Abstract
Controlling neuropathic pain is an unmet medical need and we set out to identify new therapeutic candidates. AV411 (ibudilast) is a relatively nonselective phosphodiesterase inhibitor that also suppresses glial-cell activation and can partition into the CNS. Recent data strongly implicate activated glial cells in the spinal cord in the development and maintenance of neuropathic pain. We hypothesized that AV411 might be effective in the treatment of neuropathic pain and, hence, tested whether it attenuates the mechanical allodynia induced in rats by chronic constriction injury (CCI) of the sciatic nerve, spinal nerve ligation (SNL) and the chemotherapeutic paclitaxel (Taxol®). Twice-daily systemic administration of AV411 for multiple days resulted in a sustained attenuation of CCI-induced allodynia. Reversal of allodynia was of similar magnitude to that observed with gabapentin and enhanced efficacy was observed in combination. We further show that multi-day AV411 reduces SNL-induced allodynia, and reverses and prevents paclitaxel-induced allodynia. Also, AV411 cotreatment attenuates tolerance to morphine in nerve-injured rats. Safety pharmacology, pharmacokinetic and initial mechanistic analyses were also performed. Overall, the results indicate that AV411 is effective in diverse models of neuropathic pain and support further exploration of its potential as a therapeutic agent for the treatment of neuropathic pain.
Keywords: ibudilast, mechanical allodynia, nerve injury, spinal cord, glia
INTRODUCTION
Chronic neuropathic pain is treated inadequately in the clinic because available drugs either fail to provide pain relief or are often associated with unacceptable side-effects (Rice and Hill, 2006). Currently available therapies primarily target neurons. However, activated spinal microglia and astrocytes each make crucial contributions to neuropathic pain through the release of proinflammatory cytokines and other substances that facilitate pain transmission (Watkins and Maier, 2003; Marchand et al., 2005; Moalem and Tracey, 2005). In experimental models of pain, either disruption of spinal glial activation or antagonism of substances released by activated glia either prevent or reverse pain hypersensitivity (Watkins and Maier, 2003). Pharmacological attenuation of glial activation in the spinal cord might, therefore, represent a novel approach for controlling neuropathic pain.
AV411 (ibudilast [3-isobutyryl-2-isopropylpyrazolo-[1,5-a]pyridine]) is marketed in Asia to treat bronchial asthma and post-stroke dizziness, based on its anti-inflammatory and cerebral vaso-relaxant properties (Kishi et al., 2001). It is considered a relatively nonselective phosphodiesterase (PDE) inhibitor, although it inhibits PDE types 3, 4, 10 and 11 more potently (Suzumura et al., 1999; Kishi et al., 2000; Gibson et al., 2006; Huang et al., 2006). In activated glial cells in vitro, ibudilast suppresses, in a concentration-dependent manner, the production of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β), and increases the production of the anti-inflammatory cytokine IL-10 and various neurotrophic factors (Suzumura et al., 1999; Kawanokuchi et al., 2004; Mizuno et al., 2004). Ibudilast also protects hippocampal neurons (Tominaga et al., 1996) and oligodendrocytes (Yoshioka et al., 1998; Yoshioka et al., 2000) against excitotoxity, and astrocytes against apoptosis (Takuma et al., 2001).
In animal models, ibudilast ameliorates neurological signs in experimental autoimmune encephalitis (EAE) when administered before, but not after, the onset of disease (Fujimoto et al., 1999). Ibudilast also alleviates hippocampal neuronal damage in a rat model of transient cerebral ischemia (Yoshioka et al., 2002), and reduces white matter lesions and microglial activation induced by chronic cerebral ischemia (Wakita et al., 2003). Furthermore, ibudilast treatment reduces oligodendrocyte apoptosis and demyelination in a mouse model of Krabbe's disease (Kagitani-Shimono et al., 2005), and reduces the expression of proinflammatory cytokines in patients with multiple sclerosis (Feng et al., 2004).
OBJECTIVES
Based on the anti-inflammatory and glial modulatory activities of ibudilast, we hypothesized that AV411 might provide pain relief in conditions of neuropathic pain. Therefore, in the present studies we evaluated the efficacy of systemic administration of AV411 in several neuropathic pain models. It was tested for attenuation of low-threshold mechanical allodynia induced by chronic constriction injury (CCI) of the sciatic nerve, L5/L6 spinal nerve ligation (SNL), and repeated injections of the chemotherapy drug paclitaxel. In addition, we compared the effects of AV411 to those of gabapentin, which is currently widely used for clinical neuropathic pain control, and rolipram, an anti-inflammatory PDE4 inhibitor. Lastly, we investigated AV411-mediated modulation of glial activation in vitro, and whether AV411 alters the effects of morphine and/or influenced morphine tolerance in nerve-injured rats.
METHODS
Subjects
Pathogen-free adult male Sprague-Dawley rats (250−375 g; Harlan Labs) were used in all experiments except in the SNL study and Irwin test, where male Wistar (Han) rats (160−200 g; Elevage Janvier) were used (this is the preferred strain at Porsolt & Partners, where these two studies were performed). Rats were housed in temperature- (23±3°C) and light- (12 h:12 h light:dark cycle; lights on at 7 AM) controlled rooms with standard rodent chow and water available ad libitum. Behavioral testing and drug administration were performed during the light cycle. All procedures were approved by the Institutional Animal Care and Use Committees of the University of Colorado at Boulder, Avigen Inc., or Porsolt and Partners. All experiments adhered to the guidelines of the Committee for Research and Ethical Issues of IASP (Zimmermann, 1983).
Compounds
AV411 (Haorui Inc. and Sigma) was dissolved in 35% polyethylene glycol (PEG) 400 (Sigma) in sterile physiological saline, except for the tissue-distribution study where the vehicle was 15% EtOH in saline. Fresh solutions were made every other day and stored at room temperature (RT). Gabapentin (Neurontin®; Parke-Davis/Pfizer) was formulated in sterile saline at a concentration of 10 mg ml−1 and stored at RT. Rolipram (Sigma) was formulated in a vehicle of 35% PEG 400 in saline and stored at RT. Morphine sulfate (NIDA) was dissolved in sterile saline at a concentration of 1 mg ml−1 for an injection volume of 1 ml kg−1. Paclitaxel (Taxol®; 6 mg ml−1 in Cremophor EL:absolute ethanol, 1:1; Bristol-Myers Squibb) was diluted in sterile saline to a concentration of 1 mg ml−1 before each injection. The vehicle was a 1:1 mixture of Cremophor:ethanol diluted in saline as above.
Surgical procedures
CCI
A CCI was created at mid-thigh level of the left hind leg as described previously (Bennett and Xie, 1988). Four, sterile, absorbable, chromic gut sutures (cuticular 4−0, 27"; Ethicon) were tied loosely around the isolated sciatic nerve under isoflurane anesthesia (induction 4−5%, maintenance 2−3% in oxygen; Phoenix Pharm.). The sciatic nerves of sham-operated rats were exposed identically but not ligated.
SNL (Chung model)
The surgical procedure was performed according to the method described by Kim and Chung (1992). Rats were anesthetized with isoflurane, and a small incision was made in the skin overlaying L4–S2, followed by retraction of the paravertebral musculature from the vertebral transverse processes. The transverse process was partially removed to expose the left L5 and L6 spinal nerves. A 6−0 silk suture was tied tightly around each nerve, and the wound was closed in two layers. The rats received an injection of the antibiotic clamoxyl and were allowed to recover. Sham controls were subjected to the same surgical procedure except the nerves were not ligated.
Intrathecal injections
Acute, intrathecal (i.t.) injections were performed under brief isoflurane anesthesia, using an 18-gauge sterile needle, inserted temporarily between lumbar vertebrae L5 and L6. This needle was used as a guide for a PE10 injection catheter to allow injection of compounds at the level of the lumbosacral enlargement. AV411 was injected over ∼3 sec in a dose of 25 μg in 10 μl followed by a 2 μl saline flush.
Pharmacological procedures
Reversal of CCI-induced mechanical allodynia by AV411
The details for each experiment with the CCI model are listed in Table 1. In all experiments, rats underwent either CCI or sham surgery on day 0, and either AV411 or vehicle was administered on the days indicated. Response thresholds of the hind paws to touch/pressure stimuli (von Frey test) were assessed as indicated in the figures for each experiment. In experiment 3, rats were administered vehicle, AV411, gabapentin, or AV411 plus gabapentin. The dose of 50 mg kg−1 gabapentin was selected based on literature reports (De Vry et al., 2004; Chen and Pan, 2005) and our own dose-range-finding studies in which we found unacceptable side-effects with doses of 100 mg kg−1 (data not shown). In experiment 6, rats were administered either s.c. morphine or vehicle 30 min after the i.p. injections, and responses to the von Frey test were assessed on days 12 and 16 up to 3.5 hours after the morning s.c. injections.
Table 1.
