Abstract
TGF-β inhibits adipocyte differentiation, yet is expressed by adipocytes. The function of TGF-β in adipogenesis, and its mechanism of action, is unknown. To address the role of TGF-β signaling in adipocyte differentiation, we characterized the expression of the TGF-β receptors, and the Smads which transmit or inhibit TGF-β signals, during adipogenesis in 3T3-F442A cells. We found that the cell-surface availability of TGF-β receptors strongly decreased as adipogenesis proceeds. Whereas mRNA levels for Smads 2, 3, and 4 were unchanged during differentiation, mRNA levels for Smads 6 and 7, which are known to inhibit TGF-β responses, decreased severely. Dominant negative interference with TGF-β receptor signaling, by stably expressing a truncated type II TGF-β receptor, enhanced differentiation and decreased growth. Stable overexpression of Smad2 or Smad3 inhibited differentiation and dominant negative inhibition of Smad3 function, but not Smad2 function, enhanced adipogenesis. Increased Smad6 and Smad7 levels blocked differentiation and enhanced TGF-β–induced responses. The inhibitory effect of Smad7 on adipocyte differentiation and its cooperation with TGF-β was associated with the C-domain of Smad7. Our results indicate that endogenous TGF-β signaling regulates the rate of adipogenesis, and that Smad2 and Smad3 have distinct functions in this endogenous control of differentiation. Smad6 and Smad7 act as negative regulators of adipogenesis and, even though known to inhibit TGF-β responses, enhance the effects of TGF-β on these cells.
Keywords: inhibitory Smads, PPARγ, C/EBP, 3T3-F442A, retroviral vectors
Introduction
TGF-β regulates the differentiation program of a variety of cell types (for review see Derynck and Choy 1998), including mesenchymal cells. For example, myogenesis is inhibited or stimulated by TGF-β depending on the cell type and the culture conditions. The addition of TGF-β generally inhibits myoblast differentiation (Massagué et al. 1986; Olson et al. 1986), but stimulates differentiation of embryonic myoblasts (Slager et al. 1993), whereas inhibition of type II TGF-β receptor signaling in C2C12 myoblasts blocks myogenic differentiation (Filvaroff et al. 1994). These data suggest that TGF-β signaling is required and provides competence for the early stages of myogenic differentiation, but inhibits later stages in the differentiation process. Such a model may also explain the many findings on stimulatory and inhibitory effects of TGF-β on chondrocyte and osteoblast differentiation, which likewise depend on culture conditions and the differentiation state of the cells (Kulyk et al. 1989; Ballock et al. 1993; Centrella et al. 1994). These dynamic responses of muscle and bone cells to TGF-β may relate to changes in receptor levels and ratios during differentiation (Hu and Olson 1990; Centrella et al. 1995), and may highlight the complexity of differentiation responses of mesenchymal cells to TGF-β.
While the effects of TGF-β signaling on muscle, bone, and cartilage cell differentiation are well studied, little is known about how TGF-β regulates adipocyte differentiation. Furthermore, the mechanism by which TGF-β stimulates proliferation of mesenchymal cells (Sporn et al. 1986), including preadipocytes (Jeoung et al. 1995), is also poorly understood. It is known that TGF-β inhibits adipose differentiation of preadipocyte cell lines and primary cultures (Ignotz and Massagué 1985; Sparks and Scott 1986; Torti et al. 1989; Petruschke et al. 1994). In some preadipocyte cell lines, TGF-β must be administered before, or concomitant with, the induction of differentiation, after which the cells are refractory to TGF-β and do not revert to the undifferentiated state (Ignotz and Massagué 1985; Sparks et al. 1992). However, TGF-β treatment can, in other cells, reverse the adipocyte phenotype (Torti et al. 1989) or reduce expression of differentiation markers in fully differentiated adipocytes (Petruschke et al. 1994). TGF-β also blocks adipogenesis in vivo. Transgenic overexpression of TGF-β1 in adipose tissue severely reduces both white and brown adipose tissue masses, and results in the failure of adipocytes to differentiate (Clouthier et al. 1997). In spite of its ability to inhibit adipocyte differentiation, TGF-β is expressed in cultured adipocytes (Sparks et al. 1993; Bortell et al. 1994), and adipose tissue (Samad et al. 1997). The relative increase of TGF-β levels in obese adipose tissue may result from increased expression of tumor necrosis factor α (Samad et al. 1999), another cytokine that, like TGF-β, inhibits adipocyte differentiation in vitro (Torti et al. 1985).
Collectively, these observations suggest a role for endogenous TGF-β in the development and function of adipose tissue. However, very little is known about the signaling mechanisms that lead to the differentiation responses to TGF-β. TGF-β family factors bind to a heteromeric cell-surface complex of two type II and two type I receptors (for reviews see Derynck and Feng 1997; Heldin et al. 1997; Massagué 1998). Both receptor types are transmembrane serine/threonine kinases, and ligand binding induces phosphorylation and activation of the type I receptors by the type II receptor kinases. The Smad proteins act as effectors of the signaling by activated TGF-β receptors. Upon COOH-terminal phosphorylation by type I TGF-β receptors, Smad2 and/or Smad3 heteromerize with the common partner Smad4 and are translocated into the nucleus (for reviews see Heldin et al. 1997; Derynck et al. 1998; Massagué 1998; Whitman 1998). Once there, they act as transcriptional coactivators or corepressors, through interactions with other transcription factors such as FAST-1/2 (Chen et al. 1997; Liu et al. 1999) c-Jun/c-Fos (Zhang et al. 1998), coactivators such as CBP (Feng et al. 1998; Janknecht et al. 1998; Topper et al. 1998), or corepressors such as TGIF (Wotton et al. 1999) or c-Ski (Stroschein et al. 1999; Sun et al. 1999). The TGF-β response can also be regulated by Smad6 and Smad7, which inhibit the TGF-β–induced activation of Smad2 and Smad3 (Hayashi et al. 1997; Imamura et al. 1997). The complexity of the TGF-β responses and their dependence on the physiological context and cell type, may relate to the relative levels of the different Smads, and their cooperativity with a variety of transcription factors, which themselves may be regulated by different signaling pathways (Zhang and Derynck 1999).
The expression and function of these components of the TGF-β signaling pathway during adipocyte differentiation, and the role of endogenous TGF-β expression and TGF-β responsiveness in the regulation of adipocyte differentiation, are unknown. It is known that the block in differentiation induced by TGF-β is accompanied by decreased mRNA levels for C/EBPα and PPARγ (Stephens et al. 1993; Xing et al. 1997), transcription factors which are critical for adipogenesis (Grégoire et al. 1998). However, the mechanism of this suppression is unknown, and, since these transcription factors are subject to positive autoregulation and positive cross-regulation (Tontonoz et al. 1994; Clarke et al. 1997; Tang et al. 1999), it is unclear whether this repression is a cause or an effect of blocked differentiation. To begin to elucidate the mechanism of TGF-β signaling in adipocyte differentiation, we have now characterized the expression of the TGF-β receptors and Smads during differentiation of the murine preadipocyte cell line, 3T3-F442A. To determine the role of these signaling mediators, we altered TGF-β receptor signaling by stably overexpressing a dominant negative mutant of the type II TGF-β receptor, thereby inhibiting the TGF-β responsiveness, or by overexpressing wild-type or dominant negative mutants of Smad2 or Smad3, wild-type Smad6 or Smad7, or Smad7 mutants. The consequences of these changes in signaling on adipogenesis strongly suggest that endogenous TGF-β responsiveness regulates adipocyte differentiation, and that Smad2 and Smad3, and the inhibitory Smad6 and 7, have distinct roles in the adipogenic differentiation process.
Materials and Methods
Construction of Expression Plasmids
Expression plasmids for the cytoplasmically truncated, dominant negative version of the type II TGF-β receptor (dnTβRII; Chen et al. 1993) or for Smad2 or Smad3 (Zhang et al. 1998) were described previously. The coding sequence for the Smad2ΔSSMS mutant was generated by removing the COOH-terminal sequence of Smad2 at the BamHI site, which is located 33 bp upstream of the stop codon, and replacing it with annealed oligonucleotides encoding the identical DNA sequence but lacking the last four codons. The cDNA for Smad3ΔSSVS was created by PCR amplification of the human Smad3 sequence without the last four codons, and was obtained from Y. Zhang (University of California, San Francisco, San Francisco, CA). The cDNAs for mouse Smad6 and mouse Smad7 were obtained from K. Miyazono (The Cancer Institute of Japanese Foundation for Cancer Research, Tokyo, Japan) and P. ten Dijke (Ludwig Institute for Cancer Research, Uppsala, Sweden), respectively. Mutant versions of mouse Smad7 containing a 19–amino acid deletion at the COOH terminus (Smad7ΔC) or consisting only of the C-domain, amino acids 204–426 (Smad7C), were obtained from P. ten Dijke.
