Abstract
Aerenchyma tissues form gas-conducting tubes that provide roots with oxygen under hypoxic conditions. Although aerenchyma have received considerable attention in Zea mays, the signaling events and genes controlling aerenchyma induction remain elusive. Here, we show that Arabidopsis thaliana hypocotyls form lysigenous aerenchyma in response to hypoxia and that this process involves H2O2 and ethylene signaling. By studying Arabidopsis mutants that are deregulated for excess light acclimation, cell death, and defense responses, we find that the formation of lysigenous aerenchyma depends on the plant defense regulators LESION SIMULATING DISEASE1 (LSD1), ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1), and PHYTOALEXIN DEFICIENT4 (PAD4) that operate upstream of ethylene and reactive oxygen species production. The obtained results indicate that programmed cell death of lysigenous aerenchyma in hypocotyls occurs in a similar but independent manner from the foliar programmed cell death. Thus, the induction of aerenchyma is subject to a genetic and tissue-specific program. The data lead us to conclude that the balanced activities of LSD1, EDS1, and PAD4 regulate lysigenous aerenchyma formation in response to hypoxia.
INTRODUCTION
Each year, flooding greatly reduces global land crop harvests (Dennis et al., 2000; Setter and Waters, 2003). Since flooding leads to the depletion of soil oxygen, the main strategy for improving flooding and/or waterlogging tolerance in land crops has been directed toward improving the tolerance to hypoxia (<4% [O2]) and anoxia (close to 0% [O2]). The pores in aerated soils contain the same level of oxygen as the atmosphere (21% [O2]). However, during flooding, the air in these pores is replaced by water, and because of the slow diffusion rates of oxygen in water and the aerobic activities of plant roots and microorganisms, the oxygen levels quickly fall (Pezeshki, 1994).
Adaptation to low oxygen levels in plants occurs in three stages. Initially, the plant rapidly induces a set of signal transduction components (Dennis et al., 2000). This is followed by metabolic adaptation involving fermentation pathways and, finally, depending on plant species, by morphological changes such as aerenchyma and adventitious root formation (Dennis et al., 2000). In species such as Arabidopsis thaliana, Zea mays, and Iris pseudacorus, hypoxia responses involve signaling pathways controlled by ethylene, reactive oxygen species (ROS), and abscisic acid (Monk et al., 1987; He et al., 1996; de Bruxelles et al., 1996; Peng et al., 2001; Baxter-Burrell et al., 2002; Klok et al., 2002; Fukao and Bailey-Serres, 2004). Physiological responses to hypoxia include wilting and stomata closure (Pezeshki et al., 1996a), Ca2+ signaling (Subbaiah and Sachs, 2003), and increased levels of hemoglobins (Dordas et al., 2003). The levels of hemoglobins may be affected by auxins (Watts et al., 2001) and may directly affect ethylene signaling (Manac'h-Little et al., 2005).
Hypoxia-induced aerenchyma occurs in some non-wetland plants to improve the aeration of the rhizosphere (Jackson and Armstrong, 1999). Plants form aerenchyma using two different processes, either schizogeny or lysigeny, or by their combination (Drew et al., 2000). Schizogenous aerenchyma involves cell wall reorganization and cell separation, whereas lysigenous aerenchyma is formed as a consequence of programmed cell death (PCD) and cell wall autolysis (Campbell and Drew, 1983; Gunawardena et al., 2001b; Evans, 2003). In Z. mays, ethylene has been shown to be associated with lysigenous aerenchyma formation (Jackson et al., 1985; He et al., 1996).
To date, studies have shown that Arabidopsis seedlings do not form aerenchyma in response to hypoxia. However, since waterlogging causes stratified oxygenation in soils, and the top layer maintains high oxygen levels, it would not be advantageous for small Arabidopsis seedlings to form aerenchyma. (Evans, 2003; Yu and Patrick, 2003; Colmer et al., 2004; Fukao and Bailey-Serres, 2004). However aerenchyma formation may be of importance in adult Arabidopsis plants, which have deeper root systems and larger rhizospheres.
Genetic and mechanistic links were found between light acclimation processes and defense against pathogen infection in studies of the LESION SIMULATING DISEASE1 (LSD1), PHYTOALEXIN DEFICIENT4 (PAD4), and ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1) functions (Mateo et al., 2004) that control ROS and regulate PCD in Arabidopsis leaves (Jabs et al., 1996; Rusterucci et al., 2001). Consistent with its role in both initiating and propagating foliar PCD, loss-of-function lsd1 mutants exhibit runaway cell death (RCD) in response to light stress or pathogen infection in leaves. RCD is a laterally spreading, unchecked cell death that affects a major part of the leafy rosette. (Rusterucci et al., 2001; Mateo et al., 2004). LSD1 is an important regulator of plant PCD through several interactions (Jabs et al., 1996; Rusterucci et al., 2001). LSD1 sequence predicts that the encoded protein functions as a transcription factor of the C2C2 zinc finger family. These types of proteins were shown to link different environmental stimuli to caspase activity in many organisms (Uren et al., 2000). We were able to link RCD in lsd1 to the activity of photosystem II (RCD was induced by 680-nm light but not by 700-nm light), stomatal conductance, and ultimately to photorespiratory H2O2 (Mateo et al., 2004). Null mutations in PAD4 and EDS1 block lsd1-conditioned RCD triggered by long photoperiods, high light, photorespiratory conditions, pathogen inoculation, ROS provision, or supply of the phenolic signaling molecule, salicylic acid (SA) (Jabs et al., 1996; Rusterucci et al., 2001; Mateo et al., 2004). The results point to multiple roles of LSD1 in reducing cellular ROS content (1) by controlling PAD4- and EDS1-dependent stomatal closure and consequently foliar (photoresiratory) H2O2 production during excess excitation energy (EEE) and (2) by regulating the H2O2 scavenging capacity (Mateo et al., 2004).
The SA pathway leading to systemic immunity is under negative control by mitogen-activated protein kinase 4 (Petersen et al., 2000; Rusterucci et al., 2001; Brodersen et al., 2006). Current evidence suggests that EDS1 and PAD4 amplify ethylene and SA signals by processing ROS-derived molecules that are essential for expression of cellular immunity against biotrophic pathogens (Rusterucci et al., 2001; Brodersen et al., 2006). EDS1 signaling complexes are nucleocytoplasmic, and evidence suggests that dynamic interactions between cell compartments are important for effective stress signal relay (Feys et al., 2005). Two new components of Arabidopsis immunity were discovered using gene expression microarrays combined with reverse genetics (Bartsch et al., 2006). A flavin-dependent monooxygenase (FMO1) positively regulates the EDS1 pathway, and one member (NUDT7) of a family of cytosolic Nudix hydrolases exerts negative control of EDS1 signaling. A common theme underlying the functions of counterparts of these proteins in animals and yeast is in redox stress responses.
However, the role of LSD1, PAD4, and EDS1 in acclimation to EEE, in regulation of PCD, and in defense responses was assessed only in ambient oxygen concentration and only in foliar tissues. We observed that LSD1 is a negative regulator of ethylene signaling. Therefore, we wondered if the above genetic system controls other PCD responses, for example, lysigenous aerenchyma formation, in different tissues than that observed in foliar tissues during defense and EEE acclimatory responses. Here, we report that LSD1 and its associated genetic system also specifically control the induction of lysigenous aerenchyma in Arabidopsis hypocotyls under root hypoxia.
