Abstract
JIL-1 is a novel chromosomal kinase that is upregulated almost twofold on the male X chromosome in Drosophila. Here we demonstrate that JIL-1 colocalizes and physically interacts with male specific lethal (MSL) dosage compensation complex proteins. Furthermore, ectopic expression of the MSL complex directed by MSL2 in females causes a concomitant upregulation of JIL-1 to the female X that is abolished in msl mutants unable to assemble the complex. Thus, these results strongly indicate JIL-1 associates with the MSL complex and further suggests JIL-1 functions in signal transduction pathways regulating chromatin structure.
Keywords: protein kinase, dosage compensation, Drosophila, chromatin, MSL complex
Introduction
DNA tightly packed together with histones and other proteins into nucleosomes is not easily accessible to the enzymes that use it as a template for transcription or replication. Consequently, remodeling of chromatin structure at promoter regions may play an essential role in regulation of gene expression (Wolffe and Hayes 1999). Structural changes in chromatin may also form the basis for dosage compensation mechanisms that have evolved to equalize levels of X-linked gene products between males and females. In humans, one of the two X chromosomes in females is inactivated by condensation of the chromosome into a Barr body and in C. elegans, association of condensin-like molecules with the two female X chromosomes reduces transcription levels from each by about half (Lucchesi 1998). However, in contrast to these mechanisms, both female X chromosomes in Drosophila are actively expressed. Consequently, in order to achieve equal levels of expression of genes on the X chromosome, a twofold hyper-transcription of the male's single X chromosome occurs relative to the female's two X chromosomes and the autosomes (Baker et al. 1994; Kelley and Kuroda 1995; Lucchesi 1998). This increased transcriptional activity correlates with a more diffuse chromosome structure, such that despite the fact that it contains half the DNA content, the male X chromosome appears to be the same width as the paired female X chromosomes (Gorman and Baker 1994).
In an effort to determine the molecular basis for dosage compensation in Drosophila, a number of genetic screens were performed that have identified several genes necessary for achieving equal levels of most X-linked transcription (Fukunaga et al. 1975; Belote and Lucchesi 1980). The products of these genes assemble into a complex termed MSL (male specific lethal) that is thought to be responsible for targeting a histone acetyltransferase that acetylates histone H4 (H4Ac16) to the upregulated male X chromosome, which in turn leads to altered chromatin structure (Smith et al. 2000). The MSL holocomplex is known to include MSL1, a novel acidic protein (Palmer et al. 1993); MSL2, a RING finger protein (Bashaw and Baker 1995; Kelley et al. 1995; Zhou et al. 1995); MSL3, a chromodomain protein (Gorman et al. 1995); MLE, an RNA helicase (Kuroda et al. 1991); MOF, a histone acetyltransferase (Hilfiker et al. 1997); and two nontranslated RNAs, roX1 and roX2 (Meller et al. 1997; Franke and Baker 1999). The MSL complex preferentially associates at hundreds of sites on the male X but fails to assemble in females due to a Sex lethal–regulated block in translation of the MSL2 subunit (reviewed in Bashaw and Baker 1996). The MSL complex colocalizes with the H4Ac16 pattern on the male X chromosome, and absence of any of the MSL complex subunits prevents both MSL complex assembly as well as the enhanced H4Ac16 modification on the male X chromosome (Turner et al. 1992; Bone et al. 1994). Furthermore, studies have shown that ectopic expression of MSL2 in females results in formation and targeting of the MSL complex to both female X chromosomes with a concomitant upregulation of H4Ac16 levels (Kelley et al. 1995; Bashaw and Baker 1995).
Thus, in order to understand the mechanisms of dosage compensation occurring in Drosophila it is necessary to identify all the components of the MSL complex and to determine how they may interact to mediate upregulation of transcription on the male X. Recently, we have characterized a novel tandem kinase, JIL-1, which is enriched almost twofold on the male larval polytene X-chromosome (Jin et al. 1999). In this study, we demonstrate (a) that JIL-1 colocalizes with the MSL complex proteins on the male X, (b) that JIL-1 can molecularly interact with MSL complex proteins, (c) that ectopic expression of MSL2 in females causes a concomitant upregulation of JIL-1 to the female X, and (d) that this upregulation is abolished in msl mutants that are unable to assemble the complex. Thus, these results strongly indicate that JIL-1 can associate with the MSL dosage compensation complex. The ability of JIL-1 to phosphorylate histone H3 in vitro (Jin et al. 1999) further suggests a model where JIL-1's role in the MSL complex is to assist in regulating transcription possibly through modification of chromatin.
