Abstract
We have expressed dominant-active and dominant-negative forms of the Rho GTPases, Cdc42 and Rac, using vaccinia virus to evaluate the effects of these mutants on the signaling pathway leading to the degranulation of secretory granules in RBL-2H3 cells. Dominant-active Cdc42 and Rac enhance antigen-stimulated secretion by about twofold, whereas the dominant-negative mutants significantly inhibit secretion. Interestingly, treatment with the calcium ionophore, A23187, and the PKC activator, PMA, rescues the inhibited levels of secretion in cells expressing the dominant-negative mutants, implying that Cdc42 and Rac act upstream of the calcium influx pathway. Furthermore, cells expressing the dominant-active mutants exhibit elevated levels of antigen-stimulated IP3 production, an amplified antigen-stimulated calcium response consisting of both calcium release from internal stores and influx from the extracellular medium, and an increase in aggregate formation of the IP3 receptor. In contrast, cells expressing the dominant-negative mutants display the opposite phenotypes. Finally, we are able to detect an in vitro interaction between Cdc42 and PLCγ1, the enzyme immediately upstream of IP3 formation. Taken together, these findings implicate Cdc42 and Rac in regulating the exocytosis of secretory granules by stimulation of IP3 formation and calcium mobilization upon antigen stimulation.
Keywords: Cdc42p, Rac, calcium signaling, degranulation, signal transduction
Introduction
Antigen stimulation of the IgE receptor, FcεRI, triggers a signal transduction cascade leading to the degranulation of preformed secretory granules containing a variety of potent inflammatory mediators in mast cells and basophils during the allergic response. The initial stimulus, aggregation of IgE bound to FcεRI in response to multivalent ligand, elicits the phosphorylation of FcεRI by the tyrosine kinase, Lyn, recruitment of the kinase, Syk, to the plasma membrane, and subsequent phosphorylation and activation of many signaling proteins, including phosphatidylinositol 3-kinase (PI3-K), the guanine nucleotide exchange factor Vav, focal adhesion kinase (FAK), and phospholipase C-γ (PLCγ; for review, see Beaven and Baumgartner 1996). Activated PLCγ cleaves the membrane-bound lipid phosphatidylinositol-4,5-bisphosphate (PIP2-4,5) into diacylglycerol, a stimulator of protein kinase C (PKC), and inositol 1,4,5-trisphosphate (IP3), the ligand for the IP3 receptor calcium channel in the ER membrane (for review, see Berridge 1993). Binding of IP3 to its receptor releases the intraluminal calcium from the ER into the cytoplasm, which in turn causes the influx of extracellular calcium into the cell through the ICRAC plasma membrane calcium channel (Hoth and Penner 1992). The combination of both PKC activation and the increase in intracellular calcium is sufficient for promoting the targeting and fusion of secretory granules with the cell surface (Katakami et al. 1984; Sagi-Eisenberg et al. 1985).
A role for GTP-binding proteins in the signaling cascade leading to degranulation was originally put forth based on the observation that treatment of permeabilized mast cells with the nonhydrolyzable analogue, GTPγS, activated secretion, implying the involvement of either heterotrimeric or small G proteins, or both, in the secretory response (Howell et al. 1987). Introduction of inhibitory or stimulatory peptides into mast cells have implicated both the heterotrimeric G protein, Gi3 (Aridor et al. 1993), and the small G protein, Rab3 (Oberhauser et al. 1992), in the latter stages of membrane fusion during exocytosis.
Several lines of evidence have suggested that members of the Rho family of small GTP-binding proteins, Cdc42, Rac, and Rho, also participate in regulating degranulation in mast cells and RBL-2H3 cells, a well-characterized rat mucosal mast cell line. First, secretion efficiency in permeabilized mast cells was enhanced upon incubation with recombinant activated forms of Rho and Rac and reduced under conditions that inhibit Rho and Rac activity (Price et al. 1995; Norman et al. 1996). In a similar system, recombinant Rac and Cdc42 were shown to delay the onset of the progressive decrease in secretion efficiency normally observed after streptolysin-O treatment (Brown et al. 1998). The ability to retard the gradual loss of responsiveness to stimulation indicated that Rac and Cdc42 were able to functionally replace proteins that had diffused out of the permeabilized cell, either as regulators or direct components of degranulation. A Rac/RhoGDI complex purified from bovine brain cytosol was also found to retard the loss of the secretory response (O'Sullivan et al. 1996). However, when RhoGDI, a guanine nucleotide dissociation inhibitor that negatively regulates the Rho family of GTP-binding proteins, was introduced separately, exocytosis was inhibited (Mariot et al. 1996).
Second, stable expression of the dominant-negative forms of Cdc42 and Rac in RBL-2H3 cells inhibited antigen-induced degranulation and displayed different effects on cell morphology (Guillemot et al. 1997). The Rho family has been implicated in playing critical regulatory roles in cell motility, morphology, and cytoskeletal reorganization (for review, see Hall 1998). Studies examining the redistribution of actin filaments from the cortical region to the cell interior upon antigen stimulation suggested that Rho and Rac mediate the concomitant changes in cell shape and actin reorganization during degranulation in mast cells (Norman et al. 1996).
Third, treatment of RBL-2H3 cells with Clostridium difficile toxin B, which modifies RhoA and Cdc42 by monoglucosylation, completely blocked degranulation. In contrast, degranulation was unaffected in cells treated with Clostridium botulinum C3 transferase, which selectively impairs RhoA, thus implying that toxin B inhibited exocytosis by inactivating Cdc42 (Prepens et al. 1996).
To further define at which step in the signaling cascade or the exocytosis process the Rho proteins may be acting, we have constructed a vaccinia virus expression system to produce dominant-active and dominant-negative mutants of Cdc42 and Rac. Using a vaccinia virus infection scheme offers several advantages over the strategies that have been previously used to investigate degranulation. The infection process maintains an in vivo state and avoids the cell membrane disruption and subsequent leakage of soluble protein inherent in the permeabilized cell system. Permanently transformed cell lines expressing dominant-active or dominant-negative mutants are difficult to propagate, most likely owing to the many critical cellular functions mediated by the GDP/GTP cycling of the GTPases. Thus, the transient nature of viral infection over the course of several hours circumvents the continual overexpression of cytotoxic proteins in stable cell lines. Proteins expressed by vaccinia virus are properly modified and localized in the cell, an important requirement for activity since Rho family members are dependent on a lipid modification for proper membrane localization. Finally, applying a high multiplicity of infection ensures uniform expression levels within a whole cell population, hence leading to more consistent effects in biological assays.
Using this system, we have confirmed that activation of Cdc42 and Rac participates in FcεRI-mediated activation of degranulation and have traced a role for the GTPases to an early step in the signaling cascade. We demonstrate that Cdc42 and Rac can regulate the production of the second messenger IP3 and the consequent rise in intracellular calcium required for exocytosis. Furthermore, we have detected an in vitro biochemical interaction between activated Cdc42 and PLCγ1, suggesting that the small GTPases may feed into the signal transduction pathway at the level of PLCγ1 and the production of IP3.
Materials and Methods
Cells and Reagents
The RBL-2H3 cell line, IgE, and DNP/BSA were gifts from B. Baird and D. Holowka (Cornell University, Ithaca, NY). RBL cells were maintained in monolayer culture in MEM (Life Technologies) supplemented with 20% fetal bovine serum (Atlanta Biologicals, Inc.), 2% l-glutamine (Life Technologies) and 50 μg/ml gentamicin sulfate (Sigma) at 37°C in a 5% CO2 incubator. Cells were harvested with 0.05% trypsin, 0.53 M EDTA· 4Na (Life Technologies). Experiments were generally performed 3–4 d after passage.
Construction of Recombinant Vaccinia Viruses and Infection Procedure
Wild-type and mutant forms of Cdc42 and Rac bearing a myc-tag at the NH2 terminus were cloned into pSC65 (a gift from B. Moss, NIH) using the SalI and XmaI restriction sites. Screening, isolation and amplification of the recombinant viruses were performed essentially as described (Chen and Padmanabhan 1997) with the following exceptions. During construction of the recombinant virus, the pSC65 constructs bearing Cdc42 and Rac were transfected into CV-1 cells using LipofectAMINE reagent (Life Technologies). Infected cells were incubated with 0.33 mg/ml X-Gal during the screening procedure to detect viruses that had successfully incorporated the LacZ marker gene from pSC65 into the viral genome. Identification of viruses expressing recombinant protein was confirmed by performing Western blots on lysates of infected Hutk-143B cells with the anti-myc 9E10 antibody (American Type Culture Collection). Viruses that exhibited a high level of expression were further amplified in HeLa cells and stored in small aliquots at −80°C. Titers of amplified viral stocks generally ranged from 106–107 pfu/μl.
RBL-2H3 cells were infected with recombinant vaccinia virus at 20 pfu/cell in MEM media supplemented with 5% fetal bovine serum, 2% glutamine, 50 μg/ml gentamicin sulfate, and 1 μg/ml IgE for 6–7 h. Before infection, viruses were sonicated briefly, incubated with an equal volume of 0.25 mg/ml trypsin in 0.9% NaCl (Sigma) for 30 min at 37°C, and resonicated to disrupt aggregates of viruses that had formed during storage at −80°C.
Cell Lysis, Immunoblotting, and GST Binding Experiments
RBL-2H3 cells were washed with phosphate-buffered saline and lysed in buffer A (40 mM Hepes, pH 7.4, 1% Triton X-100, 1 mM EDTA, 150 mM NaCl, 50 mM NaF, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF) by rocking for 30 min at 4°C. Lysates were precleared by centrifugation at 12,000 g for 5 min and solubilized by boiling for 3 min in SDS sample buffer. Whole cell lysates were then resolved by SDS-PAGE and transferred to Immobilon P membranes (Millipore). For Western blot detection, the following primary antibodies were used at 1:1,000 dilution: rabbit anti-Cdc42 (Erickson et al. 1996), mouse anti-Rac (Santa Cruz Biotechnology), mouse anti-myc 9E10, rabbit anti-PLCγ1-1249 (Santa Cruz Biotechnology), and rabbit anti-IQGAP (a gift from M. Hart, Onyx Pharmaceuticals). Blots were developed using either sheep anti–rabbit or sheep anti–mouse coupled to horseradish peroxidase at a dilution of 1:10,000 and the ECL detection system (Amersham Corp.).
