Abstract
Gene expression in chloroplasts is regulated mainly at the posttranscriptional level. In the green alga Chlamydomonas reinhardtii, synthesis of the D2 protein (PsbD), which is the rate-determining subunit for the assembly of photosystem II, depends on the RNA stability factor Nac2. In addition, the RNA binding protein RBP40 has been implicated in translational control via a U-rich element in the 5′ untranslated region (5′UTR) of the psbD mRNA. Here, we report the identification of the RBP40 gene based on mass spectrometric analysis of its purified product. Unexpectedly, this was found to be identical to the previously described RNA binding protein RB38, which had been suggested to be involved in the regulation of D1 protein synthesis. However, we show that RBP40 binds to the psbD 5′UTR in a Nac2-dependent fashion both in vitro and in vivo. Molecular characterization of RBP40 RNA interference lines confirmed that RBP40 specifically affects the initiation of D2 synthesis. Native polyacrylamide gel electrophoresis, coimmunoprecipitation, and sedimentation analyses revealed that Nac2 and RBP40 form parts of a complex of 550 kD that is displaced from the psbD mRNA prior to polysome assembly. Together, these data indicate that the processes of 5′UTR-mediated RNA stabilization and translation initiation are tightly coupled in Chlamydomonas.
INTRODUCTION
Chloroplasts originated from a cyanobacterium that formed an endosymbiotic relationship with a heterotrophically growing eukaryote. The gradual transformation of the endosymbiont into the chloroplast involved extensive gene transfer from the developing organelle to the nuclear genome of the host. Nevertheless, the modern chloroplast genome has retained a set of ∼100 protein-coding genes and encodes the basic machinery for their expression, which is essentially of prokaryotic origin. Since the chloroplast-encoded gene products form multisubunit complexes with imported nucleus-encoded proteins, the requirement for tight coordination of gene expression in these two cellular compartments has resulted in the development of an intracellular communication system. This comprises nucleus-encoded regulatory factors that regulate almost all stages of chloroplast gene expression, including transcription, RNA metabolism, and splicing, as well as translation and protein complex assembly.
Translational regulation plays a central role in determining the levels of the various chloroplast proteins (Bruick and Mayfield, 1999; Zerges, 2000). The application of in vitro and in vivo approaches has allowed several cis-acting determinants for translation initiation to be mapped in chloroplast RNAs (Hirose and Sugiura, 1996; Bruick and Mayfield, 1999; Higgs et al., 1999; Nickelsen et al., 1999; Yukawa et al., 2007), and these probably represent the target sites for translational regulatory factors (Zerges, 2000; Manuell et al., 2004).
To date, genetic analyses have identified only a few nuclear genes whose products play a role in protein synthesis in the chloroplast. The CRP1 gene is required for translation of petA/petD mRNA in maize (Zea mays) (Barkan et al., 1994; Schmitz-Linneweber et al., 2005), and HCF107 and HCF173 from Arabidopsis thaliana participate in psbB and psbA mRNA translation, respectively (Sane et al., 2005; Schult et al., 2007). Recently, a fourth factor from vascular plants, named ATAB2, was characterized in detail. ATAB2 is a novel, blue light–induced, RNA binding protein that activates the synthesis of plastid-encoded subunits of both photosystems I and II (PSI and PSII) (Barneche et al., 2006). In the green alga Chlamydomonas reinhardtii, its ortholog Tab2 specifically recognizes the 5′ untranslated region (5′UTR) of the psaB mRNA and, as a consequence, controls PsaB synthesis (Dauvillee et al., 2003). Furthermore, the algal proteins Tbc2 and Tba1 are required for the translation of psbC and psbA mRNAs, respectively (Auchincloss et al., 2002; Somanchi et al., 2005).
In vitro interaction assays using plastid RNA probes have also contributed to the identification of proteins capable of specifically recognizing distinct RNA elements within the 5′UTR of chloroplast mRNAs, and these represent good candidates for translational control factors (Bruick and Mayfield, 1999; Nickelsen, 2003). The genes for a few of these biochemically identified RNA binding proteins have been cloned, but no mutants are currently available that substantiate their functional assignments.
One of the best-characterized examples is represented by a multisubunit complex from Chlamydomonas that binds to the psbA 5′UTR in a redox-regulated fashion (Danon and Mayfield, 1991, 1994). This complex is composed of four major polypeptides: the 63-kD protein disulfide isomerase cPDI (Kim and Mayfield, 1997), the 47-kD poly(A) binding protein cPAB1 (Yohn et al., 1998a), the novel 38-kD RNA binding protein RB38 (Barnes et al., 2004), and a 55-kD protein (RB55). RNA binding activity of the complex correlates directly with rates of D1 synthesis, and in several nuclear mutants that exhibit reduced accumulation/activity of some of these subunits, the translation of psbA mRNA is perturbed (Yohn et al., 1998b).
The expression of the related psbD gene encoding the D2 subunit of the PSII reaction center is particularly interesting, because D2 represents the starting point for the assembly of PSII as a whole (de Vitry et al., 1989; Komenda et al., 2004; Minai et al., 2006). According to the CES (for control by epistasis of synthesis) model for the temporal sequence of PSII assembly, the amount of D2 available directly determines the levels of the other component subunits of PSII via feedback control mechanisms (Minai et al., 2006).
In Chlamydomonas, Nac2, a 140-kD tetratricopeptide repeat protein, is strictly required for the stabilization of the psbD mRNA via its 5′UTR (Kuchka et al., 1989; Nickelsen et al., 1994; Boudreau et al., 2000). Furthermore, a second factor, named RBP40, binds to a U-rich translational element located 15 nucleotides upstream of the AUG start codon in vitro (Nickelsen et al., 1999; Ossenbühl and Nickelsen, 2000). Recently, the analysis of cis-acting suppressor mutations suggested that RBP40 functions by inducing conformational changes within the RNA region encompassing the AUG start codon and thereby regulates the early steps in translation initiation on the psbD message (Klinkert et al., 2006).
Here, we report the identification and characterization of the RBP40 gene. Our genetic and biochemical data strongly suggest that the psbD mRNA is the primary target for RBP40 function. Furthermore, RBP40 is shown to be part of a chloroplast multisubunit complex that also contains the RNA stability factor Nac2.
RESULTS
Identification of the RBP40 Gene
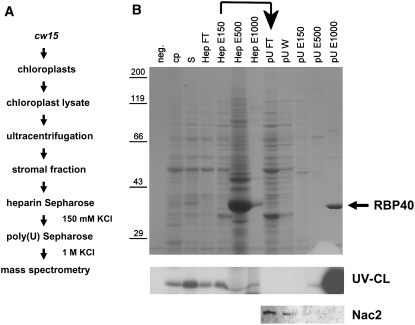
RBP40 is a soluble protein found in the stroma of chloroplasts from Chlamydomonas, where it forms part of a high molecular mass complex and recognizes a U-rich sequence in the 5′UTR of the psbD mRNA (Ossenbühl and Nickelsen, 2000). In order to isolate RBP40, a stromal fraction prepared from wild-type chloroplasts (Figure 1A) was loaded onto a heparin-Sepharose column, and bound proteins were eluted stepwise with KCl. Each fraction was tested for the ability to bind a radiolabeled RNA probe derived from the psbD 5′UTR (Figure 1B). Most of the binding activity was found in the fraction eluted with 150 mM KCl (Figure 1B, HepE150). This material was then subjected to affinity chromatography on a poly(U)-Sepharose column, with stepwise elution, and a major RNA binding activity, in the size range of ∼40 kD, was eluted with 1 M KCl, indicating tight binding to the homopolymeric RNA matrix (Figure 1B, pUE1000). Gel electrophoresis and Coomassie blue staining revealed that this fraction contained one major protein of 40 kD as well as smaller amounts of material at 60 and 80 kD (Figure 1B, pUE1000).
