Abstract
Plants have circadian oscillations in the concentration of cytosolic free calcium ([Ca2+]cyt). To dissect the circadian Ca2+-signaling network, we monitored circadian [Ca2+]cyt oscillations under various light/dark conditions (including different spectra) in Arabidopsis thaliana wild type and photoreceptor and circadian clock mutants. Both red and blue light regulate circadian oscillations of [Ca2+]cyt. Red light signaling is mediated by PHYTOCHROME B (PHYB). Blue light signaling occurs through the redundant action of CRYPTOCHROME1 (CRY1) and CRY2. Blue light also increases the basal level of [Ca2+]cyt, and this response requires PHYB, CRY1, and CRY2. Light input into the oscillator controlling [Ca2+]cyt rhythms is gated by EARLY FLOWERING3. Signals generated in the dark also regulate the circadian behavior of [Ca2+]cyt. Oscillations of [Ca2+]cyt and CHLOROPHYLL A/B BINDING PROTEIN2 (CAB2) promoter activity are dependent on the rhythmic expression of LATE ELONGATED HYPOCOTYL and CIRCADIAN CLOCK-ASSOCIATED1, but [Ca2+]cyt and CAB2 promoter activity are uncoupled in the timing of cab1 (toc1-1) mutant but not in toc1-2. We suggest that the circadian oscillations of [Ca2+]cyt and CAB2 promoter activity are regulated by distinct oscillators with similar components that are used in a different manner and that these oscillators may be located in different cell types in Arabidopsis.
INTRODUCTION
In plants, cytosolic free Ca2+ ([Ca2+]cyt) can oscillate in response to extracellular stimuli and circadian signals. Stimulus-induced oscillations of [Ca2+]cyt with periods of minutes occur in root hairs in response to NOD factors (Ehrhardt et al., 1996; Sun et al., 2006) and in guard cells in response to external Ca2+, abscisic acid, cold, fungal elicitors, and CO2 (McAinsh et al., 1995; Staxén et al., 1999; Klüsener et al., 2002; Young et al., 2006). Cytosolic and chloroplastic [Ca2+] also have oscillations with a period of ∼24 h (Johnson et al., 1995). Circadian oscillations of [Ca2+]cyt persist in constant light and light/dark cycles, are phase-shifted by light/dark signals, and have different patterns in amplitude, phase, and waveform according to photoperiod and light intensity (Johnson et al., 1995; Love et al., 2004). Oscillations in [Ca2+]cyt are thought to be important in encoding information in Ca2+ signaling networks, and it has been suggested that circadian oscillations of [Ca2+]cyt might encode temporal information to regulate cellular physiology (Johnson et al., 1995; Webb, 2003; Dodd et al., 2005a; Gardner et al., 2005) and/or contribute to the control of the photoperiodic regulation of flowering (Johnson et al., 1995; Love et al., 2004).
The function of circadian oscillations of [Ca2+]cyt is not known, but this is a common feature of many frequently measured circadian outputs in plants, such as CHLOROPHYLL A/B BINDING PROTEIN2 (CAB2) promoter activity, leaf movement, and hypocotyl elongation. It is also often a matter of speculation how these circadian outputs contribute to the competitive advantage conferred by a correctly functioning circadian clock (Dodd et al., 2005b; Hotta et al., 2007). In order to understand further the role of circadian oscillations of [Ca2+]cyt, we wished to establish the genetic basis for the control of these oscillations and their relationship with both the core components of the plant circadian oscillator and photoreceptor pathways. These experiments were designed to determine whether Ca2+ functions as an input or output of the circadian system. In addition, we wished to determine whether the same genetic oscillator regulates [Ca2+]cyt and CAB2 promoter activity, because there are differences in the circadian regulation of these outputs; most notably, CAB2:luciferase (CAB2:luc) rhythms can persist in the absence of [Ca2+]cyt rhythms in undifferentiated tobacco (Nicotiana tabacum) calli (Sai and Johnson, 1999).
In Arabidopsis thaliana, a feedback loop composed of CIRCADIAN CLOCK-ASSOCIATED1 (CCA1), LATE ELONGATED HYPOCOTYL (LHY), and TIMING OF CAB1 (TOC1) is thought to underlie most circadian behavior (Alabadí et al., 2001; Gardner et al., 2006; for review, see McClung, 2006). CCA1 and LHY are related MYB-like transcription factors whose protein and mRNA abundance have a circadian rhythm that peaks around dawn. Overexpression of either CCA1 or LHY disrupts circadian clock function, including leaf movement and transcription of CAB2, CATALASE3, and COLD AND CIRCADIAN REGULATED2 (CCR2), and delays flowering (Schaffer et al., 1998; Wang and Tobin, 1998). The expression of TOC1, which encodes a pseudo-response regulator (PRR), peaks around dusk and indirectly promotes CCA1/LHY expression at dawn (Alabadí et al., 2001). toc1-1 shortens the period of circadian rhythms, while overexpression of TOC1 causes arrhythmia (Millar et al., 1995; Más et al., 2003a). CCA1 and LHY downregulate their own expression by repressing that of TOC1 (Alabadí et al., 2001). Therefore, CCA1, LHY, and TOC1 could constitute a negative-feedback loop that can act as a circadian timing mechanism. Degradation of TOC1, an essential requirement for rhythmicity, is thought to be mediated by ZEITLUPE (ZTL) (Somers et al., 2000; Más et al., 2003b; Kevei et al., 2006). In agreement with this hypothesis, ztl-1 lengthens circadian period in a light-dependent manner (Más et al., 2003b). The circadian oscillator is entrained by red and blue light via the PHYTOCHROMES (PHY) and CRYPTOCHROMES (CRY) (Millar, 2004; Salomé and McClung, 2005). Light input to the clock is gated by EARLY FLOWERING3 (ELF3) to ensure correct entrainment of the clock to dawn (Hicks et al., 1996; McWatters et al., 2000).
Genetic and mathematical studies have elaborated the CCA1/LHY/TOC1 model, identifying new components and suggesting a system of interlocking loops. One of the interconnecting loops may involve ELF4. ELF4 is necessary for light-induced expression of both CCA1 and LHY, and ELF4 is expressed with a similar phase as TOC1, suggesting that ELF4, together with CCA1/LHY, might form yet another negative-feedback loop (Kikis et al., 2005). On the basis of mathematical modeling, a hypothetical light-induced factor Y has been proposed to form a loop with TOC1 (Locke et al., 2005a, 2005b). Y is thought to both activate TOC1 expression and be required for TOC1-induced expression of LHY. Y is subsequently repressed by TOC1 and CCA1/LHY. GIGANTEA is thought to fulfill at least some of the roles of Y (Locke et al., 2005a, 2005b; Gould et al., 2006). Recently, two different groups independently suggested that there is a further negative-feedback loop between CCA1/LHY and PRR7/PRR9 (Locke et al., 2006; Zeilinger et al., 2006). Finally, a MYB transcription factor, LUX ARRHYTHMO, can also be part of the central oscillator (Hazen et al., 2005; Onai and Ishiura, 2005). Thus, the Arabidopsis circadian system comprises more than one transcriptional feedback loop to regulate different clock output pathways and provide robustness.
In this study, we analyzed the pattern of circadian oscillations of [Ca2+]cyt in wild-type and mutant lines for photoreceptor and clock genes of Arabidopsis carrying the transgenic bioluminescent reporter of Ca2+, aequorin (Knight et al., 1991). Our data indicate that Ca2+ is an output of the circadian clock, which is regulated by similar clock genes and photoreceptors to other outputs. Differences in the regulation of [Ca2+]cyt by light, dark, and clock gene mutations compared with other circadian outputs in Arabidopsis indicate that multiple circadian oscillators with common components are present.
RESULTS
Light-Dependent Circadian [Ca2+]cyt Oscillations in Arabidopsis Seedlings
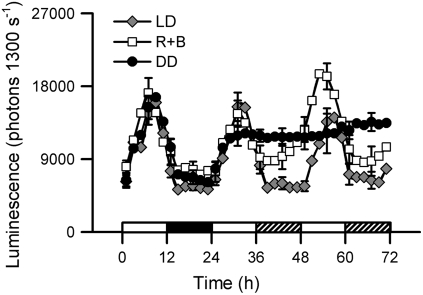
[Ca2+]cyt oscillated with a period close to 24 h (23.6 to 24.6 h, depending on ecotype) in 12-h light/12-h dark (LD) cycles or constant red/blue light (R+B; 100 μmol·m−2·s−1, equal amounts of red and blue light) (Figure 1). Arabidopsis of different ecotypes had slightly different circadian periods and phases of the [Ca2+]cyt oscillation (see Supplemental Figure 1 and Supplemental Table 1 online). As aequorin levels were different in each ecotype, oscillations were observed in different luminescence levels that may not reflect differences in [Ca2+]cyt levels (see Supplemental Figure 1 online). The period of the C24 ecotype was significantly longer than those of the other ecotypes in a one-way analysis of variance test followed by a Newman-Keuls multiple comparison test (24.6 ± 0.8 h [nonweighted mean ± sd; n = 35]; P < 0.001). Ecotype Wassilewskija (Ws) had the shortest period (23.6 ± 0.8 h; n = 62), but it was not significantly different from those of ecotypes Columbia-0 (Col-0; 23.8 ± 1.0 h; n = 66) and Landsberg erecta (Ler; 24.1 ± 1.0 h; n = 24) (see Supplemental Figure 1 and Supplemental Table 1 online). The peak of [Ca2+]cyt was 7 h after true dawn or subjective dawn, which was approximately the middle of the subjective day (Figure 1). The trough of the oscillation was 3 h after true dusk in LD and 5 h after subjective dusk in R+B. Transfer from LD to R+B caused sustained oscillations of [Ca2+]cyt upon an elevated baseline. The troughs of the [Ca2+]cyt oscillations in subjective night in continuous light (LL) were higher than the troughs in the night in LD (Figure 1).
Figure 1.
Light-Dependent Circadian [Ca2+]cyt Oscillations in Arabidopsis Seedlings.
Seedlings were entrained in LD (60 μmol·m−2·s−1) for 10 d before release to constant R+B (100 μmol·m−2·s−1) and measured using photon-counting imaging. Aequorin luminescence rhythms from transgenic seedlings were recorded under LD (gray diamonds), R+B (white squares), and DD (black circles). The final LD cycle is shown. se bars are shown every 6 h (n = 4). Values are means of one representative experiment of at least four independent replicates with the same result. The white and black bars before 24 h indicate the light and dark periods of LD. Subsequently, white bars indicate subjective day and hatched bars indicate subjective night.
