Abstract
Differentiation of skeletal muscle myoblasts follows an ordered sequence of events: commitment, cell cycle withdrawal, phenotypic differentiation, and finally cell fusion to form multinucleated myotubes. The molecular signaling pathways that regulate the progression are not well understood. Here we investigate the potential role of calcium and the calcium-dependent phosphatase calcineurin in myogenesis. Commitment, phenotypic differentiation, and cell fusion are identified as distinct calcium-regulated steps, based on the extracellular calcium concentration required for the expression of morphological and biochemical markers specific to each of these stages. Furthermore, differentiation is inhibited at the commitment stage by either treatment with the calcineurin inhibitor cyclosporine A (CSA) or expression of CAIN, a physiological inhibitor of calcineurin. Retroviral-mediated gene transfer of a constitutively active form of calcineurin is able to induce myogenesis only in the presence of extracellular calcium, suggesting that multiple calcium-dependent pathways are required for differentiation. The mechanism by which calcineurin initiates differentiation includes transcriptional activation of myogenin, but does not require the participation of NFAT. We conclude that commitment of skeletal muscle cells to differentiation is calcium and calcineurin-dependent, but NFAT-independent.
Keywords: calcium, myogenesis, signal transduction, calcineurin, myogenin
Introduction
Development of skeletal muscle consists of a highly regulated, temporally distinct sequence of events. A model for myogenesis has been proposed by Andres et al. (1996) based on immunofluorescence studies of single cells. In this model, myoblasts first commit to the differentiation pathway, a step marked by the expression of the bHLH transcription factor myogenin, followed by terminal cell cycle withdrawal and the induction of the cell cycle inhibitor p21. In later stages of myogenesis, the cells phenotypically differentiate, marked by contractile gene expression, and then fuse into multinucleated myotubes. That commitment and cell cycle withdrawal is required before phenotypic differentiation and cell fusion is supported by both in vivo and in vitro studies. Mice lacking myogenin have a severe reduction in the appearance of muscle-specific proteins and myotubes (Hasty et al. 1993). Inactivation of two apparently redundant cell cycle inhibitors, p21 and p57, results in a phenotype nearly identical to the inactivation of myogenin (Zhang et al. 1999). Treatment of BC3H-1 cells with a myogenin antisense oligomer blocks myoblast fusion and results in nearly complete inhibition of acetylcholine receptor protein expression (Brunetti and Goldfine 1990). That phenotypic differentiation precedes cell fusion is supported by numerous studies showing expression of differentiation specific proteins such as acetylcholine receptors (Merlie and Gros 1976; Bar-Sagi and Prives 1983), creatine kinase (Merlie and Gros 1976; Morris and Cole 1979), myosin heavy chain (Merlie and Gros 1976; Andres and Walsh 1996), p21, and myogenin (Andres and Walsh 1996) in mononucleated cells. In addition, fusion-arrested myoblasts have excitation and contraction properties characteristic of myotubes (Constantin et al. 1995).
Calcium regulates several of the later steps required for muscle development. Phenotypic differentiation can be inhibited by lowering extracellular calcium concentration (Shainberg et al. 1969; Morris and Cole 1979), the addition of EGTA (Morris and Cole 1979; Salzberg et al. 1995), and the addition of L-type calcium channel inhibitors or the depletion of intracellular calcium stores (Seigneurin-Venin et al. 1996). The molecular mechanisms by which calcium regulates phenotypic differentiation are unknown. Myoblast fusion also demonstrates a requirement for calcium (Shainberg et al. 1969; Knudsen and Horwitz 1977), and several molecular targets for calcium have been identified. Calcium is required for glycoprotein interactions at the cell surface (Knudsen 1985; Knudsen et al. 1990), regulation of cell surface protein phosphorylation (Lognonne and Wahrmann 1986), and regulation of calpain-induced proteolysis of proteins involved in membrane-cytoskeleton stability (Barnoy et al. 1997, Barnoy et al. 1998; Temm-Grove et al. 1999).
We propose that calcineurin, a calcium-calmodulin–regulated serine/threonine protein phosphatase, is a molecular target of calcium in the regulation of skeletal muscle differentiation at a step before phenotypic differentiation. In previous work, we demonstrated that the calcineurin inhibitor, CSA, blocks differentiation based on the dose-dependent inhibition of creatine kinase and embryonic myosin heavy chain (EMyHC) expression and the formation of multinucleated myotubes (Abbott et al. 1998), suggesting a role for calcineurin in the early stages of myogenesis. However, other cellular targets besides calcineurin may exist for CSA in muscle cells (Lo Russo et al. 1996, Lo Russo et al. 1997). Calcineurin-dependent pathways involving fiber type determination and hypertrophy have been previously described in skeletal muscle. Enhancer elements responding to the calcineurin-regulated transcription factor nuclear factor of activated T cells (NFAT) have been identified in the promoters of slow fiber-specific genes (Chin et al. 1998) and transgenic mice expressing an activated form of calcineurin display an increase in the number of slow fibers (Naya et al. 2000). Hypertrophy of skeletal muscle in response to IGF-1 (Musaro et al. 1999; Semsarian et al. 1999) and to functional overload (Dunn et al. 1999) requires calcineurin activity.
In this study, we test the hypothesis that calcineurin is a key mediator of calcium signals early in the myogenic program using biochemical and genetic modulators of calcineurin activity. We show that in addition to phenotypic differentiation and cell fusion, calcium is required for the commitment to the differentiation pathway. Calcineurin is a necessary molecular target of calcium at the commitment stage and is sufficient, in the presence of adequate calcium, to induce skeletal muscle cells to initiate differentiation. We find that transcriptional activation of myogenin is a component of calcineurin signaling during differentiation, but that NFAT is not a required element of the signaling pathway. We conclude that myogenesis is initiated by a calcineurin-dependent, NFAT-independent pathway.
Materials and Methods
Antisera and Reagents
Mouse monoclonal antibodies against α-sarcomeric actin (s-actin) and α-tubulin were purchased from Sigma-Aldrich as ascites fluid. Mouse monoclonal antibodies against myogenin (Wright et al. 1991) and EMyHC (Webster et al. 1988) were obtained from the Developmental Studies Hybridoma Bank at the University of Iowa (Iowa City, IA) and used as hybridoma supernatants. Secondary antibodies were purchased from The Jackson Laboratory. CSA was a gift of Sandoz (Basil, Switzerland). Ionomycin and PMA were purchased from Sigma-Aldrich. Amphotropic retroviral producer cells (SD-3443) were obtained from the American Type Culture Collection. All cell culture reagents were purchased from Life Technologies, except where noted. Fetal bovine serum (FBS) was purchased from Atlanta Biologicals. Basic fibroblast growth factor (bFGF) was purchased from Promega. A plasmid containing an activated form of calcineurin (pCI-neo-CnA*) was a gift of Dr. Rhonda Bassel-Duby (Chin et al. 1998). A plasmid containing a dominant-negative inhibitor of NFAT (pGFP-VIVIT) was a gift of Dr. Anjana Rao (Aramburu et al. 1999). Plasmids containing portions of the myogenin promoter were obtained from Drs. Helen Blau, Nadia Rosenthal, and Rhonda Bassel-Duby.