Figure 1.
Isolation of RBP40.
(A) Flow chart listing the steps used to purify RBP40.
(B) SDS-PAGE and Coomassie blue staining of proteins at various stages of purification (top panel), UV cross-linking of RBP40 to radiolabeled psbD 5′UTR RNA (middle panel), and immunodetection of Nac2 in selected fractions by protein gel blot analysis (bottom panel). neg., negative control for RNA binding (no protein loaded); cp, chloroplast lysate; S, stromal protein fraction; HepFT, flow-through fraction from heparin-Sepharose column; HepE150, -500, and -1000, eluates obtained with 150, 500, and 1000 mM KCl, respectively; pUFT/W, flow-through/wash fraction from poly(U)-Sepharose column; pUE150, -500, and -1000, eluates obtained with 150, 500, and 1000 mM KCl, respectively. The RBP40 that eluted with high salt from poly(U)-Sepharose is marked by the arrow.
To test whether this 40-kD protein represents RBP40, binding assays with the psbD probe were performed in the presence of increasing concentrations of homologous or heterologous unlabeled RNA probes as competitors. As shown in Figure 2, the 40-kD activity bound the psbD probe. However, in contrast with what one would expect for RBP40 (Ossenbühl and Nickelsen, 2000), it also recognized both a mutant psbD probe lacking the U-rich RBP40 target sequence and a psbA 5′UTR probe with almost equal affinity. In vivo, RBP40 activity is known to require the RNA stability factor Nac2 (Ossenbühl and Nickelsen, 2000). Therefore, we tested for the presence of Nac2 in the pUE1000 fraction using antibodies (Figure 1B, pUE1000), but none was detected. Instead, Nac2 was observed in the flow-through material containing 50 mM KCl, which itself has no RBP40 activity (Figure 1B, pUFT/W). Thus, in contrast with heparin-Sepharose, poly(U)-Sepharose chromatography does not allow copurification of the two factors.
Figure 2.
Nac2 Confers RNA Binding Specificity on RBP40.
(A) The pUE1000 fraction containing the purified RBP40 (see Figure 1A) alone, in combination with the wild-type flow-through fraction from the poly(U)-Sepharose column (pUE+WTFT) containing Nac2, or with the same fraction from the mutant nac2 (pUE+nac2FT) was incubated with radiolabeled psbD 5′UTR RNA in the presence of a 5-, 50-, or 500-fold excess of the indicated competitor RNA and analyzed by UV cross-linking.
(B) In the graphs, the intensities of the RBP40 signals are plotted against the relative levels of the indicated competitor RNAs in the reactions, based on densitometric scanning of the autoradiograms shown in (A).
Addition of the poly(U) flow-through to the pUE1000 fraction indeed restored high-affinity binding of RBP40 to the psbD 5′UTR (Figure 2). To verify that Nac2 represents the affinity-conferring component of the pUFT/W fraction, we also analyzed stromal proteins obtained from the nuclear mutant nac2, which lacks the Nac2 factor (Boudreau et al., 2000). When the poly(U)-Sepharose flow-through material obtained from this preparation was added to purified RBP40, the affinity of RBP40 for the psbD 5′UTR was reduced significantly, confirming that Nac2 is essential for high-affinity binding (Figure 2). Nevertheless, in contrast with purified RBP40 alone, the addition of nac2 mutant material still resulted in a slight increase in the affinity of RBP40 for the psbD probe relative to the other two RNAs. This might indicate the presence of additional unknown factors that facilitate recognition of the psbD 5′UTR by RBP40. However, the data confirm that the 40-kD protein in the pUE1000 fraction represents RBP40, which is separated from the Nac2 factor during poly(U)-Sepharose chromatography.
To identify the RBP40 gene, we subjected the 40-kD protein band to proteolytic digestion and analyzed the resulting peptides by mass spectrometry (Table 1). Surprisingly, the peptide analysis indicated that the 40-kD protein from the 1 M KCl poly(U) eluate fraction is identical to the previously described chloroplast RNA binding protein RB38, which has been implicated in regulating the translation of psbA mRNA (Barnes et al., 2004). That highly purified RBP40 recognizes RNA molecules in an unspecific manner (Figure 2) is consistent with the report that recombinant RB38 expressed in Escherichia coli binds to the 5′UTRs of several RNAs (Barnes et al., 2004). Since neither of these sources of RBP40/RB38 contains Nac2, these results are compatible with our finding that RBP40 only recognizes the psbD 5′UTR with high affinity in the presence of Nac2.
Table 1.
Mass Spectrometric Identification of RB40
| Peptide Mass (D) | Predicted Sequencea | Database Hit (Swissprot Accession No.) |
|---|---|---|
| 999 | AFALWLDGR | Q6EMK7 |
| 1060 | NSALWLDSR | Q6EMK7 |
| 1199 | SAAPSTPELEAK | Q6EMK7 |
| 1294 | SNPDEWYDNR | Q6EMK7 |
| 1328 | QAAEAANWEALR | Q6EMK7 |
Note that Leu (L) and Ile (I) cannot be distinguished.
The psbD mRNA Is the Target of RBP40 in the Chloroplast
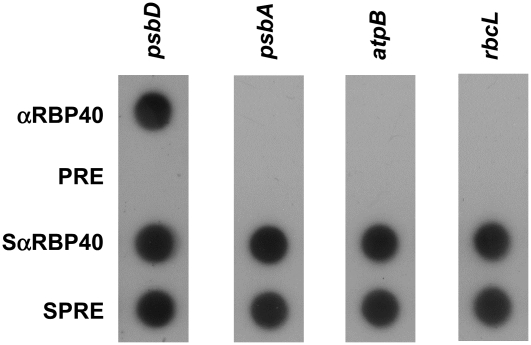
To identify target mRNAs for RBP40, immunoprecipitates obtained using an αRBP40 antiserum were probed with radiolabeled DNA probes derived from the 5′UTR regions of the psbD, psbA, rbcL, and atpB genes (Figure 3). All of these probes detected the corresponding transcripts in the supernatant after immunoprecipitation. However, only the psbD mRNA was found in substantial amounts in the precipitate, indicating that native RBP40 indeed forms a complex with the psbD mRNA, but not—or only to a very limited extent—with any of the other transcripts tested, including psbA. The preimmune serum used as a further negative control was unable to precipitate psbD mRNA, as expected (Figure 3).
Figure 3.
Coimmunoprecipitation of RBP40 and psbD mRNA.
Chloroplast stromal proteins were used for immunoprecipitation reactions with an αRBP40 antiserum or the preimmune serum (PRE). RNAs were extracted from precipitates (αRBP40 and PRE) and supernatants (SαRBP40 and SPRE), and equal proportions were subjected to dot-blot hybridization using the radiolabeled DNA probes indicated at top.