Retroviral vectors were used to establish stable cell populations overexpressing components of the TGF-β signaling pathway. The coding sequence for the dominant negative type II TGF-β receptor, along with its COOH-terminal Flag tag, was subcloned into the HpaI site of the LNCX vector, which allows selection in the neomycin derivative G418 (Miller and Rosman 1989). Smad2 and Smad3 and their dominant negative mutants were expressed from a new derivative of LNCX, which we named LPCX, and allows for selection in puromycin instead of G418. This plasmid was made by replacing the neor gene of LNCX with the puromycin resistance gene from pBabepuro (Morgenstern and Land 1990). The coding regions for NH2 terminally Flag-tagged Smad2, Smad2ΔSSMS, Smad3, and Smad3ΔSSVS were inserted into the HpaI site of LPCX. The coding regions for the NH2 terminally Flag-tagged Smad6, Smad7ΔC, and Smad7C were cloned into the BamHI-XhoI sites of pBabepuro3, whereas the coding region of Smad7 were cloned into the BamHI/blunt-ended EcoRI site of pBabepuro3.
Cell Culture and Generation of Stable Cell Lines
The preadipocyte cell line 3T3-F442A (Green and Kehinde 1976) was obtained from H. Green (Harvard Medical School, Boston, MA). The cells were grown and differentiated as described (Dobson et al. 1987), except that incubators were set at 5% rather than 10% CO2. To study differentiation in the presence of TGF-β, TGF-β was added to the medium 2 d before confluence, and again at every medium change (three times per week). The ecotropic retroviral packaging cell line Phoenix E was obtained from G. Nolan (Stanford University, Stanford, CA), and maintained in DME with 3 g/liter glucose (Cellgro), 10% FBS (Hyclone Laboratories Inc.), 10 U/ml penicillin, and 10 μg/ml streptomycin.
To generate retroviruses, Phoenix E cells were plated at 2.7 × 106 cells/60-mm tissue culture dish 24 h before transfection. Transfection was done using the calcium phosphate method (Gorman et al. 1983), using 10 μg DNA per plate. 48 h after transfection, the conditioned medium containing recombinant retroviruses was collected and filtered through 0.45-μm sterilization filters. 1–1.3 ml of these supernatants were applied immediately to 3T3-F442A cells, which had been plated 18 h before infection at a density of 5.1 × 104 cells/well of 6-well dishes. Polybrene (Sigma Chemical Co.) was added to a final concentration of 8 μg/ml, and the supernatants were incubated with the cells for 3–5 h. Alternatively, cells overlaid with viral supernatants plus polybrene were centrifuged for 45 min at room temperature at 1,800 rpm in a Beckman GPR centrifuge. The medium was aspirated and replaced with fresh viral supernatant, and the procedure was repeated. After infection, the cells were placed in fresh growth medium and cultured as usual. Selection with 1 mg/ml G418 (Life Technologies, Inc.) or 2 μg/ml puromycin (Calbiochem) was initiated 48 h after infection.
Assay of Cell Growth Rates
Cells were trypsinized, resuspended in growth medium without selective antibiotic, and 2 × 104 cells were plated per well of 24-well dishes. The cells were washed twice with PBS on the following day, and then overlaid with DME containing 0.5% BSA (Sigma Chemical Co.) either without or with the indicated concentrations of TGF-β. The next day, [3H]thymidine (2 Ci/mmol; NEN) was added to the medium at a concentration of 4 μCi/ml. Uptake of the label proceeded for 4–5 h. Cells were washed twice with PBS, fixed for 20 min with 10% TCA, washed twice with water, and solubilized for 20 min in 1 N NaOH. An equal volume of 1 N HCl was added, and the resulting lysate was subjected to liquid scintillation counting.
Analysis of Lipid Accumulation, RNA, and Protein
Neutral lipid accumulation was visualized by washing cell monolayers once with PBS, fixing for 15 min with buffered formalin, and staining them for 1 h in a freshly made solution containing four parts water mixed with six parts 0.5% Oil Red O (Sigma Chemical Co.) in isopropanol. Excess stain was removed, and the cells were washed several times with water.
RNA was isolated using the SV RNA isolation kit (Promega). 10 μg total RNA was denatured and electrophoresed in 1% formaldehyde gels, blotted to Biotrans nylon membranes (ICN), and hybridized to 32P-labeled cDNA probes, as described previously (Ausubel et al. 1994). DNA probes were labeled to a specific activity of at least 109 dpm/μg with [32P]α-dCTP (6,000 Ci/mmol; NEN) by the random priming method (Feinberg and Vogelstein 1983). The cDNAs for C/EBPα, PPARγ2, ADD-1/SREBP1, aP2 and adipsin were obtained from B. Spiegelman (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA). The cDNAs for C/EBPs β and δ were provided by S. McKnight (University of Texas, Southwestern Medical Center, Dallas, TX).
125I-labeled TGF-β1 was purchased from NEN. Cross-linking to [125I]TGF-β was performed as described (Gazit et al. 1993). Mild acetic acid washes and suramin (Calbiochem) treatment of cell monolayers, to remove any potentially endogenously produced bound TGF-β, were done as previously described (Beauchamp et al. 1992; Wade et al. 1992). Cell lysates were prepared as previously described (Chen and Derynck 1994). For analysis of endogenous receptors, equal amounts of protein (400 μg of each sample) were loaded in each lane. For analysis of receptor expression in stable dnTβRII expressing and control cells, 20% of the total lysates was reserved for direct loading on the gel, while the remaining 80% was subjected to immunoprecipitation with anti-FlagM2 IgG (Sigma Chemical Co.) as described previously (Chen and Derynck 1994). For analysis of Smad expression in the Smad-expressing stable cell lines, Flag-tagged proteins were detected in whole cell lysates by immunoprecipitation using anti-FlagM2–conjugated beads (Sigma Chemical Co.), followed by SDS-PAGE and Western blotting, using the enhanced chemiluminescence method (Amersham Pharmacia Biotech) with anti-FlagM2 IgG as the primary antibody.
Results
Cell-Surface TGF-β Receptors Decrease during Adipogenesis
3T3-F442A cells spontaneously undergo adipose differentiation, after reaching confluence in culture in the presence of FBS and insulin (Green and Kehinde 1976). Typically, 5–10% of the cells have accumulated lipid droplets at 1 d after confluence, 30–40% by 3 d, and by 8 d after confluence, ∼90% of the cells have become adipocytes. To examine the role of endogenous TGF-β signaling in adipogenesis, we first compared the mRNA expression levels for the type II and the type I TGF-β receptor (TβRII and TβRI, respectively) in confluent preadipocytes versus differentiated adipocytes (Fig. 1 A). Both the preadipocytes and the adipocytes expressed TβRII and TβRI mRNA. Whereas the TβRII mRNA level was similar in preadipocytes and adipocytes, TβRI mRNA levels increased in differentiated cells.
Figure 1.
TGF-β receptor expression during adipocyte differentiation of 3T3-F442A cells. (A) Northern analysis of TβRII and TβRI mRNA expression on 2 μg of poly(A)+ RNA from preadipocytes (pread) or 8-d after confluent adipocytes (ad). Levels of C/EBPβ mRNA are shown as a loading control. (B) Cell-surface availability of TGF-β receptors, as assessed by chemical cross-linking to [125I]TGF-β, decreases during differentiation. TGF-β binding was assessed in confluent preadipocytes (pread) and at 1, 3, and 10 d after confluence. The approximate positions of type III (RIII), type II (RII), and type I (RI) receptors are indicated.
The expression of TβRII and TβRI at the cell surface was assessed by cross-linking 125I-labeled TGF-β to the cells (Fig. 1 B). While preadipocytes expressed type III TGF-β receptor (βglycan), TβRII, and TβRI at the cell surface, the availability of these receptors strongly decreased during differentiation. These decreased levels were not a consequence of receptor occupation by unlabeled TGF-β preventing [125I]TGF-β binding since removal of endogenous ligand by suramin or acid washes did not enhance receptor binding of [125I]TGF-β (data not shown). The total expression levels of the receptors could not be determined because of the low expression levels and limited quality of the available antibodies (data not shown). The strong decrease in cell-surface availability of the receptors during adipose differentiation, in contrast to the mRNA levels for TβRII and TβRI, indicates that the cells posttranscriptionally downregulate TGF-β binding as they differentiate.
