Summary
Lipid metabolism is of particular interest due to its high concentration in CNS. The importance of lipids in cell signaling and tissue physiology is demonstrated by many CNS disorders and injuries that involve deregulated metabolism. The long suffering lipid field is gaining reputation and respect as evidenced through the Center of Biomedical Research Excellence in Lipidomics and Pathobiology (COBRE), Lipid MAPS (Metabolites And Pathways Strategy) Consortium sponsored by NIH, European initiatives for decoding the lipids through genomic approaches, and Genomics of Lipid-associated Disorder (GOLD) project initiated by Austrian government. This review attempts to provide an overview of the lipid imbalances associated with neurological disorders (Alzheimer’s, Parkinson’s; Niemann-Pick; Multiple sclerosis, Huntington, amyotrophic lateral sclerosis, schizophrenia, bipolar disorders and epilepsy) and CNS injury (Stroke, traumatic brain injury; and spinal cord injury) and a few provocative thoughts. Lipidomic analyses along with RNA silencing will provide new insights into the role of lipid intermediates in cell signaling and hopefully open new avenues for prevention or treatment options.
Keywords: Alzheimer’s disease, Arachidonic acid, CDP-choline, CNS injury, Docosahexaenoic acid, Huntington disease, Lipid peroxidation, Neurodegenerative diseases, Phospholipases, Phospholipids, Stroke
Lipids and the Central Nervous System (CNS)
Phospholipids are important components of all mammalian cells and have a variety of biological functions: 1) formation of lipid bilayers that provide structural integrity necessary for protein function 2) function as an energy reservoir (for example triglycerides) and 3) serve as precursors for various second messengers such as arachidonic acid (ArAc), docosahexaenoic acid (DHA), ceramide, 1,2-diacylglycerol (DAG), phosphatidic acid, and lyso-phosphatidic acid. Lipids comprise a large number of chemically distinct molecules arising from combinations of fatty acids with various backbone structures. Overall, mammalian cells may contain ~1000-2000 lipid species [1]. Lipids are classified into eight categories (fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides) [2]. Subtypes of these eight lipid classes are given in Table 1. Lipid metabolism may be of particular importance for the CNS, as this organ has a high concentration of lipids, second only to adipose tissue.
Table 1.
The major lipid classes and representative subtypes. A comprehensive listing of lipids is provided in Fahy, et al. [2].
| Lipid class | Subtypes |
|---|---|
| Fatty acyls | Free fatty acids and conjugates; eicosanoids; docosanoids; fatty alcohols, aldehydes and esters |
| Glycerolipids | Mono-, di-, and triacylglycerols |
| Glycerophospholipids | Phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol |
| Sphingolipids | Ceramide, sphingobases, sphingomyelin, glycosphingolipids (gangliosides) |
| Sterol lipids | Sterols including cholesterol, steroids, bile acids |
| Prenol lipids | Isoprenoids, polyprenols, quinones, hopanoids |
| Saccharolipids | acylaminosugars, acylaminosugar glycans |
| Polyketides | Macrolide and Aromatic polyketides |
Neurodegenerative diseases, mental disorders, stroke and CNS traumas are problems of vast clinical importance. Currently there exists no cure for these CNS injuries and disorders, resulting in a huge impact on quality of life and an economic burden on society (Table 2, based on the US population statistics). The crucial role of lipids in tissue physiology and cell signaling is demonstrated by the many neurological disorders, including bipolar disorders and schizophrenia, and neurodegenerative diseases such as Alzheimer’s, Parkinson’s, Niemann-Pick and Huntington diseases, that involve deregulated lipid metabolism [1]. Altered lipid metabolism is also believed to be a key event which contributes to CNS injury [3].
Table 2.
Annual cost* of selected CNS disorders** and injuries in USA
| CNS disorders or injury | Cases
(in millions) |
Costs/year
(in $ billions) |
|---|---|---|
| Neurological illnesses | 50 | $ 400 |
|
| ||
| Mental disorders, (in addition to neurological illnesses above but excluding alcohol and drug problems) | 44 | $ 148 |
|
| ||
| Alzheimer’s Disease (AD) | 4.5 | $ 100 |
| Stroke | 4.7 | $ 57 |
| Traumatic Brain Injury (TBI) | 5 | $ 56.3 |
| All Depressive Disorders | 20.5 | $ 44 |
| Schizophrenia | 2 | $ 32.5 |
| Spinal Cord Injury (SCI) | 0.25 | $ 10 |
| Multiple Sclerosis (MS) | 2.5 | $ 9.5 |
| Parkinson’s Disease (PD) | 1 | $ 5.6 |
| Huntington’s Disease (HD) | 0.3 | $ 2 |
| Amyotrophic Lateral Sclerosis (ALS, Lou Gehrig’s disease) | 0.03 | - |
Reactive Oxygen Species (ROS) and Lipid Peroxidation
ROS including superoxide anion radical and hydrogen peroxide are produced by a number of cellular oxidative metabolic processes including oxidative phosphorylation by the mitochondrial respiratory chain, xanthine oxidase, NAD(P)H oxidases, monoamine oxidases and metabolism of ArAc by cyclooxygenases/lipoxygenases (COX/LOX) [4]. However, recent literature suggests that COX does not directly produce ROS during ArAc oxidative metabolism, but does form free radicals (i.e., carbon-centered radicals on ArAc). Although there are many reports in the literature on ROS production by COX, this is probably due to secondary ROS generation induced by various eicosanoids. Disruption of mitochondria during COX-2-associated apoptosis is a likely source of ROS production as has been established for a number of different cells [5-7]. ROS then cause oxidative damage to nucleic acids, proteins, carbohydrates, and lipids. Beyond damage to membranes, lipid peroxides give rise to reactive α,β-unsaturated aldehydes including malondialdehyde, 4-hydroxynonenal (HNE) and acrolein. These aldehydes covalently bind to proteins through reaction with thiol groups and alter their function. Although there are intracellular defenses against ROS, increased production of ROS or loss of antioxidant defenses leads to progressive cell damage and decline in physiological function. “Oxidative stress” results when generation of ROS exceeds the cell’s capacity to detoxify them. The brain is believed to be particularly vulnerable to oxidative stress as it contains high concentrations of PUFAs that are susceptible to lipid peroxidation, consumes relatively large amounts of oxygen for energy production, and has lower antioxidant defenses compared to other organs.
Lipids in CNS Disorders
A summary of CNS disorders, their symptoms and lipid systems involved is presented in Table 3 as a quick reference guide.
Table 3.
Lipid systems affected by the CNS disorders and injuries
| Disorder/injury | Symptoms/pathologic features | CNS region(s) affected | Mechanism of damage | Possible Treatments |
|---|---|---|---|---|
| Alzheimer Disease [10] |
|
|||
| Parkinson’s Diseases [27] |
|
Substantia nigra | ||
| Niemann-Pick Disease [30,31,33] | Type A: progressive loss of early motor skills, early fatality typically within the first few Years | Lysosomal storage disorder that leads to SM and cholesterol accumulation. | Type A: <1% of normal A-SMase activity leads to lysosomal sphingomyelin accumulation | Enzyme replacement was shown to be effective in a mouse model for Type B [108]. |
| Type B: enlarged liver, spleen; and respiratory problems | Type B: <10% of normal A-SMase | D609, a SM synthase inhibitor may be an option not yet explored [32] | ||
| Type C: Inability to move eyes up and down. Learning and cognitive impairment |
|
Inhibition of glycosphingolipid synthesis prolonged lifespan in mouse model of type C [33] | ||
| Multiple Sclerosis-Experimental Autoimmune Encephalomyelitis (MS-EAE) [38,109] |
|
Demyelination of axons | ||
| Huntington Disease [43,110] |
|
|
Trinucleotide CAG repeat expansion in the Huntingtin (Htt) gene is responsible for This polyglutamine disease. | |
| Amyotrophic Lateral Sclerosis | Limb and muscle weakness, twitching and cramping of muscles | Progressive loss of cortical and spinal cord motorneurons | Lipid peroxidation [56] | |
| Lewy body like hyaline inclusion/SOD1 mutation | ||||
| Schizophrenia | Disturbances in thinking, emotional reactions, and social behavior | Dorsalateral prefrontal cortex | Altered lipid metabolism may be responsible for defects in neurological development [57,58] |
|
| Bipolar disorder (manic depression illness) | Mood disorders characterized by episodes of mania and major depression | - | - | Combination of EPA and DHA [57] |
| Epilepsy |
|
Focal cortical area, later transferred to the Thalamus | DHA levels ↓ [106] | |
| Stroke [69] | Sudden weakness on one side of the body, loss of balance and coordination, trouble with cognition |
|
||
| Traumatic Brain Injury [113] | Loss of CA3 hippocampal neurons | Aβ deposition, tau pathology [84] | ||
| Spinal Cord Injury [89] | Weakness and sensory loss; paralysis | Activation of PLA2, COX/LOX pathways. Corticosteroids inhibit These activations [81] | High-dose methylprednisolone in clinical use [89]; DHA treatment is beneficial [106] |
Alzheimer’s Disease (AD), the most common form of dementia, is a progressive brain disorder affecting regions that control memory and cognitive functions, gradually destroying a person’s memory and ability to learn, reason, communicate and carry out daily activities. AD is broadly divided into sporadic or late onset AD (90-95% of AD) and early-onset (occurring in persons under age 65, 5-10% of AD). One of the hallmarks of AD is overproduction of a 4-kDa peptide, amyloid β-peptide or Aβ, resulting in the formation of plaques. AD encompasses a range of phenotypes, extending from cases with exclusively aggregated Aβ42 to cases with, in addition, large quantities of insoluble Aβ40 species [8]. A two-step cleavage of the neuronal membrane protein amyloid precursor protein (APP) [9] results in two products, Aβ40 and Aβ42. Strong evidence for the role of Aβ in the pathogenesis of AD was provided by the observation that, in familial or early onset AD, mutations in APP or the enzymes that cleave it lead to overproduction of Aβ42 and rapid progression of the disease [10]. Late onset AD is also characterized by accumulation of Aβ and formation of plaques but is not inherited in any simple pattern. The second hallmark of AD is formation of neurofibrillary tangles due to hyperphosphorylation of tau protein.