Analysis of RBP40 RNA Interference Lines
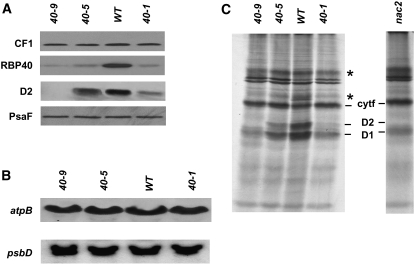
To confirm that RBP40 is specifically involved in regulating the synthesis of D2, RBP40 RNA interference (RNAi) lines were generated according to Rohr et al. (2004). As described in Methods, an inverted repeat structure comprising the RBP40 coding region was cloned into the vector NE537 (Rohr et al., 2004), and 2000 transformants harboring this construct were selected on paromomycin. Of these, 63 were resistant to 10 μM 5-fluoroindole, indicating efficient silencing of the vector-encoded Maa7 gene (Rohr et al., 2004). Subsequent chlorophyll fluorescence measurements on the 63 lines (data not shown) then identified three lines, named 40-1, 40-5, and 40-9, that exhibited the most pronounced effects on photosynthetic activity. In lines 40-5 and 40-1, the steady state levels of RBP40 protein were equivalent to 20 and 10%, respectively, of that accumulating in the wild type (Figure 4A). The greatest reduction was observed in line 40-9, which contained <10% of the wild-type level (Figure 4A). Concomitantly, levels of D2 were reduced to 80, 30, and <5% in lines 40-5, 40-1, and 40-9, respectively, indicating a strong effect of RBP40 knockdown on PSII. Other protein complexes in the chloroplast were not affected by the RNAi construct, as judged by parallel monitoring of the steady state levels of the PsaF and AtpB subunits of PSI and ATP synthase, respectively (Figure 4A).
Figure 4.
Molecular Characterization of RBP40 RNAi Lines.
(A) Protein gel blot analysis of total proteins (10 μg) isolated from the indicated RBP40 RNAi lines and the wild type was performed using antibodies raised against the proteins indicated at left.
(B) RNA gel blot analysis of psbD mRNA accumulation in RBP40 RNAi lines.
(C) Total proteins from the indicated strains were pulse-labeled for 20 min with [35S]sulfate and subsequently fractionated by SDS-PAGE. The positions of cytochrome f, D2, and D1 proteins are indicated (Klinkert et al., 2006). Fluctuations of signal intensities marked with asterisks were not seen reproducibly.
Interestingly, the reductions in D2 levels were not associated with any significant change in concentrations of psbD mRNA, indicating that RBP40 affects the synthesis or stability of PSII subunits (Figure 4B). To distinguish between these two possibilities, pulse labeling of chloroplast proteins was performed after inhibition of cytoplasmic translation with cycloheximide. D2 synthesis rates were found to be drastically reduced in RNAi lines (Figure 4C), and the degree of reduction correlated with the levels of D2 accumulation revealed by protein gel blot analysis (Figure 4A). Like the nac2 mutant, RNAi lines also showed a reduction in labeling of the D1 protein, albeit less pronounced than in the case of D2 (Figure 4C) (Kuchka et al., 1988). Effects related to light-dependent PSII repair synthesis were minimized during these experiments, because cells from RNAi lines generally were kept in very low light. However, the virtual absence of D2 synthesis excludes the possibility that the primary effect of RBP40 deficiency is a defect in translation of the psbA mRNA, since, based on previous reports, D1 deficiency does not compromise the synthesis of D2 (Kuchka et al., 1988; de Vitry et al., 1989; Minai et al., 2006).
To further confirm a role for RBP40 in translation of the psbD message, polysomal loading of atpB, psbA, and psbD mRNAs was assayed (Figure 5). In the wild type, all three mRNAs migrated into the lower regions of a sucrose gradient, indicating that they were associated with polysomes and were thus being actively translated. Overall, the polysomal association of all three mRNAs in the wild type was lower than reported previously (Minai et al., 2006). However, this is likely due to the very low light conditions used in these experiments. Disassembly of polysomes by treatment with EDTA resulted in the accumulation of all three transcripts, mainly in fractions 1 to 3 at the top of the gradient; these fractions contain ribosomal subunits and smaller RNP particles (Figure 5, WT+EDTA). When the RNAi line 40-9 was analyzed, psbD mRNA was detected only in fractions 1 to 4, even after extended exposure of autoradiograms; none was found at polysome positions within the gradient. This finding indicates that the psbD mRNA is not or almost not translated in 40-9. By contrast, atpB mRNAs migrated into the 40-9 gradient, revealing that they are actively translated in this RNAi line. The loading of psbA mRNA with polysomes was affected by the silencing of the RBP40 gene in line 40-9, although to a weaker extent compared with that of the psbD mRNA (Figure 5). Some mRNA was detected in the high molecular mass fraction corresponding to polysomes, but especially in fractions 4 to 6 containing monosomes, a significant decrease in psbA signal intensity was detectable compared with the wild type (Figure 5). This corresponds to the pulse-labeling data presented in Figure 4C, which also indicate a less-pronounced effect on D1 synthesis in the RNAi line 40-9 compared with that of D2.
Figure 5.
Polysomal Loading of psbD mRNA.
Whole-cell extracts from the wild type (WT+MgCl2) and the RNAi line 40-9 (40-9+MgCl2) were fractionated on 15 to 40% sucrose gradients by ultracentrifugation. As a negative control, polysomes were destabilized by the addition of EDTA (WT+EDTA). At the top of each of the three panels, the ethidium bromide–stained rRNA patterns before blotting of the gels are shown. Below, hybridization signals are shown that were obtained with the radiolabeled probes indicated at right. The sedimentation behavior of RBP40 in the wild type was followed by protein gel blot analysis of proteins from the same gradient fractions.
Together, these data strongly support the idea that RBP40 is a psbD-related translation factor that acts together with the RNA stabilization factor Nac2 to regulate psbD gene expression at the posttranscriptional level. Furthermore, the accumulation of wild-type levels of psbD mRNA in the RBP40 RNAi lines indicates that, although translational control via RBP40 requires Nac2-mediated RNA stabilization, RBP40 deficiency does not affect RNA stabilization by Nac2.
RBP40 Is Associated with Monosomes but Not with Polysomes
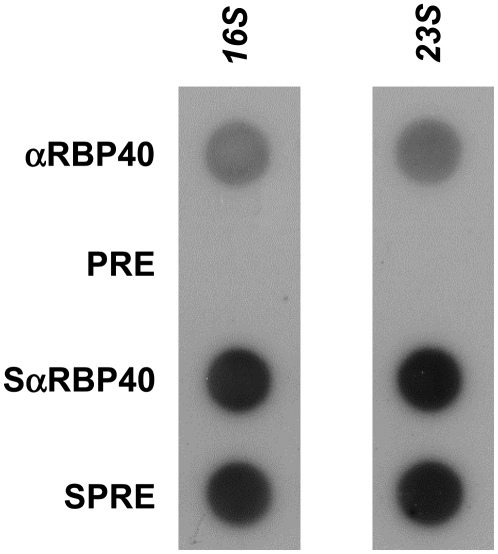
The Nac2 factor has been shown previously not to be associated with the polysomal fraction from Chlamydomonas chloroplasts, suggesting that it functions only during the early posttranscriptional stages of psbD expression (Boudreau et al., 2000). The same holds for RBP40, which was only observed in top fractions 1 to 8 of the polysome gradient (Figure 5). Moreover, treatment of samples with EDTA had no significant effect on RBP40 migration, suggesting that it sediments independently of polysomes (Figure 5, WT+EDTA). Nevertheless, the data did not exclude the possibility that RBP40 might interact with monosomes, for instance during translation initiation. To address this question, immunoprecipitates obtained following reaction with αRBP40 antiserum, similar to those shown in Figure 3, were hybridized with probes specific for either 16S or 23S rRNA (Figure 6). Indeed, both rRNAs were precipitated by the αRBP40 antiserum, suggesting that both ribosomal subunits, and thus monosomes, are assembled on the psbD mRNA while RBP40 is bound to its 5′UTR (Figure 6). However, as soon as polysomal assembly has started, RBP40 appears to leave the psbD message.