Materials and Methods
Drosophila Stocks
Fly stocks were maintained according to standard protocols (Roberts 1986). Oregon-R was used for wild-type preparations. The w; msl3 [w+ H83M2-6I]/TM6, Tb e and w; msl1L60/CyO; [w+ H83M2-6I] stocks (Kelley et al. 1995) were the generous gift of Drs. M. Kuroda and R. Kelley (Baylor College of Medicine, Houston, TX).
Immunohistochemistry
Anti–JIL-1 IgY antibodies were purified from eggs of chickens (Aves Labs) that had been injected with pGEXFI, a GST (glutathione-S-transferase)–JIL-1 fusion protein containing residues 886–1,013 (Jin et al. 1999). A JIL-1–specific monoclonal antibody, 5C9, was made to pGEXFI using standard procedures (Harlow and Lane 1988). Affinity-purified anti-MSL1 (rabbit), -MSL2 (rabbit), and -MSL3 (goat) antibodies were the generous gift of Drs. M. Kuroda and R. Kelley. Polytene chromosome squash preparations from late third instar larvae were double immunostained using FITC- and TRITC-conjugated species-specific secondary antibodies as previously described by Jin et al. 1999.
Confocal microscopy was performed with a Leica confocal TCS NT microscope system equipped with separate Argon-UV, Argon, and Krypton lasers and the appropriate filter sets for Hoechst, FITC, and TRITC imaging. A separate series of confocal images for each fluorophor of double labeled preparations was obtained simultaneously with z intervals of typically 0.5 μm. An average projection image for each of the image stacks was obtained using the NIH-image software. These were subsequently imported into Photoshop where they were pseudocolored, image processed, and merged.
JIL-1 Fusion Protein Constructs
An inducible full-length JIL-1 fusion protein with COOH-terminal V5 and His6 tags for expression in S2 cells was constructed by site-directed mutagenesis using the Transformer kit (CLONTECH Laboratories, Inc.) and inserted into the pMT/V5-His6 B vector (Invitrogen). The resulting construct was verified by sequencing and stably transformed into S2 cells using standard procedures for transfection and S2 cell line culture (Invitrogen).
A nearly full-length GST–JIL-1 fusion protein (residues 34–1,207) as well as various truncated GST–JIL-1 fusion proteins were constructed by PCR and inserted into the pGEX4T-2 (Amersham Pharmacia) vector for expression in E. coli. The following four truncated GST–JIL-1 fusion proteins were made: NTD (residues 1–211), KDI (residues 251–554), KDII (residues 615–917), and CTD (residues 927–1,207). The GST fusion protein constructs were verified by sequencing and fusion proteins purified using glutathione agarose beads (Sigma-Aldrich) following standard protocols (Amersham Pharmacia).
Coimmunoprecipitation and In Vitro Protein Interaction Assays
For immunoprecipitation (ip) experiments with V5-tagged JIL-1, 3 μg anti-V5 monoclonal antibody (Invitrogen) was coupled with 5 μl protein G–Sepharose beads (Amersham Pharmacia) for 2 h at 4°C on a rotating wheel in 50 μl ip buffer (20 mM Tris-HCl, 150 mM NaCl, 10 mM EDTA, 1 mM EGTA, 0.2% Triton X-100, 0.2% NP-40, 2 mM Na3VO4, 1 mM PMSF and 1.5 μg/ml aprotinin, pH 8.0) and then incubated overnight at 4°C with S2 cell lysate (106 cells/200 μl ip buffer) from cells stably expressing a V5–JIL-1 fusion protein or from control cells not expressing V5–JIL-1. For coimmunoprecipitation experiments with MSL proteins, 1 μg anti-MSL1, 1 μg anti-MSL2, 2 μg anti-MSL3, or identical amounts of the appropriate control antibodies (rabbit preimmune sera or normal goat sera from GIBCO BRL) were coupled with 5 μl protein G–Sepharose beads (Amersham Pharmacia) for 2 h at 4°C on a rotating wheel in 50 μl ip buffer. S2 cell lysate from V5–JIL-1–expressing cells was prepared in ip buffer (106 cells/200 μl ip buffer) and precleared with 5 μl normal sera and 20 μl protein G beads for 2 h at 4°C. The precleared lysate and protein G beads preloaded with the appropriate antibody were combined and incubated overnight at 4°C with continuous mixing. Beads were then washed four times for 10 min each with 1 ml of ip buffer. The resulting immunocomplexes were analyzed by SDS-PAGE and Western blotting according to standard techniques as described in Jin et al. 1999. In some experiments, endogenous JIL-1 and MSL proteins were immunoprecipitated from lysate of untransfected S2 cells using the same procedure. For in vitro protein–protein interaction assays, ∼0.5 μg of GST or the appropriate GST fusion protein were coupled with glutathione agarose beads and incubated with 300 μl of S2 cell lysate (from ∼106 cells) at 4°C for 6 h on a rotating wheel. The beads were washed 4 times for 10 min each with 1 ml ip buffer, and proteins retained on the glutathione agarose beads were analyzed by SDS-PAGE and Western blotting with signals detected by ECL chemiluminescence (Amersham Pharmacia).
