Abstract
In Arabidopsis thaliana, the female gametophyte is a highly polarized structure consisting of four cell types: one egg cell and two synergids, one central cell, and three antipodal cells. In this report, we describe the characterization of a novel female gametophyte mutant, eostre, which affects establishment of cell fates in the mature embryo sac. The eostre phenotype is caused by misexpression of the homeodomain gene BEL1-like homeodomain 1 (BLH1) in the embryo sac. It is known that BELL-KNAT proteins function as heterodimers whose activities are regulated by the Arabidopsis ovate family proteins (OFPs). We show that the phenotypic effect of BLH1 overexpression is dependent upon the class II knox gene KNAT3, suggesting that KNAT3 must be expressed and functional during megagametogenesis. Moreover, disruption of At OFP5, a known interactor of KNAT3 and BLH1, partially phenocopies the eostre mutation. Our study indicates that suppression of ectopic activity of BELL-KNOX TALE complexes, which might be mediated by At OFP5, is essential for normal development and cell specification in the Arabidopsis embryo sac. As eostre-1 embryo sacs also show nuclear migration abnormalities, this study suggests that a positional mechanism might be directing establishment of cell fates in early megagametophyte development.
INTRODUCTION
In Arabidopsis thaliana, the female gametophyte is a seven-cell structure consisting of four cell types: one egg cell and two synergids localized at the micropylar end of the mature embryo sac, one central cell, and three antipodal cells of undetermined function at the chalazal end (Figures 1A and 1B). All of the cells within the embryo sac are highly polarized. While the egg cell's nucleus is located toward the chalazal end of the embryo sac, the synergid and central cells have the opposite polarity (Willemse and van Went, 1984; Huang and Russell, 1992). The formation of the egg cell, synergids, and one of the polar nuclei can be traced back to the four-nucleate stage of embryo sac development, when the two pairs of nuclei migrate to opposite ends of the coenocytic embryo sac, remaining separated by a large vacuole (Pagnussat et al., 2005). At the chalazal pole, the nuclei are positioned one above the other with respect to the micropylar-chalazal axis. At the micropylar end, the nuclei generally locate side by side (Christensen et al., 1997; Pagnussat et al., 2005). A second round of mitotic nuclear division generates the eight nuclei of the mature embryo sac (Pagnussat et al., 2005). No genetic predisposition seems to guide the fate for any of the embryo sac nuclei to form the egg cell. However, the precise migration and positioning of the nuclei along the embryo sac at the four to eight nucleate stages suggests an early distinction between these nuclei as the third mitotic division progresses and the embryo sac undergoes cellularization (Webb and Gunning, 1994). Although the mechanism of cellularization remains not well understood (Russell, 1993; Brukhin et al., 2005), the microtubular cytoskeleton appears to establish and maintain organelle polarity and to function in migration and arrangement of the nuclei during embryo sac development (Webb and Gunning, 1990, 1994). In agreement with this model, radiating perinuclear microtubules have been observed during megagametophyte cellularization (Russell, 1993; Webb and Gunning, 1994).
Figure 1.
eostre Embryo Sacs Show Two Egg Cells and Only One Synergid Cell.
(A) Differential interference contrast (DIC) image showing a wild-type embryo at stage FG7. Nuclei have been artificially colored in red.
(B) Scheme indicating the different cell types present in a wild-type embryo sac at stage FG7.
(C) DIC image showing eostre mutant embryo sac phenotype at FG7. Nuclei have been artificially colored in red.
(D) Scheme indicating the cells that morphologically resemble egg cells and synergid cells in eostre mutant embryo sacs.
(E) Expression of the specific egg cell marker ET1119 in a wild-type embryo sac.
(F) The egg cell marker ET1119 is expressed in two micropylar cells in eostre embryo sacs.
(G) Distribution of ovules showing different ET1119 expression patterns in eostre-1/EOSTRE pistils. Error bars indicate se (n = 168). The white bar represents the percentage of ovules showing no GUS staining. The bar labeled “Gus (+) 1 Ec” represents the percentage of ovules showing GUS expression in only one cell of the embryo sac. The bar labeled “Gus (+) 2 Ec” represents the percentage of ovules showing GUS expression in two cells of the embryo sac. When ovules from wild-type pistils were analyzed, GUS staining was always detected in only one micropylar cell. Out of 264 observed, 47.8% of the ovules showed GUS staining.
(H) Expression of the specific synergid cell marker ET884 in a wild-type embryo sac.
(I) The synergid cell marker ET884 is expressed in only one micropylar cell in eostre embryo sacs.
(J) Distribution of ovules showing different ET884 expression patterns in eostre-1/EOSTRE pistils. Error bars indicate se (n = 127). The white bar represents the percentage of ovules showing no GUS staining. The bar labeled “Gus (+) 1 syn” represents the percentage of ovules showing GUS expression in only one cell of the embryo sac. The bar labeled “Gus (+) 2 syn” represents the percentage of ovules showing GUS expression in two cells of the embryo sac. When ovules from wild-type pistils where analyzed, GUS staining was detected in two micropylar cells in 46.8% of the ovules observed and in only one of the micropylar cells in 1.1% of the ovules (n = 247).
Ccn, central cell nucleus; Ec, egg cell; EcL, egg cell–like cell; mi, micropylar pole; S, synergide; Slc, synergid-like cell.
Although in the last few years a large number of mutants showing defects during megagametogenesis have been isolated in Arabidopsis (Christensen et al., 1997, 1998, 2002; Moore et al., 1997; Pagnussat et al., 2005), the cellular and molecular basis of cell specification in the embryo sac remains unknown. Recently, a mutant called lachesis (lis), which affects cell specification, has been described (Gross-Hardt et al., 2007). In lis mutant embryo sacs, accessory cells (synergids and antipodals) begin to display characteristics of gametic cells (egg cell and central cell). It has been proposed that LIS, which is homologous to the yeast splicing factor PRP4, might be involved in a signaling pathway operating in gametic cells that prevents accessory cells from adopting a gametic cell fate. Although the lis study indicates that accessory cells in the embryo sac might have the competence to differentiate into gametic cells, the mechanisms underlying the initial establishment of cell identity in the female gametophyte are not known. In this report, we describe the characterization of a novel female gametophyte mutant, eostre, which affects establishment of cell fates in the mature embryo sac.
RESULTS
The Cell Identity of Micropylar Cells Is Altered in the eostre Mutant
The eostre-1 mutant was identified from a large collection of female gametophyte mutants generated by Ds insertions and was originally called EDA12 (for Embryo Sac Development Arrest) (Pagnussat et al., 2005). As the Ds insertion carries an NPTII gene, gametophytic defects can be identified by the decreased transmission of resistance to kanamycin. The EDA12/eostre-1 mutant showed no sporophytic defects but exhibited an aberrant Kanr:Kans segregation ratio of 0.5513:1 in selfed progeny (Pagnussat et al., 2005). As this low ratio (<1:1) suggested that both female and male gametophytes might be affected by the Ds insertion, we performed reciprocal crosses between eostre-1 and Landsberg erecta (Ler) wild-type plants. Our results show that both the female and the male gametophytes were severely affected by the mutation (Table 1). The defect in the male transmission was shown to arise from a defect in pollen development (L. Boavida and S. McCormick, personal communication). To analyze the basis of the female transmission deficiency, we examined the terminal phenotype of the gametophytes by emasculating flowers from plants heterozygous for the Ds insertion, when wild-type embryo sacs are at the final developmental stage (stage FG7; Christensen et al., 1998; Figure 1) by emasculating heterozygous flowers. We found that the mutant gametophytes showed diverse defects ranging from embryo sacs completely collapsed showing absolute degeneration (see Supplemental Figure 1 online) or arrested very early (at FG1 to FG2), to embryo sacs that progressed to stage FG7, but presented an abnormal display of cells at the micropylar end. Specifically, we found that 29% of the embryo sacs in heterozygous pistils exhibited two cells with a polarity that resembles wild-type egg cells, while only one of the micropylar cells showed the morphology that corresponds to a synergid cell (Figures 1A to 1D, Table 2). Due to the excess of presumptive egg cells, the EDA12 mutant was renamed eostre, after the Anglo-Saxon goddess of the spring.
Table 1.
Transmission Efficiency of eostre Mutants
| Parental Genotypes (Female × Male)a | Kanr | Kans | Transmission Efficiencyb |
|---|---|---|---|
| eostre-1/EOSTRE × EOSTRE/EOSTRE | 77 | 137 | 35.98% |
| EOSTRE/EOSTRE × eostre-1/EOSTRE | 56 | 272 | 17.07% |
| eostre-3/EOSTRE × EOSTRE/EOSTRE | 76 | 83 | 47.79% |
| EOSTRE/EOSTRE × eostre-3/EOSTRE | 34 | 144 | 19.10% |
Plants were crossed manually, and seeds of the resulting cross were collected and grown on selective Murashige and Skoog (MS) plates to determine the efficiency in which the mutant allele was transmitted to the next generation by the male or female gametes.