Figure 6.
Association of RBP40 with Ribosomes.
Dot-blot hybridization with radiolabeled 16S and 23S rDNA probes was performed on immunoprecipitates similar to those shown in Figure 3.
RBP40 and Nac2 Form Part of the Same High Molecular Mass Complex
Based on earlier sedimentation analyses, it had been speculated that Nac2 and RBP40 might form parts of the same high molecular mass complex (Boudreau et al., 2000; Ossenbühl and Nickelsen, 2000). This idea can now be substantiated by data from three independent assays. First, high molecular mass material at 550 kD is detectable using αRBP40 and αNac2 antisera after colorless native PAGE of wild-type stromal proteins (Figure 7). Furthermore, the functional interdependence of the two factors is correlated with the ability to form the 550-kD complex, as the complex could not be detected by the αRBP40 antibody in the nac2 mutant (Figure 7).
Figure 7.
Native PAGE of RBP40 and Nac2.
Stromal protein fractions from wild-type and nac2 chloroplasts were subjected to native PAGE on 8% gels and transferred to nitrocellulose filters, which were immunolabeled with either αNac2 or αRBP40 antibodies. The arrow indicates the 550-kD Nac2/RBP40 complex. Equal loading was confirmed by Ponceau red staining of ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco). The sizes of marker proteins are given at left.
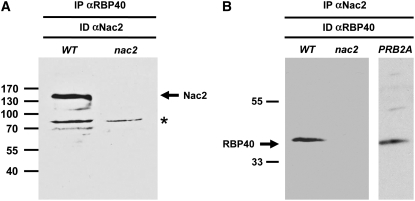
Second, coimmunoprecipitation experiments clearly demonstrated that both factors are part of the same complex. When RBP40 was immunoprecipitated from the stromal protein extract by the αRBP40 antiserum, the Nac2 protein was detected in the precipitate (Figure 8A). Conversely, RBP40 is present in the immunoprecipitate formed upon incubation with an αNac2 antiserum (Figure 8B). As a further control, immunoprecipitations were performed using stromal proteins from the nac2 mutant. In that case, no RBP40 protein was precipitated with αNac2 antiserum, ruling out the possibility that RBP40 might bind to the protein G–Sepharose via unrelated proteins (Figure 8B). To test whether the association of Nac2 and RBP40 depends on the presence of psbD mRNA, stromal proteins from a chloroplast cis-acting mutant called PRB2A were immunoprecipitated. In PRB2A, the PRB2 element of the psbD 5′UTR is mutated and, as a consequence, psbD transcripts do not accumulate because they are destabilized (Nickelsen et al., 1999) (see Figure 10). As shown in Figure 8B, the αNac2 antiserum was capable of precipitating RBP40 from PRB2A, indicating that the formation of a Nac2/RBP40 complex does not require any psbD mRNA.
Figure 8.
Coimmunoprecipitation of RBP40 and Nac2.
Stromal protein fractions from wild-type and nac2 chloroplasts were incubated with either αRBP40 antibody coupled to protein A–Sepharose (IP αRBP40) (A) or αNac2 antibody coupled to protein G–Sepharose (IP αNac2) (B). After elution from the matrix, the material was subjected to SDS-PAGE and immunolabeled using the same antibodies (ID αNac2 or ID αRBP40). The asterisk marks material that cross-reacts with the αNac2 antiserum.
Figure 10.
Working Model for the Posttranscriptional Control of psbD Gene Expression.
The sequence of the psbD 5′UTR from Chlamydomonas is given with the PRB2 site, the U-rich translational element boxed in gray, and the putative Shine-Dalgarno element (PRB1) (Nickelsen et al., 1999). The AUG start codon (gray letters) is marked by Met. Putative additional components of the Nac2/RBP40 complex are indicated by a question mark. The closed arrow represents the change in RNA conformation induced by RBP40, and the open arrows stand for the subsequent binding of components of the translational machinery. For further explanation, see text.
Finally, the existence of a 550-kD complex containing both Nac2 and RBP40 was verified by demonstrating the cosedimentation of both factors after centrifugation of wild-type stromal proteins in glycerol gradients (Figure 9). When the nac2 mutant was analyzed in the same way, RBP40 was found only in the low molecular mass fraction at the top of the gradient, again indicating that Nac2 is strictly required for the formation of the 550-kD complex and perhaps even for the assembly of subcomplexes (Figure 9; see also Figure 7). In the RNAi line 40-9, which contains only minute amounts of RBP40 (cf. Figure 4A), a high molecular mass complex that appears slightly smaller (Figure 9, fractions 10 to 13) than the wild-type form (Figure 9, fractions 11 to 14) was observed with the αNac2 antibody. Under standard conditions, no RBP40 was detectable anywhere on the gradient. However, extended exposure of immunoblots led to the detection of tiny amounts of RBP40 in fractions 11 to 14 in line 40-9 (Figure 9, RBP40ee). This strongly suggests that loss of the 40-kD RBP40 subunit, in contrast with Nac2, does not completely destabilize the complex; instead, a smaller, RBP40-less complex of ∼500 kD is observed, which contains active Nac2 and is capable of stabilizing the psbD mRNA (Figure 9; see also Figure 4B). However, the residual RBP40 protein still present in line 40-9 appears to associate with the 500-kD Nac2 complex, resulting in very low amounts of the normal 550 kD complex.
Figure 9.
Formation of an RBP40/Nac2 Complex in the Absence of psbD mRNA.
Stromal chloroplast proteins from the strains indicated at left were centrifuged through 15 to 35% glycerol gradients. The distribution of the Nac2 and RBP40 proteins after centrifugation (at right) was monitored by protein gel blot analysis of the fractions marked below each filter strip. RBP40ee represents an extended exposure (>100-fold) of the blot shown directly above it.
Earlier gel filtration analyses had revealed that psbD RNA–containing material was detectable throughout the 200- to 2000-kD range (Boudreau et al., 2000). This is compatible with the idea of a 550-kD complex containing Nac2, RBP40, and psbD RNA sequences. Treatment of the complex with RNase resulted in a reduction in its size to ∼450 kD (Boudreau et al., 2000). The same shift following incubation with RNase A was observed in glycerol gradients by monitoring the distribution of both Nac2 and RBP40 using the appropriate antibodies (Figure 9). This suggests that the 550-kD complex represents a ribonucleoprotein complex. To test whether the associated RNA is derived from the psbD gene, we analyzed the above-mentioned mutant PRB2A, which does not accumulate any psbD transcripts. Sedimentation analysis of stromal proteins from PRB2A showed that the Nac2-containing complex is of the same size (450 kD) as that seen after treatment with RNase. This strongly suggests that the size reduction to 450 kD is due to the removal of psbD mRNA regions that remain associated with the complex during sample preparation (Figure 9). That the entire psbD mRNA forms an integral part of the 550-kD complex appears unlikely, because its loss would be expected to cause a larger change in molecular mass than the observed 100 kD. Most likely, endogenous RNase activities cleave away most of the complexed RNA during sample preparation and/or gradient centrifugation. However, it cannot be totally excluded that, apart from the psbD mRNA, other RNA molecules might be present in the Nac2/RBP40 complex, since RNase treatment also resulted in complexes that are slightly smaller than those observed in PRB2A (cf. fractions 6 in Figure 9).