GST alone, GST fused to the limit Cdc42/Rac (p21)-binding domain from PAK3 (GST-PBD), and GST fusions to the wild-type and mutant forms of Cdc42 were expressed in E. coli and purified by affinity binding to agarose beads cross-linked to glutathione (Hart et al. 1994). Immobilized GST-PBD (∼50 μg) was incubated with RBL-2H3 cell lysates for 1 h at 4°C, and precipitates were then washed three times with buffer A. Bound proteins were eluted by boiling in SDS sample buffer and subjected to Western blot analysis with the anti-myc antibody. Immobilized GST alone, GST-Cdc42, GST-Cdc42L61, and GST-Cdc42N17 (∼50 μg) were incubated in 10 mM Tris-Cl, pH 7.5, 100 mM NaCl, 10 mM EDTA, and 1 mM PMSF for 15 min at room temperature to release prebound nucleotide, loaded with 0.5 mM GTPγS for 30 min at room temperature, and supplemented with 10 mM MgCl2. The GST proteins were then incubated with lysates generated from either nonstimulated RBL-2H3 cells or cells stimulated for 5 min with 100 ng/ml DNP/BSA for 1 h at 4°C in buffer B (10 mM Tris-Cl, pH 7.5, 100 mM NaCl, 0.5% Triton X-100, 1 mM PMSF, and 5 mM MgCl2). Protein precipitates were washed two times in buffer B supplemented with an additional 100 mM NaCl and once in buffer B minus the Triton X-100. Bound proteins were eluted by boiling in SDS sample buffer and subjected to Western blot analysis with the anti-PLCγ1 or anti-IQGAP antibody. Binding experiments between the GST fusion proteins and purified PLCγ1 from insect cell lysates (a gift from Fujio Sekiya and Sue Goo Rhee, NIH) were performed in a similar manner.
β-Hexosaminidase Secretion Assay
RBL-2H3 cells were plated in 48-well plates at a density of 2.5 × 105 cells/well overnight at 37°C and infected the following day. Infected cells were washed twice with Tyrode's buffer (135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 1 mg/ml BSA, and 20 mM Hepes, pH 7.4), and stimulated with 100 ng/ml DNP/BSA for 1 h at 37°C. The β-hexosaminidase content of samples was determined as previously described (Pierini et al. 1996). In brief, 50 μl of sample supernatant were incubated with 200 μl of 1 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide (Sigma) in 0.05 M citrate buffer (pH 4.5) for 1 h at 37°C. After quenching of the reaction with the addition of 500 μl of 0.05 M sodium carbonate and 0.05 M sodium bicarbonate, the β-hexosaminidase release was determined by comparing the OD400 measurements of the samples to the total amount of enzyme in cells lysed by 0.5% Triton X-100. For assays in which antigen stimulation was bypassed, cells were incubated in 20 nM PMA for 5 min, and then stimulated with either 1 μM A23187 or 500 nM thapsigargin (Calbiochem) for 1 h at 37°C.
Calcium Fluorescence
RBL-2H3 cells were plated in 100-mm plates at a density of 4 × 106 cells/plate overnight at 37°C and infected the following day. Infected cells were harvested, washed twice, and resuspended in Tyrode's buffer supplemented with 0.25 mM sulfinpyrazone at 1 × 106 cells/ml, and then loaded with 0.5 μM Indo-1/AM (Calbiochem) for 1 h with constant agitation at 37°C. Cells were then washed twice and resuspended in Tyrode's buffer and 0.25 mM sulfinpyrazone at 1 × 106 cells/ml. 3-ml aliquots of cell suspension were added to acrylic cuvettes maintained at 37°C and constantly stirred during the course of the experiment. DNP/BSA was added at 100 ng/ml to initiate calcium signaling. For experiments that specifically evaluate release of calcium from ER stores, 4 mM EGTA was added to cells before DNP/BSA stimulation. Indo-1 was excited at 330 nm and fluorescence emission was monitored at 400 nm with a Perkin-Elmer LS-5 fluorescence spectrophotometer.
IP3 Determination
The amount of IP3 in cells was determined using the [3H] radioreceptor assay kit (Dupont-NEN). RBL-2H3 cells were plated in 70 mm plates at a density of 3 × 106 cells/ plate overnight and infected the following day. Infected cells were washed twice with Tyrode's buffer, stimulated for 5 min with 100 ng/ml DNP/BSA, and lysed according to the manufacturer's instructions. The aqueous phases of the IP3 samples were ultrafiltered through a Centricon concentrator (Amicon) with a membrane cut-off of 10,000 mol wt to exclude proteoglycans that interfere with the assay (Choi et al. 1993).
Immunofluorescence
RBL-2H3 cells were plated on glass coverslips in 6-well plates at a density of 2 × 105 cells/well overnight and infected the following day. Infected cells were stimulated with 100 ng/ml DNP/BSA for 30 min, fixed in PBS/3.7% formaldehyde for 8 min, and permeabilized in PBS/0.1% Triton X-100 for 5 min. Incubations with primary and secondary antibody were performed for one hour each in a dark, humid chamber at 25°C. IP3 receptors were detected using a 1:100 dilution of rabbit anti-IP3 receptor antibody (Calbiochem) followed by a 1:200 dilution of anti-rabbit IgG conjugated to the Bodipy fluorophore (Molecular Probes). Cells were mounted in non-fade medium (Kirkegaard and Perry) and photographed on a Zeiss axioscope (Carl Zeiss, Inc.) with T-Max 400 film (Eastman Kodak Co., Inc.).
Results
Expression of Functional Wild-Type and Mutant Forms of Cdc42 and Rac Using a Vaccinia Virus Expression System
We have employed the vaccinia virus expression system to introduce dominant-active and dominant-negative mutants of Cdc42 and Rac, tagged with the myc epitope, into RBL-2H3 cells. To investigate whether the virus-expressed GTPases would exhibit the expected properties-dictated by the different mutations, in vitro binding experiments were performed between lysates from infected RBL-2H3 cells expressing the various mutants and GST fused to the GTPase (p21)-binding domain (PBD) of the p21-activated kinase, PAK3 (Bagrodia et al. 1995). The PBD, or CRIB motif, for Cdc42/Rac interactive binding, is conserved in a number of different effector proteins and mediates their interactions with Cdc42 and Rac in a GTP-dependent manner.
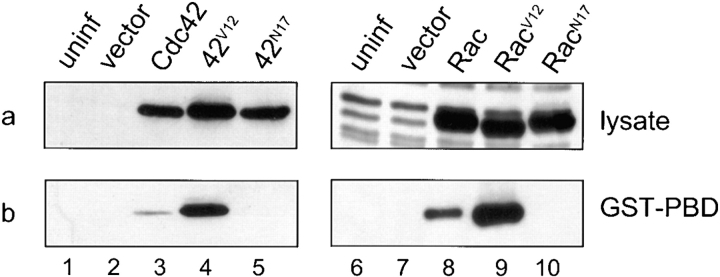
Examination of the virus-expressed mutant protein bound to GST-PBD by Western blotting using the anti-myc antibody demonstrated that both of the dominant-active mutants, Cdc42V12 and RacV12, interacted with GST-PBD (Fig. 1 b, lanes 4 and 9). In contrast, the two dominant-negative mutants, Cdc42N17 and RacN17, did not exhibit any detectable binding to the fusion protein (Fig. 1 b, lanes 5 and 10), as was the case with lysates from uninfected cells (Fig. 1 b, lanes 1 and 6) and cells infected with empty vector (Fig. 1 b, lanes 2 and 7). The wild-type forms of Cdc42 and Rac displayed relatively weaker binding, presumably reflecting a subpopulation of the wild-type GTPases that had acquired an activated state (Fig. 1 b, lanes 3 and 8). Protein expression levels from infections between the three forms of Cdc42 (Fig. 1 a, lanes 3–5) and Rac (Fig. 1 a, lanes 8–10) were comparable, indicating that uniform expression with different vaccinia viruses can be achieved. These results indicate that the virus-expressed forms of Cdc42 and Rac displayed the behavior predicted by their respective mutations and can be used to functionally interfere with the endogenous GTPases in RBL-2H3 cells.
Figure 1.
Wild-type and mutant forms of Cdc42 and Rac expressed using vaccinia virus are functionally active. (a) RBL-2H3 cells were infected with vaccinia virus containing empty vector (lanes 2 and 7), or a vector expressing Cdc42 (lane 3), Cdc42V12 (lane 4), Cdc42N17 (lane 5), Rac (lane 8), RacV12 (lane 9), or RacN17 (lane 10) for 6 h at 37°C, or were uninfected (lanes 1 and 6). Proteins were solubilized, resolved by SDS-PAGE, and the recombinant proteins were detected by Western blotting using anti-myc antibody. The additional protein bands present in the lanes containing lysates of the different forms of Rac are nonspecific (lanes 8–10), since they also appear in the uninfected and empty vector lanes (lanes 6 and 7). (b) Uninfected and infected RBL-2H3 whole cell lysates were incubated with immobilized GST-PBD for 1 h at 4°C. Bound proteins were washed and subjected to Western analysis using the anti-myc antibody.
Antigen-stimulated Degranulation Is Enhanced by Dominant-Active Cdc42 and Rac and Inhibited by Dominant-Negative Cdc42 and Rac
The effects of expressing dominant-active or dominant-negative mutants of Cdc42 and Rac on degranulation were examined by measuring the secretion efficiency of infected RBL-2H3 cells after antigen stimulation. Since any perturbation in the degranulation process will require sufficient expression of the recombinant protein in order to compete with endogenous wild-type protein for target binding, the relative levels of the virus-expressed and endogenous proteins in infected RBL-2H3 cells were determined. The recombinant proteins migrated with a slower mobility on Western blots probed with anti-Cdc42 and anti-Rac antibodies, compared with endogenous protein, due to the presence of the myc epitope (Fig. 2 a, lanes 3–5 and 7–9) and were absent in uninfected cells (Fig. 2 a, lane 1) and cells infected with empty vector (Fig. 2 a, lanes 2 and 6). Comparison of the protein levels between the two different forms revealed that approximately ≥50% of the total Cdc42 and Rac in the cell was derived from the virus-expressed source.