Results
MSL Complex Proteins on the Male X Chromosome Colocalize with JIL-1
JIL-1 kinase localizes to interband regions on both male and female polytene chromosomes but is upregulated almost twofold on the male X chromosome (Jin et al. 1999) in a pattern reminiscent of antibody labeling of proteins in the MSL complex (Kuroda et al. 1991; Gorman et al. 1995; Zhou et al. 1995). Thus, in order to determine the relationship of JIL-1 localization with respect to the MSL dosage compensation complex proteins, chromosomal squashes prepared from larval salivary glands were double labeled with JIL-1 antisera and with either MSL1, MSL2, or MSL3 antibody. Fig. 1 shows an example of such a double labeling for JIL-1 and MSL1-specific antibodies. JIL-1 localization is shown in green, MSL1 protein localization in red, and colocalization is indicated by yellow regions in the composite image. The predominantly yellow labeling in the composite image indicates that JIL-1 colocalizes with MSL1 along the entire length of the male X chromosome except at the tip (Fig. 1 A, arrow). At some band locations in the composite image the color is more green or orange than pure yellow due to slight differences in the relative staining intensity of the two antibodies that can vary from preparation to preparation. However, in all cases examined, the banding patterns themselves overlapped (except at the tip; Fig. 1B and Fig. C). Identical results were obtained in double labelings with JIL-1 and MSL2 and MSL3 antibodies (data not shown). In contrast with JIL-1 (Jin et al. 1999) neither MSL1, MSL2, nor MSL3 are found at significant levels on the autosomes (Bashaw and Baker 1996).
Figure 1.
Colocalization of JIL-1 with MSL1 protein on the male X chromosome. Double labeling of male polytene chromosomes with antibodies against JIL-1 (B) and MSL1 (C) reveals identical banded staining patterns on the X chromosome. The composite image (A) shows the extensive overlap between JIL-1 (green) and MSL1 (red) labeling as indicated by the predominantly yellow banding. The only exception to the colocalization between JIL-1 and MSL1 is found at the tip of the X (arrow) that specifically stains for JIL-1 only. Unlike JIL-1, MSL1 was not detectable on the autosomes (B and C).
JIL-1 Upregulation on X Chromosomes Depends on the Presence of the MSL Complex
The observed colocalization of JIL-1 with the MSL proteins led us to ask whether JIL-1 would also be inappropriately upregulated on the female X chromosomes in the presence of ectopic MSL2. This has been demonstrated to be the case for other proteins in the MSL complex (Bashaw and Baker 1995; Kelley et al. 1995). Chromosomal squashes prepared from salivary glands of third instar female larvae that ectopically express MSL2 in a heterozygous msl1 or msl3 background and thus have assembled MSL complexes on their paired X chromosomes were immunostained with JIL-1 antibody. When the MSL complex is targeted to the female X chromosomes, it is accompanied by a concomitant increase of JIL-1 localization (Fig. 2A and Fig. C). That this increase is dependent on the presence of the MSL complex is supported by JIL-1 immunostainings of female polytene chromosome squashes ectopically expressing MSL2 in an msl1/msl1 or msl3/msl3 homozygous background incapable of MSL complex assembly due to absence of the MSL1 or MSL3 subunit, respectively. In these females JIL-1 is no longer upregulated on the paired X chromosomes (Fig. 2B and Fig. D) but now is found distributed on both X chromosomes and throughout all of the autosomes in a pattern consistent with its normal wild-type localization. These results suggest that the upregulation of JIL-1 on the male X chromosome is directly correlated with the presence of the MSL complex. There are two possible scenarios that can account for these observations: either JIL-1 is an integral component of the MSL complex itself or the activity of the MSL complex directs the upregulation of JIL-1 to specific sites on the X chromosome.
Figure 2.