DISCUSSION
RBP40 Specifically Recognizes the 5′UTR of the psbD mRNA
Here, we report the purification of the RNA binding protein RBP40 from Chlamydomonas, which was found to be identical to the previously described RNA binding protein RB38 (Barnes et al., 2004). Notable structural features of RBP40/RB38 include a putative chloroplast transit sequence and four repeats of a motif comprising 70 amino acids with a high percentage of basic residues. Despite limited primary sequence homology between them, these repeats appear to fold into a tertiary structure that resembles well-known RNA binding domains such as the RNA recognition motif domain (Barnes et al., 2004). As implied by these features, RB38 was shown to be imported into chloroplasts, and the recombinant protein expressed in E. coli exhibited RNA binding activity, showing a selective affinity only for U-rich regions (Barnes et al., 2004). Like recombinant RB38, highly purified RBP40 showed low-specificity RNA binding in vitro. However, when the RNA stability factor Nac2 was present, RBP40 bound preferentially to the 5′UTR of psbD RNA, suggesting that Nac2 recruits the protein into a psbD-specific complex or modifies the RNA binding surface of RBP40 directly (Figure 2).
Further lines of evidence indicate that RBP40 indeed represents a trans-acting factor required for psbD gene expression. First, RBP40 was shown to form a structural and functional unit with the psbD-specific RNA stabilization factor Nac2. By contrast, RB38 has been postulated to form part of a complex of four subunits, namely RB60, RB55, RB47, and RB38, which specifically recognizes the psbA 5′UTR and mediates the redox control of D1 protein synthesis (Barnes et al., 2004). This conclusion was based solely on the coelution of the four proteins from an RNA affinity column bearing psbA 5′UTR sequences following a single application of 0.55 M KOAc (Danon and Mayfield, 1991, 1994). An interaction between RB60 and RB47 has been demonstrated: both polypeptides were isolated from an RNase T1-resistant psbA 5′UTR RNA/protein complex that had been cut out of a native mobility-shift gel (Danon and Mayfield, 1991). However, neither RB38 nor RB55 was detected in this psbA-specific complex, arguing against the formation of a complex containing all four proteins. In this study, we show that RBP40/RB38 cosediments with Nac2 in an ∼550-kD complex. Previously, we showed that the Nac2 complex is distinct from a significantly smaller complex of ∼440 kD containing the RB60 protein (Boudreau et al., 2000). This indicates that RBP40/RB38 and the psbA-specific factor RB60 do not form parts of the same complex; therefore, it seems questionable whether RB38 is indeed involved in RB60/RB47-mediated psbA gene expression.
Second, psbD, but little or no psbA, atpB, or rbcL, mRNA was immunoprecipitable in substantial amounts with αRBP40 antiserum. Although we have not precisely quantitated the RNA amounts detected in these immunoprecipitates (Figure 3), the dramatically obvious differences in precipitation efficiencies indicate a clear preference of RBP40 for complex formation with the psbD mRNA.
Finally, molecular characterization of RBP40 RNAi lines revealed severe defects in D2 synthesis. Nevertheless, minor effects on D1 synthesis were also observed. This suggests that RBP40 might—besides psbD—also be involved in psbA gene expression. Alternatively, and more likely, reduced D1 synthesis in the RBP40 RNAi lines is caused by a secondary effect reflecting the mode of regulation of PSII assembly/synthesis known as the CES principle (Minai et al., 2006). For instance, a strong effect of D2 deficiency on D1 synthesis has been reported for the nac2 mutant (Kuchka et al., 1989; Nickelsen et al., 1999) (Figure 4C) and chloroplast psbD deletion mutants (Minai et al., 2006). This reduction is attributed to a feedback control mechanism that depends on nonassembled D1 protein accumulating in the absence of its assembly partner D2. Nonassembled D1 is sensed by an unknown mechanism, and as a consequence, D1 synthesis is reduced by processes mediated via the psbA 5′UTR (Minai et al., 2006). A similar situation is expected to occur in the RBP40 RNAi lines, with partial (40-4 and 40-1) or almost complete (40-9) loss of D2 resulting in partial feedback inhibition of psbA translation. Indeed, an effect of RBP40 deficiency on D1 synthesis during protein pulse labeling and polysomal loading was observed (Figures 4C and 5). This is in agreement with previous reports revealing only a limited, although still significant, effect of D2 deficiency on polysomal loading of the psbA mRNA in a chloroplast psbD deletion mutant of Chlamydomonas (Minai et al., 2006).
Thus, together, our data support a scenario in which RBP40 is specifically or at least preferentially required for the posttranscriptional regulation of psbD gene expression. Whether the Nac2/RBP40 complex or parts of it are also involved in psbA gene expression—for instance, during feedback inhibition of psbA gene expression during CES regulation—remains to be clarified.
Aspects of Nac2/RBP40 Complex Formation
Three different approaches—native PAGE, coimmunoprecipitation, and sedimentation analysis—were utilized successfully to document that Nac2 and RBP40 form parts of a single complex of 550 kD. However, whether the two factors interact directly or via adapter components remains an open question. Yeast two-hybrid analysis, in vitro glutathione S-transferase pull down, and chemical cross-linking experiments have provided no evidence for such a direct physical interaction (data not shown). This suggests that formation of the Nac2/RBP40-containing complex requires one or more additional components that can serve as a molecular bridge between the two proteins. The sedimentation data (Figure 9) demonstrate that the psbD mRNA itself does not supply this function, because complex formation still occurs in the psbD mRNA–deficient mutant PRB2A. Two other lines of evidence suggest that additional polypeptides are present in the 550-kD complex. (1) Previous studies have revealed the existence of three unlinked nuclear loci that, when mutated, can suppress a defect in the RNA stability element PRB2 (Nickelsen, 2000). It was speculated that these loci encode subunits of a Nac2 complex that recognize the PRB2 element, which is located immediately upstream of the U-rich RBP40 target region in the psbD 5′UTR (Nickelsen, 2000) (Figure 10). (2) Treatment with RNase reduces the apparent size of the 550-kD complex by only ∼100 kD, suggesting a stable protein complex of 450 kD (Figure 9).
One other aspect of Nac2/RBP40 complex formation concerns the different requirements for Nac2 and RBP40. While lack of Nac2 prevents the formation of stable high molecular mass complexes containing RBP40, drastic reduction of RBP40 has only a minor effect on the Nac2-containing complex. Thus, RBP40 might be less tightly associated with the complex and/or might not represent an essential structural subunit. Whether this finding has functional implications remains to be elucidated.
Consistent with the data presented here, previous sedimentation analyses using either sucrose or glycerol gradients had revealed a Nac2 complex size of 500 to 600 kD (Boudreau et al., 2000; Ossenbühl and Nickelsen, 2000). However, additional, less-abundant Nac2 complexes of 700 to 2000 kD in size were observed using fast protein liquid chromatography gel filtration for the separation of complexes (Boudreau et al., 2000). In view of the finding that the Nac2/RBP40 complex might be associated with monosomes (Figure 6), these supercomplexes might still contain ribosomes or ribosomal subunits, thereby explaining their huge size. However, these associations are probably destabilized during the relatively long-lasting sedimentation analysis compared with the fast protein liquid chromatography procedure.
RBP40 Is Involved in Controlling the Translation of psbD mRNA
RBP40 recognizes a U-rich region located 15 nucleotides upstream of the psbD AUG start codon (Ossenbühl and Nickelsen, 2000) (Figure 10). Deletion of this U-rich stretch leads to the complete loss of D2 synthesis, while mRNA accumulation is compromised to only a minor extent. Both findings suggest that this element is required for translational control (Nickelsen et al., 1999). Moreover, detailed analyses of cis-acting second-site suppressor mutants have revealed a functional relationship between the U-rich stretch and an RNA stem-loop structure containing the AUG codon within the stem region. It was hypothesized that RBP40 binding alters the conformation of this region, thereby giving the ribosomal subunits access to the initiation codon (Klinkert et al., 2006) (Figure 10).