Figure 2.
Dominant-active Cdc42 and Rac enhance secretion, whereas dominant-negative Cdc42 and Rac inhibit secretion. (a) RBL-2H3 cells were infected with vaccinia virus containing empty vector (lanes 2 and 6), or a vector expressing Cdc42 (lane 3), Cdc42V12 (lane 4), Cdc42N17 (lane 5), Rac (lane 7), RacV12 (lane 8), or RacN17 (lane 9) for 6 h at 37°C, or were uninfected (lane 1). Proteins were solubilized and resolved by SDS-PAGE. Cdc42 and Rac were detected by Western blotting using either anti-Cdc42 or anti-Rac antibody, respectively. Virus-expressed proteins migrate at a slightly higher molecular mass compared with the endogenous protein due to the presence of the myc epitope. (b) β-Hexosaminidase secretion was measured in RBL-2H3 cells infected with the different forms of Cdc42 and Rac upon stimulation with 100 ng/ml DNP/BSA for 1 h at 37°C and under nonstimulated conditions. Degranulation is given as the percent of total cellular β-hexosaminidase released, as measured by treatment of RBL-2H3 cells in parallel with 0.5% Triton X-100. Error bars represent the standard deviation of four independent experiments. The paired t test was used to evaluate the statistical significance between cells infected with empty vector and Cdc42 (0.0264), empty vector and Cdc42N17 (0.0023), empty vector and RacN17 (0.0191), Cdc42 and Cdc42N17 (0.0437), and Rac and RacN17 (0.0424). A score of ≤0.05 denotes statistical significance. The comparison between cells infected with empty vector and Rac was not statistically significant.
Secretion assays were performed in infected RBL-2H3 cells by measuring the relative amounts of β-hexosaminidase, a secretory granule enzyme that is released into the extracellular media upon antigen stimulation with DNP/BSA. The infection process itself produced a modest inhibitory effect on degranulation, as evidenced by cells infected with empty vector exhibiting ∼26% secretion, a one-quarterfold decrease compared with the ∼35% secretion observed with uninfected cells (Fig. 2 b, +DNP/BSA). Strikingly, infection with the dominant-active mutants, Cdc42V12 and RacV12, enhanced the antigen-stimulated secretion to ∼46% and ∼53%, respectively, thus causing roughly a twofold increase compared with the release exhibited by cells infected with empty vector. In contrast, expression of the dominant-negative mutants, Cdc42N17 and RacN17, produced an inhibitory effect, reducing the levels of secretion to one-half of that of the empty vector control (i.e., values of ∼13% and ∼15%, respectively). The effects exhibited by the Cdc42 and Rac mutants confirmed that the levels of recombinant protein were sufficient to interfere with the activity of the endogenous wild-type protein. Overall, the ability of dominant-active Cdc42V12 and RacV12 to enhance antigen-stimulated secretion, together with the inhibitory effects caused by the corresponding dominant-negative mutants, Cdc42N17 and RacN17, strongly implicate Cdc42 and Rac in the signal transduction pathway leading to exocytosis.
Infection with wild-type Cdc42 and Rac resulted in levels of secretion comparable to those obtained with an empty vector control, albeit at slightly inhibited levels of ∼18% and ∼23%, respectively. The wild-type GTPases might have been predicted to either slightly stimulate secretion, since a fraction of each GTPase is present in an activated state (Fig. 1 b, lanes 4 and 9), or not affect secretion levels at all compared with the empty vector control. Instead, we have consistently observed that the wild-type Cdc42 or Rac has a slight inhibitory effect, which suggests that the wild-type GTPases enter into nonproductive binding interactions with other proteins that function downstream in this signaling pathway leading to degranulation. One possibility is that the binding of GDP-bound Cdc42 and/or GDP-bound Rac to a target/effector occurs with a measurable affinity, perhaps not significantly different from that for the GTP-bound forms of these proteins, but that the GDP-bound Cdc42/Rac interaction does not activate the target/effector. In such a case, the over-expression of wild-type Cdc42 or Rac would competitively inhibit the binding of the activated GTP-binding protein to the target and yield an inhibitory effect.
Cells containing the empty vector or expressing the wild-type and mutant forms of Cdc42 and Rac exhibited similar levels of basal secretion (≤5%) in the absence of antigen treatment (Fig. 2 b, −DNP/BSA). It is significant to note that RBL-2H3 cells expressing the dominant-active mutants, Cdc42V12 and RacV12, cannot bypass the requirement for DNP/BSA to effect degranulation. Thus, antigen binding may generate multiple signals in order to trigger degranulation, at least one of which involves a pathway that is not dependent on the activation of Cdc42 or Rac.
Cdc42 and Rac Function Upstream of the Calcium Influx Step and PKC Activation in Signaling Degranulation
To locate the step that Cdc42 and Rac may be modulating in the antigen stimulation pathway, infected cells were induced to degranulate by treatment with a combination of the calcium ionophore A23187 and phorbol 12-myristate 13-acetate (PMA). These agents synergistically act to bypass the requirement for antigen by directly initiating calcium influx into the cytoplasm, and by activating PKC, respectively (Katakami et al. 1984; Sagi-Eisenberg et al. 1985). Under these conditions, the continued maintenance of a secretion defect in cells expressing the dominant-negative mutants would indicate that the inhibition imparted by Cdc42N17 and RacN17 occurs downstream of calcium influx and PKC activation, whereas the ability to restore secretion would indicate that dominant-negative Cdc42 or Rac intervene at an upstream step.
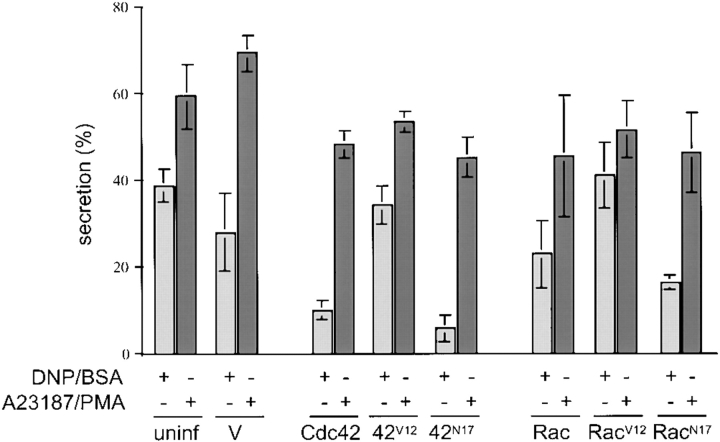
Treatment of control cells with A23187 and PMA activated degranulation more strongly than DNP/BSA, increasing the relative amount of β-hexosaminidase released from ∼39% to ∼59% in uninfected cells (Fig. 3). In cells infected with empty vector, degranulation was elevated from ∼28% in antigen-stimulated cells to ∼68% in A23187/PMA-treated cells, thus demonstrating that the slight decrease in secretion caused by vaccinia virus infection could be bypassed by treatment with A23187 and PMA. Secretion was substantially less inhibited in cells infected with either Cdc42N17 or RacN17 upon stimulation with A23187 and PMA, with percent degranulation increased from ∼6% and ∼16% in antigen-stimulated cells to ∼45% and ∼46% in A23187/PMA-treated cells, respectively. These levels of secretion were similar to those exhibited by cells expressing the dominant-active Cdc42V12 and RacV12 mutants upon A23187 and PMA treatment. The more modest inhibition that had been observed upon expression of wild-type Cdc42 or Rac was also relieved, with percent degranulation increased from ∼10% and ∼23% in antigen-stimulated cells to ∼48% and ∼45% in A23187/PMA-treated cells, respectively. Both A23187 and PMA were required to achieve substantial rescue from the inhibition. Stimulation with the ionophore alone restored secretion to only half that observed with both treatments in cells infected with the dominant-negative mutants, whereas PMA by itself did not activate degranulation above basal levels (data not shown).
Figure 3.
Treatment with the calcium ionophore A23187 and the PKC activator PMA rescues the secretion defect exhibited by antigen-stimulated cells expressing dominant-negative Cdc42 and Rac. RBL-2H3 cells were infected with vaccinia virus containing empty vector, the different forms of Cdc42 and Rac, or uninfected, for 6 h at 37°C. Cells were then stimulated with either 20 nM PMA for 5 min followed by 1 μM A23187 for 1 h or 100 ng/ml DNP/BSA for 1 h. Degranulation is given as the percent of total cellular β-hexosaminidase released, as measured by treatment of RBL-2H3 cells in parallel with 0.5% Triton X-100. Error bars represent the standard deviation of four independent experiments.
It should be noted that we have consistently found that treatment of cells expressing any form of Cdc42 or Rac with ionophore plus PMA yields a final level of secretion slightly below the levels measured in control cells (vector alone or uninfected). At the present time, we do not know the explanation for the slight dampening of the secretory response that is caused by expressing either of these GTPases. However, the fact that it is independent of their state of activation suggests that the dampening effect may reflect a perturbation of the GTP-binding/GTPase cycle of Cdc42 or Rac that occurs as an outcome of the general overexpression of any of these GTPases.
An alternative strategy to activating calcium influx with an ionophore is to treat cells with thapsigargin, a sesquiterpene lactone inhibitor of the ER Ca2+-ATPase pump, which normally maintains an intracellular calcium gradient by transporting free calcium from the cytoplasm back into the ER lumen (Lytton et al. 1991). Thus, blocking the function of the pump with thapsigargin effectively increases the cytoplasmic levels of free calcium. Using thapsigargin and PMA to stimulate degranulation, cells infected with Cdc42N17 and RacN17 also exhibited elevated levels of secretion, such that the percent degranulation was comparable to cells infected with empty vector (data not shown). The considerable stimulation of secretion in cells expressing the dominant-negative and wild-type Cdc42/Rac proteins implies that the combined effects of calcium mobilization and PKC activation are able to bypass the defect, thereby placing Cdc42 and/or Rac upstream of these two events in the antigen signaling pathway.