Regulation of Smad Expression during Adipocyte Differentiation
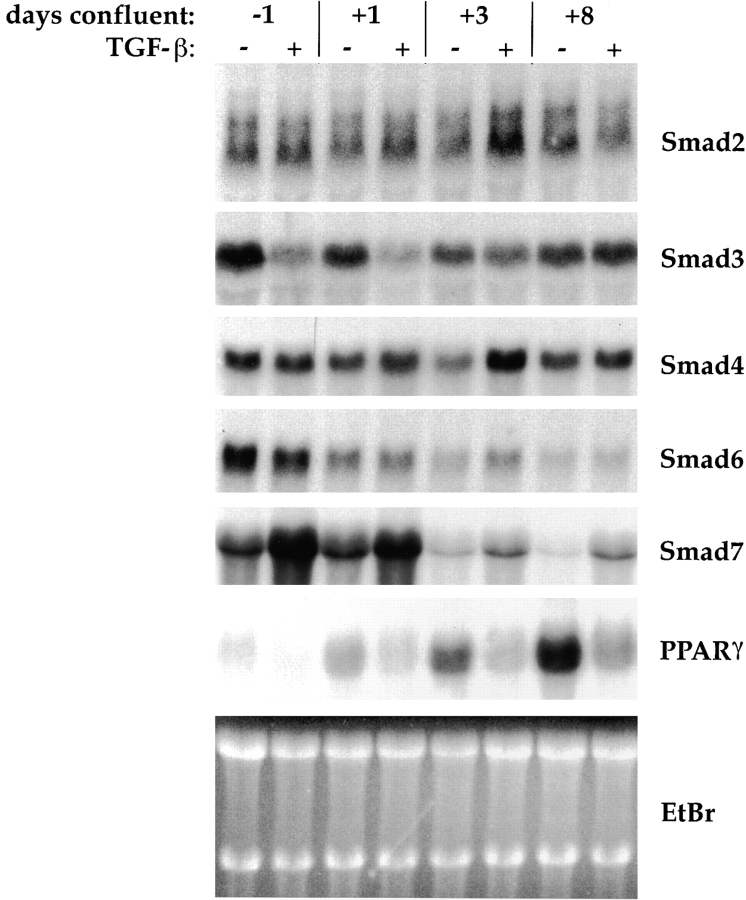
Smads act as intracellular effectors of TGF-β signaling. Smad2 and Smad3 are activators of TGF-β responses, and form heteromeric complexes with the common mediator Smad4. In contrast, Smad6 and Smad7 block TGF-β responses. We assessed the mRNA expression of these Smads at different stages of adipose differentiation, both in TGF-β–treated and untreated cells (Fig. 2). In these experiments, the TGF-β–treated cells failed to differentiate, as judged from the absence of lipid accumulation, although analysis of PPARγ mRNA expression indicated that differentiation was not entirely blocked, but strongly decreased and delayed. Thus, PPARγ expression increased with time in TGF-β–treated cells, but never achieved the expression level seen in untreated cells (Fig. 2). All five Smads were expressed at all stages of differentiation of these cells. The Smad2 or Smad4 mRNA levels were unaffected by the differentiation stage or by TGF-β, whereas Smad3 mRNA levels did not significantly change during differentiation, but were decreased by TGF-β. This TGF-β–induced repression decreased with the progression of adipose differentiation. Both Smad6 and Smad7 mRNAs decreased during differentiation, with Smad7 mRNA levels decreasing more abruptly than Smad6. In addition, TGF-β increased Smad7 expression, but did not have a significant effect on Smad6 mRNA levels. We were unable to clearly detect endogenous Smad proteins, most likely because of poor antibody quality and/or low protein abundance (data not shown). Collectively, our data show that all TGF-β Smads are expressed in 3T3-F442A cells, and that expression of the inhibitory Smads decreases during adipogenesis.
Figure 2.
Northern analysis of Smad mRNA expression during differentiation and in response to TGF-β treatment. Cells were allowed to differentiate without added TGF-β, or in the presence of 5 ng/ml TGF-β beginning at 2 d preconfluence, and RNA was harvested at the days indicated. 10 μg total RNA was blotted and sequentially hybridized to cDNA probes for Smad2, Smad3, Smad4, Smad6, and Smad7, and for PPARγ (as marker for adipogenic conversion), as indicated. Ethidium bromide staining of the gel (EtBr) is shown as a loading control. All autoradiograms were exposed for 24 h except for PPARγ, which was exposed for 8 h.
Decreased TGF-β Receptor Signaling Results in Accelerated Adipogenesis
Since the levels of cell-surface TGF-β receptors and inhibitory Smads decreased during adipocyte differentiation, we determined the role of endogenous TGF-β signaling and Smads in the growth and differentiation of these cells. We created cell lines, via retroviral transduction, which stably expressed individual components of the TGF-β signaling system. First, we attempted to generate cells that would stably overexpress wild-type TβRII, to counteract the differentiation-linked suppression of cell-surface TGF-β receptors. However, the virally expressed TβRII levels, while not decreased during differentiation as the endogenous receptors (data not shown), were too low to significantly change the overall receptor level or profile during differentiation. In contrast, we successfully generated cells that expressed a cytoplasmically truncated TβRII (dnTβRII), which acts as a dominant negative inhibitor of TGF-β signaling (Chen et al. 1993). Similar to what we observed for wild-type TβRII, the expression of dnTβRII was lower than the endogenous TβRII level. Whereas the dnTβRII protein could not be convincingly detected in metabolically labeled lysates (data not shown), it could be detected in undifferentiated and differentiated dnTβRII cells by cross-linking [125I]TGF-β to the cell-surface receptors (Fig. 3A and Fig. B). The low dnTβRII levels, when compared with endogenous TβRII, suggested that the TGF-β response in the dnTβRII cells would only be partially inhibited.
Figure 3.
Expression of cytoplasmically truncated dnTβRII in stably infected preadipocytes (A) and adipocytes (B). Cell-surface availability of TGF-β receptors was assessed by binding and chemical cross-linking to [125I]TGF-β. The first two lanes of each panel contain samples of total lysates, whereas the last two lanes contain immunoprecipitations using anti-Flag antibody, which recognizes the Flag epitope tag of dnTβRII. Positions of TβRII (RII), TβRI (RI), and dnTβRII are indicated. Endogenous TβRI coprecipitated with the dnTβRII, as expected. The mobility of the receptors was slightly different in total lysates versus immunoprecipitates because of differing salt conditions and/or the presence of IgG in the immunoprecipitates. Exposure times were identical for all panels.
Using these cells, we evaluated the effect of dnTβRII expression on adipose differentiation, and in parallel, tested the effect of TGF-β at 1 ng/ml, added 2 d before confluence and continuously present thereafter. At this dose, TGF-β confers incomplete inhibition of adipose conversion (Ignotz and Massagué 1985; Choy, L., unpublished data), which should enable us to detect the effects of enhancement or inhibition of TGF-β signaling. By 8 d after confluence, the dnTβRII cells differentiated similarly to the parental cells in the absence of added TGF-β, but were partially resistant to the differentiation-inhibiting effect of TGF-β, as judged by neutral lipid staining (Fig. 4 A). Northern analysis of adipocyte differentiation markers was performed at various points during differentiation. We analyzed the expression of the five transcription factors that are known to play important roles in adipogenesis (Grégoire et al. 1998), C/EBPβ, C/EBPδ, ADD-1, PPARγ, and C/EBPα, as well as the later markers aP2 and adipsin. This study indicated that, while the extent of differentiation of dnTβRII and control cells was similar at day 8 after confluence, the rate of differentiation of dnTβRII cells was enhanced when compared with the parental cells (Fig. 4 B). Thus, at day 3 after confluence, the mRNA levels for PPARγ, C/EBPα, aP2, and adipsin were significantly higher in dnTβRII cells than in control cells. In contrast, the mRNA levels of C/EBPβ, C/EBPδ, and ADD-1, earlier markers of adipogenic differentiation, were similar in both cell lines. The increased mRNA levels for PPARγ, C/EBPα, aP2, and adipsin in dnTβRII cells are consistent with the ability of TGF-β to repress expression of these markers (Torti et al. 1989; Stephens et al. 1993; Xing et al. 1997) and suggest that endogenous TGF-β responsiveness regulates adipocyte differentiation. Accordingly, TGF-β–treated control cells at day 8 after confluence exhibited decreased expression of these four later markers, as well as ADD-1, although they expressed C/EBPβ and C/EBPδ at similar levels as untreated control cells. Consistent with their impaired TGF-β responsiveness, TGF-β–treated dnTβRII cells showed only slight inhibition of the later differentiation markers.
Figure 4.

Effects of expression of dnTβRII on adipogenesis and cell proliferation. (A) Adipogenesis of control (vector) and dnTβRII cells was assessed by the extent of lipid accumulation, visualized by Oil Red O staining after 8 d in differentiation medium, either without or with 1 ng/ml TGF-β. (B) Northern analysis of adipocyte marker mRNA expression. Vector control or dnTβRII cells, grown in differentiation medium without or with 1 ng/ml TGF-β, as indicated, were harvested at the indicated days after confluence. 10 μg total RNA was blotted and sequentially hybridized to the cDNA probes as shown. Ethidium bromide staining of the gel (EtBr) is shown to illustrate the similar RNA loading of the lanes. (C) Proliferation rate of vector control and dnTβRII cells, as assessed by the incorporation of [3H]thymidine, either without treatment or in response to 5 ng/ml TGF-β. Values are expressed as fold induction relative to the untreated vector control.