RESULTS
Arabidopsis Hypocotyls Form Aerenchyma in Response to Hypoxia
To investigate whether hypoxia response in Arabidopsis includes lysigenous aerenchyma induction, we used 4- and 12-week-old flowering plants grown in soil. We submerged the soil entirely in water for a period of 8 d. After 7 d, the solution surrounding the pots had reached hypoxic levels (∼4% O2) (see Supplemental Figure 1 online). The hypocotyl-root axis was examined under the microscope each day during waterlogging for signs of aerenchyma formation. The hypocotyl-root axis of 4- and 12-week-old plants was mostly composed of secondary tissues. All primary extrastellar tissues had been shed, and new tissues had been initiated from the cork cambium (cork and secondary cortex) and the vascular cambium (secondary phloem and secondary xylem). In the secondary xylem of 12-week-old plants, two distinct zones were observed, termed xylem I and xylem II (Chaffey et al., 2002) (Figure 1A). Xylem I contained vessel elements surrounded by axial parenchyma, whereas xylem II was composed of vessel elements and lignified fibers. However, only xylem I was produced in 4-week-old plants.
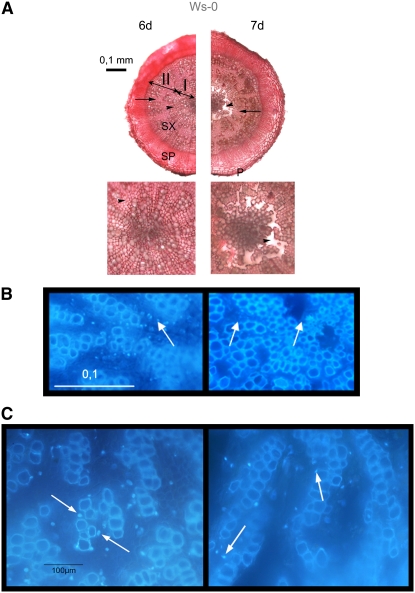
Figure 1.
Arabidopsis Hypocotyls Form Aerenchyma in Response to Root Hypoxia.
(A) Anatomy of the root-hypocotyl axis in 12-week-old plants waterlogged for 6 and 7 d as seen in ruthenium red–stained cross sections. Representative samples from 6 and 7 d of waterlogging (n = 8). Mostly secondary tissues are seen, including secondary phloem (SP), secondary xylem (SX), and periderm (P) in the top panel. In secondary xylem, there is an inner zone with vessel elements and axial parenchyma cells (arrowhead) called xylem I (I). This zone is magnified in the bottom panel. Note a disappearance of axial parenchyma cells between days 6 and 7. The outer zone of secondary xylem is composed of vessel elements and fibers (arrow) called xylem II (II).
(B) DAPI-stained xylem I in control and waterlogged plants forming aerenchyma. Representative samples from 6 and 7 d of waterlogging (n = 3). Note the presence of whole nuclei on the sixth day of waterlogging and DNA condensation and cell walls lysis (arrows) visible in plants waterlogged for 7 d.
(C) Difference in nuclei from representative samples on the sixth and seventh days of waterlogging. Normal nuclei can be observed in plant waterlogged for 6 d, while condensated and moon-shaped nuclei were found in plants waterlogged for 7 d (arrows).
No aerenchyma was observed in 4-week-old plants during the period of waterlogging and reduction of oxygen concentration to 4% (see Supplemental Figure 2 online). The 12-week-old plants did not show any anatomical changes during the first 6 d of waterlogging. After 7 d of waterlogging, the axial parenchyma cells of the secondary xylem I in the central part of the hypocotyl disappeared, forming air spaces (Figure 1A). Parenchyma cells of periderm or secondary phloem appeared normal. In the air spaces that formed in the central part, no sign of cell separation was detected. By contrast, the cell walls apparently vanished, indicating that the formation of aerenchyma was due to cell lysis. The air spaces formed all along the shoot-root axis (Figure 2). Lysigeny was confirmed by the disappearance of nuclei of these cells as evidenced by 4′-6-diamidino-2-phenylindole (DAPI) staining (Figure 1B). Analysis of the DAPI-stained nuclei revealed characteristics of PCD, such as moon-shaped nuclei and DNA condensation (Figure 1C) (Pennell and Lamb, 1997).
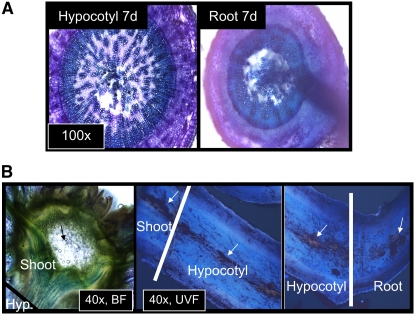
Figure 2.
Aerenchyma Stretches from Shoot to Root.
(A) Aerenchyma was detected at several different distances from the shoot in both hypocotyl and in root tissues.
(B) Aerenchchyma (white arrows) stretches continuously from the naturally occurring cavities of the Arabidopsis shoot (black arrow) through the unexposed parts of the hypocotyl to hypoxia-exposed hypocotyl and root tissues. BF, bright-field microscopy; UVF, UV field microscopy; x indicates objective magnification.
Since it has been shown that responses to hypoxia involve closure of leaf stomata and reduction of photosynthetic capacity in several angiosperm species (Pezeshki et al., 1996a, 1996b), we monitored stomatal conductance during the 8 d of waterlogging. Stomata closure decreased continuously during the 8 d of waterlogging, indicating that the plants were responding to the rhizospheric hypoxia (Figure 3A). Reduced stomatal conductance is known to play an important role in increased formation of H2O2 that functions as EEE acclimatory systemic signal (Karpinski et al., 1999; Mateo et al., 2004). Thus, we hypothesized that the same elements of systemic signaling might be used to induce PCD signals in axial parenchyma cells. Consistent with this hypothesis, we have observed that aerenchyma could be induced faster when plants were exposed to EEE (high light 500 μE or long photoperiod 18 h) (see Supplemental Table 1 online). These data suggest that light conditions affect formation of lysigenous aerenchyma during waterlogging. Thus, we propose that the plant induces an interactive systemic signaling network during waterlogging and light acclimation.
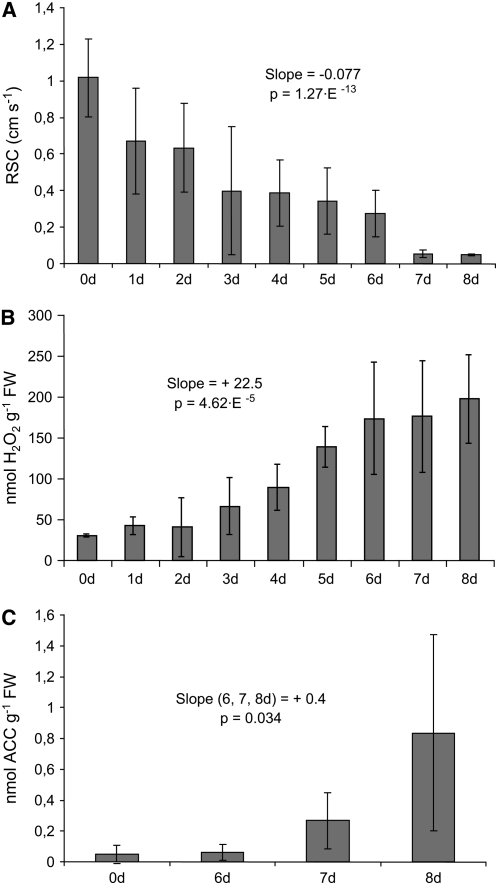
Figure 3.
Stomatal Conductance Decreases and Hydrogen Peroxide and Ethylene Are Increased during the Course of Aerenchyma Formation in Arabidopsis.
(A) A gradual decrease in relative stomatal conductance (RSC) was detected during 8 d of waterlogging in Arabidopsis leaves (slope value and P value of simple linear regression is presented in the chart, n > 6 for each time point, error bars are ± sd).
(B) During the same period and treatments, there was a gradual increase in hydrogen peroxide (slope value and P value of simple linear regression is presented in the chart, n = 4 sets of at least four grouped samples for each time point, error bars are ± sd). FW, fresh weight.
(C) The ethylene precursor ACC increased in the Arabidopsis hypocotyls after 6 d of waterlogging (slope value and P value of simple linear regression is presented in the chart, n = 3 sets of at least four grouped samples for each time point, error bars are ± sd). Observe that decrease in stomatal conductance and increase in hydrogen peroxide level precede the increase of ACC by several days.