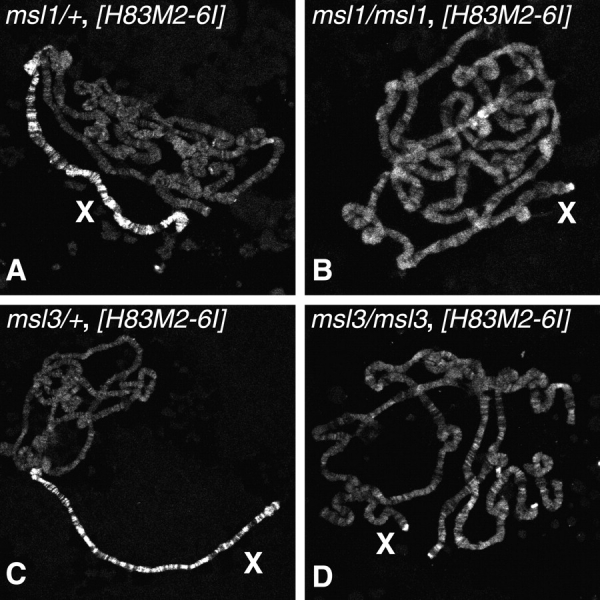
JIL-1 is upregulated on female X chromosomes by the presence of ectopically expressed MSL complex. Polytene chromosomes labeled with JIL-1 antibody from female larvae expressing the MSL complex on the X chromosomes (X) due to induced ([H83M2-6I]) ectopic MSL2 protein expression. In msl1 (A) and msl3 (C) heterozygote backgrounds the MSL complex is inappropriately assembled on the female X (Kelley et al. 1995) with a concomitant nearly twofold ectopic upregulation of JIL-1 as compared with the female autosomes. In contrast, in msl1/msl1 (B) and msl3/msl3 (D) homozygous backgrounds that are incapable of MSL complex assembly (Kelley et al. 1995) due to the absence of the MSL1 and MSL3 proteins, respectively, JIL-1 is equally distributed on both X chromosomes and autosomes in a pattern consistent with its wild-type distribution in females.
JIL-1 Molecularly Interacts with the MSL Complex
To address whether JIL-1 may associate with the MSL dosage compensation complex, we performed coimmunoprecipitation experiments designed to test for molecular interactions. We used protein extracts from the Drosophila S2 cell line that expresses both the MSL complex (Copps et al. 1998) as well as JIL-1 (Jin et al. 1999). To facilitate the cross-immunoprecipitation experiments, we introduced a V5-epitope–tagged full-length JIL-1 construct into stably transfected S2 cells. The V5-tagged JIL-1 fusion protein can be detected in Western blot analysis (Fig. 3 A, S2 lysate) and retains its kinase activity (data not shown). For immunoprecipitation experiments, proteins were extracted from the transfected S2 cells, fractionated on SDS-PAGE after ip, Western blotted, and probed with the appropriate antibodies. Fig. 3 A shows ip experiments using either anti-MSL1 (top panel), anti-MSL2 (middle panel), or anti-MSL3 (bottom panel). All three antibodies were able to coimmunoprecipitate V5-tagged JIL-1 as shown on Western blots probed with anti-V5 antibody. The V5-tagged JIL-1 can be detected in the total S2 lysate, but is not immunoprecipitated by any of the normal sera control ips. Fig. 3 B shows the converse experiment: anti-V5 monoclonal antibody was able to coimmunoprecipitate MSL1 (top panel) and MSL2 (bottom panel) as well as MSL3 (data not shown) from S2 cells that were stably expressing V5-tagged JIL-1, but not from control untransfected S2 cells that do not express the V5–JIL-1 fusion protein. Fig. 3 C shows that endogenous JIL-1 can be immunoprecipitated by MSL1 antibody and, conversely, MSL1 can be immunoprecipitated by JIL-1 antibody from untransfected S2 cell lysate. These results demonstrate a molecular interaction of JIL-1 with the MSL complex. However, since immunoprecipitation of any one of the MSL complex proteins will co-ip the other proteins (Copps et al. 1998), it does not indicate whether any of these proteins interact directly with JIL-1.
Figure 3.