There has been debate concerning the roles of Aβ vs tau protein in AD. Transgenic mouse models of AD require two or more mutations to reproduce all the physical features of AD (Aβ plaques and tau tangles), however these models have not provided any leads to the relationship between Aβ plaques and tau tangles. Transgenic mice that over-express APP do not form neurofibrillary tangles and more closely resemble age-related memory impairment than AD, Some tau models express little amyloid but develop severe memory problems associated with AD [10]. So far most of the efforts in developing treatments for AD are directed to attenuating Aβ plaques. Mouse models based on over-expression of Aβ may be useful in developing strategies to limit formation of Aβ plaques, but these models are considered limited/incomplete models for AD. APP and APP/PS1 mutant mouse models develop amyloid deposition at a young age; but fail to develop neurofibrillary tangles that are essential hall mark of AD. Neuritic atrophy is found in some transgenics, but of nearly one dozen mouse models, only one has reported loss of neurons that are characteristic of AD [11], which may limit the usefulness of these models for developing therapeutic strategies for AD [12]. Development of therapeutic strategies directed to tau pathology will require identifying which phosphorylation sites are linked to tau aggregation and filament formation, and the specific kinases and phosphatases involved [13,14].
While the etiology of late-onset AD is not clearly understood, there is growing evidence that cholesterol is of particular importance in development and progression of the disease. Apolipoprotein E (ApoE) is one of the major apolipoproteins in plasma and the principal cholesterol carrier protein in the brain. Identification of the gene encoding the variant ApoE4 (APOE ε4 allele) as a significant risk factor for late-onset AD provided evidence for a role of cholesterol in the pathogenesis of AD [15]. Elevated cholesterol levels increase Aβ in cellular and animal models, and drugs such as statins that inhibit cholesterol synthesis lower Aβ levels [14,16]. Statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase that initiates cholesterol and isoprenoid lipid synthesis. Cholesterol is needed to make the cellular membrane micro-domains referred to as lipid rafts. APP, β-secretase, γ-secretase complex, and neutral sphingomyelinase (N-SMase) are present in the lipid rafts that are rich in cholesterol and sphingomyelin (SM). Genetic mutations in APP or presenilins (part of the in γ-secretase complex) increase production of Aβ42. Recent studies suggest that Aβ40 inhibits HMG-CoA reductase while Aβ42 activates N-SMase and increases ceramide production, which can accelerate the neurodegenerative process [17,18]. However it is unclear what regulates the cleavage to Aβ42 vs Aβ40 and whether the ratio of Aβ42 to Aβ40 can be lowered such that attenuation of HMG-CoA reductase and N-SMase will be simultaneously achieved. Prospective trials evaluating statin therapy did not demonstrate improvement in cognitive function in AD patients [19]. It is also unknown whether genetic differences such as presence of the APOE ε4 allele affects clinical outcome.
Recent studies have shown that mRNA expression of pro-inflammatory secretory phospholipase A2 IIA (sPLA2 IIA) was up-regulated in AD brains compared to non-dementia elderly brains and also found sPLA2 IIA immunoreactive astrocytes in AD hippocampus that were associated with Aβ plaques [20].
AD, oxidative stress and lipid peroxidation: A number of studies demonstrating increased lipid peroxidation in AD provide increasing evidence supporting a role for oxidative damage in this disorder [21]. Recent studies demonstrated increased levels of HNE and acrolein [21] in the brain tissue from patients affected by mild cognitive disorder and early AD, indicating that lipid peroxidation occurs early in the pathogenesis of AD [21]. Acrolein, by far the strongest electrophile among all α,β-unsaturated aldehydes, reacts with DNA bases including guanine, adenine, cytosine and thymidine to form cyclic adducts, the major exocyclic adduct being acrolein-deoxyguanosine. Increased levels of acrolein-deoxyguanosine adducts were recently demonstrated in brain tissue from AD patients [22]. ROS may also play a role in amyloid deposition in AD as oxidizing conditions cause protein cross-linking and aggregation of Aβ peptides, and also contribute to tau protein aggregation [23]. Aβ aggregation has been shown to induce accumulation of ROS, which may lead to cyclic or self-propagating oxidative damage. AD and Mild Cognitive Disorder subjects also showed lower levels of antioxidant defense systems [23].
There may not be any simple answer for the complexity of AD. Are the familiar and sporadic forms of AD distinct? Is the research field on a wrong track studying the familial form and not paying more attention to sporadic form? Are the animal models good enough to provide concrete answers? Mouse models are based on mutations in familial AD (the rare form of the disease) and may not model the more common sporadic form. Furthermore, most models do not exhibit the extent of neurodegeneration seen in AD patients [10]. The amyloid vaccine AN-1792 effectively cleared plaques and improved memory in animal model, while phase 2 trials were abandoned after some participants developed meningoencephalitis, a potentially fatal inflammation of the brain. Are these treatments (for eg; AN-1792) more compatible with the familial form compared to sporadic? The triple transgenic mouse (APP/PS1/tau) studies raise the possibility that a multi-antibody-based approach (i.e. one targeted against Aβ and one against tau) may provide the most significant clinical benefit for the treatment of AD [24]. The real issue will be how the patients respond to the combination treatment without developing clinical complications.
Parkinson’s Disease (PD) is characterized by selective degeneration of dopaminergic neurons of the substantia nigra, resulting in bradykinesis, tremor and rigidity. The etiology of PD is unknown. Oxidative stress caused by free radical generation and lipid peroxidation plays a significant role in pathogenesis of PD. One of the factors responsible for this stress is believed to be phospholipases activation in substantia nigra, supported by the fact that cPLA2 null mice are resistant to 1-methyl-4-phenyl-1,2,3,6-tetrohydropyridine (MPTP) induced neurotoxicity [25]. The MPTP metabolite MPP+ is taken up by nigrostriatal neurons where it inhibits mitochondrial oxidative phosphorylation and causes neuronal death. MPTP neurotoxicity has been used as an animal model for PD. It should be noted that, while MPTP is capable of producing virtually all the signs and symptoms of PD, strictly speaking, it is not PD and is a separate disease.
Treatment of PD: No therapy has been demonstrated to slow the loss of dopamine neurons in PD. The dopamine prodrug levodopa remains the gold standard for the treatment of PD. However, long-term levodopa therapy leads to development of dyskinesia. Alternatives to the use of levodopa in early PD include monoamine oxidase B inhibitors, dopamine agonists, catechol-O-methyltransferase inhibitors, and amantadine [26]. The mechanism of action of amantadine remains unknown; however, it has been suggested to have anticholinergic properties in addition to acting as a NMDA receptor antagonist to increase dopamine release and inhibit its reuptake. These treatments, however, remain focused on achieving symptomatic relief while minimizing adverse effects.
PD, oxidative stress and lipid peroxidation: In PD, the accelerated metabolism of dopamine by monoamine-oxidase-B may result in excessive ROS formation. A role for oxidative stress in PD was demonstrated by marked increases in 8-hydroxy-2’-deoxyguanosine, a hydroxyl radical-damaged guanine nucleotide commonly used to evaluate oxidative damage to DNA. Furthermore, several markers of lipid peroxidation were found to be significantly increased in PD brain regions [23].
PD and α-synuclein: PD is associated with the presence of Lewy bodies containing insoluble aggregates of α-synuclein in association with other proteins. Recently the presence of polyunsaturated fatty acids (PUFA) was linked to the appearance of soluble oligomers of α-synuclein that ultimately promote the formation of insoluble α-synuclein aggregates [27]. Docosahexaenoic acid (DHA) was shown to stimulate oligomerization of α-synuclein. Recent studies have shown increased levels of docosahexaenoic acid (DHA) in PD brains compared to controls [28], suggesting that DHA could have a role in formation of α-synuclein aggregates. Interestingly, DHA reduced levodopa-induced dyskinesias in MPTP-treated monkeys, indicating that DHA can reduce the severity or delay the development of levodopa-induced dyskinesias [29]. It was suggested that DHA may represent a new approach to improve the quality of life of Parkinson’s disease patients. This apparent discrepancy for the role of DHA in PD (beneficial or harmful?) could be due to the use of MPTP animal models and warrants further investigation.
Niemann-Pick (NP) Diseases are genetic pediatric neurodegenerative conditions characterized by specific disorders in lipid metabolism and are categorized as types A, B and C. NP Types A and B (NPA and NPB) are caused by deficiencies in acidic sphingomyelinase (A-SMase) [30]. NPA, the most common type, is caused by a nearly complete lack of A-SMase (less than 1% of normal activity) and is characterized by jaundice, an enlarged liver, and profound brain damage. Infants with NPA rarely live beyond 18 months. Since A-SMase is localized to the lysosomes, NPA results in accumulation of sphingomyelin (SM) and is classified as a lysosomal storage disorder [31]. People with NPB have approximately 10% of the normal level of A-SMase, generally have little or no neurologic involvement and may survive into late childhood or adulthood. Tricyclodecan-9-yl potassium xanthate (D609), a widely known phosphatidylcholine-phospholipase C (PC-PLC) inhibitor, also inhibits SM synthase [32] and may help prevent accumulation of SM in NPB, a therapeutic possibility not yet explored.
