Abstract
The Arabidopsis thaliana REVOLUTA (REV) protein is a member of the class III homeodomain-leucine zipper (HD-ZIPIII) proteins. REV is a potent regulator of leaf polarity and vascular development. Here, we report the identification of a gene family that encodes small leucine zipper–containing proteins (LITTLE ZIPPER [ZPR] proteins) where the leucine zipper is similar to that found in REV, PHABULOSA, and PHAVOLUTA proteins. The transcript levels of the ZPR genes increase in response to activation of a steroid-inducible REV protein. We show that the ZPR proteins interact with REV in vitro and that ZPR3 prevents DNA binding by REV in vitro. Overexpression of ZPR proteins in Arabidopsis results in phenotypes similar to those seen when HD-ZIPIII function is reduced. We propose a negative feedback model in which REV promotes transcription of the ZPR genes. The ZPR proteins in turn form heterodimers with the REV protein, preventing it from binding DNA. The HD-ZIPIII/ZPR regulatory module would serve not only to dampen the effect of fluctuations in HD-ZIPIII protein levels but more importantly would provide a potential point of regulation (control over the ratio of inactive heterodimers to active homodimers) that could be influenced by other components of the pathway governing leaf polarity.
INTRODUCTION
Early in their development, leaf primordia become polarized along their adaxial/abaxial axes. The adaxial domain of the leaf primordium is closest to the center of the meristem and develops into the upper half of the leaf. The abaxial domain of the leaf primordium is furthest from the center of the meristem and develops into the lower half of the leaf (for reviews, see Canales et al., 2005; Carraro et al., 2006)
The establishment of ad/abaxial polarity is fundamental to several aspects of shoot development. First, it is critical for establishing the cellular specializations important for leaf function. The upper portion of the leaf, with its tightly packed, chloroplast-rich palisade cells, is optimized for light capture, while the lower portion of the leaf, with its loosely packed spongy mesophyll cells, is optimized for gas exchange. Second, the establishment of ad/abaxial polarity in the leaf is critical for blade outgrowth. The absence of a properly specified ad/abaxial boundary in the leaf primordium results in rod-shaped leaves that lack blade (Waites and Hudson, 1995; McConnell and Barton, 1998; Eshed et al., 2004). Third, correct ad/abaxial polarization of the leaf is required for the formation of meristems. In Arabidopsis thaliana, failure to specify adaxial fates results in failure to make axillary meristems and, in extreme cases, failure to make the shoot apical meristem in embryogenesis (Talbert et al., 1995; Kerstetter et al., 2001; Otsuga et al., 2001; Emery et al., 2003). Conversely, the development of ectopic adaxial fates results in the formation of supernumerary, ectopic buds (McConnell and Barton, 1998).
Class III homeodomain-leucine zipper (HD-ZIPIII) proteins are conserved plant proteins that act as potent regulators of ad/abaxial polarity in Arabidosis (Talbert et al., 1995; McConnell et al., 2001; Otsuga et al., 2001; Emery et al., 2003; Prigge et al., 2005). HD-ZIPIII protein activity promotes the development of adaxial leaf fates and meristem formation; in its absence, lower abaxial leaf fates develop and meristems fail to form. In maize (Zea mays) and tobacco (Nicotiana tabacum), HD-ZIPIII proteins appear to play a similar role in the establishment of leaf polarity (Juarez et al., 2004; McHale and Koning, 2004). HD-ZIPIII proteins are highly conserved among land plants and are found in basal land plants as well as in angiosperms and gymnosperms (Aso et al., 1999; Sakakibara et al., 2001; Floyd et al., 2006; Prigge and Clark, 2006). However, their functions in basal land plants remain to be determined.
There are five HD-ZIPIII genes encoded in the Arabidopsis genome: PHABULOSA (PHB)/ATHB14, REVOLUTA (REV), PHAVOLUTA (PHV)/ATHB9, INCURVATA4/CORONA/ATHB15, and ATHB8 (Sessa et al., 1994, 1998; Zhong and Ye, 1999; Ratcliffe et al., 2000; Baima et al., 2001; McConnell et al., 2001; Green et al., 2005; Prigge and Clark, 2006). Loss-of-function mutations in four of these (PHB, PHV, INCURVATA4/CORONA, and ATHB8) have subtle to indistinguishable mutant phenotypes. Only loss-of-function mutations in REV result in a clear mutant phenotype. rev mutants exhibit narrow, curled back leaves and frequent failure of axillary meristem formation (Talbert et al., 1995; Otsuga et al., 2001). However, when higher-order mutants are made, it becomes apparent that the HD-ZIPIII genes are required for establishing ad/abaxial polarity early in development. In the most severe cases, leaves lack adaxial characters and the main shoot apical meristem fails to form (for instance, as seen in some phb phv rev triple mutants; Emery et al., 2003). Moreover, overexpression or ectopic expression of all five genes results in clear mutant phenotypes (Baima et al., 2001; McConnell et al., 2001; Mallory et al., 2004; Ochando et al., 2006). In all cases, overexpression causes some degree of adaxialization of the leaf. The mildest forms of leaf adaxialization appear as upward curling; more severe forms include trumpet-shaped leaves with adaxial traits on the outside of the bell- and rod-shaped leaves with adaxial characters around the circumference. These phenotypes are in direct contrast to the phenotypes of abaxialized leaves. In their mildest form, abaxialized leaves are curled downward. More severely abaxialized mutants form trumpet-shaped leaves with abaxial characters on the outside of the bell- or rod-shaped leaves with abaxial traits around their circumference.
Consistent with their redundant action in the plant, the HD-ZIPIII genes are expressed in overlapping patterns. Expression is found principally in the adaxial domains of primordia and in the immature vasculature (Baima et al., 1995; McConnell et al., 2001; Otsuga et al., 2001; Kang et al., 2003; Prigge et al., 2005). REV has the broadest expression pattern. It encompasses the adaxial half of the leaf primordium as well as the immature vasculature. By contrast, PHB becomes limited to a subset of adaxial cells. This likely explains why only rev single mutants show a clear mutant phenotype.
In several cases, mutants in the HD-ZIPIII genes cause alterations in vascular development. For instance, ectopic and overexpression of ATHB8 results in increased expression of xylem (Baima et al., 2001). In cases where disruption or misregulation of the HD-ZIPIII genes causes adaxialization or abaxialization of the leaf, the vasculature is similarly affected. In wild-type leaves, the xylem strand is located adaxial to (on top of) the phloem strand. In adaxialized leaves, xylem surrounds phloem; conversely, in abaxialized leaves, phloem surrounds xylem (McConnell and Barton, 1998; Emery et al., 2003).
The HD-ZIPIII proteins contain four recognizable domains. From N terminus to C terminus, these are a DNA binding homeodomain followed immediately by a leucine zipper domain (Ruberti et al., 1991; Schena and Davis, 1992), a START domain (these bind small hydrophobic molecules such as steroids, phospholipids, or carotenoids; Ponting and Aravind, 1999; Schrick et al., 2004), and a PAS domain (sites of protein–protein interaction; Mukherjee and Burglin, 2006).