A different pattern of responses occurred following transfer from LD to continuous dark (DD). Unlike tobacco seedlings (Johnson et al., 1995), circadian oscillations of [Ca2+]cyt in Arabidopsis damp very rapidly in DD (Figure 1). During the first ∼16 h in DD, [Ca2+]cyt followed the pattern predicted from LD and LL: [Ca2+]cyt was at the trough in the night and then at subjective dawn began to increase in the same manner as in R+B and LD (Figure 1). This demonstrates that an increase in [Ca2+]cyt was not light-dependent in Arabidopsis. However, if DD was maintained for >16 h, [Ca2+]cyt ceased to rise and remained at a level that was intermediate between the trough and peak values observed in LD and R+B (Figure 1). Thus, there is circadian control of [Ca2+]cyt and extended light elevates the baseline of [Ca2+]cyt, but light is not required for an increase in [Ca2+]cyt and the machinery that returns [Ca2+]cyt to resting values does not operate in extended dark.
PHYB Regulates Circadian Oscillations of [Ca2+]cyt
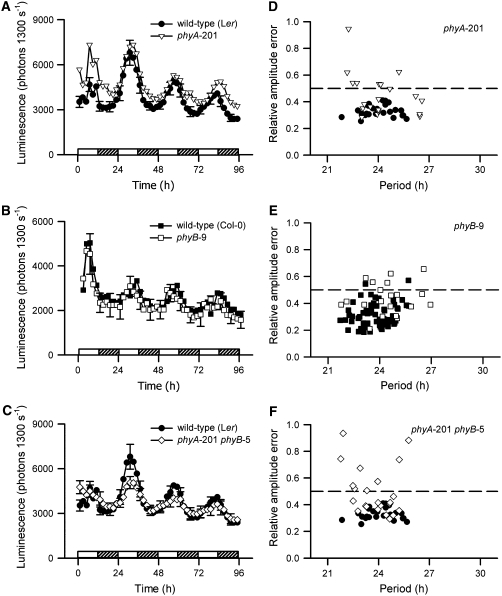
We wished to identify the relative contribution of the red and blue light signaling pathways to the generation and maintenance of the [Ca2+]cyt oscillations, because circadian oscillations of [Ca2+]cyt are light-dependent. In seedlings, red light is perceived mainly by PHY, especially PHYA and PHYB. To test the role of red light in the control of circadian [Ca2+]cyt rhythms, we used several red light photoreceptor mutants: phyA-201 (Nagatani et al., 1993), phyB-1, phyB-9 (Reed et al., 1993), phyA-201 phyB-5 double mutant (Reed et al., 1994), and PHYB-overexpressor (PHYB-ox) (Wagner et al., 1991). We have measured at least two independent transformants for each new line transformed with aequorin (35S:AEQ). The line that contained the closest levels of AEQ to the respective wild type was used as representative in each figure, unless significant differences between lines were observed. Light effects are shown for high fluence of R+B (100 μmol·m−2·s−1) (Figure 2) and intermediate fluence of red light (40 μmol·m−2·s−1) (Figure 3) conditions. First, we observed that both phyA-201 and phyB-9 mutants had wild-type periods of [Ca2+]cyt oscillations under R+B (24.1 ± 1.4 h [n = 20] and 24.4 ± 1.4 [n = 20], respectively) (Figures 2A and 2B), albeit with higher relative amplitude error (RAE) values (Figures 2D and 2E) (0.45 ± 0.16 [n = 20] and 0.44 ± 0.12 [n = 20], respectively) than the Ler or Col-0 wild types (0.33 ± 0.04 [n = 24] and 0.31 ± 0.08 [n = 66], respectively). The RAE is a measure of how well the data fit a sine curve generated by a fast Fourier transform. A RAE close to 0 indicates a good fit, and a RAE approaching 1.0 is a poor fit, indicating no rhythmic activity. We selected RAE = 0.5 as a cutoff between rhythms that were as robust as the wild type and rhythms with poor robustness. This value is approximately equal to the mean RAE of [Ca2+]cyt rhythms in wild-type background plants plus twice the sd. Thus, the higher RAE of the phy mutants suggests that the robustness of circadian [Ca2+]cyt oscillations was reduced by mutation of PHY genes (Figures 2D to 2F). The phyA-201 phyB-5 double mutant had both lower amplitude and less robust [Ca2+]cyt oscillations in high-fluence R+B (RAE = 0.51 ± 0.19; n = 24), but the period of the oscillations was similar to that in the Ler wild type (23.6 ± 1.4 h; n = 24) (Figures 2C and 2F).
Figure 2.
Phytochromes Are Required for Robust Circadian [Ca2+]cyt Oscillations.
(A) to (C) Aequorin luminescence rhythms from phyA-201, phyB-9, and phyA-201 phyB-5 mutants were measured under R+B (100 μmol·m−2·s−1) using photon-counting imaging. Mean traces, representative of three independent experiments and at least two independent transformants, show aequorin luminescence in phyA-201 (A), phyB-9 (B), and phyA-201 phyB-5 double mutant (C) lines with Ler or Col-0 wild-type control. se bars are shown every 6 h (n = 4). Seedlings were entrained under L/D (60 μmol·m−2·s−1) for 10 d before transfer to R+B. Hatched bars at bottom represent subjective night.
(D) to (F) Plots showing the FFT-NLLS analysis of luminescence rhythms. Each value indicates the analysis of one trace for Ler wild type (closed circles; n = 20), Col-0 wild type (closed squares; n = 44), phyA-201 mutant (open inverted triangles; n = 20), phyB-9 mutant (open squares; n = 20), and phyA-201 phyB-5 double mutant (open diamonds; n = 24). The dotted lines show RAE = 0.5.
Figure 3.
Red Light Modulation of Circadian [Ca2+]cyt Oscillations.
[Ca2+]cyt oscillations were recorded in the Ler wild-type background ([A]; n = 22), phyB-1 ([B]; n = 26), phyA-201 ([D]; n = 24), and the phyA-201 phyB-5 double mutant ([E]; n = 28) using photon-counting luminescence. Mean traces (n = 4) are representative of three independent experiments and at least two independent transformants. Seedlings were entrained under LD for 7 d before transfer to RR (gray bars; 40 μmol·m−2·s−1) for 3 d followed by 3 d in LL (white bars; 40 μmol·m−2·s−1). In (C), PHYB-ox lines (n = 28) containing 35S:AEQ were entrained under LD for 7 d before release to LL for 2.5 d followed by ∼2.5 d of RR (40 μmol·m−2·s−1 for all light conditions). Each value indicates the analysis of the mean trace for Ler wild type (closed circles), No-0 wild type (closed triangles), phyA-201 mutant (open inverted triangles), phyB-1 mutant (open squares), phyA-201 phyB-5 double mutant (open diamonds), and PHYB-ox (open circles).
Under intermediate fluence rates of constant red light (RR; 40 μmol·m−2·s−1), Ler wild-type plants had robust circadian [Ca2+]cyt rhythms (Figure 3A). The observed increase in luminescence levels is probably due to plant growth, but a small sustained [Ca2+]cyt increase cannot be excluded. If there is a sustained [Ca2+]cyt increase in the Ler wild type in RR, the magnitude is much smaller than that observed in DD (Figure 1) or that observed in the transition from RR to LL (Figure 3A). In the same conditions, the phyA-201 mutant had no effect on the period or amplitude of the [Ca2+]cyt rhythm (Ler wild type, 24.0 ± 0.2 h [n = 22]; phyA-201, 24.0 ± 0.1 h [n = 24]), while phyB-1 had very low amplitude rhythms in RR (Figures 3A, 3B, and 3D). No [Ca2+]cyt oscillation was detected in RR in the phyA-201 phyB-5 double mutant (Figure 3E). The very low amplitude in RR in phyB-1 and phyA-201 phyB-5 but not phyA-201 indicates that red light controls the [Ca2+]cyt oscillation mainly through PHYB, rather than through PHYA. PHYB-ox had no effect on the period of [Ca2+]cyt rhythms in LL (Nossen [No-0] wild type, 23.9 ± 0.2 h [n = 31]; PHYB-ox, 23.8 ± 0.3 h [n = 28]) or RR (No-0 wild type, 23.9 ± 0.11 h [n = 28]; PHYB-ox, 23.8 ± 0.2 h [n = 25]). However, PHYB-ox increased the amplitude of circadian [Ca2+]cyt rhythms in RR compared with the No-0 wild type (Figure 3C). These data suggest that PHYB is involved in the red light regulation of the amplitude of [Ca2+]cyt oscillation, but not the period or phase. Plants with phyB mutations in RR had an apparently unregulated increase in [Ca2+]cyt (Figures 3B and 3E). This is analogous to the situation in DD, in which [Ca2+]cyt is elevated from resting. Together, these data suggest that [Ca2+]cyt becomes unregulated in the prolonged absence of a light signal.
Redundant Roles for CRY1 and CRY2 in Blue Light Modulation of [Ca2+]cyt Oscillations
CRY1 and CRY2, the principal photoreceptors for blue light entrainment of the oscillator, appear to act to control circadian oscillations of [Ca2+]cyt (Figures 4 and 5). The [Ca2+]cyt oscillation was examined in cry1 (hy4-223N) (Ahmad and Cashmore, 1993; Baum et al., 1999), cry2-1 (Guo et al., 1998), and cry1 cry2 double mutant (hy4-1 fha-1) (El-Assal et al., 2003) seedlings in continuous blue (BB; 40 μmol·m−2·s−1) and R+B (40 μmol·m−2·s−1) light. In both cry1 single mutant and cry1 cry2 double mutant, [Ca2+]cyt oscillated with a lower amplitude that quickly damped in R+B, resulting in high RAE values (cry1, 0.57 ± 0.10 [n = 11]; cry1 cry2, 0.61 ± 0.20 [n = 24]) compared with the Ler wild type (0.33 ± 0.04; n = 24) or the Col-0 wild type (0.31 ± 0.08; n = 66) (Figure 4). There was no effect of the cry mutations on the period of the circadian oscillations of [Ca2+]cyt in R+B (cry1, 24.6 ± 2.5 h [n = 11]; cry2-1, 24.1 ± 0.7 h [n = 27]; cry1 cry2, 23.7 ± 1.4 h [n = 24]) compared with the Ler wild type (24.1 ± 1.0 h; n = 24) or the Col-0 wild type (23.8 ± 1.0; n = 66). Under BB, circadian oscillations of [Ca2+]cyt were undetectable in cry1 cry2 (Figure 5F) and were less robust in the same conditions in cry1 and cry2-1 (RAE, 0.54 ± 0.30 [n = 18] and 0.55 ± 0.40 [n = 18], respectively) than in the Ler and Col-0 wild types (RAE, 0.39 ± 0.01 [n = 20] and 0.36 ± 0.17 [n = 23], respectively) (Figures 5A to 5D).
