Abstract
Background
Tuberculosis (TB) of the central nervous system (CNSTB) is associated with higher mortality rates than other forms of TB. Epidemiologic associations with and prognostic indicators of CNSTB have not been assessed in a large US population-based study.
Methods
Between 1995 and 2004 and using a population-based active surveillance study, we compared patients with CNSTB to patients with TB affecting sites other than CNS (non-CNSTB) with respect to sociodemographic, clinical and Mycobacterium tuberculosis genotype variables. Risk factors associated with mortality at 180 days were compared between the two groups.
Results
We enrolled 92 patients with CNSTB and 3,570 with non-CNSTB. HIV co-infection was present in 31 (33.7%) of the CNSTB cases. In a Cox proportion hazard model, we found that CNSTB patients who died at 180 days were more likely to be older (HR 1.06, 95% CI 1.02–1.10), have a positive MTB culture from a CNS source (HR 5.11, 95% CI 1.06–24.62) and have hydrocephalus (HR 10.62, 95% CI 3.28–34.36) than patients who survived CNSTB. HIV co-infection association with mortality was not statistically significant (HR 1.74, 95% CI 0.35–8.62).
Conclusions
In our cohort, hydrocephalus was the most important predictor of mortality post CNSTB diagnosis.
Keywords: tuberculosis, meningitis, epidemiology, mortality, CNS
The difference in the tuberculosis disease burden between the industrialized world and the rest of the globe is stark: while the disease incidence in the US is at an all-time low (4.60 cases per 100,000 population in 2006), one third of the earth’s population is infected with Mycobacterium tuberculosis (MTB) and more than 8 million individuals have tuberculous disease annually 1–3. Even within the US, there are important differences in the epidemiology of the disease between various states and cities. In 2005, the City of Houston, Texas had a TB case rate of 9.10 cases per 100,000 population (more than twice the national average) and within Houston, certain inner city neighborhoods had a TB incidence that reached up to 300/100,000 persons, as estimated by the kernel density estimation and geocoding analysis 4,5. Hence, efforts at the elimination of tuberculosis in the US would necessitate and benefit from detailed traditional and molecular epidemiologic analysis of TB in high incidence areas within the US, such as Houston, Harris County, Texas.
One of the most feared complications of TB is tuberculosis of the central nervous system (meningitis, tuberculomas, radiculomyelitis; collectively referred to as CNSTB) due to the higher mortality rates and the disabling neurological sequelae 6. The incidence of TB meningitis is relatively low: in 2005, only 1.3% and 1.6% of TB cases were classified as meningeal TB in the US and Texas, respectively 4. The TB resurgence in the US between 1985–1992 due to weakened public health infrastructure, increased immigration and the human immunodeficiency virus (HIV) epidemic has spurred interest and analyses of the epidemiologic characteristics of and mortality from TB meningitis (TBM) 7–9. In a study published in 1987, Ogawa et al. performed a retrospective review of 45 TBM cases diagnosed between 1968 and 1983 at a New York City medical center and found a mortality of 31% 10. Only one patient was known to have HIV co-infection. In 1996, and using similar methodologies, Yechoor et al found a mortality of 41% among 31 patients with TBM identified over a 12-year period in a medical center in Houston, Texas 11. HIV co-infection was present in 65% of these patients, but did not alter the clinical presentation of TBM. Porkert et al reviewed the medical records of 34 patients with culture-positive TBM between 1984 and 1995 and identified 34 patients with TBM in Atlanta, Georgia 12. HIV co-infection was present in 47% of these patients, but did not affect the clinical presentation or the mortality. The abovementioned studies reviewed retrospectively a relatively small number of patients due to a multitude of reasons: restricting the data collection to one medical center, the low incidence of TBM or the exclusion of culture-negative disease. These limitations have made it difficult to generalize the respective conclusions and to appreciate the impact of HIV on clinical presentation of and mortality from TBM. In Vietnam, Thwaites et al prospectively followed 528 adult TBM cases, 18.2% of whom were HIV co-infected, and found that co-infection with HIV was associated with decreased survival at 9 months (relative risk of death 2.91, 95% CI 2.14–3.96) 13. The discrepancy in these findings is possibly due to geographical differences translating into differences in healthcare standards and comorbidities; or due to the small sample size of the studies performed in the US.