TGF-β stimulates the proliferation of many types of mesenchymal cells, including 3T3-F442A preadipocytes (Jeoung et al. 1995). Therefore, we compared the growth rates of control preadipocyte cells and dnTβRII cells, in the absence or presence of added TGF-β. Growth was assayed by [3H]thymidine incorporation, a measure of DNA synthesis, under subconfluent conditions. In the absence of added TGF-β, the growth rate of dnTβRII cells was 25–30% lower than that of the control cells, and in the presence of TGF-β, the mitogenic response of dnTβRII cells was only half of that of the control cells (Fig. 4 C). These results show that, despite the modest dnTβRII expression level, the TGF-β response was significantly affected. Collectively, these results show that the TGF-β responsiveness regulates adipocyte differentiation in culture, and that impaired TGF-β responsiveness results in accelerated differentiation and slower growth.
Smad2 and Smad3 Differentially Regulate Adipogenesis and Cell Proliferation
To characterize the roles of Smad2 and Smad3 in adipocyte differentiation of 3T3-F442A cells, we generated two pairs of cell lines. One pair overexpressed wild-type Smad2 or Smad3, while the complementary pair expressed mutant versions of Smad2 (Smad2ΔSSMS) or Smad3 (Smad3ΔSSVS). These mutants lacked the last four amino acids, including the COOH-terminal serines, and, thus, cannot be phosphorylated by the activated TβRI. Similarly to the 3SA derivatives of Smad2 and Smad3, in which the last three serines are mutated to alanine (Macias-Silva et al. 1996; Liu et al. 1997), overexpression of Smad2 ΔSSMS or Smad3ΔSSVS resulted in dominant negative interference of signaling by Smad2 or Smad3 in transfection/reporter assays (data not shown). Expression of wild-type and mutant Smad2 or Smad3 proteins could be readily detected in the stable cell populations (Fig. 5 A). While we were unable to clearly detect endogenous Smad proteins, the viral transcripts were expressed at a >100-fold higher level than the endogenous Smad2 and Smad3 mRNAs (data not shown).
Figure 5.

Effect of Smad2, Smad3, or their dominant negative mutants on adipogenesis. (A) Expression of Flag-tagged Smads in their respective stable cell lines. Whole cell lysates were immunoprecipitated using anti-Flag–conjugated beads, followed by Western blotting with anti-Flag antibody. Position of background IgG is indicated. (B and C) The effect of overexpression of Smad2, Smad3, or their dominant negative mutants on lipid accumulation in adipogenic differentiation. Untreated cells (B) and cells treated with 1 ng/ml (C) at 8 d after confluence are shown.
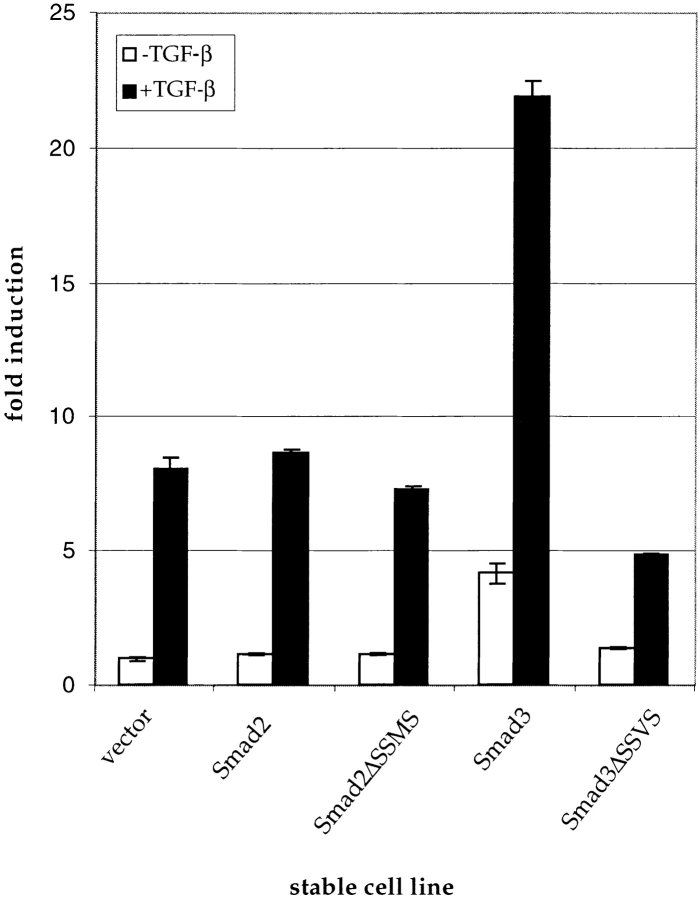
We tested the abilities of these stable cell lines to undergo adipocyte differentiation and the ability of TGF-β to inhibit their differentiation. Expression of either Smad2 or Smad2ΔSSMS inhibited lipid accumulation, although the effect of Smad2ΔSSMS was weaker than Smad2 (Fig. 5 B). Enhanced Smad3 expression resulted in a stronger inhibitory effect than Smad2 overexpression, while cells expressing Smad3ΔSSVS differentiated similarly to vector control cells (Fig. 5 B). In the presence of 1 ng/ml TGF-β, Smad2 or Smad3 expression both significantly augmented the differentiation-inhibiting effect of TGF-β (Fig. 5 C). However, the effect of Smad3 was stronger than Smad2; no lipid-accumulating cells were found in the Smad3-expressing cultures, whereas a few could be seen among the cells overexpressing Smad2. Cells expressing Smad3 ΔSSVS differentiated to a much greater extent than control cells in the presence of TGF-β, demonstrating that this Smad3 mutant blocked the differentiation-inhibitory effect of TGF-β. In contrast, expression of Smad2ΔSSMS did not block this effect of TGF-β, and slightly enhanced the inhibitory effect of TGF-β (Fig. 5 C).
The effect of these Smads on lipid accumulation was reflected in the expression pattern of adipocyte differentiation mRNAs (Fig. 6 A). The induction of PPARγ, ADD-1, C/EBPα, aP2, and adipsin was strongly reduced in cells with increased Smad3 expression, whereas the differentiation-dependent suppression of C/EBPδ was abrogated. Smad2 or Smad2ΔSSMS expression resulted in only a slight decrease in the differentiation-dependent induction of the differentiation markers, whereas Smad3ΔSSVS expression did not significantly alter their expression. In the presence of TGF-β, the Smad3ΔSSVS cells expressed PPARγ, C/EBPα, aP2, and adipsin at higher levels than in TGF-β–treated control cells, suggesting that impaired Smad3 signaling suppresses the responsiveness to TGF-β. In contrast, Smad2ΔSSMS expression enhanced the TGF-β–mediated suppression of these differentiation markers, although to a lesser extent than in cells overexpressing Smad2 or Smad3.
Figure 6.
Effect of Smad2, Smad3, or their dominant negative mutants on differentiation-dependent gene expression and proliferation in the absence and presence of TGF-β. (top) Northern analysis of adipocyte marker gene expression. Total RNA from stable cell lines was isolated on the indicated days and processed as in Fig. 4 B. −TGF-β lanes: Smad2Δ, Smad2ΔSSVS, Smad3Δ, and Smad3ΔSSVS. All RNA samples of cells without TGF-β treatment were on the same membrane, but the autoradiogram in the figure has been cut into panels for easier viewing. +TGF-β lanes: v, vector; 2, Smad2; 2Δ, Smad2ΔSSMS; 3, Smad3; and 3Δ, Smad3ΔSSVS. (bottom) Proliferation rate of vector control cells and cells overexpressing Smad2, Smad3, Smad2ΔSSMS, or Smad3ΔSSVS are as shown. Cells were either untreated or treated with 5 ng/ml TGF-β, and the level of [3H]thymidine incorporation was measured. Values are expressed as fold induction relative to the untreated vector control.
We also measured the effect of overexpression of Smad2 or Smad3 or their dominant negative mutants on the growth rate (Fig. 6 B). Expression of Smad2 or Smad2 ΔSSMS had little if any effect on cell proliferation or its stimulation by TGF-β. In contrast, Smad3 overexpression stimulated cell growth about threefold, both in the presence or absence of TGF-β. Expression of Smad3ΔSSVS inhibited the growth rate in the presence of TGF-β, and was ∼30% less than the vector alone (Fig. 6 B).
Finally, we evaluated the effect of TGF-β on the morphology of these cell lines (Fig. 7). Adipogenic conversion of control cells in the absence of TGF-β resulted in rounding, accumulation of lipid droplets, and a consequent enlargement of the cells, whereas nearby cells that had not yet undergone differentiation maintained a fibroblastic appearance (Fig. 7 A). TGF-β treatment prevented cell rounding and lipid accumulation and conferred a more densely packed, spindly morphology to the cells (Fig. 7 B). Increased expression of Smad2 (Fig. 7 C) or Smad3 (Fig. 7 D) enhanced these typical TGF-β–induced morphological changes; however, the effect of Smad3 was stronger than that of Smad2, which is consistent with the milder effects of Smad2 versus Smad3 on TGF-β–induced inhibition of lipid accumulation (Fig. 5 C). Expression of Smad2ΔSSMS conferred a morphology intermediate between the vector control and Smad2 overexpressing cells (Fig. 7 E). In contrast, Smad3ΔSSVS expression counteracted the cell shape changes induced by TGF-β and allowed a level of lipid accumulation (Fig. 7 F), which approached that of control cells in the absence of TGF-β (Fig. 7 A). In the absence of TGF-β, the morphology of the different Smad-expressing cell lines was similar to each other, except that the Smad3-expressing cells were somewhat more spindly and densely packed (data not shown), which is consistent with a constitutive level of TGF-β signaling. These results identify Smad3 as a major regulator of adipogenic differentiation and of TGF-β–mediated inhibition of adipogenic conversion and growth stimulation. The Smad2 effects appear to be more complex and may reflect its involvement in only some TGF-β effects, yet suggest that Smad2 is also an important regulator of normal adipocyte differentiation.