Figure 4.
Cryptochromes Are Required for Robust Circadian Oscillations of [Ca2+]cyt.
(A) to (C) Aequorin luminescence rhythms from cry1, cry2-1, and cry1 cry2 mutants were measured under R+B (100 μmol·m−2·s−1) using photon-counting imaging. Mean traces, representative of three independent experiments and at least two independent transformants, show the aequorin luminescence in cry1 (A), cry2-1 (B), and cry1 cry2 double mutant (C) with Ler or Col-0 wild-type control. se bars are shown every 6 h (n = 4). Seedlings were entrained under LD for 10 d before transfer to R+B. Hatched bars at bottom represent subjective night.
(D) to (F) Plots showing the FFT-NLLS analysis of the luminescence rhythms. Each value indicates the analysis of one trace for Ler wild type (closed circles; n = 24), Col-0 wild type (closed squares; n = 44), cry1 mutant (open inverted triangles; n = 11), cry2-1 mutant (open squares; n = 27), and cry1 cry2 double mutant (open diamonds; n = 24).
Figure 5.
Blue Light Modulation of Circadian [Ca2+]cyt Oscillations.
(A) to (F) Circadian [Ca2+]cyt oscillations were measured in Ler wild type ([A]; closed circles), Col-0 wild type ([B]; closed squares), cry1 ([C]; open inverted triangles), cry2-1 ([D]; open triangles), and cry1 cry2 double mutant ([F]; open diamonds) using photon-counting luminescence. Mean traces are representative of three independent experiments and at least two independent transformants. Seedlings were entrained under LD for 7 d before transfer to LL (white bars; 40 μmol·m−2·s−1 for all light conditions) for 2 d followed by 3 d in BB (dark gray bars) and 3 d in R+B (cross-hatched bars). In (E), Ler wild type was entrained under LD for 7 d before transfer to LL (white bar) followed by RR (light gray bar) and R+B (hatched bar; 40 μmol·m−2·s−1 for all light conditions).
(G) and (H) PHYB-ox (open circles) and phyB-1 (open squares) mutants transgenic with 35S:AEQ were entrained under LD for 7 d before release to LL for 2 d followed by ∼3 d of BB and R+B (40 μmol·m−2·s−1 for all light conditions).
n = 12 to 46 for LL and n = 20 to 23 for all other light conditions except cry1 cry2 in monochromatic light (n = 9) (F).
Blue light also regulated the basal level of the circadian [Ca2+]cyt oscillations. [Ca2+]cyt oscillated with a lower amplitude when Ler wild-type seedlings were transferred from LL to RR, and the amplitude could be restored when the light conditions were changed to R+B (40 μmol·m−2·s−1; Figure 5E). Under BB, the basal level of the [Ca2+]cyt oscillation in Ler and Col-0 wild-type seedlings increased progressively; when the light condition was changed from BB to R+B, the basal [Ca2+]cyt reverted to a lower level (Figures 5A and 5B). BB did not elevate the basal [Ca2+]cyt in the cry1 and cry2-1 single mutants (Figures 5C and 5D), suggesting that both CRY1 and CRY2 are involved in the blue light regulation of the basal level of circadian [Ca2+]cyt oscillations.
PHYB in Addition to CRY1 and CRY2 Regulates the Basal Level of Circadian [Ca2+]cyt Oscillations in Blue Light
We next investigated whether PHYB may contribute alongside CRY1 and CRY2 to blue light regulation of the circadian oscillations of [Ca2+]cyt, due to the role of PHYB as the principal photoreceptor for red light–mediated circadian oscillations of [Ca2+]cyt. Both phyB-1 single mutant and PHYB-ox had sustained oscillations of [Ca2+]cyt in LL, BB, and R+B, suggesting that PHYB does not contribute to the robustness of the oscillations in blue light (Figures 5G and 5H). For example, the RAE of [Ca2+]cyt rhythms in phyB-1 (0.36 ± 0.09; n = 11) and PHYB-ox (0.35 ± 0.12; n = 8) were the same as those for Ler and No-0 wild type in BB (0.39 ± 0.01 [n = 20] and 0.31 ± 0.14 [n = 21], respectively). However, PHYB may form part of the phototransduction pathway by which blue light elevates the basal level of the circadian [Ca2+]cyt oscillations, because BB had no effect on basal [Ca2+]cyt in phyB-1 (Figure 5H).
ELF3 Is Required to Maintain Circadian [Ca2+]cyt Oscillations
To assess the role of ELF3 in the light-dependent circadian [Ca2+]cyt oscillations, we monitored [Ca2+]cyt in an elf3-1 mutant. In elf3-1 (Hicks et al., 1996), [Ca2+]cyt was rhythmic for the first 36 h in constant light (R+B, LL, BB, and RR) before becoming arrhythmic, indicating that ELF3 is necessary to maintain the [Ca2+]cyt oscillation (Figure 6). In R+B, 85% of the traces were arrhythmic and the remaining traces had a very high RAE (0.82 ± 0.13; n = 20). Transfer of elf3-1 to BB (Figure 6B) or DD (Figure 6C) resulted in an increase in the basal level of [Ca2+]cyt similar to that of the Col-0 wild type, suggesting that the mechanisms that regulate the basal level of [Ca2+]cyt in prolonged blue light or dark do not require a functional rhythmic circadian oscillator. Moreover, the delay before the increase of [Ca2+]cyt observed in Col-0 wild type in DD is not observed in elf3-1. [Ca2+]cyt starts increasing immediately after the transfer to dark in elf3-1 (Figure 6A; see Supplemental Figure 2D online).
Figure 6.
ELF3 Is Required to Maintain Circadian [Ca2+]cyt Oscillations.
[Ca2+]cyt in elf3-1 (open squares) and Col-0 wild type (closed squares) was measured under R+B (100 μmol·m−2·s−1) (A), RR or BB (40 μmol·m−2·s−1 for both conditions) (B), and DD (C) using photon-counting luminescence (B) or photon-counting imaging ([A] and [C]). Seedlings were entrained under LD for 7 d before release to constant conditions. White bars indicate subjective day in R+B, light gray bars indicate RR, dark gray bars indicate BB, and black bars indicate DD. Hatched bars represent subjective night in R+B and subjective day in DD. se bars are shown every 6 h (n = 4). At least three replicates and three independent transformants were used for each experiment.
Circadian Ca2+ Oscillations Are Downstream of a CCA1/LHY/TOC1 Oscillator
Arrhythmia of [Ca2+]cyt in elf3-1 after the first 36 h in continuous light suggested that circadian [Ca2+]cyt oscillations might act downstream of an oscillator comprising the Arabidopsis core clock genes CCA1, LHY, and TOC1. ELF3 gates light input into the CCA1/LHY/TOC1 oscillator, and the oscillator is arrhythmic in elf3-1 after 10 h in LL (McWatters et al., 2000). A functional CCA1/LHY/TOC1 oscillator is required for circadian [Ca2+]cyt oscillations since, under R+B, [Ca2+]cyt was arrhythmic in seedlings overexpressing CCA1 (CCA1-ox) (Figure 7A) (Wang and Tobin, 1998) or LHY (LHY-ox) (Figure 7C) (Schaffer et al., 1998). In CCA1-ox lines, 47% of the traces had no detectable rhythms and the remaining traces had high RAE (0.69 ± 0.20; n = 36), while in LHY-ox lines, 50% of traces had no detectable rhythm and the remaining traces had a RAE of 0.70 ± 0.17 (n = 28).
Figure 7.
Circadian Ca2+ Oscillations Are Downstream of CCA1/LHY.
Aequorin luminescence in CCA1-ox (A), cca1-1 (B), and LHY-ox (C) under LL (100 μmol·m−2·s−1) measured using photon-counting luminescence. se bars are shown every 6 h (n = 4). Mean traces are representative of three independent experiments and at least two independent transformants. Seedlings were entrained under LD for 7 d before transfer to LL. Hatched boxes indicate subjective night in LL. n > 40 for (A) to (C). In (D), plots show the FFT-NLLS analysis of rhythms in leaf movement. Each value indicates the analysis of one trace for Ws wild type (n = 16) and cca1-1 (n = 16). Plants were grown under LD for 7 d and then transferred to LL for 5 d for assessment of leaf movement rhythms as described by Michael et al. (2003). Closed squares, Col-0 wild type; closed triangles, Ws wild type; closed circles, Ler wild type; open diamonds, CCA1-ox; open circles, cca1-1; open triangles, LHY-ox.
CCA1 is required for circadian oscillations of [Ca2+]cyt, since in R+B, [Ca2+]cyt was mainly arrhythmic in a cca1-1 null mutant (Figure 7B; see Supplemental Figure 3 online) (Green and Tobin, 1999). Four of five independently transformed lines had no detectable rhythms of [Ca2+]cyt (see Supplemental Figures 3A, 3B, 3D, and 3E), while they had short-period rhythms in leaf movement (cca1-1, 21.9 ± 1.1 h [n = 16]; Ws wild type, 23.6 ± 1.0 h [n = 16]) (Figure 7D). Short-period rhythms of [Ca2+]cyt that damped rapidly were detected in the other independent transformant line (50% of the traces were rhythmic in this line, with a mean period of 20.1 ± 0.9 h; n = 4) (see Supplemental Figure 3C online). In LD cycles, we detected rhythms of [Ca2+]cyt in all four circadian arrhythmic lines—cca1-1, CCA1-ox, LHY-ox, and elf3-1—demonstrating that the aequorin reporter was functional in these lines and thus that the arrhythmia of [Ca2+]cyt in LL was due to clock gene misexpression (see Supplemental Figure 2 online).