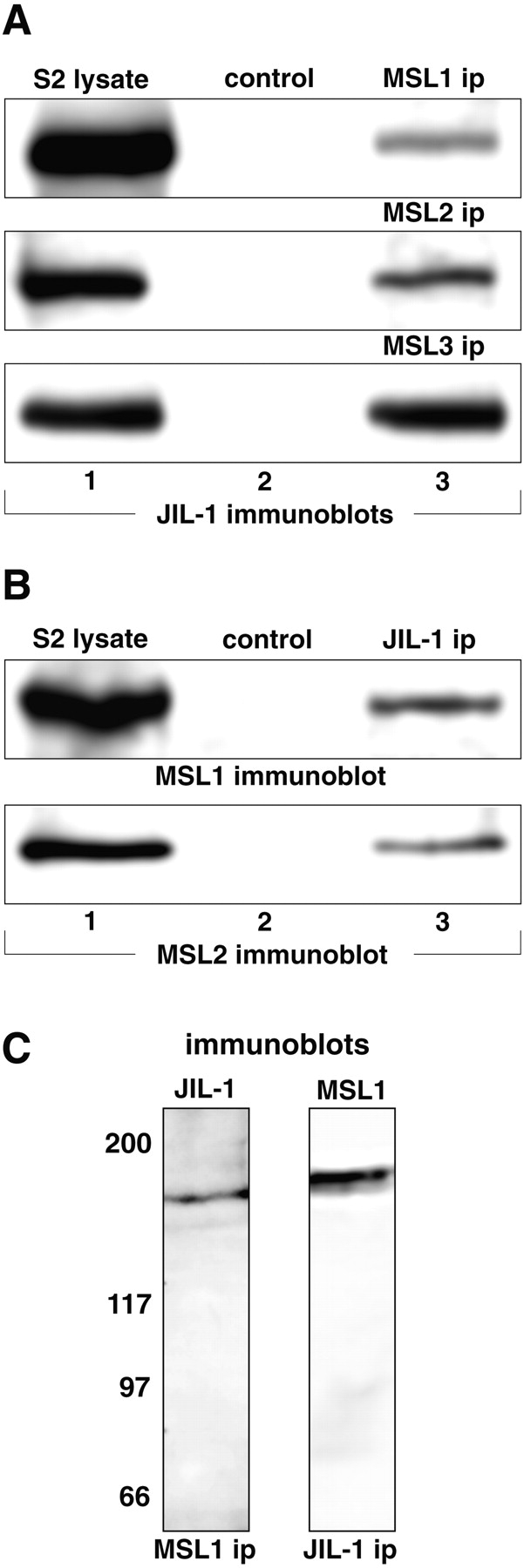
JIL-1 physically interacts with the MSL complex. (A) Immunoprecipitation of lysates from S2 cells stably expressing a V5-tagged JIL-1 fusion protein was performed using either anti-MSL1 (top panel), -MSL2 (middle panel), or -MSL3 (bottom panel) antibodies or the appropriate control serum antibody and analyzed by SDS-PAGE and Western blotting using anti-V5 antibody for detection of the V5-tagged JIL-1 fusion protein. V5-tagged JIL-1 is detected in the S2 cell lysate (lane 1) as well as in the anti-MSL1, -2, and -3 immunoprecipitated samples (lane 3), but is not detected in the control sera-immunoprecipitated samples (lane 2). (B) Anti-V5 monoclonal antibody was used to immunoprecipitate protein from S2 cell lysate from transfected cells expressing a V5-tagged JIL-1 fusion protein or from control, nontransfected S2 cells. The immunoprecipitated proteins were fractionated by SDS-PAGE, Western blotted, and probed with anti-MSL1 (top panel) or anti-MSL2 (bottom panel) antibodies. Both MSL1 and MSL2 were detected in the S2 cell lysate (lane 1) as well as coimmunoprecipitated with the V5-tagged JIL-1 protein (lane 3) from the V5–JIL-1–transfected cells, but could not be detected in the untransfected control S2 cell ip samples (lane 2). (C) Lane 1 shows endogenous JIL-1 immunoprecipitated by MSL1 antibody from lysate of untransfected S2 cells. Lane 2 shows MSL1 immunoprecipitated from S2 cells by the JIL-1 monoclonal antibody, 5C9. The relative migration of molecular markers is indicated to the left in kD.