To investigate if aerenchyma induction in Arabidopsis involves ethylene and ROS signaling, we monitored the levels of 1-aminocyclopropane-1-carboxylic acid (ACC), the precursor of de novo ethylene synthesis (Adams and Yang, 1979), and hydrogen peroxide (H2O2) during the 8-d waterlogging period leading to aerenchyma formation in hypocotyls. The levels of H2O2 increased in a gradual manner from day 1 to day 8 (i.e., before the formation of aerenchyma was detected) (Figure 3B). ACC is the immediate biosynthetic precursor of ethylene and is a reliable marker of ethylene levels in the plant (Adams and Yang, 1979). ACC level in the waterlogged plants remained unchanged for 6 d, but the level strongly increased after 6 d (Figure 3C), notably coinciding with the formation of the lysigenous aerenchyma (Figure 1A).
ASCORBATE PEROXIDASE1 (APX1) is a ubiquitous H2O2 scavenging enzyme that has been shown to be regulated by H2O2 and ABA (Karpinski et al., 1997, 1999; Storozhenko et al., 1998). To analyze induction of APX1 expression, we have used transgenic Arabidopsis plants that harbor the APX1-promoter fused to the firefly luciferase gene (APX1:LUC). Luciferase activity in this transgenic line mirrors induction of the native APX1 gene (Karpinski et al., 1997, 1999). We observed that the APX1:LUC transgene was induced by 7 d of waterlogging (Figure 4A). We also found that the APX1:LUC transgene was induced more in roots than in shoots (Figure 4B), indicating root-specific ROS (H2O2) signaling. Altogether, these data show that Arabidopsis plants induce lysigenous aerenchyma in secondary xylem I of the hypocotyl-root axis in response to root hypoxic conditions and that this response may involve H2O2, ethylene, systemic root-to-shoot signaling, and PCD (Figures 1 to 4).
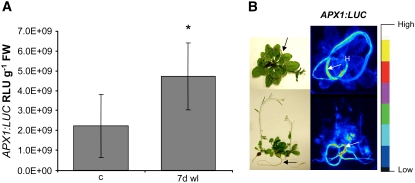
Figure 4.
Analyses of APX1 Expression Reveals Root-Specific Signals.
(A) Quantification of luciferase level controlled by the APX1 promoter in transgenic APX1promoter:LUC Arabidopsis Col-0 rosettes, shoots, hypocotyls, and roots (Karpinski et al., 1999; Karpinska et al., 2000) showed that 7 d of waterlogging [7 d wl]) induced APX1:LUC expression compared with untreated plants (c) (*P < 0.05, Students t test, n = 5, error bars are ± sd).
(B) Imaging of luciferase level in the same transgenic lines revealed a significantly higher luciferase activity in roots than in shoots during waterlogging. Arrows indicate root systems, and H indicates hypocotyls of the investigated plants.
LSD1, EDS1, and PAD4 Control Lysigenous Aerenchyma Formation
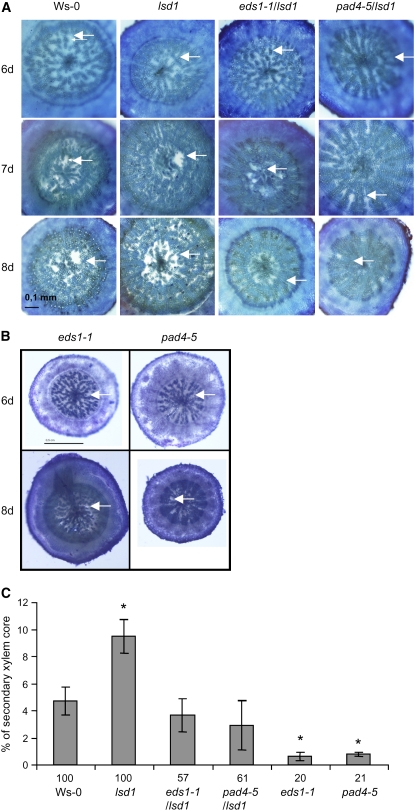
Previously, we have shown that LSD1 inhibits stomatal closure and ROS signaling during acclimation to EEE (Mateo et al., 2004). We were interested if the same genetic system was involved in regulation of the aerenchyma formation and ethylene signaling. Therefore, we compared the aerenchyma formation in the wild type (Wassilewskija [Ws-0]) and in the lsd1 null mutant. Image analysis was used to quantify the area of induced aerenchyma in cross sections of lsd1 plants and the corresponding Ws-0 ecotype plants that had been waterlogged for 7 d. While Ws-0 ecotype produced on average 4.7% of aerenchyma per unit area of the secondary xylem core, the lsd1 mutant had an on average 9.6% of aerenchyma (Figures 5A and 5C) even though the amount of parenchyma was similar in the lsd1 mutant as in the wild-type plants (see Supplemental Figure 3 online). This indicates that LSD1 acts as a negative regulator of lysigenous aerenchyma formation, and this is in agreement with the previously described role of LSD1 role in PCD (Jabs et al., 1996; Rusterucci et al., 2001; Mateo et al., 2004).
Figure 5.
LSD1, EDS1, and PAD4 Control Aerenchyma Formation in Arabidopsis.
(A) Toluidine blue–stained cross sections of Arabidopsis Ws-0, lsd1, lsd1 eds1-1, and lsd1 pad4-5 hypocotyls of plants subjected to waterlogging for 6, 7, and 8 d. Representative samples are shown (n = 8). Arrows indicate parenchyma (6 d) and aerenchymatous lacunae (7 and 8 d).
(B) Toluidine blue–stained cross sections of Arabidopsis eds1-1 and pad4-5 hypocotyls waterlogged for 7 d. Representative samples are shown (n = 8). White arrows indicate parenchyma (6 d waterlogged) and aerenchymatous lacunae (7 d).
(C) Quantification of cross-section areas of aerenchymatous lacunae in Arabidopsis Ws-0, lsd1, eds1-1 lsd1, pad4-5 lsd1, eds1-1, and pad4-5 hypocotyls waterlogged for 7 d (*P < 0.05, Students t test, n = 8, error bars are ± se). The numbers beneath each column denote the percentage of plants that formed aerenchyma.
RCD in lsd1 leaves is dependent on the functions of EDS1 and PAD4 (Rusterucci et al., 2001; Mateo et al., 2004). Therefore, we quantified formed aerenchyma per secondary xylem area formation in eds1-1 lsd1 and pad4-5 lsd1 double mutants and in eds1-1 and pad4-5 single null mutants (all in the Ws-0 ecotype), thinking that they may revert the lsd1 phenotype. Interestingly, only 60% of the eds1-1 lsd1 and pad4-5 lsd1 double mutant plants induced aerenchyma at days 7 and 8 compared with 100% plants in the wild-type and lsd1 plants (Figures 5A and 5C). The plants that formed aerenchyma had the area of aerenchymatous lacunae comparable to that of the wild type (i.e., two times less than the lsd1 mutant) (Figure 5C). Consistently, only 20% of eds1-1 and pad4-5 mutant plants induced aerenchyma at days 7 and 8 (Figures 5B and 5C). In these lines (eds1-1 and pad4-5), the plants that did induce aerenchyma had only 0.6 and 0.75% of aerenchyma per unit area of the secondary xylem core, respectively, which was significantly less than the wild type or the eds1-1 lsd1 and pad4-5 lsd1 double mutants (Figure 5C). These data indicate that EDS1 and PAD4 positively regulate both induction and the amount of this tissue counteracting the inhibitory action of LSD1.