Experimental design of CCI studies
| Exp | n/group | Treatment (route of administration, frequency, days of dosing)1 | Dose |
|---|---|---|---|
| 1 | 3 (sham), 5 (CCI) | AV411 (i.p., QD, days 10−14 post-surgery) | 3, 15 mg kg−1 |
| 2 | 5−8 | AV411 (i.p., BID, days 10−14 post-surgery) | 2.5, 7.5, 10 mg kg−1 |
| 3 | 4−5 | AV411 ((i.p., BID, days 10−14 post-surgery) | 7.5 mg kg−1 |
| Gabapentin (i.p., BID, days 10−14 post-surgery) | 50 mg kg−1 | ||
| 4 | 7−8 | AV411 (p.o., BID, days 15−19 post-surgery) | 22 mg kg−1 |
| 5 | 4−7 | AV411 (i.t., single, day 10) | 25 μg rat−1 |
| 6 | 5−6 | AV411 (i.p., BID, days 10−16 post-surgery) | 7.5 mg kg−1 |
| Morphine (s.c., BID, days 12−16 post-surgery) | 1 mg kg−1 |
Abbreviations: i.p., intraperitoneal; p.o., per os; i.t., intrathecal; s.c., subcutaneous; QD, once daily; BID, twice daily.
Reversal of SNL-induced mechanical allodynia by AV411
On day 0, rats underwent either SNL surgery (n=5/group) or sham surgery (n=3−4/group). On days 12−14 post-surgery, rats were administered i.p. AV411 (10 mg kg−1) or vehicle twice daily. Responses to the von Frey test were assessed as indicated in the figure for this experiment. Although a negative-control group (CCI/vehicle) was not run simultaneously in this particular experiment, Porsolt and Partners have a history of performing this type of surgery, wherein rats develop allodynia within 2 weeks after surgery that persists for at least 3 weeks (M. Lemaire, personal communication).
Effect of AV411 on paclitaxel-induced mechanical allodynia
Rats (n=5−6/group) received four i.p. injections of paclitaxel (1 mg kg−1 per injection; cumulative dose 4 mg kg−1) on alternate days, as described previously (Polomano et al., 2001). Rats were administered i.p. AV411 (7.5 mg kg−1) or vehicle twice daily on days 19−25 after the first injection of paclitaxel. In a separate study, rats received paclitaxel (cumulative dose 4 mg kg−1; n=5/group) or vehicle (n=2/group) as above, and were administered i.p. AV411 (7.5 mg kg−1) or vehicle twice daily on days 12−18 after the first paclitaxel injection. Responses to the von Frey test were assessed as indicated in the figures for each experiment.
Tissue distribution and plasma concentration of AV411
Naïve Sprague-Dawley rats (n=2/group) received a single dose of 5 mg kg−1 AV411 i.p. and were sacrificed either 1 hour or 7 hours after dosing. Brain (striatum), muscle (quadriceps), and spinal cord (lumbar region) tissues were collected, weighed and flash-frozen in liquid nitrogen and stored at −80°C until assayed. Whole-blood samples were collected by cardiac punctures into EDTA-coated tubes and centrifuged at 6500 rpm for 15 min at 4°C. Plasma was removed and stored at −80°C until assayed. In a separate experiment, naïve Sprague-Dawley rats (n=3/group) were administered either a single i.p. or oral dose of AV411 (7.5 mg kg−1). Blood was drawn from the tail vein into EDTA-coated tubes and centrifuged at 6500 rpm for 15 min at 4°C. Plasma was removed and stored at −80°C until assayed. Concentrations of AV411 were quantitated by LC/MS/MS by Integrated Analytical Solutions Inc. Briefly, samples were precipitated with ice cold acetonitrile containing 100 ng ml−1 diphendyramine as the internal standard. Following centrifugation, the supernatant fractions were diluted in 0.2% formic acid in water and analyzed by high performance liquid chromatography (HPLC) in conjunction with a triple-quadrupole mass spectrometer that uses electrospray ionization in tandem with positive ionization (MS/MS). On the basis of data obtained with quality control samples, the accuracy and precision were within 6%. The lower limit of quantitation was 1 ng ml−1.
Behavioral measurements
von Frey test for mechanical allodynia
The von Frey test (Chaplan et al., 1994) was performed within the sciatic innervation area of the hind paws as described previously (Milligan et al., 2000). Briefly, calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting) were applied randomly to left and right hind paws to elicit paw withdrawal responses. The monofilaments used ranged from 3.61 (0.407 g) to 5.18 (15.136 g) bending force. Assessments were made before (baseline) and at specific times after surgery or drug injections, as detailed for each experiment. Behavioral testing was performed blind with respect to drug administration. The behavioral responses were used to calculate the 50% paw withdrawal threshold (absolute threshold), by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method, allowing parametric statistical analyses (Harvey, 1986; Treutwein and Strasburger, 1999), as described in detail (Milligan et al., 2000). In the CCI and SNL experiments, rats were tested only on the ipsilateral hind paw. In the paclitaxel experiments, rats were tested on both hind paws because systemic paclitaxel treatment results in bilateral allodynia. Thresholds in these rats did not differ between left and right hind paws at any time point in any group, so the data are presented as average values from both hind paws. Baseline and post-surgery (pre-treatment) threshold values vary between experiments because of differences in experimental testing conditions (notably, a difference in ambient temperature at different research sites) at the time these studies were performed.
Primary observation (Irwin) test
Naïve Sprague-Dawley rats (n=3/group) received AV411 (7.5 mg kg−1) or vehicle i.p. twice daily for five days, and were assessed for behavior and physiological function in the Irwin test on day 1 and day 5 of treatment, immediately, 15, 30, 60, 120 and 180 min, and 24 hours after the morning injection. The methodology described by Irwin (1968) was utilized. Rats were administered either AV411 or vehicle and observed simultaneously with a control group given physiological saline. Behavioral modifications, physiological and neurotoxicity symptoms, rectal temperature and pupil diameter were recorded according to a standardized observation grid derived from that of Irwin. The grid contains the following items: death*, convulsions*, tremor*, Straub tail*, sedation, excitation, jumping*, abnormal gait* (rolling, tiptoe), motor incoordination*, altered muscle tone, loss of grasping, akinesia, catalepsy, loss of traction, loss of balance*, fore-paw treading*, writhing*, piloerection*, stereotypies* (sniffing, chewing, head movements), head-twitches*, scratching*, altered respiration*, aggression*, altered fear, altered reactivity to touch, ptosis, exophthalmia, loss of righting reflex, loss of corneal reflex, analgesia, defecation/diarrhea, salivation, lacrimation, rectal temperature (hypothermia/hyperthermia) and pupil diameter (myosis/mydriasis). Observations were performed 15, 30, 60, 120, 180 min and 24 hours after administration of the test substance. The symptoms marked (*) were observed continuously from 0 to 15 min after administration.
Rat primary microglial cell cultures
Primary mixed glial cell cultures were prepared from newborn (<3-day-old) Sprague-Dawley rats (Harlan) using the trypsin dissociation method as described previously (Ledeboer et al., 2000) and grown in 75 cm2 culture flasks coated with 10 μg ml−1 poly-D-lysine. Enriched microglial cell cultures were obtained from the mixed glial cultures by shaking 10−14 day-old cultures for 2 hours at 37°C at 175 rpm on an orbital shaker. The detached microglial cells were washed and allowed to adhere for 1 hour in 24-well plates (1 × 105 cells well−1) coated with poly-D-lysine before treatments. Cultures were treated in duplicate with AV411 (1−100 μM) or vehicle (0.1% DMSO [Sigma D2650]) in DPBS) for 30 min and then co-stimulated with 2 ng ml−1 lipopolysaccharide (LPS; Sigma L4391) and 100 ng ml−1 rat interferon γ (IFN-γ) (RandD Systems, 585-IF). Culture supernatants were collected 6 hours and 21 hours post LPS and stored at −20°C until assayed. The concentration of rat TNF-α and rat MCP-1 in culture supernatants were determined by ELISA (RandD Systems, SRTA00 and Assay Designs, 900−077, respectively) according to the manufacturer's instructions. Detection limits for the assay were 5 pg ml−1 for TNF-α and 20 pg ml−1 for MCP-1.
Receptor activity screen
The activity of AV411 was evaluated in radioligand binding and enzyme assays conducted by MDS Pharma Services (Taipei). Detailed methods (specific conditions, cellular sources, substrates and reference standards) for individual assays are available from MDS Pharma Services. Targets were of human or rat origin as described in the MDS procedures. AV411 was screened at 10 μM. Results are presented as the % inhibition of specific binding or activity.
Statistical analysis
Statistical comparisons were computed using either Statview 5.0.1 or GraphPad Prism 4.00. Data from the von Frey test were analyzed as the interpolated 50% threshold (absolute threshold) in log10 of stimulus intensity (monofilament stiffness in mg × 10). Baseline measures for the von Frey test were analyzed by one-way ANOVA. Time-course measures were analyzed by repeated measures ANOVA followed by Fisher's protected least significant difference post-hoc comparisons, where appropriate. Data from the SNL study were analyzed using Bonferroni's Multiple Comparison Test. P<0.05 was considered to indicate a significant difference.