Circadian Ca2+ Oscillations Are Insensitive to the toc1-1 Mutation
Having established the effects of the misexpression of CCA1/LHY on the regulation of circadian oscillations of [Ca2+]cyt, we next investigated the role of TOC1 because it is thought to be part of the positive arm regulating CCA1 and LHY expression in the circadian clock (Alabadí et al., 2001). In toc1-1 mutants, the circadian clock runs faster, shortening the free-running period of circadian outputs, including CAB2:luc luminescence and leaf and stomatal movements, by ∼3 to 3.5 h compared with the C24 wild type (Figure 8E) (Millar et al., 1995; Somers et al., 1998a). In our experimental conditions, the period of oscillations in CAB2:luc luminescence in toc1-1 was 21.8 ± 0.2 h (n = 12), compared with the C24 wild-type period of 23.9 ± 0.6 h (n = 28) (Figures 8D and 8E). By contrast, we were unable to detect a period difference between the [Ca2+]cyt oscillations of toc1-1 (24.6 ± 1.3 h; n = 46) and the C24 wild type (24.6 ± 0.8 h; n = 35) (Figures 8A and 8D). We were also unable to detect any phase difference between toc1-1 and the C24 wild type in R+B for at least 7 d in LL (see Supplemental Figure 4A online). Three toc1-1 lines independently transformed with 35S:AEQ were used (see Supplemental Figures 4B and 4C online). Each line contained different levels of aequorin, which resulted in different luminescence levels that are not necessarily related to the [Ca2+]cyt levels. Importantly, in these experiments, the toc1-1 lines had both the CAB2:luc and 35S:AEQ constructs, and seedlings generated from the same lines were simultaneously imaged side by side following treatment with either luciferin or coelenterazine to monitor either luciferase or aequorin luminescence, respectively.
Figure 8.
toc1-1 Does Not Affect the Period of Circadian [Ca2+]cyt Oscillations.
Luminescence of aequorin ([A] to [C]) and luciferase ([E] to [G]) was measured using photon-counting imaging from C24 wild type (black symbols), toc1-1 (white symbols), toc1-2 (light gray symbols), and ztl-1 (dark gray symbols) lines transformed with both 35S:AEQ and CAB2:luc transgenes. Seedlings were entrained in LD for 10 d before transfer to R+B (100 μmol·m−2·s−1 ). Measurements of aequorin (circles) and luciferase (inverted triangles) luminescence were made simultaneously using photon-counting imaging of sibling seedlings treated with luciferin and coelenterazine, respectively. se bars are shown every 6 h (n = 4). At least three replicates and three independent transformants were used for each experiment. (D) shows FFT-NLLS analysis of 35S:AEQ and CAB2:luc luminescence in C24 wild type, toc1-1, toc1-2, and ztl-1. The data are means ± sd from one experiment representative of at least seven independent replicates.
The measurement of luciferase and aequorin in mutant lines with the same toc1-1 background allowed the confirmation of differential effects of the mutation on CAB2 promoter activity and [Ca2+]cyt. In these conditions, the luciferase signal was at least 1 order of magnitude greater than the aequorin luminescence signal. To confirm our finding and avoid any possibility of contamination of the aequorin signal with reflected light from the luciferase emission, we independently measured the effect of toc1-1 on [Ca2+]cyt in the absence of luciferase luminescence and obtained the same result (see Supplemental Figure 4D online). Thus, toc1-1 was without effect on circadian oscillations of [Ca2+]cyt. toc1-1 is a semidominant allele with a base pair change that leads to a conservative amino acid change from Ala to Val in the second half of the highly conserved CCT (for CONSTANS, CONSTANS-LIKE, and TOC1) domain (Strayer et al., 2000). The biochemical function of the CCT domain is still unknown, but it might involve protein–protein interaction (Laubinger et al., 2006; Wenkel et al., 2006) and nuclear localization (Robson et al., 2001).
To check whether circadian oscillations of [Ca2+]cyt are independent of TOC1 or just insensitive to the toc1-1 mutation, we measured circadian oscillations of [Ca2+]cyt and CAB2 promoter activity concurrently in the toc1-2 mutant (Strayer et al., 2000). toc1-2 is a recessive allele that has the last nucleotide in exon 1 altered, resulting in changes to preferential mRNA splicing. The incorrectly spliced transcripts, which total 94% of the toc1-2 spliced transcripts, result in a truncated protein of only 59 residues (Strayer et al., 2000). Unlike toc1-1, toc1-2 mutants had short-period rhythms of both circadian oscillations of [Ca2+]cyt and CAB2 promoter activity (22.7 ± 1.4 h [n = 8] and 21.6 ± 0.1 h [n = 4], respectively) (Figures 8B, 8D, and 8F). These data demonstrate that our techniques allowed the detection of changes in the period of circadian oscillations of [Ca2+]cyt when present, confirming that the toc1-1 mutation does not affect the period of [Ca2+]cyt oscillations.
We next investigated the effect of the long-period mutant ztl-1 on circadian oscillations of [Ca2+]cyt, because the degradation of TOC1 involves ZTL, an F-box protein that is negatively regulated by light (Más et al., 2003b). ztl-1 (Más et al., 2003b) lengthened the period of oscillations of both CAB2:luc luminescence (C24 wild type, 23.9 ± 0.6 [n = 28]; ztl-1, 26.6 ± 0.17 h [n = 4]) (Figures 8D and 8G) and [Ca2+]cyt luminescence (C24 wild type, 24.4 ± 0.7 h [n = 27]; ztl-1, 30.8 ± 0.4 h [n = 4]) (Figures 8C and 8D) by >3 h. This suggests that ZTL participates in the circadian control of [Ca2+]cyt.
DISCUSSION
Our data demonstrate that in Arabidopsis seedlings, the circadian oscillation of [Ca2+]cyt is regulated by red and blue light through CRY1, CRY2, PHYB, and, possibly, PHYA. Red and blue light regulate both the amplitude of the oscillations and also the basal level of [Ca2+]cyt. Additionally, unknown signals regulate the basal level of [Ca2+]cyt at night and in prolonged darkness. Circadian oscillations of [Ca2+]cyt are regulated by several core clock genes (LHY, CCA1, TOC1, ELF3, and ZTL), which contribute to the control of a wide range of circadian outputs, including leaf and stomatal movements, gene expression, and rhythms of photosynthesis (Gardner et al., 2006). Surprisingly, [Ca2+]cyt rhythms are unaffected by toc1-1; this and other genetic differences in the regulation of circadian oscillations of [Ca2+]cyt from other circadian outputs suggest the presence of multiple circadian oscillators in Arabidopsis.
Light-Dependent [Ca2+]cyt Oscillations in Arabidopsis
In Arabidopsis, circadian oscillations of [Ca2+]cyt were robust in LL but were absent after the first 24 h in DD (Figure 1) (Johnson et al., 1995; Sai and Johnson, 1999; Love et al., 2004). Therefore, light input is required for the circadian oscillations of [Ca2+]cyt in Arabidopsis. In tobacco seedlings, the [Ca2+]cyt rhythm can persist in DD, albeit with a decreasing amplitude (Johnson et al., 1995). Since [Ca2+]cyt increased in the dark in anticipation of dawn in LD in wild-type plants (Figure 1) (Love et al., 2004) and in a cca1-1 null mutant (see Supplemental Figure 2A online) and at subjective dawn in DD (Figure 1), light input appears to be required to maintain rhythmicity but light is not the signal that elevates [Ca2+]cyt.
After the first 12 h in DD, [Ca2+]cyt increased in anticipation of subjective dawn, then remained elevated at an essentially constant value that was intermediate between the peak and trough in DD (Figure 1). Based on our current knowledge of Ca2+ homeostasis, the decline of [Ca2+]cyt to resting at night might have been assumed to be through the action of a default Ca2+ homeostatic apparatus that maintains [Ca2+]cyt at the usual unstimulated levels. However, the persistent elevation of [Ca2+]cyt in DD suggests that homeostasis alone is not sufficient to return [Ca2+]cyt to resting. One possibility among others is that an induced mechanism (such as a Ca2+ pump) is activated during the dark period that actively keeps the basal level of [Ca2+]cyt low. Under DD, this mechanism would keep [Ca2+]cyt low during the first 12 h (night) and then would be inactivated, allowing [Ca2+]cyt to increase and reach a new constant level. Therefore, there may be a negative regulator of [Ca2+]cyt that is an output of the clock in the night but that is not rhythmic in persistent dark. Alternatively, a signal in response to stress in DD may elevate [Ca2+]cyt in persistent dark. In DD, [Ca2+]cyt reaches its maximum level at the same time that DARK-INDUCED2 (DIN2) and DIN9 are induced (Fujiki et al., 2001). The relationship, if any, between the increase in [Ca2+]cyt and DIN2/9 in DD is unclear, because the DIN genes are negatively regulated by Ca2+/calmodulin signaling (Fujiki et al., 2001).
The circadian clock is entrained mainly by light and temperature signals in plants (Johnson et al., 1998; Millar, 2004; Salomé and McClung, 2005). Light entrainment of the clock involves resetting and circadian clock period changes through the action of PHY and CRY in Arabidopsis (Somers et al., 1998b; Devlin and Kay, 2000; Yanovsky et al., 2001; Millar, 2003). We also find that PHY and CRY are important in the maintenance and control of circadian oscillations of [Ca2+]cyt. Rhythms of [Ca2+]cyt were almost undetectable in phyA-201 phyB-5 mutants in RR (Figure 3E) and in cry1 cry2 in BB or R+B (Figures 4C and 5F). These data suggest that red light signals to the circadian Ca2+ oscillation mainly through PHYB but not PHYA, because the [Ca2+]cyt rhythm in phyB-1 was very weak in RR but persisted in phyA-201 (Figures 3B and 3D). PHYB also plays a role in the amplitude regulation of the [Ca2+]cyt oscillation, because overexpression of PHYB caused a higher amplitude of the oscillation than that of No-0 wild type in red light (Figure 3C). Our studies were in high-fluence red light (40 μmol·m−2·s−1). Under these conditions, phyB single mutants and phyA-201 phyB-5 double mutants have been reported to have periods of CAB2:luc luminescence 1.5 to 2 h longer than the wild type due to reduced sensitivity to red light (Somers et al., 1998b; Devlin and Kay, 2000; Yanovsky et al., 2001). The lengthening of the period of CAB2:luc luminescence in phy mutants in red light demonstrates a role for the PHY in entraining the oscillator that regulates CAB2 promoter activity. By contrast, [Ca2+]cyt oscillations had very low amplitude in phyB-1 and phyA-201 phyB-5 in RR, due to the dependence of the circadian [Ca2+]cyt oscillation on light input (Figures 3B and 3E).
