SUMMARY
We have previously demonstrated that DHA at low micromolar concentrations has a remarkable effect on morphological differentiation of hippocampal neurons by increasing the population of neurons with more branches and longer neurites. In this study, possible involvement of the retinoid X receptor (RXR) in the DHA-induced hippocampal neurite outgrowth was evaluated as DHA is an endogenous ligand for RXR. Immunocytochemical examination revealed that all RXR isoforms, RXR-alpha, -beta 1, -beta 2 and -gamma, are expressed exclusively in neurons with distinctive intracellular distribution. The cell-based dual luciferase reporter assay indicated that DHA activates RXRα at or above 10 μM but not at 1.5 μM where DHA induces neurite outgrowth. Arachidonic acid also activated RXRα in a similar concentration range but with lower efficacy. Our results suggest that DHA-induced neurite outgrowth may not be mediated by direct activation of RXRα, although involvement of other isoforms or DHA metabolites can not be excluded.
Keywords: RXR, docosahexaenoic acid, arachidonic acid, hippocampal, neuronal cells
INTRODUCTION
Docosahexaenoic Acid (22:6n-3;DHA), a long chain polyunsaturated fatty acid, is particularly enriched in brain, and is essential for normal brain development and function (1,2). We have previously shown that DHA uniquely promotes neurite outgrowth in vitro by increasing the total neurite length and number of branches per neuron (3). It has been established that the signaling of retinoid X receptor (RXR) is involved in the nervous system development (4) and is essential for normal development (5). In addition, it has been demonstrated that DHA is an endogenous ligand for the RXR that functions as ligand-activated transcription factor (6), which in turn could lead to gene expression.
RXR belongs to the family of nuclear hormone receptors that are ligand-activated transcription factors. RXR is an obligatory component of various nuclear receptor heterodimers such as retinoic acid receptor, vitamin D receptor, thyroid hormone receptor and PPARs. RXR is activated in vitro by 9-cis-retinoic acid (9-cis-RA), a vitamin A metabolite, which binds with high affinity to the RXR ligand binding domain (7,8). Since 9-cis-RA is not detected in vivo (9), RXR has been considered as an orphan receptor lacking an identified endogenous ligand until a few years ago. Nevertheless, ligand-induced activation has been demonstrated with transgenic mice (4,5), indicating the presence of endogenous ligand in vivo. In 2000, Perlmann and collaborators demonstrated that DHA is an endogenous ligand for RXR (6). The DHA concentration required to reach half of the maximum activation (EC50) of RXRα was in the 50–100 μM range (6), however, depending on the method to prepare the DHA-containing medium the EC50 reported for RXRα activation was in the range of 5–10 μ M (10). The purpose of this work is to evaluate the possible role of RXR in the DHA-induced neurite outgrowth. We first examined the expression of RXR isoforms in the hippocampal neurons and subsequently RXR activation using Neuro 2A cell-based dual luciferase reporter assay. We found isoform-distinctive intracellular distribution of RXR in neurons. Although RXRα was activated by 10μ M DHA, no activation was observed at lower DHA concentrations such as 1.5 μ M, where the DHA promotes neurite outgrowth.
2. Materials and methods
2.1. Materials
Neuro 2A cell line was obtained from ATCC (Manassas, VA). Fetal bovine serum (FBS), Neurobasal medium, N2 supplements, DMEM and MEM were purchased from Invitrogen (Carlsbad, CA). Anti RXRα, β1, β2, and γ are rabbit polyclonal antibodies obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). 9-cis-Retinoic acid (9-cis-RA) and anti-MAP 2ab, mouse monoclonal were obtained from Sigma (St. Louis, MO). Rabbit polyclonal Anti-GFAP antibody and Dual Luciferase® Reporter Assay System were obtained from Promega (Madison, WI). Cy2-, Cy3- and HRP-conjugated secondary antibodies were acquired from Jackson Immunoresearch Laboratories, Inc (West Grove, PA). Docosahexaenoic acid (22:6n-3, DHA), oleic acid (18:1n-9, OA) and arachidonic acid (20:4n-6, AA) were obtained from Nu-Chek-Prep (Elysian, MN). Docosapentaenoic acid (22:5n-6, DPA) was a generous gift from Omegatech (currently incorporated Martek, Columbia, MD). Chambered slides were purchased from Lab-Tek (Naperville, IL), and 3,3’-diaminobenzidine (DAB) substrate was obtained from Roche (Indianapolis, IN). The Translucent RXR Reporter Vector reporter vector for RXR and control vector were obtained from Panomics (Fremont, CA). The vector CMX-GAL4-hRXRα was a generous gift from Dr. Thomas Perlmann at Ludwig Institute for Cancer Research (Stockholm, Sweden).