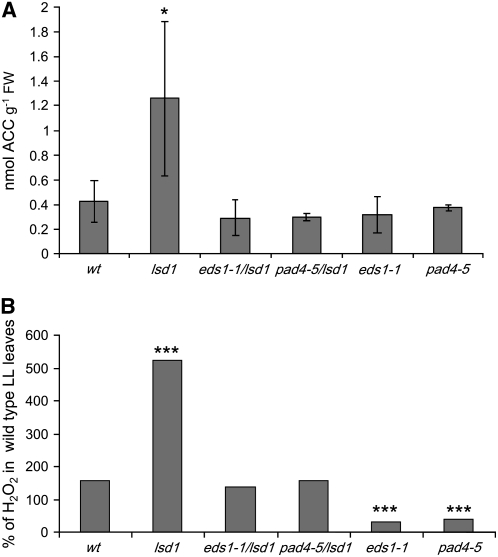
To further investigate the signaling network that regulates aerenchyma formation, we measured ACC and H2O2 levels during EEE exposure of wild-type and mutant plants (Figure 6A). EEE exposure caused higher increases of ACC levels in lsd1 leaves compared with the wild type (twofold versus sixfold, respectively; Figure 6A). Analysis of the single eds1-1 and pad4-5 mutants as well as eds1-1 lsd1 and pad4-5 lsd1 double mutants revealed that the increase in ACC under EEE conditions in lsd1 requires the defense regulators EDS1 and PAD4 (Figure 6B). Similar results were obtained for H2O2 levels (Figure 6B). Thus, EDS1 and PAD4 operate upstream of ethylene and ROS production in EEE stress signaling, leading to propagation of cell death in lsd1.
Figure 6.
LSD1, EDS1, and PAD4 Control Stress Induced Ethylene and Hydrogen Peroxide Levels in Arabidopsis.
(A) EEE treatment (2000 ± 250 μmol m2 s−1) induces significantly higher production of ACC in Ws-0 lsd1 mutants than in the Ws-0 wild type (Student's t test P < 0.05*, n = 4 ± sd for each treatment). lsd1 mutants produce threefold higher amounts of the ethylene precursor ACC in response to EEE compared with wild-type plants. Therefore, LSD1 is a negative regulator of stress ethylene. Ethylene signaling in the absence of functional LSD1 was positively dependent on EDS1 and PAD4 since eds1-1 lsd1 and pad4-5 lsd1 double mutants reverted to wild-type levels of ACC in EEE and single pad4-4 and eds1-1 mutants could not increase ACC levels. Control levels of ACC in Ws-0 and all mutants vary between 0.15 and 0.24 nmol ± 0.12 sd of ACC per g FW.
(B) EEE treatment induces significantly higher production of H2O2 in Ws lsd1 mutants than in the Ws-0 wild type (Student's t test; ***P < 0.001, n = 4 ± sd for each treatment). The lsd1 mutant produce twofold higher amounts of H2O2 in response to EEE compared with wild-type plants. Therefore, LSD1 is a negative regulator of stress H2O2. H2O2 signaling in absence of functional LSD1 was positively dependent on EDS1 and PAD4 since eds1-1 lsd1 and pad4-5 lsd1 double mutants reverted to wild-type levels of H2O2 levels in EEE, and single pad4-5 and eds1-1 actually had decreased H2O2 levels (Student's t test; ***P < 0.001, n = 4 ±S.D. for each treatment). Control levels of H2O2 in Ws-0 and all mutants were set to 100%
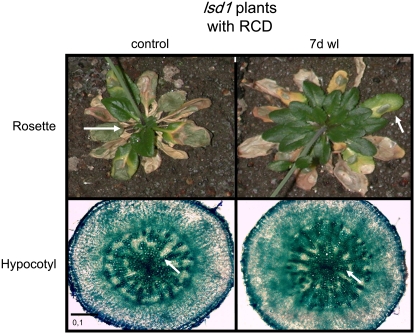
We wondered if the development of foliar RCD had an impact on aerenchyma formation and vice versa. Development of RCD in older leaves does not induce aerenchyma in hypocotyls under normal oxygen concentration (Figure 7). In addition, plants that developed foliar RCD and then were exposed to waterlogging for 7 d could also not develope lysigenous aerenchyma (Figure 7). This observation indicated that the control exerted by LSD1 in lysigenous aerenchyma formation was separated from that of foliar PCD. Additionally, these results indicated that the systemic signals produced during RCD blocked the competence for lysigenous aerenchyma formation (Figure 7).
Figure 7.
Development of Aerenchyma and Foliar RCD in lsd1 Plants Follows Tissue-Specific Regulation.
In lsd1 plants cultivated in normal oxygen concentration and with strongly induced foliar RCD, we could not detect aerenchyma in hypocotyls of 12-week-old control plants. Waterlogging for 7 d of such plants also did not induce aerenchyma after 7 d of waterlogging (7d wl). Arrows indicate leaves in lsd1 in the top panels with RCD and parenchyma in hypocotyl cross sections of the same plants in the bottom panels. It is concluded that development of foliar RCD before root hypoxia restricts aerenchyma formation.
We also analyzed LSD1, EDS1, and PAD4 expression and biologically active cis-regulatory elements (CREs) in the promoter region for these genes. LSD1 was upregulated by high CO2 levels, and EDS1 and PAD4 were both upregulated by short exposures to drought (Table 1). Further analysis revealed that all three genes were regulated by phosphate deficiency and heat (Table 1). This analysis revealed a similar regulation of EDS1 and PAD4 and partially overlapping regulation of LSD1. We have also used the PLACE algorithm (Prestridge, 1991; Higo et al., 1999) and our new algorithm (Geisler et al., 2006) for CRE analysis. All three genes contain possible CREs involved in drought, heat, light, ROS/sugar, abscissic acid, ethylene, gibberellin, and auxin responses. These data suggest that factors that directly influence plant metabolism are integrated into LSD1-, EDS1-, and PAD4-mediated redox and cell death signaling.
Table 1.
Expression of LSD1, EDS1, and PAD4 after Different Treatments of Wild-Type and PHO1 and PHO3 Null Mutant Plants
| Gene
|
|||
|---|---|---|---|
| Treatment | LSD1 | EDS1 | PAD4 |
| Phosphate starvation 24 h | Induced, P = 0.006520 | Not changed | Not changed |
| pho1 mutant | Not changed | Induced, P = 0.000419 | Induced, P = 0.000419 |
| pho3 mutant | Not changed | Induced, P = 0.000697 | Induced, P = 0.000697 |
| Elevated CO2, 0.08% | Induced, Pearson correlation >0.8 | Not changed | Not changed |
| Heat shock shoot 15 min | Induced, P = 0.0011 | Inhibited, P = 0.009515 | Inhibited, P = 0.009515 |
| Drought <1 h | Not changed | Induced, P = 0.000259 | Induced, P = 0.000184 |
Significant changes in transcript levels (indicated as induced or inhibited) 24 h after phosphorous starvation in pho1 or pho3 null mutants, 48 h after treatment with elevated CO2 levels (0.08%) in wild-type plants, 15 min after heat shock treatment in wild-type plants, and 1 h after drought stress in wild-type plants. Plants were fumigated with 0.08% CO2 in ambient oxygen and nitrogen concentration for 2 d. Experiments were performed in triplicate on pooled samples (n = 3, ±sd).
DISCUSSION
Arabidopsis Forms Lysigenous Aerenchyma in Secondary Xylem I in Response to Flooding
We found that flooding of 12-week-old Arabidopsis plants induces autolysis of the axial parenchyma cells in secondary xylem I. This tissue is formed during the secondary growth of the root-hypocotyl axis, and it is composed of axial parenchyma cells and vessel elements (Chaffey et al., 2002; A. Banasiak and E. Mellerowicz, unpublished data). The autolysis creates air spaces continuously throughout the stem-root axis where vessel elements with remaining parenchyma cells are apparently suspended. By definition, the aerenchyma is a tissue frequently induced by flooding where enlarged air spaces between cells create gas exchange channels (Evans, 2003; Evert, 2006); therefore, in Arabidopsis, we called this tissue aerenchyma.