This requirement for PHYB in monochromatic red light could be indicative of a role for PHYB in generating the oscillations or entraining the oscillator; however, because of the lack of a detectable rhythm, we could not test the latter. At the fluence rates used in our experiments, PHYB-ox is without significant effect on the period of rhythms of [Ca2+]cyt (Figure 3C). At lower fluence rates, PHYB-ox shortens the period of CAB2:luc (Somers et al., 1998b), but at similar fluence rates to those used in this study, PHYB-ox has little effect on the period of CAB2:luc rhythms, similar to [Ca2+]cyt. phyA-201 was without significant effect on the period of [Ca2+]cyt rhythms compared with Ler in 100 μmol·m−2·s−1 R+B (Figure 2A). At the same fluence, phyA-201 also does not significantly affect the period of CAB2:luc rhythms (Devlin and Kay, 2000).
In Arabidopsis, CRY1, CRY2, and PHYA can mediate blue light input to entrain the circadian clock that regulates CAB2:luc rhythms (Somers et al., 1998b; Devlin and Kay, 2000; Yanovsky et al., 2001). Our data demonstrate that CRY1 and CRY2 have redundant roles in regulating [Ca2+]cyt rhythms, because damped rhythms persisted in cry1 and cry2-1 in BB, R+B, and LL (Figures 4A to 4C, 5C, and 5D), but rhythms were absent in cry1 cry2 double mutants in BB (Figure 5F). The period of the oscillation of [Ca2+]cyt in cry1 in 100 μmol·m−2·s−1 R+B was <1 h longer than that in the Ler wild type (Figures 4A and 4D). This finding is consistent with previous studies of the effects of cry1 on CAB2:luc rhythms in the same fluence of white light, in which the period of the CAB2:luc rhythm was also <1 h longer than in the wild type, although the effect was statistically different (Devlin and Kay, 2000). Again, the arrhythmia of [Ca2+]cyt in the cry1 cry2 double mutant in BB confirms a requirement for light input to maintain circadian oscillations of [Ca2+]cyt. Rhythms of CAB2:luc luminescence in cry1 cry2 in BB have a long period, providing evidence for a role for blue light in entraining the oscillator (Devlin and Kay, 2000), but the absence of a rhythm of [Ca2+]cyt in these conditions made it difficult to test for a role of blue light in entraining [Ca2+]cyt rhythms.
An important result from our study is that blue light caused an increase in the basal level of the circadian [Ca2+]cyt oscillation, mediated by CRY1, CRY2, and PHYB (Figure 5). The elevation in basal [Ca2+]cyt stimulated by prolonged blue light appears to be independent of the circadian system, because blue light elevated [Ca2+]cyt in elf3-1, which is arrhythmic for Ca2+ (Figure 6B). Strikingly, the photoreceptors in this blue light–induced increase in basal [Ca2+]cyt do not appear to function redundantly (Figures 5C and 5D). This contrasts with their roles in maintaining circadian rhythms. The mechanisms by which CRY1, CRY2, and PHYB increase the basal level of [Ca2+]cyt in continuous light are unknown. Ca2+ signaling has been implicated in both CRY- and phototropin-mediated blue light signaling (Long and Jenkins, 1998; Baum et al., 1999). The mechanisms by which CRY activation increases [Ca2+]cyt are a matter of speculation, but phototropin activates plasma membrane phototropin-activated calcium channels (PACC) and phospholipase C to cause transient increases in [Ca2+]cyt in response to blue light pulses (Baum et al., 1999; Babourina et al., 2002; Harada et al., 2003; Stoelzle et al., 2003). However, the PACC is unlikely to be responsible for either the blue light–mediated increase in basal [Ca2+]cyt or the circadian oscillations of [Ca2+]cyt, because PACC activation was unaffected by cry1-304 cry2-1 (Stoelzle et al., 2003) and transient increases in [Ca2+]cyt in response to blue light were unaffected by cry1 or cry2 mutations (Baum et al., 1999).
Our data also suggest a new role for PHYB in blue light signaling: cooperating with CRY1 and/or CRY2 to regulate the amplitude of clock-controlled [Ca2+]cyt oscillations. cry1, cry2-1, and phyB-1 reduced the amplitude of circadian oscillations of [Ca2+]cyt (Figures 5C, 5D, and 5H), while PHYB-ox increased the amplitude in BB (Figure 5G). The effects seen in the phyA-201 mutants in R+B (Figures 2A and 2D) might be due to the effect of this mutant on blue light signaling. This would explain why no effect was seen in constant red light (Figure 3D). PHYs have previously been suggested to interact with CRY, and PHYA was previously demonstrated to be required for normal blue light signaling in low-fluence blue light (<3 to 5 μmol·m−2·s−1) (Devlin and Kay, 2000). An in vivo interaction between PHYB and CRY2 has been demonstrated (Más et al., 2000). Therefore, it appears that both PHYA and PHYB can participate in blue light signaling via interactions with CRY1 and CRY2. These interactions may be significant for Ca2+ signaling, because it has been suggested that SHORT UNDER BLUE LIGHT1, which encodes a Ca2+ binding protein of unknown function, acts downstream of CRY and may be a point of crosstalk between CRY and PHYA signal transduction pathways (Guo et al., 2001).
Light input into the circadian system is thought to be regulated by ELF3, a negative regulator of light resetting in Arabidopsis (McWatters et al., 2000; Covington et al., 2001). [Ca2+]cyt was arrhythmic in LL in elf3-1 mutants, as would be predicted from the arrhythmia of leaf movements and CAB2:luc luminescence in elf3-1 (Hicks et al., 1996, 2001; McWatters et al., 2000; Covington et al., 2001; Liu et al., 2001) (Figure 6A). This suggests that light input into the circadian oscillator regulating [Ca2+]cyt could be modulated by ELF3, which is essentially as predicted by the current conceptual models of light input to the circadian clock (Millar, 2004; Salomé and McClung, 2005).
Light and dark signals can entrain the oscillator regulating [Ca2+]cyt (Johnson et al., 1995). In addition to entraining the circadian oscillator, light and dark signals also appear to affect the basal level of [Ca2+]cyt independently of a functional circadian system. In the wild type and arrhythmic elf3-1, both prolonged blue light and dark elevate basal [Ca2+]cyt. The blue light–induced increase in basal [Ca2+]cyt requires CRY1, CRY2, and PHYB, but the mechanisms regulating [Ca2+]cyt in the dark are unknown.
Modulation of Circadian [Ca2+]cyt Oscillations by Arabidopsis Core Clock Components
Circadian oscillations of [Ca2+]cyt have been observed in tobacco and Arabidopsis seedlings, but the relationship with known core clock genes is unclear (Johnson et al., 1995; Love et al., 2004; Dodd et al., 2005a; Gardner et al., 2005). We investigated whether circadian oscillations of [Ca2+]cyt are an output of the proposed core circadian feedback loop consisting of CCA1/LHY/TOC1 (Alabadí et al., 2001). Circadian oscillations of [Ca2+]cyt require oscillations of CCA1 and LHY expression, because [Ca2+]cyt was arrhythmic in CCA1-ox and LHY-ox lines (Figures 7A and 7C). Circadian oscillations of [Ca2+]cyt may specifically require CCA1, as [Ca2+]cyt was arrhythmic or had very low-amplitude oscillations in a cca1-1 mutant (Figure 7B), which has short-period oscillations of CAB2 expression and rhythms of leaf movement (Green and Tobin, 1999; Mizoguchi et al., 2005) (Figure 7D). This suggests that CCA1 modulates circadian [Ca2+]cyt oscillations differently than oscillations of CAB2 expression or rhythms of leaf movement.
The toc1-1 mutation had no effect on the period of [Ca2+]cyt, unlike the short period observed in all other outputs measured, including leaf and stomatal movements and CAB2 and CCR2 expression (Millar et al., 1995; Somers et al., 1998a; Strayer et al., 2000) (Figure 8E). In our experimental conditions, the circadian period for [Ca2+]cyt and CAB2:luc in the C24 wild type was essentially the same (∼24 h), but in the toc1-1 plants, the periods for these two outputs was significantly different, as the CAB2:luc rhythm was 2.5 h shorter than the [Ca2+]cyt rhythm in plants of the same lineage (Figures 8A, 8D, and 8E). The difference between luminescence levels in the C24 wild type and toc1-1 (Figure 8A) is likely to reflect different aequorin levels, as seen in the independent transformants, and not different levels of [Ca2+]cyt (see Supplemental Figures 4B and 4C online). As we are unable to calibrate aequorin levels into [Ca2+]cyt (Johnson et al., 1995; Love et al., 2004), luminescence levels and oscillation amplitude are less informative than the dynamics of oscillations. The toc1-1 experiments were performed with plants that contained both the 35S:AEQ and CAB2:luc transgenes. The period of rhythms of CAB2:luc and [Ca2+]cyt were confirmed by measuring both reporters side by side from the same transgenic lines, by measuring the lines for 7 d in R+B, by using two different techniques (photon counting imaging and photon counting luminescence), and by repeating the experiments independently in the laboratories of the University of Cambridge and Vanderbilt University (Figure 8A; see Supplemental Figure 4 online). A similar experiment with the null allele, toc1-2, demonstrated that, unlike in toc1-1, both CAB2:luc rhythms and [Ca2+]cyt rhythm periods were shortened by the toc1-2 mutation (Figures 8B and 8F). These data also demonstrate that we could detect short-period rhythms of [Ca2+]cyt when present, confirming the lack of effect of toc1-1 on the period of free-running rhythms of [Ca2+]cyt. Our data are consistent with the differential effects of toc1-2 and toc1-1 on hypocotyl elongation in red light, despite the short circadian period of CAB2 expression in both alleles (Somers et al., 1998a; Más et al., 2003a).
The periods of both CAB2:luc rhythms and [Ca2+]cyt rhythms were longer in ztl-1 compared with the C24 wild type (Figures 8C and 8G). Both ztl-1 and toc1-2 have altered levels of TOC1 protein that result in altered periods of both [Ca2+]cyt oscillations and CAB2 expression. The mutant toc1-2 has very low levels of TOC1 (Strayer et al., 2000), which shortens the period of the rhythms. ztl-1, on the other hand, has high levels of TOC1, which leads to period lengthening (Más et al., 2003b). By contrast, toc1-1 is thought to have normal levels of TOC1 protein but with a mutation in the CCT domain of the C terminus (Strayer et al., 2000). This indicates that [Ca2+]cyt oscillations are dependent on TOC1 levels but not on a functional CCT domain. The biochemical function of the CCT domain is still unknown, but it might be involved in protein–protein interaction (Laubinger et al., 2006; Wenkel et al., 2006) and nuclear localization (Robson et al., 2001). The mutated CCT domain results in the shortening of the period of CAB2 expression, but the period of [Ca2+]cyt oscillations remains unaltered.