Unlike animal homeodomain proteins, such as ANTENNAPEDIA, which can bind DNA as monomers, HD-ZIP DNA binding requires dimerization through the leucine zipper domain (Sessa et al., 1993, 1998; Tron et al., 2001, 2004, 2005). In vitro, dimeric HD-ZIPIII proteins have been shown to bind to a pseudopalindromic sequence (Sessa et al., 1997); however, the in vivo targets of HD-ZIPIII transcription factors remain unknown.
The HD-ZIPIII proteins are part of a larger network of regulatory factors that establish ad/abaxial leaf fates. The ASYMMETRIC LEAVES1 (AS1) protein, a MYB-domain containing protein, and the AS2 protein, a LOB domain–containing plant-specific protein, act to promote adaxial fates in this network (Byrne et al., 2000; Iwakawa et al., 2002; Lin et al., 2003). LOB domain proteins have been shown to behave as transcription factors (Borghi et al., 2007; Husbands et al., 2007). The KANADI and YABBY proteins along with the AUXIN RESPONSE FACTOR3 (ARF3) and ARF4 transcription factors, operate on the abaxial side of the leaf (Kerstetter et al., 2001; Eshed et al., 2004; Pekker et al., 2005), although the YABBY gene product appears to play a role in adaxial development as well (Nole-Wilson and Krizek, 2006). While it is generally believed that adaxial and abaxial factors act antagonistically in this network, little is known of the physical interactions among these proteins, if any, or of the set of transcriptionally regulated downstream target genes.
A subset of the transcription factors involved in the establishment of ad/abaxial polarity is controlled by small RNAs. mRNAs encoding the HD-ZIPIII factors are targeted by microRNAs 165 and 166 (Reinhart et al., 2002; Mallory et al., 2004; Kim et al., 2005; Williams et al., 2005b). The ARF3 and ARF4 loci are controlled by tasiRNAs. (tasiRNAs operate similarly to microRNAs but are generated through a distinct pathway involving double-stranded RNA rather than hairpin-containing transcripts) (Williams et al., 2005a; Fahlgren et al., 2006; Garcia et al., 2006; Hunter et al., 2006). Interestingly, both the mir165/166–HD-ZIPIII pathway and the tasi–ARF pathways are widely conserved in land plants (Floyd and Bowman, 2004; Axtell et al., 2007).
Here, we report the discovery of a new family of plant genes, the LITTLE ZIPPER (ZPR) genes, which are transcriptionally upregulated by HD-ZIPIII activity. The ZPR proteins consist principally of a stretch of leucine zipper similar to the leucine zipper in HD-ZIPIII proteins. Furthermore, we show that the ZPR proteins interact with and repress HD-ZIPIII activity, thus forming a negative feedback loop. The balance between HD-ZIPIII:HD-ZIPIII homodimers and HD-ZIPIII:ZPR heterodimers provides a potential point of regulation. We speculate that the START and PAS domains of the HD-ZIPIII protein may affect this balance through interaction with small molecule ligands or protein–protein interactions.
RESULTS
A System for Inducible HD-ZIPIII Function
To identify genes whose transcript levels are affected by HD-ZIPIII transcription factor activity, we generated an HD-ZIPIII protein with inducible activity. One of the five Arabidopsis HD-ZIPIII proteins, REV, was placed under glucocorticoid control by fusing the glucocorticoid receptor (GR) domain to its N terminus (Figure 1A). The GR domain prevents the GR-REV protein from acting in the absence of exogenously added glucocorticoid. The GR-REV gene fusion was placed under the control of the highly and constitutively expressed viral cauliflower mosaic virus 35S promoter (CaMV35S) promoter. Since the HD-ZIPIII genes are negatively controlled by microRNAs 165/166, it was also necessary to introduce mutations that disrupted the microRNA target sequence. Transgenic Arabidopsis seedlings carrying this construct, called 35S:GR-REVd, developed normal leaves in the absence of the glucocorticoid dexamethasone. However, when plate-grown seedlings were flushed with 50 micromolar dexamethasone for 5 min and then transplanted to soil, they developed adaxialized, trumpet-shaped leaves (Figure 1B). Thus, the 35S:GR-REVd fusion construct is capable of directing adaxial leaf development in response to dexamethasone treatment. Subsequent leaves developed normally (Figure 1C), indicating that HD-ZIPIII activity does not persist after this short dexamethasone treatment. (Similar constructs with the GR domain fused to the C terminus of the REV protein, or with a wild-type microRNA complementary site, did not cause adaxialization in this assay; data not shown).
Figure 1.
Dexamethasone-Inducible HD-ZIPIII Activity Leads to Leaf Adaxialization and Upregulation of a Family of Small Leucine Zipper Proteins.
(A) Diagram of the dexamethasone-inducible REV fusion protein. Transcription is driven by the widely expressed CaMV35S promoter. GR, glucocorticoid binding domain; HD, homeodomain; LZ, leucine zipper domain; START, domain with similarity to steroid binding domain; PAS, similar to PAS domain; MTS*, mutant microRNA target sequence.
(B) and (C) Two views of a seedling treated briefly (5 min) with dexamethasone (50 μM) 1 week after germination.
(B) Two leaves developed a trumpet shape with adaxial characters on the outside of the trumpet bell.
(C) Leaves that developed subsequently developed with normal ad/abaxial polarity. rlf, radial leaf; lf, normal leaf.
(D) Real-time RT-PCR shows increase in transcript levels of four ZPR genes following dexamethasone induction of 35S:GR-REVd transgenic plants. Seedlings were treated with dexamethasone for 2 h before tissue was harvested. Bars indicate se for at least three measurements (technical replicates). The experiment was repeated three times with similar results (data not shown).
(E) Real-time RT-PCR detection of ZPR3 mRNA in shoots from three self progeny (biological replicates) with strong abaxialized phenotypes from parents of the genotype phv; phb; rev/+. Bars indicate se for at least three measurements (technical replicates).
(F) Real-time RT-PCR of phb-d mutants (shoots from three pooled homozygotes) and phv-d mutants (shoots from two individuals [biological replicates]). Bars indicate se for at least three measurements (technical replicates).
Identification of ZPR Genes as Targets of REV Control
Using genome-wide transcriptional profiling, we screened for genes whose transcripts were rapidly upregulated following dexamethasone treatment of 35S:GR-REVd plants. RNA was harvested from 35S:GR-REVd and wild-type Landsberg erecta (Ler) plants after 1 h of dexamethasone treatment or after 1 h of mock treatment. The RNA was labeled and hybridized to Affymetrix microarrays. In this screen, At2g45450 (subsequently named ZPR1) was the most highly induced transcript in 35S:GR-REVd plants in the presence of dexamethasone (see Supplemental Table 1 online for levels of ZPR1 and ZPR3 relative to actin. Data for the entire experiment can be retrieved from the Gene Expression Omnibus repository (series record number GSE7003).
Although ZPR1 was annotated in the Arabidopsis database as encoding a chloroplast protein, more intensive comparison of the protein sequence showed that it encodes a leucine zipper–containing protein where the leucine zipper shows similarity to the leucine zipper in the REV protein (see below).