Figure 7.

Morphology of cells overexpressing Smad2 or Smad3 or their dominant negative mutants. Except for A, all cells were treated with 1 ng/ml TGF-β. (A) Vector control cells, untreated; (B) vector control cells; (C) Smad2-expressing cells; (D) Smad3 expressing cells; (E) Smad2ΔSSMS-expressing cells; and (F) Smad3 ΔSSVS-expressing cells.
Overexpression of Smad6 or Smad7 Blocks Adipogenesis and Enhances TGF-β–induced Growth and Inhibition of Differentiation
In contrast to the roles of Smad2 and Smad3 as TGF-β signaling effectors, Smad6 and Smad7 inhibit signaling by TGF-β family members. We hypothesized that the overexpression of these proteins might result in a more complete blockade of TGF-β signaling than expression of dominant negative Smad2 or 3 mutants expressed alone, or of dnTβRII. Furthermore, the downregulation of both Smad6 and Smad7 during adipocyte differentiation (Fig. 2) suggested a function for these Smads in the differentiation process. Therefore, we evaluated the consequences of stable overexpression of these two inhibitory Smads. Retroviral expression of Smad6 exceeded by far the expression level of Smad7 (Fig. 8 A). Expression of the viral mRNAs for these Smads was much higher than the corresponding endogenous Smad transcripts (data not shown).
Figure 8.

Generation and differentiation of Smad6 or Smad7 overexpressing cell lines. (A) Whole cell lysates of vector control, Smad6, or Smad7 expressing cells were analyzed by anti-Flag immunoprecipitation and anti-Flag Western blotting. (B) Lipid accumulation in Smad6 and Smad7 overexpressing cell lines. Vector control and Smad6 or Smad7-overexpressing cell lines were grown under differentiation conditions in the absence or presence of 1 ng/ml TGF-β. At 8 d after confluence, the cells were fixed and stained with Oil Red O.
These cells were cultured under differentiation conditions, in the presence or absence of TGF-β, to assess the effects of Smad6 or Smad7 on adipogenic conversion, and on the ability of TGF-β to block differentiation. Expression of either Smad protein resulted in a profound blockage of lipid accumulation (Fig. 8 B). Despite its lower expression level (Fig. 8 A), the differentiation blockage induced by Smad7 was greater than that induced by Smad6. In the presence of TGF-β, this inhibition was even more complete, and no adipocytes could be found in Smad7 overexpressing cell cultures treated with TGF-β. These effects of Smad6 and Smad7 on adipose conversion were also apparent in the analysis of adipocyte marker expression (Fig. 9 A). While C/EBPβ levels were not strongly affected, Smad6 or Smad7 overexpression increased the levels of C/EBPδ, which is normally downregulated during differentiation. These cells also showed strongly reduced PPARγ, C/EBPα, aP2, and adipsin mRNAs, and a moderately reduced ADD-1 mRNA level. In the presence of TGF-β, all the TGF-β–induced changes in mRNA expression were enhanced by overexpression of Smad6 or Smad7.
Figure 9.
Analysis of differentiation marker expression and proliferation in Smad6- and Smad7-overexpressing cells. (top) Northern analysis of adipocyte marker gene expression. Total RNA was isolated on the indicated days from vector control and Smad6- or Smad7-expressing cells and processed as in Fig. 4 B. All RNA samples were on the same membrane, but the autoradiogram in the figure has been cut into panels for easier viewing. (bottom) Cell proliferation in the absence or presence of TGF-β. TGF-β was added to 5 ng/ml, and [3H]thymidine incorporation was measured. Values are expressed as fold induction relative to the untreated vector control.
We next tested the effect of Smad6 or Smad7 overexpression on cell proliferation in the absence or presence of TGF-β. Smad6 or Smad7 overexpression both greatly potentiated TGF-β–induced growth stimulation (Fig. 9 B) to a similar magnitude as Smad3 overexpression (Fig. 6 B). However, in contrast to the effect of Smad3 overexpression, the basal growth rate in the absence of added TGF-β was unchanged. Therefore, we conclude that Smad6 or Smad7 expression cooperates with TGF-β to enhance cell proliferation, but not in the same manner as Smad3.
The Smad6- or Smad7-expressing cells had an unusual morphology. When subconfluent, the cells were overall similar in size, but the Smad6 and Smad7 cells had a more cuboidal appearance and smoother edges, and seemed more contact-inhibited than control cells (Fig. 10 A). After reaching confluence under conditions that normally result in adipocyte differentiation, Smad6 and especially Smad7-overexpressing cells became very large and flat, in contrast to the compact piles of rounded, lipid-filled cells in control cultures (Fig. 10 B). The Smad7-overexpressing cells were generally larger and flatter than the Smad6-expressing cells, and had a higher incidence of giant cells, which were about fivefold larger than the surrounding cells (Fig. 10 D). The few adipocytes that did appear in the Smad6 or Smad7 cell cultures were very small in comparison to control adipocytes, and did not accumulate much lipid (Fig. 10 B, arrow). In the presence of TGF-β, all cell lines had a similar morphology, with the exception of the Smad6 or Smad7 cell cultures, which contained a few of the remnant larger cells (Fig. 10 C), and appeared larger overall.
Figure 10.

Morphology of vector control cells versus Smad6- and Smad7-overexpressing cells. (A) Cells during active growth phase. (B) 4 d after confluence in differentiation medium. [Arrowhead in Smad6 panel indicates cluster of small adipocytes.] (C) 4 d after confluence in differentiation medium with 1 ng/ml TGF-β. (D) Same as B except that an example of giant Smad7 cells is shown.
To identify the domain of Smad7 that is required for its effects on adipogenesis, we evaluated the effects of expression of two different Smad7 mutants. The Smad7ΔC mutant has a 19–amino acid deletion at the COOH terminus, a mutation which abrogates the ability of Smad7 to block TGF-β receptor signaling (Hayashi et al. 1997). Conversely, expression of only the C-domain of Smad7, i.e., the Smad7C protein, is sufficient to block TGF-β receptor signaling similarly to full-length Smad7 (Souchelnytskyi et al. 1998). We stably expressed these mutant Smad7 proteins in 3T3-F442A cells, at levels similar to wild-type Smad7 (Fig. 11 A). The Smad7ΔC expressing cells accumulated lipid similarly as the vector control cells, whereas lipid accumulation was blocked in the Smad7C expressing cells (Fig. 11 B). Differentiation, as assessed by expression of PPARγ mRNA, also indicated that Smad7C, but not Smad7ΔC, decreased differentiation (Fig. 11 C). Staining and PPARγ mRNA expression also revealed that Smad7C, but not Smad7ΔC, cooperated with TGF-β in blocking differentiation (Fig. 11B and Fig. C). Smad7C, but not Smad7ΔC, also synergized with TGF-β in stimulating DNA synthesis (Fig. 11 D). Finally, the phenotype of the Smad7C cells resembled that of cells expressing wild-type Smad7, whereas Smad7ΔC-expressing cells resembled vector control cells (Fig. 11 E). While Smad7C was qualitatively identical to wild-type Smad7 in our assays, it was quantitatively a weaker effector in comparison to full-length Smad7.
Figure 11.


Effect of expression of Smad7ΔC versus Smad7C on differentiation, proliferation, and cell morphology. (A) Expression of Smad7C and Smad7ΔC in stable 3T3-F442A cell lines, as determined by Flag immunoprecipitation and Western blotting. The expression of wild-type Smad7 in the corresponding stable 373-F442A cell line is shown alongside to enable a comparison of the expression levels. (B) Lipid accumulation in Smad 7ΔC- and Smad7C-expressing cells. Vector control and Smad7ΔC- or Smad7C-expressing cell lines were grown as in Fig. 8 B, and stained with Oil Red O. (C) Northern analysis of PPARγ expression in Smad7ΔC- and Smad7C-expressing cells. Total RNA was isolated on the days indicated, blotted, and hybridized as in Fig. 4 B. (D) Cell proliferation in the absence or presence of TGF-β. Cells were untreated, or TGF-β was added to 5 ng/ml, and [3H]thymidine incorporation was measured. Values are expressed as fold induction relative to the untreated vector control. (E) Cell morphology of Smad7ΔC- and Smad7C-expressing cells at 4 d after confluence in differentiation medium without (top row) or with (bottom row) 1 ng/ml TGF-β.