Cdc42 and Rac Regulate the Intracellular Calcium Response after Antigen Stimulation
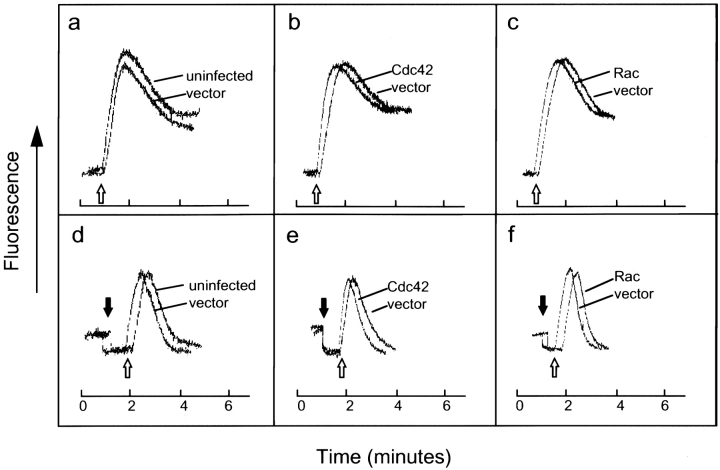
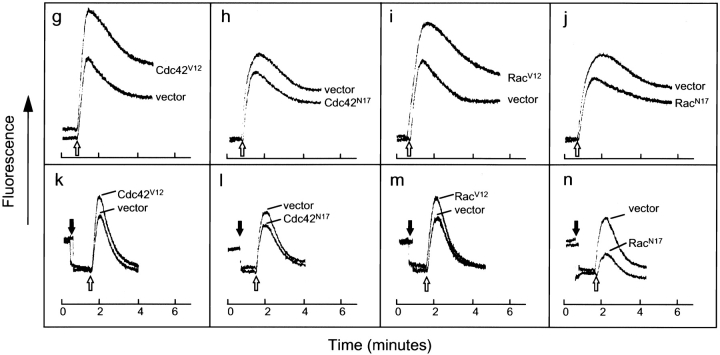
In light of our finding that direct mobilization of calcium into the cell can synergize with PKC activation to bypass the inhibitory effects on degranulation caused by the dominant-negative mutants, we examined the possibility that Cdc42 and Rac may regulate the calcium influx pathway. This could occur either by modulating the release of calcium from ER stores or by directly activating plasma membrane calcium channels. The calcium responses of RBL-2H3 cells infected with the Cdc42 or Rac mutants were compared with those of cells infected with empty vector, in order to control for variations in cell number and loading efficiency of the fluorescent dye Indo-1. Upon antigen stimulation (Fig. 4, unfilled arrows), cells infected with either Cdc42V12 or RacV12 exhibited an enhancement of both phases of the calcium response, namely the initial, rapid peak contributed by release from ER stores and the influx pathway, and the more sustained plateau derived primarily from influx (Fig. 4g and Fig. i). Expression of either dominant-negative mutants Cdc42N17 or RacN17, produced the opposite effect, dampening the two phases of calcium signaling (Fig. 4h and Fig. j). The calcium response of cells infected with empty vector was slightly inhibited, but did not significantly differ from that of uninfected cells compared with the effects generated by the Cdc42 and Rac mutants (Fig. 4 a). Surprisingly, calcium signaling in cells expressing wild-type Cdc42 and Rac was very similar to that of cells expressing the empty vector (Fig. 4b and Fig. c). We had expected the calcium response in the cells expressing the wild-type GTPases to be less robust compared with that of the vector control, consistent with our observation that expression of wild-type Cdc42 and Rac produced a small inhibition of antigen-stimulated secretion. The absence of an effect on calcium signaling suggested the possibility that the inhibitory effects produced by the wild-type GTPases during antigen-stimulated secretion may be manifested subsequent to calcium signaling. Rho GTPases have previously been suggested to play a role in the latter stages of degranulation (Norman et al. 1996).
Figure 4.
Dominant-active Cdc42 and Rac enhance calcium signaling, while dominant-negative Cdc42 and Rac dampen calcium signaling. The calcium responses of uninfected RBL-2H3 cells (a and d), cells infected with vaccinia virus expressing wild-type Cdc42 (b and e), wild-type Rac (c and f), Cdc42V12 (g and k), Cdc42N17 (h and l), RacV12 (i and m), and RacN17 (j and n), were compared with those of cells containing empty vector. Cells were infected for 6 h at 37°C and loaded with Indo-1. Overall calcium responses were measured in a fluorescence spectrophotometer in response to stimulation with 100 ng/ml DNP/BSA (a–c and g–j, unfilled arrows). To specifically measure the release of calcium from ER stores (d–f and k–n), loaded cells were treated with 4 mM EDTA (filled arrows) and then stimulated with 100 ng/ml DNP/BSA (unfilled arrows). For each form of Cdc42 and Rac, measurements of the calcium responses in the absence and presence of EGTA were performed concurrently. Traces shown are representative of three independent experiments.
To specifically evaluate release of calcium from intracellular stores, fluorescence assays performed in the presence of EGTA, to chelate total extracellular calcium, were carried out in parallel with experiments that contained free calcium in the medium. The release of calcium from ER stores has been implicated in directly activating the calcium influx pathway (Hoth and Penner 1992). Thus, an alteration in the amount of calcium exiting the ER may provide an explanation for the stimulation in the calcium response observed with Cdc42V12 and RacV12, as well as the inhibition obtained with Cdc42N17 and RacN17. Addition of EGTA decreased calcium fluorescence to a lower resting level, presumably due to a small amount of extracellular Indo-1 releasing its bound calcium (Fig. 4, d–f and k–n, filled arrows). Subsequent antigen stimulation (Fig. 4, d–f and k–n, unfilled arrows) triggered a sharp peak that quickly returned close to the starting baseline fluorescence, confirming that the sustained plateau phase of the calcium response seen in the absence of EGTA represents the influx of extracellular calcium. Expression of the dominant-active mutants, Cdc42V12 and RacV12, elevated the peak of the calcium response compared with infection with empty vector (Fig. 4k and Fig. m). In contrast, infection of cells with either Cdc42N17 or RacN17 led to a reduced amplitude in calcium signaling (Fig. 4l and Fig. n). Although expression of RacN17 generally produced a more pronounced decrease in the release of calcium from intracellular stores compared with Cdc42N17, the calcium responses in the presence of extracellular calcium are very comparable. Since expression of the two dominant-negative GTPases inhibited overall calcium signaling to a very similar extent, it may be that there is a threshold level of calcium release from the ER, below which an inhibited calcium signal is produced. Finally, the release of calcium from ER stores in uninfected cells or cells expressing wild-type Cdc42 and Rac did not significantly differ from the empty vector control (Fig. 4, d–f)
The opposing effects produced by the dominant-active and dominant-negative mutants indicate that Cdc42 and Rac are able to directly modulate calcium signaling in response to antigen stimulation. The finding that these opposing effects are also maintained in the presence of EGTA strongly suggests that Cdc42 and Rac act upstream of the release of calcium from the ER lumen rather than directly on plasma membrane calcium channels. Altering the ability of calcium to be released from stores would subsequently influence the extent of calcium influx from the extracellular medium.
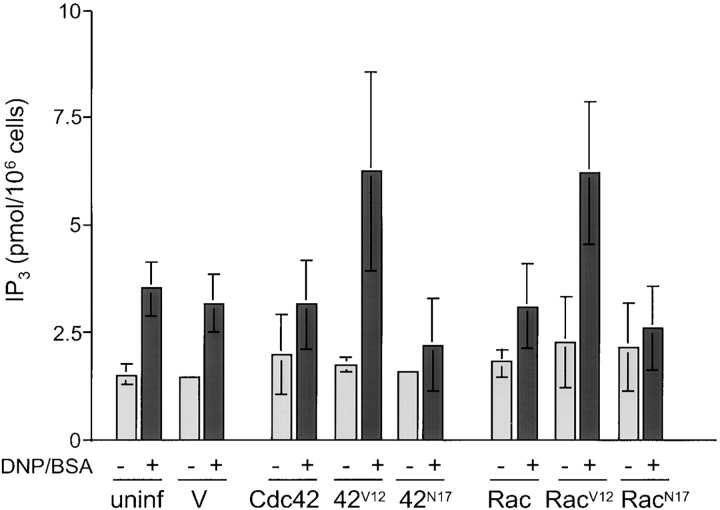
Cellular IP3 Levels Are Enhanced in Cells Expressing Dominant-Active Cdc42V12 or RacV12 upon Antigen Stimulation
We next investigated the step immediately before the release of calcium from the ER stores, that is, production of the second messenger IP3. IP3, generated by the PLCγ-mediated cleavage of PIP2-4,5 in response to antigen stimulation, binds to the IP3 receptor, a ligand-gated calcium channel in the ER membrane. This activates channel opening and calcium efflux into the cytoplasm (Ferris et al. 1989; Mignery and Sudhof 1990). The addition of DNP/BSA to uninfected cells and to cells infected with empty vector increased IP3 production by approximately twofold compared with nonstimulated cells (Fig. 5). The dominant-active mutants Cdc42V12 and RacV12 further enhanced the antigen-promoted IP3 production, such that the stimulation was approximately threefold relative to nonstimulated cells. Wild-type Cdc42 or Rac displayed a modest (∼1.5-fold) inhibition in antigen-stimulated IP3 production, while expression of the dominant-negative mutants Cdc42N17 and RacN17 prevented any significant increase in IP3 levels upon antigen stimulation, compared with that of nonstimulated cells. We suspect that the levels of antigen-stimulated IP3 production in cells expressing dominant-negative Cdc42 or Rac are reduced relative to cells expressing wild-type protein and in fact, this appears to be the trend in any given experiment. However, when averaging the experiments, the deviations are sufficiently large to make it difficult to detect these differences and to make a definitive conclusion.
Figure 5.
Dominant-active Cdc42 and Rac enhance IP3 levels in the cell. RBL-2H3 cells were infected with vaccinia virus containing empty vector, a vector expressing different forms of Cdc42 and Rac, or uninfected, for 6 h at 37°C. The IP3 levels in the cells were determined according to the procedure described in Materials and Methods for both nonstimulated cells and cells stimulated with 100 ng/ml DNP/BSA for 5 min. Error bars represent the standard deviation of four independent experiments. The standard deviations for unstimulated cells infected with empty vector is ±0.06 and with Cdc42N17 is ±0.12.