ZPR1 is one of four members of a family of small genes, only two of which are represented on the Affymetrix microarray (ZPR1 and ZPR3). To verify that ZPR1 is upregulated in response to increased HD-ZIPIII activity and to test the other three family members for induction, we performed quantitative RT-PCR experiments comparing RNA harvested from treated and untreated seedlings. We found that all four ZPR genes were upregulated in 35S:GR-REVd plants within 2 h of dexamethasone treatment (Figure 1D).
Expression of ZPR Genes in Planta Correlates with HD-ZIP Gene Activity
Analysis of ZPR gene expression in wild-type and mutant Arabidopsis plants supports the role of HD-ZIPIII genes in promoting ZPR transcriptional activation. Triple mutants with reduced function for three of five HD-ZIPIII genes, PHV, PHB, and REV, showed reduced levels of ZPR3 mRNA (Figure 1E). Conversely, gain-of-function PHV and PHB mutants (microRNA-resistant mutants) show increased levels of ZPR3 mRNA (Figure 1F). The dominant PHV mutant showed substantially higher levels of ZPR3 mRNA than the dominant PHB mutant. This was surprising to us because gain-of-function mutations in PHB cause a much more severe phenotype than gain-of-function mutations in PHV. One possible explanation is that the PHV HD-ZIPIII protein is a more potent activator of ZPR3 expression than the PHB HD-ZIPIII protein. Another possibility is that the PHV gain-of-function mutants are substantially healthier and closer to the wild type in their morphology than PHB gain-of-function mutants. For instance, phv-d mutant leaf blades and petioles are more well developed than those of phb-d. If these tissues are the site of ZPR3 transcription (ZPR3 is expressed in the petiole; see next section), then one would expect more ZPR transcription in phv-d plants.
In situ hybridization experiments show that ZPR3 is expressed within the domain known to express REV and are therefore consistent with HD-ZIPIII control of ZPR transcription. ZPR1 and ZPR3 mRNAs are expressed within the domain of HD-ZIPIII–expressing cells; both are expressed in the adaxial epidermis of the cotyledons and in the vascular cylinder of wild-type torpedo stage embryos (Figures 2A to 2D and 2G). In these experiments, we did not observe ZPR1 or ZPR3 expression in earlier stages (late globular to early torpedo) of cotyledon development.
Figure 2.
Analysis of Spatial Expression Patterns of ZPR1 and ZPR3.
(A) Wild-type torpedo stage embryo hybridized to antisense probe for ZPR1. Expression is principally seen in the L1, although it may extend into the subepidermal layer in some regions. Expression is typically patchy and does not necessarily extend throughout all adaxial epidermal cells.
(B) Wild-type torpedo stage embryo hybridized to antisense probe for ZPR3.
(C) Wild-type torpedo stage embryo hybridized to sense probe for ZPR1.
(D) Wild-type torpedo stage embryo hybridized to sense probe for ZPR3.
(E) Torpedo stage embryo expressing ZPR3-GUS reporter.
(F) Mature embryo expressing ZPR3-GUS reporter.
(G) Three torpedo stage embryos from one silique hybridized to the antisense probe for ZPR1 showing similar patterns of expression.
(H) Cross section through petiole of leaf expressing ZPR3-GUS. Expression (GUS activity) is seen in the medial epidermis and in cells adaxial to the midvein.
(I) Longitudinal section through the shoot apex of a plant expressing the ZPR3-GUS expression. Expression is found in the adaxial epidermis of young leaves.
Bars = 50 μm, except in (F) where it is 100 μm.
Because ZPR3 is expressed at very low levels, we constructed a reporter to analyze the spatial distribution of the ZPR3 protein. Therefore, we fused 2.3 kb of 5′ regulatory sequences and the ZPR3 coding sequence (including introns) in frame to the bacterial uidA gene (β-glucuronidase [GUS]). Similar to the in situ hybridization signal, the expression of this reporter was limited to the adaxial cotyledon domain and the vascular cylinder (Figures 2E and 2F; note that the dark outline surrounding the torpedo stage embryo in Figure 2E is a shadow around the whole-mount embryo and does not reflect GUS expression). We also examined expression of the GUS transgene in vegetatively growing plants (Figures 2H and 2I). Similar to our findings in cotyledons, expression was seen principally in the adaxial epidermis of leaves. Expression in subepidermal layers occurred in the area over the midvein. In the petiole, expression was seen more extensively in the adaxial domain.
Structure of ZPR Proteins Suggests a Model for Their Action
When ZPR1 amino acid sequence was used as a query in a psi-BLAST search (Altschul et al., 1997), it was found to contain a leucine zipper domain similar to the leucine zipper domain found in HD-ZIPIII proteins but not similar to the leucine zipper domain found in class I, II, or IV HD-ZIP proteins (Figures 3A and 3B). The ZPR proteins vary in the placement of the leucine zipper within the protein: the leucine zipper is central in ZPR1 and ZPR2 and N-terminal in ZPR3 and ZPR4 (Figure 3A). The ZPR proteins are widely conserved in the angiosperms with five homologs found in rice (Oryza sativa; see Supplemental Figure 1 online; Figure 3D). Inspection of the phylogenetic tree indicates that the difference between ZPR1/ZPR2 types and ZPR3/ZPR4 types of ZPR proteins predates the split of monocotyledonous (e.g., rice) and eudicotyledonous (e.g., Arabidopsis) plants.
Figure 3.
ZPR Family of Genes.
(A) Placement of leucine zipper (blue) in each of the four Arabidopsis ZPR proteins.
(B) Alignment of leucine zippers from the Arabidopsis REV (class III HD-ZIP), ZPR1 (At2g45450), ZPR2 (At3g60890), ZPR3 (At3g52770), ZPR4 (At2g36307), HD-ZIP1 (class I HD-ZIP protein), HD-ZIP2 (class II HD-ZIP), and GL2 (class IV HD-ZIP) proteins. REV and ZPR proteins have leucine zippers that are six heptads in length. The class IV HD-ZIP proteins have an insertion in the leucine zipper relative to the class III HD-ZIP proteins.
(C) Helical wheel model for interactions between the leucine zipper in the ZPR proteins and the REV protein. Proteins would be arranged in a parallel conformation. h1, h2, etc., indicate heptad 1, heptad 2, etc.
(D) Phylogenetic tree of all four Arabidopsis, all five rice, and two maize ZPR proteins. Alignment of the ZPR proteins was performed using the ClustalW algorithm. Bayesian phylogenetic analysis was conducted on the aligned protein data set using MrBayes version 3.1.2 (Huelsenbeck and Ronquist, 2001). Default settings were used, and the program was allowed to run for 100,000 generations. Trees were summarized after discarding the first 25,000 generations. The number above each branch corresponds to the posterior probability for that node.