In the present study, we use a population-based approach to investigate the traditional and molecular epidemiology of CNSTB in Harris County, Texas, as well as the risk factors associated with mortality following CNSTB diagnosis, including HIV co-infection.
Methods
In this 9-year cohort study (10/01/1995-09/30/2004), patients were selected from the Houston Tuberculosis Initiative (HTI) database. The HTI is a population-based, active surveillance and molecular epidemiology study of TB in Harris County, Texas. Patient interviews and medical record review were used to gather pertinent sociodemographic and clinical data from TB patients. All available isolates of MTB were genotyped using 3 methods: major genetic group designation, spoligotyping, and Restriction Fragment Length Polymorphism (RFLP) of the IS6110 insertion element 14–16. Between 10/1/1995 and 9/30/2004 the HTI has systematically enrolled approximately 85% of reported TB cases in Harris County. In addition, basic sociodemographic and clinical data were available on non-enrolled patients for comparison from public health surveillance data. The protocol was approved by the Institutional Review Board of the participating institutions and enrolled subjects (or proxy) were appropriately consented.
Definitions
CNSTB diagnosis
The diagnosis was ascertained from public health surveillance data supplemented by medical record review for enrolled subjects. Patients with clinical presentation compatible with meningitis or radiculomyelitis, cerebrospinal fluid (CSF) studies compatible with TB and one of the following: 1) positive culture for MTB from a CNS source (CSF, biopsy), 2) positive culture for MTB from a non-CNS source, 3) response to antituberculous therapy or 4) positive MTB polymerase chain reaction (PCR) from a CNS source, were considered to have tuberculosis of the central nervous system. Patients with CNSTB who were enrolled by HTI and reported between 10/01/1995 and 09/30/2004 were included in the analysis.
Control group
Patients with TB other than CNS (pulmonary and/or extrapulmonary), enrolled in the same time period in the HTI were included in the analysis as controls, and referred to as non-CNSTB.
Cluster
Strains with at least 5 IS6110 copies were considered to be clonally related (and part of the same “print” or “cluster”) if they have exact matching of their IS6110 RFLP patterns. Strains with fewer than 5 IS6110 copies were considered clustered if they had exactly matched IS6110 RFLP patterns, spoligotypes and major genetic group designations.
Statistical analysis
Enrolled CNSTB cases were compared to the control group with respect to sociodemographic, clinical and strain genotype variables using univariate logistic regression. A multivariate logistic model was constructed using the variables associated with CNSTB at a P value of ≤0.20. Variables that significantly limited the sample size or that showed a colinearity coefficient of 0.30 or more with another model covariate were not considered for inclusion in the base multivariate model. In the final model, P values of ≤0.05 were considered significant.
Results
Between 10/01/1995 and 09/30/2004, there were 108 cases of CNSTB reported in Houston of whom 92 (85.2%) were enrolled in the HTI; and 4,204 reported cases of non-CNSTB of whom 3,570 (84.9%) were enrolled in the HTI. Comparing enrolled and non-enrolled TB patients, we found enrollees were more likely to be African-American and of a pediatric age group but less likely to be Asian and foreign born (p<0.05). However, enrollees and non-enrollees did not significantly differ with regards to disease site (CNSTB vs non-CNSTB) or gender (Table 1). The reasons for non-enrollment included: patient refusal, patient relocating out of area and patient death with a lack of proxy. CNSTB diagnosis was ascertained by a positive MTB culture from a CNS source in 53 cases (57.6%), from a non-CNS source in 11 additional cases (12.0%), response to antituberculous treatment in 23 cases (25.0%) and positive PCR from CSF in four patients (4.3%). Concomitant pulmonary TB was diagnosed in 39 (42.4%) patients with CNSTB and at least one other site of extrapulmonary TB was identified for 16 (17.4%). The number of CNSTB cases per study year varied between 5 and 16 per year (1.4–3.4% of all TB cases in the study) with no significant decreasing or increasing incidence trend. The racial distribution of the CNSTB cases was as follows: 39 (42.4%) were African-Americans, 37 (40.3%) were Hispanic, 8 (8.7%) were Caucasians and 8 (8.7%) were Asians. Sixty-four (69.6%) of the CNSTB cases were male. The majority (67 patients, 72.8%) of CNSTB patients were US born, a proportion that did not significantly change during the 9 years of the study period (data not shown). HIV co-infection was present in 31 (33.7%) of the CNSTB cases, 44 (47.8%) were HIV seronegative and for 17 (18.5%) no HIV test results were available. The CSF findings were as follows: mean white blood cell count of 196 cells/microliter (median 131, range 0–675, n=43), mean glucose level of 45 mg/dl (median 36, range 2–163, n=44), mean protein level of 233 mg/dl (median 141, range 17–2,280, n=44), mean percentage of lymphocytes 60% (median 76%, range 3–98%, n=33).