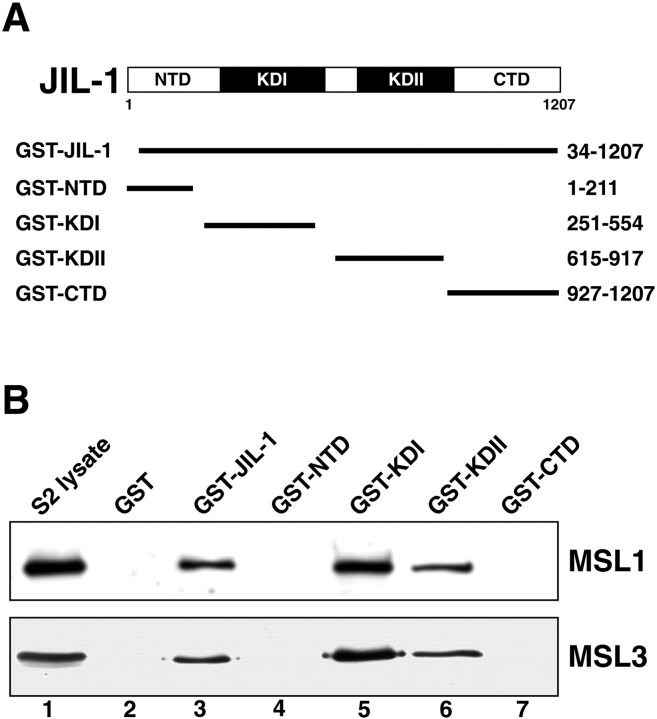
To further characterize which region(s) of the JIL-1 kinase is likely to interact with other components of the MSL dosage compensation complex, we generated GST–JIL-1 fusion proteins covering different domains of JIL-1 including a nearly full-length JIL-1 construct (GST–JIL-1), the NH2-terminal domain (GST-NTD), the first kinase domain (GST-KDI), the second kinase domain (GST-KDII), and the COOH-terminal domain (GST-CTD) (Fig. 4 A). These GST–JIL-1 fusion proteins or a GST-only control were coupled with glutathione agarose beads, incubated with S2 lysate, washed, fractionated by SDS-PAGE, and analyzed by Western blot analysis using antibodies specific for MSL1 or MSL3 (Fig. 4 B). Whereas the GST-only control showed no pull-down activity for either MSL protein, GST–JIL-1 was able to pull down both MSL1 and MSL3. Furthermore, we found that this pull-down activity was specific to the kinase domains of JIL-1: both GST-KDI and GST-KDII were able to pull down both MSL1 and MSL3, but neither GST-NTD nor GST-CTD showed such activity. These results suggest that the direct molecular interaction of JIL-1 with the MSL complex is mediated by the kinase domains and not the NH2- or COOH-terminal sequences.
Figure 4.
JIL-1 interactions with the MSL complex are mediated by the kinase domains. (A) Diagram of the 1,207-residue JIL-1 double kinase and the five GST fusion constructs used for pull-down assays. A nearly full-length (GST–JIL-1) and four truncated (GST-NTD, GST-KDI, GST-KDII, and GST-CTD) GST–JIL-1 fusion proteins were generated. The JIL-1 amino acid residues included in each construct are indicated at the right. (B) S2 cell lysate incubated with each of the JIL-1 GST fusion constructs diagrammed in (A) or a GST-only control was pelleted with glutathione-agarose beads and the interacting proteins fractionated by SDS-PAGE, Western blotted, and probed with anti-MSL1 (top panel) or anti-MSL3 (bottom panel) antibody. Unincubated S2 cell lysate was included as a control (lane 1). GST–JIL-1 (lane 3) as well as constructs containing each of the kinase domains, GST-KDI (lane 5) and GST-KDII (lane 6), were able to pull down the MSL complex as indicated by detection with both MSL1 and MSL3 antibody, while no interaction was observed with the GST-only control (lane 2) or the GST-NTD (lane 4) or GST-CTD (lane 7) constructs.
Discussion
In this study, we present evidence that JIL-1 can associate with the MSL dosage compensation complex by showing that JIL-1 colocalizes with MSL proteins on the polytene chromosomes and that JIL-1 physically interacts with the MSL complex in cross-immune precipitation and pull-down assays. Furthermore, ectopic expression of MSL2 in females, known to inappropriately recruit the MSL complex to the female X, causes a concomitant recruitment of JIL-1 to the female X. This upregulation is abolished in msl mutants that are unable to assemble the complex strongly indicating that the upregulation of JIL-1 on the male X is dependent on the presence of a functional MSL complex.
The MSL complex is believed to promote dosage compensation in males by targeting MOF, the histone acetylase responsible for the increased H4Ac16 modification found on the male X chromosome (Smith et al. 2000). This modification is thought to lead to a more diffuse chromatin structure and enhanced accessibility of the DNA for transcription. However, it is becoming increasingly evident that in addition to acetylation, phosphorylation may also play a key role not only at the level of modulation of transcription factor activity, but also more generally at the level of chromatin structure. For example, histone H3 phosphorylation has been found to occur in a small subset of nucleosomes in mitogenically stimulated cells (Barratt et al. 1994; Sassone-Corsi et al. 1999). This phosphorylation appears to be regulated by mitogen-activated signal transduction cascades indicating a direct link between signal transduction pathways and chromatin structure. JIL-1 has been shown to phosphorylate histone H3 in vitro (Jin et al. 1999) suggesting that the MSL complex may affect chromatin structure not only by histone acetylation, but also by histone phosphorylation. However, the finding that JIL-1 interacts with the MSL complex through its kinase domains also raises the possibility that one or more of the proteins in the MSL complex itself could be a substrate for JIL-1. Regardless of whether the substrate(s) for JIL-1 is histones or components of the MSL complex or both it is likely that the JIL-1 kinase serves as a regulator of MSL complex function through site-specific phosphorylation.