RESULTS
Reversal of CCI-induced mechanical allodynia by once daily i.p. AV411
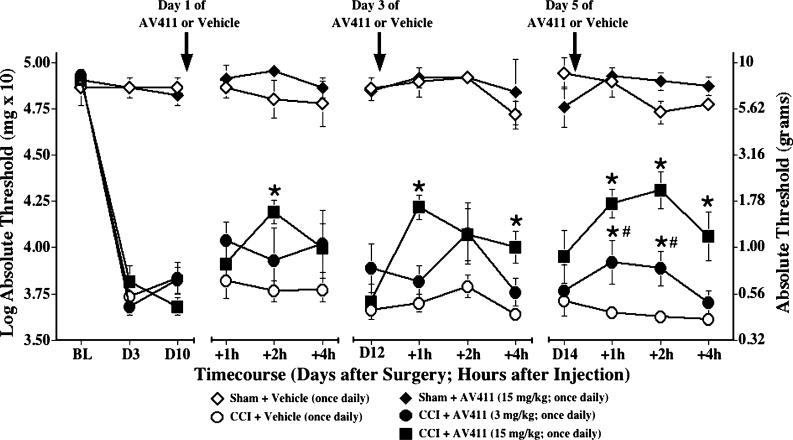
Initial efficacy of AV411, administered once daily i.p. at either a low or a high dose, was assessed in the rat CCI model, a classic model of neuropathic pain. As shown in Fig. 1, pre-surgery baseline responses on the von Frey test did not differ between groups. CCI surgery induced reliable, low-threshold mechanical allodynia in the ipsilateral hind paw by day 3 post-surgery compared to sham surgery. AV411 injections (15 mg kg−1, i.p.) transiently attenuated CCI-induced allodynia. This effect was apparent as early as 1 hour post-injection, peaked at ∼2 hours, and was still evident at 4 hours post-injection. Repeated dosing over 2−3 days increased the magnitude and duration of reversal. Using a lower dose of AV411 (3 mg kg−1), an attenuating effect was not significant until treatment was maintained for 4 days. Following injections of the high dose of AV411, some transient (prior to allodynia testing) subdued activity and occasional diarrhea were observed. However, these effects diminished with repeated administration. In sham-operated animals, treatment with AV411 did not affect basal response thresholds.
Fig. 1. Once daily i.p. AV411 transiently attenuates CCI-induced mechanical allodynia.
Rats received either sham or CCI surgery on day 0 and were administered either vehicle or AV411 (3 or 15 mg kg−1) once daily from days 10−14 post-surgery. Low-threshold mechanical sensitivity was assessed by the von Frey test, before surgery (baseline, BL), on day 3 post-surgery, and on days 10, 12, and 14 before, 1, 2, and 4 hours after the i.p. injection. Data are mean±SEM. *P<0.05 vs CCI + vehicle; #P<0.05 vs CCI + 15 mg kg AV411.
Reversal of CCI-induced mechanical allodynia by twice daily i.p. AV411
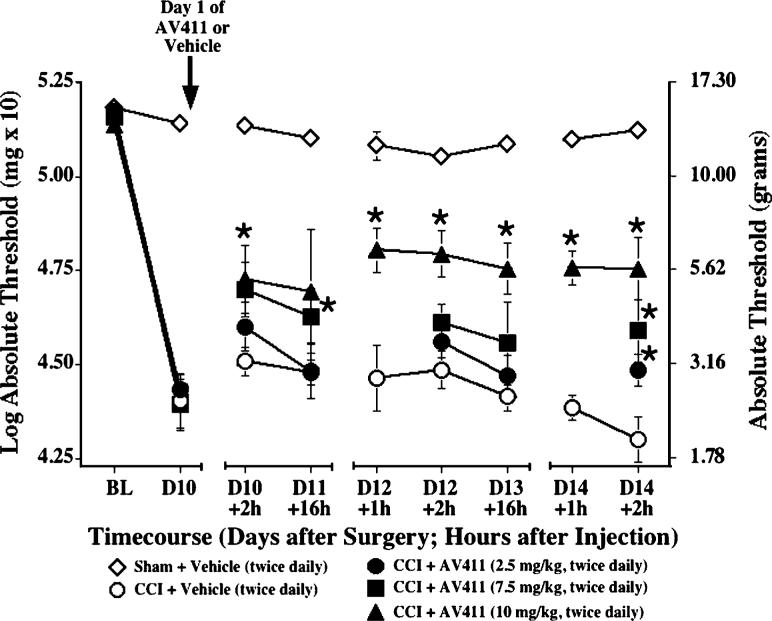
Because the rats receiving the dose of 15 mg kg−1 in the previous experiment exhibited some side-effects in the first hour after injection, we utilized a range of lower doses to further assess AV411 efficacy, and switched to a paradigm of twice daily administration, based on pharmacokinetic observations (see later). As shown in Fig. 2, pre-surgery baseline responses on the von Frey test did not differ between groups, and CCI induced allodynia by day 10 post-surgery. Twice daily i.p. delivery of AV411 attenuated mechanical allodynia in a dose-related fashion. The attenuation of allodynia by administration of AV411 at the 10 mg kg−1 dose, and to a lesser extent at 7.5 mg kg−1, was sustained for up to 16 hours post-administration. Preliminary investigations show that the attenuation of allodynia by AV411 is associated with a shift in weight bearing on the first and third days of treatment. Rats treated with AV411 (7.5 mg kg−1) exhibited increased weight bearing on the ipsilateral hind limb to 10−12% of their body weight compared with 3−4% body weight in vehicle-treated rats. This is similar to the effect observed following treatment with 3 mg kg−1 morphine. In addition, preliminary observations indicate that the behavioral efficacy of AV411 is related to decreased immunoreactivity for glial fibrillary acidic protein (GFAP; a marker for astrocyte activation) in the ipsilateral lumbar spinal cord of CCI rats treated with AV411 (7.5 mg kg−1) for 5 days. In a separate study, thermal hyperalgesia (Hargreaves) was assessed in CCI rats treated with 10 mg kg−1 AV411, but no significant differences were observed in hind-paw latencies compared to vehicle controls after multiple days of AV411 treatment (data not shown).
Fig. 2. Twice daily i.p. AV411 attenuates CCI-induced mechanical allodynia.
Rats received either sham or CCI surgery on day 0, and either vehicle or AV411 (2.5, 7.5 and 10 mg kg−1) were administered twice daily from days 10−14 post-surgery. Low-threshold mechanical sensitivity was assessed by the von Frey test before surgery (baseline, BL) and from days 10−14 either before, 1 hour or 2 hours after the morning administration. Data are mean±SEM. *P<0.05 vs CCI + vehicle; +2 hours, with respect to first dose of that day; +16 hours, with respect to second dose of the previous day. N.B. Baseline and post-surgery threshold values are higher because of different experimental testing conditions (see text).
In contrast to the effects of AV411, treatment of CCI rats with rolipram, a selective inhibitor of PDE4 that partitions to the CNS, using the same paradigm of twice daily i.p. administration for multiple days, did not attenuate CCI-induced mechanical allodynia at doses ranging from 0.1 mg kg−1 to the maximum tolerated dose of 0.6 mg kg−1 (data not shown).
Reversal of CCI-induced mechanical allodynia by twice daily i.p. injection of AV411, gabapentin, and combination of AV411 and gabapentin
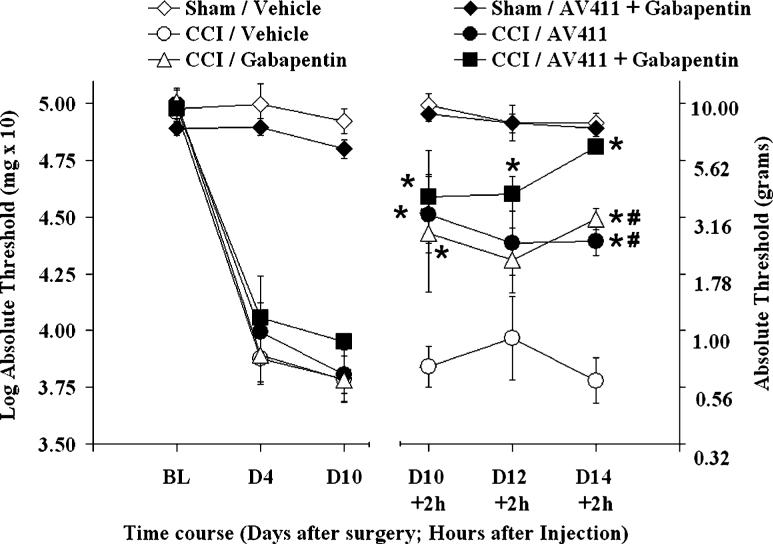
The multiday efficacy of AV411 was also compared to that of gabapentin, a commonly prescribed drug with proven analgesic efficacy. As shown in Fig. 3, twice daily administration of gabapentin (50 mg kg−1 i.p.) attenuated CCI-induced allodynia to a similar extent as AV411 (7.5 mg kg−1). Treatment with AV411 and gabapentin in combination resulted in an enhanced anti-allodynic effect on the fifth day of treatment compared with either drug alone, which indicates that the two drugs have additive effects.
Fig. 3. Twice daily i.p. AV411 and gabapentin attenuate CCI-induced mechanical allodynia similarly, and efficacy is enhanced when combined.
Rats received either sham or CCI surgery on day 0, and were administered vehicle, AV411 (7.5 mg kg−1), gabapentin (50 mg kg−1) or both from days 10−14 post-surgery. Low-threshold mechanical sensitivity was assessed by the von Frey test before surgery (baseline, BL) and from day 10−14 either before or 2 hours after the morning administration. Data are mean±SEM. *P<0.05 vs CCI + vehicle; #P<0.05 vs CCI + AV411 + gabapentin.