Table 1.
Comparison of basic demographic characteristics between enrolled and non-enrolled subjects.
| ENROLLED PATIENTS (N=3,662) | NON-ENROLLED PATIENTS (N=650) | P VALUE | |
|---|---|---|---|
| CNS disease | 92 (2.5%) | 16 (2.5%) | 0.939 |
| Pediatric Age Group | 315 (8.6%) | 18 (2.8%) | <0.001 |
| Male Gender | 2,468 (67.4%) | 433 (66.6%) | 0.696 |
| Foreign Born | 1,349 (36.8%) | 289 (44.4%) | <0.001 |
| Race
Black Hispanic White Asian |
1,399 (38.2%) 565 (15.4%) 1,220 (33.3%) 463 (12.6%) |
210 (32.3%) 116 (17.8%) 197 (30.3%) 127 (19.5%) |
Referent 0.013 0.494 <0.001 |
Patients with CNSTB were compared to patients with non CNSTB in a univariate analysis with regard to demographic, clinical and MTB isolates’ genotype data (Table 2). In a multivariate logistic regression model we found the following variables to be independently associated with CNS-TB in our population: younger age (OR 0.97, 95% CI 0.96–0.98; analyzed in continuous form), HIV co-infection (OR 2.13, 95% CI 1.23–3.72), negative MTB culture (OR 2.18, 95% CI 1.30–3.64), and fever on presentation (OR 7.30, 95% CI 3.81–13.97). However, patients with CNSTB were less likely to present with sweats (OR 0.39, 95% CI 0.25–0.62) than non-CNSTB patients. Current smoking, prison history, and a history of liver disease were not significantly associated with CNSTB after controlling for the other model covariates. When the multivariate logistic model was restricted to persons with MTB genotype information, MTB isolates belonging to genetic group 3 (compared to genetic groups 1 or 2) were significantly less likely to be isolated from CNSTB patients than non CNSTB patients (OR 0.19, 95% CI 0.05–0.81).
Table 2.
