Abstract
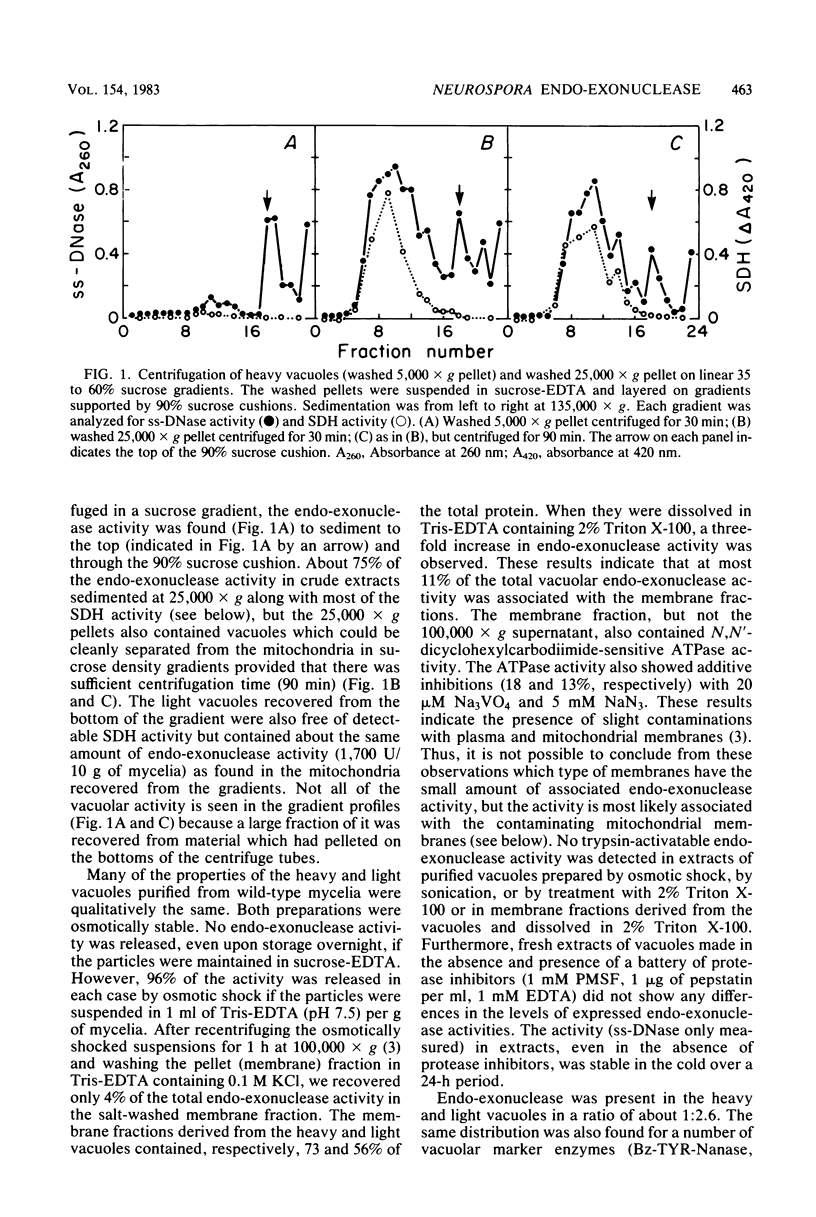
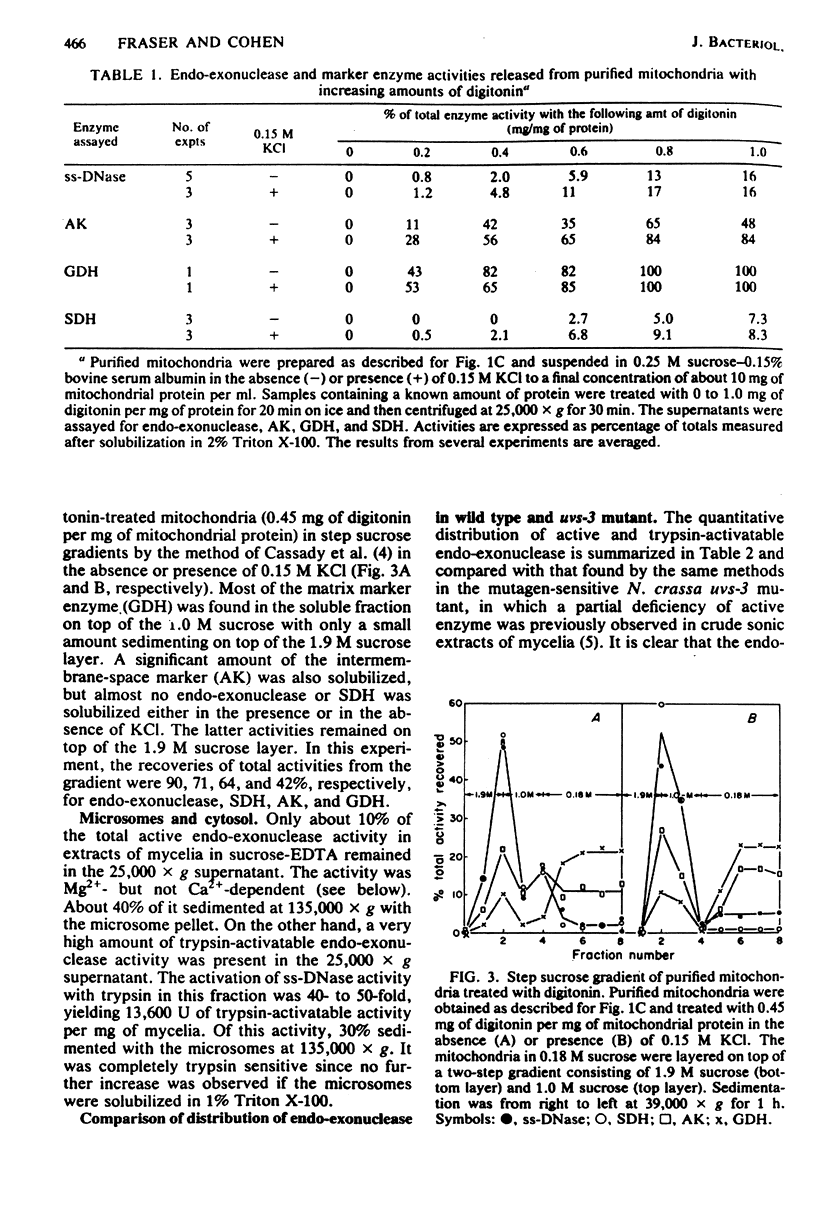
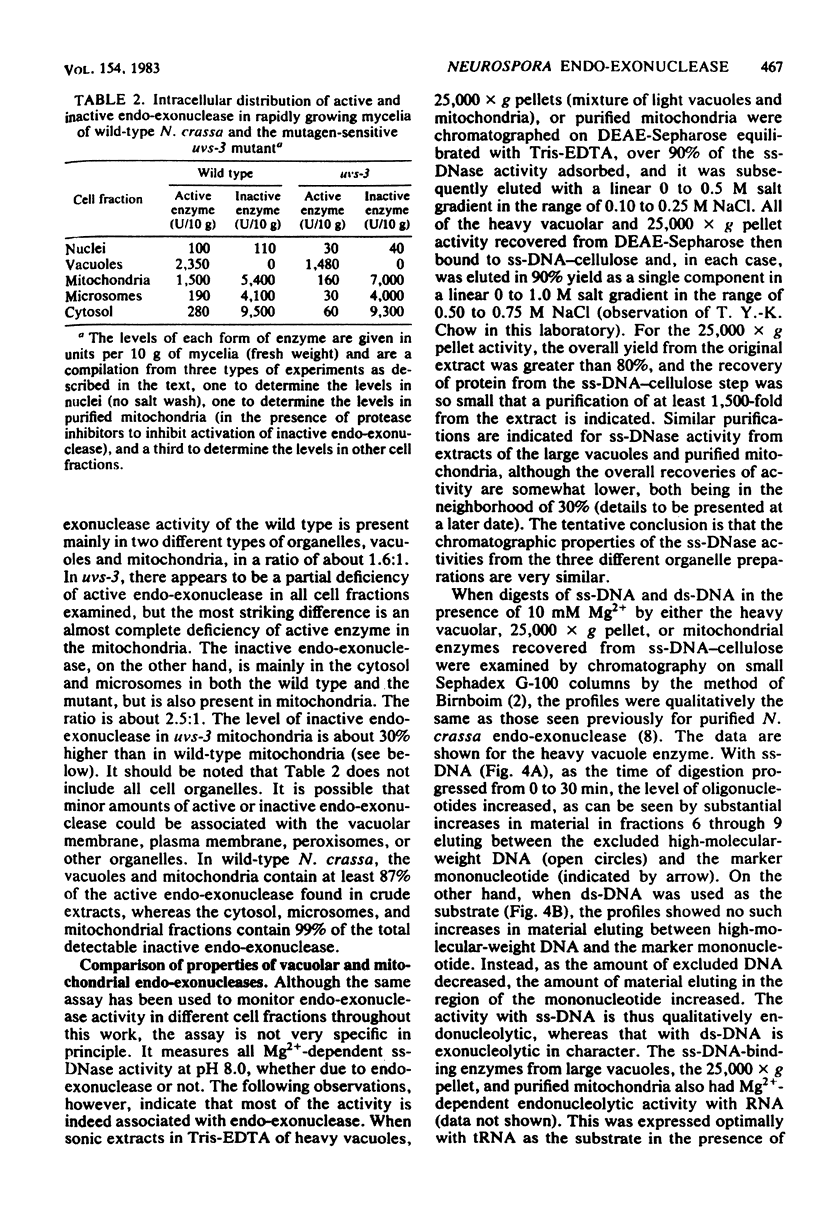
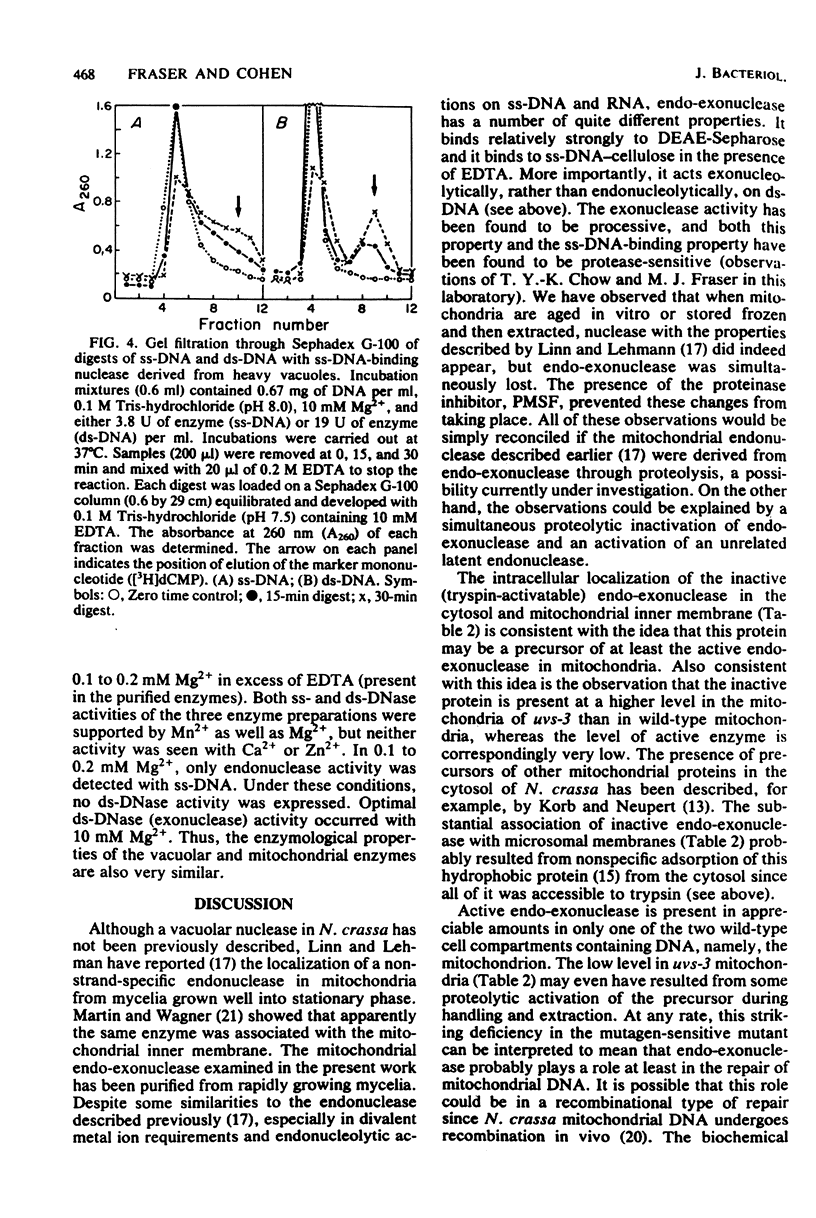
Endo-exonuclease of rapidly growing mycelia of Neurospora crassa was found to be distributed in a ratio of about 1.6:1 in vacuoles and in mitochondria where it is associated with the inner membrane. Although the activity in vacuoles was readily released by osmotic shock, very little of that in mitochondria was released by this method. The mitochondrial activity was partially (60 to 70%) released by sonication, and the remaining activity was solubilized in the presence of Triton X-100. An inactive form of endo-exonuclease, activated in vitro by treatment with trypsin, is present in mycelia at a level over four times that of active enzyme. It was found to be distributed in a ratio of about 2.5:1 in the cytosol and in the inner membrane of mitochondria. The mitochondrial protein was more tightly bound than the active enzyme. Very little of the inactive enzyme was released by sonication, but it was solubilized in the presence of Triton X-100. The intracellular distribution of active and inactive forms of endo-exonuclease differs in a mutagen-sensitive mutant of Neurospora crassa (uvs-3) which shows many pleiotropic effects. The most striking difference in distribution is in the mitochondria where endo-exonuclease is present almost entirely in the inactive form at a level 30% higher than in wild-type mitochondria.