Journal of Physiology-London. 1998;507:881–894. doi: 10.1111/j.1469-7793.1998.881bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han HC, Lee DH, Chung JM. Characteristics of ectopic discharges in a rat neuropathic pain model. Pain. 2000;84:253–261. doi: 10.1016/s0304-3959(99)00219-5. [DOI] [PubMed] [Google Scholar]
- 35.Govrin-Lippmann R, Devor M. Ongoing activity in severed nerves: source and variation with time. Brain Res. 1978;159:406–410. doi: 10.1016/0006-8993(78)90548-6. [DOI] [PubMed] [Google Scholar]
- 36.Wall PD, Devor M. Sensory afferent impulse originate from dorsal root ganglia as well as from the periphery in normal and nerve injury rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- 37.Amir R. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 39.Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: Early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whim MD, Lloyd PE. Frequency-dependent release of peptide cotransmitters from identified cholinergic motor neurons in Aplysia. Proc Natl Acad Sci U S A. 1989;86:9034–9038. doi: 10.1073/pnas.86.22.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramer MS, Thompson SW, McMahon SB. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain. 1999;(Suppl 6):S111–120. doi: 10.1016/S0304-3959(99)00144-X. [DOI] [PubMed] [Google Scholar]
- 43.Ramer MS, Murphy PG, Richardson PM, Bisby MA. Spinal nerve lesion-induced mechanoallodynia and adrenergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout mice. Pain. 1998;78:115–121. doi: 10.1016/S0304-3959(98)00121-3. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, Richmond A, Strieter R, Dey SK, DuBois RN. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM, Cassatella MA. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. 2004;172:5034–5040. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- 46.Zhang JM, Li H, Munir MA. Decreasing sympathetic sprouting in pathologic sensory ganglia: a new mechanism for treating neuropathic pain using lidocaine. Pain. 2004;109:143–149. doi: 10.1016/j.pain.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 47.McLachlan EM, Jang W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 48.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 49.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 50.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 51.Tabo E, Jinks SL, Eisele JH, Jr., Carstens E. Behavioral manifestations of neuropathic pain and mechanical allodynia, and changes in spinal dorsal horn neurons, following L4-L6 dorsal root constriction in rats. Pain. 1999;80:503–520. doi: 10.1016/S0304-3959(98)00243-7. [DOI] [PubMed] [Google Scholar]