(E) Model for a negative feedback loop between HD-ZIPIII proteins and ZPR proteins. Active HD-ZIPIII dimers induce the transcription of ZPR genes. The ZPR proteins dimerize with the HD-ZIPIII proteins, preventing them from forming homodimers and thus inactivating them. (In this model, we do not distinguish between active HD-ZIPIII dimers that are true homodimers, e.g., REV homodimers, or HD-ZIPIII dimers that consist of two types of HD-ZIPIII proteins, e.g., REV/PHB dimers. The extent to which mixed HD-ZIPIII dimers are important is not currently known.)
Leucine zippers are coiled-coil domains consisting of heptad repeats with a Leu residue in the “d” position (Landschulz et al., 1988). Each heptad is two turns of the α-helix. In plants, the related amino acids Ile and Met are often found in place of Leu residue at the “d” position (Deppmann et al., 2004). Amino acids at both the “a” and “d” positions are at the interhelical interface (Figure 3C). The “a” position is occupied by Asn in heptads two, three, four, and six in the leucine zippers of both ZPR and HD-ZIPIII proteins. Asn at the “a” position interacts favorably with Asn and unfavorably with aliphatic amino acids at the “a” position in the partner helix (Acharya et al., 2002). Based on the similar pattern of Asn residues at “a” positions in both the ZPR and HD-ZIPIII leucine zippers, the ZPR and HD-ZIPIII proteins have the potential to form parallel heterodimers with one another (Figure 3C).
Unlike many animal homeodomain proteins that bind DNA as monomers, HD-ZIP proteins bind DNA as dimers (Sessa et al., 1993). In fact, amino acid residues in the loop between helix one and helix two of the homeodomain in HD-ZIP proteins prevent binding of the homeodomain to DNA as a monomer (Tron et al., 2004). Hence, heterodimerization of ZPR proteins, which lack a homeodomain, with HD-ZIPIII proteins is predicted to either alter or abolish DNA binding of the HD-ZIPIII proteins. This prediction is clearest for the ZPR3/ZPR4 type of ZPR protein that lacks an extension N-terminal to the leucine zipper and is less clear for the ZPR1/ZPR2 type, which carries an extension N-terminal to the leucine zipper and could potentially contact the DNA backbone.
The structural features of the ZPR proteins, and their induction by HD-ZIPIII activity, suggest a model for the action of the ZPR genes as part of a negative feedback loop on HD-ZIPIII function (Figure 3E). In the proposed regulatory module, HD-ZIPIII proteins activate transcription of the ZPR genes. The ZPR proteins then form heterodimers with the HD-ZIPIII proteins, preventing or altering their DNA binding.
In Vitro Studies of the Interaction between REV and ZPR Proteins
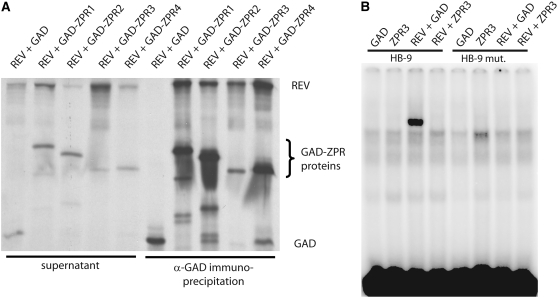
To test the model proposed above, we assayed the ability of the REV HD-ZIPIII protein to interact with ZPR proteins in vitro. ZPR proteins fused to the GAL4 activation domain (GAD) epitope and untagged REV proteins were synthesized in a cell-free system in the presence of 35S-Met (Figure 4A; see Supplemental Figure 2 online). Immunoprecipitation of any of the four GAD-ZPR proteins, using an anti-GAD antibody, from a mixture with REV precipitated the REV protein (Figure 4A). Immunoprecipitation of the GAD protein from a mixture with REV did not precipitate the REV protein. We conclude that the REV and ZPR proteins have the ability to physically interact in vitro. This experiment was repeated more than three times with similar results.
Figure 4.
ZPR Protein Interactions with REV.
(A) PAGE analysis of in vitro, 35S-synthesized proteins from supernatants (left) and pellets (right) of anti-GAD immunoprecipitates. GAD:ZPRx, in vitro–synthesized, 35S-labeled ZPRx protein with GAD domain fused to the N terminus; REV, in vitro–synthesized, 35S-labeled REV protein. The image of the SDS gel has been modified; it was stretched ∼20% in the direction of the y axes. The photo was cropped at the bottom; no bands below those shown were seen. The top of the gel photo was not cropped.
(B) Gel electrophoresis of 32P-labeled oligos containing an HD-ZIPIII DNA binding site (HB-9) and a mutated binding site (HB-9mut). In the presence of in vitro–synthesized REV protein, migration of the HB-9 oligonucleotide is retarded due to REV binding. This binding is abolished in the presence of ZPR3.
As a further test of the model, we asked whether ZPR3 could prevent DNA binding by REV (Figure 4B). REV protein in the presence of GAD protein bound to a radiolabeled oligonucleotide carrying one HD-ZIPIII binding site causing this oligonucleotide to migrate slower in an acrylamide gel. REV protein did not bind to a similar oligonucleotide in which the sequence was scrambled. In the presence of ZPR3, the ability of REV to bind DNA was abolished.
Overexpression of ZPR1 and ZPR3 Genes
If the ZPR proteins form heterodimers with HD-ZIPIII proteins and prevent DNA binding in vivo, we expect this interaction to result in inhibition of HD-ZIPIII function. To test this hypothesis, we overexpressed ZPR3 and ZPR1 in Arabidopsis from the viral CaMV35S promoter. Transgenic plants overexpressing ZPR3 did indeed show abaxialized leaf phenotypes (Figure 5). Strongly affected plants showed meristem termination with abaxialized rod-shaped leaves (Figure 5A). We also observed abaxialized, trumpet-shaped leaves and downward curled leaves (Figures 5B and 5C). Downward curled leaves are also seen when HD-ZIPIII gene activity is decreased by increased microRNA 166 levels (Williams et al., 2005b) and is opposite to the upward curling when HD-ZIPIII activity is increased (Mallory et al., 2004; Ochando et al., 2006). Leaves from 35S:ZPR3 plants have vasculature typical of abaxialized plants with phloem nearly surrounding xylem (Figures 5K and 5L). Transgenic plants overexpressing ZPR1 also made downward curled leaves (Figure 5D), but this phenotype was less penetrant than for plants overexpressing ZPR3 (downward curled leaves were seen in 2/12 35S:ZPR1 T1 plants compared with 6/13 T1 35S:ZPR3 plants). No trumpet-shaped leaves were seen in the 35S:ZPR1 transgenic plants (seen in 0/12 35S:ZPR1 T1 plants compared with 4/13 35S:ZPR3 T1 plants). Importantly, we did not see glabrous plants in either the 35S:ZPR1 or 35S:ZPR3 transformants. If ZPR proteins could bind to and inhibit the GL2 class IV HD-ZIP protein, this should have resulted in hairless plants.
Figure 5.
Overexpression of ZPR Proteins Causes Leaves to Develop Abaxialized Characters.
(A) ZPR3-overexpressing plant showing meristem termination phenotype.
(B) Abaxialized trumpet-shaped leaf from ZPR3-overexpressing plant.