NP Type C (NPC) also is always fatal but it differs from NPA and NPB at the biochemical and genetic level. NPC is caused by mutations in either the NPC1 gene (95% of cases) or NPC2 gene (5% of cases) [33]. While the precise functions of the NPC1 and NPC2 proteins are not clear, these are involved in transport of lipids, particularly cholesterol, from the late endosomes/lysosomes [34]. Deficiencies in these proteins result in lysosomal accumulation of cholesterol and other lipids. In NPC, cholesterol accumulates in all tissues except the brain, where cholesterol levels decrease with age [33]. Since 70-80% of cholesterol in the brain is contained in myelin, the extensive demyelination that occurs in NPC probably accounts for the loss of cholesterol in the brain, which would likely mask accumulation of cholesterol in neurons or astrocytes. Currently there are no treatments for NPC. Despite the accumulation of cholesterol in late endosomes/lysosomes, neither cholesterol lowering agents nor dietary measures slowed the progression of the disease. Neurosteroids and enzymes involved in steroid synthesis were significantly reduced in NPC1-deficient mice. Administration of the neurosteroid allopregnanolone alleviated progression of the disease [33], suggesting that neurosteroid therapy might be a treatment option for NPC. Since NP diseases have a known genetic origin, it is possible that gene therapy to replace the defective genes may eventually provide a cure since that would be directed to the root causes of the diseases.
Multiple Sclerosis (MS) is an inflammatory demyelinating autoimmune disease affecting the CNS, but its underlying cause remains elusive. Symptoms range from relatively benign to severely disabling, in which communication between the brain and other parts of the body is disrupted, rendering a person unable to write, speak, or walk. In MS, the immune system attacks the myelin sheath of nerve cell fibers in the brain and spinal cord. MS is predominantly a T-lymphocyte mediated disorder, and cytokines may therefore have a key role in the pathogenesis of the disease. MS is the only neurological disorder where therapeutic manipulation of the cytokine system influences development of the disease [35]. Thiobarbituric acid reactive substances and F2-isoprostane levels were shown to be elevated in CSF of MS patients, and HNE was associated with MS lesions, indicative that lipid peroxidation also occurs in MS [36].
Experimental autoimmune encephalomyelitis (EAE) can be induced in animals by immunizing against myelin antigens [37] and is an animal model for MS due to similarities in its histopathology and clinical course. Recent studies demonstrated a key role for cPLA2 in EAE [37,38]. cPLA2, which can be induced by the cytokine tumor necrosis factor-α (TNF-α) [39,40], was highly expressed in EAE lesions. Blocking cPLA2 showed a remarkable decrease in both the onset and progression of the disease [37], indicating that cPLA2 has a significant role in both the induction and effector phases of EAE. A second study showed that cPLA2 null mice were resistant to EAE [38]. It should be noted that these studies were conducted using C57BL/6 or SV127 mouse strains which are naturally deficient in inflammatory PLA2 or sPLA2 IIA [4]. Treatment of EAE rats with the nonapeptide sPLA2 inhibitor CHEC-9 (CHEASAAQC) significantly attenuated sPLA2 activity, EAE symptoms, and ED-1 positive microglia/macrophages [41]. Recently, MS patients were shown to have elevated sPLA2 activity [41]. These studies suggest that both cPLA2 as well as sPLA2 inhibition may be treatment options for MS.
Previous studies have focused on the immunological aspects of MS and the immune system’s involvement in the disease has been elucidated. Future studies seeking deeper insights into the molecular mechanisms of T cell activation (what causes the immune system to attack the myelin?) and the signaling cascades responsible for neurodegeneration will help to further identify molecular targets for efficient treatment strategies [42].
Huntington’s Disease (HD) is a relatively rare (8 per 100,000) inherited neurological disorder characterized by abnormal body movements (chorea, or jerky, random, uncontrollable movements) and a lack of coordination; cognition and personality may also be affected. HD is caused by a trinucleotide repeat expansion in the Huntingtin (Htt) gene. The normal gene has fewer than 36 repeats, whereas the mutated Htt gene has 40 or more CAG repeats. Since CAG is the codon for glutamine, HD is one of the polyglutamine diseases.
Endocannabinoids are endogenous agonists of cannabinoid receptors comprised of amides, esters and ethers of long chain PUFAs. N-Arachidonoylethanolamine (AEA, anandamide) and 2-arachidonylglycerol (2-AG) are well characterized lipid mediators of the endocannabinoid system [43]. The endocannabinoid system has been found to have an important neuroprotective role in CNS injury and neurodegenerative diseases and an extensive review of these studies was recently published [44].
Endocannabinoids act as retrograde messengers and, upon release from postsynaptic neurons, regulate further neurotransmitter release by activating presynaptic cannabinoid receptors [45]. Cannabinoid receptor 1 (CB1) expression in the brain is highest in the basal ganglia, globus pallidus, and substantia nigra; moderate in the cerebellum, hippocampus, caudate nucleus, putamen, hypothalamus, and amygdala. The endocannabinoid system is hypoactive in HD [43], which may underlie the neurotransmission abnormalities of HD and may be the cause of the clinical manifestations of the disease. Inhibition of fatty acid amide hydrolase, monoacylglycerol lipase or the endocannabinoid membrane transporter can enhance endocannabinoid levels and counteract neurochemical deficits and the hyperkinetic effects of HD [46]. Other studies have shown that dietary supplementation with essential fatty acids protected against motor deficits in a transgenic mouse model of HD [47]. Recent studies using ethyl-eicosapetaenoate (Ethyl-EPA, LAX-101 or Miraxion) showed promise in clinical trials and its action is presumed to be through c-Jun amino-terminal kinase (JNK) pathway [48]. HD is associated with up-regulated transglutaminase activity in selectively vulnerable brain regions and transglutaminase-catalyzed cross-links co-localize with hunttingtin (htt) protein aggregates [49]. Combination therapy using minocycline and coenzyme Q10 (CoQ10) in R6/2 transgenic HD mouse model also provided benefit by acting synergistically (minocycline attenuated microglia proliferation and CoQ10 reduced htt protein aggregation)[50].
HD and lipid peroxidation [23]: In HD, one study reported no increase in 8-hydroxy-2’-deoxyguanosine or other markers of DNA oxidation, and no change in lipid peroxidation (as measured by thiobarbituric acid reactive substances, a relatively non-specific marker for lipid peroxidation). In contrast, other studies have shown increases in the lipid peroxidation markers F2-isoprostane and malondialdehyde in HD.
Amyotrophic Lateral Sclerosis (ALS) is an adult-onset neurodegenerative disease characterized by progressive loss of spinal cord and cortical motorneurons and is usually fatal within 2-5 years of diagnosis. Approximately 10% of ALS cases are familial (inherited), with the remaining 90% of cases being sporadic in origin [51]. The underlying cause of sporadic ALS still remains elusive. Of the familial cases, approximately 20% (i.e., 2% of all ALS) are due to mutations in the gene for the cytosolic copper-zinc superoxide dismutase (SOD1). SOD is responsible for detoxifying superoxide radicals to hydrogen peroxide. Expression of a mutant SOD1 protein, with or without residual SOD activity, is necessary to cause ALS phenotype, suggesting a dominant negative mechanism [51]. There is strong evidence that the toxicity of mutant SOD1 in ALS is not due to loss of activity, but to the gain of one or more toxic functions that are independent of SOD1 activity [52]. It is hypothesized that mutant SOD1 stimulates oxidative stress and induces mitochondrial dysfunction, excitotoxicity, inflammation, and protein aggregation [51].
The role of glutamate-mediated excitotoxicity in ALS was supported by the efficacy of riluzole, (a benzothiazole derivative that acts by reducing glutamate excitotoxicity) in slowing the progression of ALS [51]. Riluzole is the only available treatment for ALS, but is now known to have limited therapeutic benefits with minimal effects on survival [53]. Several inflammatory markers such as caspase 1, cyclooxygenase 2 (COX-2) and TNF-α are increased in spinal cord tissue in transgenic mouse models of ALS. Inhibition of COX-2 reduced spinal neurodegeneration and prolonged the survival of ALS transgenic mice [54]. The role of COX-2 in ALS as well as the presence of TNF-α suggests that cPLA2 and/or sPLA2 may also be up-regulated in ALS to supply the ArAc substrate to the COX pathway. TNF-α induces cPLA2, sPLA2 as well as COX-2. This suggests that anti-TNF-α therapy could attenuate the progression of ALS, an option that does not appear to have been tested.
ALS and lipid peroxidation: Evidence of increased oxidative DNA damage in ALS was indicated by elevated levels of 8-hydroxy-2’-deoxyguanosine in plasma, urine and CSF [23]. Several studies have shown increased lipid peroxidation and DNA damage in transgenic mice expressing mutant SOD1 (an animal model for ALS) and in neural tissue or sera from ALS patients [55,56].
Although studies demonstrate that oxidative damage occurs in a number of neurodegenerative diseases, in chronic conditions such as these it is difficult to determine if this damage relates directly to the underlying cause of the disease or represents secondary damage in cells with otherwise compromised function [55]. Interpretation of data from patients is complicated by the fact that observations are usually obtained at the end stage of neurological disease and thus the time course of oxidative damage relative to the progression of the disease is not obtained. Also, these oxidative modifications are not disease specific but occur in a number of neurodegenerative diseases.