Cell Culture
Primary myoblast cultures were prepared from SJL mice and purified to >99% as previously described (Abbott et al. 1998; Rando and Blau 1994). Growth media (GM) consisted of Ham's F10, 20% FBS, 5 ng/ml bFGF, 200 U/ml penicillin G, and 200 μg/ml streptomycin. The myoblast cell line L6 was grown in L6 Growth Media (LGM) consisting of DMEM, 10% FBS, 200 U/ml penicillin G, and 200 μg/ml streptomycin. Differentiation was induced by changing primary cells grown on E-C-L (Upstate Biotechnology)–coated dishes or L6 cells on uncoated dishes to a low serum, low mitogen differentiation media (DM: DME (1.4 mM Ca2+), 2% horse serum (HS), 200 U/ml penicillin G, 200 μg/ml streptomycin) for 24–48 h. Calcium-free differentiation media (DM-CF) was prepared using CaCl2-free DME and HS that had been dialyzed twice against PBS using a membrane with a 3,500-kD molecular mass cutoff. DM-CF has a residual calcium concentration of at least 8 μM due to calcium pantothenate in the DME and calcium remaining in dialyzed HS. When necessary, CaCl2 was added to DM-CF to the desired calcium concentration. Cells were photographed on a Nikon TMS inverted microscope with a Polaroid MicroCam.
Retroviral Plasmids, Production and Infection, and FACS® Sorting
The retroviral NFAT-responsive plasmid (pKA7) contains a luciferase coding sequence under the control of a minimal IL-2 promoter with an upstream triplex of the distal IL-2 gene NFAT response element (Abbott et al. 1998; Boss et al. 1998). The activated calcineurin A (aCnA) expression plasmid was constructed in the retroviral plasmid pTJ66 in which expression of the cDNA insert is driven by the viral 5′-LTR promoter (Murphy et al. 2000). pTJ66 contains a chimeric GFP-zeocin selectable marker driven by IRES-dependent translation that allows the efficiency of retroviral mediated gene delivery to be assessed by fluorescence microscopy. An aCnA cDNA was obtained by EcoRI digestion of the plasmid pCI-neo-CnA* (Chin et al. 1998). The insert was subcloned into SfiI sites in pTJ66 after blunt-end ligation of SfiI adapters to the insert. The CAIN retroviral expression plasmid was made by using RT-PCR of mouse skeletal muscle RNA to generate a cDNA corresponding to amino acids 1,989–2,182 of the rat CAIN sequence, a portion adequate to inhibit calcineurin activity (Taigen et al. 2000). RT-PCR primers were made corresponding to amino acids 1,989–1,994 for the forward primer and 2,182–2,178 for the reverse primer of the rat sequence. The cDNA was subcloned into the retroviral vector pCL1 (Murphy et al. 2000) in which expression of CAIN is driven by the 5′ LTR. The GFP-VIVIT retroviral expression plasmid was made by restriction digest of the vector pGFP-VIVIT (Aramburu et al. 1999) to generate a cDNA that was subcloned into pCL1. A retroviral reporter plasmid was created using a portion of the myogenin promoter containing 1,565-bp of upstream promoter sequence (pMyogLuc). A promoter fragment was generated by restriction digest of the plasmid pMyo1565lacZ and was subcloned into the vector pCatCul (Murphy et al. 2000). The myogenin promoter drives the transcription of a luciferase gene.
Production of infectious retrovirus and infection of primary myoblasts were performed as previously described (Abbott et al. 1998). The efficiency of one round of retroviral infection was estimated at >60% and >90% after two rounds, based on visualization of GFP-positive cells. In some experiments, GFP-positive cells were further purified by flow cytometry. After infection, cells were grown for 2–3 passages, and the top 50% of GFP expressing cells were sorted and used for further experiments.
Reporter Assays
NFAT Reporter Assays.
Primary or L6 myoblasts were plated at 4 × 104 cells per well of 24-well dishes. Cells were infected by two rounds of infection with retroviruses appropriate to each experiment. After 24 h, the medium was replaced with DM and the cells were allowed to differentiate for 48 h. The medium was replaced with the appropriate drug containing media and incubated for 5–6 h at 37°C. Cells were washed twice with PBS and 75 μl of Luciferase Cell Culture Lysis Reagent (Promega) was added to each well. The cell lysates were collected and spun at 12,000 g for 30 s. 100 μl of Luciferase Assay Reagent (Promega) was injected into 20 μl of cell lysate and light output was measured after a 5-s delay over a 10-s window using a Turner TD-20e luminometer (Turner Designs).
Myogenin Promoter Reporter Assays.
Primary myoblasts that had been previously infected with pMyogLuc retrovirus were plated as above and infected with two rounds of control or aCnA retroviruses. After 48 h in GM, the cells were lysed and luciferase levels were determined as above.
Immunoblotting
Cells were lysed with RIPA-2 (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS) containing protease inhibitors (Mini Complete; Boehringer). Equal amounts of protein (5–10 μg per lane; Bradford 1976) were separated by SDS-PAGE and transferred electrophoretically to a PVDF membrane (Immobilon P; Millipore). After nonspecific binding was blocked in 5% nonfat milk in TBS for 30 min, the membrane was incubated overnight in 0.5% nonfat milk in TBS containing primary antibodies. Primary antibody dilutions were as follows: anti-EMyHC, 1:4; anti–s-actin 1:1,000; anti–α-tubulin, 1:1,000; anti-myogenin, 1:10. Blots were washed extensively in TBS containing 0.1% Tween-20 (TBS-T) and then incubated with a goat anti–mouse HRP-conjugated secondary antibody (1:10,000) in 0.5% nonfat milk in TBS-T. Blots were washed in TBS-T and antibody binding was detected using ECL reagents (Amersham Pharmacia Biotech). To demonstrate relative protein loading, SDS-PAGE gels or membranes were stained with Coomassie blue (Bio-Rad) or membranes were stained with colloidal gold (Bio-Rad).
Northern Blotting
RNA was prepared from cells using Trizol Reagent (Life Technologies) following the manufacturer's protocol. RNA was separated on 1% agarose-formaldehyde gels and transferred to Nytran SPC membranes (Schleicher & Schuell). Membranes were probed with random-primed cDNA (Rediprime II; Amersham Pharmacia Biotech) labeled with 32P in Rapid-hyb buffer (Amersham Pharmacia Biotech). After high-stringency washing, membranes were visualized by autoradiography.
Results
Calcium Is Required for Multiple Phases of Myogenesis
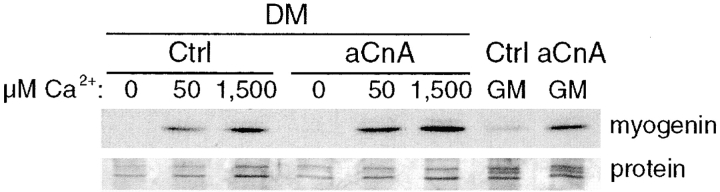
To investigate the requirement for calcium at each phase of myogenesis (commitment, phenotypic differentiation, and cell fusion), myoblasts were induced to differentiate in a calcium-free DM containing different concentrations of exogenously added calcium. As shown in Fig. 1 a, primary myoblasts show a dose-dependent requirement of calcium for myoblast alignment and fusion. At 50 μM exogenously added Ca2+, rounded, mononucleated cells are the predominant cell type. As the calcium is increased to 300 μM, the cells are still predominantly mononucleated, but the cells have elongated and aligned next to each other. Significant fusion of myoblasts is not seen until 1,500 μM Ca2+. To determine the requirement for calcium in myogenic phases that can not be distinguished morphologically, we performed immunoblot analyses using antibodies against myogenin, and the sarcomeric proteins EMyHC and actin, markers of commitment and phenotypic differentiation, respectively (Fig. 1 b). All of these markers display a dose-dependent increase in expression in response to increasing concentrations of extracellular Ca2+. Myogenin shows significant expression at 25–50 μM Ca2+, whereas EMyHC and s-actin are not expressed until 150 μM Ca2+. As shown in Fig. 1 c, the inhibition of differentiation in calcium-free media is reversible, suggesting that it is not due to cytotoxicity. After readdition of calcium to the media, the cells express myogenin and EMyHC. Thus, these data suggest that at least three distinct steps in myogenesis are regulated by calcium based on the differing calcium requirements of commitment (25–50 μM), phenotypic differentiation (150 μM), and cell fusion (1,500 μM).