Demographic, clinical and Mycobacterium tuberculosis genotypic characteristics of patients with CNS-TB and non-CNS TB in Houston, Texas 1995–2004
| CNS –TB (N=92) | NON-CNS TB (N=3,570) | P VALUE | |
|---|---|---|---|
| Age group
0–17 years old 18–59 years old ≥ 60 years old |
27 (29.4%) 60 (65.2%) 5 (5.4%) |
288 (8.1%) 2,691 (75.4%) 591 (16.6%) |
<0.001 Referent 0.038 |
| Age** | 32.5 (0–66) | 42 (0–93) | <0.001 |
| Male Gender | 64 (69.6%) | 2,404 (67.3%) | 0.651 |
| Race
Black Hispanic White Asian |
39 (42.4%) 8 (8.7%) 37 (40.2%) 8 (8.7%) |
1,360 (38.1%) 557 (15.6%) 1,183 (33.1%) 455 (12.7%) |
0.077 0.048 Referent 0.688 |
| Foreign born | 25 (27.2%) | 1,324 (37.1%) | 0.047 |
| Negative MTB culture | 28 (30.4%) | 569 (15.9%) | 0.001 |
| Print 12 MTB isolate *
Spoligotype 777776777760601 |
2 (3.4%) | 6 (0.2%) | 0.009 |
| MTB Genetic Group *
Group 1 Group 2 Group 3 |
19 (34.5%) 34 (61.8%) 2 (3.6%) |
853 (32.6%) 1,307 (50.0%) 455 (17.4%) |
Referent 0.592 0.030 |
| Prison history | 6 (6.5%) | 422 (11.8%) | 0.091 |
| Daily alcohol use | 24 (26.7%) | 1,199 (33.7%) | 0.156 |
| Current smoker | 27 (29.3%) | 1,485 (41.7%) | 0.016 |
| History of illicit drug use | 21 (22.8%) | 1,199 (33.6%) | 0.025 |
| Directly Observed Therapy | 74 (84.1%) | 3,031 (85.6%) | 0.690 |
| Symptoms on presentation
Cough Fatigue Fever Sweats Chest pain Headaches Altered mental status Nausea/vomiting Syncope Dizziness Changes in vision Seizure Stiff Neck |
39 (42.4%) 25 (27.5%) 79 (85.9%) 39 (42.4%) 7 (7.6%) 44 (47.8%) 39 (42.4%) 35 (38.0%) 2 (2.2%) 13 (14.1%) 9 (9.8%) 16 (17.4%) 14 (15.2%) |
2,613 (73.8%) 1,395 (39.5%) 2,072 (58.5%) 1,821 (51.5%) 903 (25.5%) 160 (4.5%) 50 (14.0%) 296 (8.3%) 16 (0.4%) 91 (2.6%) 17 (0.5%) 15 (0.4%) 15 (0.4%) |
<0.001 0.017 <0.001 0.084 <0.001 <0.001 <0.001 <0.001 0.082 <0.001 <0.001 <0.001 <0.001 |
| HIV co-infection | 31 (33.7%) | 583 (16.3%) | <0.001 |
| CD4 count within 90 days** | 82 (8–441) | 99.5 (1–1551) | 0.135 |
| HIV viral load
<400 copies 401–10,000 copies 10,001–100,000 copies 100,000–750,000 copies >750,000 copies |
2 (12.5%) 2 (12.5%) 3 (18.8%) 7 (43.8%) 2 (12.5%) |
24 (7.9%) 34 (11.1%) 69 (22.6%) 131 (43.0%) 47 (15.4%) |
0.514 0.752 0.982 0.781 Referent |
| Liver disease | 25 (27.2%) | 570 (16.0%) | 0.007 |
| Positive TB skin test | 39 (51.3%) | 2,279 (70.6%) | 0.001 |
| BCG vaccination | 15 (16.9%) | 932 (27.4%) | 0.018 |
| Days in hospital** | 24 (3–396) | 3 (0–784) | 0.004 |
| Death at 30 days after diagnosis | 9 (9.8%) | 143 (4.0%) | 0.018 |
| Death at 180 days after diagnosis | 16 (17.39%) | 304 (8.52%) | 0.008 |
| Completion of TB treatment | 56 (67.5%) | 2,868 (84.3%) | <0.001 |
Analysis performed only on patients with culture- positive TB from whom an MTB isolate was available for analysis (58 MTB isolates from CNS-TB and 2,749 isolates from non-CNS TB)
The variables were analyzed as continuous, the median (range) values are provided for clarification purposes.
HIV co-infection
HIV co-infection was present in a third (33.7%) of the patients with CNSTB in our population. We compared clinical, sociodemographic and MTB genotype characteristics in HIV-seronegative CNSTB and HIV-seropositive CNSTB patients. HIV-seropositive CNSTB patients were more likely to be older (median age 41 versus 25, p<0.001, ranges 23–61 and 0–66, respectively), male (83.9% vs 62.3% p=0.028), have a positive MTB culture from any source (83.9 % vs 62.3%, p=0.028), have liver comorbidities (67.7% vs 6.6% p<0.001), have altered mental status on presentation (67.7% vs 29.5% p<0.001) and have died within 180 days post-diagnosis (29.0% vs 11.5%, p=0.041) than HIV-seronegative CNSTB patients. CSF parameters, tuberculin skin test positivity (36.4% vs 57.4%, p=0.095), concomitant pulmonary disease (45.2% vs 41.0%, p=0.7), concomitant extrapulmonary disease (25.8% vs 13.1%, p=0.137) were not significantly different between HIV-seropositive and HIV-seronegative CNSTB patients, respectively.