The analysis of RBP40 RNAi lines supports the idea that RBP40 is a translational activator. In particular, the pulse labeling and polysomal loading analyses underline its significance for translation initiation (Figures 4C and 5). Interestingly, psbD mRNA accumulation was not affected in RBP40-deficient cell lines, indicating that Nac2 is still fully functional and, hence, can operate independently of RBP40. This is consistent with the accumulation of high molecular mass Nac2 complexes in the absence of RBP40. By contrast, absence of Nac2 leads to the loss of translational activity, even when the psbD mRNA is artificially stabilized by the insertion of poly(G) stretches into the psbD 5′UTR (Nickelsen et al., 1999), indicating that Nac2 is required for the RBP40 function. Thus, the Nac2/RBP40 complex fulfills two distinct functions. First, a subcomplex containing Nac2 but lacking RBP40 is sufficient to stabilize the psbD mRNA, probably via the PRB2 element (Figure 10). Then, the complete Nac2/RBP40 holocomplex of 550 kD mediates the subsequent steps of psbD gene expression (i.e., translation initiation via an interaction with the U-rich element) (Klinkert et al., 2006). Future work will focus on the identification of the predicted additional subunits of the complex by genetic and biochemical means, with a view to obtaining a more complete picture of the regulatory network controlling psbD gene expression in Chlamydomonas.
Strikingly, a recent characterization of the spatial organization of PSII synthesis and assembly processes has revealed that RBP40 is localized to a specialized chloroplast subcompartment near the pyrenoid, called the T zones (Uniacke and Zerges, 2007). This further underlines the role that RBP40 plays for the de novo biosynthesis of PSII.
METHODS
Culture Conditions
Chlamydomonas reinhardtii strains were grown to a density of 2 × 106 cells/mL in Tris-acetate-phosphate (TAP) medium (Harris, 1989) containing 1% sorbitol. RNAi lines were grown in low light (<5 mE·m−2·s−1). Chlorophyll content was determined following acetone extraction as described before (Klinkert et al., 2006).
Purification of RBP40 and Peptide Sequencing by Mass Spectrometry
Chloroplasts from cell wall–deficient strains carrying the cw15 mutation were isolated from a discontinuous Percoll gradient (45 to 75%) as described previously (Zerges and Rochaix, 1998). To prepare chloroplast stromal fractions, isolated chloroplasts were osmotically lysed in hypotonic buffer (10 mM Tricine/KOH, pH 7.8, 10 mM EDTA, and 5 mM 2-mercaptoethanol) by repeated pipetting. Insoluble material was removed by ultracentrifugation for 30 min at 100,000g through a 1 M sucrose cushion in hypotonic buffer in an SW40 rotor (Beckman).
An aliquot of the supernatant containing 10 to 15 mg of stromal protein was then applied to a 5-mL heparin–Sepharose 4B (GE Healthcare) column equilibrated with buffer I (50 mM KCl, 10 mM Tricine/KOH, pH 7.8, and 10 mM EDTA). Bound proteins were eluted using a discontinuous salt gradient (150 mM, 550 mM, and 1 M KCl in buffer I). Proteins eluting at 150 mM KCl were desalted using Amicon Ultra centrifugal filtration devices (Millipore) with a 10-kD molecular mass cutoff according to the manufacturer's instructions.
The protein solution (in buffer I) was then applied to a 2-mL poly(U)–Sepharose 4B (GE Healthcare) column equilibrated with buffer I. The column was washed with 3 volumes of buffer I, and bound proteins were eluted with a discontinuous salt gradient (150 mM, 550 mM and 1 M KCl in buffer I).
The different fractions were tested for the presence of RNA binding activity by assessing their ability to bind to 5′UTR sequences from psbD RNA in UV cross-linking assays. Prior to use in UV cross-linking assays (see below), all protein fractions were dialyzed against RNA binding buffer (30 mM Tris-HCl, pH 7.0, 50 mM KCl, 5 mM MgCl2, and 5 mM 2-mercaptoethanol). Protein concentrations were determined using the Bradford assay (Bio-Rad).
For mass spectrometric peptide sequencing, RBP40-containing gel pieces were treated with trypsin (sequencing grade; Promega) and the resulting peptides were analyzed on a Q-TOF2 mass spectrometer (Micromass) as described (Piotrowski and Volmer, 2006).
Production of Antiserum against RBP40
An RBP40 cDNA was isolated after screening of a cDNA library prepared from wild-type cells (Boudreau et al., 2000). A DNA fragment encoding the first 191 amino acids of RBP40 was amplified from this cDNA by PCR with the primers 5′-GGATCCGCCGCGGCGCACCCCCCTGG-3′ (RBP40-BamHI-5′) and 5′-GTCGACGCTGTCCAGCCACAGCG-3′ (RBP40-SalI-3′). The fragment was cloned into the expression vector pGEX4T1 (via the BamHI and SalI restriction sites present in the two primers). Overexpression of this construct in the Escherichia coli strain BL21 and purification of the resulting RBP40–glutathione S-transferase fusion protein were performed according to the manufacturer's protocol using glutathione–Sepharose 4B (GE Healthcare). A polyclonal antiserum was produced by immunizing rabbits with this protein fraction (Biogenes).
Analysis of Nucleic Acids and Proteins
Pulse labeling of total cell proteins with [35S]sulfate, isolation of algal nucleic acids and proteins, and RNA gel blot and protein gel blot analyses were performed essentially as described previously (Klinkert et al., 2006). Signal intensities in four independent protein gel blot analyses were densitometrically quantified using Scion Image software (http://www.scioncorp.com).
Generation of RBP40 RNAi Lines
To create RBP40-deficient mutants of Chlamydomonas, we used the RNAi system described previously by Rohr et al. (2004). For the generation of an inverted repeat construct specific for RBP40 RNA, a 573-bp fragment corresponding to the 5′ coding sequence of RBP40 was amplified with PCR using the RBP40 cDNA as a template with the primers RBP40-BamHI-5′ and RBP40-SalI-3′, which add BamHI and SalI restriction sites, respectively. A longer 800-bp fragment containing an additional 227 bp of the coding sequence that functioned as a spacer for the inverted repeat was amplified using the primers RBP40 RNAi-H-E-S (5′-AAGCTTGAATTCCTGACCTTGAGACGTGC-3′) and RBP40 RNAi3′ (5′-GAGCTCGTCGACCTAGTAGCGGGCGC-3′), adding HindIII, EcoRI, SalI, and SacI restriction sites to the sequences. These two fragments were ligated and cloned as an inverted repeat (with the central spacer) into the EcoRI site of the vector NE537 (which is located in the inverted repeat of the Maa7 gene for the β-subunit of Trp synthase) using the E. coli strain XL-1 Blue as a host (Rohr et al., 2004).
Cells of the cw15 strain were transformed with the resulting construct, kept for 2 d in liquid culture (TAP + 1.5 mM l-Trp) in dim light, and then plated on TAP plates containing 5 μg/mL paromomycin (to select for transformants) and 1.5 mM l-Trp. At intervals of 2 weeks, colonies were transferred to TAP plates containing 5 and then 10 μM 5-fluoroindole in the dark. 5-Fluoroindole–resistant clones were screened for high chlorophyll fluorescence phenotypes and then subjected to molecular analysis.