Figure 1.
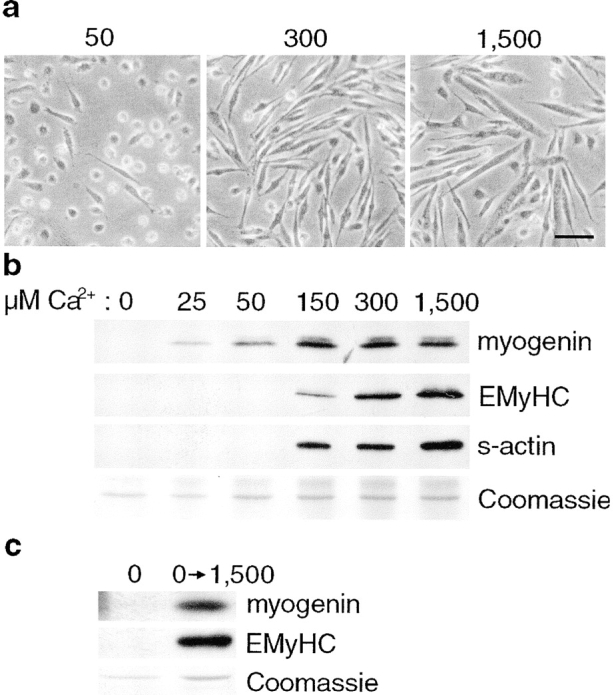
Extracellular calcium is required for myoblast fusion and the expression of markers of myogenic commitment and differentiation. Primary myoblasts were induced to differentiate in DM-CF containing the indicated concentrations of exogenously added calcium for 24 h. (a) Phase-contrast images of cells grown in 50, 300, and 1,500 μM added calcium. Mononucleated cells are seen at both 50 and 300 μM Ca2+, but the cells become elongated and align at 300 μM. At 1,500 μM Ca2+, mononucleated cells fuse into multinucleated myotubes. (b) Immunoblots were performed using antibodies against markers of distinct myogenic phases. Extracellular calcium is required for significant expression of all the markers tested. A portion of a Coomassie-stained gel demonstrates relative protein loading. These results are representative of three independent experiments. (c) After 24 h in DM-CF containing no exogenously added calcium, cells were either maintained in the same media or changed to DM-CF containing 1,500 μM Ca2+ for an additional 24 h. Immunoblots demonstrate that the cells retain the ability to differentiate after the readdition of calcium. A portion of a Coomassie-stained gel demonstrates relative protein loading. Bar, 30 μm.
Calcineurin Activity Is Necessary for Myogenic Commitment
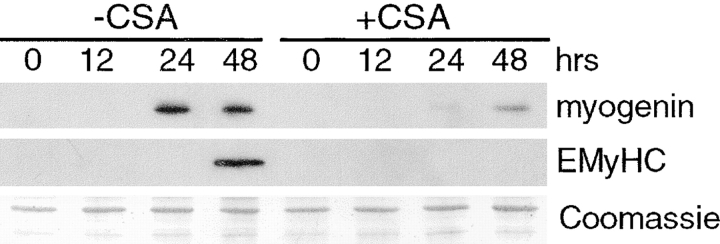
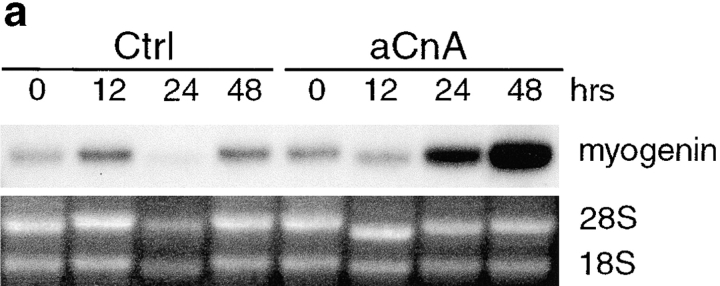
Calcineurin is a potential downstream effector of calcium during myogenesis. The importance of calcineurin in muscle differentiation is suggested by our previous studies demonstrating that CSA blocks phenotypic differentiation and fusion. CSA could directly inhibit each of these phases, or CSA could act earlier at the commitment stage. To determine the phase of myogenesis in which CSA exerts its effect, we induced L6 myoblasts to differentiate in media containing CSA (Fig. 2). At various times after changing cells to DM, proteins were collected and analyzed by immunoblotting using antibodies against myogenin and EMyHC. Myogenin is expressed in vehicle-treated cells at 24 h after inducing differentiation, whereas EMyHC is first detected at 48 h. CSA reduces the level of myogenin expression compared with control cells to ∼11% at 24 h and to ∼35% at 48 h. EMyHC expression is undetectable in CSA-treated cells. Since CSA inhibits the expression of myogenin, then calcineurin is a likely component of the signaling pathways that initiate commitment, the first step of myogenesis.
Figure 2.
CSA inhibits myogenic commitment and differentiation. L6 myoblasts were induced to differentiate in DM containing either vehicle or 1 μM CSA. Cellular proteins were collected at various time points and analyzed for expression of myogenic markers. In control cells, expression of myogenin and EMyHC is first detected at 24 and 48 h, respectively. In CSA-treated cells, the expression of myogenin is decreased, and EMyHC is undetectable. A portion of a Coomassie-stained gel demonstrates relative protein loading.
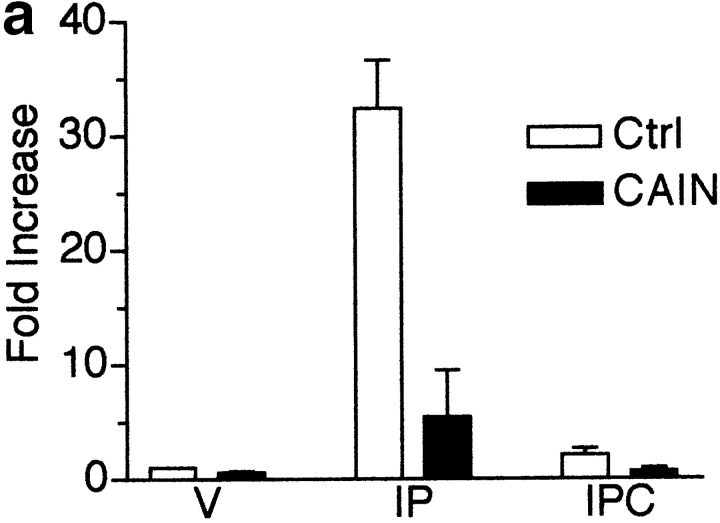
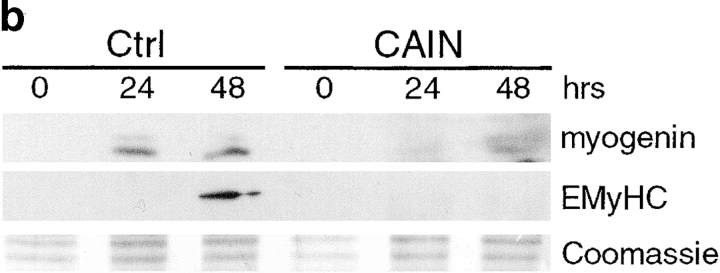
CSA may have effects on skeletal muscle cells distinct from its actions on calcineurin. To conclusively demonstrate that calcineurin activity is required for the commitment stage, we tested the effect of a novel protein inhibitor of calcineurin on myogenesis. CAIN is a natural protein product identified by its ability to bind to calcineurin. It has been shown to inhibit calcineurin activity using in vitro phosphatase assays (Lai et al. 1998) and by indirectly inhibiting transcription from a reporter responding to the calcineurin target NFAT (Taigen et al. 2000). We first expressed CAIN in the presence of an NFAT reporter to test for inhibition of calcineurin activity in skeletal muscle cells (Fig. 3 a). This reporter requires the calcineurin-induced nuclear translocation of NFAT and the induction of AP-1 for transcription of the luciferase cDNA. Stimulation with ionomycin, to activate calcineurin, and PMA, to induce AP-1, in control cells results in an ∼32-fold increase in luciferase levels compared with vehicle-treated control cells. In cells expressing CAIN, ionomycin and PMA treatment yields only an approximately fivefold increase. Thus, CAIN effectively inhibits calcineurin in skeletal muscle cells. In further experiments, L6 myoblasts were infected with a CAIN retrovirus and changed to DM to test whether myogenesis would be inhibited (Fig. 3 b). In L6 cells infected with the CAIN retrovirus, myogenin expression is reduced to ∼7% of control cell levels at 24 h and to ∼39% at 48 h. EMyHC was undetectable in CAIN-expressing cells. We obtained similar results in primary muscle cells (data not shown). As both CSA and CAIN inhibit myogenin and EMyHC expression, we conclude that calcineurin is an essential mediator of the signaling required for myogenesis to occur.