Reversal of CCI-induced mechanical allodynia by twice daily oral AV411
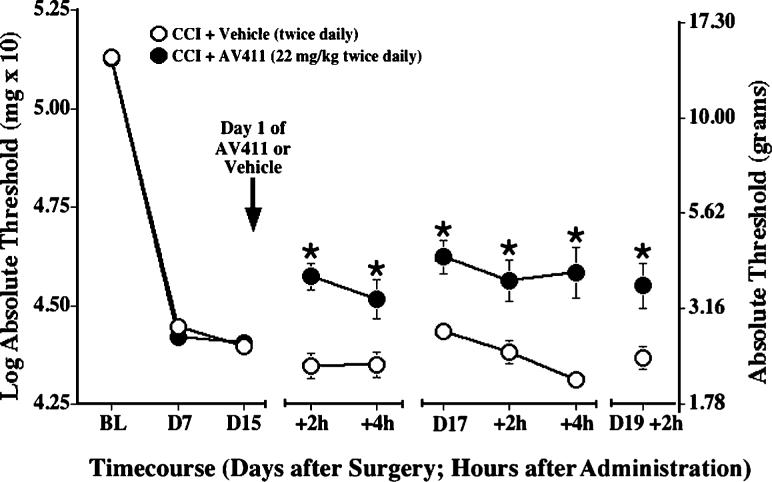
The efficacy of oral administration of AV411 was also assessed in the CCI model. At a dose comparable to that given i.p. (7.5 mg kg−1), oral AV411 did not attenuate CCI-induced allodynia (data not shown). However, this is probably because of low oral bioavailability of AV411 in rats, as indicated by pharmacokinetic data (see later) so, subsequently, we assessed the effectiveness of a higher dose (22 mg kg−1). The dose of 22 mg/kg was used because AV411 solubility and the tolerability of this PEG formulation is dose-limiting. Pre-surgery baseline responses to the von Frey test did not differ between groups, and CCI induced reliable allodynia by day 7 post-surgery (Fig. 4). Twice daily AV411 (22 mg kg−1; p.o.) attenuated CCI-induced allodynia as early as 2 hours post-administration, compared to vehicle controls. Attenuation was sustained at 4 hours and throughout subsequent testing. Moreover, AV411 treatment was well-tolerated with no adverse effects observed.
Fig. 4. Twice daily oral administration of AV411 attenuates CCI-induced mechanical allodynia.
Rats received CCI surgery on day 0, and were administered either vehicle or AV411 (22 mg kg−1) twice daily from days 15−19 post-surgery. Low-threshold mechanical sensitivity was assessed by the von Frey test before surgery (baseline, BL) on day 7 post-surgery, and on days 15, 17, and 19 either before, 2 hours or 4 hours after the morning administration. Data are mean±SEM. *P<0.05 vs CCI + vehicle. N.B. Baseline and post-surgery threshold values are higher because of different experimental testing conditions (see text).
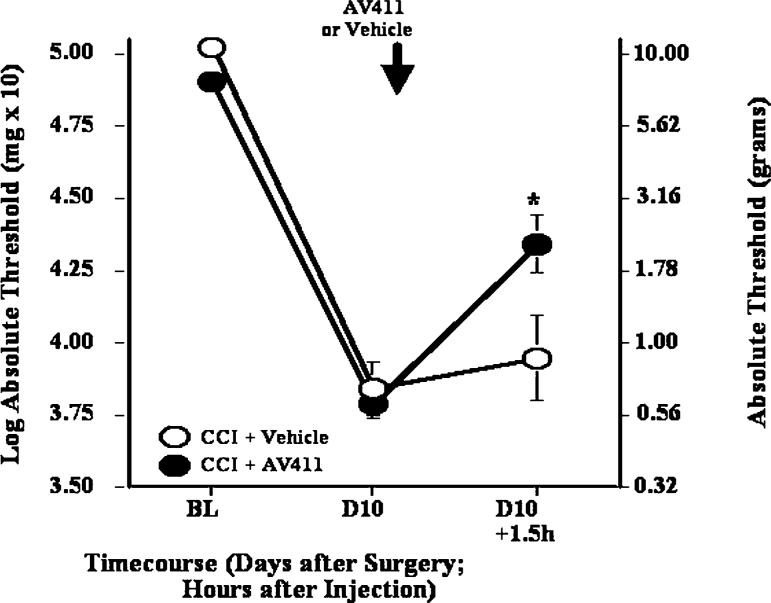
Reversal of CCI-induced mechanical allodynia by a single i.t. AV411 injection
To gain more insight about the site of action of AV411, we also assessed the effect of i.t. administration of AV411. AV411 was given at a dose of 25 μg rat−1, based on pilot studies. As shown in Fig. 5, a single i.t. injection of AV411 reversed CCI-induced mechanical allodynia 1.5 hours after injection, with a magnitude of reversal comparable to that seen after acute i.p. injection. These data indicate that AV411 might act, at least in part, at the level of the spinal cord.
Fig. 5. A single i.t. dose of AV411 attenuates CCI-induced mechanical allodynia.
Rats received CCI surgery on day 0, and either vehicle or AV411 (25 μg) were administered on day 10 post-surgery. Low-threshold mechanical sensitivity was assessed by the von Frey test before surgery (baseline, BL), on day 10 post-surgery, and 1.5 hours after i.t. administration. Data are mean±SEM. *P<0.05 vs CCI + vehicle.
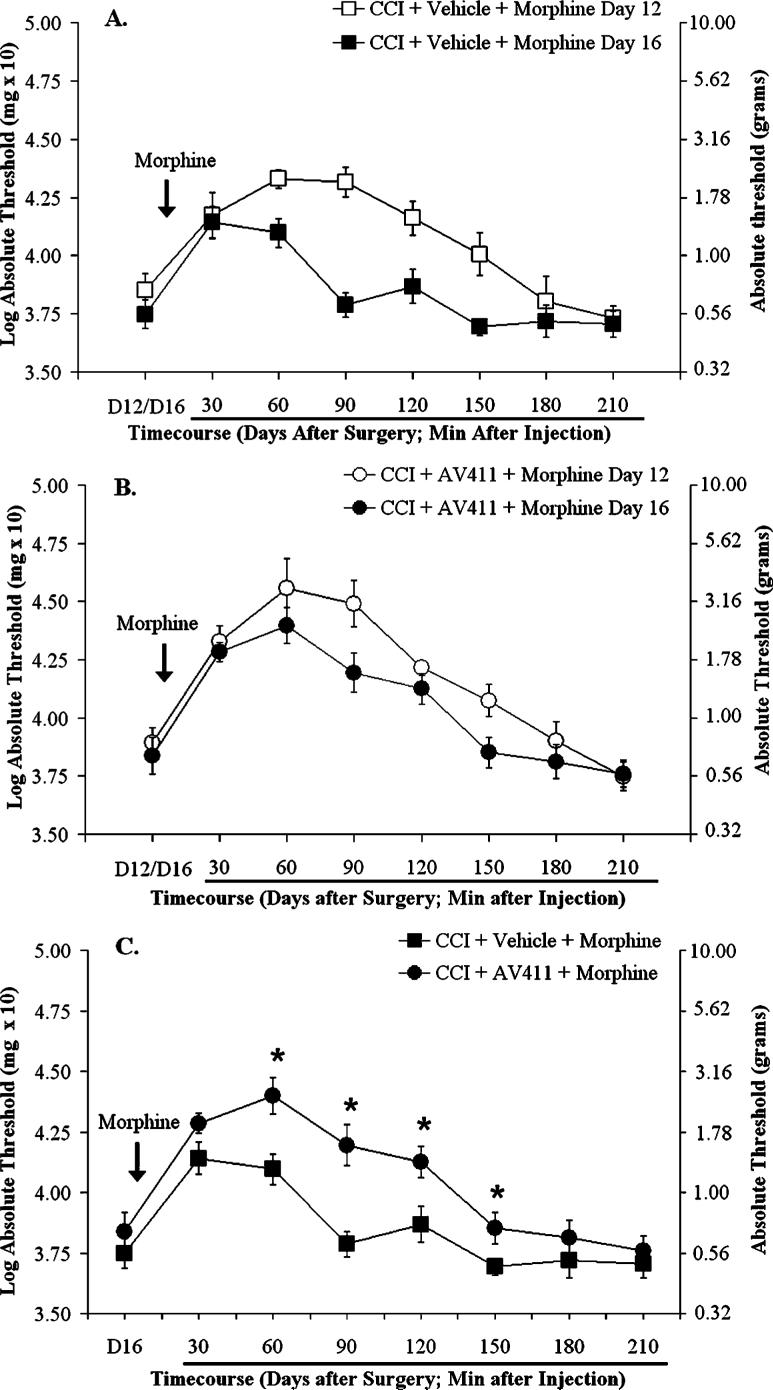
Effects of AV411 treatment on acute anti-allodynic action of morphine and morphine tolerance following CCI
To test if AV411 affects the anti-allodynic effect of morphine and/or tolerance, rats with CCI received i.p. AV411 (7.5 mg kg−1) or vehicle on days 10−16 post-surgery, and s.c. morphine (1 mg kg−1) or vehicle on days 12−16 post-surgery. We chose this low dose of morphine so that potential modulatory effects of AV411 would not be obscured. Time-courses were assessed on day 12 and day 16, following the first and the last administration of morphine, respectively (Fig. 6). Following morphine administration on day 12, rats showed an attenuation of allodynia that peaked at ∼1−1.5 hours post-injection and lasted >2 hours (Fig. 6A). Following morphine administration on day 16, there was a decrease in both the magnitude and duration of the anti-allodynic response to morphine, indicating that the animals developed tolerance (Fig. 6A). Cotreatment with AV411 did not significantly affect the magnitude of reversal of allodynia by morphine on day 12 (Fig. 6A,B). The AV411 cotreated rats exhibited similar responses on day 16 and day 12 (Fig. 6B), indicating markedly less tolerance to morphine (Fig. 6C).