Our results indicate that Smad6 and Smad7 enhance, rather than inhibit, the effects of TGF-β signaling on adipogenic differentiation and cell proliferation. The C-domain of Smad7, which has been shown to be required for inhibition of TGF-β signaling in other experimental systems, is necessary and sufficient for its effects on adipocytic differentiation in 3T3-F442A cells.
Discussion
The ability of TGF-β and TGF-β–related factors to regulate mesenchymal differentiation is well documented. Nevertheless, little is known about the role of endogenous signaling by TGF-β, or its related factors, or the Smads, in cell differentiation. In this study, we have investigated the functions of endogenous TGF-β and Smad signaling in adipocyte differentiation, using a well-known model system for adipocyte differentiation. While exogenous TGF-β is known to inhibit adipocyte differentiation, TGF-β also has been shown to be endogenously produced by adipocytes. Furthermore, preadipocytes secrete and activate TGF-β, whereas mature adipocytes do not (Rahimi et al. 1998). These observations suggested a role for autocrine TGF-β signaling in adipocyte differentiation. We now show that endogenous TGF-β receptor signaling is regulated during adipocyte differentiation, that autocrine TGF-β signaling modulates differentiation, and that Smad2 and Smad3 have distinct roles in the differentiation of these cells. Finally, our results indicate that Smad6 and Smad7 act as negative regulators of adipocyte differentiation, and enhance, rather than inhibit, TGF-β signaling in these cells.
Regulation and the Role of TGF-β Receptor Signaling in Adipogenesis
To characterize the potential role of autocrine TGF-β in adipose differentiation, we assessed the availability of receptors for TGF-β binding during differentiation. We found that the cell-surface expression of the TGF-β receptors is strongly downregulated during adipose differentiation. Our results are in accordance with the strong decrease in ligand binding during differentiation of primary rat adipocytes (Serrero and Mills 1991) and of HIB-1B brown adipocytes (data not shown), but contrast with the apparent lack of repression of TGF-β binding in differentiating 3T3-L1 preadipocytes (Ignotz and Massagué 1985; data not shown). Since TGF-β inhibits adipocyte differentiation, the repression of TGF-β receptor availability may enable the cells to withdraw from the autocrine, differentiation inhibitory activity of TGF-β. This downregulation may also explain why cells become refractory to the differentiation-inhibitory effect of TGF-β once differentiation has commenced. Accordingly, TGF-β did not inhibit 3T3-F442A adipose conversion at 3–4 d after confluence, by which time there is a significant loss of TGF-β binding (data not shown).
The downregulation of cell-surface TGF-β receptors in 3T3-F442A adipocytes is reminiscent of observations in other types of mesenchymal differentiation. Differentiation of myoblasts into myocytes is accompanied by a strong decrease in TGF-β binding sites at the cell surface (Hu and Olson 1990), whereas the availability of type II but not type I TGF-β receptors is downregulated during osteoblastic differentiation (Centrella et al. 1995). These observations suggest that autocrine TGF-β signaling controls mesenchymal cell differentiation, and that these cells alter their autocrine TGF-β response during differentiation to allow for efficient maturation. Other mechanisms in addition to receptor availability may also be involved; e.g., 3T3-L1 adipocytes differentiate without repressing TGF-β binding (Ignotz and Massagué 1985), but differentiation is accompanied by a block in activation of autocrine latent TGF-β (Rahimi et al. 1998).
The differentiation-inhibiting role of autocrine TGF-β in adipogenesis was confirmed in cells expressing a dominant negative version of the type II TGF-β receptor. Even though we did not obtain high levels of dnTβRII expression, the cells showed a decreased response to TGF-β. The dnTβRII cells had accelerated differentiation, suggesting that autocrine TGF-β responsiveness regulates adipose conversion. These observations suggest that decreased receptor availability during adipogenesis allows these cells to escape the autocrine differentiation-inhibitory and growth-promoting roles of TGF-β. The observation that retrovirally expressed cell-surface wild-type or dnTβRII were not decreased during differentiation, whereas endogenous cell-surface TβRII was decreased without a decrease in mRNA levels, suggests that the differentiation-dependent decrease in receptor availability results from translational repression.
Regulation and the Roles of Smad2 and Smad3 in Adipogenesis
Smad2 and Smad3 are both activated in response to TGF-β and act, in cooperation with Smad4, as effectors of the TGF-β response. All three Smads are expressed in 3T3-F442A cells, and their mRNA levels do not change significantly during differentiation. Whether Smad protein levels or their activation are altered during differentiation is currently unknown and awaits the availability of better antibodies. Our data also show that TGF-β represses Smad3 but not Smad2 mRNA expression, and that this TGF-β–induced repression decreases with differentiation, which is consistent with the downregulation of the cell-surface TGF-β receptors. Whether cells alter Smad expression and activation as a mechanism to regulate TGF-β responsiveness during differentiation is currently unclear. However, a recent report suggests differential expression of Smads at different stages during maturation of chondrocytes (Sakou et al. 1999).
To characterize the roles of Smad2 and Smad3, we generated 3T3-F442A cells that stably overexpress either wild-type or dominant negative versions of Smad2 or Smad3. We assessed the effect of these manipulations on preadipocyte growth and differentiation, and the TGF-β response. This study revealed different roles for Smad2 and Smad3. Overexpression of Smad3 decreased differentiation and increased proliferation in the absence or presence of TGF-β, suggesting that Smad3 overexpression mimicked and enhanced the TGF-β response. Conversely, dominant negative interference with Smad3 signaling enhanced the rate and extent of differentiation and inhibited cell proliferation. These results suggest that Smad3 mediates the differentiation-inhibiting and proliferative responses to TGF-β.
In contrast to Smad3, alterations of Smad2 function did not affect cell proliferation, in the absence or presence of TGF-β. However, increased Smad2 levels inhibited differentiation, albeit to a milder extent than Smad3, and enhanced the differentiation inhibitory response to TGF-β. Remarkably, expression of dominant negative Smad2 had a similar, but smaller, effect on differentiation as wild-type Smad2. These observations were not peculiar to the ΔSSXS mutations, since cells stably expressing dominant negative versions of Smad2 or Smad3 with the last three serines mutated to alanines (Macias-Silva et al. 1996; Liu et al. 1997), behaved identically to the ΔSSXS mutants (data not shown). In principle, this effect could be explained by a possible weak residual activity of the mutated Smad2ΔSSMS or Smad2SA; however, this seems unlikely since we did not observe such behavior with the corresponding mutants of Smad3. Since Smad2 is also activated by activin, dominant negative interference with Smad2 signaling may primarily inhibit activin signaling, and this effect could explain the ability of Smad2ΔSSMS to inhibit differentiation and to enhance the TGF-β response. Whether activin signaling contributes to adipogenesis remains to be explored since nothing is yet known about the function of activin in adipocyte differentiation.
Regulation and the Roles of Smad6 and Smad7 in Adipogenesis
While Smad2 and Smad3 are effectors of the TGF-β response, Smad6 and Smad7 have been shown to act as inhibitors of TGF-β responses. In contrast to Smad2 and Smad3, Smad6 and Smad7 mRNA expression is strongly repressed as adipogenesis proceeds. Since TGF-β can induce Smad6 and Smad7 expression (Nakao et al. 1997; Afrakhte et al. 1998; Chen et al. 1999), it is possible that the loss of Smad6 and Smad7 expression results from the downregulation of cell-surface receptors for TGF-β and, consequently, a lack of autocrine TGF-β stimulation.
The downregulation of Smad6 and Smad7 suggested that these Smads normally exert an inhibitory effect on adipocyte differentiation, which is suppressed by the cells to allow full differentiation. Alternatively, the inhibitory effect of Smad6 and Smad7 on the TGF-β response could suggest that increased Smad6 or Smad7 expression would favor adipogenic differentiation. Remarkably, Smad6 or Smad7 overexpression blocked adipogenesis and enhanced the inhibitory effect of TGF-β on differentiation. In addition, Smad6 or Smad7 overexpression also enhanced the TGF-β–induced stimulation of cell proliferation, without changing the growth rate in the absence of exogenous TGF-β. This effect contrasts with the growth stimulatory effect of Smad3 overexpression, which enhances cell proliferation both in the absence or presence of TGF-β. While the activities of Smad6 and Smad7 appeared qualitatively the same, Smad7 was more potent than Smad6, in spite of its lower level of overexpression.
The mechanisms by which Smad6 and Smad7 block adipocyte differentiation and enhance the TGF-β response are unclear. However, we determined that the C-domain of Smad7 is necessary and sufficient for these effects in 3T3-F442A cells. This region is also required for the inhibitory effect of Smad7 on TGF-β receptor signaling (Hayashi et al. 1997; Souchelnytskyi et al. 1998), suggesting that the mechanism could be receptor-mediated. Since the inhibitory effects of Smad6 and Smad7 on ligand-induced responses are not restricted to TGF-β, it is conceivable that the observed phenotype results from inhibition of autocrine responsiveness to another TGF-β family member, such as BMP or activin. Accordingly, BMPs have been shown to regulate adipogenic differentiation: BMP-7 stimulates (Asahina et al. 1996) whereas BMP-2 has been reported to stimulate or inhibit adipogenic differentiation (Ahrens et al. 1993; Wang et al. 1993; Gimble et al. 1995). The differentiation stage and relative expression of the two BMP type I receptors, BMP-RIA and -RIB, may determine the nature of the autocrine response. Interference with BMP-RIA signaling can inhibit adipocyte conversion of mouse calvarial 2T3 cells, whereas a block in BMP-RIB signaling promotes adipocyte differentiation of these cells (Chen et al. 1998). Whether the differentiation and proliferation phenotypes of our Smad6 or Smad7 overexpressing cells can be explained through inhibition of BMP signaling remains to be investigated.