Transmission efficiency = Kanr/Kans × 100%.
Table 2.
Analysis of eostre/EOSTRE Pistils at Different Stages of Development
| Stage and Phenotype of Ovules found in the Pistils Analyzed
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pistil Stagea | Collapsed Ovules | FM | FG2-3 | FG4 | FG5 | FG6 | FG7 | Total Analyzed | Abnormal (%) |
| FG1 | 0 | 125 | 0 | 0 | 0 | 0 | 0 | 125 | 0.00 |
| FG2-3 | 3 | 0 | 96 (0) | 12 (0) | 0 | 0 | 0 | 111 | 2.70 |
| FG3-4 | 0 | 33 | 56 (0) | 32 (6) | 0 | 0 | 0 | 121 | 4.95 |
| FG4 | 10 | 1 | 5 (3) | 21 (7) | 2 (1) | 0 | 0 | 49 | 42.85 |
| FG4-5 | 7 | 0 | 2 (1) | 13 (5) | 27 (6) | 1 (0) | 0 | 41 | 46.34 |
| FG6 | 23 | 5 | 5 (5) | 17 (14) | 27 (14) | 55 (6) | 0 | 132 | 46.96 |
| FG7 | 47 | 0 | 0 | 0 | 0 | 21 (21) | 296 (107) | 364 | 48.07 |
Assignment of pistil stage was done according to Christensen et al. (1998). An FG stage was assigned to pistils showing the majority (>75%) of the wild-type female gametophytes at that particular stage. If a single stage does not predominate, a transition between two stages was assigned.
Values within parenthesis indicate number of abnormal embryo sacs observed.
To further characterize the identities of the cells present in eostre-1 embryo sacs, we tested the expression of different cell-specific molecular markers. The marker lines were crossed with both wild-type plants and eostre-1 mutant plants, and the F1 progeny was analyzed for expression of the marker gene. The expression of an egg cell–specific marker (ET119; Gross-Hardt et al., 2007) was tested both in wild-type and in eostre-1 embryo sacs. While wild-type embryo sacs express the marker only in the egg cell, the marker was found to be strongly expressed in two of the eostre-1 micropylar cells in ∼15% of the embryo sacs (Figures 1E to 1G). This result suggested that two of the eostre-1 embryo sac cells might have egg cell attributes. On the other hand, while two micropylar cells were β-glucuronidase (GUS) positive when the expression of a synergid-specific marker (ET884; Gross-Hardt et al., 2007) was tested in wild-type embryo sacs, its expression was downregulated in many eostre-1 embryo sacs and ∼13% of the female gametophytes of eostre-1 pistils showed GUS expression in just one of the cells composing the embryo sac (Figures 1H to 1J), indicating that only one of the micropylar cells of the eostre-1 embryo sac might present synergid features. Markers for the central cell and the antipodal cells were also tested in eostre-1 embryo sacs. For both cases, similar GUS staining patterns were observed in wild-type and eostre-1 mutant embryo sacs (data not shown).
Fertilization of eostre Embryo Sacs Reveals Two Functional Egg Cells
In view of the above results, we decided to test whether the eostre-1 mutation was affecting embryo sac functions such as pollen tube guidance or fertilization. Fertilization in Arabidopsis requires controlled growth of the pollen tube until it enters the micropyle to penetrate the female gametophyte. Since the synergid cells are responsible for pollen tube attraction (Ray et al., 1997; Shimizu and Okada, 2000) and eostre-1 mutants appear to have only one synergid cell instead of the two present in wild-type embryo sacs, we analyzed the pollen tube growth patterns in eostre-1 by aniline blue staining. Although ∼80% of the mutant ovules were able to attract pollen tubes, abnormal pollen tube growth patterns (i.e., spinning and curling pollen tubes) were observed in ∼22% of the ovules in eostre-1/EOSTRE pistils (Figures 2A and 2B; see Supplemental Table 1 online). Also, ∼11% of the ovules showed a pollen tube on the funiculus but not at the micropyle. (The same situation could also be observed when wild-type pistils were analyzed, but the percentage of ovules without pollen tubes was only of 5.72; see Supplemental Table 1 online). Furthermore, a significantly higher fraction of mutant ovules was found to attract two pollen tubes to the micropyle compared with wild-type ovules (4.26 versus 0.96%, respectively; see Supplemental Table 1 online; P < 0.001, χ2 test). Together, the results indicated a defect in pollen tube attraction and guidance, and as a consequence, part of the pollen tubes either cannot reach the micropyles of the ovules or growth toward them following a wavering pattern. These defects were also observed when wild-type plants were used as pollen donors (see Supplemental Table 1 online; no significant differences χ2 test), indicating that the problem arises due to a disruption of the female gametophytic functions.
Figure 2.
Functional Study of Egg Cells and Synergids in eostre Embryo Sacs.
(A) Abnormal pollen tube growth patterns observed at the micropyle of an eostre mutant ovule.
(B) Pollen tube growing toward the micropyle of a wild-type embryo sac present in the pistil of eostre-1/EOSTRE plants.
(C) eostre embryo sac after fertilization showing one zygote-like cell, an egg cell–like cell, and endosperm development (endosperm nuclei are indicated by arrowheads).
(D) eostre embryo sac after fertilization showing two zygote-like cells and no endosperm development.
(E) to (H) GUS expression in both wild-type ([E] and [G]) and eostre ([F] and [H]) ovules after an eostre-1/EOSTRE plant was pollinated with a transgenic plant carrying the PFAC1IE:GFP-GUS:TFAC1 construct.
(E) GUS expression observed in the zygote of a wild-type ovule.
(F) GUS expression observed in both of the zygote-like cells present in this eostre embryo sac.
(G) GUS expression observed in the embryo and in the endosperm of a wild-type ovule.
(H) GUS expression observed in one zygote-like cell and in the endosperm of an eostre embryo sac. The arrows point at endosperm nuclei.
Ec, egg cell; EcL, egg cell–like cell; En, endosperm; EP, embryo proper; F, funiculus; mi, micropylar pole; PT, pollen tube; Z, zygote; ZL, zygote like.
To study if the two micropylar cells expressing the specific egg cell marker in the eostre-1 embryo sac (Figure 1F) are able to become fertilized, we studied the phenotype of eostre-1 embryo sacs after pollinating eostre-1 pistils using wild-type plants as pollen donors. When pistils were analyzed 24 h after pollination, 49.7% of the ovules showed an elongated zygote and endosperm development, 3.28% of the ovules were found unfertilized, 4.22% showed a zygote but have endosperm development affected, and ∼24% of the ovules looked fertilized, showing two zygote-like cells, characterized by a relatively rounded shape and a centrally located nucleus (Figure 2D). There were also ovules showing only one zygote-like cell and an egg cell that also exhibited some extent of endosperm development (four to six endosperm nuclei, Figure 2C). For those ovules showing two zygote-like cells, they did not show any endosperm development (Figure 2D). Also, 18.3% of the ovules were found collapsed at this stage. Although the two micropylar cells observed in the mutant ovules after fertilization resemble the characteristics of zygotic cells, we decided to investigate whether those cells were actually fertilized by pollinating eostre-1 pistils using transgenic plants carrying the PFAC1IE:GFP-GUS:TFAC1 construct as pollen donors. FAC1 (Embryonic Factor 1) encodes a putative AMP deaminase (EC:3.5.4.6) and is expressed in both the developing embryo and endosperm from the paternal allele (Xu et al., 2005). Two different patterns of GUS expression were observed in eostre-1 mutants after pollination: (1) GUS staining was observed either in both of the zygote- like cells or (2) GUS staining was found in only one of the zygote-like cells and in the endosperm. These results suggested that either fertilization of both of the egg cells or fertilization of only one of the egg cells and of the central cell can occur (Figures 2F and 2H; see Supplemental Table 2 online). GUS expression was not detected in both zygote-like cells and endosperm at the same time, suggesting that even when some eostre-1 mutant sacs are able to attract more than one pollen tube (see Supplemental Table 1 online), only one of them would deliver sperm cells into the embryo sac. On the other hand, no autonomous endosperm development was observed when the two egg cells are fertilized, suggesting that the unfertilized central cell is not dividing after the fertilization event. This result was intriguing since it was previously shown that a positive signal from the fertilization of the egg cell initiates proliferation of the unfertilized central cell in wild-type plants (Nowack et al., 2006). We therefore considered whether the fertilization of two egg cells rather than one in the embryo sac was interfering with the signaling after fertilization, preventing both embryo and endosperm development in the eostre-1 mutant. To test that possibility, we used the cdc-2 mutant, carrying a mutation in the Arabidopsis Cdc2 homolog CDC2A, to pollinate eostre-1/EOSTRE siliques. In cdc2a mutant pollen, only one sperm cell, instead of two, is produced. Mutant pollen is viable and the single sperm fertilizes only the egg cell, but this single fertilization event appears to trigger endosperm development in the central cell (Nowack et al., 2006). If the response of the central cell of an eostre-1 embryo sac is similar to that of a wild-type embryo sac, fertilization with cdc2a pollen should result in an increase in the fraction of ovules that show both embryo development and some extent of endosperm development. However, we did not observe any difference in endosperm development in the siliques analyzed after pollinating eostre-1 with cdc2a pollen (see Supplemental Table 3 online).
eostre-1 Shows Nuclei Migration and Cellularization Abnormalities during Megagametogenesis
To analyze the developmental stage at which eostre-1 female gametophytes start to show defects, we observed the embryo sacs of heterozygous pistils at different phases of development. Since female gametophyte development within a pistil is generally synchronous (Christensen et al., 1997), we used the wild-type embryo sacs siblings as a guide to ascribe delays to the mutant embryo sacs present within the same pistil.