Mortality of CNSTB
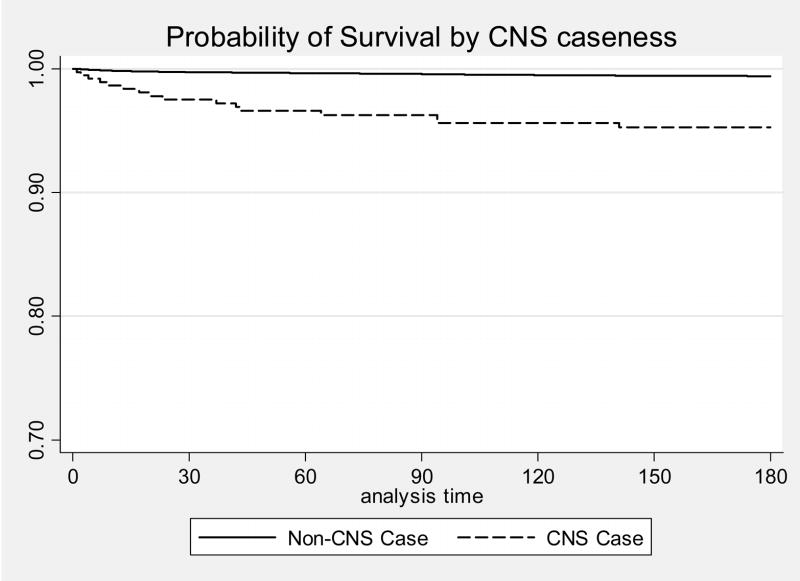
At 180 days post TB diagnosis, CNSTB patients were significantly more likely to die than non-CNSTB patients (17.4% vs 8.5%, p=0.006) (Figure 1). Among patients with TB (CNS and non-CNS disease combined) CNS disease and HIV co-infection were associated with increased mortality (HR 2.35, 95% CI 1.41–3.92 and HR 2.01, 95% CI 1.58–2.56 respectively) while pediatric age group was associated with decreased mortality at 180 days (HR 0.07. 95% CI 0.02–0.28) in a Cox proportion hazard model. Similar results were obtained when mortality data was analyzed at 30 days post diagnosis.
Figure 1.
Probability of survival by disease site: CNSTB vs non- CNSTB, adjusted for age and HIV status. Number of patients at risk: 3,662 patients at 0 day; 3,499 patients at 30 days; 3,431 patients at 60 days; 3,386 patients at 90 days; 3,322 patients at 120 days, 3,276 patients at 150 days, 3,232 patients at 180 days.