The exact mechanism by which release of calcium from the ER stores elicits calcium influx from the extracellular medium remains poorly understood. However, the enhancement in IP3 levels is consistent with the observed stimulation of the calcium response in cells expressing dominant-active Cdc42V12 or RacV12, supporting our model that Cdc42 and Rac act upstream of IP3 production to regulate calcium signaling and ultimately, degranulation.
Immunolocalization of the IP3 Receptor to Aggregates in the Cell Body Is Enhanced by Dominant-Active and Inhibited by Dominant-Negative Cdc42 or Rac
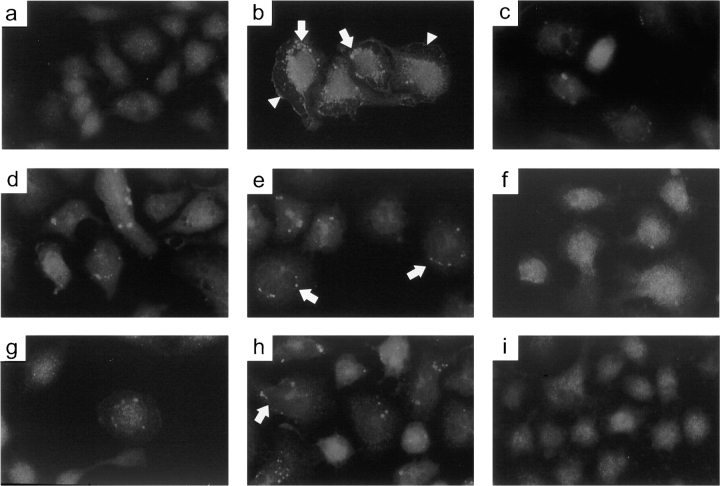
The IP3 receptor has been demonstrated by immunolocalization studies to redistribute from a diffuse staining pattern to large, distinct aggregates after antigen stimulation in RBL-2H3 cells (Wilson et al. 1998). The functional significance of the IP3 receptor clustering is unclear, although models in which aggregation modulates channel opening or IP3 receptor interaction with resident ER calcium-binding proteins have been proposed. Formation of these clusters is highly dependent on elevated levels of intracellular calcium, since treatment of cells with thapsigargin or the ionophore ionomycin also produced cluster formation. We have used this immunofluorescence assay to determine whether the Cdc42 and Rac mutants and their concomitant effects on IP3 production and calcium signaling could also influence IP3 receptor clustering.
Upon antigen stimulation, permeabilized RBL-2H3 cells incubated with anti-IP3 receptor antibody displayed an average of 4.5 small brightly labeled aggregates per cell that localized to the periphery of the dense cell body (Fig. 6 b, see arrows and Table ), whereas nonstimulated cells exhibited an extremely low level of cluster formation (Fig. 6 a and Table ). It seems likely that the cellular structure outlined by the ring of aggregates represents the nuclear envelope and the contiguous ER membranes, the primary location of the IP3 receptor (Ross et al. 1989). This staining pattern differs from an earlier study in which the aggregates were more evenly distributed in the cytoplasm (Wilson et al. 1998). However, the discrepancy may be explained by the different sources of IP3 receptor antibody used in both analyses.
Figure 6.
Dominant-negative Cdc42 and Rac inhibit aggregation of the IP3 receptor. RBL-2H3 cells were infected with vaccinia virus containing (c) empty vector, a vector expressing (d) Cdc42, (e) Cdc42V12, (f) Cdc42N17, (g) Rac, (h) RacV12, or (i) RacN17 for 6 h at 37°C, or were (a and b) uninfected. The cellular distribution of IP3 receptor in cells stimulated with 100 ng/ml DNP/BSA for 30 min (b–i) and in nonstimulated cells (a) was examined by indirect immunofluorescence using rabbit anti-IP3 receptor antibody and a Bodipy-conjugated anti-rabbit secondary antibody. Stimulation of uninfected cells with antigen (b) induces cell spreading (arrowheads) and IP3 receptor aggregate formation in a ring-like structure (arrows). IP3 receptor aggregate formation is also indicated in cells infected with Cdc42V12 and RacV12 (e and h, arrows).
Table 1.
Distribution of IP3 Receptor Clusters in RBL-2H3 Cells Infected with Different Forms of Cdc42 and Rac
| Virus | DNP/BSA | Number of clusters/cell |
|---|---|---|
| Uninfected | − | 0.04 |
| Uninfected | + | 4.50 |
| Vector | + | 3.74 |
| Cdc42 | + | 3.22 |
| Cdc42V12 | + | 5.12 |
| Cdc42N17 | + | 0.18 |
| Rac | + | 3.04 |
| RacV12 | + | 5.60 |
| RacN17 | + | 0.12 |
IP3 receptor clusters were counted from ∼50 cells.
Antigen stimulation also produced a dramatic increase in cell spreading, so that the flattened membrane extensions (Fig. 6 b, see arrowheads) are clearly delineated from the more central cell body (Fig. 6, compare a with b; Pfeiffer et al. 1985). This morphological change was not as pronounced in stimulated cells that underwent viral infection, presumably because the infection process itself induces a rounding-up effect on cells (Elroy-Stein and Moss 1991). Thus, stimulated cells infected with the different forms of Cdc42 and Rac did not exhibit as flattened an appearance as stimulated, uninfected cells (Fig. 6, compare b with c–i).
Expression of dominant-active Cdc42V12 and RacV12 induced a similar ring-like staining pattern of IP3 receptor upon antigen addition, displaying an average of 5.12 and 5.60 IP3 receptor clusters per cell, respectively (Fig. 6e and Fig. h, see arrows and Table ). Nonstimulated cells that were infected with the dominant-active mutants did not exhibit this clustering phenotype, indicating that virus infection itself did not elicit a redistribution of the IP3 receptor (data not shown). Brightly labeled clusters were also visible in cells infected with empty vector (3.74 clusters/cell), wild-type Cdc42 (3.22 clusters/cell), and wild-type Rac (3.04 clusters/cell), although to a lesser extent than seen for Cdc42V12 or RacV12 (Fig. 6c, Fig. d, and Fig. g and Table ). However, cluster formation was nearly eliminated in cells expressing the dominant-negative mutants, Cdc42N17 and RacN17, except for the occasional single aggregate (Fig. 6f and Fig. i and Table ).
Given the requirement for the calcium influx pathway in IP3 receptor redistribution, the observed increase in aggregate formation is consistent with the stimulated IP3 production and subsequent enhanced calcium signaling exhibited by cells infected with either Cdc42V12 or RacV12. Interestingly, RBL-2H3 cells infected with either Cdc42N17 or RacN17 displayed a striking decrease in aggregate formation, despite the fact that the calcium responses in these cells were inhibited, but not completely abolished, compared with infection with empty vector. This suggests that a threshold level exists for cytoplasmic calcium above which IP3 receptors undergo aggregation. The IP3 receptor contains several calcium-binding sites through which calcium may mediate its effects on IP3 receptor redistribution (Sienaert et al. 1997).
Cdc42 Interacts with PLCγ In Vitro in a GTP-dependent Manner
The combined effects on calcium signaling, IP3 production, and IP3 receptor aggregation strongly argue that Cdc42 and/or Rac function upstream of IP3 formation. Additionally, the observation that PMA synergizes with A23187 to strongly stimulate degranulation in cells infected with the dominant-negative mutants suggests that Cdc42 and/or Rac also act upstream of diacylglycerol formation, which normally activates PKC. In an effort to begin to identify the target that Cdc42 and Rac may be modulating in the IP3/calcium pathway, we focused on PLCγ, the enzyme responsible for cleaving PIP2-4,5 into IP3 and diacylglycerol. A recent study had demonstrated that both Cdc42 and Rac can stimulate the activity of phospholipase Cβ2 in a purified protein system (Illenberger et al. 1998), thus providing a precedent for small G proteins to interact with members of the PLC family.
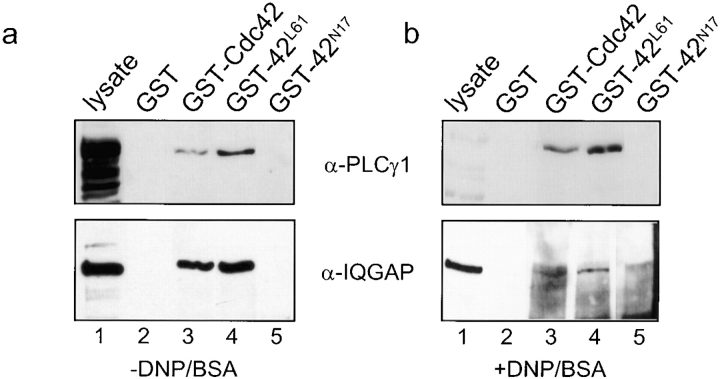
Binding studies were performed by incubating lysates from nonstimulated (Fig. 7 a) and stimulated (Fig. 7 b) RBL-2H3 cells with GST fusions to wild-type and mutant forms of Cdc42. The dominant-active Cdc42L61, and to a lesser extent, wild-type Cdc42, interacted with a protein that migrated with the expected mobility (∼145 kD) of PLCγ1 and was recognized by an anti-PLCγ1 antibody on Western blots for both nonstimulated (Fig. 7 a, upper panel, lanes 3 and 4) and stimulated (Fig. 7 b, upper panel, lanes 3 and 4) lysates. This protein did not bind to the dominant-negative mutant Cdc42N17 (Fig. 7, a and b, upper panels, lane 5) nor to GST alone (Fig. 7, a and b, upper panels, lane 1). Probing these Western blots with antibody to IQGAP, a known target/effector protein for Cdc42 (Kuroda et al. 1996; McCallum et al. 1996), exhibited a very similar binding profile (Fig. 7, a and b, lower panels). Thus, these results indicate that PLCγ1 may in fact serve as a target for activated Cdc42 and could represent a focal point from which Cdc42 influences calcium signaling in RBL-2H3 cells.
Figure 7.