Figure 3.
Inhibition of calcineurin activity by CAIN inhibits myogenesis in L6 muscle cell cultures. (a) L6 myoblasts were infected with an NFAT reporter and either control (Ctrl) or CAIN retroviruses. The cells were induced to differentiate in DM for 48 h before drug treatment. Luciferase assays were performed on vehicle (V), ionomycin (I, 1 μM) plus PMA- (P, 10 nM), or IP plus CSA- (C, 1 μM) treated cultures. CAIN inhibits calcineurin in skeletal muscle cultures based on the reduction in luciferase activity in IP-treated cultures. Data are reported as the fold increase over vehicle-treated control cells. Each bar represents the mean ± SEM of three independent experiments each performed in triplicate. (b) L6 myoblasts were infected with either control or CAIN expression retroviruses and induced to differentiate in DM. Cellular proteins were collected at various time points and analyzed by immunoblotting. CAIN reduces the expression level of both myogenic markers. A portion of a Coomassie-stained membrane demonstrates relative loading of proteins. The blot shown is representative of two independent experiments.
Calcineurin Activity Is Sufficient to Induce Differentiation
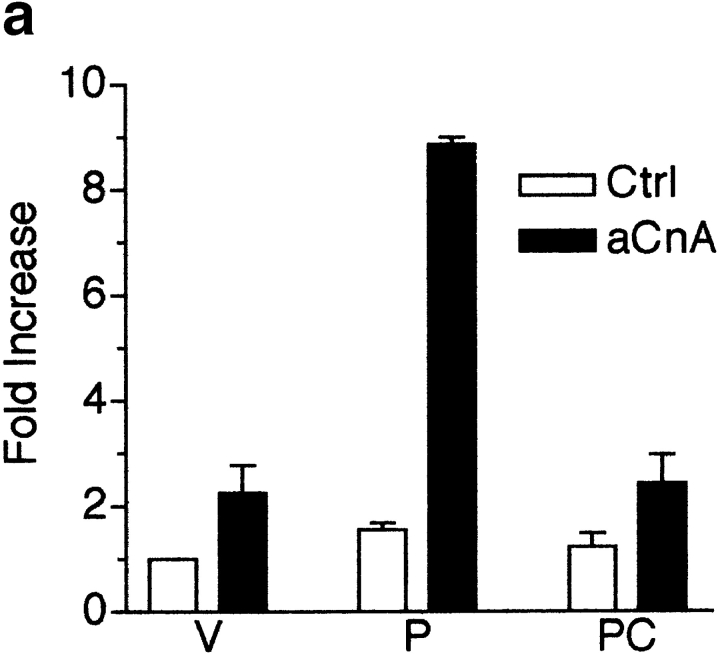
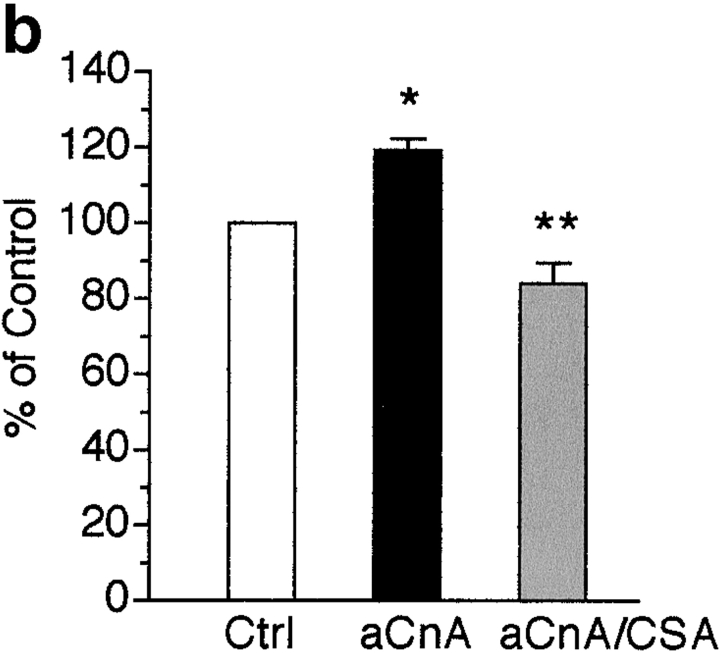
To determine if calcineurin activity is sufficient to induce myogenic differentiation, we constructed a retroviral vector that allows for high efficiency gene transfer of a constitutively active form of calcineurin (aCnA). Originally described as a proteolytic fragment of calcineurin, aCnA retains its catalytic activity and sensitivity to CSA, but no longer requires calcium for activity (Manalan and Klee 1983; O'Keefe et al. 1992). An NFAT responsive luciferase reporter was used to indirectly test the activity of aCnA in skeletal muscle cells. Cells infected with either control or aCnA retroviruses were induced to differentiate and then treated with vehicle or PMA. Some cultures were pretreated with CSA. As seen in Fig. 4 a, luciferase activity is increased by only ∼1.6-fold in control cells with PMA treatment. By contrast, luciferase activity is increased in aCnA-infected cells by approximately ninefold. The response to PMA is blocked by the addition of CSA. These results confirm the activity of aCnA in skeletal muscle cells.
Figure 4.