2.2. Cell Culture
Embryonic hippocampal cells were obtained from E18 rat hippocampi, and cultured as previously described (3). Briefly, hippocampi were dissected in CMF-HBSS, washed in the same solution and trypsinized (0.25%) for 15 min at 37°C. After washing with CMF-HBSS the tissue was resuspended in MEM supplemented with 1mM glutamine, 1mM sodium pyruvate, 100U/ml penicillin, 100μg/ml streptomycin and 10% heat inactivated horse serum. After 4 h, culture medium was changed to Neurobasal medium containing N2 supplements, 1mM glutamine, 100U/ml penicillin and 100μg/ml streptomycin. Hippocampal cells were seeded on poly-D-lysine chambered slides at a density of 30,000 cells/cm2. All cultures were maintained in humidified atmosphere of 5% CO2 at 37° C. The procedures employed in this study were approved by the National Institute on Alcohol Abuse and Alcoholism (LMBB-HK11). Neurite measurements were performed as previously described (3). Briefly, cells were stained with anti-MAP2 and HRP-conjugated secondary antibody after 6 days in vitro. Neurons were traced with a camera Lucida using a 40x oil objective and the Olympus B41 upright microscope from Olympus (Center Valley, PA). The neurite length was determined with the NIH image software Image J 1.25s. Fifty neurons were evaluated for each sample.
2.3. Immunocytochemistry
Cells were fixed with 0.4% paraformaldehyde in PBS at pH 7.4 for 30min. After permeabilization with 0.1% Triton X-100, cells were blocked for 2h with PBS containing 10% goat serum, and then incubated with the primary antibody against MAP-2ab (mouse monoclonal 1:250) and/or GFAP (rabbit polyclonal 1:1000). After washing with PBS, cells were incubated with the corresponding Cy2-, Cy3- or horseradish peroxidase-conjugated secondary antibody. Horseradish peroxidase (HRP) was developed using 3,3’-diaminobenzidine (DAB) following the manufacturer’s instructions.
2.4. Neuro2A transfection and Dual Luciferase Assay (DLR)
Mouse neuroblastoma cells (Neuro 2A) were seeded in 24 well plates and cultured in DMEM containing 5%FBS, 100U/ml penicillin and 100μg/ml streptomycin. Transfections were performed in triplicates with Lipofectamine 2000. The Translucent RXR and control luciferase reporter vectors were tested first. As an alternate approach, the CMX-GAL4-hRXRα encoding the hybrid GAL4 followed by the RXRα ligand binding domain (GAL4-hRXRα )(11), pFR-Luc, the firefly luciferase reporter plasmid containing the GAL4 binding site sequence, and phRL-TK, the renilla luciferase plasmid were used. At 24h after transfection, cells were stimulated with DHA, AA or 9-cis-RA in DMEM containing 0.2% FBS, 0.1% BSA and 40 μMα-tocopherol. After 24h stimulation cells were lysed and the RXR activation was assayed using the Dual Luciferase® Reporter Assay. The firefly luciferase expression was normalized to renilla luciferase and reported as relative luciferase acitivty.