In Vitro Synthesis of RNA and UV Cross-Linking of RNA to Proteins
DNA templates for the in vitro synthesis of psbD and psbA leader RNA probes were generated by PCR using appropriate primers: for psbD RNA (the wild-type and the ΔU mutant sequences of the psbD mRNA corresponding to positions −74 to +18 relative to the AUG), 5′-ACCGATCGCAATTGTCAT-3′ (3131) and 5′-TAATACGACTCACTATAGGGACACAATGATTAAAATTAAA-3′ (2126); and for psbA RNA (the wild-type sequence of the psbA mRNA corresponding to positions −91 to +13 relative to the AUG), 5′-GTAATACGACTCACTATAGGGTACCATGCTTTTAATAGAAG-3′ (T7-psbA5′) and 5′-GATCCATGGTCATATGTTAATTTTTTTAAAG-3′ (2054). In vitro transcription of RNA, UV cross-linking of RNA to protein, and quantification of binding signals were performed as described previously (Klinkert et al., 2006). Radiolabeled RNA and nonlabeled competitors were mixed prior to the addition of proteins to competition experiments. Quantification of competitor RNAs was performed by measuring the incorporation of low levels of radioactivity into transcripts. Signal intensities in competition experiments were densitometrically quantified using Scion Image software.
Coimmunoprecipitations of Proteins and RNA
Chlamydomonas chloroplasts were isolated as described above and resuspended in lysis buffer (10 mM Tricine/KOH, pH 7.8, 10 mM EDTA, 5 mM 2-mercaptoethanol, and Roche Complete mini protease inhibitor cocktail). Membranes were pelleted by centrifugation for 30 min at 100,000g through a 1 M sucrose cushion in an SW40 rotor (Beckman). The resulting supernatant constituted the stromal fraction used for subsequent immunoprecipitation experiments. To minimize nonspecific interactions, this supernatant was first incubated with 250 μL of protein A–Sepharose (GE Healthcare) in lysis buffer for 1 h at 4°C. For coimmunoprecipitations, αRBP40 IgGs cross-linked to 10 mg of protein A–Sepharose were added to the pretreated stromal fraction, and the mixture was incubated overnight at 4°C. The beads were then washed 10 times in Tris-buffered saline–BSA (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% BSA, and Roche Complete mini protease inhibitor cocktail). Bound proteins were released from the beads in 5× SDS loading buffer (50% glycerol, 125 mM Tris-HCl, pH 7.0, 5% SDS, 0.05% bromophenol blue, and 150 mM 2-mercaptoethanol) for 15 min and subsequently subjected to immunoblotting analysis. For immunoprecipitations using αNac2 IgGs, the same protocol was followed, except that protein G–Sepharose was substituted for protein A–Sepharose.
Coimmunoprecipitations of RNA were performed in the presence of 0.5 μg/μL yeast tRNA and 1 unit/μL RNasin (Promega). The RNA was isolated by extraction with phenol-chloroform after the addition of SDS to 0.5%. Equal proportions of RNA samples were transferred to nylon membranes using a dot-blot manifold (Schleicher and Schüll). To avoid membrane saturation effects, total RNA amount per dot was restricted to 2.5 μg. Subsequently, membranes were hybridized with radiolabeled DNA probes comprising the 5′UTRs of the indicated genes, which were PCR-amplified using appropriate oligonucleotides. As a loading control, all blots were finally hybridized with the psbD probe.
Sedimentation Analysis in Glycerol Gradients
For sedimentation analysis, isolated chloroplasts were hypotonically lysed in 20 mM Tricine/KOH, pH 7.8, 55 mM KCl, 3 mM EDTA, 5 mM ɛ-amino caproic acid, 5 μg/μL tRNA, 80 units of RNasin, and 0.05% BSA. Aliquots equivalent to 1.5 mg of stromal proteins were then loaded onto 15 to 35% glycerol gradients and centrifuged for 18 h at 180,000g in an SW40 rotor (Beckman). The gradient was fractionated into 18 0.5-mL samples, and 40 μL of each was used for immunoblotting analysis.
Polysome Purification in Sucrose Gradients
Polysomes were purified as described previously (Mussgnug et al., 2005). Cells (7.4 × 108) were recovered by centrifugation, broken in a freeze–thaw cycle, resuspended in 1 mL of polysome extraction buffer (200 mM Tris-HCl, pH 9.0, 200 mM KCl, 35 mM MgCl2, 25 mM EGTA, 0.2 M sucrose, 1% Triton X-100, and 2% polyethylene-10-tridecyl-ether), supplemented with inhibitors (0.5 mg/mL heparin, 100 mM 2-mercaptoethanol, 100 mg/mL chloramphenicol, 1 mM 1,10-phenanthroline, and 0.5% [v/v] Complete mini protease inhibitor cocktail either with or without EDTA [Roche]), and immediately centrifuged at 4°C for 20 min at 10,000g. The supernatant was supplemented with sodium deoxycholate to a final concentration of 0.5% and layered onto a linear gradient composed of 15 to 40% sucrose in cushion buffer (40 mM Tris-HCl, pH 9.0, 20 mM KCl, 30 mM MgCl2 or 1 mM EDTA, and 5 mM EGTA). The gradients were centrifuged for 225 min at 100,000g at 4°C. Aliquots of collected fractions were either used for immunoblot analysis or supplemented with 0.5% SDS and 20 mM EDTA before RNA isolation by phenol/chloroform extraction. RNA was dissolved in nuclease-free water and separated on a 1% agarose-formaldehyde gel.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AJ271460 (Nac2) and AY124882 (RPB40/RB38).
Acknowledgments
We thank U. Aschke and K. Schmieja for skilled technical assistance and O. Kruse for help with chlorophyll fluorescence measurements on RNAi lines. This work was supported by a grant from the Deutsche Forschungsgemeinschaft to J.N. (Grant Ni390/4-1)
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jörg Nickelsen (joerg.nickelsen@lrz.uni-muenchen.de).