We have shown that rhythms of [Ca2+]cyt and CAB2:luc are separable genetically. The most striking differences are observed in lines carrying mutations in CCA1 and TOC1, which are both considered oscillator genes (Alabadí et al., 2001). We found that mutations in CCA1 or TOC1 have different effects on these two outputs. Since the period of a rhythmic output is coupled to the period of the oscillator driving the rhythm, it is very unlikely that a mutation in an oscillator gene would affect differently two outputs coupled directly to the same oscillator. Therefore, our data provide genetic evidence for the presence of multiple circadian oscillators in plants. It is already assumed that each plant cell contains an independent oscillator (Webb, 2003; Dodd et al., 2004, 2005a). Our data suggest that these oscillators may be regulated differently. Since toc1-1 shortens the period of circadian expression of both CCA1 and LHY (Alabadí et al., 2001) and we demonstrate that circadian oscillations of [Ca2+]cyt are dependent on CCA1/LHY but unaffected by the toc1-1 mutation, we suggest that the oscillators regulating [Ca2+]cyt and CAB2 might be localized in functionally separate compartments and are possibly in different cell types. This conclusion does not exclude the possibility that in some cells both CAB2 and [Ca2+]cyt are controlled by the same oscillator, but the predominant luminescent signals for [Ca2+]cyt and CAB2:luc measured from whole seedlings may be generated from different cell populations.
Alternatively, the [Ca2+]cyt- and CAB2-controlling oscillators could be localized in the same cell, but the altered responses to toc1-1 and cca1-1 demonstrate that the mechanism of the clock-controlled [Ca2+]cyt oscillations in Arabidopsis is different from that of some other circadian outputs, including CAB2 gene expression and leaf movement. This suggests that the circadian oscillators regulating [Ca2+]cyt and CAB2 have different molecular architectures or regulation but also share many common components, such as CCA1, LHY, and TOC1 and are both apparently regulated by ZTL, ELF3, CRY1, CRY2, and PHYs. The conclusion that circadian oscillations of [Ca2+]cyt and CAB2 derive from oscillators of different molecular architectures, possibly in different cell populations, is consistent with previous reports that [Ca2+]cyt and CAB2:luc rhythms can be uncoupled in tobacco (Sai and Johnson, 1999), that the phase of oscillation of [Ca2+]cyt may differ between cell types (Wood et al., 2001), and that many rhythms have different free-running periods (Hall et al., 2002; Dodd et al., 2004, 2005a). Uncoupling of period rhythms can indicate that the rhythms occur in different tissues receiving varying levels of light or that different oscillator structures are present (Dodd et al., 2004, 2005a; Gardner et al., 2006).
Our genetic data provide evidence for the presence of multiple oscillator types in Arabidopsis. Multiple oscillator types have been proposed for other organisms. For example, in the single-celled alga Gonyaulax, at least two oscillators appear to coexist, one driving a rhythm of bioluminescence and the other regulating cell motility/aggregation (Roenneberg and Morse, 1993). Moreover, it has been suggested that at least three types of oscillators are present in Neurospora: the first based on a feedback loop between FREQUENCY (FRQ), WHITE COLLAR-1 (WC-1), and WC-2; a FRQ-less oscillator; and a WC-less and FRQ-less oscillator (de Paula et al., 2006). Mutations in frq can affect the period of the WC-less and FRQ-less oscillator, suggesting that oscillators can run independently but may interact. Recently, it was shown that Clock knockout mice still had oscillatory behavior, despite the previous understanding that the CLOCK gene was essential to the mammalian clock (Asher and Schibler, 2006; Debruyne et al., 2006). In plants, it appears that an oscillator containing CCA1 and LHY could regulate [Ca2+]cyt. This oscillator is insensitive to the toc1-1 mutation but not to the toc1-2 mutation and is arrhythmic in cca1-1, suggesting that, in spite of sharing the same components with the oscillator regulating CAB2 promoter activity, the oscillator that regulates [Ca2+]cyt uses these components in a different manner (see Supplemental Figure 5 online). Our data suggest that protein–protein interactions with TOC1, specifically with its CCT domain, may be involved in the differential regulation of circadian outputs by cell-specific oscillators. Unraveling the nature of these interactions will be necessary to fully describe the differences between the oscillators regulating CAB2 and [Ca2+]cyt.
METHODS
Plant Materials
The Arabidopsis thaliana mutant lines toc1-1 (background ecotype C24) (Millar et al., 1995) and ztl-1 (C24) (Somers et al., 2000) containing the CAB2:luc construct were a gift from A. Millar (University of Edinburgh). The toc1-2 mutant (C24) (Strayer et al., 2000) was a gift from A. Hall (University of Liverpool). The CCA1-ox lines (Col-0) (Wang and Tobin, 1998) and the cca1-1 null mutant (Ws) (Green and Tobin, 1999) were a gift from E. Tobin (University of California). lhy-1 (LHY-ox; Ler) (Schaffer et al., 1998) was gift from T. Mizoguchi (University of Tsukuba). elf3-1 (Col-0) (Hicks et al., 1996) was a gift from I. Carré (University of Warwick) and K. Hicks (Kenyon College). cry1 (hy4-223N; Ler) (Ahmad and Cashmore, 1993), a fast-neutron irradiation deletion mutant that has an ∼300-bp deletion resulting in reduced levels of a truncated transcript transformed with aequorin (Baum et al., 1999), and cry2-1 (Col-4) were gifts from Gideon Baum (Weizmann Institute). cry1 cry2 (hy4-1 fha1-1; Ler) (El-Assal et al., 2003) was a gift from K. Halliday (University of Bristol). phyA-201 (Ler) (Nagatani et al., 1993), phyB-9 (Col-0) (Reed et al., 1993), phyA-201 phyB-5 (Ler) (Reed et al., 1994), and cry2-1 (Col-4) (Guo et al., 1998) were obtained from the Nottingham Arabidopsis Stock Centre. The Ws wild type carrying the 35S:AEQ transgene was originally a gift from M. Knight (University of Durham).
Plasmid Construction and Transformation
A plasmid (pJAAAEQ) used to transform the Arabidopsis lines with APOAEQUORIN cDNA under the control of a 35S cauliflower mosaic virus promoter (35S) was constructed using a modified Gateway (Invitrogen) destination vector from pJawohl8-RNAi (AF408413; B. Ülker, T. Lipka, V. Rademacher, and I. Somssich, Max-Planck Institute for Plant Breeding Research), in which one of the two inverted Gateway cassettes was removed. A sequence containing a ribosomal recognition sequence (RRS; 5′-GCCACC-3′) fused to the 5′ terminus of the APOAEQUORIN cDNA sequence (AEQ) from pMAQ2 (Molecular Probes) and flanked by the AttB1 and AttB2 cloning adaptors was engineered using high-fidelity thermostable DNA polymerase (Roche) with PCR and forward (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGCCACCATGACCAGCGAAC-3′) and reverse (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTAGGGGACAGCTCCACCG-3′) primers. The product was cloned into pDONR201 (Invitrogen), using the BP reaction, according to the manufacturer's instructions. Reactions were transfected into Escherichia coli strain DH5α. Bacterial colonies were selected by overnight growth at 37°C on plates containing Luria-Bertani (LB) agar (1% [w/v] NaCl, 1% [w/v] tryptone, 0.5% [w/v] yeast extract, and 1.5% [w/v] bacto-agar, pH 7.0) with 50 mg/L kanamycin. The colonies were cultured overnight at 37°C in 5 mL of LB broth (1% [w/v] NaCl, 1% [w/v] tryptone, and 0.5% [w/v] yeast extract, pH 7.0) containing 50 mg/L kanamycin. Recombined plasmids containing the PCR construct were purified from bacterial suspensions using the Qiagen miniprep kit and verified by sequencing. A single plasmid (pDONRAEQ) containing the correct AttB1:RRS:AEQ:AttB2 sequence was selected.
A Gateway binary vector for transgene expression in plants was constructed from pJAWOL18-RNAi, which has a PAT (dl-PHOSPHINOTHRICIN ACETYL TRANSFERASE) plant resistance cassette that confers resistance to the herbicide dl-phosphinothricin (PPT) and the inverted repeat AttR1:cmR:ccdB:AttR2 downstream of a 35S promoter that contains a duplicated enhancer sequence (35SS) and upstream of the cauliflower mosaic virus terminator (pA35S). The XhoI and BamHI restriction endonucleases were used to excise the antisense AttR1:cmR:ccdB:AttR2 sequence from pJAWOL18-RNAi. The linearized plasmid was blunt-ended using Pfu polymerase (Promega) and religated using T4 DNA ligase, making the binary vector pJAA containing a bacterial ampicillin resistance gene.
pJAA was linearized using MLuI and purified. The RRS:AEQ PCR sequence from pDONRAEQ was engineered between the AttR1 and AttR2 sites in pJAA using LR recombinase (Invitrogen) as recommended by the supplier. Reactions were transformed into E. coli strain DH5α. Bacterial colonies were selected by plating on LB agar containing 50 mg/L ampicillin and overnight growth at 37°C before overnight incubation in 5 mL of LB broth with 50 mg/L ampicillin. Purified plasmids were verified by digestion with EcoRV and BamHI. A plasmid (pJAAAEQ) containing the RRS:AEQ sequence was selected.
pJAAAEQ was electroporated into Agrobacterium tumefaciens strain GV3101 containing the pMP90RK helper plasmid. Bacteria were suspended in 1 mL of ice-cold SOC medium (2% [w/v] tryptone, 5% [w/v] yeast extract, 20 mM glucose, 10 mM NaCl, 10 mM MgCl2, and 10 mM MgSO4) and incubated for 3 h at 30°C, then transferred to 4 mL of YEB medium (5% [w/v] beef extract, 5% [w/v] peptone, 5% [w/v] sucrose, and 1% [w/v] yeast extract, pH 7.2) and incubated overnight at 30°C. Thirty microliters of this suspension was plated onto LB agar containing 50 mg/L rifampicin, 25 mg/L kanamycin, 25 mg/L gentamycin, and 25 mg/L carbenicillin and incubated for 2 d at 30°C. Colonies were picked into 5 mL of YEB medium containing the same antibiotics, except that gentamycin was not included, and incubated with shaking at 30°C for 2 to 3 d. Five hundred microliters of this suspension was transferred to 5 mL of YEB medium containing 100 mg/L rifampicin, 50 mg/L kanamycin, 50 mg/L gentamycin, and 50 mg/L carbenicillin and incubated for 3 d at 30°C with shaking. Five hundred microliters of bacterial suspension was inoculated into 500 mL of LB with identical concentrations of each antibiotic and incubated for 3 d at 30°C with shaking. Agrobacterium was harvested by centrifugation and resuspended in 0.22% (w/v) Murashige and Skoog salts, 5% (w/v) sucrose, and 0.05% Silwet L-77 (Lehle). Arabidopsis was transformed using the floral-dip method. Transformed lines were selected by growing plants in half-strength Murashige and Skoog salts, 1% (w/v) sucrose, 1% (w/v) agar, and 5 mg/mL PPT or spraying plants germinated on a 1:1 vermiculite:compost mixture with 200 mg/mL PPT.