To examine prognostic factors associated with mortality among patients diagnosed with CNSTB, we compared demographic, clinical and MTB genotype variables between patients with CNSTB who died within 180 days and those who survived (Table 3). In a Cox proportion hazard model, we found that CNSTB patients who died were more likely to be older (HR 1.06, 95% CI 1.02–1.10), have a positive MTB culture from a CNS source (HR 5.11, 95% CI 1.06–24.62) and have hydrocephalus (HR 10.62, 95% CI 3.28–34.36) than patients who survived CNSTB. HIV co-infection, altered mental status, and pulmonary TB involvement were not significantly associated with an increased mortality among patients with CNSTB (HR 1.74, 95% CI 0.35–8.62, HR 1.30 95% CI 0.30–5.58, HR 0.78 95% CI 0.23–2.63, respectively) in the multivariate logistic model. There was one death and no HIV co-infected CNSTB cases in our pediatric cohort, so we performed an analysis restricted to adult CNSTB patients (18 years of age or older, n=65). Of 31 patients with HIV co-infection, 9 died (29.0%) and of 34 HIV seronegative patients, 6 died (17.6%) by 180 days. Among adults with CNSTB, age, HIV status, and altered mental status were not significantly associated with mortality in univariate Cox regression (p-values 0.45, 0.30, and 0.22, respectively). A Cox proportion hazard model showed that a positive MTB culture from CNS source (HR 6.04, 95% CI 1.34–27.31), hydrocephalus (14.71, 95% CI 4.10–52.79), and additional extrapulmonary site(s) of TB involvement (HR 4.80 95% CI 1.19–19.44) were predictive of mortality at 180 days, while concomitant pulmonary TB was not (HR 0.98, 95% CI 0.34–2.77). Information on HIV treatment during TB therapy was available on 19 co–infected patients: 11 patients received highly active antiretroviral therapy (HAART) of whom 1 (9.1%) died, and 8 patients did not receive HAART, of whom 3 (37.5%) died eventually.
Table 3.
Selected demographic and clinical characteristics of patients who died within 180 days after diagnosis of CNSTB and those who survived, Texas 1995–2004 (p≤0.2)
| DECEASED (N=16) | SURVIVORS (N=76) | P VALUE | |
|---|---|---|---|
| Age group
0–17 years old 18–59 years old ≥ 60 years old |
1 (6.3%) 13 (81.3%) 2 (12.5%) |
26 (34.2%) 47 (61.8%) 3 (3.9%) |
0.074 Referent 0.254 |
| Male | 11 (68.8%) | 53 (69.7%) | 0.958 |
| Race
Black Hispanic White Asian |
9 (56.3%) 5 (31.3%) 2 (12.5%) 0 |
30 (39.5%) 32 (42.1%) 6 (7.9%) 8 (10.5%) |
0.858 0.410 Referent - |
| HIV co-infection | 9 (56.3%) | 22 (28.9%) | 0.044 |
| Culture-negative disease | 1 (6.3%) | 27 (35.5%) | 0.010 |
| Days from symptoms to treatment* | 37 (3–802) | 29 (2–396) | 0.165 |
| Military service | 4 (25.0%) | 8 (10.5%) | 0.148 |
| High School Graduate | 11 (68.8%) | 28 (38.9%) | 0.040 |
| Men who have sex with men | 3 (18.8%) | 6 (8.3%) | 0.155 |
| Contact with known TB patient | 1 (6.3%) | 29 (38.2%) | 0.027 |
| Altered mental status | 10 (62.5%) | 29 (38.2%) | 0.078 |
| Positive MTB culture from CNS | 14 (87.5%) | 39 (51.3%) | 0.005 |
| Concomitant pulmonary TB | 9 (56.3%) | 30 (39.5%) | 0.261 |
| Concomitant extrapulmonary TB | 5 (31.3%) | 11 (14.5%) | 0.109 |
| Hydrocephalus | 9 (56.3%) | 17 (22.4%) | 0.007 |
The variables were analyzed as continuous, the median (range) values are provided.
Discussion
We have analyzed the epidemiology of TB of the central nervous system in Houston over a 9-year time period. The study was performed in a county-wide, population-based approach; thus minimizing inclusion bias. The number of CNSTB patients included in the study is 92 patients, the largest in any epidemiologic survey in the US. We have found that pediatric age group, HIV co-infection and culture negative disease to be significantly associated with CNS disease site; and that mortality among subjects with CNSTB was significantly associated with hydrocephalus, older age, positive MTB culture from a CNS source, but not HIV co-infection. One important limitation of our study is that disease grade was not included as a variable in the various models. This is due to the fact that upon the initial design of the study in 1995, a specific analysis of TBM was not accounted for.