Schizophrenia, Epilepsy, and Bipolar disorders. Schizophrenia is marked by disturbances in thinking, emotional reactions, social behavior, with delusions and hallucinations. Drugs that block dopamine receptors alleviate symptoms of schizophrenia, indicative of excess dopaminergic function, while agents that block glutamate receptors induce some of the symptoms of schizophrenia in otherwise normal persons [57].
Recent theories on the neurological deficits of schizophrenia have focused on abnormalities in phospholipid metabolism, particularly increased activity of PLA2 enzymes and reduced activity of the system which incorporates PUFAs into phospholipids (a simultaneous increase in phospholipid hydrolysis and decrease in synthesis) [57,58]. Neither abnormality alone produces schizophrenia but the presence of both does [57]. These abnormalities lead to changes in membrane structure and thus the function of membrane-bound proteins, availability of cell signaling molecules, and the behavior of neurotransmitter systems. This hypothesis is supported by animal studies demonstrating that application of PLA2 into the brain produces alterations in the dopamine system [57]. Also, since phospholipid metabolism has a crucial role in neuronal and synaptic growth and remodeling, it is plausible that defects in this system result in failure of normal neurodevelopment in schizophrenia. There is also evidence that schizophrenia is associated with alterations in lipid transport proteins and membrane phospholipid composition (increase in phosphatidylserine and decrease in phosphatidylcholine and phosphatidylethanolamine) [58]. Genome studies have found that several genes involved in myelination have decreased expression levels in schizophrenia [59].
From a therapeutic perspective, a number of reports indicate that at least a portion of schizophrenic patients have reduced levels of PUFAs, particularly ArAc and DHA, in red cell phospholipids, with low levels particularly associated with negative symptoms [57]. ArAc, DHA and eicosapentaenoic acid (EPA) are important for monoaminergic neurotransmission, brain development, and synaptic functioning [58]. This suggests that supplementation with essential fatty acids could alleviate symptoms of schizophrenia. In preliminary studies, however, DHA essentially had no effect and ArAc appeared to worsen symptoms in some schizophrenia patients. Unexpectedly, EPA provided significant improvement, comparable in magnitude to that produced by new atypical antipsychotic drugs, without any of the side effects characteristic of drug treatment. The combination of EPA and DHA was also beneficial in bipolar disorder (manic-depressive illness) [57].
Epilepsy is a neurological disorder characterized by recurrent spontaneous seizures due to an imbalance between cerebral excitability and inhibition, with a tendency towards uncontrolled excitability [60]. Recurrent severe seizures can lead to death of brain cells. The usual treatment for epilepsy is administration of anti-epileptic drugs. Phenytoin (Dilantin, Phenytek, US brand names) is the generic name of a widely used anti-seizure medicine [61]. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited possibly by promoting sodium efflux from neurons. Phenytoin tends to stabilize the threshold against hyper-excitability caused by excessive stimulation. The current status of new (second generation) antiepileptic drugs has been recently reviewed [61-63].
The ketogenic diet is an established and effective non-pharmacological symptomatic treatment for epilepsy that has been in clinical use for more than 80 years [60,64,65]. The ketogenic diet is a high fat, low protein and low carbohydrate diet in which >90% of calories are derived from fat and dietary availability of glucose is minimal [60,64,65]. The hallmark feature of the ketogenic diet is production of ketone bodies (β-hydroxybutyrate, acetoacetate, and acetone) in the liver with a concomitant rise in plasma levels. Since glucose (the preferred source of energy, particularly in the brain) is severely restricted, the ketone bodies are used as the energy source in extrahepatic tissues [64]. Despite its many years of use, there is still considerable debate over how the ketogenic diet works; several major hypotheses have been advanced, but none are widely accepted.
Lipids in CNS Injury
Focal Cerebral Ischemia (Stroke), caused by obstruction of blood flow (ischemia) to the brain, is the first leading cause of long-lasting disability and third leading cause of death. Presently, tissue plasminogen activator (tPA) is the only FDA approved drug for the treatment of ischemic stroke. tPA administration beyond 3 hr presents hemorrhagic risk. The recent failure of NXY-059 (a nitrone spin trap agent developed by AstraZeneca) in phase III stroke clinical trials emphasizes the need for careful pre-clinical scrutiny of test agents before embarking into mega clinical trials [66].
The initial event in cerebral ischemia is energy failure, resulting in excessive release of the neurotransmitters such as dopamine and glutamate [67]. Over-stimulation of glutamate receptors results in elevated intracellular Ca++ and activation of phospholipases (A2, C, and D) (Fig. 1A) and sphingomyelinases (Fig. 1B). Activation of these phospholipases causes hydrolysis of membrane phospholipids and release of second messengers [4,68,69]. TNF-alpha and IL-1, major pro-inflammatory cytokines, are rapidly up-regulated in brain after cerebral ischemia and activate phospholipases [70-73]. The nature of the inflammatory response after stroke suggests that cytokines, particularly TNF-alpha and IL-1alpha/beta, affect the phospholipid metabolism and subsequent production of eicosanoids, ceramide and free radicals (ROS, RNS and other radicals) that may potentiate brain injury [74].
Figure 1. A) Lipid metabolism in ischemic neuronal death.

Activation of phospholipases (PLA2, PC-PLC, PI-PLC and PLD) following cerebral ischemia results in release of lipid second messengers 1,2-diacylglycerol (DAG), phosphatidic acid (PA), lyso-phosphatidic acid (lyso-PA), docosahexaenoic acid (DHA), and arachidonic acid (ArAc). PA and DAG can be readily inter-converted by phosphohydrolases and DAG-kinases. ArAc undergoes further metabolism by cyclooxygenases/lipoxygenases (COX/LOX) to generate important signaling and vasoactive eicosanoids. Free radicals are formed during ArAc metabolism by COX/LOX and free radical generation can be induced by eicosanoids. ArAc generates pro-inflammatory prostaglandins, leukotrienes, and thromboxanes as well as LOX-generated anti-inflammatory lipoxins. Through the LOX pathway, DHA is metabolized to anti-inflammatory resolvins and protectins, including 10,17S-docosatriene (Neuroprotectin D1), an endogenous neuroprotectant. B). Glycerophospholipid and sphingolipid relationship. Tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) activate neutral sphingomyelinase (N-SMase) and acidic sphingomyelinase (A-SMase) through stimulation of PLA2 and PC-PLC and release of ArAc and DAG, respectively. N-SMase and A-SMase hydrolyze sphingomyelin (SM) to liberate ceramide. SM synthase transfers the phosphocholine head-group of PC to ceramide to form SM and DAG.
CDP-choline, the rate-limiting intermediate in phosphatidylcholine (PC) biosynthesis, has shown beneficial effects in cerebral ischemia, TBI, hypoxia, AD, PD, learning and memory disorders, alcoholism, drug addiction, amblyopia and glaucoma, and has undergone several clinical trials for stroke treatment worldwide [67] (see the boxed text “CDP-choline and stroke: An issue to be revisited”). An extensive review of CDP-choline studies was recently published [75]. Due to the complexity of stroke biochemistry, targeting a single pathway may never prove efficacious. Drug cocktails or combinational therapies have been suggested but have not yet been initiated in clinical trails. In experimental paradigms, CDP-choline in combination with urokinase, basic fibroblast growth factor, or nimodipine provided synergistic benefits [67]. The failure of NXY-059 SAINT II trails [76] reiterates need for combinational treatment. CDP-choline is a safe drug (virtually no side effects even when given at 2 grams/day in clinical trials) and should be considered as one component in combinational therapy.
Stroke, ROS and lipid peroxidation: Until now, it has been generally accepted that the oxidative metabolism of ArAc thorough COX generates prostaglandins and ROS. Recent studies have clarified that COX-2 produces tyrosyl radicals on the protein and carbon centered radicals on the substrate ArAc, but does not generate ROS [5,6]. It has been shown recently that ROS production was elevated in a stroke model but that COX-2 inhibition did not attenuate ROS production. Also, ROS generation was not reduced in COX-2 deficient mice [7]. These studies indicated that NADPH oxidase was a significant source of ROS in the stroke model [7]. The role of ROS in activation of various signaling pathways such as p38, JNK, p53, ERK1/2, Akt, NF-κB [77], matrix mettaloproteinases [78] and stroke injury [4,79] have been recently reviewed.
Generation of HNE has been demonstrated by us and others in brain tissue after stroke [4]. Recently, elevated levels of an acrolein-protein conjugate (Nε-(3-formyl-3,4-dehydropiperidino)-lysine, FDP-lysine) were demonstrated in plasma of stroke patients [80]. The time-course of alterations in lipid metabolism and formation of lipid metabolites and lipid peroxidation products after transient cerebral ischemia is presented in Fig. 2.
Figure 2. Time course of changes related to cytokines, lipid metabolism and oxidative stress after transient brain ischemia.

↑=increase, ↓=decrease, compared to control. ArAc: arachidonic acid; CCT: cytidine triphosphate:phosphocholine cytidylyltransferase; HNE: 4-hydroxynonenal; IL-1β: interleukin-1β; MDA: malondialdehyde; •OH: hydroxyl radical; PLA2: phospholipase A2; PC-PLC: PC-phospholipase C; PLD2: phospholipase D2; PLA2 enzyme activity, sPLA2 mRNA and protein expression, PC-PLC activity and PLD2 protein expression were increased after stroke. CCT catalyzes the rate-limiting step in the biosynthesis of PC. CCT activity and protein expression decreased following stroke. Activation of phospholipases and loss of CCT collectively resulted in loss of PC.