As shown in Table 2, eostre-1 female gametophytes begin to develop normally, as no differences can be observed at FG1 stage among any of the embryo sacs present in the heterozygous pistils analyzed. However, from stage FG3 on, the developmental asynchrony was evident. While the majority of the embryo sacs was already between FG3 and FG4 stages, ∼27% of the embryo sacs were still at FG1 stage. Moreover, some of the embryo sacs already at FG4 stage showed abnormal nuclear position. While in the wild-type embryo sacs at stage FG4 the two pairs of nuclei are separated by a large central vacuole and showed a typical arrangement (Figures 3A and 3C), in eostre-1 female gametophytes, the vacuole is positioned on a side of the embryo sac, the two pairs of nuclei are not completely separated, and they look aligned along the coenocytic embryo sac (Figure 3I). When pistils were analyzed at stages FG4 to FG5, 10% of the embryo sacs showed this defect (Table 2). Approximately 18% of the embryo sacs also showed to be collapsed at this stage (Table 2) probably arising from those that were previously observed to be severely delayed. During the cellularization process that begins following the third round of mitosis (FG5 and FG6), not only the developmental asynchrony was obvious but also ∼30% of the embryo sacs showed abnormal distribution of nuclei along the embryo sac or cells with aberrant shapes and polarities at the micropylar end (Table 2). At mid stage FG5, a wild-type embryo sac shows four micropylar nuclei with a distinctive distribution, in which the two nuclei that will further become synergid nuclei are located side by side at the embryo sac mycropylar edge, while the future egg cell nuclei is located toward the embryo sac micropylar half (Figure 3D). However, an atypical arrangement of nuclei at the micropylar pole was observed in eostre-1 embryo sacs at this stage (Figure 3J). Only one nuclei instead of the two observed in wild-type embryo sac is located at the micropylar embryo sac edge, two nuclei are located side by side at the middle region of the micropylar pole, and one nucleus is located closer to the center of the embryo sac (Figures 3D and 3J). As cellularization progresses in wild-type embryo sacs, cellular morphology in the cells at the embryo sac's micropylar pole become clearly identifiable. At FG6 stage, the egg cell shows a characteristic pear shape and a highly polarized cytoplasm; its nucleus and most of its cytoplasm are at its chalazal end and a large vacuole occupies the remaining space of the cell (Figures 3A and 3E). Similarly, wild-type synergid cells also have a distinguishing shape and polarity, which is the opposite of the egg cell cytoplasm (Figures 3A and 3E). By contrast, eostre-1 female gametophytes showed two cells in which the nuclei occupied a chalazal position and only one cell in which the nucleus showed a micropylar orientation. Thus, at the end of FG7, eostre-1 embryo sacs appear to contain two egg cells instead of one and only one synergid cell (Figures 3K and 3L). This asynchrony in embryo sac development was not observed when wild-type pistils were analyzed, and abnormal or aborted embryo sacs were rarely observed (only 1 to 2.3%; see Supplemental Table 4 online).
Figure 3.
Wild-Type and eostre Female Gametophyte Development in Arabidopsis.
(A) Scheme showing the developmental stages leading to the wild-type embryo sac formation.
(B) Wild-type embryo sac at FG3 stage, showing two nuclei separated by a vacuole.
(C) Wild-type embryo sac at FG4 stage, showing two pairs of nuclei separated by a vacuole.
(D) Eight-nucleated wild-type embryo sac. One of the chalazal end nuclei is migrating toward the micropylar end (indicated by arrows).
(E) Wild-type embryo sac containing seven cells and eight nuclei.
(F) Wild-type embryo sac containing seven cells and seven nuclei.
(G) Scheme showing the developmental stages leading to the eostre mutant female gametophyte formation.
(H) eostre embryo sac at FG3 stage, showing two nuclei separated by a small vacuole.
(I) eostre embryo sac at FG4 stage. The vacuole is positioned on a side of the embryo sac, the two pairs of nuclei are not completely separated, and they look aligned along the embryo sac.
(J) eostre embryo sac at FG5 stage showing an atypical arrangement of nuclei at the micropylar end. One of the chalazal end nuclei is migrating toward the micropylar end (indicated by arrows).
(K) eostre embryo sac containing seven cells and eight nuclei. eostre embryo sacs appear to contain two egg cells instead of one and only one synergid cell.
(L) eostre embryo sac at FG7 stage. Nuclei have been artificially colored in red.
Cc, central cell; Ccn, central cell nucleus; Ch, chalazal pole; Ec, egg cell; EcL, egg cell–like cell; mi, micropylar pole; Pn, polar nuclei; S, synergide; SL, synergid-like cell.
A Ds Element Is Inserted Inside an Intergenic Region and Promotes Misexpression of a BEL1-Like Gene in eostre Embryo Sacs
Thermal asymmetric interlaced PCR (Liu et al., 1995) was used to identify the flanking sequence surrounding the Ds insertion in eostre-1 mutants using Ds-specific primers and arbitrary degenerated primers. The flanking sequence obtained revealed that the single Ds insertion was inserted in the intergenic region between the genes At2g35940 (BEL1-like homeodomain 1 [BLH1]) and At2g35950 (unknown protein) 1862 nt upstream of the At2g35950 start codon (Figure 4A). This location was further confirmed by PCR using sequence-specific primers for the putative insertion site in combination with the corresponding Ds element primers. A DNA fragment of the predicted size was amplified from the genomic DNA of eostre-1 but not from wild-type Arabidopsis genomic DNA. The DNA sequence of the PCR product verified the junction between the genomic DNA and the Ds element and showed a direct repeat of 8 bp at the point of insertion (Figure 4A). To verify if this insertion site was responsible for the phenotype observed in the eostre-1 mutant, we obtained lines containing T-DNA insertions around this region from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003). We analyzed two independent T-DNA alleles with insertions in the intergenic region between At2g35940 and At2g35950 that were named eostre-2 and eostre-3. In eostre-2, the T-DNA is inserted 1161 nucleotides upstream of the At2g35950 start codon, and in eostre-3, the T-DNA is inserted 3132 nucleotides upstream of the At2g35950 start codon. No homozygous plants could be detected for either of these alleles. However, the mutation seemed to be less penetrant in these mutants. eostre-2 siliques contained ∼27% of aborted/desiccated ovules, while eostre-3 siliques contained ∼32% of aborted ovules (Figures 4B and 4C). To study whether the eostre allele mutations affect the female gametophyte as in the original eostre-1 mutation, we analyzed the phenotype of embryo sacs in both eostre-2 and eostre-3 pistils. In both cases, ∼15% of the mutant ovules showed micropylar cells with abnormal polarities as was observed for eostre-1 (Figures 4D and 4E). A fraction of the ovules collapsed very early during development (∼30% for both alleles; see Supplemental Table 5 online). The remaining fraction, even when they looked normal at stage FG7, seemed to have problems attracting pollen tubes and remain unfertilized (see Supplemental Table 5 online). We also noticed that the number of unfertilized or aborted ovules in eostre-2 and eostre-3 varied widely from silique to silique even within the same plant, a characteristic that was not observed in the eostre-1 mutant. Also, eostre-3 exhibited reduced transmission through both the female and the male gametophyte (Table 1).
Figure 4.
The eostre Mutant Phenotype Is Caused by a Ds Insertion within the Intergenic Region between the Genes At2g35940 and At2g35950.
(A) Position of the Ds element in eostre-1 and of the T-DNA insertion in eostre-2 and eostre-3. The arrow indicates the GUS gene within the Ds insertion. The Ds insertion is located 11,272 bp upstream of the At2g35940 (BLH1) start codon.
(B) DIC image showing eostre-2 mutant embryo sac phenotype at FG7.
(C) DIC image showing eostre-3 mutant embryo sac phenotype at FG7.
(D) Phenotype of an eostre-2/EOSTRE silique. The insert shows an enlarged section of the silique, and the arrow points to an aborted ovule.
(E) Phenotype of an eostre-3/EOSTRE silique. The insert shows an enlarged section of the silique, and the arrowhead points to an aborted ovule.
Ccn, central cell nucleus; EcL, egg cell–like cell; mi, micropylar pole of the embryo sac; SL, synergid-like cell.