3. Results
3.1. Effect of fatty acids on neurite outgrowth
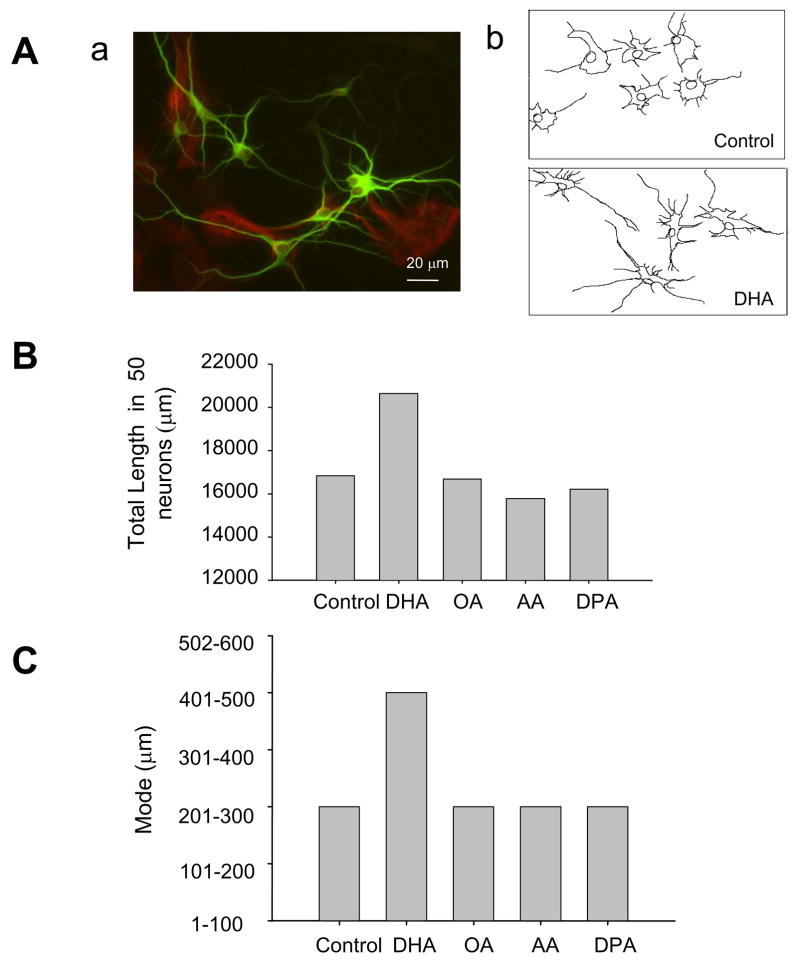
We have previously demonstrated that DHA increases the neurite outgrowth in hippocampal neurons by increasing the population of neurons with longer neurites and higher number of branches (3). This effect is unique for DHA as other fatty acids failed to promote neurite outgrowth (3). Hippocampal cultures grown for 6 days in vitro with N2 supplements contained neuronal and glial cells approximately in 1:1 ratio as shown in a representative photomicrograph in Figure 1Aa, where neurons and glial cells were specifically stained by MAP2 and glial fibrillary acidic protein (GFAP) antibodies, respectively. Incubation of hippocampal cultures with 1.5μ M individual fatty acids such as DHA, DPA, AA and OA for 6 days in chemically defined medium confirmed that DHA uniquely increases the total neurite length in hippocampal neurons (Figs. 1Ab, 1B, 1C). The sum of all neurites in 50 neurons and the mode of the total neurite length/neuron increased with DHA supplementation but not with DPA, AA or OA supplementation.
Figure 1.
Hippocampal cultures after 6 days in vitro. Aa) Representative microphotograph showing MAP2 immunofluorescence staining for neuronal cells in green and GFAP staining for glial cells in red. Bar = 20μ m. Ab) Representative tracings of hippocampal neurons processed for immunocytochemistry with HRP after 6 days of incubation with or without 1.5μ M DHA. B) Sum of the total neurite length of all neurons after 6 days incubation with 1.5 μ M of the indicated fatty acids. Fifty neurons were counted for each fatty acid group. C) Mode of the neuronal population classified by the total neurite length per neuron (sum of all the individual neurites per neuron) in a population of 50 neurons incubated with the indicated fatty acids.