Traumatic Brain Injury (TBI) is usually associated with significant neuropsychological deficits, primarily in the domains of attention, executive functioning and memory. In TBI, the initial traumatic event is shearing, laceration, and/or contusion of brain tissue resulting from a physical impact. Secondary injury after the initial trauma results from ischemia, alterations in ion and neuromodular levels, oxidative stress caused by ROS, edema and axonal swelling [81]. Similar to stroke, pathways of secondary injury that occur subsequent to the initial trauma present targets for therapeutic intervention. Corticosteroids have been proposed as therapies to reduce the secondary injuries following TBI. Corticosteroids inhibit the PLA2/COX/LOX pathways, thus limiting ArAc release and metabolism, down-regulating pro-inflammatory cytokines and attenuating the inflammatory response. However, large scale clinical trials of corticosteroids and lazaroids (21-aminosteroids) for treatment of TBI have either failed to demonstrate efficacy or found increased risk of mortality [81].
TBI and ApoE. While the APOE ε4 allele was first implicated as a significant risk factor for AD, a number of clinical studies have indicated that performance on neuropsychological tasks is worse in TBI patients with the APOE ε4 allele than those without it [82]. While other clinical studies did not find an association of APOE ε4 allele with poorer outcome after TBI, these differences could be due to the severity of the TBI (no association in some studies with predominantly mild TBI), the neurological evaluation methods that assess the involvement of different brain regions, and the evaluation time post injury (up to 25 years after TBI). Fluid percussion brain injury up-regulated both ApoE mRNA and protein expression in rats [83]. Both human postmortem and experimental studies have shown Aβ deposition and tau pathology after TBI [84]. The development of AD-like neuropathological and biochemical changes after severe TBI suggested that TBI may be a risk factor for subsequent development of dementia [85]. Epidemiological studies have provided discrepant findings and the relationship between TBI and dementia remains a topic for further investigation. It has been suggested that TBI may lower the threshold for dementia among predisposed individuals. In other studies, the presence of the APOE ε4 allele has been associated with poorer outcome after cardiopulmonary resuscitation and intracerebral hemorrhage, but not after ischemic stroke [86]. Statins have shown benefit in experimental TBI [87], but it is unknown if statin treatment affected Aβ levels.
ApoE is an important mediator of cholesterol and lipid transport in the brain and is encoded by the polymorphic gene APOE. ApoE has been shown to reduce glial activation and CNS inflammatory response. While it may be reasonable to relate effects of ApoE to cholesterol transport, the mechanism whereby ApoE elicits these effects has not been elucidated. This action is isoform-specific with the ApoE4 isoform being less effective at down-regulating inflammatory cytokines [88]. These studies suggest that ApoE could be a therapeutic target for treatment of TBI, although administration of the full protein would be impractical due to its large size and inability to cross the blood-brain barrier. A small peptide apoE(133-149) was created from the receptor binding region that retains the ability of the native protein in down-regulating inflammatory responses. Administration of apoE(133-149) was shown to significantly improve histological and functional outcome after experimental TBI [88].
Spinal Cord Injury (SCI). Similar to TBI, SCI is the result of an initial physical trauma followed by a secondary degenerative process. The majority of SCI do not involve physical transaction of the spinal cord; instead, the cord is injured as a result of contusive, compressive, or stretch injury. Residual white matter containing portions of sensory and motor tracts remain intact, suggestive that pharmacological interventions aimed at the secondary injury cascade could promote neurological recovery and preserve function [89]. The initial event after SCI is depolarization and opening of voltage-dependent ion channels, and consequent massive release of neurotransmitters including glutamate. This leads to accumulation of intracellular calcium, initiating a number of damaging events: mitochondrial dysfunction, activation of nitric oxide synthase (NOS) and PLA2. PLA2 releases ArAc into the COX/LOX pathways to generate eicosanoids. One consequence of mitochondrial dysfunction, activation of NOS, and COX/LOX activity is generation of free radicals (comprised of different species including reactive nitrogen species (RNS), ROS and other radicals) and subsequent lipid peroxidation, believed to be a major pathway of secondary injury in SCI [89].
The glucocorticoid steroids dexamethasone and methylprednisolone have been extensively used in clinical treatment of SCI. The initial rationale was that, since these compounds reduced brain edema in brain tumor patients, they would also reduce edema in SCI. With the knowledge of the lipid peroxidation mechanism, later attention focused on the potential of glucocorticoid steroids to inhibit post-SCI lipid peroxidation, based on their ability to intercalate into artificial membranes and limit propagation of lipid peroxidation chain reactions [89]. In animal studies, it was demonstrated that high dose of methylprednisolone did inhibit post-traumatic lipid peroxidation in spinal cord tissue. Beneficial effects secondary to inhibition of lipid peroxidation included preservation of ion homeostasis, mitochondrial energy metabolism, and attenuation of delayed glutamate release [89]. It is believed that inhibition of lipid peroxidation is the principle neuroprotective mechanism of high-dose methylprednisolone and that glucocorticoid receptor-mediated anti-inflammatory effects have only a minor role [89]. In clinical trials, high-dose methylprednisolone was shown to improve outcome in SCI if treatment was initiated within 8 hr of the initial trauma.
Another agent that has undergone Phase III clinical trials for SCI is GM1 ganglioside. Since high-dose methylprednisolone had become widely accepted for treatment of SCI, GM1 was administered after the completion of the 24 hr methylprednisolone dosing protocol. The results indicated that GM1 did not provide greater functional improvement compared to methylprednisolone alone [89].
DHA generates anti-inflammatory resolvins and protectins including Neuroprotectin D1
Release of lipid second messengers after neuronal injury can either promote further neuronal injury or neuroprotection. Cerebral ischemia results in rapid accumulation of free fatty acids, including ArAc (20:4) and DHA (22:6). Through the COX/LOX pathways, ArAc is metabolized to pro-inflammatory and vasoactive eicosanoids (prostaglandins, leukotriens, thromboxanes). ArAc is also metabolized to anti-inflammatory lipoxins through the LOX pathway (Fig. 1A).
DHA is the most abundant polyunsaturated fatty acid in the brain, and is concentrated in aminophospholipids of cell membranes. Although the underlying mechanisms of its essential function are not clear, emerging evidence suggests that unique metabolism of DHA plays an important role [90]. Studies on the role of PLA2 and PLA2 hydrolysis products have identified the DHA metabolites resolvins and protections including 10,17S-docosatriene (Neuroprotectin D1) following cerebral ischemia/reperfusion in mouse [91] (Fig. 1A). Neuroprotectin D1 was found to serve an endogenous neuroprotective role by inhibiting apoptotic DNA damage, up-regulating anti-apoptotic proteins Bcl-2 and BclxL, and down-regulating pro-apoptotic Bax and Bad expression [92]. Neuroprotectin D1 also inhibited oxidative stress-induced caspase-3 activation and IL-1β-stimulated COX-2 expression [93 and references cited therein]. Administration of albumin causes systemic mobilization of n-3 PUFAs including DHA, and provides substantial neuroprotection in models of brain ischemia and trauma. DHA-albumin administration increased Neuroprotectin D1 in the ipsilateral brain after transient cerebral ischemia in rat [94]. Recent studies also showed that Neuroprotectin D1 promoted neuronal survival by the induction of neuroprotective gene expression and anti-apoptotic pathways that attenuated Aβ42-induced neurotoxicity [95].
Future Trends: Lipidomics and RNA Silencing
The emerging field of lipidomics tries to define the crucial role of lipids in the cell; the research is aimed at mapping the entire lipid population in a biological system, describing the composition and biological function [1,96,97]. The main progress that has spurred recent advances in lipid analysis was the development of new mass spectroscopic techniques, particularly the “soft ionization” techniques electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI). Lipidomics seeks to provide a molecular signature to a certain pathway or a disease condition. The role of lipids in formation of cell membranes makes them both ligand and substrate for proteins, suggesting that advances in lipidomics could have far reaching implications in genomic, proteomic and metabolomic fields. Impressive achievements and advancements have surfaced during the last few years [91]. Lipidomics holds the promise of characterizing complex mixtures of lipids, identifying previously unknown changes in lipid metabolism [67].
RNA interference (RNAi) [98-100] takes advantage of a naturally occurring process to degrade RNA. RNAi selectively and temporarily switches off specific genes, providing a powerful tool to analyze protein function and biochemical pathways. Lipidomic analyses together with RNAi may provide a powerful tool to elucidate the specific roles of lipid intermediates in cell signaling. These approaches have provided crucial information that was previously unachievable [95]. A deeper knowledge of the complexity of lipid signaling will elevate our understanding of the role of lipid metabolism in various CNS disorders, opening new opportunities for drug development and therapies for neurological diseases.
CDP-choline and Stroke: An Issue to be Revisited
The therapeutic action of CDP-choline is thought to be due to stimulation of phosphatidylcholine (PC) synthesis in the brain after stroke. However to the best of our knowledge there were no reports in stroke models demonstrating that loss of PC occurs, and CDP-choline treatment restores PC levels. CDP-choline has also been termed a ‘membrane stabilizer’, ‘free radical scavenger’ and ‘antioxidant’ without any experimental evidence. Recent research on this drug revealed some interesting findings [68,101].