As At2g35950 was the nearest gene to these intergenic insertions, we examined two lines obtained from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003) carrying a T-DNA insertion within this gene to study if a putative deficiency in At2g35950 expression was causing the observed phenotype. For both lines, we were able to identify homozygous null mutants plants for At2g35950, as no transcripts for this gene were detected by RT-PCR (data not shown), indicating that disruption of At2g35950 does not lead to gametophytic defects.
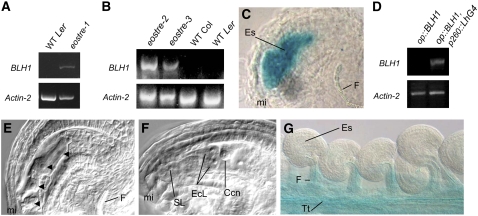
In an attempt to identify genes of noncoding RNAs, the intergenic region was screened for the presence of microRNAs using the microRNA candidates database (http://sundarlab.ucdavis.edu/mirna/search_candidates.html/; Adai et al., 2005) and for the presence of small RNAs using the small RNA database (http://asrp.cgrb.oregonstate.edu/db), but no matches were found around the insertion site. We also looked for transcription units across the intergenic region in an effort to detect the expression of nonannotated genes using the Arabidopsis Transcriptome Genomic Express Database (http://signal.salk.edu/cgi-bin/atta; Yamada et al., 2003) and by checking the putative transcripts by RT-PCR, without positive results. As no new genes or noncoding RNAs across the region of the insertions studied were detected, the expression of the upstream gene At2g35940 (BLH1) and the expression of two additional downstream genes (At2g35960 and At2g35970, both encoding a harpin-induced family protein) was studied in wild-type and eostre-1 ovules by RT-PCR experiments using ovule total mRNA as a template. For the downstream genes studied, we were not able to detect any expression in wild-type or eostre-1 ovules (data not shown). Although no expression of BLH1 was detected in wild-type ovules either, its transcripts were consistently detected in eostre-1 ovules (Figure 5A). This result suggested that BLH1 might be misexpressed in the mutant ovules. Accordingly, expression of BLH1 was also found in the eostre-1 alleles eostre-2 and eostre-3 (Figure 5B). To study if the overexpression detected in eostre-1 was ovule specific, BLH1 expression was also analyzed in leaves. BLH1 expression was higher in eostre-1 leaves compared with the wild-type ones (Figure 7D), although no defects were observed in leaf development or morphology. This result indicated that although the deregulation of BLH1 gene might be general, it only causes a defect in reproductive tissues. We used a gene trap line (GT9784) carrying an insertion in BLH1 gene from the Cold Spring Harbor collection (http://genetrap.cshl.org/) to analyze the BLH1 expression pattern in Arabidopsis. As observed in Figure 5G, when dissected pistils of heterozygous plants were analyzed for GUS expression, GUS staining was only detected in the transmitting tract and in the base of the funiculus but not in the ovule or the embryo sac. Thus, the normal BLH1 expression pattern seems to exclude both the ovule and the female gametophyte.
Figure 5.
Misexpression of BLH1 in the Embryo Sac Recapitulates the eostre Phenotype.
(A) RT-PCR showing BLH1 overexpression and actin expression as a positive control (bottom) in eostre-1 ovules.
(B) Overexpression of BLH1 was also observed in eostre-2 and eostre-3 ovules in the Col background. Actin expression is shown as a positive control (bottom).
(C) GUS staining pattern in a pES1≫BLH1 ovule showing that pES1 specifically directs expression of the reporter genes to the female gametophyte.
(D) BLH1 expression in pES1≫BLH1 ovules detected by RT-PCR. Actin expression is shown as a positive control (bottom).
(E) Phenotype of a pES1≫BLH1 embryo sac at FG4 stage. Arrowheads indicate the unusual position of nuclei.
(F) Phenotype of a pES1≫BLH1 embryo sac at FG7 stage.
(G) Expression pattern of BLH1 in the pistil of the BLH1 gene trap line GT9784.
Ccn, central cell nucleus; EcL, egg cell–like cell; Es, embryo sac; F, funiculus; mi, micropylar pole of the embryo sac; SL, synergid-like cell; Tt, transmitting track.
Figure 7.
At OFP5, an Interactor of BLH1 and KNAT3, Is Required for Normal Embryo Sac Development.
(A) At OFP5-1 mutant embryo sac at FG7 showing a phenotype similar to the one observed in eostre-1 ovules.
(B) RT-PCR showing KNAT3 expression in wild-type, eostre-1, and spl ovules. Actin expression is also shown as a positive control (bottom). The picture corresponds to 20 PCR cycles.
(C) RT-PCR showing At OFP5 expression in wild-type and spl ovules. Actin expression is shown as a positive control (bottom). The picture corresponds to 20 PCR cycles. At OFP5 expression was detected in spl ovules after 25 PCR cycles.
(D) RT-PCR showing BLH1 expression in wild-type and eostre-1 leaves. The picture corresponds to 25 PCR cycles. BLH1 expression was detected in wild-type leaves at 30 cycles.
(E) RT-PCR showing KNAT3 expression in wild-type and eostre-1 leaves. The picture corresponds to 25 PCR cycles.
Ccn, central cell nucleus; EcL, egg cell–like cell; mi, micropylar pole of the embryo sac; SL, synergid-like cell.
The eostre Phenotype Is Caused by Misexpression of BLH1 in the Embryo Sac
As BLH1 was shown to be misexpressed in eostre ovules, we further investigated if this might be the cause of the observed phenotype by driving the expression of BLH1 in the embryo sacs of wild-type plants using the pOp/LhG4 transactivation system (Moore et al., 1998). We generated transgenic lines expressing the chimeric transcription factor LhG4 under the control of two different promoters: At5g40260 (pES1 [for Embryo Sac promoter 1]), which encodes a protein of the nodulin MtN3 family and is expressed in the embryo sac from FG1 to FG7 (Yu et al., 2005), and At1g26795 (pES2 [for Embryo Sac promoter 2]), which encodes a self-incompatibility protein and it is strongly expressed from FG3 in the dividing nuclei of the embryo sac to FG7 (Yu et al., 2005). A reporter construct carrying the BLH1 coding region under the control of the p10Op promoter, which is activated by LhG4, was introduced in plants that were crossed to pES:LhG4 plants. As the pES:LhG4 constructs were directly introduced into transgenic plants carrying a pOp-GUS reporter construct, we were able to confirm that the promoters used were directing the expression of the reporter genes to the embryo sacs by studying GUS staining patterns (Figure 5C). To verify if BLH1 was in fact expressed in these plants, RT-PCR experiments were performed using total RNA from ovules of plants carrying the Op:BLH1 reporter construct alone as well as plants carrying both the driver and reporter constructs. BLH1 expression was only detected in ovules from plants carrying both constructs (Figure 5D). These results indicated that BLH1 was successfully and specifically expressed in the embryo sac of the plants under study. When the pistils of F1 plants expressing BLH1 in the embryo sacs were analyzed, approximately one-fourth of the ovules showed to be defective, which was the proportion expected for a double heterozygote for the driver and reporter construct. The defects observed range, as in eostre-1 mutants, from early aborted embryo sacs to embryo sacs that progress to the terminal stage showing two egg cells and a single synergid (Figures 5E and5F). When the phenotype of these embryo sacs was studied after fertilization, both embryo sacs showing only one arrested zygote, one egg cell–like cell and four to six endosperm nuclei or two zygotic-like structures without endosperm development were observed (see Supplemental Figure 2 online). Plants carrying the reporters or the driver constructs alone did not show any phenotype, indicating no background activity.
blh1 Loss-of-Function Mutants Have Normal and Functional Gametophytes
To examine the effects of loss of function of BLH1, we analyzed three independent T-DNA lines with insertions in At2g35940 (BLH1) that are predicted to be null alleles. For all three lines, we were able to identify homozygous mutant plants with blh1 null alleles (verified by RT-PCR; data not shown), indicating that BLH1 alone is not essential for gametophyte development or function. As BLH1 belongs to the BELL family of homeodomain transcription factors (Chan et al., 1998; Roeder et al., 2003), a large family that includes 13 proteins, we could not eliminate the possibility that a functional redundancy might be obscuring BLH1 function in the embryo sac. As BLH5 is the BEL1-like protein that shows the closest homology to BLH1 (Roeder et al., 2003), double gene knockout lines were generated and were statistically analyzed in segregating F2 populations (blh1 × blh5). Homozygous plants for both insertions were recovered, and plants were found to look like wild-type plants, showing no obvious defects either in the sporophytic or the gametophytic organs (data not shown). This result suggested either that BLH1 or BLH5 is not involved in gametophyte development or that a broader functional redundancy among the members of the BELL1 family exists, requiring additional mutations in other BLH genes to reveal their functions in the embryo sac.