Activated Cdc42 interacts with PLCγ1 in vitro. Lysates from (a) unstimulated RBL-2H3 cells and (b) cells stimulated with 100 ng/ml DNP/BSA for 5 min were incubated with immobilized GST (lane 2), GST-Cdc42 (lane 3), GST-Cdc42L61 (lane 4), and GST-Cc42N17 (lane 5) for 1 h at 4°C. Lane 1 contains whole cell lysate used in the binding reactions. Bound proteins were washed, resolved by SDS-PAGE, and analyzed by Western blots using either anti-PLCγ1 antibody (upper panel) or anti-IQGAP antibody (bottom panel).
The observation that activated Cdc42 binds to PLCγ1 from unstimulated lysates suggests that the phosphorylation state of PLCγ1 is unimportant in the binding interaction. It is important to note that these in vitro binding results may not necessarily reflect the in vivo spatial and timing events that most likely regulate the binding of PLCγ1 and Cdc42 at the plasma membrane. Although activated Cdc42 appears to bind equally well to PLCγ1 from either stimulated or unstimulated RBL-2H3 lysates in vitro, the Cdc42/PLCγ1 interaction may still be carefully regulated by antigen stimulation in the cell, which signals translocation of PLCγ1 to the plasma membrane for activation via phosphorylation.
We have not found that activated Cdc42 stimulates PLCγ1 activity, upon mixing the two purified recombinant proteins. However, experiments using purified PLCγ1 from insect cells suggest that activated Cdc42 binds preferentially to a form of PLCγ1 that migrates with a slightly faster mobility compared with the majority of the PLCγ1 protein (data not shown). Interestingly, this more mobile form of PLCγ1 from insect cells comigrated with the protein from RBL-2H3 lysates that interacted with activated GST-Cdc42 and was recognized by the anti-PLCγ1 antibody. At present, we do not know if this altered mobility reflects a specific posttranslational modification of PLCγ1 that is necessary for efficient binding to Cdc42, but the inability to assay a population of fully modified PLCγ1 may explain the lack of a Cdc42-mediated effect in activity assays.
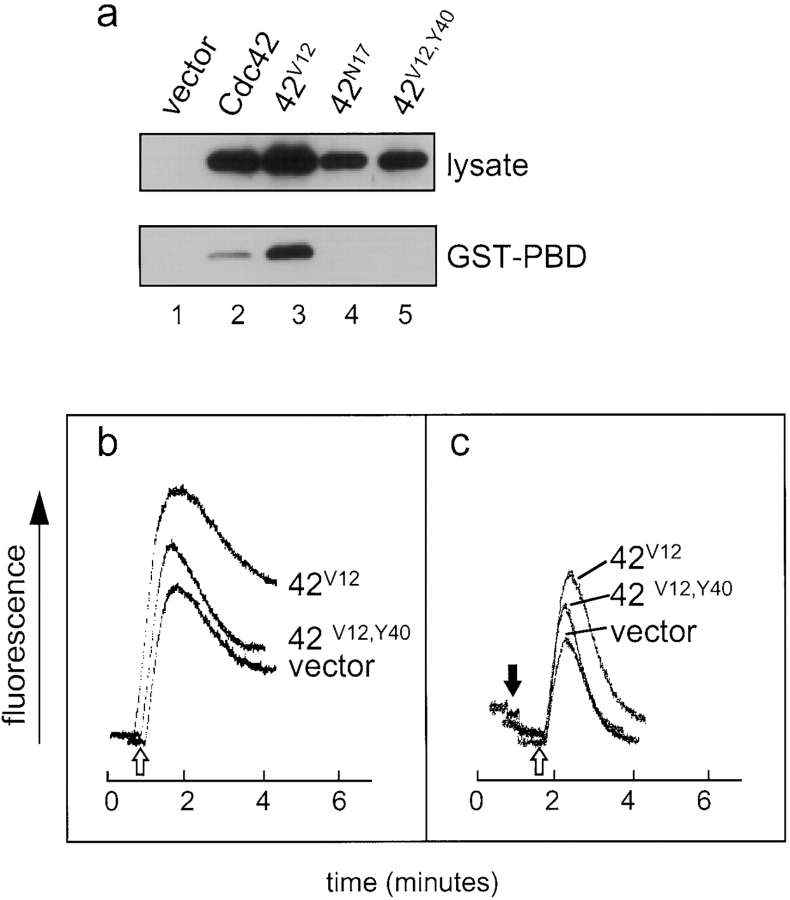
Introduction of the Y40 Effector Domain Mutation into Cdc42V12 Decreases the Enhanced Calcium Signaling Exhibited by Cells Infected with Cdc42V12 Alone
The GTPase family members contain a short effector binding domain from residues 32–40, termed the Switch I region, that mediates binding to a variety of target proteins in a GTP-dependent manner. To obtain further support that Cdc42 plays a role in degranulation through the signaling of downstream effector targets, we have constructed a virus expressing the Cdc42V12,Y40 double mutant to determine whether disruption of Cdc42 binding to effector proteins would affect the enhanced calcium signaling and secretory response in cells infected with Cdc42V12 alone. The Y40 mutation has been demonstrated to prevent Cdc42 interaction with targets containing the CRIB domain, which leads to defective signaling in the JNK transcriptional activation pathway (Lamarche et al. 1996). The virus-expressed Cdc42V12,Y40 did not bind to the GST-PBD fusion protein (Fig. 8, lane 5), indicating that introduction of the Y40 mutation disrupted the ability of Cdc42V12 to interact with GST-PBD (Fig. 8, lane 3). Upon antigen stimulation (Fig. 8 b, unfilled arrow), cells expressing Cdc42V12,Y40 exhibited a reduction in both the initial peak and sustained plateau phases of the calcium response, compared with cells expressing Cdc42V12 alone. The Y40 mutation did not completely inhibit the stimulatory effects of the dominant-active V12 mutation, since cells expressing Cdc42V12,Y40 still displayed an enhancement of calcium signaling over cells infected with empty vector. Furthermore, expression of Cdc42V12,Y40 also dampened the release of calcium from ER stores compared with cells expressing Cdc42V12, suggesting that the Y40 mutation most likely impaired a step before the initiation of calcium signaling (Fig. 8 c). Finally, expression of Cdc42V12,Y40 exhibited ∼32% secretion, compared with ∼40% in cells expressing Cdc42V12 and ∼25% in cells expressing empty vector (data not shown). Taken together, the inhibition of calcium signaling and secretion produced by the Cdc42V12,Y40 double mutant are consistent with a defect in IP3 production and further supports our hypothesis that Cdc42/Rac interaction with downstream effectors is a key step in the pathway leading to calcium mobilization and degranulation.
Figure 8.
Cells infected with the effector domain mutant Cdc42V12,Y40 exhibit impaired calcium signaling compared with cells infected with Cdc42V12. (a) Lysates from RBL-2H3 cells infected with vaccinia virus containing empty vector (lane 1), or a vector expressing Cdc42 (lane 2), Cdc42V12 (lane 3), Cdc42N17 (lane 4), and Cdc42V12,Y40 (lane 5) were treated as described in the legend to Fig. 1, a and b. (b) The calcium responses of RBL-2H3 cells infected with Cdc42V12,Y40 were compared with those of Cdc42V12 and empty vector upon antigen stimulation (unfilled arrows) in the absence or presence (c) of 4 mM EGTA (filled arrow). Traces shown are representative of three independent experiments.
Discussion
The Rho family of small GTP-binding proteins plays critical regulatory roles in a variety of cellular processes, including cytoskeletal organization, cell motility, transcriptional signaling, and cell cycle progression (for review, see Hall 1998). In this study, we have implicated Cdc42 and/or Rac in a novel biological function to signal the degranulation of secretory vesicles in RBL-2H3 cells. By expressing dominant-active and dominant-negative mutants using vaccinia virus, we have demonstrated that Cdc42 and Rac act early in this signaling pathway by activating IP3 production and the subsequent rise in intracellular calcium upon antigen stimulation. Viral expression vectors for a third member of the Rho family, RhoA, were also constructed. However, infection of HeLa cells with dominant-active RhoA induced dramatic cellular events, such as a misshapen cell morphology and abnormal actin localization. Since these phenotypes were indicative of aberrant cellular behavior and physiology, we discontinued further study of RhoA in the context of degranulation in RBL-2H3 cells.
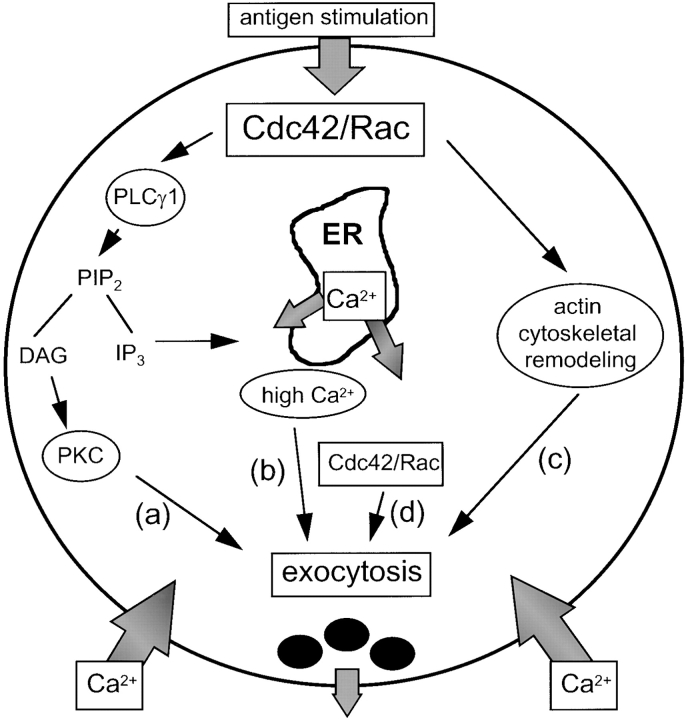
A role for the small GTPases, Cdc42, Rac, and Rho, in exocytosis had been proposed based on earlier work in which the addition of activated, recombinant GTPases enhanced degranulation in permeabilized cells (Norman et al. 1994; Price et al. 1995; Brown et al. 1998). The majority of these studies had made the presumption that the GTPases were stimulating degranulation by primarily inducing the actin cytoskeletal rearrangements that normally accompany secretion. Filamentous actin depolymerizes in the cortical region underneath the plasma membrane and repolymerizes in the cell interior upon antigen stimulation (Koffer et al. 1990; Norman et al. 1994). This redistribution is thought to be required for relieving steric hindrance at the cell periphery, allowing secretory granules to gain access to the plasma membrane for fusion and release of their contents (Lelkes et al. 1986). In addition, recombinant Rac and Rho have been shown to enhance secretion in permeabilized mast cells that had been stimulated with GTPγS in the presence of high calcium, suggesting that the Rho GTPases also perform as yet unknown functions in later stages of the signaling pathway following the calcium influx step (Norman et al. 1996). Our observation that expression of wild-type Cdc42 or Rac did not appear to inhibit calcium signaling suggested the possibility that the inhibitory effects produced during antigen-stimulated secretion may be manifested after the activation of the calcium influx pathway. Although it remains unclear why wild-type Cdc42 or Rac produces an inhibitory effect on secretion, we speculate that overexpression of these proteins, through competition with the activated forms of GTP-binding protein, apparently can form a nonproductive complex with a target-effector that is important for secretion and thereby inhibits this signaling response. Finally, our demonstration that Cdc42 and Rac stimulate IP3 formation and calcium signaling upon antigen stimulation leads us to favor a model in which the GTPases play a role in at least three processes that are able to influence degranulation: (a) stimulation of calcium signaling and PKC activation (Fig. 9, a and b), (b) actin cytoskeletal changes (Fig. 9 c), and (c) an unidentified function subsequent to calcium influx (Fig. 9 d).