The molecular basis for the remarkable phenotype of the large cells in the Smad6 or Smad7 overexpressing cell lines also remains to be determined. It is conceivable that this phenotype results from an indiscriminate block in autocrine responsiveness to the different TGF-β–related factors by Smad6 or Smad7. This blockage may disable the mesenchymal differentiation program, which would be consistent with the ability of Smad7 to block mesoderm differentiation and to induce formation of neural tissue in Xenopus (Bhushan et al. 1998; Casellas and Hemmati-Brivanlou 1998). Similar data have been presented for Smad6 (Nakayama et al. 1998). However, the phenotype of our Smad6 and Smad7 stable cell lines does not suggest acquisition of a neuronal phenotype, and indeed, development of neural tissue in Xenopus is believed to be a default developmental response. Thus, Smad6 or 7 overexpression may prevent differentiation along the mesenchymal lineage.
In summary, our results suggest that autocrine TGF-β regulates the rate of adipose conversion, and that cells reduce their autocrine response to TGF-β by repressing the availability of the TGF-β receptors as they differentiate. The effects of TGF-β on preadipocyte differentiation are mediated by Smad2 and Smad3, which have distinct functions in differentiation. The mechanisms by which Smad2 and Smad3 block differentiation may be related to their well-known ability to interact with and modify the activity of transcription factors. Whether these Smads target the transcription factors that drive adipose differentiation is currently under investigation. Since TGF-β did not affect the induction of C/EBPβ and -δ, but blocked the induction of ADD-1, PPARγ, and C/EBPα, Smad2 or Smad3 may exert their effects at some point upstream of ADD-1 and PPARγ induction. The function of Smad6 and Smad7 may be to block premature differentiation (akin to the function of autocrine TGF-β production), possibly by blocking several TGF-β family signaling pathways. The current observations provide a basis for the investigation of the molecular mechanisms whereby these Smads regulate adipocyte differentiation.
Acknowledgments
We thank the following investigators for plasmids and cell lines: K. Easley (Harvard Medical School, Boston, MA), H. Green, S. Itoh, S. McKnight, K. Miyazono, G. Nolan, B. Spiegelman, P. ten Dijke, and X.-F. Wang (Duke University Medical School, Durham, NC). We also thank Y. Zhang for providing the Smad3ΔSSVS mutant construct, and X.-H. Feng for rounding up many other constructs from outside investigators. We are grateful to E. Filvaroff and M. Bauzon (both from University of California, San Francisco, San Francisco, CA) for help with obtaining the retroviral packaging cell lines, and to T. Alliston, J. Qing, and H. Fan for helpful discussions and encouragement.
This research was supported by a grant from the Arthritis Foundation and the National Institutes of Health grants P50-DE10306 (Project IV) and P60-DE1305 (Project III) to R. Derynck, and postdoctoral fellowships from the American Cancer Society and the American Heart Association to L. Choy.
Footnotes
Abbreviations used in this paper: dnTβRI and II, type I and II TGF-β receptor, respectively.
References
- Afrakhte M., Morén A., Jossan S., Itoh S., Sampath K., Westermark B., Heldin C.H., Heldin N.E., ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem. Biophys. Res. Commun. 1998;249:505–511. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- Ahrens M., Ankenbauer T., Schröder D., Hollnagel A., Mayer H., Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- Asahina I., Sampath T.K., Hauschka P.V. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp. Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Current Protocols of Molecular Biology. John Wiley & Sons; New York: 1994. [Google Scholar]
- Ballock R.T., Heydemann A., Wakefield L.M., Flanders K.C., Roberts A.B., Sporn M.B. TGF-β1 prevents hypertrophy of epiphyseal chondrocytesregulation of gene expression for cartilage matrix proteins and metalloproteases. Dev. Biol. 1993;158:414–429. doi: 10.1006/dbio.1993.1200. [DOI] [PubMed] [Google Scholar]
- Beauchamp R.D., Sheng H.-M., Bascom C.C., Miller D.A., Lyons R.M., Torre-Amione G., Moses H.L. Phenotypic alterations in fibroblasts and fibrosarcoma cells that overexpress latent transforming growth factor-β1. Endocrinology. 1992;130:2476–2486. doi: 10.1210/endo.130.5.1374006. [DOI] [PubMed] [Google Scholar]
- Bhushan A., Chen Y., Vale W. Smad7 inhibits mesoderm formation and promotes neural cell fate in Xenopus embryos. Dev. Biol. 1998;200:260–268. doi: 10.1006/dbio.1998.8965. [DOI] [PubMed] [Google Scholar]
- Bortell R., Owen T.A., Ignotz R., Stein G.S., Stein J.L. TGF-β1 prevents the down-regulation of type I procollagen, fibronectin, and TGF-β1 gene expression associated with 3T3-L1 pre-adipocyte differentiation. J. Cell. Biochem. 1994;54:256–263. doi: 10.1002/jcb.240540214. [DOI] [PubMed] [Google Scholar]
- Casellas R., Hemmati-Brivanlou A. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev. Biol. 1998;198:1–12. doi: 10.1006/dbio.1998.8893. [DOI] [PubMed] [Google Scholar]
- Centrella M., Casinghino S., Kim J., Pham T., Rosen V., Wozney J., McCarthy T. Independent changes in type I and type II receptors for transforming growth factor-β induced by bone morphogenetic protein 2 parallel expression of the osteoblast phenotype. Mol. Cell. Biol. 1995;15:3273–3281. doi: 10.1128/mcb.15.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., Horowitz M.C., Wozney J.M., McCarthy T.L. Transforming growth factor-β gene family members and bone. Endocrine Rev. 1994;15:27–39. doi: 10.1210/edrv-15-1-27. [DOI] [PubMed] [Google Scholar]
- Chen D., Ji X., Harris M.A., Feng J.Q., Karsenty G., Celeste A.J., Rosen V., Mundy G.R., Harris S.E. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J. Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.-H., Derynck R. Homomeric interactions between the type II TGF-β receptors. J. Biol. Chem. 1994;269:22868–22874. [PubMed] [Google Scholar]
- Chen R.-H., Ebner R., Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-β activities. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- Chen S.J., Yuan W., Mori Y., Levenson A., Trojanowska M., Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-βinvolvement of Smad 3. J. Invest. Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Chen X., Weisberg E., Fridmacher V., Watanabe M., Naco G., Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- Clarke S.L., Robinson C.E., Gimble J.M. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor-γ2 promoter. Biochem. Biophys. Res. Commun. 1997;240:99–103. doi: 10.1006/bbrc.1997.7627. [DOI] [PubMed] [Google Scholar]
- Clouthier D.E., Comerford S.A., Hammer R.E. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-β1 transgenic mice. J. Clin. Invest. 1997;100:2697–2713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Feng X.-H. TGF-β receptor signaling. Biochim. Biophys. Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- Derynck R., Choy L. Transforming growth factor-β and its receptors. In: Thomsen A., editor. The Cytokine Handbook. Academic Press, Inc; Orlando, FL: 1998. pp. 593–636. [Google Scholar]
- Derynck R., Zhang Y., Feng X.-H. Smadstranscriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- Dobson D.E., Groves D.L., Spiegelman B.M. Nucleotide sequence and hormonal regulation of mouse glycerophosphate dehydrogenase mRNA during adipocyte and muscle cell differentiation. J. Biol. Chem. 1987;262:1804–1809. [PubMed] [Google Scholar]
- Feinberg A.P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feng X.-H., Zhang Y., Wu R.-Y., Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for Smad3 in TGF-β-induced transcriptional activation. Genes Dev. 1998;12:2153–2163. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filvaroff E.H., Ebner R., Derynck R. Inhibition of myogenic differentiation in myoblasts expressing a truncated type II TGF-β receptor. Development. 1994;121:185–195. doi: 10.1242/dev.120.5.1085. [DOI] [PubMed] [Google Scholar]
- Gazit D., Ebner R., Kahn A., Derynck R. Modulation of expression and cell surface binding of members of the transforming growth factor-β superfamily during retinoic acid-induced osteoblastic differentiation of multipotential mesenchymal cells. Mol. Endocrinol. 1993;7:189–198. doi: 10.1210/mend.7.2.8385738. [DOI] [PubMed] [Google Scholar]
- Gimble J.M., Morgan C., Kelly K., Wu X., Dandapani V., Wang C.S., Rosen V. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J. Cell. Biochem. 1995;58:393–402. doi: 10.1002/jcb.240580312. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B.H. High efficiency DNA-mediated transformation of primate cells. Science. 1983;221:551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion of 3T3 cells. Cell. 1976;7:105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Grégoire F.M., Smas C.M., Sul H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Abdollah S., Qiu Y., Cai J., Xu Y.Y., Grinnell B.W., Richardson M.A., Topper J.N., Gimbrone M.A., Jr., Wrana J.L., Falb D. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Heldin C.-H., Miyazono K., ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hu J.S., Olson E.N. Functional receptors for transforming growth factor-β are retained by biochemically differentiated C2 myocytes in growth factor-deficient medium containing EGTA but down-regulated during terminal differentiation. J. Biol. Chem. 1990;265:7914–7919. [PubMed] [Google Scholar]