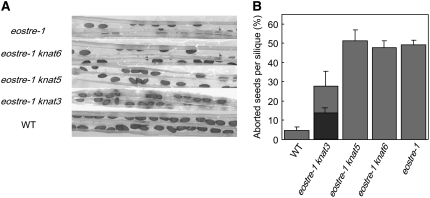
A Mutation in the Class II Knox Gene KNAT3 Reverts the eostre Phenotype
Biochemical studies have shown that BELL proteins associate with KNOX proteins to form heterodimers that are thought to compose functional complexes regulating plant development (Muller et al., 2001). Specifically, BLH1 has been reported to interact with the KNOX proteins KNAT3, KNAT5, and KNAT6 (Hackbusch et al., 2005). If overexpression of BLH1 in the embryo sac requires these KNOX proteins to cause the eostre phenotype, we might expect that loss of function of the corresponding KNAT gene would suppress the eostre mutation. To test this possibility, we generated double mutants by crossing knox gene knockout lines to eostre-1. The segregating F1 population was analyzed by genotyping and analysis of the siliques. No significant differences in the number of aborted seeds per silique were observed between eostre-1 single mutant and eostre-1 knat5 or eostre-1 knat6 double mutants (Figures 6A and 6B). On the other hand, heterozygous plants for both a T-DNA insertion in knat3 gene and for the eostre-1 Ds insertion showed a significant reduction in the number of aborted seeds per silique when compared with the eostre-1 single mutant (Figure 6; t test, P < 0.005). The suppression of eostre-1 cannot be attributed to the different Arabidopsis ecotype of the knat3 mutant (Columbia [Col]), as no suppression was detected when eostre-1 (Ler background) was crossed to the knat5 or knat6 mutants (Col background). When the F2 population was analyzed, plants that were homozygous for the T-DNA insertion in the knat3 gene and heterozygous for the eostre-1 Ds insertion were recovered and its siliques studied. Only ∼13% of the ovules observed looked aborted in these siliques, a number significantly lower than the one observed for pistils from heterozygous plants for both the knat3 gene and eostre-1 insertions (Figure 6; t test, P < 0.05). Thus, the knat3 mutation is able to suppress the eostre phenotype, but the suppression is not complete, suggesting the possibility that other KNATs might be interacting with BLH1 in the embryo sac. Previous results have reported KNAT3 expression during early organ development in leaves, buds, and pedicels at the junction between two organs during development, including the ovule-funiculus boundaries, and in maturing tissues, such as siliques, petioles of maturing leaves, and the root (Serikawa et al., 1997; Truernit et al., 2006). Although no data regarding KNAT3 expression in Arabidopsis embryo sac have been reported so far, the closely related class II knox gene KNOX6 was shown to be expressed in the maize (Zea mays) embryo sac after cellularization (Evans, 2007). To examine KNAT3 expression in Arabidopsis ovules, total RNA from wild-type ovules and from spl mutant ovules, which lack embryo sacs (Yang et al., 1999), was used to study KNAT3 expression by RT-PCR. KNAT3 expression was consistently detected in both wild-type and spl mutant ovules, indicating that KNAT3 is at least expressed sporophytically in the ovule, although expression in the embryo sac remains to be confirmed (Figure 7). Furthermore, and to test the possibility of an upregulation of KNAT3 in eostre ovules, the level of KNAT3 expression was assessed in eostre-1, wild-type, and spl ovules by RT-PCR. No differences were found among the samples, indicating that KNAT3 is not upregulated in eostre-1 ovules (Figure 7B). As BLH1 overexpression was also detected in somatic tissues (Figure 7D), we also studied KNAT3 expression in wild-type and eostre-1 leaves by RT-PCR. Again, no differences were detected (Figure 7E).
Figure 6.
A Mutation in the Class II Knox Gene KNAT3 Suppresses the eostre Phenotype.
(A) Mature siliques of eostre-1/EOSTRE plants and double mutants eostre1 knat6, eostre1 knat5, and eostre1 knat3. The bottom panel shows a mature silique of the wild type.
(B) The aborted seeds per silique were quantified for each genetic background. Values represent average number of aborted seeds per silique (mean ± se, n = 20). Light-gray bars correspond to ovules from plants heterozygous for the insertion in knox genes. The dark-gray bar shows data corresponding to ovules from plants that were homozygous for the insertion in the knat-3 gene.
At OFP5, a Putative Regulator of BLH1 Activity, Is Required for Normal Embryo Sac Development
An investigation of Arabidopsis TALE protein interactions revealed a heavily connected network of interactions of TALE proteins with each other and with members of the recently discovered OVATE protein family (At OFP) (Hackbusch et al., 2005). Particularly, it was shown that members of this family interact both with KNAT3 and BLH1 in a yeast two-hybrid system (Hackbusch et al., 2005). Furthermore, At OFP1 and At OFP5 regulate subcellular localization of BLH1, relocalizing it from the nucleus, where it would be active as a transcription factor, to the cytoplasmic space (Hackbusch et al., 2005). Although little information is available about the specific expression patterns of both TALE and At OFP genes in the female gametophyte, the results with eostre and knat3 suggested that complexes involving both TALE and At OFP might be functional during embryo sac development. To study this possibility, we analyzed Arabidopsis lines with T-DNA insertions in four At OFP genes (At OFP1-1, At OFP1-2, At OFP2-1, At OFP4-1, and At OFP5-1) obtained from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003). Although homozygous plants were recovered from selfing of heterozygous plants for At OFP1-1, At OFP1-2, At OFP2-1, and At OFP4-1 mutants, no homozygous plants were detected for At OFP5-1 mutants, suggesting that At OFP5 might be required for essential processes in gametophyte or sporophyte development. When female gametophyte development was investigated in At OFP5-1 pistils, out of 256 embryo sacs studied, 54% looked normal, while ∼38% of the ovules seemed to collapse very early during development around stage FG2. The remaining ∼8% of the embryo sacs (20/256) showed abnormal micropylar cells, with embryo sacs showing two egg cells as was observed in eostre-1 mutants (Figure 7). These results strongly indicate a requirement for At OFP5 during female gametophyte development and also that TALE-OVATE protein complexes might be functional during embryo sac early developmental stages. RT-PCR experiments were performed to evaluate At OFP5 expression in the embryo sac. At OFP5 expression levels were found to be higher in wild-type ovules compared with spl ovules, indicating that At OFP5 is expressed both in the embryo sac and in the sporophytic tissues of the ovule (Figure 7).
DISCUSSION
Regulation of BELL-KNOX TALE Complexes Is Essential for Normal Development of the Arabidopsis Embryo Sac
The eostre gametophytic mutant shows synergid to egg cell fate switch in Arabidopsis embryo sacs, a phenotype that could be recapitulated when BLH1 was misexpressed in the embryo sac. Hence, misexpresion of BLH1 seems sufficient to generate the eostre phenotype, probably by interfering with the normal patterning during female gametophyte development. Although no loss-of-function mutants were found for BELL proteins that might be involved in the normal embryo sac development pathway, functional redundancy might be obscuring a substantial role for these proteins during normal megagametogenesis. BELL and KNOX proteins are members of the MEINOX-TALE superfamily of eukaryotic transcriptional regulators that function as heterodimers and are known to regulate developmental processes both in plants and animals (Knoepfler et al., 1997; Rieckhof et al., 1997; Bellaoui et al., 2001; Muller et al., 2001; Smith et al., 2004). Biochemical studies have shown that BELL proteins associate with KNOX proteins to form functional complexes regulating plant development (Muller et al., 2001). In particular, BLH1 has been reported to interact with the KNOX proteins KNAT3, KNAT5, and KNAT6 (Hackbusch et al., 2005). As a common feature of KNOX-BELL interactions is that the KNOX protein partner often interacts with a subset of BELL proteins (Cole et al., 2006), BLH1 misexpression in the embryo sac might be preventing functional KNOX–BELL interactions to take place and, as a result, interfering with normal patterning. Supporting this idea, KNAT3, a gene belonging to the class II subfamily of knox genes (Reiser et al., 2000) and an already described interactor of BELL proteins (including BLH1; Hackbusch et al., 2005), appears to be essential for the phenotypic effect of BLH1 overexpression in the embryo sac (Figure 6). This result suggested that KNAT3 must be expressed and functionally competent in the embryo sac. In agreement, KNAT3 expression was detected in Arabidopsis ovules (Figure 7B). In addition, the class II gene Knox6, which is the nearest maize homolog of KNAT3, was the only Knox gene found to be consistently expressed in isolated maize embryo sacs (Evans, 2007). As no functions for class II Knox genes in plant development have been reported in any plant species to date (Reiser et al., 2000), knat3 suppression of the embryo sac defects of the eostre mutant revealed a phenotype for a class II Knox mutant. Emphasizing the importance of regulation of knox genes during megagametophyte development, the maize gene INDETERMINATE GAMETOPHYTE1 (IG1), which represses the expression of meristem-specific knox genes in leaf primordia, has been shown to be expressed during early embryo sac development, and ig1 mutants exhibit embryo sac abnormalities that include extra egg cells, extra polar nuclei, and extra synergids (Evans, 2007). In Arabidopsis, mutation of the putative Ig1 ortholog AS2 does not result in embryo sac defects (Semiarti et al., 2001). However, At OFP5, a member of the Arabidopsis ovate family, which was shown to interact with KNAT3 and to regulate subcellular localization of BLH1 (Hackbusch et al., 2005), appears to play an essential role during embryo sac development (Figure 7). At ofp5 embryo sacs displayed phenotypes that resemble those observed in eostre-1 mutants, consistent with At OFP5 acting as a key negative regulator of BELL-KNOX activity during early embryo sac development in Arabidopsis.