Figure 9.
Model for the roles that Cdc42 and Rac play in stimulating exocytosis of secretory granules in RBL-2H3 cells. Upon antigen stimulation, Cdc42 and/or Rac activate at least three processes that are required for degranulation: (a and b) stimulation of calcium signaling and PKC activation, (c) actin cytoskeletal remodeling, and (d) an as yet unidentified function subsequent to calcium influx during the later stages of degranulation.
So far, we have not been able to detect Rho GTPase-mediated effects on events upstream of IP3 production in the signaling pathway. Antigen stimulation induces the activation of many signaling proteins via tyrosine phosphorylation events in RBL-2H3 cells (Li et al. 1992). Expression of dominant-negative Cdc42 did not significantly alter the pattern of enhanced phosphorylation in stimulated RBL-2H3 cell lysates on Western blots probed with anti-phosphotyrosine antibody (data not shown). In COS7 monkey kidney cells and NIH-3T3 mouse fibroblast cells infected with dominant-negative Cdc42, immunoprecipitated PLCγ1 was phosphorylated to the same extent as cells infected with empty vector, indicating that dominant-negative Cdc42 did not interfere with events leading up to the phosphorylation of PLCγ1 in these cell types (data not shown).
In addition, we do not think that it is likely that cytoskeletal changes induced by viral infection caused any dramatic effect to the signaling pathway leading to degranulation since cells that were infected with empty vector still exhibited a strong level of secretion in response to antigen stimulation. However, cytoskeletal changes produced by viral infection may be one of the factors that contributed to the modest decrease in secretion efficiency upon infection with the vector control compared with uninfected cells. We have attempted to look at the consequence of vaccinia infection on FcεRI-induced cytoskeletal reorganization by staining cells with phalloidin and performing immunofluorescence. Unfortunately, viral infection induced a rounding up of the cells during the course of the infection, thus making it more difficult to distinguish the fine structure of cytoskeletal elements.
We suspect that the observed changes in IP3 formation and calcium signaling are not merely secondary phenotypes induced by Cdc42 and Rac effects on actin remodeling. Treatment of cells with the phorbol ester, PMA, induced cytoskeletal changes similar to those resulting from incubation with both PMA and the calcium ionophore A23187. However, the combination of PMA and A23187 strongly stimulated degranulation, whereas PMA alone did not, indicating that cytoskeletal rearrangement is insufficient to induce the signaling pathway leading to secretion (Ludowyke et al. 1994). In addition, our in vitro binding studies identifying an interaction between Cdc42 and PLCγ1 suggest that Cdc42 feeds directly into the IP3/calcium signaling pathway.
PLCγ hydrolyzes PIP2-4,5 to form the two second messengers, IP3, which binds to the IP3 receptor in the ER membrane to release internal calcium stores, and 1,2-diacylglycerol, which stimulates PKC activity. Thus, activation of PLCγ is instrumental in initiating a signaling cascade in a wide variety of cell types that responds to receptor–ligand interactions at the cell surface. Three major families of PLC have been identified: β, γ, and δ (for review, see Rhee and Bae 1997). PLCβ and PLCγ stimulation are linked to receptors coupled to heterotrimeric G proteins and tyrosine kinases, respectively, whereas a role for PLCδ activation by cell surface receptors remains unclear. PLCβ2 has been characterized as a target for Cdc42 and Rac in a study describing the isolation of a complex between Cdc42 and LyGDI, which was found to mediate GTP-dependent activation of PLCβ2 in vitro (Illenberger et al. 1998). Rac, but not Rho, was also capable of stimulating PLCβ2 activity. This activation was further observed in a system composed of purified recombinant proteins, suggesting that the interaction between Cdc42/Rac and PLCβ2 was direct. Cdc42/Rac activation of PLCβ2 appeared to be very specific since the GTPases could not enhance the activity of PLCδ1 or a second PLCβ isoform, PLCβ1 (Illenberger et al. 1997).
We had originally focused on PLCγ1 as a possible target for the Rho GTPases in stimulating the IP3/calcium pathway based on the work examining Cdc42/Rac-mediated activation of PLCβ2. We have established an in vitro interaction between purified, recombinant GST-Cdc42 and a protein from RBL-2H3 cell lysates that closely matched the expected molecular mass (∼145 kD) of PLCγ1 and was recognized by a polyclonal antibody raised against amino acids 1249–1262 at the COOH terminus of PLCγ1, but migrated slightly faster than the majority of the PLCγ1 purified from insect cells. The purified PLCγ1 contained a component protein that both comigrated with the interacting protein from RBL-2H3 cell lysates and bound to GST-Cdc42 in a GTP-dependent manner. This suggests that it may be a modified form of PLCγ1 that binds most effectively to activated Cdc42, and perhaps results in a specialized regulatory function that links Cdc42 to signaling events that lead to secretion. We attempted to determine whether the small increase in mobility observed for the PLC-immunoreactive species that binds Cdc42 may have been caused by hypophosphorylation of PLCγ1. In both quiescent NIH-3T3 cells and A431 human epidermoid cells, PLCγ1 in its basal state has been demonstrated to contain phosphoserine (Meisenhelder et al. 1989). Thus, it seemed possible that a percentage of the total purified, insect cell-expressed PLCγ1 may contain fewer phosphate groups on serine residues, and thus account for the faster mobility in protein gels. However, treatment of purified or immunoprecipitated PLCγ1 with potato acid phosphatase to remove susceptible phosphate groups did not yield a lower molecular mass protein (data not shown). There are a number of other possibilities for Cdc42/PLCγ1 interactions that remain to be addressed. For example, it is possible that the association between activated Cdc42 and an unmodified PLCγ1 is transient, as thus far, we have been unable to detect a stable in vivo complex between PLCγ1 and Cdc42 using coimmunoprecipitation methods.
In addition to possibly regulating PLCγ1 directly and remodeling the cytoskeletal architecture, the Rho GTPases may also modulate the activities of other signaling molecules that play a role in triggering degranulation. Some potential candidates belong to the lipid kinase family, which generates lipid products that perform key signaling tasks after antigen stimulation. First, phosphoinositide 3-kinase (PI3-K) phosphorylates PIP2-4,5 to form phosphatidylinositol-3,4,5-trisphosphate (PIP3-3,4,5), which can induce PLCγ1 translocation to the plasma membrane via interactions with its pleckstrin homology and SH2 domains (Bae et al. 1998; Falasca et al. 1998). Since Cdc42 and Rac have been shown to stimulate PI3-K activity in a GTP-dependent manner (Zheng et al. 1994; Tolias et al. 1995), the GTPases may be exerting their influence on PLCγ1 indirectly by stimulating PI3-K to generate higher levels of PIP3-3,4,5. Furthermore, treatment of RBL-2H3 cells with the PI3-K inhibitor, wortmannin, attenuated the secretory response, suggesting that PI3-K activity is essential for degranulation to occur (Yano et al. 1993). Second, the combined activities of phosphatidylinositol phosphate 4-kinase (PIP4-K) and phosphoinositide 4,5-kinase (PI5-K) generate PIP2-4,5, the target lipid that is cleaved by PLCγ1 (for review, see Toker 1998). Both Rac and Rho have been implicated in activating these lipid kinases to increase production of PIP2-4,5 (Chong et al. 1994; Hartwig et al. 1995), which may subsequently upregulate the signaling pathway leading to degranulation by providing more substrate for PLCγ1. Finally, sphingosine kinase activity has been shown to mobilize calcium upon antigen stimulation (Choi et al. 1996), suggesting that multiple signals may be activated to elevate intracellular levels of calcium. The implication of sphingosine lipids in mediating calcium signaling identifies them as possible targets for regulation by the Rho GTPases.
In summary, we now present findings that highlight an important role for the Cdc42 and/or Rac GTPase in mediating calcium changes that are critical for the exocytosis of secretory granules. These regulatory events occur at a step immediately upstream of calcium release from stores and appear to impact on IP3 production and on the interplay between this second messenger and the aggregation of its ER receptor/calcium channels. The ability of activated Cdc42 and/or Rac to bind PLCγ1 may underlie a key step in what is likely to be a complex regulation of calcium signaling via antigen binding and the resultant activation of Rho GTPases. Future efforts will be directed at better understanding the mechanistic basis by which Cdc42 and Rac influence calcium changes and whether other aspects of antigen-mediated secretion are also affected by these GTPases.
Acknowledgments
We thank D. Holowka for helpful discussions throughout the course of this work and for critically reading this manuscript. We also thank C. Fewtrell for the use of her fluorescence spectrophotometer, D. Holowka, B. Baird, B. Moss, F. Sekiya, and S.G. Rhee for sharing reagents, and C. Westmiller for secretarial assistance.