References
- Auchincloss, A.H., Zerges, W., Perron, K., Girard-Bascou, J., and Rochaix, J.D. (2002). Characterization of Tbc2, a nucleus-encoded factor specifically required for translation of the chloroplast psbC mRNA in Chlamydomonas reinhardtii. J. Cell Biol. 157 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan, A., Walker, M., Nolasco, M., and Johnson, D. (1994). A nuclear mutation in maize blocks the processing and translation of several chloroplast mRNAs and provides evidence for the differential translation of alternative mRNA forms. EMBO J. 13 3170–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barneche, F., Winter, V., Crevecoeur, M., and Rochaix, J.-D. (2006). ATAB2 is a novel factor in the signalling pathway of light-controlled synthesis of photosystem proteins. EMBO J. 25 5907–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, D., Cohen, A., Bruick, R.K., Kantardjieff, K., Fowler, S., Efuet, E., and Mayfield, S.P. (2004). Identification and characterization of a novel RNA binding protein that associates with the 5′ untranslated region of the chloroplast psbA mRNA. Biochemistry 43 8541–8550. [DOI] [PubMed] [Google Scholar]
- Boudreau, E., Nickelsen, J., Lemaire, S.D., Ossenbuhl, F., and Rochaix, J.D. (2000). The Nac2 gene of Chlamydomonas encodes a chloroplast TPR-like protein involved in psbD mRNA stability. EMBO J. 19 3366–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick, R.K., and Mayfield, S.P. (1999). Light-activated translation of chloroplast mRNAs. Trends Plant Sci. 4 190–195. [DOI] [PubMed] [Google Scholar]
- Danon, A., and Mayfield, S.P. (1991). Light regulated translational activators: Identification of chloroplast gene-specific mRNA binding proteins. EMBO J. 10 3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon, A., and Mayfield, S.P. (1994). Light-regulated translation of chloroplast messenger RNAs through redox potential. Science 266 1717–1719. [DOI] [PubMed] [Google Scholar]
- Dauvillee, D., Stampacchia, O., Girard-Bascou, J., and Rochaix, J.D. (2003). Tab2 is a novel conserved RNA binding protein required for translation of the chloroplast psaB mRNA. EMBO J. 22 6378–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry, C., Olive, J., Drapier, D., Recouvreur, M., and Wollman, F.A. (1989). Posttranslational events leading to the assembly of photosystem II protein complex: A study using photosynthesis mutants from Chlamydomonas reinhardtii. J. Cell Biol. 109 991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, E.H. (1989). The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. (San Diego, CA: Academic Press). [DOI] [PubMed]
- Higgs, D.C., Shapiro, R.S., Kindle, K.L., and Stern, D.B. (1999). Small cis-acting sequences that specify secondary structures in a chloroplast mRNA are essential for RNA stability and translation. Mol. Cell. Biol. 19 8479–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose, T., and Sugiura, M. (1996). Cis-acting elements and trans-acting factors for accurate translation of chloroplast psbA mRNAs: Development of an in vitro translation system from tobacco chloroplasts. EMBO J. 15 1687–1695. [PMC free article] [PubMed] [Google Scholar]
- Kim, J., and Mayfield, S.P. (1997). Protein disulphide isomerase as a regulator of chloroplast translational activation. Science 278 1954–1957. [DOI] [PubMed] [Google Scholar]
- Klinkert, B., Elles, I., and Nickelsen, J. (2006). Translation of chloroplast psbD mRNA in Chlamydomonas is controlled by a secondary RNA structure blocking the AUG start codon. Nucleic Acids Res. 34 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komenda, J., Reisinger, V., Muller, B.C., Dobakova, M., Granvogl, B., and Eichacker, L.A. (2004). Accumulation of the D2 protein is a key regulatory step for assembly of the photosystem II reaction center complex in Synechocystis PCC 6803. J. Biol. Chem. 279 48620–48629. [DOI] [PubMed] [Google Scholar]
- Kuchka, M.R., Goldschmidt-Clermont, M., van Dillewijn, J., and Rochaix, J.-D. (1989). Mutation of the Chlamydomonas nuclear Nac2 locus specifically affects stability of the chloroplast psbD transcript encoding polypeptide D2 of PS II. Cell 58 869–876. [DOI] [PubMed] [Google Scholar]
- Kuchka, M.R., Mayfield, S.P., and Rochaix, J.-D. (1988). Nuclear mutations specifically affect the synthesis and/or degradation of the chloroplast-encoded D2 polypeptide of photosystem II in Chlamydomonas reinhardtii. EMBO J. 7 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuell, A., Beligni, M.V., Yamaguchi, K., and Mayfield, S.P. (2004). Regulation of chloroplast translation: Interactions of RNA elements, RNA-binding proteins and the plastid ribosome. Biochem. Soc. Trans. 32 601–605. [DOI] [PubMed] [Google Scholar]
- Minai, L., Wostrikoff, K., Wollman, F.A., and Choquet, Y. (2006). Chloroplast biogenesis of photosystem II cores involves a series of assembly-controlled steps that regulate translation. Plant Cell 18 159–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussgnug, J.H., et al. (2005). NAB1 is an RNA binding protein involved in the light-regulated differential expression of the light-harvesting antenna of Chlamydomonas reinhardtii. Plant Cell 17 3409–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickelsen, J. (2000). Mutations at three different nuclear loci of Chlamydomonas suppress a defect in chloroplast psbD mRNA accumulation. Curr. Genet. 37 136–142. [DOI] [PubMed] [Google Scholar]
- Nickelsen, J. (2003). Chloroplast RNA-binding proteins. Curr. Genet. 43 392–399. [DOI] [PubMed] [Google Scholar]
- Nickelsen, J., Fleischmann, M., Boudreau, E., Rahire, M., and Rochaix, J.-D. (1999). Identification of cis-acting RNA leader elements required for chloroplast psbD gene expression in Chlamydomonas. Plant Cell 11 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickelsen, J., van Dillewijn, J., Rahire, M., and Rochaix, J.-D. (1994). Determinants for stability of the chloroplast psbD RNA are located within its short leader region in Chlamydomonas reinhardtii. EMBO J. 13 3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenbühl, F., and Nickelsen, J. (2000). Cis- and trans-acting determinants for translation of psbD mRNA in Chlamydomonas reinhardtii. Mol. Cell. Biol. 20 8134–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski, M., and Volmer, J.J. (2006). Cyanide metabolism in higher plants: Cyanoalanine hydratase is a NIT4 homolog. Plant Mol. Biol. 61 111–122. [DOI] [PubMed] [Google Scholar]
- Rohr, J., Sarkar, N., Balenger, S., Jeong, B.R., and Cerutti, H. (2004). Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 40 611–621. [DOI] [PubMed] [Google Scholar]
- Sane, A.P., Stein, B., and Westhoff, P. (2005). The nuclear gene HCF107 encodes a membrane-associated R-TPR (RNA tetratricopeptide repeat)-containing protein involved in expression of the plastidial psbH gene in Arabidopsis. Plant J. 42 720–730. [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber, C., Williams-Carrier, R., and Barkan, A. (2005). RNA immunoprecipitation and microarray analysis show a chloroplast pentatricopeptide repeat protein to be associated with the 5′ region of mRNAs whose translation it activates. Plant Cell 17 2791–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schult, K., Meierhoff, K., Paradies, S., Töller, T., Wolff, P., and Westhoff, P. (2007). The nuclear-encoded factor HCF173 is involved in the initiation of translation of the psbA mRNA in Arabidopsis thaliana. Plant Cell 19 1329–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanchi, A., Barnes, D., and Mayfield, S.P. (2005). A nuclear gene of Chlamydomonas reinhardtii, Tba1, encodes a putative oxidoreductase required for translation of the chloroplast psbA mRNA. Plant J. 42 341–352. [DOI] [PubMed] [Google Scholar]
- Uniacke, J., and Zerges, W. (2007). Photosystem II assembly and repair are differentially localized in Chlamydomonas. Plant Cell 19 3640–3654. [DOI] [PMC free article] [PubMed]
- Yohn, C.B., Cohen, A., Danon, A., and Mayfield, S.P. (1998. a). A poly(A) binding protein functions in the chloroplast as a message-specific translation factor. Proc. Natl. Acad. Sci. USA 95 2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn, C.B., Cohen, A., Rosch, C., Kuchka, M.R., and Mayfield, S.P. (1998. b). Translation of the chloroplast psbA mRNA requires the nuclearencoded poly(A) binding protein RB47. J. Cell Biol. 142 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa, M., Kuroda, H., and Sugiura, M. (2007). A new in vitro translation system for non-radioactive assay from tobacco chloroplasts: Effect of pre-mRNA processing on translation in vitro. Plant J. 49 367–376. [DOI] [PubMed] [Google Scholar]
- Zerges, W. (2000). Translation in chloroplasts. Biochimie 82 583–601. [DOI] [PubMed] [Google Scholar]
- Zerges, W., and Rochaix, J.D. (1998). Low density membranes are associated with RNA-binding proteins and thylakoids in the chloroplast of Chlamydomonas reinhardtii. J. Cell Biol. 140 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]