Aerenchyma is commonly induced in young roots of hydrophytes and mezophytes but is has not been previously observed in Arabidopsis (Justin and Armstrong, 1987; Evans, 2003; Seago et al., 2005). Most commonly it has been found in the cortical parenchyma close to the root apical meristem (Justin and Armstrong, 1987; Seago et al., 2005). Relatively little is known about the occurrence of aerenchyma in older roots with secondary growth where the primary cortex has been destroyed. In soybean (Glycine max), the secondary aerenchyma developed in the secondary cortex of roots and hypocotyls (Laan et al., 1989; Shimamura et al., 2003). Lythrum salicaria and some other species form the aerenchyma in the phellem (Stevens et al., 2002). By contrast, pea (Pisum sativum; Gladish et al., 2006) and lodgepole pine (Pinus contorta; Topa and McLeod, 1986) induced aerenchyma in root stele when exposed to waterlogging. Arabidopsis plants also formed aerenchyma within the secondary tissues of the vascular cylinder, but in contrast with the species mentioned above, the aerenchymatous lacunae were mostly restricted to xylem I (Figure 1A). The younger plants that did not develop lignified secondary xylem II failed to develop aerenchyma under hypoxia conditions. The failure of younger plants to develop aerenchyma (see Supplemental Figure 2 online) might be related to the lack of a lignified xylem core that could isolate the tissues by creating compartments that entrap ethylene (Evans, 2003). In young roots of wetland plants, the compact cell layers of endodermis and exodermis always surround the developing aerenchyma tissues (Seago et al., 2005), whereas suberized or lignified tissues surround aerenchyma in secondary growth (Stevens et al., 2002; Shimamura et al., 2003). Thus, the developmental stage of the plants appears to play a role in their ability to form aerenchyma.
Classically, two basic mechanisms of aerenchyma formation have been described: cell lysis (lysigeny) and cell separation (shizogeny) (Justin and Armstrong, 1987; Evans, 2003; Evert, 2006). In addition, cell division and/or differential cell expansion (expansigeny) is involved in many but not in all species studied to date (Seago et al., 2005). In hypocotyls of 12-week-old Arabidopsis plants, no signs of cell expansion, division, necrosis, or cell separation were observed. Instead, the cells disappeared completely by lysigeny (Figure 1).
The cell wall autolysis in Arabidopsis plants was rapid. During the first 6 d of flooding, no signs of any differentiation were observed at the light microscopy level, and at day 7, condensed and moon-shaped nuclei were detected in not fully digested cells, while other parenchyma cells were completely digested, including their walls and protoplasts (Figures 1A and 1B). Similar timing of lysigenous aerenchyma formation has been reported in maize and rice (Oryza sativa; Evans, 2003). The rapidity of the autolysis process during aerenchyma development resembled that during the PCD of tracheary elements, except that in the latter case, the lignified walls and primary cell walls that are in contact with them remained intact (Ohdaira et al., 2002). However, when the deposition of secondary walls is prevented as in the gapped xylem mutant, the entire cell with its primary walls is autolysed (Kozela and Regan, 2003) in an apparently similar manner to the lysigenous aerenchyma.
A Genetic System That Controls Aerenchyma Formation in Arabidopsis
From the analysis of stomatal conductance and H2O2 and ACC levels, the following sequence of events leading to aerenchyma formation in Arabidopsis is proposed (Figures 1 to 8). First, the stomatal conductance in leaves decreases, which we propose results from a systemic signaling of hypoxia, for example, due to reduced root pressure, followed by the initiation of a gradual increase in H2O2 in hypocotyls for 6 d and an increase in ACC in hypocotyls preceding the aerenchyma formation after 7 d (Figures 1 and 5). In previous work, we have found that photoproduced H2O2 is strongly correlated with a decrease in leaf stomatal conductance and foliar RCD (Mateo et al., 2004). Additionally, we showed that light sensitivity, stomatal conductance, and ROS accumulation are controlled by LSD1, EDS1, and PAD4 in foliar tissues (Figure 6; Mateo et al., 2004). Therefore, we tested if this genetic system regulated the aerenchyma formation. Here, we show that LSD1, PAD4, and EDS1 also control lysigenous aerenchyma formation via ethylene and ROS (Figures 4 to 7). It is important to note, however, that the PCD of lysigenous aerenchyma in hypocotyls occurs in a similar but independent manner to the foliar PCD when roots are exposed to hypoxia. This is indicated by the observations that simultaneous exposure of rosettes to mild EEE and hypoxia stress induce RCD (Mateo et al., 2004) and accelerate aerenchyma formation (see Supplemental Table 1 online). However, it is important to note that under root normoxia and induced foliar RCD or under root hypoxia of plants that already developed RCD in majority of leaves, aerenchyma was not formed (Figure 7). In addition, the observation that APX1 was induced more in roots than shoots (Figure 5) confirms that root-specific signaling is a prerequisite for aerenchyma formation. Thus, the induction of aerenchyma is subject to a genetic and tissue-specific program. Moreover, roots and shoots respond differently to waterlogging (Liao and Lin, 2001) in several plant species as well as in Arabidopsis (Ellis et al., 1999; Liao and Lin, 2001), and our results confirm this observation.
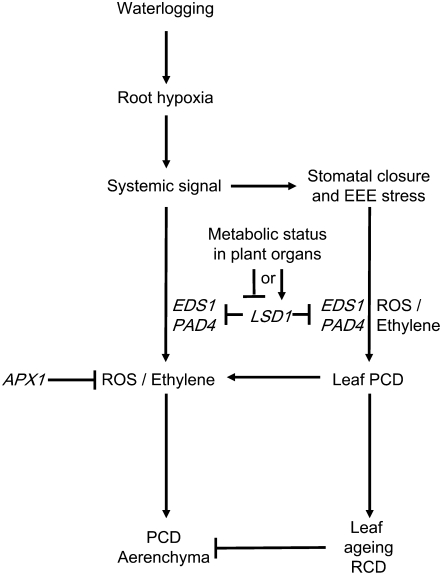
Figure 8.
Proposed Working Model for Signaling Pathways That Regulate Aerenchyma Formation in Arabidopsis.
Root hypoxia produces a systemic signal that promotes ROS/ethylene formation, leading to the induction of aerenchyma and stomatal closure. Stomatal closure leads to EEE stress, which enhance ROS/ethylene systemic signaling and PCD in leaves (Mateo et al., 2004) and aerenchyma formation (Figures 1 to 7; see Suplemental Table 1 online). ROS/ethylene signaling operates in a positive feed-forward loop, which is negatively controlled by LSD1 and positively controlled by PAD4 and EDS1 (Figures 5 to 7). PCD during aerenchyma formation is independent from that in leaves and needs a tissue-specific signal. Severe PCD development (RCD) in leaves somehow restricts the development of aerenchyma.
We suggest that several environmental factors are integrated for the induction of lysigenous aerenchyma. First, light quantity has been shown to affect stellar development (Wahl et al., 2001), and we observed that stellar development determines the ability of Arabidopsis hypocotyls to produce aerenchyma. We also observed that different light conditions affect the timing of aerenchyma induction (see Supplemental Table 1 online). Second, CO2 has been shown to accumulate in waterlogged soils to levels (Drew et al., 1979; Jackson, 1985) that are ∼5 times higher than what is needed to promote expression of LSD1 (Table 1). High CO2 levels are also known to affect both ethylene signaling and aerenchyma formation (Drew et al., 1979; Jackson, 1985; Constable and Longstreth, 1994; Bragina et al., 2001; Liao and Lin, 2001). Third, root hypoxia reduces phosphorus accumulation in roots and reduces total phosphorus uptake in the plant (Drew et al., 1979; Topa and Cheeseman, 1994). Phosphorous deficiency also induces aerenchyma formation and enhances production and sensitivity to ethylene and other hormones (such as auxin) involved in hypoxia (He et al., 1992; Fan et al., 2003). Our transcript level analysis and meta-analysis of DNA microarray experiments show that phosphorous deficiency strongly influences LSD1, EDS1, and PAD4 gene expression. Phosphorous deficiency caused either by limitation of Pi in the growth medium or through mutation in the PHOSPHOLIPIDS DEFICIENT1 (PHO1) or PHO3 genes that cause phosphate-deficient phenotypes induce LSD1, EDS1, and PAD4 (Abel et al., 2002; Table 1). Additionally, reduced respiration is known to be a common denominator for phosphorous deficiency, hypoxia, and high CO2 (Qi et al., 1994; Fan et al., 2003), indicating that the metabolic status in the plant is an important determinant for aerenchyma formation. Therefore, we propose that metabolic signals derived from hypoxia upstream of LSD1, EDS1, and PAD4 contribute to the regulation of the cellular redox status during aerenchyma formation as suggested in the proposed model (Figure 8).