This work is supported by National Institutes of Health grant GM47458 to R.A. Cerione and an American Cancer Society post-doctoral fellowship to E. Hong-Geller.
Footnotes
Abbreviations used in this paper: FAK, focal adhesion kinase; IP3, inositol 1,4,5-trisphosphate; PBD, Cdc42/Rac (p21)-binding domain; PI3-K, phosphoinositide 3-kinase; PIP2-4,5, phosphatidylinositol-4,5-bisphosphate; PKC, protein kinase C; PLCγ, phospholipase C-γ.
References
- Aridor M., Rajmilevich G., Beaven M.A., Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3 . Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- Bae Y.S., Cantley L.G., Chen C., Kim S., Kwon K., Rhee S.G. Activation of phospholipase C-γ by phosphoinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Bagrodia S., Derijard B., Davis R.J., Cerione R.A. Cdc42 and PAK-mediated signaling leads to jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- Beaven M.A., Baumgartner R.A. Downstream signals initiated in mast cells by FcεRI and other receptors. Curr. Opin. Immunol. 1996;8:766–772. doi: 10.1016/s0952-7915(96)80002-1. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Brown A.M., O'Sullivan A.J., Gomperts B.D. Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol. Biol. Cell. 1998;9:1053–1063. doi: 10.1091/mbc.9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., and R. Padmanabhan. 1997. A simplified method of screening for isolation of recombinant vaccinia virus. In Methods in Molecular Biology. R. Tuan, editor. Humana Press, Inc., Totowa, NJ. 62:199–206. [DOI] [PubMed]
- Choi O.H., Kim J., Kinet J. Calcium mobilization via sphingosine kinase in signaling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Choi O.H., Lee J.H., Kassessinoff T., Cunha-Melo J.R., Jones S.V., Beaven M.A. Antigen and carbachol mobilize calcium by similar mechanisms in a transfected mast cell line (RBL-2H3 cells) that expresses m1 muscarinic receptors. J. Immunol. 1993;151:5586–5595. [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan A., Bokoch G.M., Schwartz M.A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein, O., and B. Moss. 1991. Expression of proteins in mammalian cells using vaccinia viral vectors. Curr. Prot. Mol. Biol. 16.15.11–16.19.19.
- Erickson J.W., Zhang C., Kahn R.A., Evans T., Cerione R.A. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J. Biol. Chem. 1996;271:26850–26854. doi: 10.1074/jbc.271.43.26850. [DOI] [PubMed] [Google Scholar]
- Falasca M., Logan S.K., Lehto V.P., Baccante G., Lemmon M.A., Schlessinger J. Activation of phospholipase Cγ by PI3-kinase-induced PH domain-mediated membrane targeting. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.D., Huganir R.L., Supattapone S., Solomon S.H. Purified inositol-1,4,5-trisphosphate receptor mediates calcium influx in reconstituted lipid vesicles. Nature. 1989;342:87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Guillemot J., Montcourrier P., Vivier E., Davoust J., Chavrier P. Selective control of membrane ruffling and actin plaque assembly by the Rho GTPases Rac1 and Cdc42 in FcεRI-activated rat basophilic leukemia (RBL-2H3) cells. J. Cell Sci. 1997;110:2215–2225. doi: 10.1242/jcs.110.18.2215. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hart M.J., Eva A., Zangrilli D., Aaronson S.A., Evans T., Cerione R.A., Zheng Y. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J. Biol. Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- Hartwig J.H., Bokoch G.M., Carpenter C.L., Janmey P.A., Taylor L.A., Toker A., Stossel T.P. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Howell T.W., Cockcroft S., Gomperts B.D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J. Cell Biol. 1987;105:191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illenberger D., Schwald F., Gierschik P. Characterization and purification from bovine neutrophils of a soluble guanine-nucleotide-binding protein that mediates isozyme-specific stimulation of phospholipase C-β2. Eur. J. Biochem. 1997;246:71–77. doi: 10.1111/j.1432-1033.1997.t01-1-00071.x. [DOI] [PubMed] [Google Scholar]
- Illenberger D., Schwald F., Pimmer D., Binder W., Maier G., Dietrich A., Gierschik P. Stimulation of phospholipase C-β2 by the Rho GTPases Cdc42Hs and Rac1. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:6241–6249. doi: 10.1093/emboj/17.21.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakami Y., Kaibuchi K., Sawamura M., Takai Y., Nishizuka Y. Synergistic action of protein kinase C and calcium for histamine release from rat peritoneal mast cells. Biochem. Biophys. Res. Commun. 1984;121:573–578. doi: 10.1016/0006-291x(84)90220-1. [DOI] [PubMed] [Google Scholar]
- Koffer A., Tatham P.E.R., Gomperts B.D. Changes in the state of actin during the exocytotic reaction of permeabilized rat mast cells. J. Cell Biol. 1990;111:919–927. doi: 10.1083/jcb.111.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S., Fukata M., Kobayashi K., Nakafuku M., Nomura N., Iwamatsu A., Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J. Biol. Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- Lamarche N., Tapon N., Stowers L., Burbelo P.D., Aspenstrom P., Bridges T., Chant J., Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65 PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lelkes P.I., Friedman J.E., Rosenheck K., Oplatka A. Destabilization of actin filaments as a requirement for the secretion of catecholamines from permeabilized chromaffin cells. FEBS Lett. 1986;208:357–363. doi: 10.1016/0014-5793(86)81049-3. [DOI] [PubMed] [Google Scholar]
- Li W., Deanin G.G., Margolis B., Schlessinger J., Oliver J.M. FcεRI-mediated tyrosine phosphorylation of multiple proteins, including phospholipase Cγ1 and the receptor βγ2 complex, in RBL-2H3 rat basophilic leukemia cells. Mol. Cell Biol. 1992;12:3176–3182. doi: 10.1128/mcb.12.7.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludowyke R.I., Kawasugi K., French P.W. PMA and calcium ionophore induce myosin and F-actin rearrangement during histamine secretion from RBL-2H3 cells. Cell. Motil. Cytol. 1994;29:354–365. doi: 10.1002/cm.970290408. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- Mariot P., O'Sullivan A.J., Brown A.M., Tatham P.E.R. Rho guanine nucleotide dissociation inhibitor protein (RhoGDI) inhibits exocytosis in mast cells. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6476–6482. [PMC free article] [PubMed] [Google Scholar]
- McCallum S.J., Wu W.J., Cerione R.A. Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2. J. Biol. Chem. 1996;271:21732–21737. doi: 10.1074/jbc.271.36.21732. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P., Rhee S.G., Hunter T. Phospholipase C-γ is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro . Cell. 1989;57:1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mignery G.A., Sudhof T.C. The ligand binding site and transduction mechanism in the inositol-1,4,5-trisphosphate receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J.C., Price L.S., Ridley A.J., Hall A., Koffer A. Actin filament organization in activated mast cells is regulated by heterotrimeric and small GTP-binding proteins. J. Cell Biol. 1994;126:1005–1015. doi: 10.1083/jcb.126.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J.C., Price L.S., Ridley A.J., Koffer A. The small GTP-binding proteins, Rac and Rho, regulate cytoskeletal organization and exocytosis in mast cells by parallel pathways. Mol. Biol. Cell. 1996;7:1429–1442. doi: 10.1091/mbc.7.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A.J., Brown A.M., Freeman H.N.M., Gomperts B.D. Purification and identification of FOAD-II, a cytosolic protein that regulates secretion in streptolysin-O permeabilized mast cells, as a Rac/RhoGDI complex. Mol. Biol. Cell. 1996;7:397–408. doi: 10.1091/mbc.7.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser A.F., Monck J.R., Balch W.E., Fernandez J.M. Exocytotic fusion is activated by Rab3a peptides. Nature. 1992;360:270–273. doi: 10.1038/360270a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer J.R., Seagrave J.C., Davis B.H., Deanin G.G., Oliver J.M. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J. Cell Biol. 1985;101:2145–2155. doi: 10.1083/jcb.101.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini L., Holowka D., Baird B. FcεRI-mediated association of 6-μm beads with RBL-2H3 mast cells results in exclusion of signaling proteins from the forming phagosome and abrogation of normal downstream signaling. J. Cell Biol. 1996;134:1427–1439. doi: 10.1083/jcb.134.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prepens U., Just I., von Eichel-Streiber C., Aktories K. Inhibition of FcεRI-mediated activation of rat basophilic leukemia cells by Clostridium difficile toxin B (monoglucosyltransferase) J. Biol. Chem. 1996;271:7324–7329. doi: 10.1074/jbc.271.13.7324. [DOI] [PubMed] [Google Scholar]
- Price L.S., Norman J.C., Ridley A.C., Koffer A. The small GTPases Rac and Rho as regulators of secretion in mast cells. Curr. Biol. 1995;5:68–73. doi: 10.1016/s0960-9822(95)00018-2. [DOI] [PubMed] [Google Scholar]
- Rhee S.G., Bae Y.S. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Ross C.A., Meldolesi J., Milner T.A., Satoh T., Supattapone S., Snyder S.H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989;339:468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Lieman H., Pecht I. Protein kinase C regulation of the receptor-coupled calcium signal in histamine-secreting rat basophilic leukemia cells. Nature. 1985;313:59–60. doi: 10.1038/313059a0. [DOI] [PubMed] [Google Scholar]
- Sienaert I., Missiaen L., De Smedt H., Parys J.B., Sipma H., Casteels R. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1997;272:25899–25906. doi: 10.1074/jbc.272.41.25899. [DOI] [PubMed] [Google Scholar]
- Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr. Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- Tolias K.F., Cantley L.C., Carpenter C.L. Rho family GTPases bind to phosphoinositide kinases. J. Biol. Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- Wilson B.S., Pfeiffer J.R., Smith A.J., Oliver J.M., Oberdorf J.A., Wojcikiewicz R.J.H. Calcium-dependent clustering of inositol 1,4,5-trisphosphate receptors. Mol. Biol. Cell. 1998;9:1465–1478. doi: 10.1091/mbc.9.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Nakanishi S., Kimura K., Hanai N., Saitoh Y., Fukui Y., Nonomura Y., Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J. Biol. Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- Zheng Y., Bagrodia S., Cerione R.A. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J. Biol. Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]