3.2. Expression of RXR isoforms in hippocampal cells
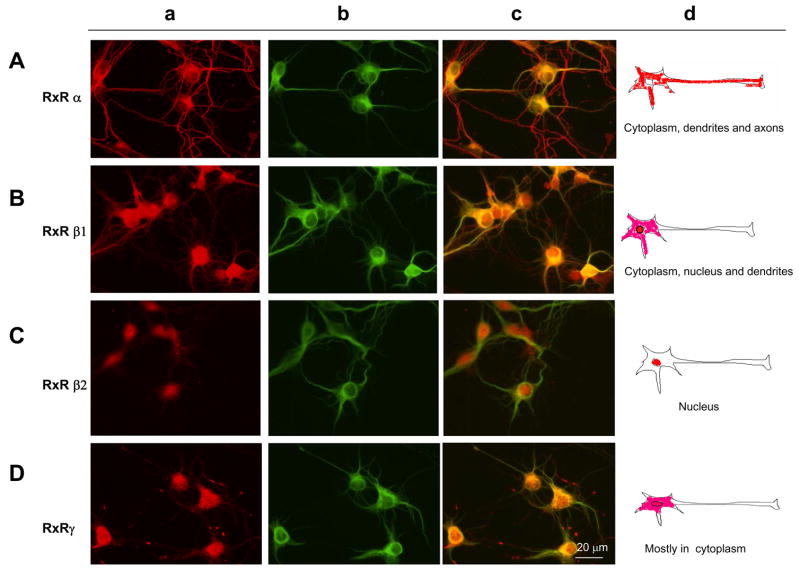
The expression of RXR isoforms was evaluated in hippocampal cultures after 6 days in vitro by double immunofluorescence. Immunoreactivity against all the RXR isoforms, alpha, beta, and gamma in hippocampal culture was detected exclusively in neurons in different patterns. RXRα immunoreactivity was distributed in the cytoplasm of soma and dendrites (Fig. 2Aa), as can be seen by the RXR immunoreactivity overlapping with MAP2 staining (Fig. 2Ab,c), a somatodendritic marker. RXRα also distributed in the axons (Fig. 2Aa,c). RXRβ 1 distributed in soma and dendrites, not only in the cytoplasm but also in the nucleus (Fig. 2Ba,c) and no distribution in the axon was observed. Overlapping of RXRβ 1 with MAP2 is shown in Figure 2Bc. RXRβ 2 showed an exclusive nuclear distribution in hippocampal neurons (Fig. 2Ca) confirmed by the lack of overlapping immunoreactivity with MAP2 (Fig 2Cc). RXRγ distributed within the soma, both in the cytosol and nucleus, (Fig. 2Da) and a weak staining was observed in dendrites (2Da). A punctated staining was observed around the neurons outside soma and dendrites, probably located in the axons. A scheme summarizing the distribution of RXR isoforms is shown in Figure 2d. Remarkably, immunoreactivity of all RXR isoforms was detected only in neuronal cells showing MAP2-positive staining. No RXR immunoreactivity was observed in glial cells which would have been confirmed by glial morphology and MAP2-negative staining.
Figure 2.
Immunnofluorescence of hippocampal cultures after 6 days in vitro. Staining for RXRα , RXRβ 1, RXRβ 2, and RXRγ is visualized in red (a), for MAP2 in green (b). Merged images from (a) and (b) are shown in (c). Bar = 20μ m (d) Schematic drawings showing the distribution of RXR isoforms in hippocampal neurons. All the RXR isoforms were expressed exclusively in neurons, and no detection was observed in glial cells.
3.3. Transcirptional activation of RXR by DHA and AA
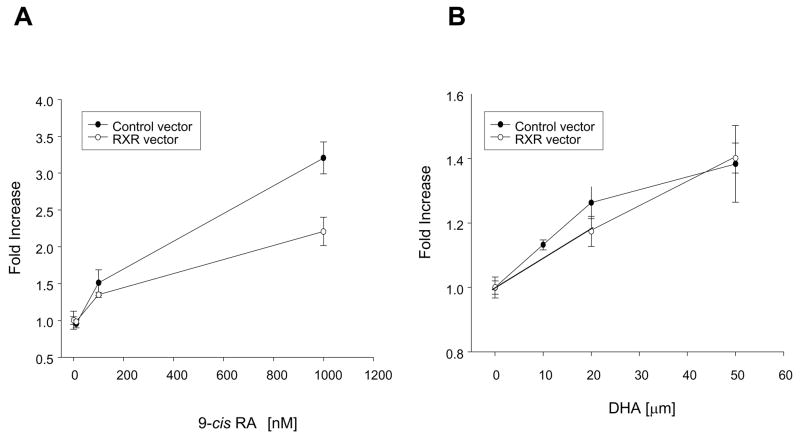
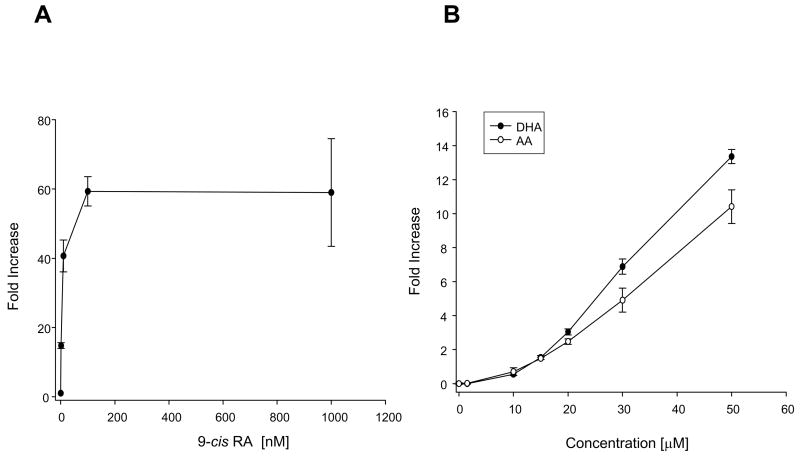
To evaluate the effect of DHA on transcriptional activity of RXR we have used a luciferase reporter assay. We first tested the RXR reporter vector which contained 5 repeats of the RXR binding element. Binding of the activated RXR at this site should result in the expression of the luciferase which in turn generates bioluminescence when exposed to its substrate, luciferin. We used the Dual Luciferase® Reporter Assay system that refers to the simultaneous expression and measurement of the experimental reporter vector that expresses firefly luciferase and the reference reporter vector that expresses Renilla luciferase, providing an internal control for the baseline response. Neuro2A cells transfected with the control and RXR reporter vectors, were evaluated for RXR transcriptional activity. Stimulation with cis-retinoic acid (9-cis-RA), a specific ligand for RXR showed a dose-dependent responses for both RXR and control reporter vectors (Fig. 3A), suggesting a lack of specificity of the RXR reporter vector in response to 9-cis-RA. Stimulation with different concentrations of DHA also showed lack of specificity as both RXR and control reporter vectors responded to DHA (Fig. 3B). As