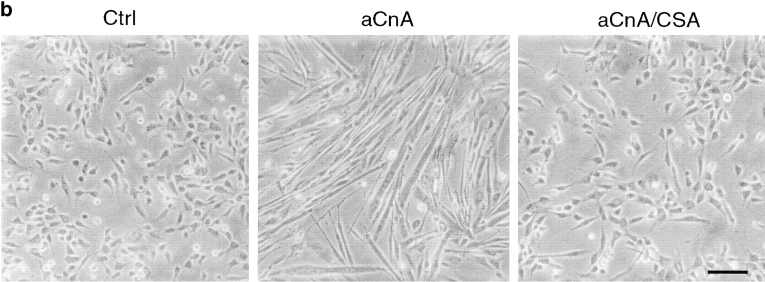
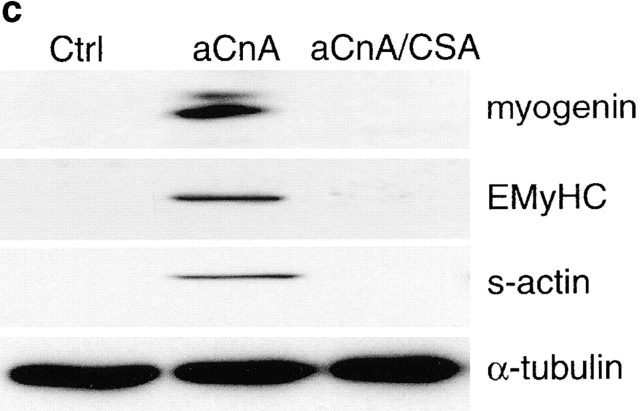
A constitutively active form of calcineurin induces myogenic differentiation in primary myoblasts under growth promoting conditions. (a) Primary myoblasts containing an NFAT responsive reporter construct were infected with either control (Ctrl) or aCnA retroviruses. After 48 h, the cells were induced to differentiate by changing to DM for 24 h. Luciferase assays were performed on vehicle- (V) or PMA- (P, 10 nM) treated cultures. Some cultures were pretreated with 1 μM CSA. aCnA expression increases luciferase levels compared with control cells in PMA-treated cultures. aCnA is sensitive to inhibition by CSA. Data are reported as the fold increase over vehicle-treated control cells. Each bar represents the mean ± SEM of three independent experiments each performed in duplicate. (b and c) Primary myoblasts underwent two rounds of infection with either control (Ctrl) or aCnA retroviruses. CSA (1 μM) was added to some cultures after the second infection. (b) Phase-contrast images of cells 48 h after infection. aCnA induces morphologic changes indicative of myogenic differentiation, including cell elongation, cell alignment, and occasional cell fusion. The morphologic changes are blocked by the addition of CSA to the media. (c) 48 h after infection, proteins were collected and analyzed by immunoblotting for markers of commitment and differentiation. Expression of aCnA results in induction of multiple myogenic markers, which can be blocked with CSA. The expression of the nonmyogenic protein α-tubulin demonstrates equivalent loading of proteins. These results are representative of three independent experiments. Bar, 30 μm.
To examine the effect of aCnA on induction of myogenic differentiation, primary myoblasts were infected with either control or aCnA retroviruses and maintained in GM that contains 300 μM Ca2+ and mitogenic stimuli from both FBS and exogenously added bFGF. Within 48 h after infection, aCnA-infected cells undergo morphological changes consistent with differentiation, including alignment and elongation (Fig. 4 b). The majority of the cells are mononucleated, but occasional multinucleated cells are observed. The cells appear similar to myoblasts induced to differentiate in DM-CF containing 300 μM Ca2+ (Fig. 1 a). The morphological changes are blocked by the addition of CSA to the media, indicating that the effect is specific to the expression of aCnA and not an artifact of the infection protocol. The biochemical differentiation of the aCnA-infected cells was assayed by immunoblot analyses using antibodies against markers of myogenic commitment and differentiation (Fig. 4 b). aCnA-infected cells express myogenin, EMyHC and s-actin. None of these markers are detected in either control cells or in aCnA-infected cells in the presence of CSA. Thus, calcineurin activity is sufficient to initiate differentiation even in the presence of mitogenic stimuli.
Multiple Calcium-dependent Pathways Initiate Myogenesis
Myogenesis initiated by changing cells from GM to DM requires extracellular calcium (Fig. 1). If calcineurin was the only calcium-dependent pathway required for differentiation, aCnA should allow myogenesis to occur in calcium-free DM. To test this hypothesis, primary myoblasts were infected with either control or aCnA retroviruses and induced to differentiate in calcium-free DM in the presence of different concentrations of exogenously added calcium. Immunoblots were performed using an antibody against myogenin (Fig. 5). At 0 μM Ca2+, neither control nor aCnA-infected cells express myogenin. At 50 μM Ca2+ and 1,500 μM Ca2+, myogenin is expressed at equivalent levels in cells infected with either virus. To confirm that the absence of myogenin expression at 0 μM Ca2+ was due to the inability of aCnA to substitute for the calcium requirement and not due to inefficient infection, some cultures were maintained in GM. aCnA induces the expression of myogenin in these cells compared with control cells. Myogenin is detected in control cells maintained in GM (compare to Fig. 4 c), most likely due to spontaneous differentiation as a result of cell confluency. Therefore, aCnA is not able to substitute for the calcium requirement during commitment to differentiation, indicating that calcium is necessary for multiple downstream molecular targets. Thus, calcineurin activity is sufficient to induce differentiation only in the presence of adequate levels of calcium.
Figure 5.
Multiple calcium-dependent pathways are necessary for initiating myogenesis. Myoblasts were infected with either control (Ctrl) or aCnA retroviruses. Cells were maintained in GM for 6 h and then induced to differentiate in DM-CF containing different concentrations of exogenously added calcium for 36 h. Some cultures were kept in GM for the entire experiment. Cellular proteins were analyzed by immunoblotting using an antibody against myogenin. aCnA-infected cells demonstrate a calcium-dependent expression pattern of myogenin similar to control cells. The absence of the expression of myogenin at 0 μM Ca 2+ is not due to inefficient infection of the cells, because aCnA-infected cells maintained in GM express myogenin at higher levels than control cells. A portion of a membrane stained for total protein with colloidal gold is shown to indicate relative protein loading. The data are representative of three independent experiments.
Transcriptional Activation of Myogenin Is a Component of aCnA-induced Differentiation
Expression of myogenin mRNA and protein mark the transition from a proliferative myoblast to a cell committed to the differentiation pathway. Expression of aCnA can induce differentiation and results in an increase in myogenin protein expression. The increase in the level of myogenin protein could be accounted for by stabilization of either the mRNA or the protein, or by an increase in transcription or translation. To determine the mechanism for the increase in myogenin protein we performed Northern blot analyses on RNA isolated from primary muscle cells infected with either control or aCnA retroviruses (Fig. 6 a). Compared with control cells, the levels of myogenin mRNA increase in aCnA-infected cells at both 24 and 48 h after infection. Therefore, mRNA stabilization or transcriptional activation could account for the increase in myogenin protein levels.
Figure 6.
aCnA increases levels of myogenin mRNA and activates transcription from a myogenin reporter construct. (a) Primary myoblasts were infected with either control (Ctrl) or aCnA retroviruses and maintained in GM. RNA samples were collected at various time points and analyzed by Northern blotting for myogenin mRNA. Expression of aCnA results in an increase in myogenin mRNA compared with control cultures. A portion of an ethidium bromide–stained gel containing 28S and 18S rRNAs demonstrates relative RNA loading. The blot shown is representative of two independent experiments. (b) Primary myoblasts containing a myogenin promoter reporter construct were infected with either control or aCnA retroviruses. After 48 h in GM, the cultures were analyzed for luciferase activity. Expression of aCnA results in a CSA-sensitive activation of the myogenin promoter. Each bar represents the mean ± SEM of 3–4 independent experiments each performed in triplicate. Statistical analyses were performed using one-way ANOVA with Bonferroni's multiple comparison test. *P < 0.01 vs Ctrl; **P < 0.001 vs aCnA.
To examine transcriptional activation of the myogenin promoter by aCnA, we constructed a retroviral reporter plasmid that contains 1,565 bp of upstream promoter sequence from the myogenin gene driving luciferase expression. Primary muscle cells containing pMyogLuc were infected with either control or aCnA retroviruses (Fig. 6 b). Luciferase assays were performed on cells after 48 h in GM. Compared with control cells, luciferase levels increase by ∼20% in aCnA-infected cells. The increase was not seen with CSA treatment demonstrating that the increase is specific to the expression of aCnA. We conclude that transcriptional activation of the myogenin gene represents a downstream target of calcineurin activity in myogenesis.