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. The use of gel filtration to distinguish between endonucleolytic and exonucleolytic types of degradation. Biochim Biophys Acta. 1966 Apr 18;119(1):198–200. doi: 10.1016/0005-2787(66)90052-9. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J. Identification and properties of an ATPase in vacuolar membranes of Neurospora crassa. J Bacteriol. 1982 Sep;151(3):1326–1337. doi: 10.1128/jb.151.3.1326-1337.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CODDINGTON A., FINCHAM J. R. PROOF OF HYBRID ENZYME FORMATION IN A CASE OF INTER-ALLELIC COMPLEMENTATION IN NEUROSPORA CRASSA. J Mol Biol. 1965 May;12:152–161. doi: 10.1016/s0022-2836(65)80289-3. [DOI] [PubMed] [Google Scholar]
- Chow T. Y., Fraser M. J. The major intracellular alkaline deoxyribonuclease activities expressed in wild-type and Rec-like mutants of Neurospora crassa. Can J Biochem. 1979 Jun;57(6):889–901. doi: 10.1139/o79-109. [DOI] [PubMed] [Google Scholar]
- Fraser M. J. Alkaline deoxyribonucleases released from Neurospora crassa mycelia: two activities not released by mutants with multiple sensitivities to mutagens. Nucleic Acids Res. 1979 Jan;6(1):231–246. doi: 10.1093/nar/6.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M. J., Tjeerde R., Matsumoto K. A second form of the single-strand specific endonuclease of Neurospora crassa which is associated with a double-strand exonuclease. Can J Biochem. 1976 Nov;54(11):971–980. doi: 10.1139/o76-140. [DOI] [PubMed] [Google Scholar]
- Hautala J. A., Conner B. H., Jacobson J. W., Patel G. L., Giles N. H. Isolation and characterization of nuclei from Neurospora crassa. J Bacteriol. 1977 May;130(2):704–713. doi: 10.1128/jb.130.2.704-713.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- Korb H., Neupert W. Biogenesis of cytochrome c in Neurospora crassa. Synthesis of apocytochrome c, transfer to mitochondria and conversion to Holocytochrome c. Eur J Biochem. 1978 Nov 15;91(2):609–620. doi: 10.1111/j.1432-1033.1978.tb12714.x. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Marzluf G. A. Characterization of the sequence complexity and organization of the Neurospora crassa genome. Biochemistry. 1979 Aug 21;18(17):3705–3713. doi: 10.1021/bi00584a011. [DOI] [PubMed] [Google Scholar]
- Kwong S., Fraser M. J. Neurospora endoexonuclease and its inactive (precursor?) form. Can J Biochem. 1978 Jun;56(6):370–377. doi: 10.1139/o78-059. [DOI] [PubMed] [Google Scholar]