an alternative approach to evaluate the RXR activation, we used the pFR-Luc reporter vector which contains five GAL4 binding sites upstream of the promoter region of the firefly luciferase, and the CMX-GAL4-hRXRα encoding the hybrid GAL4-hRXRα ligand binding domain (GAL4-hRXRα ) (11). The negative control was the background luciferase activity measured in the absence of the GAL4-hRXRα , which resulted in no increase in luciferase activity as expected (data not shown). The activation of the RXRα ligand binding domain by its ligand resulted in an increase in luciferase expression. Stimulation with different concentrations of 9-cis-RA induced a concentration-dependent increase of the response in the nanomolar range (Fig. 4A) as expected, demonstrating specific activation of RXRα by this ligand. Stimulation with increasing concentrations of DHA showed activation of RXRα at or above 10 μ M (Fig. 4B). DHA concentrations above 50 μ M were toxic in this experimental condition using a low level of serum (data not shown). Concentrations below 10 μ M did not show significant stimulation of RXRα . To compare the effect of DHA stimulation of RXRα in relation to other fatty acids, AA in increasing concentrations was evaluated for its capability to stimulate the RXR. As seen from the dose-response curve in Figure 4B, AA and DHA similarly stimulated the RXR at 10 and 15μ M but at higher concentrations such as 20, 30 and 50 μ M, AA was not as effective as DHA.
Figure 3.
Stimulation of RXR evaluated by the Translucent RXR luciferase reporter assay. Neuro2A cells were transfected with the RXR luciferase reporter vector and the control vector. Cells were stimulated 24h later. A) Stimulation with different concentrations of 9-cis-RA. Non-specific activation of luciferase expression was observed with the RXR and control vectors. B) Stimulation with different concentrations of DHA. Non-specific activation of luciferase expression was observed with RXR and control vectors.
Figure 4.
Stimulation of RXRα evaluated by the GAL4-hRXRα luciferase reporter assay. Neuro2A cells were transfected with GAL4-hRXRα and pFR-Luc reporter vectors and stimulated at 24h after transfection. The control lacked the GAL4-hRXRα vector. A) Stimulation with different concentrations of 9-cis-RA. A dose-dependent response of luciferase expression was observed in the nanomolar range. B) Stimulation with different concentrations of DHA or AA. Dose-dependent activation of luciferase expression was observed in the micromolar range.
4. Discussion
In this study, we investigated the possible participation of RXR in the stimulating effect of DHA on neurite outgrowth. We found that hippocampal neurons express all the isoforms of RXR, RXR-α, -β1,- β 2 and -γ, with different patterns of distribution. The RXRα isoform was the only one distributed along the axon but expressed in negligible levels in the nucleus. Nuclear expression was seen for other isoforms, RXR-β 1, -β 2 and -γ. The β 2 isoform showed an exclusive nuclear distribution while RXR-β 1 also distributed cytoplasm of soma and dendrites. The expression of the γ isoform appeared to be restricted to the soma and possibly in some portions of the axons. The finding that only neurons but not glia expressed RXR suggests a specific role of RXR in neurons.