The finding that JIL-1 associates with the MSL complex is based on physical interaction assays such as coimmunoprecipitation and GST pull-down experiments. These approaches require a fairly stable interaction between JIL-1 and the MSL complex making it unlikely that this association depends on the presence of the roX RNAs. This is in contrast to the MLE product that is not found as part of the MSL complex during chromatographic separation (Copps et al. 1998), a finding consistent with it being the only one of the known MSL complex proteins to be lost from the male X chromosomes upon treatment with RNase (Richter et al. 1996). Moreover, the finding that addition of GST–JIL-1 fusion protein to S2 cell extracts can be used to pull down the MSL complex indicates that this association can occur in vitro and suggests that the JIL-1 kinase can interact with the preassembled MSL complex. However, since this preassembled complex may already contain endogenous JIL-1 it does not address whether the formation of the MSL complex requires the presence of JIL-1 protein or whether JIL-1 is present in the MSL complex in stoichiometric amounts.
In contrast to the other MSL complex proteins, JIL-1 is also present in female chromosomes and male autosomes (Jin et al. 1999), all of which are void of MSL complexes (Bashaw and Baker 1996). Future experiments will determine whether JIL-1's more general distribution on the autosomes and on the female X chromosomes may reflect JIL-1's participation in other transcriptional regulator complexes. The deployment of certain proteins into several different chromatin remodeling complexes is emerging as a common theme in the composition of various chromatin remodeling machines and may be a way to accomplish hierarchical levels of gene regulation (Holstege et al. 1998). Here we have shown that JIL-1 associates with at least one known chromatin remodeling machine, the MSL complex, suggesting a direct link between the JIL-1 kinase and the signal transduction pathways regulating transcription and chromatin structure.
Acknowledgments
We wish to thank Drs. Mitzi Kuroda and Richard Kelley for valuable discussions and suggestions as well as for their generous gift of anti-MSL antibodies and [H83M2-6I] stocks. We also wish to thank Ms. Anna Yeung for expert technical assistance and Ms. Virginia Lephart for maintenance of fly stocks.
This work was supported by NSF Grant MCB-9600587.
Footnotes
The first two authors contributed equally to this work.
Abbreviations used in this paper: GST, glutathione-S-transferase; ip, immunoprecipitation; MSL, male specific lethal.
References
- Baker B.S., Gorman M., Marin I. Dosage compensation in Drosophila . Annu. Rev. Genet. 1994;28:491–521. doi: 10.1146/annurev.ge.28.120194.002423. [DOI] [PubMed] [Google Scholar]
- Barratt M.J., Hazzalin C.A., Cano E., Mahadevan L.C. Mitogen-stimulated phosphorylation of histone H3 is targeted to a small hyperacetylation-sensitive fraction. Proc. Natl. Acad. Sci. USA. 1994;91:4781–4785. doi: 10.1073/pnas.91.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashaw G.J., Baker B.S. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal . Development. 1995;121:3245–3258. doi: 10.1242/dev.121.10.3245. [DOI] [PubMed] [Google Scholar]
- Bashaw G.J., Baker B.S. Dosage compensation and chromatin structure in Drosophila . Curr. Opin. Genet. Dev. 1996;6:496–501. doi: 10.1016/s0959-437x(96)80073-6. [DOI] [PubMed] [Google Scholar]
- Belote J.M., Lucchesi J.C. Male-specific lethal mutations of Drosophila melanogaster . Genetics. 1980;96:165–186. doi: 10.1093/genetics/96.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone J.R., Lavender J., Richman R., Palmer M.J., Turner B.M., Kuroda M.I. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila . Genes Dev. 1994;8:96–104. doi: 10.1101/gad.8.1.96. [DOI] [PubMed] [Google Scholar]
- Copps K., Richman R., Lyman L.M., Chang K.A., Rampersad-Ammons J., Kuroda M.I. Complex formation by the Drosophila MSL proteinsrole of the MSL2 RING finger in protein complex assembly. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5409–5417. doi: 10.1093/emboj/17.18.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A., Baker B.S. The rox1 and rox2 RNAs are essential components of the compensasome, which mediates dosage compensation in Drosophila . Mol. Cell. 1999;4:117–122. doi: 10.1016/s1097-2765(00)80193-8. [DOI] [PubMed] [Google Scholar]