PC is a major membrane phospholipid and its loss threatens cell viability. PC homeostasis is regulated by the balance between hydrolysis and synthesis [67]. PC is hydrolyzed by phospholipase A2 (PLA2) [4], PC-phospholipase C (PC-PLC) and phospholipase D (PLD). PC synthesis is rate-limited by CTP:phosphocholine cytidylyltransferase (CCT) that makes CDP-choline. Pro-inflammatory cytokines TNF-α and IL-1β are up-regulated after stroke and activate phospholipases and sphingomyelinases (SMases), generating arachidonic acid (ArAc) and pro-apoptotic ceramide, and also down-regulate CCT and sphingomyelin (SM) synthase (Fig. 3).
In rat stroke model, the up-regulation of phospholipases and down-regulation of CCT collectively resulted in the loss of PC [68]. CDP-choline treatment significantly attenuated phospholipase induction, loss of CCT and PC, and infarction volume. CDP-choline also attenuated cytokine expression (TNF-α and IL-1β) after stroke [102], suggesting that CDP-choline may affect PC synthesis and hydrolysis through proinflammatory pathways.
CDP-choline in stroke clinical trials. Clinical trials in Japan and Europe used intravenous administration in contrast to oral route in US trials, which gave ambiguous results [67,101]. It was unclear why the oral route was chosen in USA trials (to the best of our knowledge, this is the only drug given orally to stroke patients; if the patient was incapable of swallowing the drug was administered nasally). Animal studies have shown brain uptake of CDP-choline increased to 2% when administered intravenous compared to 0.5% by oral route. The following points are worth considering in future CDP-choline clinical trials:
CDP-choline efficacy may depend on the formulation and route of delivery [103]. Liposome encapsulation of CDP-choline provided more efficient delivery of the drug to the brain and significantly decreased infarction (by 62%) compared to the equivalent dose of free CDP-choline (26% reduction) after stroke [101]. Blood-brain barrier permeability after stroke allows passage of liposomes <100 nm directly through the compromised vasculature [101]. Advantages of CDP-choline liposomes in stroke include: 1) effective at low doses (18 mg/kg in CDP-choline liposomes vs 500 mg/kg free CDP-choline), 2) the presence of GM1 ganglioside confers long-circulating properties by suppressing phagocytosis, 3) brain uptake of the drug is increased to ~23% of the injected dose, and 4) exogenous CDP-choline is rapidly hydrolyzed and absorbed as cytidine and choline and needs to be re-synthesized by CCT [101]. However it should be noted that CCT expression is significantly reduced after stroke [68]. CDP-choline liposomes deliver the agent intact to the brain, circumventing the rate-limiting CCT step [101] (Fig. 4).
Conclusions: CDP-choline beneficial effects could be attributed to interactions at TNF-α/IL-1 mediated events, differentially affecting phospholipases and CCT [102]. This limits phospholipid hydrolysis and increases synthesis, thereby restoring PC and SM levels (Fig. 3). Our recent studies also showed that PC loss is a cause rather than a result of cell death [32]. Since all cells (neurons, astrocytes, and endothelial cells etc., called the ‘neurovascular unit’) contain PC as a membrane building block, CDP-choline protection may arise from stabilizing the ‘neurovascular unit’. These studies provide an integration of CDP-choline effects with cytokine biology and lipid metabolism in stroke [67,74,102].
IVAX Corporation, a Florida based company has entered into a licensing agreement with Grupo Ferrer, Barcelona, Spain, for the final development of CDP-choline in oral neuroprotective therapy for the treatment of stroke.
Figure 3. CDP-choline: Integration of cytokine response and lipid metabolism after stroke.
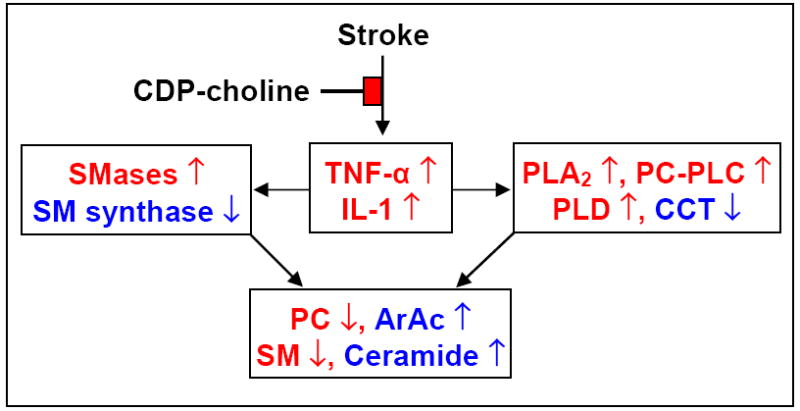
[74] Upregulation of TNF-α and IL-1 differentially affects phospholipases, SMases; CCT and SM synthase in collectively causing loss of PC and SM and release of ArAc and ceramide. CDP-choline partially prevents loss of these phospholipids by attenuating the inflammatory response.
Figure 4. CDP-choline and PC synthesis.

CCT is down-regulated after stroke, impeding PC synthesis. CDP-choline liposomes deliver the drug intact to the brain, bypassing the CCT step. CCT: cytidine triphosphate:phosphocholine cytidylyltransferase. CPT: CDP-choline:1,2-diacylglycerol cholinephosphotransferase. The Western blot shows loss of CCT protein after stroke.
Acknowledgments
This work was supported by grants from NIH/NINDS (NS42008), American Heart Association Greater Midwest Affiliate Grant-in-Aid (0655757Z), UW-School of Medicine and Public Health and UW-Graduate school (to RMA), and laboratory resources provided by William S. Middleton VA Hospital. We would like to thank Ms. Shah for help with preparation of Table 1. Due to space limitations, we sincerely apologize for those important contributions that could not be included. We would like to express our sincere thanks to the anonymous reviewers for their excellent suggestions that have improved the quality of this review article.
List of abbreviations
- Aβ
Amyloid beta
- AD
Alzheimer’s disease
- ALS
Amyotrophic Lateral Sclerosis
- APP
Amyloid precursor protein
- ArAc
Arachidonic acid
- CDP-choline
Cytidine-5’-diphosphocholine
- CNS
Central Nervous System
- COX/LOX
Cyclooxygenase/lipoxygenase
- DAG
1,2-diacylglycerol
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FFA
Free fatty acid
- HD
Huntington disease
- HNE
4-Hydroxynonenal
- MS-EAE
Multiple sclerosis-Experimental Autoimmune Encephalomyelitis
- NP
Niemann-Pick disease
- PC
Phosphatidylcholine
- PD
Parkinson’s disease
- PLA2
Phospholipase A2
- PLC
Phospholipase C
- PUFA
Polyunsaturated fatty acid
- ROS
Reactive Oxygen Species
- SCI
Spinal Cord Injury
- SM
Sphingomyelin
- SMase
Sphingomyelinase
- TBI
Traumatic Brain Injury
Footnotes
Financial Disclosure The authors do not have any financial interests relating to this manuscript.
Biblography
Papers of special note have been highlighted as either of interest (●) or of considerable interest (●●) to readers.
- 1.Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4(7):594–610. doi: 10.1038/nrd1776. ●● An excellent comprehensive review on various aspects of lipidomics and bioinformatics.
- 2.Fahy E, Subramaniam S, Brown HA, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46(5):839–862. doi: 10.1194/jlr.E400004-JLR200. ● A comprehensive classification of lipids with a common platform that is compatible with informatics requirements for the hard-core lipid community.
- 3.Adibhatla RM, Hatcher JF, Dempsey RJ. Lipids and lipidomics in brain injury and diseases. AAPS J. 2006;8(2):E314–E321. doi: 10.1007/BF02854902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adibhatla RM, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40(3):376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Borisenko GG, Osipov A, et al. Arachidonic acid-induced carbon-centered radicals and phospholipid peroxidation in cyclo-oxygenase-2-transfected PC12 cells. J Neurochem. 2004;90(5):1036–1049. doi: 10.1111/j.1471-4159.2004.02577.x. [DOI] [PubMed] [Google Scholar]
- 6.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56(3):387–437. doi: 10.1124/pr.56.3.3. ● A comprehensive review on COX enzymes and prostaglandin synthesis.
- 7.Kunz A, Anrather J, Zhou P, Orio M, Iadecola C. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007;27(3):545–551. doi: 10.1038/sj.jcbfm.9600369. ●● A first report showing that COX-2 does not contribute to ROS production.
- 8.Delacourte A, Sergeant N, Champain D, et al. Nonoverlapping but synergetic tau and APP pathologies in sporatic Alzheimer’s disease. Neurology. 2002;59(3):398–407. doi: 10.1212/wnl.59.3.398. ● Highlights the missing link between synergetic tau and Aβ pathologies.
- 9.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160(1):113–123. doi: 10.1083/jcb.200207113. ● Suggests that cleavage of the different APP pools by α- vs β-secretases depends on distribution of APP to lipid rafts.
- 10.Mandavilli A. The amyloid code. Nat Med. 2006;12(7):747–751. doi: 10.1038/nm0706-747. ● A provocative commentary on the current amyloid theories in AD.
- 11.Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: Cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24(42):9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. ●● A critical review on neuronal cell cycle and death and transgenic models in AD.
- 12.Schwab C, Hosokawa M, McGeer PL. Transgenic mice over-expressing amyloid beta protein are an incomplete model of Alzheimer disease. Exp Neurol. 2004;188(1):52–64. doi: 10.1016/j.expneurol.2004.03.016. ● Transgenic models over-expressing Aβ alone are incomplete models for AD and have limited use.