Fig. 6. AV411 reduces tolerance to morphine.
Rats received CCI surgery on day 0. Either AV411 (7.5 mg kg−1) or vehicle was administered i.p. twice daily on days 10−16 post-surgery, and either s.c. morphine (1 mg kg−1) or saline was administered on days 12−16 post-surgery. Low-threshold mechanical sensitivity was assessed by the von Frey test on days 12 and 16 post-surgery, before, and 30−210 min after the morning s.c. injection (A,B). Graphs comparing the time courses following morphine on day 12 and 16 in vehicle- and AV411-treated groups are shown in panels A and B, respectively. (C). The time course following morphine on day 16 for both groups. Data from rats treated with saline are omitted for clarity. Data are mean±SEM. *P<0.05 vs vehicle + morphine.
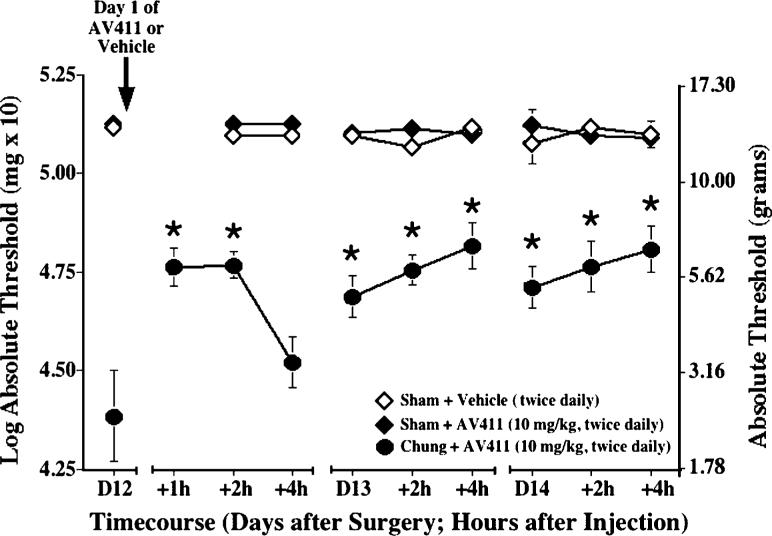
Reversal of SNL-induced mechanical allodynia by twice daily i.p. AV411
AV411 efficacy was also evaluated in the rat L5/L6 SNL model of neuropathic pain (Chung model). Because AV411 7.5−10 mg kg−1 i.p. twice daily was efficacious and relatively well-tolerated in the CCI model, these doses were used for subsequent experiments. As shown in Fig. 7, on day 12 post-surgery, rats with ligated spinal nerves showed marked mechanical allodynia compared with sham-operated controls. Twice daily AV411 administration (10 mg kg−1 i.p.) attenuated SNL-induced allodynia (i.e. mean thresholds following AV411 treatment differed significantly from thresholds in the same rats before treatment). Although the effect was transient on the first day, by the second day of treatment the attenuation of allodynia was sustained through the next morning, similar to that observed in the CCI model.
Fig. 7. Twice daily i.p. AV411 attenuates SNL-induced mechanical allodynia.
Rats received either sham or SNL surgery on day 0, and either vehicle or AV411 (10 mg kg−1) were administered twice daily from days 12−14 post-surgery. Low-threshold mechanical sensitivity was assessed by the von Frey test on days 12−14 post-surgery, before, 1, 2, and/or 4 h after the morning administration. Data are mean±SEM. *P<0.05 vs pretreatment (day 12) values. N.B. Baseline and post-surgery threshold values are higher due to different experimental testing conditions (see text).
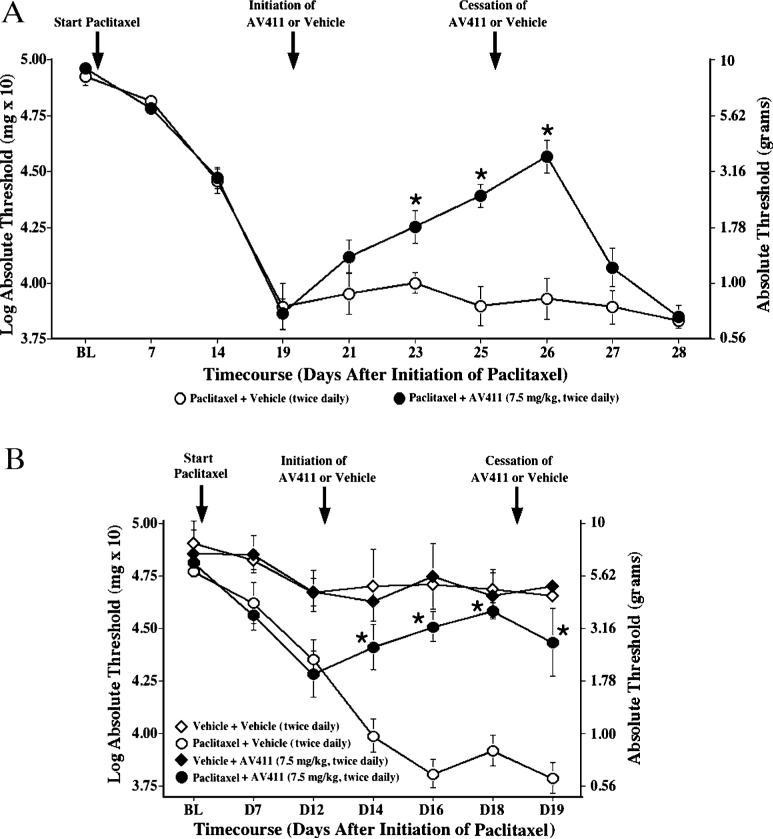
Reversal of paclitaxel-induced mechanical allodynia by twice daily i.p. AV411
In cancer chemotherapy regimens, the development of painful peripheral neuropathy can be dose-limiting (Dougherty et al., 2004). To assess the potential efficacy of systemic AV411 in a clinically relevant, different model of neuropathic pain than the nerve trauma models, it was assessed in rats with neuropathic pain induced by the chemotherapeutic drug paclitaxel. Baseline responses to the von Frey test did not differ between groups (Fig. 8). Repeated injections of paclitaxel induced bilateral allodynia 2−3 weeks after the first i.p. injection. Following AV411 administration (7.5 mg kg−1 i.p.) twice daily starting on day 19 after the first day of paclitaxel treatment, paclitaxel-treated rats showed a partial reversal of allodynia with a progressively greater magnitude of reversal across days (Fig. 8A). On testing days, allodynia was assessed 16 hours after AV411 administration, indicating that, also in this model, the anti-allodynic effects of AV411 are sustained through the next morning. Rats returned to allodynia within 2 days after stopping AV411 treatment. Thus, AV411 was efficacious in reversing paclitaxel-induced allodynia.
Fig. 8. Twice daily i.p. AV411 progressively attenuates and prevents the development of paclitaxel-induced mechanical allodynia.
Rats received four i.p. injections of paclitaxel (1 mg kg−1; cumulative dose 4 mg kg−1) on alternate days, and either vehicle or AV411 (7.5 mg kg−1) was administered twice daily from day 19−25 (A) or from Day 12−19 (B) after the first injection of paclitaxel. Low-threshold mechanical sensitivity was assessed by the von Frey test before the first paclitaxel injection (baseline, BL), and throughout the experiment before the morning injection. Data are mean±SEM. *P<0.05 vs paclitaxel + vehicle.
Prevention of paclitaxel-induced mechanical allodynia by twice daily i.p. AV411
We further explored whether AV411 treatment might also arrest the development of allodynia after it was initially observed (i.e. when neuropathic pain presented). In this experiment AV411 therapy (7.5 mg kg−1, i.p. twice daily) was initiated on day 12 after the first paclitaxel injection, when rats were beginning to exhibit allodynia. As shown in Fig. 8B, vehicle-injected controls progress to a more severe allodynia, whereas thresholds in AV411-treated rats remain stable. Thus, AV411 either prevents or delays the onset of paclitaxel-induced mechanical allodynia. Treatment of vehicle-injected animals with AV411 did not significantly affect basal response thresholds.