Factors Affecting Cell Fate Specification during Female Gametophyte Development
The atypical migration and positioning of nuclei observed in eostre mutant embryo sacs lead to a variety of morphological and functional defects, including the production of an extra functional egg cell and only one synergid cell. Our observations suggest that the specification of cell fate, as egg cell or synergid, appears to rely on a position-based mechanism. However, we cannot rule out that the altered migration of nuclei could also be a consequence of an altered fate. At FG5, only one nucleus is located at the micropylar edge of eostre mutant embryo sacs, a position at which the pair of nuclei that will further form part of the synergid cells are normally positioned in wild-type embryo sacs. Furthermore, two nuclei are located side by side at the middle region of the micropylar pole, where typically the future egg cell nucleus is placed in wild-type embryo sacs. As cellularization progresses, eostre embryo sacs clearly showed an abnormal pattern of cells at their micropylar edge, with only one cell displaying synergid features and two cells showing egg cell characteristics (Figure 1). These specific cells were also shown to be functional, as the synergid cell was able to attract pollen tubes and both egg cells in eostre mutant embryo sacs are able to become fertilized (Figure 2). Recently, the description of the lis mutant, which shows extra egg cells in detriment of both synergids and central cell identities, has led to a model that upon differentiation, gametic cells would generate an inhibitory signal that transmitted to the adjacent cells would prevent excess gametic cell formation (Gross-Hardt et al., 2007). Although this mechanism of lateral inhibition can explain the maintenance of only one egg cell in the embryo sac after the initial specification of cell fate, the mechanisms involved in the early establishment of different cell fates in the embryo sac are not addressed by the model. A positional mechanism would explain how cell fate is specified in early megagametophyte development. Although when all the nuclei present in the embryo sac might be competent to form gametic cells upon cellularization, those nuclei that are at the micropylar or chalazal poles acquire the accessory cell fates. However, once the egg cell is differentiated, a lateral inhibition mechanism seems to be necessary to maintain cell fates, as accessory cells can yet differentiate later on into gametic cells if this inhibitory mechanism is not present (Gross-Hardt et al., 2007). The idea of a positional mechanism is also supported by studies in maize, where ig1 embryo sacs undergo extra rounds of free nuclear divisions, resulting in extra egg cells, extra central cells, and extra polar nuclei (Guo et al., 2004; Evans, 2007).
The migration and position of nuclei during megagametogenesis in Arabidopsis has been shown to be highly regular (Webb and Gunning, 1990, 1994). Previous studies by Brown and Lemmon (1991, 1992) suggested that cytoplasmic domains may determine the fate of cells during cell partitioning. This idea is supported by our findings studying eostre mutants, as the unusual position of the nuclei along the embryo sac might expose them to different cytoplasmic domains compared with the wild-type embryo sac nuclei. An abnormal microtubular behavior (i.e., a change in microtubule dynamics) during and following the second and third rounds of mitotic divisions (FG2 to FG4) or a failure in the nuclei interactions with components of the microtubular cytoskeleton might explain the unusual pattern of nuclear migration and the resulting abnormal nuclear positioning observed in eostre embryo sacs. The importance of specific positions of nuclei along the embryo sac for cell fate determination might rely on the asymmetric distribution of still unknown morphogenetic determinants, perhaps in the form of gradients, that might be responsible for determining the fate of the different cells composing the embryo sac as described for numerous multicellular systems (Adler, 2000; Betschinger and Knoblich, 2004; Harris and Peifer, 2005).
METHODS
Plant Growth Conditions
The origin of the Ds insertion lines used in this study has been previously described (Sundaresan et al., 1995). Typically 50 to 200 seeds were sterilized in 20% (v/v) sodium hypochlorite, washed with sterile water, and plated on MS medium with 50 mg L−1 kanamycin in Percival growth chambers (Percival Scientific), with a 16-h-light/8-h-dark cycle at 22°C. Resistant (green) seedlings were then transferred onto soil and grown under the conditions described above with 60% relative humidity. For crosses, flowers of the female parent were manually emasculated 2 d before anthesis and cross-pollinated 2 d later.
Histology and Microscopy
To prepare cleared whole-mount preparations, pistils containing at least 20 ovules were dissected and cleared overnight in Hoyer's solution (Liu and Meinke, 1998). The dissected pistils were observed on a Zeiss Axioplan imaging 2 microscope under DIC optics. Images were captured on an Axiocam HRC CCD camera (Zeiss) using the Axiovision program (version 3.1). For GUS staining, developing carpels and siliques were dissected and incubated in GUS staining buffer [5 mM EDTA, 0.1% Triton X-100, 5 mM K4Fe(CN)6, 0.5 mM K3Fe (CN)6, and 1 mg mL−1 X-Gluc [Rose Scientific] in 50 mM sodium phosphate buffer, pH 7.0) overnight at 37°C. Individual ovules were dissected from the pistils/siliques and cleared overnight with Hoyer's solution. The ovules were observed under DIC optics. For pollen tube staining, 10 pistils containing at least 20 ovules each were manually pollinated and opened longitudinally 24 h after pollination for each mutant. The pistils were cleared in 10% chloral hydrate at 65°C for 5 min and washed with water, softened with 5 M NaOH at 65°C for 5 min, and washed again with water. The pistils were then treated with 0.1% aniline blue in 0.1 M K3PO4 buffer, pH 8.3, for 3 h in darkness and washed with 0.1 M K3PO4 buffer. The pistils were mounted on a microscope slide using a drop of glycerol and carefully squashed under a cover slip. The pistils were observed using a fluorescence microscope.
Image Processing
All images were processed for publication using Adobe Photoshop CS (Adobe Systems).
Segregation Analysis
For self-cross analysis, heterozygous plants were allowed to self-pollinate and progeny seed was collected. The progeny seed was germinated on growth medium containing the proper markers (kanamycin, BASTA, or sulfadiazine) as indicated in each case. The F1 seed was germinated on growth medium containing 50 μg/mL kanamycin, and the number of resistant and sensitive plants was scored. For analysis of reciprocal crosses, we crossed the heterozygous mutant lines carrying a resistant marker gene, with wild-type plants using the insertional lines as egg donor and wild-type Ler as the sperm donor and vice versa. Quantification of resistant plants in the progeny was performed by collecting the seeds derived from these crosses and plating them in selective media according to the selection marker carried by each of the insertional lines tested.
Molecular Analysis of the Sequences Flanking the Ds Insertion
The sequences flanking the Ds element were isolated with thermal asymmetric interlaced PCR as described by Liu et al. (1995) and sequenced with an Applied Biosystems sequencer. The flanking sequences obtained were run against BLASTN to identify the genomic location of the Ds element. The junctions were then confirmed by PCR using sequence-specific primers for the putative insertion site in combination with the corresponding Ds element primers. To verify the left border–genomic sequence junction, we used the DS-specific primer Ds5′-1A (5′-ACGGTCGGGAAACTAGCTCTAC-3′) combined with the genomic sequence primer EDA12-5 (5′-CCATCCTATGTATTTAGAGTTCCTGC-3′). For the right border–genomic sequence junction, we used the DS-specific primer Ds3′-2A (5′-CGATTACCGTATTTATCCCGTTTC-3′) combined with the genomic sequence primer EDA12-3 (5′-TGATACGATGGTTAGATCACG-3′). Both junctions were corroborated by sequencing.