(C) Leaf from ZPR3-overexpressing plant showing downward curling.
(D) Leaf from ZPR1-overexpressing plant viewed from below showing downward curling. White line points to curled under margin.
(E) Wild-type leaf showing nearly flat blade.
(F) Leaf from ZPR3-overexpressing plant with roughened surface and ridges along midrib. White line points to ridge.
(G) Section of cleared wild-type leaf blade showing vein pattern.
(H) Section of cleared leaf from ZPR3-overexpressing plant showing dense pattern of veins.
(I) Close-up of (G).
(J) Close-up of (H). Note proliferation of cells around veins.
(K) Section through wild-type petiole. Note that xylem is located adaxially, above the phloem.
(L) Section through petiole from ZPR3-overexpressing plant. Xylem is nearly surrounded by phloem. x, xylem; p, phloem.
Additional phenotypic similarities between mutants overexpressing ZPR3 and lacking HD-ZIPIII activity are seen in the leaf blade. 35S:ZPR3 plants make leaves with a roughened blade texture and ridges along the midvein and blade (Figure 5F). The roughened leaf with midvein ridges has been seen in mutants with reduced activity of three of the five HD-ZIPIII genes (e.g., phv; phb/+; rev mutants; Prigge et al., 2005). 35S:ZPR3 plants with rough blades showed an increased density of veins (Figure 5H). In addition, the veins were surrounded by ridges of small cells, indicating ectopic cellular proliferation (Figure 5J). These ridges were seen on the adaxial but not the abaxial surface. This extra proliferation on the adaxial surface may be, at least in part, responsible for the downward curling of the mutant leaf. In sum, overexpressing ZPR1 and ZPR3 causes leaf abnormalities similar to those seen when HD-ZIPIII function is reduced.
Mutants showing dramatically reduced levels of ZPR2 and ZPR3 mRNAs were isolated from publicly available T-DNA insertion collections (see Supplemental Figure 3 online). These mutants were indistinguishable from the wild type. Given the high degree of similarity between the ZPR proteins, this is likely due to redundancy between members of the ZPR gene family. Determination of the exact role these genes play in leaf development will therefore likely required the generation of appropriate double and perhaps higher-order mutant combinations.
DISCUSSION
Discovery of a Family of ZPR Genes
In this article, we have described the discovery of a small family of plant-specific ZPR genes that encode small proteins (67 to 105 amino acids long) that include a stretch of leucine zipper motifs. This stretch of leucine zipper is approximately six heptads in length and is similar to the leucine zipper found in the class III HD-ZIP proteins. In particular, the pattern of Asn residues at the “a” positions of the leucine zippers is similar to that of the HD-ZIPIII proteins, and this pattern predicts that the ZPR proteins and HD-ZIPIII proteins should heterodimerize.
Both the ZPR1/ZPR2 (central leucine zipper) and ZPR3/ZPR4 (N-terminal leucine zipper) types of proteins are present in rice and maize as well as Arabidopsis, indicating that the two types of ZPR genes diverged prior to the split between monocots and eudicots. Our searches of other plant databases have yet to turn up homologs of the ZPRs, indicating that they may not be present outside of angiosperm plants. However, we cannot rule out that their small size (making them difficult to annotate) and low abundance may have prevented us from detecting them. It is currently unclear whether the ZPR genes and the HD-ZIPIII genes share a common ancestor or whether their similar leucine zipper regions are examples of convergent evolution.
There are three additional classes of HD-ZIP proteins in addition to the class III type studied here. These are the class I, II, and IV types. While these all have leucine zippers, the leucine zipper motif is distinct in all four classes, and the ZPR proteins described here are not predicted to interact with them. However, it remains possible that these families are regulated by other families of as yet undiscovered leucine zipper–containing proteins.
Our work on this gene family was prompted by the discovery that ZPR1 transcript levels are upregulated by the REV protein, a potent regulator of adaxial leaf development. Increases in ZPR transcript levels were also seen in gain-of-function mutants for two other HD-ZIPIII genes (PHB and PHV), indicating that upregulation of ZPR transcript levels may be a more general feature of HD-ZIPIII function. Given the relatively rapid upregulation of ZPR transcript levels, it is possible that HD-ZIPIII transcription factors bind to DNA at the ZPR loci and act as direct transcriptional activators of these loci. However, evidence for a role of HD-ZIPIII transcription factors as direct activators of ZPR transcription awaits further experimentation.
Interestingly, for the two ZPR genes examined, expression begins well after HD-ZIPIII expression is first established in the embryo. Whereas expression of REV, PHB, and PHV is seen in the early globular embryo (McConnell et al., 2001; Prigge et al., 2005), expression of ZPR1 and ZPR3 is first detected well after the cotyledons are initiated. Even then, expression of these genes is in a more restricted domain than REV. While REV mRNA is found in the adaxial half of the cotyledons, ZPR mRNA is principally restricted to the epidermis and appears in patches rather than throughout the adaxial epidermis. These observations argue that the presence of HD-ZIPIII proteins alone is not sufficient to increase transcript levels of the ZPR1 and ZPR3 genes. Some additional condition must also be satisfied, such as removal of a repressor at the ZPR loci or a requirement for a cell specific cofactor.
A Negative Feedback Loop
The plant-specific HD-ZIP proteins differ from animal homeodomain proteins, such as the Drosophila ANTENNAPEDIA protein, in that they cannot bind DNA as dimers. Tron et al. (2004) identified the loop between helices two and three as the determinant of this difference. Given the requirement for dimerization for DNA binding, we propose that the ZPR proteins inhibit HD-ZIPIII DNA binding activity by forming heterodimers with them. We present three experiments that support this mode of action. First, ZPR proteins and REV interact in vitro. Second, in the presence of ZPR3 protein, the REV protein can no longer bind DNA in a gel shift assay. Third, when overexpressed, the ZPR1 and ZPR3 proteins cause abaxialization consistent with inhibition of HD-ZIPIII function. ZPR3 causes a stronger phenotype, in some cases resulting in the production of a rod-shaped leaf that terminates the shoot apical meristem. This may reflect a more potent inhibitory activity of the ZPR3/ZPR4 type of ZPR. The ZPR3/ZPR4 type lacks the N-terminal extension present in ZPR1 that might allow ZPR1/HD-ZIPIII dimers to bind DNA. Alternatively, ZPR3 protein may simply be more stable than ZPR1 protein.
What then is the role of this feedback loop in the wild-type plant? The wild-type phenotype of the single mutants isolated thus far indicates that these genes are likely to act redundantly to one another. It will likely be necessary to maker higher-order mutant combinations to see a phenotype. The relatively late onset of ZPR1 and ZPR3 expression indicates that at least these two ZPR genes play a role in adaxial leaf development that occurs after the initial establishment of polarity in the primordium. Likely roles include promoting vascular development and growth in the adaxial leaf domain by preventing the action of HD-ZIPIII proteins. Such late events in leaf development would likely be important for the fine-tuning of leaf development to prevailing environmental conditions.