NFAT Is Not a Required Downstream Target of Calcineurin during Myogenesis
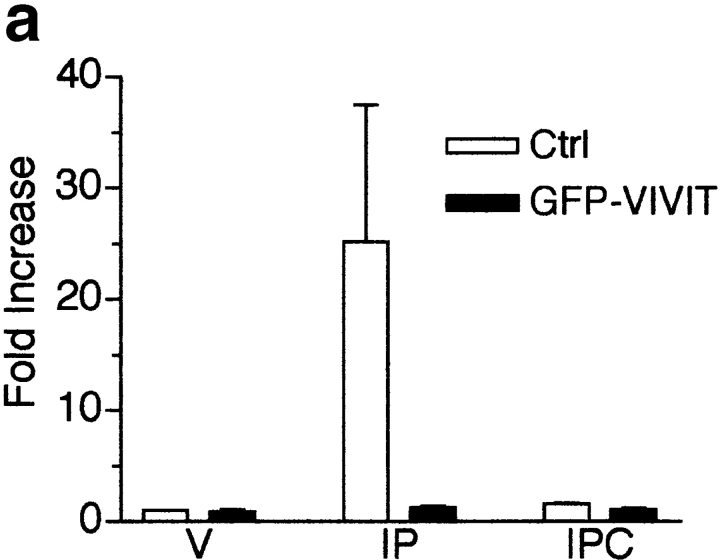
Transcriptional activation is at least one mechanism whereby calcineurin regulates differentiation. To determine if the transcription factor NFAT is a required down stream target of calcineurin during differentiation, we used a peptide inhibitor of NFAT, GFP-VIVIT (Aramburu et al. 1999). We have shown previously that three NFAT isoforms are expressed in skeletal muscle and are able to translocate to the nucleus at different stages of myogenesis (Abbott et al. 1998). To test whether GFP-VIVIT inhibits NFAT activity in skeletal muscle cells, primary myoblasts were infected with an NFAT reporter and either control or GFP-VIVIT retroviruses and sorted for GFP expression by flow cytometry (Fig. 7 a). Myoblasts were induced to differentiate in DM for 48 h, followed by treatment with ionomycin and PMA for 5 h. Some cultures were also treated with CSA. In control cells, luciferase levels increase ∼25-fold, whereas levels in GFP-VIVIT–infected cells increase only ∼1.3-fold demonstrating that GFP-VIVIT effectively inhibits NFAT activity in skeletal muscle cells.
Figure 7.
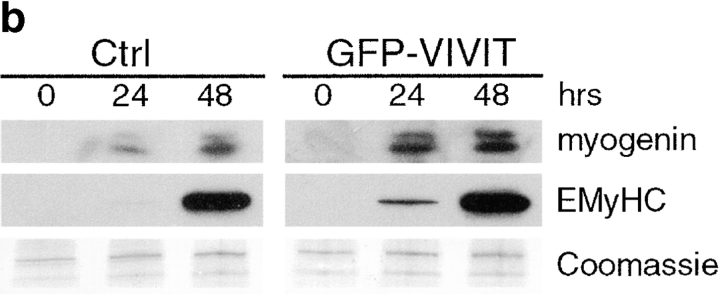
Inhibition of NFAT activity by the expression of GFP-VIVIT does not inhibit myogenesis in L6 muscle cell cultures. (a) Primary myoblasts were infected with an NFAT responsive reporter and either control (Ctrl) or GFP-VIVIT retroviruses. Cells were induced to differentiate for 48 h in DM. Luciferase assays were performed on vehicle (V), ionomycin (I, 1 μM) plus PMA- (P, 10 nM), or IP plus CSA- (C, 1 μM) treated cultures. GFP-VIVIT inhibits NFAT activity in skeletal muscle cells. Data are reported as the fold increase over vehicle-treated control cells. Each bar represents the mean ± SEM of three independent experiments each performed in triplicate. (b) L6 myoblasts were infected with either control or GFP-VIVIT retroviruses and induced to differentiate in DM. Cellular proteins were collected at various time points and analyzed by immunoblotting. GFP-VIVIT does not inhibit the expression of either myogenic marker. The blot shown is representative of two independent experiments.
To determine if NFAT activity is required during differentiation, L6 muscle cells were infected with either control or GFP-VIVIT retroviruses and induced to differentiate in DM. Immunoblot analysis was performed on cellular proteins using antibodies against myogenin and EMyHC (Fig. 7 b). GFP-VIVIT expression does not inhibit the expression of either myogenin or EMyHC. Paradoxically, we see a slight increase in expression of both markers after 24 h in DM. We obtained similar results in primary muscle cells except that the paradoxical increase was not observed (data not shown). Since GFP-VIVIT expression inhibits NFAT activity, but does not inhibit differentiation, we conclude that NFAT is not an essential downstream target of calcineurin during myogenesis.
Discussion
The molecular mechanisms that initiate differentiation of myoblasts are not well understood. Changes in muscle regulatory factors such as MyoD, Myf-5, and myogenin do occur early in differentiation (Megeney and Rudnicki 1995; Rudnicki and Jaenisch 1995; Buckingham 1996), but the signaling pathways that regulate these changes have not been delineated. A large body of work has demonstrated that calcium and calcium-dependent pathways are required for myogenesis (Shainberg et al. 1969; Knudsen and Horwitz 1977; Knudsen 1985; Lognonne and Wahrmann 1986; Przybylski et al. 1989; Cottin et al. 1994; Constantin et al. 1995; Barnoy et al. 1997, Barnoy et al. 1998; Temm-Grove et al. 1999). For the most part, these earlier studies linking calcium to differentiation focused on the clearly identifiable cell fusion event, but since myoblasts pass through multiple, temporally distinct phases before cell fusion, the results of these studies may also reflect calcium-dependent regulation at earlier stages in myogenesis. Thus, it is not exactly clear where calcium acts in the myogenic pathway and whether multiple points of regulation are present. The data presented in this paper suggest that at least three distinct steps require calcium based on their differing requirements for extracellular calcium: commitment to differentiation, phenotypic differentiation, and cell fusion.
Changes in the concentration of extracellular calcium may inhibit differentiation for several reasons. (a) Extremely low levels of extracellular calcium may be toxic to cells. This is unlikely due to the reversibility of the inhibition (Fig. 1 c) that has been noted by us as well as other groups (Shainberg et al. 1969; Przybylski et al. 1989). (b) Calcium available for intracellular signaling processes may be decreased. During differentiation, the total cell calcium increases, and decreases in extracellular calcium produce similar decreases in intracellular calcium (Przybylski et al. 1989). That extracellular calcium is directly needed for intracellular signaling is shown by the ability of calcium channel blockers to inhibit cell fusion and the expression of creatine kinase (Seigneurin-Venin et al. 1996). (c) Extracellular proteins may require calcium for normal activity. The glycoprotein interactions that mediate cell aggregation before fusion require calcium (Knudsen 1985). A combination of multiple mechanisms at multiple phases is likely to account for the calcium requirement in differentiation.
We demonstrate that calcineurin is a downstream effector for calcium in regulating myogenic commitment to differentiation. Experiments using the calcineurin inhibitor CSA demonstrated inhibition in the expression of myogenin, the earliest known marker of skeletal muscle differentiation. The effects of CSA on myogenic commitment suggest a role for calcineurin in initiating myogenesis, but do not rule out targets besides calcineurin. In smooth muscle cells, increased cytosolic calcium concentrations resulting from CSA treatment are due to a calcineurin-independent pathway (Lo Russo et al. 1997). A calcineurin-independent pathway has also been described for inhibition of nitric oxide synthase activation by CSA in a glioma cell line (Trajkovic et al. 1999). To rule out the involvement of calcineurin-independent pathways, we used both a physiological protein inhibitor and a constitutively active form of calcineurin to modulate calcineurin activity in myoblasts. Results using these methods conclusively show that calcineurin participates in initiating myogenic differentiation. Possibly, calcineurin also plays a role at later stages of myogenesis, but this would not be detected in our assays since inhibiting differentiation at the early stages would also be detected as an inhibition of the later stages. Calcineurin is unlikely to be the only downstream effector of calcium in initiating differentiation, as the constitutively active form of calcineurin could not induce differentiation in the absence of extracellular calcium.