- 13.Gotz J, Streffer JR, David D, et al. Transgenic animal models of Alzheimer’s disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004;9(7):664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 14.Puglielli L. Aging of the brain, neurotrophin signaling, and Alzheimer’s disease: Is IGF1-R the common culprit? Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nature Neurosci. 2003;6(4):345–351. doi: 10.1038/nn0403-345. ●● Presents the connection between cholesterol and Aβ and how statins lower Aβ in experimental models by inhibiting cholesterol synthesis.
- 16.Whitfield JF. Can statins put the brakes on Alzheimer’s disease? Expert Opin Investig Drugs. 2006;15(12):1479–1485. doi: 10.1517/13543784.15.12.1479. ● Review suggests more clinical trials are needed to specifically asses the statin effects on sporadic AD.
- 17.Grimm MOW, Grimm HS, Patzold AJ, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-[beta] and presenilin. Nat Cell Biol. 2005;7(11):1118–1123. doi: 10.1038/ncb1313. ● A model suggesting the combined effects of cellular cholesterol and sphingomyelin levels regulate γ-secretase activity in APP cleavage.
- 18.Mattson MP, Cutler RG, Jo DG. Alzheimer peptides perturb lipid-regulating enzymes. Nat Cell Biol. 2005;7(11):1045–1047. doi: 10.1038/ncb1105-1045. [DOI] [PubMed] [Google Scholar]
- 19.Caballero J, Nahata M. Do statins slow down Alzheimer’s disease? A review. J Clin Pharm Ther. 2004;29(3):209–213. doi: 10.1111/j.1365-2710.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- 20.Moses GS, Jensen MD, Lue LF, et al. Secretory PLA2-IIA: A new inflammatory factor for Alzheimer’s disease. J Neuroinflammation. 2006;3(1) doi: 10.1186/1742-2094-1183-1128. ●● First report showing the expression of sPLA2 IIA inflammatory PLA2 in AD brains.
- 21.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27(8):1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Lovell MA, Lynn BC. Development of a method for quantification of acrolein-deoxyguanosine adducts in DNA using isotope dilution-capillary LC/MS/MS and its application to human brain tissue. Anal Chem. 2005;77(18):5982–5989. doi: 10.1021/ac050624t. [DOI] [PubMed] [Google Scholar]
- 23.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J Chromatog B. 2005;827(1):65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Farooqui AA, Ong W-Y, Horrocks LA. Inhibitors of brain phospholipase A2 activity: Their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58(3):591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 26.Hauser RA, Zesiewicz TA. Advances in the pharmacologic management of early Parkinson disease. Neurologist. 2007;13(3):126–132. doi: 10.1097/01.nrl.0000256433.15481.eb. [DOI] [PubMed] [Google Scholar]
- 27.Welch K, Yuan J. α-Synuclein oligomerization: a role for lipids? Trends Neurosci. 2003;26(10):517–519. doi: 10.1016/j.tins.2003.08.001. ● Discusses the relationship between PUFA and α-synuclein multimerization in PD.
- 28.Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ. The formation of highly soluble oligomers of α-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron. 2003;37(4):583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 29.Samadi P, Grégoire L, Rouillard C, et al. Docosahexaenoic acid reduces levodopa-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Ann Neurol. 2006;59(2):282–288. doi: 10.1002/ana.20738. [DOI] [PubMed] [Google Scholar]
- 30.Horinouchi K, Erlich S, Perl DP, et al. Acid sphingomyelinase deficient mice - a model of types A and B Niemann-Pick disease. Nature Genetics. 1995;10(3):288–293. doi: 10.1038/ng0795-288. ● An animal model for Niemann-Pick types A and B diseases was developed.
- 31.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5(7):554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 32.Larsen EC, Hatcher JF, Adibhatla RM. Effect of tricyclodecan-9-yl potassium xanthate (D609) on phospholipid metabolism and cell death during oxygen-glucose deprivation in PC12 cells. Neuroscience. 2007;146(3):946–961. doi: 10.1016/j.neuroscience.2007.02.022. ● Report showing that the loss of PC is a cause rather than a result of cell death.
- 33.Vance JE. Lipid imbalance in the neurological disorder, Niemann-Pick C disease. FEBS Lett. 2006;580(23):5518–5524. doi: 10.1016/j.febslet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438(7068):612–621. doi: 10.1038/nature04399. ● This review is more biophysical in nature and discusses the importance of cholesterol in membrane phospholipid organization.
- 35.Aarli JA. Role of cytokines in neurological disorders. Curr Med Chem. 2003;10:1931–1937. doi: 10.2174/0929867033456918. [DOI] [PubMed] [Google Scholar]
- 36.Carlson NG, Rose JW. Antioxidants in multiple sclerosis: do they have a role in therapy? CNS Drugs. 2006;20(6):433–441. doi: 10.2165/00023210-200620060-00001. [DOI] [PubMed] [Google Scholar]
- 37.Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron. 2004;41(3):323–335. doi: 10.1016/s0896-6273(04)00003-0. ● First report showing the cPLA2 role in MS.
- 38.Marusic S, Leach MW, Pelker JW, et al. Cytosolic phospholipase A2α-deficient mice are resistant to experimental autoimmune encephalomyelitis. J Exp Med. 2005;202(6):841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heller RA, Kronke M. TNF receptor-mediated signaling pathways. J Cell Biol. 1994;126:5–9. doi: 10.1083/jcb.126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kronke M, Adam-Klages S. Role of caspases in TNF-mediated regulation of cPLA2. FEBS Lett. 2002;531(1):18–22. doi: 10.1016/s0014-5793(02)03407-5. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham TJ, Yao L, Oetinger M, Cort L, Blankenhorn EP, Greensteinm JI. Secreted phospholipase A2 activity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroinflammation. 2006;3 doi: 10.1186/1742-2094-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aktas O, Waiczies S, Zipp F. Neurodegeneration in autoimmune demyelination: Recent mechanistic insights reveal novel therapeutic targets. J Neuroimmunol. 2007;184(12):17–26. doi: 10.1016/j.jneuroim.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 43.Maccarrone M, Battista N, Centonze D. The endocannabinoid pathway in Huntington’s disease: A comparison with other neurodegenerative diseases. Prog Neurobiol. 2007;81(56):349–379. doi: 10.1016/j.pneurobio.2006.11.006. ●● Review discusses the endocannabinoid pathway in AD, PD, HD and MS. Agonists/antagonists of endocannabinoid receptors and inhibitors of endocannabinoid metabolism are discussed in detail.
- 44.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degroot A, Nomikos GG. In vivo neurochemical effects induced by changes in endocannabinoid neurotransmission. Curr Opin Pharmacol. 2007;7(1):62–68. doi: 10.1016/j.coph.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Battista N, Fezza F, Finazzi-Agro A, Maccarrone M. The endocannabinoid system in neurodegeneration. Ital J Biochem. 2006;55(34):283–289. [PubMed] [Google Scholar]
- 47.Clifford JJ, Drago J, Natoli AL, et al. Essential fatty acids given from conception prevent topographies of motor deficit in a transgenic model of Huntington’s disease. Neuroscience. 2002;109(1):81–88. doi: 10.1016/s0306-4522(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 48.Puri BK, Leavitt BR, Hayden MR, et al. Ethyl-EPA in Huntington disease: a double-blind, randomized, placebo-controlled trial. Neurology. 2005;65(2):286–292. doi: 10.1212/01.wnl.0000169025.09670.6d. [DOI] [PubMed] [Google Scholar]
- 49.Muma NA. Transglutaminase is linked to neurodegenerative diseases. J Neuropathol Exp Neurol. 2007;66(4):258–263. doi: 10.1097/nen.0b013e31803d3b02. [DOI] [PubMed] [Google Scholar]
- 50.Stack EC, Smith KM, Ryu H, et al. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington’s disease mice. Biochim Biophys Acta. 2006;1762(3):373–380. doi: 10.1016/j.bbadis.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Kunst CB. Complex genetics of amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75(6):933–947. doi: 10.1086/426001. ● A thorough review of ALS genetics.
- 52.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Ann Rev Neurosci. 2004;27(1):723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 53.Nirmalananthan N, Greensmith L. Amyotrophic lateral sclerosis: recent advances and future therapies. Curr Opin Neurol. 2005;18(6):712–719. doi: 10.1097/01.wco.0000187248.21103.c5. ● This articles discusses clinical trials in ALS. Despite intense research, still riluzole, an anti-glutamate agent, remains the only treatment for ALS.
- 54.Minghetti l. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropath Exp Neurol. 2004;63(9):901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 55.Agar J, Durham H. Relevance of oxidative injury in the pathogenesis of motor neuron diseases. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(4):232–242. doi: 10.1080/14660820310011278. [DOI] [PubMed] [Google Scholar]
- 56.Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH. Increased lipid peroxidation in sera of ALS patients: A potential biomarker of disease burden. Neurology. 2004;62(10):1758–1765. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- 57.Horrobin D. The lipid hypothesis of schizophrenia. In: Skinner ER, editor. Brain Lipids and Disorders in Biological Psychiatry. Elsevier Science; Amsterdam: 2002. pp. 39–52. ● Role of lipids in schizophrenia is elegantly presented in this book chapter.