HIV disease is associated with increased incidence, accelerated progression and more disseminated forms of TB 17–21. In Houston, HIV co-infection was present in 33.7% of all enrolled CNSTB patients, a frequency that is relatively lower than reported in the TB meningitis cases series from Atlanta (47.0%) or previously in Houston (65%). This discrepancy is likely due to differences in study populations: the first study was restricted to one medical center and to culture-positive disease and the second study was restricted to adult patients in one medical center 11–12. We found that HIV co-infection is independently associated with increased mortality among subjects with TB in general within 180 days, but the association of HIV co-infection with mortality among subjects with CNSTB did not reach statistical significance (HR 1.74, 95% CI 0.35–8.62). Also, the clinical presentation, including CSF parameters, was not significantly different between HIV-seropositive and HIV-seronegative CNSTB patients, except for altered mental status on presentation, which was significant in a univariate but not in a multivariate logistic model. A possible explanation is that both groups had evidence of additional anatomic sites of TB morbidity: concomitant pulmonary TB disease was present in 45.2% and 41.0% and concomitant extrapulmonary TB disease was present in 25.8% vs 13.1% of HIV seropositive and HIV seronegative CNSTB patients, respectively. Moreover, some CNSTB patients had other comorbidities (cancer (3), diabetes mellitus (8) and renal insufficiency (4)). Due to the increased morbidity in the CNSTB population as a whole, a potential added negative impact of HIV immunosuppression on mortality could not be appreciated in our study. Another potential influence on mortality from CNSTB in HIV co-infected patients is improved immunological status with highly active antiretroviral therapy (HAART) 22. Some of the patients in the study were on HAART, and a trend was found for reduced mortality in patients receiving HAART (9.1% vs 37.5%). However, this finding will need to be confirmed in studies with larger sample size. In Vietnam, a country of relatively high incidence of TB, Thwaites et al similarly found no impact of HIV disease on clinical presentation of TBM in a series of 528 patients with TBM, 18.2% of whom were co-infected with HIV 13. However, HIV negatively impacted survival: 64.6% of patients co-infected with HIV died vs 28.2% of HIV seronegative patients. Of note, the mortality observed in the Vietnam series is markedly higher than the one observed in our series, regardless of HIV serostatus, making it difficult to compare the findings from both studies. The difference may be related to differences in comorbidities, standards of care, drug resistance prevalence (40% resistance to at least 1 drug in Vietnam vs 7.9% in our cohort), access to care or delay in presentation 23, 24.
The more important predictor of mortality in our cohort was the development of hydrocephalus, which was found in 26 of 92 (28.3%) of our enrollees, regardless of the statistical modeling used (HR 10.62, 95% CI 3.28–34.36). Hydrocephalus is a common finding with TB meningitis on CT scans or MRI of the brain 25–27. It is due to impaired CSF resorption by the arachnoid villi (communicating hydrocephalus) or to obstruction of interventricular passages (obstructive hydrocephalus) 28, 29. In general, hydrocephalus is a prognostic indicator of poor outcome in other types of meningitis (bacterial and fungal) 30, 31. Tan et al and Ramos et al have found hydrocephalus to be an important predictor of morbidity and mortality in TBM, in smaller case series 32, 33. Various studies have evaluated the modalities of treatment of hydrocephalus in TBM, with variable results. Schoeman et al evaluated the clinical outcome of treating hydrocephalus in pediatric with 3 different medical regimens or shunting34. They found no difference in mortality or number of disabled survivors between groups. Mathew et al evaluated the clinical outcome of patients with TBM and hydrocephalus who underwent shunting if their disease is Grade 3 or shunting following clinical response to external drainage if they had Grade 4 disease. They found that the response to external drainage did not predict the response to shunting. The more important predictors of clinical outcome were the disease grade and the severity of hydrocephalus on presentation35. Steroid treatment in patients with TBM resulted in a reduction of mortality but not long-term neurologic morbidity 36. When serial magnetic resonance images were performed on patients receiving steroids or placebo, a non significant reduction in hydrocephalus at 60 days was observed among steroid recipients37. Taken together, the data suggest that hydrocephalus is a poor prognostic indicator and its treatment remains a major challenge. A pre-emptive approach of performing frequent LP on patients with increased opening pressure on initial lumbar puncture, similar to the one used in the management of cryptococcal meningitis, has not been widely studied, but the findings from this study and the abovementioned series warrant an investigation of this approach 38.