Molecular Characterization of Insertional Lines around the Ds Insertion
eostre-2 (SALK_033866) and eostre-3 (SAIL_582_F02) insertional lines were obtained from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003). For eostre-2, the left border–genomic sequence junction was determined by PCR using the T-DNA–specific primer LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′) combined with the genomic sequence-specific primer eostre1RP (5′-CAGTGGATATGGGAATGCAAC-3′). For eostre-3, the left border–genomic sequence junction was determined also by PCR in plants showing BASTA resistance using the T-DNA–specific primer 5′-GCCTTTTCAGAAATGGATAAATAGCCTTGCTTCC-3′) combined with the genomic sequence-specific primer eostre2 RP (5′-AATCCGATCGGTATTACGAGG-3′). Lines 35950-1 (SALK_086362) and 35950-2 (SALK_086364) were obtained from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003). For both lines, the left border–genomic sequence junction was determined by PCR using the T-DNA–specific primer LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′) combined with genomic sequence-specific primers. For both lines, the specific genomic primers were 35950-1/2 RP (5′-TTGTACCGAGAAAGGCTCAAG-3′) and 35950-/21 LP (5′-TGGGAATTTTGGATTTAGCCC-3′). Line blh1-1 (SALK_089095) was obtained from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003), while blh1-2 (GK-114D09) and blh1-3 (GK-475C05) were obtained from the Nottingham Arabidopsis Stock Centre (Scholl et al., 2000). For blh1-1, the left border–genomic sequence junction was determined by PCR using the T-DNA–specific primer LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′) combined with genomic sequence-specific primers blh1-1RP (5′-TTCCAGCCGCTTAAGCATAC-3′) and blh1-1LP (5′-TATGAATCCCAATCACAACGG -3′). For blh1-2, the left border–genomic sequence junction was determined by PCR using the T-DNA–specific primer LBGK (5′-TGGTTCACGTAGTGGGCCATCG-3′) combined with genomic sequence-specific primers blh1-2RP (5′-CCGTCGGATCCGGCAGAGATCT-3′) and blh1-2LP (5′-GATCTTTGAGTCTGACACAGAGACC -3′). For blh1-3, the left border–genomic sequence junction was determined by PCR using the T-DNA–specific primer LBGK (5′-TGGTTCACGTAGTGGGCCATCG-3′) combined with genomic sequence-specific primers blh1-3RP (5′-CCAGCCGCTTAAGCATACATGT-3′) and blh1-3LP (5′-GGCGTCACTGGAATGCAAGGAA-3′).
Molecular Characterization of Insertional Lines for KNOX and OVATE Genes
Insertional lines for KNAT3 (SALK_136464), KNAT5 (SALK_088589), and KNAT6 (SALK_140566) genes were obtained from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003). For the three lines, the left border–genomic sequence junction was determined by PCR using the T-DNA–specific primer LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′) combined with genomic sequence-specific primers. For knat3, the DNA-specific primers used were knat3LP (5′-GTTAAACACAGCGCTTCTTCG-3′) and knat3RP (5′-TCACAGGATTCATTTTCTCACC-3′). For knat5, the DNA-specific primers used were knat5LP (5′-ATTCGGGGTTTTGATTACCAG-3′) and knat5RP (5′-GAACAGCAACTCTTCCACGTC-3′). For knat6, the DNA-specific primers used were knat6LP (5′-GTTTTAGCCATGGGATTAGGG-3′) and knat6RP (5′-TGATTTGTTATGGCCACAATG-3′). T-DNA insertional lines for At OFP1 (SALK_111492 and SALK_127550), At OFP2 (SALK_122550), At OFP4 (SALK_014905), and At OFP5 (SALK_010386) were obtained from the Salk Institute Genomic Analysis Laboratory collection (Alonso et al., 2003). For all the lines, the left border–genomic sequence junction was determined by PCR using the T-DNA–specific primer LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′) combined with genomic sequence-specific primers. For At OFP1-1, the DNA-specific primers used were At OFP1-1LP (5′-AGAGATCCCAGATCTCGAAGC-3′) and At OFP1-1RP (5′-AAGGTTGCGGTTTTGGATAAC-3′). For At OFP1-2, the DNA-specific primers used were At OFP1-2LP (5′-ATTGGGCCGAAAACATATAGG-3′) and At OFP1-2RP (5′-AAGGTTGCGGTTTTGGATAAC-3′). For At OFP2-1, the DNA-specific primers used were At OFP2-1LP (5′-ACCAAATTCAAAGAAGCATCG-3′) and At OFP2-1RP (5′-TGGTGAGTTATGGTGAGGAGG-3′). For At OFP4-1, the DNA-specific primers used were At OFP4-1LP (5′-TCGTTAGGGTTGTGACTGACC-3′) and At OFP4-1RP (5′-CCTAGATTCAAAGGATGTGCG-3′). For At OFP5-1, the DNA-specific primers used were At OFP5-1LP (5′-GCTTCTATATCCCCATTTTAATGTG-3′) and At OFP4-1RP (5′-CCTAGATTCAAAGGATGTGCG-3′).
Molecular Characterization of a Gene Trap Line with an Insertion in BLH1
The gene trap line GT9784, carrying an insertion in the BLH1 gene, was obtained from the Cold Spring Harbor collection (http://genetrap.cshl.org/). The junctions of the Ds element with the genomic DNA were confirmed by PCR using sequence-specific primers for the insertion site in combination with Ds element primers. The DS-specific primer Ds5′-1A (5′-ACGGTCGGGAAACTAGCTCTAC-3′) was used combined with the genomic sequence primer GT9784RP (5′-TCATCAATCTTGTAAGTTGTCA-3′), and the DS-specific primer Ds3′-2A (5′-CGATTACCGTATTTATCCCGTTTC-3′) was used combined with the genomic sequence primer GT9784LP (5′-GGTGGCGTACTAAGGACTGTGGGA-3′).
Expression Analysis
Total RNA was isolated from dissected ovules using TRIzol (Invitrogen). cDNA was generated by SuperScript II reverse transcriptase (Invitrogen). This was used for RT-PCR analysis using 0.5 μL of cDNA template, and intron-spanning primer pairs were used for 30 cycles at 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. RT-PCR results were normalized using Arabidopsis thaliana actin-2 controls for PCR. BLH1 cDNA was amplified using the following primers: 5′-CCGGGTCGGGTCTGGCTCTA-3′ and 5′-CGGCTCGTTCTGGGAGACCACG-3′. ACTIN2 was amplified using 5′-CCTGAAAGGAAGTACAGTG-3′ and 5′-CTGTGAACGATTCCTGGAC-3′.
Constructs and Plant Transformation
Total RNA was isolated from dissected Ler ovules using TRIzol (Invitrogen). cDNAs of BLH1 were generated by SuperScript II reverse transcriptase (Invitrogen). After sequence verification, 10op∷BLH1 was constructed by inserting BLH1 cDNA behind an OP array (10OP-TATA-BJ36) and subsequently subcloned into the binary vector pCAMBIA 1300. The plasmid was introduced into Agrobacterium tumefaciens strain GV3101 by electroporation, and Ler wild-type plants were transformed using the floral dip method (Clough and Bent, 1998). Transformants were selected based on their ability to survive in MS medium with 20 mg L−1 hygromicyn. Resistant (green seedlings with true leaves) were then transferred onto soil and grown under the conditions described above. At5g40260 (pES1), At1g26795 (pES2), and promoter fragments were previously cloned and its activity characterized as described (Yu et al., 2005). pES:Lhg4 constructs were prepared by inserting the promoter fragments upstream the coding sequence of the quimeric transcription factor LhG4 and then subcloned into the binary vector pMLBART. The plasmid was introduced into A. tumefaciens strain GV3101 by electroporation, and wild-type Ler plants containing the transgene 6OP:GUS were transformed using the floral dip method (Clough and Bent, 1998). Transformants were selected based on their ability to survive in MS medium containing 50 mg L−1 kanamycin and 6 mg L−l of ammonium glufosinate.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: At2g35940 (BLH1), At5g40260 (pES1), At1g26795 (pES2), AT4G18830 (ATOFP5), and AT5G25220 (KNAT3).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. DIC Image Showing a Collapsed Embryo Sac.
Supplemental Figure 2. Misexpression of BLH1 Expression in pES2≫BLH1 Ovules Recapitulates the eostre Phenotype.
Supplemental Table 1. Pollen Tube Growth Patterns toward eostre and Wild-Type Female Gametophytes.
Supplemental Table 2. Patterns of GUS Expression Observed when Both Wild-Type Plants and eostre-1 Mutants Were Pollinated Using Transgenic Plants Carrying the PFAC1IE:GFP-GUS:TFAC1 Construct as Pollen Donors.
Supplemental Table 3. Embryo and Endosperm Development in Plants Fertilized with cdc2a Pollen.
Supplemental Table 4. Analysis of Wild-Type Pistils at Different Stages of Development.
Supplemental Table 5. Phenotype of Embryo Sacs in eostre-2 and eostre-3 Pistils.
Supplementary Material
Acknowledgments
We thank John Bowman for providing 10OP-TATA-BJ36 and LhG4-BJ36 vectors and seeds of 6OP-GUS transgenic plants, Joe Simorowski for providing seeds of the BLH1 gene trap line, and members of the Gasser and Sundaresan labs for helpful discussions. This work was funded by National Science Foundation 2010 Grant 0313501.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Venkatesan Sundaresan (sundar@ucdavis.edu).
Online version contains Web-only data.