It is possible that these genes in their functions as redox regulators have effects on the whole-plant metabolism that may have affected aerenchyma formation. Nevertheless, LSD1, EDS1, and PAD4 control the extent and the timing of aerenchyma formation (Figure 3). These data suggested that LSD1 is a negative regulator, while PAD4 and EDS1 are positive regulators of the amount of aerenchyma. Additionally, PAD4 and EDS1 also regulate the timing of the event (Figure 3B). Since both PAD4 and EDS1 functions are needed to initiate aerenchyma, these genes likely act in a common pathway, as previously described (Rusterucci et al., 2001; Mateo et al., 2004). These findings also indicate that the LSD1, EDS1, and PAD4 genes may have important functions in other responses to hypoxia (Mateo et al., 2004; Table 1).
We have concluded that hypocotyls and roots of the model plant species, Arabidopsis, can form lysigenous aerenchyma associated with PCD. We have also confirmed that some of the known inducers and regulators of lysigenous aerenchyma in other species, such as hypoxia, H2O2, and ethylene are involved in aerenchyma development in Arabidopsis. We have demonstrated that timing of ROS signaling is ahead of ethylene signaling in aerenchyma development. Moreover, we have identified genes essential for this process (Figures 5 to 8). Therefore, we report here that this genetic system does not only control leaf PCD during normoxia but also controls a tissue-specific PCD during hypoxia.
Future Perspectives
Our findings open new possibilities of using the genetic system of Arabidopsis to elucidate the underlying mechanism of this important process of stress adaptation in plants. An interesting aspect of the flooding response in Arabidopsis is the predictable and complete autolysis of cell walls. This gives another opportunity, namely, to use this system to study cell wall autolysis. This process is very important, for example, in the vessel differentiation of angiosperm plants, but because no good experimental system has been developed to study this process so far, the enzymes involved and their dynamics remain elusive.
Moreover, we have shown that the lysigenous aerenchyma of Arabidopsis is formed at a late stage of development when the secondary growth is advanced. It is possible that most species with profuse secondary growth, especially trees, form a similar type of aerenchyma, and this is an area that desperately needs to be investigated. Since Arabidopsis is already a well-explored model system for wood formation (Chaffey et al., 2002; Nieminen et al., 2004), our findings open up new possibilities of using the Arabidopsis model to specifically address issues relating to tree responses to flooding and hypoxia.
METHODS
Growth and Light Conditions
Arabidopsis thaliana Ws-0 and five different mutants of the same accession were used: lsd1, eds1-1, and pad4-5 mutants and the lsd1 eds1-1 and lsd pad4-5 double mutants (Mateo et al., 2004). Plants were grown in low-light chambers at PFD 100 ± 25 μmol m2 s−1, relative air humidity 60%, and temperature 20 ± 2°C in a 9-h photoperiod. Oxygen levels were analyzed using a Schott minilab 12 (Schott-Geräte). For excess light, Arabidopsis plants were treated at PFD 2000 ± 250 μmol m2 s−1 (HMI 1200 W/GS photo-optic lamp; Osram) for up to 2 h. Photoperiod, temperature (±4°C), and humidity (±5%) for all experiments were similar to those in the low-light chamber.
Stomatal Conductance
Stomatal conductance was measured in growth conditions by measuring the speed of rehydration (cm/s) of a cyclically desiccated chamber by 1-cm2 leaf areas. We used a portable AP4 Porometer (Delta-T Devices) according to the manufacturer's instructions.
Aerenchyma Detection and Quantification
Hypocotyls of waterlogged and control plants (12 weeks old) were fixed in FAA (70% ethanol, 5% acetic acid, and 1.75% formaldehyde) for 2 d. The fixed material was then transferred to 80% ethanol and gradually rehydrated before hand-sectioning with a razor blade. Cross sections were stained with 0.05% Toluidine Blue containing 1% boric acid for general anatomy staining or with 0.1% ruthenium red to visualize pectin-rich cell walls of parenchyma cells. Area analysis of the aerenchymatous lacunae and parenchymatous tissues was determined from grayscale digital images as previously described (Gunawardena et al., 2001a; Chaerle et al., 2004).
Root-shoot aerenchyma distribution was visualized by first dipping sections in red ink and then in 99.5% ethanol. The hardened ink was carefully wiped off the surface of the sections, leaving only the cavities ink filled. Sections were then analyzed under UV fluorescence for increased visibility. For DAPI staining, hypocotyls were collected and hand-sectioned with a razor blade. Sections were stained with DAPI solution (1 μg/mL) and observed under UV fluorescence.
Hydrogen Peroxide Quantification
Hydrogen peroxide was quantified as described (Guilbault et al., 1968; Jimenez et al., 2002), with the following modification: 100 mg of fresh Arabidopsis hypocotyl or leaf tissue of 12-week-old plants was used per 1 mL of extraction medium.
ACC Quantification
ACC production was determined as described by Lizada and Yang (1979) and Langebartels et al. (1991). Fresh hypocotyl or leaf tissues of 12-week-old plants were used.
Molecular Analyses
For in vitro analysis of APX1 promoter:LUC induction, hypocotyls of 12-week-old APX1promoter:LUC transgenic plants (Karpinska et al., 2000; Chang et al., 2004) were used. A luciferase assay kit (Promega) and a Berthold LB950 luminometer was used to measure the luciferase expression as previously described (Karpinski et al., 1997, 1999; Chang et al., 2004). For in vivo visualization, APX1promoter:LUC transgenic plants were grown hydroponically as described (Norén et al., 2004) in Hoagland solution modified for Arabidopsis (Gibeaut et al., 1997). Detection of luciferase was done by spraying entire plants with a 2 mM d(−)-luciferin (Promega) solution containing 0.01% Tween and imaging of luciferase activity with a Typhoon 8600 variable mode imager (Molecular Dynamics).
Quantitative PCR reactions for LSD1, PAD4, and EDS1 were performed with the following gene-specific primers: LSD1 forward, 5′-GAGGAGCATCTAATGTGCGTTGT-3′, and reverse, 5′-GAGGGTGGTGTTGAAGTTGATGTA-3′; EDS1 forward, 5′-TCAAGCTTCTGTGGAAATGG-3′, and reverse, 5′-CGCTTCCAGTCAATTCACAA-3′; PAD4 forward, 5′-AGTTAAAGATCAAGGAAGGATTGG-3′ and reverse, 5′-CCTCTGATGTTCCTCGGTG-3′ Reactions were performed as described before (Chang et al., 2004). Putative CREs of LSD1, EDS1, and PAD4 were identified by analyzing 1000-bp 5′ upstream (promoter) regions using the publicly available PLACE (Higo et al., 1999) database algorithms and verified by our algorithm (Geisler et al., 2006), which generates a list of element identities, sequence, and position on the promoter region. Sequences of LSD1, EDS1, and PAD4 were obtained from the National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
In silico analysis of gene expression was performed on publicly available microarray data as described by Geisler et al., (2006). Data for the effect of elevated CO2 (800 ppm) on LSD1 expression are publicly available at http://www.arabidopsis.org (expression of clone ID 268g12XP, locus At4g20380), and we performed our elevated CO2 experiments as described before (Mateo et al., 2004).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: At4g20380 (LSD1), At3g52430 (PAD4), and At3g48090 (EDS1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Oxygen Levels Decrease during Waterlogging.