The discovery of the ZPRs and the finding that HD-ZIPIII proteins have the potential to exist in two types of dimers, inactive heterodimers and active homodimers, adds a new point of potential regulation in which the ratio of inactive heterodimers to active homodimers could be controlled. Note that we use homodimers to refer to dimers made of two HD-ZIPIII proteins, whether these are dimers of one HD-ZIPIII (e.g., REV-REV dimer) protein or dimers of two different HD-ZIPIII proteins (e.g., REV-PHB dimer). The presence of a START domain in the HD-ZIPIII proteins has puzzled researchers because, although indicating that HD-ZIPIII activity is modulated by a small hydrophobic ligand, the role of such a ligand has eluded understanding. An attractive hypothesis that follows from the results presented here is that the ligand could control HD-ZIPIII activity by influencing the type of dimer that is formed, for instance by promoting or inhibiting the formation of either active HD-ZIPIII/HD-ZIPIII homodimers or inactive HD-ZIPIII/ZPR heterodimers. Chandler et al. (2007) have recently shown that the AP2-like transcription factors DORNROSCHEN and DORNROSCHEN-LIKE interact with the PAS domain of HD-ZIPIII proteins. It is also possible that such proteins could influence homodimerization versus heterodimerization.
The role of this feedback loop in leaf development adds a second layer of negative regulation of the HD-ZIPIIIs to that mediated by microRNAs 165 and 166. Whether the two negative regulatory loops function independently of one another or are in some way interconnected remains to be determined.
METHODS
Generation of 35S:GR-REVd Transgenic Plants
The N-terminal coding sequences of REV were amplified using PCR from the wild-type cDNA using primers REV 5′ fusion and HB8L2 with a combination of Pfu and Taq polymerases to produce a product with 3′ A overhangs. This gave the first 251 codons of the REV coding sequence and incorporated the ATG start codon into an NcoI site. This PCR product was cloned into pGEM T-EASY and cut out again with an EcoRI site in the vector polylinker and a SalI site in the REV coding sequence to isolate the 51 N-terminal-most codons. This fragment was then fused to each of three REV cDNAs; wild-type, G189D, and ∂miRNA, the latter two of which contain a gain-of-function mutation in the miR165/166 binding site as described by Emery et al. (2003). These cDNAs were then subcloned into the shuttle vector pART7 carrying a CaMV35S promoter followed by modified GR coding sequences (Wagner et al., 1999) ending in an NcoI site for in-frame fusion of the NcoI-REV cDNAs and followed by 3′ untranslated region sequences from ocs to provide a poly(A) signal. The entire 35S:GR:REV:ocs fusion was transferred into the pMLBART binary vector using two flanking NotI sites. Constructs were introduced into Agrobacterium tumefaciens strain GV3101 by electroporation and then into Arabidopsis thaliana Ler plants by floral dipping. Plasmids pART7 and pMLBART were obtained by J.E. from Bart Janssen and Kim Richardson (Horticultural and Food Research Institute of New Zealand, Auckland).
Microarray Experiment
Seedlings were grown in liquid culture, in 50 mL of Murashige and Skoog salts plus glucose in 250-mL flasks, 100 seeds per flask, two flasks per condition, with a total of eight flasks. Flasks were shaken gently on a rotator under fluorescent lights (24 h light, 22°C). Seeds were stratified at 4°C in water for 2 d prior to adding to culture medium. Four flasks were seeded with 35S:GR-REVd transgenics (in Ler), and four flasks were seeded with wild-type Ler. Ten days after the start of culturing, dexamethasone was added to two flasks of 35S:GR-REVd Ler plants and two flasks of Ler plants to a final concentration of 50 μg/mL. The other four flasks received only 0.1% ethanol (carrier). The flasks were allowed to incubate for 1 h after adding dexamethasone or mock treatment and then the medium was poured off and the seedlings briefly drained on paper towels, then wrapped in aluminum foil and flash-frozen in liquid nitrogen. One hundred milligrams of tissue was weighed out for each sample (while keeping them frozen) and RNA isolated by the Qiagen RNeasy plant mini prep kit. The data from this experiment have been deposited at the Gene Expression Omnibus repository (www.ncbi.nlm.nih.gov/geo/) under series record number GSE7003.
Histology
Petioles were fixed in 3% glutaraldehyde and 1.6% formaldehyde in 50 mM sodium phosphate buffer overnight at 4°C. Tissue was postfixed in 1% osmium tetroxide for 2 h, rinsed in phosphate buffer, dehydrated through an ethanol series, and embedded in Spurr's resin. One-micron sections were cut using a Leica microtome and stained with Toluidine Blue.
Real-Time RT-PCR
For RT-PCR, total RNA was isolated using the Qiagen RNeasy kit and DNaseI treated using the DNA-free kit from Ambion. Two micrograms of total RNA was then reverse-transcribed using the Ambion RetroScript kit with oligo(dT) primers. cDNA was diluted 10-fold, and 2 μL were used for RT-PCR reactions. PCR reactions were done using Dynamo reagents (Finnzyme). Real-time measurements of PCR product accumulation were done using a Bio-Rad Chromo 4 thermocycler and qBase software. Primers used are shown in Supplemental Table 2 online.
In Situ Hybridization
In situ hybridization experiments were done as by McConnell et al. (2001). Hybridization was at 52°C. Probes used for ZPR1 and ZPR3 corresponded to full-length cDNA sequences.
In Vitro Studies
Using primers harboring attB sites, all ZPR and REV cDNAs were amplified and subsequently recombined into pDONR201 using the BP II enzyme mix (Invitrogen). The ZPR cDNAs were then recombined into the pTNT:GAD vector (Wenkel et al., 2006) using the LR enzyme mix, providing an in-frame fusion to GAD. The cDNA of REV was introduced into pTNT, allowing the expression of the REV protein in vitro without epitope tag. Prey and bait proteins were synthesized in the presence of 35S-Met using the TNT quick coupled transcription/translation system (Promega). Coimmunoprecipitations were performed following the protocol described by Laubinger and Hoecker (2003). Gels were imaged using autoradiography.
For in vitro DNA binding assays, proteins were individually synthesized (for 45 min) using the Promega TNT T7 quick coupled transcription/translation system in the presence of nonradioactive Met. Proteins (REV + GAD; REV + ZPR3) were then mixed and incubated for another 45 min. Single-stranded oligonucleotides were denatured at 95°C for 10 min and then cooled to room temperature allowing the formation of a double strand. The DNA sequences of the duplexes were 5′-CAGATCTGTAATGATTACGAGAAT-3′ for the HB-9 oligonucleotide harboring the HD-ZIPIII binding site and 5′-CAGATCTGTATAATATACGAGAAT-3′ for the HB-9mut oligonucleotide in which the core nucleotides of the HD-ZIPIII binding site were altered.