- Ignotz R.A., Massagué J. Type β transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl. Acad. Sci. USA. 1985;82:8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T., Takase M., Nishihara A., Oeda E., Hanai J., Kawabata M., Miyazono K. Smad6 inhibits signaling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Wells N.J., Hunter T. TGF-β-stimulated cooperation of Smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeoung D.-I., Tang B., Sonenberg M. Mitogenic response to TGF-β in 3T3-F442A cells. Biochem. Biophys. Res. Commun. 1995;216:964–969. doi: 10.1006/bbrc.1995.2714. [DOI] [PubMed] [Google Scholar]
- Kulyk W.M., Rodgers B.J., Greer K., Kosher R.A. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-β. Dev. Biol. 1989;135:424–430. doi: 10.1016/0012-1606(89)90191-7. [DOI] [PubMed] [Google Scholar]
- Liu B., Dou C.L., Prabhu L., Lai E. FAST-2 is a mammalian winged-helix protein which mediates transforming growth factor-β signals. Mol. Cell. Biol. 1999;19:424–430. doi: 10.1128/mcb.19.1.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Sun Y., Constantinescu S., Karam E., Weinberg R., Lodish H. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl. Acad. Sci. USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M., Abdollah S., Hoodless P., Pirone R., Attisano L., Wrana J.L. MADR-2 is a substrate of the TGF-β receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-β signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type β transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl. Acad. Sci. USA. 1986;83:8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.D., Rosman G.J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–988. [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J.P., Land H. Advanced mammalian gene transferhigh titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A., Afrakhte M., Moren A., Nakayama T., Christian J.L., Heuchel R., Itoh S., Kawabata M., Heldin N.E., Heldin C.H., ten Dijke P. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signaling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Gardner H., Berg L.K., Christian J.L. Smad6 functions as an intracellular antagonist of some TGF-β family members during Xenopus embryogenesis. Genes Cells. 1998;3:387–394. doi: 10.1046/j.1365-2443.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- Olson E.N., Sternberg E., Hu J.S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type β transforming growth factor. J. Cell Biol. 1986;103:1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruschke T., Rohrig K., Hauner H. Transforming growth factor beta(TGF-β) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int. J. Obes. Relat. Metab. Disord. 1994;18:532–536. [PubMed] [Google Scholar]
- Rahimi N., Tremblay E., McAdam L., Roberts A., Elliott B. Autocrine secretion of TGF-β1 and TGF-β2 by pre-adipocytes and adipocytesa potent negative regulator of adipocyte differentiation and proliferation of mammary carcinoma cells. In Vitro Cell. Dev. Biol. 1998;34:412–420. doi: 10.1007/s11626-998-0023-z. [DOI] [PubMed] [Google Scholar]
- Sakou T., Onishi T., Yamamoto T., Nagamine T., Sampath T., ten Dijke P. Localization of Smads, the TGF-β family intracellular signaling components during endochondral ossification. J. Bone Miner. Res. 1999;14:1145–1152. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- Samad F., Uysal K.T., Wiesbrock S.M., Pandey M., Hotamisligil G.S., Loskutoff D.J. Tumor necrosis factor-α is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc. Natl. Acad. Sci. USA. 1999;96:6902–6907. doi: 10.1073/pnas.96.12.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad F., Yamamoto K., Pandey M., Loskutoff D.J. Elevated expression of transforming growth factor-β in adipose tissue from obese mice. Mol. Med. 1997;3:37–48. [PMC free article] [PubMed] [Google Scholar]
- Serrero G., Mills D. Decrease in transforming growth factor-β1 binding during differentiation of rat adipocyte precursors in primary culture. Cell Growth Differ. 1991;2:173–178. [PubMed] [Google Scholar]
- Slager H.G., van Inzen W., Freund E., van den Eijnden-van Raaij A.J.M., Mummery C.L. Transforming growth factor-β in the early mouse embryoimplications for the regulation of muscle formation and implantation. Dev. Genet. 1993;14:212–224. doi: 10.1002/dvg.1020140308. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S., Nakayama T., Nakao A., Morén A., Heldin C.-H., Christian J.L., ten Dijke P. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-β receptors. J. Biol. Chem. 1998;273:25364–25370. doi: 10.1074/jbc.273.39.25364. [DOI] [PubMed] [Google Scholar]
- Sparks R.L., Scott R.E. Transforming growth factor type β is a specific inhibitor of 3T3 T mesenchymal stem cell differentiation. Exp. Cell Res. 1986;165:345–352. doi: 10.1016/0014-4827(86)90588-4. [DOI] [PubMed] [Google Scholar]
- Sparks R.L., Allen B.J., Strauss E.E. TGF-β blocks early but not late differentiation-specific gene expression and morphologic differentiation of 3T3 T proadipocytes. J. Cell. Physiol. 1992;150:568–577. doi: 10.1002/jcp.1041500318. [DOI] [PubMed] [Google Scholar]
- Sparks R.L., Allen B.J., Zygmunt A.I., Strauss E.E. Loss of differentiation control in transformed 3T3 T proadipocytes. Cancer Res. 1993;53:1770–1776. [PubMed] [Google Scholar]
- Sporn M.B., Roberts A.B., Wakefield L.M., Assoian R.K. Transforming growth factor-βbiological function and chemical structure. Science. 1986;233:532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Stephens J.M., Butts M., Stone R., Pekala P.H., Bernlohr D.A. Regulation of transcription factor mRNA accumulation during 3T3-L1 preadipocyte differentiation by antagonists of adipogenesis. Mol. Cell. Biochem. 1993;123:63–71. doi: 10.1007/BF01076476. [DOI] [PubMed] [Google Scholar]
- Stroschein S.L., Wang W., Zhou S., Zhou Q., Luo K. Negative feedback regulation of TGF-β signaling by the SnoN oncoprotein. Science. 1999;286:771–774. doi: 10.1126/science.286.5440.771. [DOI] [PubMed] [Google Scholar]
- Sun Y., Liu X., Eaton E.N., Lane W.S., Lodish H.F., Weinberg R.A. Interaction of the Ski oncoprotein with Smad3 regulates TGF-β signaling. Mol. Cell. 1999;4:499–509. doi: 10.1016/s1097-2765(00)80201-4. [DOI] [PubMed] [Google Scholar]
- Tang Q.Q., Jiang M.S., Lane M.D. Repressive effect of Sp1 on the C/EBP-α gene promoterrole in adipocyte differentiation. Mol. Cell. Biol. 1999;19:4855–4865. doi: 10.1128/mcb.19.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR-γ 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Topper J.N., DiChiara M.R., Brown J.D., Williams A.J., Falb D., Collins T., Gimbrone M.A., Jr. CREB binding protein is a required coactivator for Smad-dependent, transforming growth factor-β transcriptional responses in endothelial cells. Proc. Natl. Acad. Sci. USA. 1998;95:9506–9511. doi: 10.1073/pnas.95.16.9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti F.M., Dieckmann B., Beutler B., Cerami A., Ringold G.M. A macrophage factor inhibits adipocyte gene expressionan in vitro model of cachexia. Science. 1985;229:867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Torti F.M., Torti S.V., Larrick J.W., Ringold G.M. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor-β. J. Cell Biol. 1989;108:1105–1113. doi: 10.1083/jcb.108.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade T.P., Kasid A., Stein C.A., LaRocca R.V., Sargent E.R., Gomella L.G., Myers C.E., Linehan W.M. Suramin interference with transforming growth factor-β inhibition of human renal cell carcinoma in culture. J. Surg. Res. 1992;53:195–198. doi: 10.1016/0022-4804(92)90034-w. [DOI] [PubMed] [Google Scholar]
- Wang E.A., Israel D.I., Kelly S., Luxenberg D.P. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGF-β superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Wotton D., Lo R.S., Lee S., Massagué J. A Smad transcriptional corepressor. Cell. 1999;97:29–39. doi: 10.1016/s0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Xing H., Northrop J.P., Grove J.R., Kilpatrick K.E., Su J.L., Ringold G.M. TNF-α-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPAR-γ without effects on Pref-1 expression. Endocrinology. 1997;138:2776–2783. doi: 10.1210/endo.138.7.5242. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Derynck R. Regulation of Smad signaling by protein associations and signaling crosstalk. Trends Cell Biol. 1999;9:274–279. doi: 10.1016/s0962-8924(99)01579-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Feng X.-H., Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]