The commercial RXR reporter vectors showed a concentration dependent response to 9-cis RA and DHA in the nanomolar and micromolar ranges, respectively. Unexpectedly, the control reporter vector responded to 9-cis-RA and DHA in a concentration dependent manner too, presenting a problem of non-specificity. The alternative approach using the CMX-GAL4-hRXRα produced specific dose-dependent responses from nanomolar concentrations of 9-cis RA, and micromolar concentrations of DHA. It has been previously reported that DHA induces the transcriptional activation of RXRα over 10 μ M concentrations (10) when DHA was added in the presence of 10% stripped calf serum. In the present work, we used a low serum content (0.2% fetal bovine serum plus 0.1% BSA) to be consistent with the condition used for inducing neurite outgrowth, and to avoid possible interference from animal serum by decreasing the effective concentration available to stimulate the RXRα. However, our results were similar to those previously reported in that the necessary concentrations to stimulate the RXRα were at or over 10 μ M. AA showed a similar capacity to activate the RXRα as DHA, although at higher concentrations AA was less effective. No RXRα activation was detected with DHA at 1.5 μ M, the concentration where DHA stimulated neurite outgrowth in hippocampal neurons, suggesting that RXRα activation may not be directly involved in the observed DHA-induced neurite outgrowth. Despite the lack of RXRα activation, the participation of RXR in DHA-induced neurite outgrowth cannot be ruled out. In fact, nuclear localization of RXRα in hoppocampal neurons is minimal in comparison to other isoforms, suggesting a possible involvement of other RXR isoforms in DHA-promoted neurite outgrowth. In addition, the possibility that DHA derivatives (12) are generated during the culture and activate the RXR remains to be evaluated.
Acknowledgments
We thank Dr. Henry L. Puhl III from the Section on Transmitter Signaling, Laboratory of Molecular Physiology, NIAAA for his expert assistance on the DLR assay. This work was supported by the intramural program of the National Institute on Alcohol Abuse and Alcoholism.
This work was supported by the intramural program of NIAAA, NIH.
Abbreviations used
- FBS
fetal bovine serum
- DAB
3,3’–diaminobenzidine
- RXR
Retinoid X receptor
- DHA (22, 6n-3)
docosahexaenoic acid
- AA (20, 4n-6)
arachidonic acid
- OA (18, 1n-9)
oleic acid
- DPA (22, 5n-6)
docosapentaenoic acid
- 9-cis RA
9-cis Retinoic acid
- DMEM
Dulbecco’s modified eagle’s medium
- MEM
minimum essential medium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 2.Uauy R, Calderon F, Mena P. Essential fatty acids in somatic growth and brain development. World Rev Nutr Diet. 2001;89:134–160. doi: 10.1159/000059785. [DOI] [PubMed] [Google Scholar]
- 3.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 4.Solomin L, Johansson CB, Zetterstrom RH, Bissonnette RP, Heyman RA, Olson L, Lendahl U, Frisen J, Perlmann T. Retiinoid –X receptor signaling in the developing spinal cord. Nature. 1998;395:398–402. doi: 10.1038/26515. [DOI] [PubMed] [Google Scholar]
- 5.Mascrez B, Mark M, Dierich A, Ghyselinck N>B>, Kastner P, Chambon P. the RXRalpha ligand-dependent activation function 2 (AF-2) is important ofr mouse development. Development. 1998;125:4691–4707. doi: 10.1242/dev.125.23.4691. [DOI] [PubMed] [Google Scholar]
- 6.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 7.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 8.Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, speck J, Kratzeisen C, Rosenberger M, Lovey A, Grippo JF. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXRα. Nature. 1992;355:359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 9.Horton C, Maden M. Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev Dyn. 1995;202:312–323. doi: 10.1002/aja.1002020310. [DOI] [PubMed] [Google Scholar]
- 10.Lengqvist J, de Urquiza AM, Bergman AC, Willson TM, Sjovall J, Perlmann T, Griffiths WJ. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor α ligand–binding domain. Mol Cell Proteomics. 2004;3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9:769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]