Duplex DNA was end-labeled with T4 polynucleotide kinase and [γ-32P]ATP. DNA binding reactions were performed using the Promega gel shift assay system. TNT-produced protein mixes were incubated in gel-shift binding buffer for 1 h on ice. After adding the radiolabeled nucleotides, binding reactions were incubated at room temperature for 20 min and subsequently separated on 6% DNA retardation gels (Invitrogen), dried, and exposed to a phosphor imager screen.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: ZPR1, At2g45450; ZPR2, At3g60890; ZPR3, At3g52770; ZPR4, At2g36307; REV, At5g60690; PHB, At2g34710; and PHV, At1g30490.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Alignment of LITTLE ZIPPER Proteins from Arabidopsis, Maize, and Rice.
Supplemental Figure 2. SDS-PAGE of in Vitro–Synthesized Proteins Used for in Vitro Protein Binding Assays.
Supplemental Figure 3. Effect of T-DNA Insertions in LITTLE ZIPPER Genes.
Supplemental Table 1. Expression Levels of ZPR1 and ZPR3 after Dexamethasone Treatment.
Supplemental Table 2. Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank Doris Wagner (University of Pennsylvania) for the gift of the plasmid containing the glucocorticoid response sequences and Michael Prigge (University of Indiana) for the gift of the rev/+ phv phb line. Khar-Wai Lye provided excellent technical assistance. The work was supported by a grant from the National Institutes of Health (to M.K.B.) and a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (to S.W.; DFG WE4281/1-1).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: M.K. Barton (kbarton@stanford.edu).
Online version contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Acharya, A., Ruvinov, S., Gal, J., Moll, J.R., and Vinson, C. (2002). A heterodimerizing leucine zipper coiled coil system for examining the specificity of a position interactions: Amino acids I, V, L, N, A and K. Biochemistry 41 14122–14131. [DOI] [PubMed] [Google Scholar]
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso, K., Kato, M., Banks, J.A., and Hasebe, M. (1999). Characterization of homeodomain-leucine zipper genes in the fern Ceratopteris richardii and the evolution of the homeodomain-leucine zipper gene family in vascular plants. Mol. Biol. Evol. 16 544–552. [DOI] [PubMed] [Google Scholar]
- Axtell, M.J., Snyder, J.A., and Bartel, D. (2007). Common functions for diverse small RNAs of land plants. Plant Cell 19 1750–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baima, S., Nobili, F., Sessa, G., Lucchetti, S., Ruberti, I., and Morelli, G. (1995). The expression of the ATHB-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121 4171–4182. [DOI] [PubMed] [Google Scholar]
- Baima, S., Possenti, M., Metteuci, A., Wisman, E., Altamura, M.M., Ruberti, I., and Morelli, G. (2001). The Arabidopsis ATHB-8 HD-ZIP protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 126 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi, L., Bureau, M., and Simon, R. (2007). Arabidopsis JAGGED LATERAL ORGANS is expressed in boundaries and coordinates KNOX and PIN activity. Plant Cell 19 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, M.E., Barley, R., Curtis, M., Arroyo, J.M., Dunham, M., Hudson, A., and Martienssen, R.A. (2000). Asymmetric leaves 1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408 967–971. [DOI] [PubMed] [Google Scholar]
- Canales, C., Grigg, S., and Tsiantis, M. (2005). The formation and patterning of leaves: Recent advances. Planta 221 752–756. [DOI] [PubMed] [Google Scholar]
- Carraro, N., Peaucelle, A., Laufs, P., and Traas, J. (2006). Cell differentiation and organ initiation at the shoot apical meristem. Plant Mol. Biol. 60 811–826. [DOI] [PubMed] [Google Scholar]
- Chandler, J.W., Cole, M., Flier, A., Grewe, B., and Werr, W. (2007). The AP2 transcription factors DORNROSCHEN and DORNROSCHEN-LIKE redundantly control Arabidopsis embryo patterning via interaction with PHAVOLUTA. Development 134 1653–1662. [DOI] [PubMed] [Google Scholar]
- Deppmann, C.D., Acharya, A., Rishi, V., Wobbes, B., Smeekens, S., Taparowsky, E.J., and Vinson, C. (2004). Dimerization specificity of all 67 B-ZIP motifs in Arabidopsis thaliana: A comparison to Homo sapiens B-ZIP motifs. Nucleic Acids Res. 32 3435–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery, J.F., Floyd, S.K., Alvarez, J., Eshed, Y., Hawker, N.P., Izhaki, A., Baum, S.F., and Bowman, J.L. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13 1768–1774. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Izhaki, A., Baum, S.F., Floyd, S.K., and Bowman, J.L. (2004). Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131 2997–3006. [DOI] [PubMed] [Google Scholar]
- Fahlgren, N., Montgomery, T.A., Howell, M.D., Allen, E., Dvorak, S.K., Alexander, A.L., and Carrington, J.C. (2006). Regulation of AUXIN RESPONSE FACTOR 3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr. Biol. 16 939–944. [DOI] [PubMed] [Google Scholar]
- Floyd, S.K., and Bowman, J.L. (2004). Gene regulation: Ancient microRNA target sequences in plants. Nature 428 485–486. [DOI] [PubMed] [Google Scholar]
- Floyd, S.K., Zalewski, C.S., and Bowman, J.L. (2006). Evolution of class III homeodomain-leucine zipper genes in streptophytes. Genetics 173 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, D., Collier, S.A., Byrne, M.E., and Martienssen, R.A. (2006). Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr. Biol. 16 933–944. [DOI] [PubMed] [Google Scholar]
- Green, K.A., Prigge, M.J., Katzman, R.B., and Clark, S.E. (2005). CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell 17 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck, J.P., and Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17 754–755. [DOI] [PubMed] [Google Scholar]
- Hunter, C., Willmann, M.R., Wu, G., Yoshikawa, M., Gutierrez-Nava, M.L., and Poethig, R.S. (2006). Trans-acting siRNA-mediated repression of ETTIN and ARF4 regulates heteroblasty in Arabidopsis. Development 133 2973–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands, A., Bell, E.M., Shuai, B., Smith, H.M.S., and Springer, P.S. (2007). LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res. 35 6663–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakawa, H., Ueno, Y., Semiarti, E., Onouchi, H., Kojima, S., Tsukaya, H., Hasebe, M., Soma, T., Ikezaki, M., Machida, C., and Machida, Y. (2002). The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for the formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol. 43 467–478. [DOI] [PubMed] [Google Scholar]
- Juarez, M.T., Kul, J.S., Thomas, J., Heller, B.A., and Timmermans, M.C.P. (2004). MicroRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428 84–88. [DOI] [PubMed] [Google Scholar]
- Kang, J., Tang, J., Donnelly, P., and Dengler, N. (2003). Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytol. 158 443–454. [DOI] [PubMed] [Google Scholar]
- Kerstetter, R.A., Bollman, K., Taylor, R.A., Bomblies, K., and Poethig, R.S. (2001). KANADI regulates organ polarity in Arabidopsis. Nature 411 706–709. [DOI] [PubMed] [Google Scholar]