References
- Adai, A., Johnson, C., Mlotshwa, S., Archer-Evans, S., Manocha, V., Vance, V., and Sundaresan, V. (2005). Computational prediction of miRNAs in Arabidopsis thaliana. Genome Res. 15 78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, R. (2000). A model of retinal cell differentiation in the chick embryo. Prog. Retin. Eye Res. 19 529–557. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide Insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Bellaoui, M., Pidkowich, M.S., Samach, A., Kushalappa, K., Kohalmi, S.E., Modrusan, Z., Crosby, W.L., and Haughn, G.W. (2001). The Arabidopsis BELL1 and KNOX TALE homeodomain proteins interact through a domain conserved between plants and animals. Plant Cell 13 2455–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger, J., and Knoblich, J.A. (2004). Dare to be different: Asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14 R674–R685. [DOI] [PubMed] [Google Scholar]
- Brown, R.C., and Lemmon, B.C. (1991). Pollen development in orchids 2: The cytokinetic apparatus in simultaneous cytokinesis. Protoplasma 165 155–166. [Google Scholar]
- Brown, R.C., and Lemmon, B.C. (1992). Cytoplasmic domain: A model for spatial control of cytokinesis in reproductive cells of plants. EMSA Bull. 22 48–53. [Google Scholar]
- Brukhin, V., Curtis, M.D., and Grossniklaus, U. (2005). The angiosperm female gametophyte: No longer the forgotten generation. Curr. Sci. 89 1844–1852. [Google Scholar]
- Chan, R.L., Gago, G.M., Palena, C.M., and Gonzalez, D.H. (1998). Homeoboxes in plant development. Biochim. Biophys. Acta. 1442 1–19. [DOI] [PubMed] [Google Scholar]
- Christensen, C.A., Gorsich, S.W., Brown, R.H., Jones, L.G., Brown, J., Shaw, J.M., and Drews, G.N. (2002). Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. Plant Cell 14 2215–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, C.A., King, E.J., Jordan, J.R., and Drews, G.N. (1997). Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex. Plant Reprod. 10 49–64. [Google Scholar]
- Christensen, C.A., Subramanian, S., and Drews, G.N. (1998). Identification of gametophytic mutations affecting female gametophyte development in Arabidopsis. Dev. Biol. 202 136–151. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Cole, M., Nolte, C., and Werr, W. (2006). Nuclear import of the transcription factor SHOOT MERISTEMLESS depends on heterodimerization with BLH proteins expressed in discrete sub-domains of the shoot apical meristem of Arabidopsis thaliana. Nucleic Acids Res. 34 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, M.M.S. (2007). The indeterminate gametophyte1 gene of maize encodes a LOB domain protein required for embryo sac and leaf development. Plant Cell 19 46–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Hardt, R., Kagi, C., Baumann, N., Moore, J.M., Baskar, R., Gagliano, W.B., Jurgens, G., and Grossniklaus, U. (2007). LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biol. 5 e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, F.L., Huang, B.Q., Han, Y.Z., and Zee, S.Y. (2004). Fertilization in maize indeterminate gametophyte1 mutant. Protoplasma 223 111–120. [DOI] [PubMed] [Google Scholar]
- Hackbusch, J., Richter, K., Muller, J., Salamini, F., and Uhrig, J.F. (2005). A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proc. Natl. Acad. Sci. USA 102 4908–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, T.J.C., and Peifer, M. (2005). The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 170 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, B.Q., and Russell, S.D. (1992). Female germ unit - Organization, isolation, and function. Int. Rev. Cytol. 140 233–293. [Google Scholar]
- Knoepfler, P.S., Calvo, K.R., Chen, H.M., Antonarakis, S.E., and Kamps, M.P. (1997). Meis1 and pKnox1 bind DNA cooperatively with Pbx1 utilizing an interaction surface disrupted in oncoprotein E2a-Pbx1. Proc. Natl. Acad. Sci. USA 94 14553–14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C.M., and Meinke, D.W. (1998). The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant J. 16 21–31. [DOI] [PubMed] [Google Scholar]
- Liu, Y.G., Mitsukawa, N., Oosumi, T., and Whittier, R.F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8 457–463. [DOI] [PubMed] [Google Scholar]
- Moore, I., Galweiler, L., Grosskopf, D., Schell, J., and Palme, K. (1998). A transcription activation system for regulated gene expression in transgenic plants. Proc. Natl. Acad. Sci. USA 95 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J.M., Calzada, J.P.V., Gagliano, W., and Grossniklaus, U. (1997). Genetic characterization of hadad, a mutant disrupting female gametogenesis in Arabidopsis thaliana. Cold Spring Harb. Symp. Quant. Biol. 62 35–47. [PubMed] [Google Scholar]
- Muller, J., Wang, Y.M., Franzen, R., Santi, L., Salamini, F., and Rohde, W. (2001). In vitro interactions between barley TALE homeodomain proteins suggest a role for protein-protein associations in the regulation of Knox gene function. Plant J. 27 13–23. [DOI] [PubMed] [Google Scholar]
- Nowack, M.K., Grini, P.E., Jakoby, M.J., Lafos, M., Koncz, C., and Schnittger, A. (2006). A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat. Genet. 38 63–67. [DOI] [PubMed] [Google Scholar]
- Pagnussat, G.C., Yu, H.J., Ngo, Q.A., Rajani, S., Mayalagu, S., Johnson, C.S., Capron, A., Xie, L.F., Ye, D., and Sundaresan, V. (2005). Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132 603–614. [DOI] [PubMed] [Google Scholar]
- Ray, S., Park, S.S., and Ray, A. (1997). Pollen tube guidance by the female gametophyte. Development 124 2489–2498. [DOI] [PubMed] [Google Scholar]
- Reiser, L., Sanchez-Baracaldo, P., and Hake, S. (2000). Knots in the family tree: Evolutionary relationships and functions of knox homeobox genes. Plant Mol. Biol. 42 151–166. [PubMed] [Google Scholar]
- Rieckhof, G.E., Casares, F., Ryoo, H.D., AbuShaar, M., and Mann, R.S. (1997). Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91 171–183. [DOI] [PubMed] [Google Scholar]
- Roeder, A.H.K., Ferrandiz, C., and Yanofsky, M.F. (2003). The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis fruit. Curr. Biol. 13 1630–1635. [DOI] [PubMed] [Google Scholar]
- Russell, S.D. (1993). The egg cell - Development and role in fertilization and early embryogenesis. Plant Cell 5 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl, R.L., May, S.T., and Ware, D.H. (2000). Seed and molecular resources for Arabidopsis. Plant Physiol. 124 1477–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiarti, E., Ueno, Y., Tsukaya, H., Iwakawa, H., Machida, C., and Machida, Y. (2001). The asymmetric leaves2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 128 1771–1783. [DOI] [PubMed] [Google Scholar]
- Serikawa, K.A., Martinez-Laborda, A., Kim, H.S., and Zambryski, P.C. (1997). Localization of expression of KNAT3, a class 2 knotted1-like gene. Plant J. 11 853–861. [DOI] [PubMed] [Google Scholar]
- Shimizu, K.K., and Okada, K. (2000). Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development 127 4511–4518. [DOI] [PubMed] [Google Scholar]
- Smith, H.M.S., Campbell, B.C., and Hake, S. (2004). Competence to respond to floral inductive signals requires the homeobox genes PENNYWISE and POUND-FOOLISH. Curr. Biol. 14 812–817. [DOI] [PubMed] [Google Scholar]
- Sundaresan, V., Springer, P., Volpe, T., Haward, S., Jones, J.D.G., Dean, C., Ma, H., and Martienssen, R. (1995). Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 9 1797–1810. [DOI] [PubMed] [Google Scholar]
- Truernit, E., Siemering, K.R., Hodge, S., Grbic, V., and Haseloff, J. (2006). A map of KNAT gene expression in the Arabidopsis root. Plant Mol. Biol. 60 1–20. [DOI] [PubMed] [Google Scholar]
- Webb, M.C., and Gunning, B.E.S. (1990). Embryo sac development in Arabidopsis thaliana. 1. Megasporogenesis, including the microtubular cytoskeleton. Sex. Plant Reprod. 3 244–256. [Google Scholar]
- Webb, M.C., and Gunning, B.E.S. (1994). Embryo sac development in Arabidopsis thaliana. 2. The cytoskeleton during megagametogenesis. Sex. Plant Reprod. 7 153–163. [Google Scholar]
- Willemse, M.T.M., and van Went, J.L. (1984). The Female Gametophyte. (Berlin: Springer-Verlag).
- Xu, J., Zhang, H.Y., Xie, C.H., Xue, H.W., Dijkhuis, P., and Liu, C.M. (2005). EMBRYONIC FACTOR 1 encodes an AMP deaminase and is essential for the zygote to embryo transition in Arabidopsis. Plant J. 42 743–756. [DOI] [PubMed] [Google Scholar]
- Yamada, K., et al. (2003). Empirical analysis of transcriptional activity in the Arabidopsis genome. Science 302 842–846. [DOI] [PubMed] [Google Scholar]
- Yang, W.C., Ye, D., Xu, J., and Sundaresan, V. (1999). The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 13 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H.J., Hogan, P., and Sundaresan, V. (2005). Analysis of the female gametophyte transcriptome of Arabidopsis by comparative expression profiling. Plant Physiol. 139 1853–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.