The PHYB-ox 35S:AEQ and phyB-1 35S:AEQ lines were generated by crossing a 35S:PHYB line or phyB-1 line (Sharrock et al., 2003) to the Arabidopsis No-0 transgenic line containing the MAQ 2.4 (35S:AEQ) construct (Johnson et al., 1995). F3 segregant lines that were homozygous for the transgenes, and mutations were identified by PCR.
Growth Conditions
Transgenic Arabidopsis seeds of 35S:AEQ were rinsed in 70% ethanol, sterilized in 20% (v/v) Clorox (5.25% [v/v] sodium hypochlorite) for 5 min, and washed three times with sterilized distilled water. The seeds were stratified at 4°C for 2 d in darkness and germinated on half-strength Murashige and Skoog medium (Sigma-Aldrich) and 0.8% (w/v) agar. Seedlings were grown in LD for 7 to 10 d at constant 19 or 22°C. The light source for germination and growth was cool-white fluorescent light at 40 or 60 μmol·m−2·s−1. After 7 to 10 d under 12-h photoperiods, the seedlings were transferred to continuous light conditions (white, red, blue, or red and blue mixed light). The red and blue mixed light treatment (100 μmol·m−2·s−1) was provided by equal amounts of red (630 nm) and blue (475 nm) light-emitting diodes in a mixed array. White light was provided by fluorescent tubes (40 μmol·m−2·s−1; Sylvania). Red light (40 μmol·m−2·s−1) was provided by fluorescent tubes with one layer of medium red Roscolene plastic wrap No. 823 (Rosco). Blue light (40 μmol·m−2·s−1 ) was provided by fluorescent tubes with a blue filter No. 119 (Rosco).
Measurement of Rhythms
Before measurement, aequorin was reconstituted by overnight incubation of 7- to 10-d-old seedlings in 10 μM coelenterazine free base solution (Biosynth or Nanolight) for 12 h in the darkness of the last light/dark cycle. In the case of plants expressing luc, 5 mM luciferin (Nanolight) was added to visualize luciferase activity. The luminophore substrate coelenterazine was prepared by initially dissolving in a tiny amount of methanol and then diluting with deionized sterile water to a final concentration of 10 μM.
The long-term measurement of bioluminescence from the Ca2+ reporter aequorin was monitored by either (1) an automated 30-channel photomultiplier/photocounting apparatus (Johnson et al., 1995) or (2) an ICCD 225 photon-counting camera (Love et al., 2004). The former apparatus recorded the luminescence from samples (10 to 12 seedlings per vial) every 40 min for 40 s (note: seedlings first stay in darkness for 40 s before measurement to allow decay of the chlorophyll phosphorescence) (Figures 3, 5, 6, and 7); the latter apparatus captured the photons of seedlings grown on agar plates in clusters of ∼10 to 12 seedlings with typically 12 clusters per plate. Photon-counting images were captured every 2 h for 25 min. The software of the camera apparatus discards the first 200 s of recording of luminescence to allow phosphorescence decay of chlorophyll (although 30 to 40 s would be sufficient, the camera software was set for a 200-s delay in counting) (Figures 1, 2, 4, and 8; see Supplemental Figure 1 online). In order to measure AEQ and luc lines at the same time, divided round plates (Starsted) were used to separate the plants receiving the luminophores luciferin and coelenterazine. This way, seedlings from the same lineage could be imaged side by side and receive either luciferin, to report luc, or coelenterazine, to report AEQ. Image analysis was done with Photek IFS32 software, and the luminescence rhythms were analyzed with FFT-NLLS in the BRASS interface as described (Love et al., 2004). Traces with a relative amplitude error > 0.5 were considered to have abnormal robustness compared with the wild type. At least two independent transformants were tested for each transgenic line.
35S:AEQ-positive transformed lines were screened for high aequorin content using either a photon-counting luminometer (photomultiplier tube 9899A cooled to −18°C with FACT50 housing [Electron Tubes] or a Zylux FB12 luminometer [Oak Ridge]) or a multifunctional microplate reader (FluoStar OPTIMA; BMG LabTech). When using the luminometer, the reconstituted aequorin was totally discharged at the end of the experiment by injection of 2 mL of 2 M CaCl2 dissolved in 20% (w/v) ethanol (discharge solution, 4°C) via a light-tight septum. Measurements were made until the detected luminescence reached 5% of the first peak after injection. When using the 96-well plate reader, each well contained one leaf from 3-week-old plants grown in a 1:1 vermiculite:compost mixture and functional aequorin was reconstituted by overnight incubation in 100 μL of 5 μM coelenterazine free base. The plate reader automatically measured the luminescence coming from the leaf for 120 s after 150 μL of discharge solution was injected in each well. In both methods, total aequorin activity was estimated by the integration of all luminometer counts and compared with the total aequorin activity present in established 35S:AEQ lines.
In order to measure rhythms of leaf movements, plants were growth in LD for 7 d and measured for 5 d as described by Michael et al. (2003).
Accession Number
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AF408413.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Different Wild-Type Ecotypes Show Similar Circadian [Ca2+]cyt Oscillations.
Supplemental Figure 2. Arrhythmic Lines Have [Ca2+]cyt Oscillations in Light and Dark Cycles.
Supplemental Figure 3. cca1-1 Has Arrhythmic Circadian [Ca2+]cyt Oscillations.
Supplemental Figure 4. toc1-1 Has Wild-Type Circadian [Ca2+]cyt Oscillations.
Supplemental Figure 5. A Proposed Model for the Generation of Circadian [Ca2+]cyt Oscillations.
Supplemental Table 1. Period Estimates of Circadian [Ca2+]cyt Oscillations in Different Wild-Type Ecotypes.
Supplementary Material
Acknowledgments
We thank Jiqing Sai for advice and assistance at the beginning of this project. This research was funded by the National Institute of Mental Health (Grant R01 MH-43836 to C.H.J.) and the Biotechnology and Biological Science Research Council (to A.A.R.W.). A.A.R.W. is also grateful to the Royal Society of London for the award of a University Research Fellowship. C.T.H. is supported by a Compoanha de Aperfeiçoamento ce Pessoal de Nível Superior (Brazil) Scholarship. We are grateful to the many laboratories that made their seed lines freely available to us.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Alex A.R. Webb (alex.webb@plantsci.cam.ac.uk).
Online version contains Web-only data.
References
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 162–166. [DOI] [PubMed] [Google Scholar]
- Alabadí, D., Oyama, T., Yanovsky, M.J., Harmon, F.G., Más, P., and Kay, S.A. (2001). Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293 880–883. [DOI] [PubMed] [Google Scholar]
- Asher, G., and Schibler, U. (2006). A CLOCK-less clock. Trends Cell Biol. 16 547–549. [DOI] [PubMed] [Google Scholar]
- Babourina, O., Newman, I., and Shabala, S. (2002). Blue light-induced kinetics of H+ and Ca2+ fluxes in etiolated wild-type and phototropin-mutant Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 99 2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc. Natl. Acad. Sci. USA 96 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington, M.F., Panda, S., Liu, X.L., Strayer, C.A., Wagner, D.R., and Kay, S.A. (2001). ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13 1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne, J.P., Noton, E., Lambert, C.M., Maywood, E.S., Weaver, D.R., and Reppert, S.M. (2006). A clock shock: Mouse CLOCK is not required for circadian oscillator function. Neuron 4 465–477. [DOI] [PubMed] [Google Scholar]
- de Paula, R.M., Lewis, Z.A., Greene, A.V., Seo, K.S., Morgan, L.W., Vitalini, M.W., Bennett, L., Gomer, R.H., and Bell-Pedersen, D. (2006). Two circadian timing circuits in Neurospora crassa cells share components and regulate distinct rhythmic processes. J. Biol. Rhythms 21 159–168. [DOI] [PubMed] [Google Scholar]
- Devlin, P.F., and Kay, S.A. (2000). Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 12 2499–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd, A.N., Love, J., and Webb, A.A.R. (2005. a). The plant clock shows its metal: Circadian regulation of cytosolic free Ca2+. Trends Plant Sci. 10 15–21. [DOI] [PubMed] [Google Scholar]
- Dodd, A.N., Parkinson, K., and Webb, A.A.R. (2004). Independent circadian regulation of assimilation and stomatal conductance in the ztl-1 mutant of Arabidopsis. New Phytol. 162 63–70. [Google Scholar]
- Dodd, A.N., Salathia, N., Hall, A., Tóth, E.-K., Nagy, F., Hibberd, J.M., Millar, A.J., and Webb, A.A.R. (2005. b). Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309 630–633. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Wais, R., and Long, S.R. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85 673–681. [DOI] [PubMed] [Google Scholar]
- El-Assal, S.E., Alonso-Blanco, C., Peeters, A.J., Wagemaker, C., Weller, J.L., and Koornneef, M. (2003). The role of cryptochrome 2 in flowering in Arabidopsis. Plant Physiol. 133 1504–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki, Y., Yoshikawa, Y., Sato, T., Inada, N., Ito, M., Nishida, I., and Watanabe, A. (2001). Dark-inducible genes from Arabidopsis thaliana are associated with leaf senescence and repressed by sugars. Physiol. Plant. 111 345–352. [DOI] [PubMed] [Google Scholar]
- Gardner, M.J., Hotta, C.T., Sanders, D., Dodd, A.N., and Webb, A.A.R. (2005). Circadian regulation of Ca2+ signalling. In Endogenous Plant Rhythms, A. Hall and H. McWatters, eds (Oxford, UK: Blackwell Scientific Publications), pp. 191–209.