- Fukunaga, A., A. Tanaka, and K. Oishi. 1975. Maleless, a recessive autosomal mutant of Drosophila melanogaster that specifically kills male zygotes. Genetics. 81:135–114. [DOI] [PMC free article] [PubMed]
- Gorman M., Baker B.S. How flies make one equal twodosage compensation in Drosophila . Trends Gen. 1994;10:376–380. doi: 10.1016/0168-9525(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Gorman M., Franke A., Baker B.S. Molecular characterization of the male-specific lethal-3 gene and investigation of the regulation of dosage compensation in Drosophila . Development. 1995;121:463–475. doi: 10.1242/dev.121.2.463. [DOI] [PubMed] [Google Scholar]
- Harlow E., Lane D. AntibodiesA Laboratory Manual. Cold Spring Harbor Laboratory Press, ; Cold Spring Harbor, NY: 1988. [Google Scholar]
- Hilfiker A., Hilfiker-Kleiner D., Pannuti A., Lucchesi J.C. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila . EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2054–2060. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege F.C., Jennings E.G., Wyrick J.J., Lee T.I., Hengartner C.J., Green M.R., Golub T.R., Lander E.S., Young R.A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- Jin Y., Wang Y., Walker D.L., Dong H., Conley C., Johansen J., Johansen K.M. JIL-1a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila . Mol. Cell. 1999;4:129–135. doi: 10.1016/s1097-2765(00)80195-1. [DOI] [PubMed] [Google Scholar]
- Kelley R.L., Kuroda M.I. Equality for X chromosomes. Science. 1995;270:1607–1610. doi: 10.1126/science.270.5242.1607. [DOI] [PubMed] [Google Scholar]
- Kelley R.L., Solovyeva I., Lyman L.M., Richman R., Solovyev V., Kuroda M.I. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila . Cell. 1995;81:867–877. doi: 10.1016/0092-8674(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Kuroda M.I., Kernan M.J., Kreber R., Ganetzky B., Baker B.S. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila . Cell. 1991;66:935–947. doi: 10.1016/0092-8674(91)90439-6. [DOI] [PubMed] [Google Scholar]
- Lucchesi J.C. Dosage compensation in flies and wormsthe ups and downs of X-chromosome regulation. Curr. Opin. Genet. Dev. 1998;8:179–184. doi: 10.1016/s0959-437x(98)80139-1. [DOI] [PubMed] [Google Scholar]
- Meller V.H., Wu K.H., Roman G., Kuroda M.I., Davis R.L. roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell. 1997;88:445–457. doi: 10.1016/s0092-8674(00)81885-1. [DOI] [PubMed] [Google Scholar]
- Palmer M.J., Mergner V.A., Richman R., Manning J.E., Kuroda M.I., Lucchesi J.C. The male-specific lethal-one (msl-1) gene of Drosophila melanogaster encodes a novel protein that associates with the X chromosome in males. Genetics. 1993;134:545–557. doi: 10.1093/genetics/134.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L., Bone J.R., Kuroda M.I. RNA-dependent association of the Drosophila maleless protein with the male X chromosome. Genes Cells. 1996;1:325–336. doi: 10.1046/j.1365-2443.1996.26027.x. [DOI] [PubMed] [Google Scholar]
- Roberts D.B. Drosophila: A Practical Approach 1986. IRL Press, ; Oxford, UK: pp. 295 [Google Scholar]
- Sassone-Corsi P., Mizzen C.A., Cheung P., Crosio C., Monaco L., Jacquot S., Hanauer A., Allis C.D. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- Smith E.R., Pannuti A., Gu W., Seurnagel A., Cook R.G., Allis C.D., Lucchesi J.C. The Drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol. Cell. Biol. 2000;20:312–318. doi: 10.1128/mcb.20.1.312-318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B.M., Birley A.J., Lavender J. Histone H4 isoforms acetylated at specific lysine residues define individual chromosomes and chromatin domains in Drosophila polytene nuclei. Cell. 1992;69:375–384. doi: 10.1016/0092-8674(92)90417-b. [DOI] [PubMed] [Google Scholar]
- Wolffe A.P., Hayes J.J. Chromatin disruption and modification. Nucleic Acids Res. 1999;27:711–720. doi: 10.1093/nar/27.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Yang Y., Scott M.J., Pannuti A., Fehr K.C., Eisen A., Koonin E.V., Fouts D.L., Wrightsman R., Manning J.E., Lucchesi J.C. Male-specific lethal 2, a dosage compensation gene of Drosophila, undergoes sex-specific regulation and encodes a protein with a RING finger and a metallothionein-like cysteine cluster. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2884–2895. doi: 10.1002/j.1460-2075.1995.tb07288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]