The calcium-dependent expression of myogenin and the role of calcineurin in regulating myogenic initiation suggest that changes in cytosolic calcium must occur early in the myogenic program. Few studies quantitating the level of calcium in myoblasts undergoing differentiation have been performed at early time points. In one study, changing from a growth media to a differentiation media increased cytosolic calcium in myoblasts from 90 to 128 nM after 8 h (Constantin et al. 1995). Another study showed that the exchangeable calcium content of differentiation-arrested cells in 0.04 mM Ca2+ increased significantly 2 h after increasing media calcium concentration (Przybylski et al. 1989). A study by Seigneurin-Venin et al. 1996 is particularly relevant to our work, because it demonstrated the importance of the dihydropyridine receptor in regulating calcium release from intracellular stores for creatine kinase expression. Since the dihydropyridine receptor requirement occurs at a step before phenotypic differentiation, it may therefore represent a pathway for regulating increases in cytosolic calcium necessary for the activation of calcineurin.
The downstream effectors for calcineurin in regulating the initiation of myogenesis are not known. Certainly, transcriptional activation is a component as expression of aCnA-activated transcription from pMyogLuc. However, we detected only a relatively small increase in luciferase levels after expression of aCnA, as compared with the relatively large increase in the level of myogenin mRNA. This suggests that calcineurin-dependent regulatory elements exist in the myogenin promoter outside of the region we used for our reporter. Alternatively, mRNA stabilization may occur as a result of an increase in calcineurin activity. Recently, calcineurin was shown to regulate the stability of the acetylcholinesterase mRNA in C2C12 muscle cells during differentiation (Luo et al. 1999).
The transcription factor NFAT is a potential target of calcineurin during myogenesis. We have previously shown the presence and activity of NFAT in skeletal muscle cells (Abbott et al. 1998). Muscle cells express three NFAT isoforms that differ in their ability to be activated and undergo nuclear translocation at different stages of development. However, our results show that calcineurin substrates other than NFAT are in fact required for differentiation, and novel calcineurin substrates may exist in skeletal muscle cells. The muscle regulatory factor family (Myf-5, MyoD, myogenin, MRF4) of transcription factors plays a primary role in regulating myogenesis and are themselves regulated by phosphorylation, and could therefore be potential targets for the phosphatase activity of calcineurin. All of the muscle regulatory factors are inhibited by cAMP-dependent protein kinase (Li et al. 1992), and MyoD activity is inhibited by phosphorylation of a threonine residue at position 115 (Liu et al. 1998). The myocyte enhancer factor (MEF) family of transcription factors also represent potential calcineurin targets. These factors are required for differentiation as a dominant-negative form of MEF2 inhibits differentiation (Ornatsky et al. 1997). The potential for MEFs as calcineurin targets is highlighted by work demonstrating that the myogenin gene is activated by posttranslational modifications of preexisting MEF2 (Buchberger et al. 1994). In addition, a reporter construct containing only a consensus MEF binding site and a TATA-box was activated after overexpression of a constitutively active form of calcineurin in skeletal muscle cells (Chin et al. 1998).
Our results suggest that calcineurin plays a more fundamental role in myogenesis than has been previously described. Several papers have described the necessity of calcineurin activity during skeletal muscle hypertrophy in vitro and in vivo (Dunn et al. 1999; Musaro et al. 1999; Semsarian et al. 1999). Skeletal muscle hypertrophy using in vivo models requires activation and differentiation of resident satellite cells (Rosenblatt and Parry 1992; Rosenblatt et al. 1994; Phelan and Gonyea 1997). The results of the in vivo studies on the role of calcineurin in hypertrophy may, therefore, be consistent with our results. If satellite cell differentiation requires calcineurin activity, then inhibition of calcineurin would also inhibit hypertrophy. However, at least one study using a model of functional overload has reported that the prevention of hypertrophy by CSA treatment was not due to an inhibition of satellite cell differentiation (Dunn et al. 1999). Our results and the models of calcineurin-dependent hypertrophy may represent distinct pathways.
In summary, we have defined a calcium-dependent pathway that regulates the commitment of myoblasts to the differentiation pathway. Calcineurin activity is both necessary and sufficient in the presence of adequate extracellular calcium to induce differentiation. This pathway is independent of NF-AT activity. Future studies will identify the upstream signals that regulate calcium changes early in myogenesis as well as the downstream targets of calcineurin in skeletal muscle.
Acknowledgments
We thank Jonathan Gephart for technical assistance, Dr. Rhonda Bassel-Duby for the activated calcineurin cDNA clone pCI-neo-CnA*, Dr. Anjana Rao for the GFP-VIVIT cDNA, and Drs. Helen Blau, Nadia Rosenthal, and Rhonda Bassel-Duby for the myogenin promoter clones. We thank Dr. Jeffery Molkentin for help with the CAIN construct.
This work was supported by grants to G.K. Pavlath from the National Institutes of Health (AR-43410 and DE13040).
Footnotes
Abbreviations used in this paper: aCnA, activated form of calcineurin A; CSA, cyclosporine A; DM, differentiation medium; DM-CF, calcium-free differentiation medium; EMyHC, embryonic myosin heavy chain; GM, growth medium; HS, horse serum; MEF, myocyte enhancer factor; NFAT, nuclear factor of activated T cells; s-actin, α-sarcomeric actin; TBS-T, TBS containing Tween 20.
References
- Abbott K.L., Friday B.B., Thaloor D., Murphy T.J., Pavlath G.K. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol. Biol. Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres V., Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J., Yaffe M.B., Lopez-Rodriguez C., Cantley L.C., Hogan P.G., Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Prives J. Trifluoperazine, a calmodulin antagonist, inhibits muscle cell fusion. J. Cell Biol. 1983;97:1375–1380. doi: 10.1083/jcb.97.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnoy S., Glaser T., Kosower N.S. Calpain and calpastatin in myoblast differentiation and fusioneffects of inhibitors. Biochim. Biophys. Acta. 1997;1358:181–188. doi: 10.1016/s0167-4889(97)00068-2. [DOI] [PubMed] [Google Scholar]
- Barnoy S., Glaser T., Kosower N.S. The calpain-calpastatin system and protein degradation in fusing myoblasts. Biochim. Biophys. Acta. 1998;1402:52–60. doi: 10.1016/s0167-4889(97)00144-4. [DOI] [PubMed] [Google Scholar]
- Boss V., Abbott K.L., Wang X.F., Pavlath G.K., Murphy T.J. The cyclosporin A-sensitive nuclear factor of activated T cells (NFAT) proteins are expressed in vascular smooth muscle cells. Differential localization of NFAT isoforms and induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors. J. Biol. Chem. 1998;273:19664–19671. doi: 10.1074/jbc.273.31.19664. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunetti A., Goldfine I.D. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J. Biol. Chem. 1990;265:5960–5963. [PubMed] [Google Scholar]
- Buchberger A., Ragge K., Arnold H.H. The myogenin gene is activated during myocyte differentiation by pre-existing, not newly synthesized transcription factor MEF-2. J. Biol. Chem. 1994;269:17289–17296. [PubMed] [Google Scholar]