- Kim, J., Jung, J.-H., Reyes, J.L., Kim, Y.-S., Kim, S.-Y., Chung, K.-S., Kim, J.A., Lee, M., Lee, Y., Kim, V.N., Chua, N.-H., and Park, C.-M. (2005). microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 42 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz, W.H., Johnson, P.F., and McKnight, S.L. (1988). The leucine zipper: A hypothetical structure common to a new class of DNA binding proteins. Science 240 1759–1764. [DOI] [PubMed] [Google Scholar]
- Laubinger, S., and Hoecker, U. (2003). The SPA1-like proteins SPA3 and SPA4 repress photomorphogenesis in the light. Plant J. 35 373–385. [DOI] [PubMed] [Google Scholar]
- Lin, W.C., Shuai, B., and Springer, P.S. (2003). The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial-abaxial patterning. Plant Cell 15 2241–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory, A.C., Reinhart, B.J., Jones-Rhoades, M.W., Tang, G., Zamore, P.D., Barton, M.K., and Bartel, D.P. (2004). MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5′ region. EMBO J. 23 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell, J.R., and Barton, M.K. (1998). Leaf polarity and meristem formation in Arabidopsis. Development 125 2935–2942. [DOI] [PubMed] [Google Scholar]
- McConnell, J.R., Emery, J., Eshed, Y., Bao, N., Bowman, J., and Barton, M.K. (2001). Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411 709–713. [DOI] [PubMed] [Google Scholar]
- McHale, N.A., and Koning, R.E. (2004). MicroRNA-directed cleavage of Nicotiana sylvestris PHAVOLUTA mRNA regulates the vascular cambium and structure of apical meristems. Plant Cell 16 1730–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, K., and Burglin, T. (2006). MEKHLA, a novel domain with similarity to PAS domains is fused to plant HD-ZIPIII homeodomain proteins. Plant Physiol. 140 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nole-Wilson, S., and Krizek, B.A. (2006). AINTEGUMENTA contributes to organ polarity and regulates growth of lateral organs in combination with YABBY genes. Plant Physiol. 141 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochando, I., Jover-Gil, S., Ripoll, J.J., Candela, H., Vera, A., Ponce, M.R., Martinez-Laborda, A., and Micol, J.L. (2006). Mutations in the microRNA complementarity site of the INCURVATA4 gene perturb meristem function and adaxialize lateral organs in Arabidopsis. Plant Physiol. 141 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuga, D., DeGuzman, B., Prigge, M.J., Drews, G.N., and Clark, S.E. (2001). REVOLUTA regulates meristem initiation at lateral positions. Plant J. 25 223–236. [DOI] [PubMed] [Google Scholar]
- Pekker, I., Alvarez, J.P., and Eshed, Y. (2005). Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17 2899–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting, C.P., and Aravind, L. (1999). START: A lipid binding domain in StAR, HD-ZIP and signaling proteins. Trends Biochem. Sci. 24 130–132. [DOI] [PubMed] [Google Scholar]
- Prigge, M.J., and Clark, S.E. (2006). Evolution of the class III HD-ZIP gene family in land plants. Evol. Dev. 8 350–361. [DOI] [PubMed] [Google Scholar]
- Prigge, M.J., Otsuga, D., Alonso, J.M., Ecker, J.R., Drews, G.N., and Clark, S.E. (2005). Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe, O.J., Riechmann, J.L., and Zhang, J.Z. (2000). INTERFASCICULAR FIBERLESS1 is the same gene as REVOLUTA. Plant Cell 12 315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart, B.J., Weinstein, E.G., Rhoades, M.W., Bartel, B., and Bartel, D.P. (2002). MicroRNAs in plants. Genes Dev. 16 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti, I., Sessa, G., Lucchetti, S., and Morelli, G. (1991). A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 10 1787–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara, K., Nishiyama, T., Kato, M., and Hasebe, M. (2001). Isolation of homeodomain-leucine zipper genes from the moss Physcomitrella patens and the evolution of homeodomain-leucine zipper genes in land plants. Mol. Biol. Evol. 18 491–502. [DOI] [PubMed] [Google Scholar]
- Schena, M., and Davis, R.W. (1992). HD-Zip proteins: Members of an Arabidopsis homeodomain superfamily. Proc. Natl. Acad. Sci. USA 89 3894–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrick, K., Nguyen, D., Karlowski, W.M., and Mayer, K.F.X. (2004). START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 5 R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa, G., Carabelli, M., Ruberti, I., Lucchetti, S., Baima, S., and Morelli, G. (1994). Identification of distinct families of HD-ZIP proteins in Arabidopsis thaliana. In Molecular-Genetic Analysis of Plant Development and Metabolism, P. Puigdomenech and G. coruzzi, eds (Berlin: Springer), pp. 411–426.
- Sessa, G., Morelli, G., and Ruberti, I. (1993). The Athb-1 and -2 HD-ZIP domains homodimerize, forming complexes of different DNA binding specificities. EMBO J. 12 3507–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa, G., Morelli, G., and Ruberti, I. (1997). DNA-binding specificity of the homeodomain-leucine zipper domain. J. Mol. Biol. 274 303–309. [DOI] [PubMed] [Google Scholar]
- Sessa, G., Steindler, C., Morelli, G., and Ruberti, I. (1998). The Arabidopsis Athb-8, -9 and -14 genes are members of a small gene family coding for highly related HD-ZIP proteins. Plant Mol. Biol. 38 609–622. [DOI] [PubMed] [Google Scholar]
- Talbert, P.B., Alder, H.T., Parks, D.W., and Comai, L. (1995). The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development 121 2723–2735. [DOI] [PubMed] [Google Scholar]
- Tron, A.E., Bertoncini, C.W., Palena, C.M., Chan, R.L., and Gonzalez, D.H. (2001). Combinatorial interactions of two amino acids with a single base pair define target specificity in plant dimeric homeodomain proteins. Nucleic Acids Res. 29 4866–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron, A.E., Comelli, R.N., and Gonzalez, D.H. (2005). Structure of homeodomain-leucine zipper/DNA complexes studied using hydroxyl radical cleavage of DNA and methylation interference. Biochemistry 44 16796–16803. [DOI] [PubMed] [Google Scholar]
- Tron, A.E., Welchen, E., and Gonzalez, D.H. (2004). Engineering the loop region of a homeodomain-leucine zipper protein promotes efficient binding to a monomeric DNA binding site. Biochemistry 43 15845–15851. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Sablowski, R.W.M., and Meyerowitz, E.M. (1999). Transcriptional activation of APETALA1 by LEAFY. Science 285 582–584. [DOI] [PubMed] [Google Scholar]
- Waites, R., and Hudson, A. (1995). phantastica: A gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121 2143–2154. [Google Scholar]
- Wenkel, S., Turck, F., Singer, K., Gissot, L., Le Gourrierec, J., Samach, A., and Coupland, G. (2006). CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18 2971–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, L., Carles, C.C., Osmont, K.S., and Fletcher, J.C. (2005. a). A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proc. Natl. Acad. Sci. USA 102 9703–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, L., Grigg, S.P., Xie, M., Christensen, S., and Fletcher, J.C. (2005. b). Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 132 3657–3668. [DOI] [PubMed] [Google Scholar]
- Zhong, R., and Ye, Z.-H. (1999). IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell 11 2139–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.