- 58.Berger GE, Smesny S, Amminger GP. Bioactive lipids in schizophrenia. Int Rev Psychiatry. 2006;18(2):85–98. doi: 10.1080/09540260600583072. [DOI] [PubMed] [Google Scholar]
- 59.Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98(8):4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papandreou D, Pavlou E, Kalimeri E, Mavromichalis I. The ketogenic diet in children with epilepsy. Br J Nutr. 2006;95(1):5–13. doi: 10.1079/bjn20051591. [DOI] [PubMed] [Google Scholar]
- 61.LaRoche SM. A new look at the second-generation antiepileptic drugs: a decade of experience. Neurologist. 2007;13(3):133–139. doi: 10.1097/01.nrl.0000256353.14257.7c. [DOI] [PubMed] [Google Scholar]
- 62.Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: A summary of the Eigth Eilat Conference (EILAT VIII) Epilepsy Research. 2007;73(1):1–52. doi: 10.1016/j.eplepsyres.2006.10.008. ● A review on the new generation anti-epileptic drugs.
- 63.Klotz U. The role of pharmacogenetics in the metabolism of antiepileptic drugs: pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 2007;46(4):271–279. doi: 10.2165/00003088-200746040-00001. [DOI] [PubMed] [Google Scholar]
- 64.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17(56):431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48(1):43–58. doi: 10.1111/j.1528-1167.2007.00915.x. ● Review presents a view of how the ketogenic diet exerts anticonvulsant effects, summarizing key insights from experimental and clinical studies. PUFAs are generated after ketogenic diet that up-regulate various energy metabolism genes and mitochondrial biogenesis that reduces ROS generation and increase energy production.
- 66.Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: A need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205(1):20–25. doi: 10.1016/j.expneurol.2007.03.003. ● A critical appraisal of failed NXY-059 SAINT II phase III stroke clinical trial.
- 67.Adibhatla RM, Hatcher JF. Cytidine 5’-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochem Res. 2005;30(1):15–23. doi: 10.1007/s11064-004-9681-8. ●● A review on CDP-choline actions in stroke and its possible effect in regulating pro-inflammatory cytokine such as TNF-α and IL-1β.
- 68.Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao F. CDP-choline significantly restores the phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP-phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281(10):6718–6725. doi: 10.1074/jbc.M512112200. ●● First report showing the loss of PC due to activation of sPLA2 and loss of CCT; CDP-choline restored PC levels by opposing these actions in stroke model.
- 69.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007:34–66. doi: 10.1016/j.brainresrev.2006.11.003. ●● Comprehensive review of molecular mechanisms in cerebral ischemia.
- 70.Liu T, Clark RK, McDonnell PC, et al. TNF-α expression in ischemic neurons. Stroke. 1994;25(7):1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 71.Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67(2):161–172. doi: 10.1016/s0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 72.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16(5):932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF-α and IL-1β mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropath. 1994;23(23):103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- 74.Adibhatla RM, Dempsey RJ, Hatcher JF. Integration of cytokine biology and lipid metabolism in stroke. Front Neurosurg Res (under the aegis of Front Biosci) 2007 doi: 10.2741/2759. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Secades JJ, Lorenzo JL. Citicoline: Pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006;28:1–56. [PubMed] [Google Scholar]
- 76.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: In the aftermath of the SAINT trials. Ann Neurol. 2007;61(5):396–402. doi: 10.1002/ana.21127. ● Critical issues and STAIR guidelines for future stroke clinical trials are discussed in the aftermath of failed SAINT-II NXY-059 stroke trails.
- 77.Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38(11):1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 78.Liu KJ, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39(1):71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 79.Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 2005;39(4):429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Tomitori H, Usui T, Saeki N, et al. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36(12):2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. ● First report showing acrolein, a highly toxic aldehyde 100 times more potent than HNE, in plasma of stroke patients. So far accumulation of acrolein in stroke brains had not been demonstrated.
- 81.Rigg JL, Zafonte RD. Corticosteroids in TBI: is the story closed? J Head Trauma Rehab. 2006;21(3):285–288. doi: 10.1097/00001199-200605000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Ariza M, Pueyo R, Matarin MdM, et al. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77(10):1191–1193. doi: 10.1136/jnnp.2005.085167. ● An interesting clinical study demonstrating that performance of neuropsychological tasks is worse in patients with APOE ε4 allele after TBI.
- 83.Iwata A, Browne KD, Chen X-H, Yuguchi T, Smith DH. Traumatic brain injury induces biphasic upregulation of ApoE and ApoJ protein in rats. J Neurosci Res. 2005;82(1):103–114. doi: 10.1002/jnr.20607. ● Fluid percussion injury showed Aβ accumulation and increased ApoE mRNA expression in rats.
- 84.Jellinger KA. Head injury and dementia. Curr Opin Neurol. 2004;17(6):719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 85.Starkstein SE, Jorge R. Dementia after traumatic brain injury. Int Psychogeriatr. 2005;17(Suppl 1):S93–107. doi: 10.1017/s1041610205001973. [DOI] [PubMed] [Google Scholar]
- 86.Smith C, Graham DI, Murray LS, Stewart J, Nicoll JAR. Association of APOE e4 and cerebrovascular pathology in traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77(3):363–366. doi: 10.1136/jnnp.2005.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Treatment of traumatic brain injury with a combination therapy of marrow stromal cells and atorvastatin in rats. Neurosurgery. 2007;60(3):546–554. doi: 10.1227/01.NEU.0000255346.25959.99. [DOI] [PubMed] [Google Scholar]
- 88.Lynch JR, Wang H, Mace B, et al. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exp Neurol. 2005;192(1):109–116. doi: 10.1016/j.expneurol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 89.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1(1):80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim H-Y. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007 doi: 10.1074/jbc R700015200. [DOI] [PubMed] [Google Scholar]
- 91.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278(44):43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 92.Bazan N. The onset of brain injury and neurodegeneration triggers the synthesis of docosanoid neuroprotective signaling. Cell Mol Neurobiol. 2006;26(46):901–913. doi: 10.1007/s10571-006-9064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28(4):176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Belayev L, Marcheselli VL, Khoutorova L, et al. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36(1):118–123. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 95.Lukiw WJ, Cui J-G, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115(10):2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balazy M. Eicosanomics: targeted lipidomics of eicosanoids in biological systems. Prostaglandins Other Lipid Mediat. 2004;73(34):173–180. doi: 10.1016/j.prostaglandins.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Hillard CJ. Lipids and drugs of abuse. Life Sci. 2005;77(14):1531–1542. doi: 10.1016/j.lfs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 98.Senn C, Hangartner C, Moes S, Guerini D, Hofbauer KG. Central administration of small interfering RNAs in rats: A comparison with antisense oligonucleotides. Eur J Pharmacol. 2005;522(13):30–37. doi: 10.1016/j.ejphar.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 99.Cejka D, Losert D, Wacheck V. Short interfering RNA (siRNA): tool or therapeutic? Clin Sci (Lond) 2006;110(1):47–58. doi: 10.1042/CS20050162. ●● This review and the following one by Dykxhorn et al. discuss the technology of RNA silencing, potential obstacles and possibilities for clinical applications.
- 100.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 101.Adibhatla RM, Hatcher JF, Tureyen K. CDP-choline liposomes provide significant reduction in infarction over free CDP-choline in stroke. Brain Res. 2005;1058(12):193–197. doi: 10.1016/j.brainres.2005.07.067. ● Efficient delivery of CDP-choline to the brain using liposome encapsulation substantially improves the outcome after stroke. The route of administration is very important in clinical trials, which has been ignored in US trials.
- 102.Adibhatla RM. Mechanistic aspects of CDP-choline (Citicoline) action in stroke. 4th Asia Pacific Conference Against Stroke (APCAS); March 30-April 1, 2007; New Delhi, India. 2007. [Google Scholar]
- 103.Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002;70(2):133–139. doi: 10.1002/jnr.10403. [DOI] [PubMed] [Google Scholar]
- 104.Wolozin B. Cholesterol, statins and dementia. Curr Opin Lipidol. 2004;15(6):667–672. doi: 10.1097/00041433-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 105.Lane RM, Farlow MR. Lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer’s disease. J Lipid Res. 2005;46(5):949–968. doi: 10.1194/jlr.M400486-JLR200. [DOI] [PubMed] [Google Scholar]
- 106.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101(3):577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 107.Mukherjee S, Maxfield FR. Lipid and cholesterol trafficking in NPC. Biochim Biophys Acta. 2004;1685(13):28–37. doi: 10.1016/j.bbalip.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Miranda SRP, He X, Simonaro CM, et al. Infusion of recombinant human acid sphingomyelinase into Niemann-Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14(13):1988–1995. doi: 10.1096/fj.00-0014com. [DOI] [PubMed] [Google Scholar]
- 109.Kanter JL, Narayana S, Ho PP, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12(1):138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 110.Centonze D, Finazzi-Agro A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol Sci. 2007;28(4):180–187. doi: 10.1016/j.tips.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Lin T-N, Wang Q, Simonyi A, et al. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90(3):637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- 112.Reid RC. Inhibitors of secretory phospholipase A2 group IIA. Curr Med Chem. 2005;12(25):3011–3026. doi: 10.2174/092986705774462860. ● This review presents the structurally diverse array of available sPLA2 group IIA inhibitors, their associated biological activity in animal models, and evaluation of therapeutic potential in phase II clinical trials.
- 113.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503–557. doi: 10.1089/089771502753754037. ● Despite promising pre-clinical data, most of the clinical TBI trials that have been performed in recent years have failed to demonstrate any significant improvement in outcomes. This report summarizes the key points of a workshop sponsored by NIH/NINDS to evaluate problems of past trials and insights into proper planning of future clinical trials.