Tissue distribution and plasma concentration of AV411
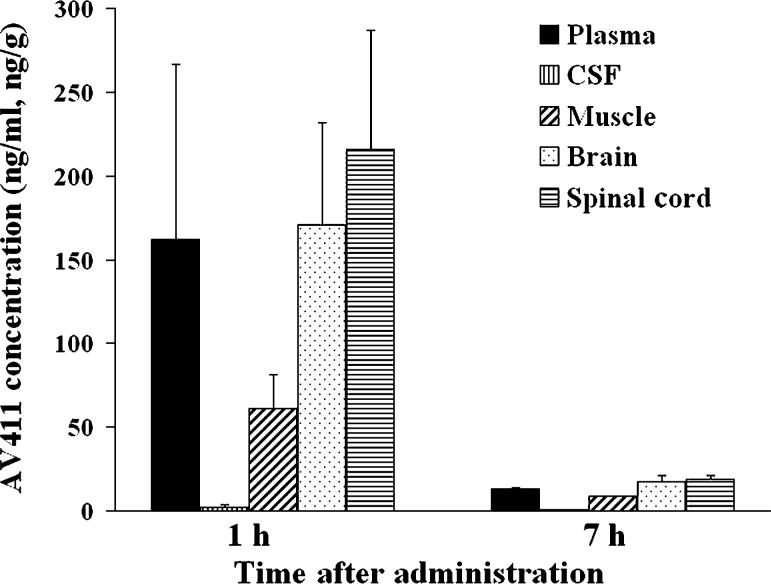
Concentrations of AV411 in various tissues following a single i.p. dose of AV411 (5 mg kg−1) to naïve animals were measured utilizing sensitive HPLC/MS to better understand partitioning of the drug. As shown in Fig. 9, concentrations of AV411 were high in plasma, spinal cord and brain 1 hour after dosing, which indicates good CNS penetration of the drug. By 7 hours after dosing, AV411 levels decreased markedly in all tissues. In addition, plasma levels following a single oral dose of 7.5 mg kg−1 AV411 were ∼3-fold lower than plasma levels following a single i.p. administration of the same dose (area under the plasma concentration-time curve [AUC] 591 ng h ml−1 and 1602 ng h ml−1, respectively).
Fig. 9. AV411 concentration in plasma, CSF and tissues.
The concentration of AV411 in plasma, CSF, muscle, spinal cord and brain, measured 1 hour and 7 hours after i.p. administration of AV411 (5 mg kg−), measured by high performance liquid chromatography/mass spectrometry. Data are mean±SEM.
AV411 tolerability
Assessment of serum chemistry and hematological parameters in CCI rats treated with 7.5 mg kg−1 AV411 twice daily for 5 days revealed no changes relative to either controls or historical data (data not shown). AV411 was also evaluated in naïve animals in the neurobehavioral Irwin test and produced early, transient (within 30 min post-dosing), slight sedation, decreased muscle tone and decreased reactivity to touch on both day 1 (3 out of 3 animals) and 5 (2 out of 3 animals). Decreased muscle tone and reactivity to touch were more marked on day 1 than on day 5. Decreased fear (3 out of 3 animals), diarrhea (1 out of 3 animals) and mild hypothermia (3 out of 3 animals) were observed on day 1 but not on day 5. Because these early, transient effects were not noted following oral administration (up to 50 mg kg−1), rapid peak-plasma levels following i.p. administration were implicated and were nonetheless accommodated to within several drug administrations. Together, these data demonstrate that AV411 was well-tolerated in general and, specifically, at doses and times when neuropathic pain efficacy was evident.
Activity of AV411 in rat microglial cell cultures
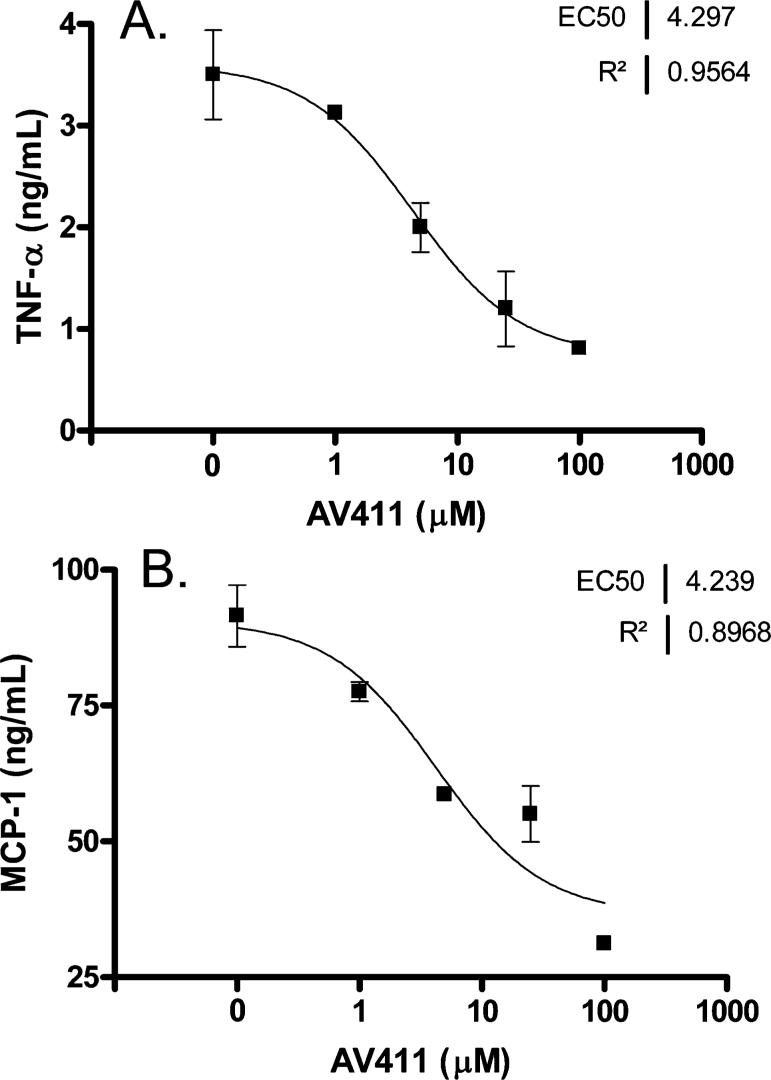
The effects of AV411 on the production of TNF-α and the chemokine monocyte chemoattractant protein 1 (MCP-1) were assessed in primary cultures of rat microglial cells treated with LPS (2 ng ml−1) in combination with IFN-γ (100 ng ml−1). LPS/IFN-γ stimulation induced TNF-α protein levels 6 hours after treatment, which was dose-dependently inhibited by AV411 at >5 μM (Fig. 10A). MCP-1 protein concentration was not changed 21 hours after treatment with LPS/IFN-γ, but was inhibited dose-dependently by AV411 with a similar EC50 as for inhibition of TNF-α (Fig. 10B).
Fig. 10. Effect of AV411 on the production of TNF-α and MCP-1 in primary cultures of rat microglia treated with LPS and IFN-γ.
Cells were treated with LPS (2 ng ml−1) and IFN-γ (100 ng ml−1) for 6 hours (A) and 21 hours (B) in either the absence or presence of AV411 (1, 5, 25 and 100 μM). The basal levels of TNF-α in untreated cultures (536 pg ml−1) were subtracted from the values in (A). Data are mean±SEM, and are representative of two independent cultures.
Receptor-activity screen
To better understand the mechanism of action of AV411, AV411 was evaluated in a screen of receptor and enzyme targets that might be linked to neurological regulation (including neuropathic pain). We used a single concentration (10 μM) of AV411, which is a standard selection for such studies and is greater than plasma concentrations associated with efficacy against neuropathic pain in rat models. Results are presented in Table 2. We observed no significant inhibitian or stimulation, which indicates that AV411 is inactive at the tested concentration against any of the targets assayed.
Table 2.
Activity of AV411 in a screening study1
| Target (enzyme/binding site) | Inhibition or stimulation (%) |
|---|---|
| Calcium channel (L-type, N-type) | 1−11 |
| Cannabinoid receptor (CB1, CB2) | −9−15 |
| Gabapentin-binding site | −8 |
| GABAA receptor | −17−9 |
| Glutamate receptors (kainate, NMDA) | −7−11 |
| MAPK (p38, ERK, JNK) | −8−16 |
| Opiate receptors (δ, κ, μ) | −5−12 |
| PKC (non-selective), PKG1α | −7−0 |
| Purinergic receptors (P2X, P2Y) | 2−16 |
| Sodium Channel (Site 2) | 2 |
AV411 was screened at 10 μM in enzyme assays (MAPK and PKC) and radioligand-binding assays (others). Negative values correspond to stimulation of either binding or enzyme activity. Data are presented as a range of values for receptors with multiple subtypes.
CONCLUSIONS
• AV411 (ibudilast) inhibits the release of LPS-stimulated TNF-α and MCP-1 in rat primary microglial cell cultures.
• AV411 is well-distributed to brain and spinal cord tissue.
• Administration of AV411 intraperitoneally attenuates mechanical allodynia in two classic rat nerve ligation neuropathic pain models and a rat model of chemotherapy-induced painful neuropathy.
• Well-tolerated, oral efficacy is validated and repeated dosing of AV411 i.p. or p.o. results in sustained attenuation of allodynia.
• AV411 does not alter morphine action and attenuates tolerance to morphine in CCI rats.