- Gardner, M.J., Hubbard, K.E., Hotta, C.T., Dodd, A.N., and Webb, A.A.R. (2006). Circadian clocks. How plants tell the time. Biochem. J. 397 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, P.D., Locke, J.C.W., Larue, C., Southern, M.M., Davis, S.J., Hanano, S., Moyle, R., Milich, R., Putterill, J., Millar, A.J., and Hall, A. (2006). The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell 18 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, R., and Tobin, E.M. (1999). Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulating gene expression. Proc. Natl. Acad. Sci. USA 96 4176–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H., Mockler, T., Duong, H., and Lin, C. (2001). SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science 291 487–490. [DOI] [PubMed] [Google Scholar]
- Guo, H., Yang, H., Mockler, T.C., and Lin, C. (1998). Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363. [DOI] [PubMed] [Google Scholar]
- Hall, A., Kozma-Bognar, L., Bastow, R.M., Nagy, F., and Millar, A.J. (2002). Distinct regulation of CAB and PHYB gene expression by similar circadian clocks. Plant J. 32 529–537. [DOI] [PubMed] [Google Scholar]
- Harada, A., Sakai, T., and Okada, K. (2003). phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc. Natl. Acad. Sci. USA 100 8583–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen, S.P., Schultz, T.F., Pruneda-Paz, J.L., Borevitz, J.O., Ecker, J.R., and Kay, S.A. (2005). LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythm. Proc. Natl. Acad. Sci. USA 102 10387–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, K.A., Albertson, T.M., and Wagner, D.R. (2001). EARLY FLOWERING 3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 13 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, K.A., Millar, A.J., Carré, I.A., Somers, D.E., Straume, M., Meeks-Wagner, D.R., and Kay, S.A. (1996). Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science 274 790–792. [DOI] [PubMed] [Google Scholar]
- Hotta, C.T., Gardner, M.J., Hubbard, K.E., Baek, S.J., Dalchau, N., Suhita, D., Dodd, A.N., and Webb, A.A.R. (2007). Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ. 30 333–349. [DOI] [PubMed] [Google Scholar]
- Johnson, C.H., Knight, M., Trewavas, A., and Kondo, T. (1998). A clockwork green: Circadian programs in photosynthetic organisms. In Biological Rhythms and Photoperiodism in Plants, P.J. Lumsden and A.J. Millar, eds (Oxford, UK: BIOS Scientific Publishers), pp. 35–50.
- Johnson, C.H., Knight, M.R., Kondo, T., Masson, P., Sedbrook, J., Haley, A., and Trewavas, A. (1995). Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269 1863–1865. [DOI] [PubMed] [Google Scholar]
- Kevei, É., et al. (2006). Forward genetic analysis of the circadian clock separates the multiple functions of ZEITLUPE. Plant Physiol. 140 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikis, E.A., Khanna, R., and Quail, P.H. (2005). ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J. 44 300–313. [DOI] [PubMed] [Google Scholar]
- Klüsener, B., Young, J.J., Murata, Y., Allen, G.J., Mori, I.C., Hugouvieux, V., and Schroeder, J.I. (2002). Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 130 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352 524–526. [DOI] [PubMed] [Google Scholar]
- Laubinger, S., Marchal, V., Gentilhomme, J., Wenkel, S., Adrian, J., Jang, S., Kulajta, C., Braun, H., Coupland, G., and Hoecker, U. (2006). Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133 3213–3222. [DOI] [PubMed] [Google Scholar]
- Liu, X.L., Covington, M.F., Fankhauser, C., Chory, J., and Wagner, D.R. (2001). ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell 13 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, J.C.W., Kozma-Bognár, L., Gould, P.D., Fehér, B., Kevei, É., Nagy, F., Turner, M.S., Hall, A. and Millar, A.J. (2006). Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol. Syst. Biol. 2 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, J.C.W., Millar, A.J., and Turner, M.S. (2005. a). Modelling genetic networks with noisy and varied experimental data: The circadian clock in Arabidopsis thaliana. J. Theor. Biol. 234 383–393. [DOI] [PubMed] [Google Scholar]
- Locke, J.C.W., Southern, M.M., Kozma-Bognár, L., Hibberd, V., Brown, P.E., Turner, M.S., and Millar, A.J. (2005. b) Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol. Syst. Biol. 1 2005.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J.C., and Jenkins, G.I. (1998). Involvement of plasma membrane redox activity and calcium homeostasis in the UV-B and UV-A/blue light induction of gene expression in Arabidopsis. Plant Cell 10 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, J., Dodd, A.N., and Webb, A.A.R. (2004). Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 16 956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más, P., Alabadí, D., Yanovsky, M.J., Oyama, T., and Kay, S.A. (2003. a). Dual role of TOC1 in the control of circadian and photomorphogenic responses in Arabidopsis. Plant Cell 15 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más, P., Devlin, P.F., Panda, S., and Kay, S.A. (2000). Functional interaction of phytochrome B and cryptochrome 2. Nature 408 207–211. [DOI] [PubMed] [Google Scholar]
- Más, P., Kim, W.Y., Somers, D.E., and Kay, S.A. (2003. b). Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426 567–570. [DOI] [PubMed] [Google Scholar]
- McAinsh, M.R., Webb, A.A.R., Taylor, J.E., and Hetherington, A.M. (1995). Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung, C.R. (2006). Plant circadian rhythms. Plant Cell 18 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWatters, H.G., Bastow, R.M., Hall, A., and Millar, A.J. (2000). The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature 408 716–720. [DOI] [PubMed] [Google Scholar]
- Michael, T.P., Salomé, P.A., Yu, H.J., Spencer, T.R., Sharp, E.L., McPeek, M.A., Alonso, J.M., Ecker, J.R., and McClung, C.R. (2003). Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302 1049–1053. [DOI] [PubMed] [Google Scholar]
- Millar, A.J. (2003). A suite of photoreceptors entrains the plant circadian clock. J. Biol. Rhythms 18 217–226. [DOI] [PubMed] [Google Scholar]
- Millar, A.J. (2004). Input signals to the plant circadian clock. J. Exp. Bot. 55 277–283. [DOI] [PubMed] [Google Scholar]
- Millar, A.J., Carré, I.A., Strayer, C.A., Chua, N.H., and Kay, S.A. (1995). Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 24 1161–1163. [DOI] [PubMed] [Google Scholar]
- Mizoguchi, T., Wright, L., Fujiwara, S., Cremer, F., Lee, K., Onouchi, H., Mouradov, A., Fowler, S., Kamada, H., Putterill, J., and Coupland, G. (2005). Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell 17 2255–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani, A., Reed, J.W., and Chory, J. (1993). Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 102 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai, K., and Ishiura, M. (2005). PHYTOCLOCK1 encoding a novel GARP protein essential for the Arabidopsis circadian clock. Genes Cells 10 963–972. [DOI] [PubMed] [Google Scholar]
- Reed, J.W., Nagatani, A., Elich, T.D., Fagan, M., and Chory, J. (1994). Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, J.W., Nagpal, P., Poole, D.S., Furuya, M., and Chory, J. (1993). Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson, F., Costa, M.M., Hepworth, S.R., Vizir, I., Pineiro, M., Reeves, P.H., Putterill, J., and Coupland, G. (2001). Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 28 619–631. [DOI] [PubMed] [Google Scholar]
- Roenneberg, T., and Morse, D. (1993). Two circadian oscillators in one cell. Nature 362 362–364. [DOI] [PubMed] [Google Scholar]
- Sai, J., and Johnson, C.H. (1999). Different circadian oscillators control Ca2+ fluxes and Lhcb gene expression. Proc. Natl. Acad. Sci. USA 96 11659–11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé, P.A., and McClung, C.R. (2005). What makes Arabidopsis tick: Light and temperature entrainment of the circadian clock. Plant Cell Environ. 28 21–38. [Google Scholar]
- Schaffer, R., Ramsay, N., Samach, A., Corden, S., Putterill, J., Carré, I.A., and Coupland, G. (1998). The LATE ELONGATED HYPOCOTYL mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93 1219–1229. [DOI] [PubMed] [Google Scholar]
- Sharrock, R.A., Clack, T., and Goosey, L. (2003). Signaling activities among the Arabidopsis phyB/D/E-type phytochromes: A major role for the central region of the apoprotein. Plant J. 34 317–326. [DOI] [PubMed] [Google Scholar]
- Somers, D., Webb, A.A.R., Pearson, M., and Kay, S.A. (1998. a). The short period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development 125 485–494. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Devlin, P.F., and Kay, S.A. (1998. b). Phytochromes and cryptochromes in the entrainment of Arabidopsis circadian clock. Science 282 1488–1490. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Schultz, T.F., Milnamow, M., and Kay, S.A. (2000). ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101 319–329. [DOI] [PubMed] [Google Scholar]
- Staxén, I., Pical, C., Montgomery, L.T., Gray, J.E., Hetherington, A.M., and McAinsh, M.R. (1999). Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA 96 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoelzle, S., Kagawa, T., Wada, M., Hedrich, R., and Dietrich, P. (2003). Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc. Natl. Acad. Sci. USA 100 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer, C., Oyama, T., Schultz, T.F., Raman, R., Somers, D.E., Más, P., Panda, S., Kreps, J.A., and Kay, S.A. (2000). Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289 768–771. [DOI] [PubMed] [Google Scholar]
- Sun, J., Cardoza, V., Mitchell, D.M., Bright, L., Oldroyd, G., and Harris, J.M. (2006). Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 46 961–970. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Tepperman, J.M., and Quail, P.H. (1991). Overexpression of phytochrome B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell 3 1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.Y., and Tobin, E.M. (1998). Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93 1207–1217. [DOI] [PubMed] [Google Scholar]
- Webb, A.A.R. (2003). The physiology of circadian rhythms in plants. New Phytol. 160 281–303. [DOI] [PubMed] [Google Scholar]
- Wenkel, S., Turck, F., Singer, K., Gissot, L., Le Gourrierec, J., Samach, A., and Coupland, G. (2006). CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18 2971–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, N.T., Haley, A., Viry-Moussaïd, M., Johnson, C.H., van der Luit, A.H., and Trewavas, A.J. (2001). The calcium rhythms of different cell types oscillate with different circadian phases. Plant Physiol. 125 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky, M.J., Mazzella, M.A., Whitelam, G.C., and Casal, J.J. (2001). Resetting of the circadian clock by phytochromes and cryptochromes in Arabidopsis. J. Biol. Rhythms 16 523–530. [DOI] [PubMed] [Google Scholar]
- Young, J.J., Mehta, S., Israelsson, M., Godoski, J., Grill, E., and Schroeder, J.I. (2006). CO2 signaling in guard cells: Calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. USA 103 7506–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilinger, M.N., Farré, E.M., Taylor, S.R., Kay, S.A., and Doyle, F.J. (2006). A novel computational model of the circadian clock in Arabidopsis that incorporates PRR7 and PRR9. Mol. Syst. Biol. 2 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.