Supplemental Figure 2. Four-Week-Old Plants Do Not Make Aerenchyma.
Supplemental Figure 3. Wild-Type (Ws-0) and lsd1 Xylem Cores Contain Similar Amounts of Parenchyma.
Supplemental Table 1. Simultaneous Exposure to EEE and Root Hypoxia Accelerates Aerenchyma Formation.
Supplementary Material
Acknowledgments
We acknowledge the financial support from the Polish Ministry of Science and Higher Education (Grants N301 075 31/2414 and N301 2550 33 to S.K.), the Swedish Research Council and the Swedish Strategic Foundation, and the Knut and Alice Wallenberg Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Stanislaw Karpinski (karpinski@ifr-pan.krakow.pl).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abel, S., Ticconi, C.A., and Delatorre, C.A. (2002). Phosphate sensing in higher plants. Physiol. Plant. 115 1–8. [DOI] [PubMed] [Google Scholar]
- Adams, D.O., and Yang, S.F. (1979). Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 76 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch, M., Gobbato, E., Bednarek, P., Debey, S., Schultze, J.L., Bautor, J., and Parker, J.E. (2006). Regulators of salicylic acid–independent EDS1 signaling in Arabidopsis immunity and cell death. Plant Cell 18 1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell, A., Yang, Z., Springer, P.S., and Bailey-Serres, J. (2002). RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296 2026–2028. [DOI] [PubMed] [Google Scholar]
- Bragina, T.V., Martinovich, L.I., Rodionova, N.A., Bezborodov, A.M., and Grineva, G.M. (2001). Ethylene-induced activation of xylanase in adventitious roots of maize as a response to the stress effect of root submersion. Appl. Biochem. Microbiol. V37 618–621. [PubMed] [Google Scholar]
- Brodersen, P., Petersen, M., Nielsen, H.B., Zhu, S., Newman, M.A., Shokat, K.M., Rietz, S., Parker, J., and Mundy, J. (2006). Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J. 47 532–546. [DOI] [PubMed] [Google Scholar]
- Campbell, R., and Drew, M.C. (1983). Electron microscopy of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to oxygen shortage. Planta 157 350–357. [DOI] [PubMed] [Google Scholar]
- Chaerle, L., Hagenbeek, D., De Bruyne, E., Valcke, R., and Van Der Straeten, D. (2004). Thermal and chlorophyll-fluorescence imaging distinguish plant-pathogen interactions at an early stage. Plant Cell Physiol. 45 887–896. [DOI] [PubMed] [Google Scholar]
- Chaffey, N., Cholewa, E., Regan, S., and Sundberg, B. (2002). Secondary xylem development in Arabidopsis: A model for wood formation. Physiol. Plant. 114 594–600. [DOI] [PubMed] [Google Scholar]
- Chang, C.C.-C., Ball, L., Fryer, M.J., Baker, N.R., Karpinski, S., and Mullineaux, P.M. (2004). Induction of ASCORBATE PEROXIDASE 2 expression in wounded Arabidopsis leaves does not involve known wound-signalling pathways but is associated with changes in photosynthesis. Plant J. 38 499–511. [DOI] [PubMed] [Google Scholar]
- Colmer, T.D., Peeters, A.J.M., Wagemaker, C.A.M., Vriezen, W.H., Ammerlaan, A., and Voesenek, L.A.C.J. (2004). Expression of alpha-expansin genes during root acclimations to O2 deficiency in Rumex palustris. Plant Mol. Biol. V56 423-437. [DOI] [PubMed] [Google Scholar]
- Constable, J.V.H., and Longstreth, D.J. (1994). Aerenchyma carbon dioxide can be assimilated in Typha latifolia L. leaves. Plant Physiol. 106 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruxelles, G.L., Peacock, W.J., Dennis, E.S., and Dolferus, R. (1996). Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 111 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, E.S., Dolferus, R., Ellis, M., Rahman, M., Wu, Y., Hoeren, F.U., Grover, A., Ismond, K.P., Good, A.G., and Peacock, W.J. (2000). Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 51 89–97. [PubMed] [Google Scholar]
- Dordas, C., Rivoal, J., and Hill, R.D. (2003). Plant haemoglobins, nitric oxide and hypoxic stress. Ann. Bot. (Lond.) 91 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, M.C., He, C.J., and Morgan, P.W. (2000). Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 5 123–127. [DOI] [PubMed] [Google Scholar]
- Drew, M.C., Jackson, M.B., and Giffard, S. (1979). Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta V147 83–88. [DOI] [PubMed] [Google Scholar]
- Ellis, M.H., Dennis, E.S., and James Peacock, W. (1999). Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 119 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, D.E. (2003). Aerenchyma formation. New Phytol. 161 35–49. [Google Scholar]
- Evert, R.F. (2006). Esau's Plant Anatomy: Meristems, Cells and Tissues of the Plant Body: Their Structure, Function and Development. (Hoboken, NJ: John Wiley & Sons).
- Fan, M., Zhu, J., Richards, C., Brown, K.M., and Lynch, J.P. (2003). Physiological roles for aerenchyma in phosphorus-stressed roots. Funct. Plant Biol. 30 493–506. [DOI] [PubMed] [Google Scholar]
- Feys, B.J., Wiermer, M., Bhat, R.A., Moisan, L.J., Medina-Escobar, N., Neu, C., Cabral, A., and Parker, J.E. (2005). Arabidopsis SENESCENCE ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17 2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao, T., and Bailey-Serres, J. (2004). Plant responses to hypoxia - Is survival a balancing act? Trends Plant Sci. 9 449–456. [DOI] [PubMed] [Google Scholar]
- Geisler, M., Kleczkowski, L.A., and Karpinski, S. (2006). A universal algorithm for genome-wide in silicio identification of biologically significant gene promoter putative cis-regulatory-elements; Identification of new elements for reactive oxygen species and sucrose signaling in Arabidopsis. Plant J. 45 384–398. [DOI] [PubMed] [Google Scholar]
- Gibeaut, D.M., Hulett, J., Cramer, G.R., and Seemann, J.R. (1997). Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 115 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladish, D.K., Xu, J., and Niki, T. (2006). Apoptosis-like programmed cell death occurs in procambium and ground meristem of pea (Pisum sativum) root tips exposed to sudden flooding. Ann. Bot. (Lond.) 97 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbault, G.G., Brignac, J.P., and Zimmer, M. (1968). Homovanillic acid as a fluorometric substrate for oxidative enzymes. Analytical applications of the peroxidase, glucose oxidase and xanthine oxidase systems. Anal. Chem. 40 190–196. [DOI] [PubMed] [Google Scholar]
- Gunawardena, A., Pearce, D.M.E., Jackson, M.B., Hawes, C.R., and Evans, D.E. (2001. a). Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize (Zea mays L.) promoted by ethylene. Plant Cell Environ. 24 1369–1375. [Google Scholar]
- Gunawardena, A.H., Pearce, D.M., Jackson, M.B., Hawes, C.R., and Evans, D.E. (2001. b). Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212 205–214. [DOI] [PubMed] [Google Scholar]
- He, C.J., Morgan, P.W., and Drew, M.C. (1992). Enhanced sensitivity to ethylene in nitrogen-starved or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiol. 98 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C.J., Morgan, P.W., and Drew, M.C. (1996). Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 112 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo, K., Ugawa, Y., Iwamoto, M., and Koranaga, T. (1999). Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 27 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs, T., Dietrich, R.A., and Dangl, J.L. (1996). Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 27 1853–1856. [DOI] [PubMed] [Google Scholar]
- Jackson, M.B. (1985). Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 36 145–174. [Google Scholar]
- Jackson, M.B., and Armstrong, W. (1999). Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1 274–287. [Google Scholar]
- Jackson, M.B., Fenning, T.M., Drew, M.C., and Saker, L.R. (1985). Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta 165 486–492. [DOI] [PubMed] [Google Scholar]