DISCUSSION
We tested whether a drug used in asthma, which inhibits PDE and has been recognized more recently as a glial attenuator, might be effective in models of neuropathic pain. Our data indicate that twice daily i.p. AV411 (ibudilast) attenuated low-threshold mechanical allodynia induced by CCI, L5/L6 SNL and treatment with the antineoplastic agent paclitaxel (Taxol®). Efficacy was dose-related, improved in magnitude and duration with repeated dosing, and apparent 16 hours after the last dose of AV411. Though AV411 did not abolish allodynia completely, its effects were significant and comparable to gabapentin, a drug used clinically to treat neuropathic pain. Its effects were qualitatively distinct from those of the PDE4 inhibitor rolipram, which indicates that PDE inhibition is not the dominant factor for efficacy. We also showed that AV411 was effective orally, and that AV411 therapy attenuated morphine tolerance. The data indicate that AV411 might be a promising therapeutic candidate for the control of neuropathic pain.
Our initial evaluation of efficacy showed that AV411 attenuates CCI-induced allodynia with a minimal efficacious dose of 2.5−3 mg kg−1. The effect improved with repeated dosing, which indicates that tolerance does not develop. Furthermore, AV411 is well-tolerated, with only transient side-effects that decreased upon repeated administration. The transient attenuation of allodynia following a single dose of AV411 correlates with similarly transient plasma levels of the drug observed in pharmacokinetic studies. Multiday treatment with AV411 resulted in a sustained (overnight) anti-allodynic effect. AV411 was as effective as gabapentin in reversing allodynia, and combined treatment with the two compounds resulted in enhanced efficacy. This indicates that co-administration of AV411 and gabapentin, possibly at lower doses than required with either compound alone, might lead to enhanced pain relief with comparable or, even, reduced side-effects.
We evaluated potential interactions between AV411 and morphine because opioid analgesics are first-line medications for neuropathic pain but the clinical use of opioids is compromised by the development of analgesic tolerance on prolonged exposure. In naïve rats, repeated administration of morphine results in progressive inhibition of antinociception, indicating the development of tolerance (Song and Zhao, 2001; Raghavendra et al., 2002; Johnston et al., 2004). Importantly, the acute antinociceptive action of morphine decreased significantly and the rate of development of tolerance increased in nerve-injured rats, compared to sham-operated rats (Raghavendra et al., 2002). Recently, a role for spinal cord glial cells and proinflammatory cytokines in the development of morphine tolerance in vivo has been recognized (reviewed in Watkins et al., 2005). Chronic treatment with morphine induces glial activation and enhances proinflammatory cytokine expression in lumbar spinal cord (Song and Zhao, 2001; Raghavendra et al., 2002; Narita et al., 2004; Raghavendra et al., 2004; Tawfik et al., 2005). Furthermore, treatments that modulate either glial activation (fluorocitrate and propentofylline) or proinflammatory cytokines (IL-1ra and IL-10) potentiate acute morphine analgesia and/or inhibit development of tolerance (Song and Zhao, 2001; Raghavendra et al., 2002; Johnston et al., 2004; Raghavendra et al., 2004; Shavit et al., 2005). Together, these data support the concept that spinal glial activation and proinflammatory cytokines contribute to the development of morphine tolerance. Our study shows that systemic treatment with AV411 does not alter morphine-induced anti-allodynia in nerve-injured rats and reduces the development of morphine tolerance in these rats. Whether the effects of AV411 depend on inhibition of glial activation and/or associated release of proinflammatory factors remains to be investigated. Regardless of the mechanism, a combination therapy of morphine and AV411 might render morphine more effective, so that lower doses of morphine can be administered to attain the same pain relief.
In addition, the efficacy of AV411 was evaluated in a model of chemotherapeutic-induced neuropathy. Paclitaxel causes dose-related, painful, peripheral neuropathies in many cancer patients, which can persist for months (Rowinsky et al., 1993; Postma et al., 1995; Dougherty et al., 2004). The mechanisms that underlie this side-effect are understood poorly. Recently, a rat model has been described in which repeated i.p. injections of relatively low doses of paclitaxel induce stable, long-lasting, mechanical allodynia without significant systemic toxicity or motor impairment (Polomano et al., 2001). Here, we show that AV411 is also efficacious in this model, and that it both reverses and prevents paclitaxel-induced mechanical allodynia. These results implicate glial activation in the pathophysiology of paclitaxel-induced neuropathic pain. This is supported by our recent data indicating that paclitaxel-induced allodynia is reversed by i.t. IL-1ra and spinal IL-10 gene therapy (Ledeboer et al., in press). As with other anti-inflammatory therapies, in both our CCI and paclitaxel studies, AV411 modulated allodynia only in nerve-injured or paclitaxel-treated rats and did not affect basal thresholds.
Furthermore, the present study shows that AV411 partitions to the spinal cord following systemic administration, and that i.t. AV411 attenuates CCI-induced allodynia. Together, these data indicate that AV411 acts, at least in part, at the level of the spinal cord. This is consistent with reports of attenuation by ibudilast, the active ingredient in AV411, of glial activation in vitro (Suzumura et al., 1999 and this study) and oligodendrocyte apoptosis in an animal model of Krabbe's disease (Kagitani-Shimono et al., 2005). Additionally, in a separate study to evaluate morphine dependence, AV411 decreased morphine-induced microglial and astroglial activation in various brain regions, as visualized by immunoreactivity to OX-42 and GFAP (Hutchinson et al., unpublished observations). Furthermore, other reports indicate systemic ibudilast affects inflammatory cells in brain and/or spinal cord. Specifically, AV411 efficacy in a model of EAE correlated with reduced infiltration of inflammatory cells in the lumbar spinal cord (Fujimoto et al., 1999), and AV411 reduced neuronal damage and microglial activation in ischemia models (Yoshioka et al., 2002; Wakita et al., 2003). However, because AV411 also inhibits peripheral immune cells (Fujimoto et al., 1999; Feng et al., 2004), it is possible that the peripheral targets of systemic AV411 contribute to its anti-allodynic effect.
Although our preliminary observations indicate that AV411 effects on glia might partly account for the anti-allodynic effects, molecular mechanisms of action in addition to inhibition of PDE remain to be elucidated. For now, the efficacy of AV411 cannot be dissociated fully from inhibition of PDE. Indeed, some PDE inhibitors have immunoregulatory activities and are potential therapeutic candidates for neuroinflammatory disorders (Bielekova et al., 2000; Burnouf and Pruniaux, 2002). Furthermore, propentofylline, a PDE3 inhibitor, administered either i.t. or i.p. to rats, is effective in reducing neuropathic pain induced by either L5 spinal nerve transection (Sweitzer et al., 2001; Raghavendra et al., 2003; Tawfik et al., 2006) or the chemotherapeutic drug vincristine (Sweitzer et al., 2006). In other animal models, the PDE4 inhibitor rolipram is effective in arthritis (Francischi et al., 2000), spinal cord injury (Nikulina et al., 2004), EAE (Genain et al., 1995; Sommer et al., 1995; Jung et al., 1996) and Alzheimer's disease (Gong et al., 2004; Comery et al., 2005). However, the lack of efficacy of rolipram in the CCI model and poor efficacy of propentofylline in CCI (unpublished observations), indicate that the effect of AV411 in neuropathic pain might not depend on inhibition of PDEs. Possibly, a unique means of glial cell regulation by AV411/ibudilast is key to its pharmacological effects; for example, in microglia AV411 is more effective in suppressing secretion of TNF-α and upregulating IL-10 than other PDE inhibitors (Suzumura et al., 1999; Yoshikawa et al., 1999). In the present study, we also found that treatment with AV411 of LPS-treated microglial cell cultures markedly inhibited the release of MCP-1, a chemokine that has been recently implicated in neuropathic pain (Tanaka et al., 2004; White et al., 2005a; White et al., 2005b). As noted in Table 2, the involvement of various other neuronal/glial targets with potential links to regulation of neuropathic pain has been ruled out.
One impediment for clinical development of PDE inhibitors in the past has been adverse effects, in particular, gastrointestinal disturbance such as nausea and emesis. However, ibudilast has been administered (as either Ketas® or generics) to patients with stroke (Fukuyama et al., 1993; Shinohara et al., 2002) and bronchial asthma (Kawasaki et al., 1992; Hoshino et al., 1993) in Japan for many years. Clinical data show that AV411 is generally well-tolerated, with few adverse effects (Ketas® package insert; Kyorin Pharmaceutical Co. Ltd, Tokyo, Japan). Extrapolating the doses from our rat studies indicate that effects might be observed at regimens similar to those reported in patients (Feng et al., 2004).
In summary, we have identified an existing drug that might be redirected for testing in neuropathic pain. Our studies show that systemic administration of AV411 is well tolerated and attenuates mechanical allodynia in several rat models of neuropathic pain. Although further studies are needed to address the mechanism of action in neuropathic pain conditions, we speculate that regulation of spinal cord glial activation by AV411 is involved. Considering that AV411 is bioavailable orally, partitions into the CNS, and has a good track record in humans, AV411 seems to be an attractive candidate to consider for clinical development in various chronic pain conditions.