- Käfer E., Fraser M. Isolation and genetic analysis of nuclease halo (nuh) mutants of Neurospora crassa. Mol Gen Genet. 1979 Jan 31;169(2):117–127. doi: 10.1007/BF00271662. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linn S., Lehman I. R. An endonuclease from mitochondria of Neurospora crassa. J Biol Chem. 1966 Jun 10;241(11):2694–2699. [PubMed] [Google Scholar]
- Mannella C. A., Pittenger T. H., Lambowitz A. M. Transmission of mitochondrial deoxyribonucleic acid in Neurospora crassa sexual crosses. J Bacteriol. 1979 Mar;137(3):1449–1451. doi: 10.1128/jb.137.3.1449-1451.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Peeters-Joris C., Vandevoorde A. M., Baudhuin P. Subcellular localization of superoxide dismutase in rat liver. Biochem J. 1975 Jul;150(1):31–39. doi: 10.1042/bj1500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M. G. Colorimetric determination of inorganic phosphate in the presence of biological material and adenosine triphosphate. Anal Biochem. 1968 Jan;22(1):27–34. doi: 10.1016/0003-2697(68)90255-8. [DOI] [PubMed] [Google Scholar]
- Vaughn L. E., Davis R. H. Purification of vacuoles from Neurospora crassa. Mol Cell Biol. 1981 Sep;1(9):797–806. doi: 10.1128/mcb.1.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]