- Jimenez, A., Creissen, G., Kular, B., Firmin, J., Robinson, S., Verhoeyen, M., and Mullineaux, P. (2002). Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 214 751–758. [DOI] [PubMed] [Google Scholar]
- Justin, S., and Armstrong, W. (1987). The anatomical characteristics of roots and plant-response to soil flooding. New Phytol. 106 465–495. [Google Scholar]
- Karpinska, B., Wingsle, G., and Karpinski, S. (2000). Antagonistic effects of hydrogen peroxide and glutathione on acclimation to excess excitation energy in Arabidopsis. IUBMB Life 50 21–26. [DOI] [PubMed] [Google Scholar]
- Karpinski, S., Escobar, C., Karpinska, B., Creissen, G., and Mullineaux, P. (1997). Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski, S., Reynolds, H., Karpinska, B., Wingsle, G., Creissen, G., and Mullineaux, P. (1999). Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284 654–657. [DOI] [PubMed] [Google Scholar]
- Klok, E.J., Wilson, I.W., Wilson, D., Chapman, S.C., Ewing, R.M., Somerville, S.C., Peacock, W.J., Dolferus, R., and Dennis, E.S. (2002). Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14 2481–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela, C., and Regan, S. (2003). How plants make tubes. Trends Plant Sci. 8 159–164. [DOI] [PubMed] [Google Scholar]
- Laan, P., Berrevoets, M.J., Lythe, S., Armstrong, W., and Blom, C. (1989). Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. J. Ecol. 77 693–703. [Google Scholar]
- Langebartels, C., Kerner, K., Leonardi, S., Schraudner, M., Trost, M., Heller, W., and Sandermann, H., Jr. (1991). Biochemical plant responses to ozone: Differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiol. 95 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, C.T., and Lin, C.H. (2001). Physiological adaptation of crop plants to flooding stress. Proc Natl Sci Counc Repub China B. 25 148–157. [PubMed] [Google Scholar]
- Lizada, M.C., and Yang, S.F. (1979). A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 100 140–145. [DOI] [PubMed] [Google Scholar]
- Manac'h-Little, N, Igamberdiev, AU, Hill, RD. (2005). Hemoglobin expression affects ethylene production in maize cell cultures. Plant Physiol. Biochem. 43 485–489. [DOI] [PubMed] [Google Scholar]
- Mateo, A., Mühlenbock, P., Rusterucci, C., Chang, C.C.-C., Miszalski, Z., Karpinska, B., Parker, J.E., Mullineaux, P.M., and Karpinski, S. (2004). LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol. 136 2818–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk, L.S., Fagerstedt, K.V., and Crawford, R.M.M. (1987). Superoxide dismutase as an anaerobic polypeptide - A key factor in recovery from oxygen deprivation in Iris pseudacorus. Plant Physiol. 85 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen, K.M., Kauppinen, L., and Helariutta, Y. (2004). A weed for wood? Arabidopsis as a genetic model for xylem development. Plant Physiol. 135 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norén, H., Svensson, P., and Andersson, B. (2004). A convenient and versatile hydroponic cultivation system for Arabidopsis thaliana. Physiol. Plant. 121 343–348. [Google Scholar]
- Ohdaira, Y., Kakegawa, K., Amino, S.-i., Sugiyama, M., and Fukuda, H. (2002). Activity of cell-wall degradation associated with differentiation of isolated mesophyll cells of Zinnia elegans into tracheary elements. Planta V215 177–184. [DOI] [PubMed] [Google Scholar]
- Peng, H.P., Chan, C.S., Shih, M.C., and Yang, S.F. (2001). Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 126 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell, R.I., and Lamb, C. (1997). Programmed cell death in plants. Plant Cell 9 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, M., et al. (2000). Arabidopsis MAP Kinase 4 negatively regulates systemic acquired resistance. Cell 103 1111–1120. [DOI] [PubMed] [Google Scholar]
- Pezeshki, S.R. (1994). Responses of Baldcypress (Taxodium distichum) seedlings to hypoxia - Leaf protein-content, Ribulose-1,5-bisphosphate carboxylase oxygenase activity and photosynthesis. Photosynthetica 30 59–68. [Google Scholar]
- Pezeshki, S.R., DeLaune, R.D., Kludze, H.K., and Choi, H.S. (1996. b). Photosynthetic and growth responses of cattail (Typha domingensis) and sawgrass (Cladium jamaicense) to soil redox conditions. Aquat. Bot. 54 25–35. [Google Scholar]
- Pezeshki, S.R., Pardue, J.H., and DeLaune, R.D. (1996. a). Leaf gas exchange and growth of flood-tolerant and flood-sensitive tree species under low soil redox conditions. Tree Physiol. 16 453–458. [DOI] [PubMed] [Google Scholar]
- Prestridge, D.S. (1991). SIGNAL SCAN: A computer program that scans DNA sequences for eukaryotic transcriptional elements. Comput Appl Biosci. 7 203–206. [DOI] [PubMed] [Google Scholar]
- Qi, J., Marshall, J.D., and Mattson, K.G. (1994). High soil carbon dioxide concentrations inhibit root respiration of Douglas fir. New Phytol. 128 435–442. [DOI] [PubMed] [Google Scholar]
- Rusterucci, C., Aviv, D.H., Holt III, B.F., Dangl, J.L., and Parker, J.E. (2001). The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13 2211–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seago, J.L.J., Marsh, L.C., Stevens, K.J., Soukup, A., Votrubova, O., and Enstone, D.E. (2005). A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Ann. Bot. (Lond.) 96 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter, T.L., and Waters, I. (2003). Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253 1–34. [Google Scholar]
- Shimamura, S., Mochizuki, T., Nada, Y., and Fukuyama, M. (2003). Formation and function of secondary aerenchyma in hypocotyl, roots and nodules of soybean (Glycine max) under flooded conditions. Plant Soil 251 351–359. [Google Scholar]
- Stevens, K.J., Peterson, R.L., and Reader, R.J. (2002). The aerenchymatous phellem of Lythrum salicaria (L.): A pathway for gas transport and its role in flood tolerance. Ann. Bot. (Lond.) 89 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storozhenko, S., De Pauw, P., Van Montagu, M., Inze, D., and Kushnir, S. (1998). The heat-shock element is a functional component of the Arabidopsis APX1 gene promoter. Plant Physiol. 118 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah, C.C., and Sachs, M.M. (2003). Molecular and cellular adaptations of maize to flooding stress. Ann. Bot. (Lond.) 91 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topa, M.A., and Cheeseman, J.M. (1994). Maintenance of 32P uptake and transport in Pinus serotina seedlings under hypoxic growth conditions. Physiol. Plant. 92 171–180. [Google Scholar]
- Topa, M.A., and McLeod, K.W. (1986). Aerenchyma and lenticel formation in pine seedlings: A possible avoidance mechanism to anaerobic growth conditions. Physiol. Plant. 68 540–550. [Google Scholar]
- Uren, A.G., O'Rourke, K., Aravind, L., Pisabarro, M.T., Seshagiri, S., Koonin, E.V., and Dixit, V.M. (2000). Identification of paracaspases and metacaspases: Two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 6 961–967. [DOI] [PubMed] [Google Scholar]
- Wahl, S., Ryser, P., and Edwards, P.J. (2001). Phenotypic plasticity of grass root anatomy in response to light intensity and nutrient supply. Ann. Bot. (Lond.) 88 1071–1078. [Google Scholar]
- Watts, R.A., Hunt, P.W., Hvitved, A.N., Hargrove, M.S., Peacock, W.J., and Dennis, E.S. (2001). A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc. Natl. Acad. Sci. USA 98 10119–10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, K., and Patrick, W.H., Jr. (2003). Redox range with minimum nitrous oxide and methane production in a rice soil under different pH. Soil Sci. Soc. Am. J. 67 1952–1958. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.