- Buckingham M. Skeletal muscle development and the role of the myogenic regulatory factors. Biochem. Soc. Trans. 1996;24:506–509. doi: 10.1042/bst0240506. [DOI] [PubMed] [Google Scholar]
- Chin E.R., Olson E.N., Richardson J.A., Yang Q., Humphries C., Shelton J.M., Wu H., Zhu W., Bassel-Duby R., Williams R.S. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin B., Cognard C., Raymond G. Myoblast fusion is not a prerequisite for the appearance of calcium current, calcium release, and contraction in rat skeletal muscle cells developing in culture. Exp. Cell Res. 1995;217:497–505. doi: 10.1006/excr.1995.1115. [DOI] [PubMed] [Google Scholar]
- Cottin P., Brustis J.J., Poussard S., Elamrani N., Broncard S., Ducastaing A. Ca(2+)-dependent proteinases (calpains) and muscle cell differentiation. Biochim. Biophys. Acta. 1994;1223:170–178. doi: 10.1016/0167-4889(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Dunn S.E., Burns J.L., Michel R.N. Calcineurin is required for skeletal muscle hypertrophy. J. Biol. Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J.H., Edmondson D.G., Venuti J.M., Olson E.N., Klein W.H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Knudsen K.A. The calcium-dependent myoblast adhesion that precedes cell fusion is mediated by glycoproteins. J. Cell Biol. 1985;101:891–897. doi: 10.1083/jcb.101.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K.A., Horwitz A.F. Tandem events in myoblast fusion. Dev Biol. 1977;58:328–338. doi: 10.1016/0012-1606(77)90095-1. [DOI] [PubMed] [Google Scholar]
- Knudsen K.A., Myers L., McElwee S.A. A role for the Ca2(+)-dependent adhesion molecule, N-cadherin, in myoblast interaction during myogenesis. Exp. Cell Res. 1990;188:175–184. doi: 10.1016/0014-4827(90)90157-6. [DOI] [PubMed] [Google Scholar]
- Lai M.M., Burnett P.E., Wolosker H., Blackshaw S., Snyder S.H. Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- Li L., Heller-Harrison R., Czech M., Olson E.N. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Mol. Cell. Biol. 1992;12:4478–4485. doi: 10.1128/mcb.12.10.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.N., Dias P., Houghton P.J. Mutation of Thr115 in MyoD positively regulates function in murine fibroblasts and human rhabdomyosarcoma cells. Cell Growth Differ. 1998;9:699–711. [PubMed] [Google Scholar]
- Lo Russo A., Passaquin A.C., Andre P., Skutella M., Ruegg U.T. Effect of cyclosporin A and analogues on cytosolic calcium and vasoconstrictionpossible lack of relationship to immunosuppressive activity. Br. J. Pharmacol. 1996;118:885–892. doi: 10.1111/j.1476-5381.1996.tb15482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Russo A., Passaquin A.C., Cox C., Ruegg U.T. Cyclosporin A potentiates receptor-activated [Ca2+]c increase. J. Recept. Signal Transduct. Res. 1997;17:149–161. doi: 10.3109/10799899709036600. [DOI] [PubMed] [Google Scholar]
- Lognonne J.L., Wahrmann J.P. Spontaneous myoblast fusion is mediated by cell surface Ca2+-dependent protein kinase(s) Exp. Cell Res. 1986;166:340–356. doi: 10.1016/0014-4827(86)90481-7. [DOI] [PubMed] [Google Scholar]
- Luo Z.D., Wang Y., Werlen G., Camp S., Chien K.R., Taylor P. Calcineurin enhances acetylcholinesterase mRNA stability during C2-C12 muscle cell differentiation. Mol. Pharmacol. 1999;56:886–894. [PubMed] [Google Scholar]
- Manalan A.S., Klee C.B. Activation of calcineurin by limited proteolysis. Proc. Natl. Acad. Sci. USA. 1983;80:4291–4295. doi: 10.1073/pnas.80.14.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney L.A., Rudnicki M.A. Determination versus differentiation and the MyoD family of transcription factors. Biochem. Cell Biol. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- Merlie J.P., Gros F. In vitro myogenesis. Expression of muscle specific function in the absence of cell fusion. Exp. Cell Res. 1976;97:406–412. doi: 10.1016/0014-4827(76)90632-7. [DOI] [PubMed] [Google Scholar]
- Morris G.E., Cole R.J. Calcium and the control of muscle-specific creatine kinase accumulation during skeletal muscle differentiation in vitro. Dev Biol. 1979;69:146–158. doi: 10.1016/0012-1606(79)90281-1. [DOI] [PubMed] [Google Scholar]
- Murphy T.J., Pavlath G.K., Wang X., Boss V., Abbott K.L., Robida A.M., Nichols J., Xu K., Ellington M., Loss J.R. Retroviral vectors applied to gene regulation studies. Methods Enzymol. 2000;In press doi: 10.1016/s0076-6879(02)45045-8. [DOI] [PubMed] [Google Scholar]
- Musaro A., McCullagh K.J., Naya F.J., Olson E.N., Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Naya F.J., Mercer B., Shelton J., Richardson J.A., Williams R.S., Olson E.N. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- O'Keefe S.J., Tamura J., Kincaid R.L., Tocci M.J., O'Neill E.A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Ornatsky O.I., Andreucci J.J., McDermott J.C. A dominant-negative form of transcription factor MEF2 inhibits myogenesis. J. Biol. Chem. 1997;272:33271–33278. doi: 10.1074/jbc.272.52.33271. [DOI] [PubMed] [Google Scholar]
- Phelan J.N., Gonyea W.J. Effect of radiation on satellite cell activity and protein expression in overloaded mammalian skeletal muscle. Anat. Rec. 1997;247:179–188. doi: 10.1002/(SICI)1097-0185(199702)247:2<179::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Przybylski R.J., MacBride R.G., Kirby A.C. Calcium regulation of skeletal myogenesis. I. Cell content critical to myotube formation. In Vitro Cell Dev. Biol. 1989;25:830–838. doi: 10.1007/BF02623667. [DOI] [PubMed] [Google Scholar]
- Rando T.A., Blau H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J.D., Parry D.J. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J. Appl. Physiol. 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J.D., Yong D., Parry D.J. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Rudnicki M.A., Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Mandelboim M., Zalcberg M., Shainberg A., Mandelbaum M. Interruption of myogenesis by transforming growth factor beta 1 or EGTA inhibits expression and activity of the myogenic-associated (2′-5′) oligoadenylate synthetase and PKR [published erratum appears in Exp. Cell Res. 220:509] Exp. Cell Res. 1995;219:223–232. doi: 10.1006/excr.1995.1222. [DOI] [PubMed] [Google Scholar]
- Seigneurin-Venin S., Parrish E., Marty I., Rieger F., Romey G., Villaz M., Garcia L. Involvement of the dihydropyridine receptor and internal Ca2+ stores in myoblast fusion. Exp. Cell Res. 1996;223:301–307. doi: 10.1006/excr.1996.0085. [DOI] [PubMed] [Google Scholar]
- Semsarian C., Wu M.J., Ju Y.K., Marciniec T., Yeoh T., Allen D.G., Harvey R.P., Graham R.M. Skeletal muscle hypertrophy is mediated by a Ca21-dependent calcineurin signalling pathway. Nature. 1999;400:576–581. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Control of myogenesis in vitro by Ca2+ concentration in nutritional medium. Exp. Cell Res. 1969;58:163–167. doi: 10.1016/0014-4827(69)90127-x. [DOI] [PubMed] [Google Scholar]
- Taigen T., De Windt L.J., Lim H.W., Molkentin J.D. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temm-Grove C.J., Wert D., Thompson V.F., Allen R.E., Goll D.E. Microinjection of calpastatin inhibits fusion in myoblasts. Exp. Cell Res. 1999;247:293–303. doi: 10.1006/excr.1998.4362. [DOI] [PubMed] [Google Scholar]
- Trajkovic V., Badovinac V., Jankovic V., Mostarica Stojkovic M. Cyclosporin A inhibits activation of inducible nitric oxide synthase in C6 glioma cell line. Brain Res. 1999;816:92–98. doi: 10.1016/s0006-8993(98)01130-5. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A.P., Blau H.M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988;52:503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Wright W.E., Binder M., Funk W. Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Mol. Cell. Biol. 1991;11:4104–4110. doi: 10.1128/mcb.11.8.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wong C., Liu D., Finegold M., Harper J.W., Elledge S.J. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]