ACKNOWLEDGEMENTS
This work was supported by Avigen and NIH grants DA015642 and DA017670. Drs Steven Maier and Ken Chahine are acknowledged for scientific and resourcing contributions, and Brian Suzuki for his technical support. Part of this work was performed at Porsolt & Partners Pharmacology (Le-Genest-Saint-Isle, France). A.L., S.V., M.I.G., L.S., M.D.C., L.M.S. and K.W.J. are currently employees and/or have stock options in Avigen, Inc. L.R.W. has received research support from Avigen, Inc.
REFERENCES
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Lincoln A, McFarland H, Martin R. Therapeutic potential of phosphodiesterase-4 and -3 inhibitors in Th1-mediated autoimmune diseases. Journal of Immunology. 2000;164:1117–1124. doi: 10.4049/jimmunol.164.2.1117. [DOI] [PubMed] [Google Scholar]
- Burnouf C, Pruniaux MP. Recent advances in PDE4 inhibitors as immunoregulators and anti-inflammatory drugs. Current Pharmaceutical Design. 2002;8:1255–1296. doi: 10.2174/1381612023394665. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Effect of systemic and intrathecal gabapentin on allodynia in a new rat model of postherpetic neuralgia. Brain Research. 2005;1042:108–113. doi: 10.1016/j.brainres.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. Journal of Neuroscience. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. European Journal of Pharmacology. 2004;491:137–148. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Feng J, Misu T, Fujihara K, Sakoda S, Nakatsuji Y, Fukaura H, et al. Ibudilast, a nonselective phosphodiesterase inhibitor, regulates Th1/Th2 balance and NKT cell subset in multiple sclerosis. Multiple Sclerosis. 2004;10:494–498. doi: 10.1191/1352458504ms1070oa. [DOI] [PubMed] [Google Scholar]
- Francischi JN, Yokoro CM, Poole S, Tafuri WL, Cunha FQ, Teixeira MM. Anti-inflammatory and analgesic effects of the phosphodiesterase 4 inhibitor rolipram in a rat model of arthritis. European Journal of Pharmacology. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Sakoda S, Fujimura H, Yanagihara T. Ibudilast, a phosphodiesterase inhibitor, ameliorates experimental autoimmune encephalomyelitis in Dark August rats. Journal of Neuroimmunology. 1999;95:35–42. doi: 10.1016/s0165-5728(98)00251-3. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Kimura J, Yamaguchi S, Yamauchi H, Ogawa M, Doi T, et al. Pharmacological effects of ibudilast on cerebral circulation: a PET study. Neurological Research. 1993;15:169–173. doi: 10.1080/01616412.1993.11740130. [DOI] [PubMed] [Google Scholar]
- Genain CP, Roberts T, Davis RL, Nguyen MH, Uccelli A, Faulds D, et al. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phosphodiesterase inhibitor. Proceedings of the National Academy of Sciences of the U.S.A. 1995;92:3601–3605. doi: 10.1073/pnas.92.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, et al. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. European Journal of Pharmacology. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. Journal of Clinical Investigation. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behavior Research Methods, Instruments, & Computers. 1986;18:623–632. [Google Scholar]
- Hoshino K, Kawasaki A, Mizushima Y, Oosaki R, Yano S, Kobayashi M. Comparison of the effects on bronchial hyperresponsiveness of antiallergic agents and beclomethasone dipropionate in long-term bronchial asthma. A retrospective study. Allergy. 1993;48:196–201. doi: 10.1111/j.1398-9995.1993.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu S, Zhang L, Salem M, Greig GM, Chan CC, et al. Preferential inhibition of human phosphodiesterase 4 by ibudilast. Life Sciences. 2006;78:2663–2668. doi: 10.1016/j.lfs.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. Journal of Neuroscience. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Zielasek J, Kollner G, Donhauser T, Toyka K, Hartung HP. Preventive but not therapeutic application of Rolipram ameliorates experimental autoimmune encephalomyelitis in Lewis rats. Journal of Neuroimmunology. 1996;68:1–11. doi: 10.1016/0165-5728(96)00051-3. [DOI] [PubMed] [Google Scholar]
- Kagitani-Shimono K, Mohri I, Fujitani Y, Suzuki K, Ozono K, Urade Y, et al. Anti-inflammatory therapy by ibudilast, a phosphodiesterase inhibitor, in demyelination of twitcher, a genetic demyelination model. Journal of Neuroinflammation. 2005;2:10. doi: 10.1186/1742-2094-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanokuchi J, Mizuno T, Kato H, Mitsuma N, Suzumura A. Effects of interferon-beta on microglial functions as inflammatory and antigen presenting cells in the central nervous system. Neuropharmacology. 2004;46:734–742. doi: 10.1016/j.neuropharm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Hoshino K, Osaki R, Mizushima Y, Yano S. Effect of ibudilast: a novel antiasthmatic agent, on airway hypersensitivity in bronchial asthma. Journal of Asthma. 1992;29:245–252. doi: 10.3109/02770909209048938. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Ohta S, Kasuya N, Sakita S, Ashikaga T, Isobe M. Ibudilast: a non-selective PDE inhibitor with multiple actions on blood cells and the vascular wall. Cardiovascular Drug Reviews. 2001;19:215–225. doi: 10.1111/j.1527-3466.2001.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Ohta S, Kasuya N, Tatsumi M, Sawada M, Sakita S, et al. Ibudilast modulates platelet-endothelium interaction mainly through cyclic GMP-dependent mechanism. Journal of Cardiovascular Pharmacology. 2000;36:65–70. doi: 10.1097/00005344-200007000-00009. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Breve JJ, Poole S, Tilders FJ, Van Dam AM. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of proinflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30:134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain, Behavior, and Immunity. doi: 10.1016/j.bbi.2006.10.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nature Reviews Neuroscience. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Research. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Research, Brain Research Reviews. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Yajima Y, Suzuki R, Shioda S, Suzuki T. Neuronal protein kinase C gamma-dependent proliferation and hypertrophy of spinal cord astrocytes following repeated in vivo administration of morphine. European Journal of Neuroscience. 2004;19:479–484. doi: 10.1111/j.0953-816x.2003.03119.x. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proceedings of the National Academy of Sciences of the U. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Annals of Oncology. 1995;6:489–494. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. Journal of Neuroscience. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Rice AS, Hill RG. New treatments for neuropathic pain. Annual Review of Medicine. 2006;57:535–551. doi: 10.1146/annurev.med.57.121304.131324. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). Seminars in Oncology. 1993;20:1–15. [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Kusunoki T, Nakashima N. Clinical efficacy of ibudilast on vertigo and dizziness observed in patients with cerebral infarction in the chronic stage - a double-blind, randomized, placebo-controlled trial with a run-in period. Shinnkeitiryougaku (Neurological Therapeutics) 2002;19:177–187. [Google Scholar]
- Sommer N, Loschmann PA, Northoff GH, Weller M, Steinbrecher A, Steinbach JP, et al. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nature Medicine. 1995;1:244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neuroscience Research. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Research. 1999;837:203–212. doi: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Pahl JL, Deleo JA. Propentofylline attenuates vincristine-induced peripheral neuropathy in the rat. Neuroscience Letters. 2006;400:258–261. doi: 10.1016/j.neulet.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. Journal of Pharmacology and Experimental Therapeutics. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Takuma K, Lee E, Enomoto R, Mori K, Baba A, Matsuda T. Ibudilast attenuates astrocyte apoptosis via cyclic GMP signalling pathway in an in vitro reperfusion model. British Journal of Pharmacology. 2001;133:841–848. doi: 10.1038/sj.bjp.0704146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neuroscience Research. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA. Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. Journal of Pharmacology and Experimental Therapeutics. 2005;313:1239–1247. doi: 10.1124/jpet.104.082420. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain, Behavior, and Immunity. 2007;21:238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Tominaga Y, Nakamura Y, Tsuji K, Shibata T, Kataoka K. Ibudilast protects against neuronal damage induced by glutamate in cultured hippocampal neurons. Clinical and Experimental Pharmacology and Physiology. 1996;23:519–523. doi: 10.1111/j.1440-1681.1996.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Perception & Psychophysics. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Lin JX, Ihara M, Ohtani R, Shibata M. Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain Research. 2003;992:53–59. doi: 10.1016/j.brainres.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends in Neurosciences. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nature Reviews Drug Discovery. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nature Reviews Drug Discovery. 2005a;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proceedings of the National Academy of Sciences of the U.S.A. 2005b;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Multiple Sclerosis. 1999;5:126–133. doi: 10.1177/135245859900500210. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Shimizu Y, Hirose G, Kitasato H, Pleasure D. Cyclic AMP-elevating agents prevent oligodendroglial excitotoxicity. Journal of Neurochemistry. 1998;70:2416–2423. doi: 10.1046/j.1471-4159.1998.70062416.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Yamaya Y, Saiki S, Kanemoto M, Hirose G, Pleasure D. Cyclic GMP/cyclic GMP-dependent protein kinase system prevents excitotoxicity in an immortalized oligodendroglial cell line. Journal of Neurochemistry. 2000;74:633–640. doi: 10.1046/j.1471-4159.2000.740633.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Suda N, Mori K, Ueno K, Itoh Y, Togashi H, et al. Effects of ibudilast on hippocampal long-term potentiation and passive avoidance responses in rats with transient cerebral ischemia. Pharmacological research: the official journal of the Italian Pharmacological Society. 2002;45:305–311. doi: 10.1006/phrs.2002.0949. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]