One epidemiologic finding of interest is that the majority of the CNSTB patients in our study were US-born individuals and the ratio of foreign born/US born did not change significantly throughout the study period of 9 years. This is in contradistinction to TB patients in the US, were foreign-born patients constitute an increasing proportion of the overall TB cases; underscoring the importance of analyzing the epidemiology of tuberculosis at a local in addition to the national levels 1,39.
In our study, MTB strains belonging to genetic group 3 were less likely to be isolated from CNSTB patients than groups 1 and 2. To our knowledge, this is a new finding pointing at the potential of particular strains of MTB to be relatively more or less neuroinvasive in humans than other strains. Confirmation of this finding and a study of the mechanisms that underlie this difference in pathogenesis would be of interest for future studies.
In conclusion, we found that while HIV is a risk factor for CNSTB it did not significantly alter the clinical presentation or mortality of the disease and that hydrocephalus remains the most important predictor of mortality from CNSTB.
Acknowledgments
The project has been funded with federal funds from The National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract N01-AO-02738.
Footnotes
Written informed consent was obtained from all subjects. Guidelines of the US Department of Health and Human Services and from the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals were followed in the conduct of the study.
The authors do not have commercial or other associations that might pose a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention (CDC) Trends in tuberculosis incidence-United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:245–50. [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3. [Last accessed May 1st, 2007]; Available at www.who.int/tb/publications/global_report/en/index.html.
- 4. [Last accessed on May 1st, 2007]; Available at www.cdc.gov/tb/surv/surv2005.
- 5.Stone ML. The utility of geographical information systems (GIS) and spatial analysis in tuberculosis surveillance in Harris County, Texas, 1995–1998 [Master’s thesis] Houston, TX: University of Texas - Houston School of Public Health; 2001. [Google Scholar]
- 6.Kennedy DH, Fallon RJ. Tuberculous meningitis. JAMA. 1979;241:264–268. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Tuberculosis morbidity-United States, 1992. MMWR Morb Mortal Wkly Rep. 1993;42:696–7. [PubMed] [Google Scholar]
- 8.Brudney K, Dobkin J. Resurgent tuberculosis in New York City: human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis. 1991;144:745–9. doi: 10.1164/ajrccm/144.4.745. [DOI] [PubMed] [Google Scholar]
- 9.Cantwell MF, Snider DE, Jr, Cauthen GM, Onorato IM. Epidemiology of tuberculosis in the United States, 1985 through 1992. JAMA. 1994;272:535–9. [PubMed] [Google Scholar]
- 10.Ogawa SK, Smith MA, Brennessel DJ, Lowy FD. Tuberculous meningitis in an urban medical center. Medicine. 1987;66:317–26. doi: 10.1097/00005792-198707000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Yechoor VK, Shandera WX, Rodriguez P, Cate TR. Tuberculous meningitis among adults with and without HIV infection. Experience in an urban public hospital. Arch Intern Med. 1996;156:1710–6. [PubMed] [Google Scholar]
- 12.Porkert MT, Sotir M, Parrott-Moore P, Blumberg HM. Tuberculous meningitis at a large inner-city medical center. Am J Med Sci. 1997;313:325–31. doi: 10.1097/00000441-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Thwaites GE, Bang ND, Dang NH. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with tuberculous meningitis. J Infect Dis. 2005;192:2134–41. doi: 10.1086/498220. [DOI] [PubMed] [Google Scholar]
- 14.Sreevastan S, Pan X, Stockbauer KE, et al. Restricted structural gene polymorphism in the M. tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Embden JDA, Cave MD, Crawford JT, et al. Strain identification of M. tuberculosis by DNA fingerprinting: recommendations for a standard methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamerbeek J, Schouls L, Kolk A, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray JF. Cursed duet: HIV infection and tuberculosis. Respiration. 1990;57:210–20. doi: 10.1159/000195844. [DOI] [PubMed] [Google Scholar]
- 18.Daley CL, Small PM, Schecter GF. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. N Engl J Med. 1992;36:231–5. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 19.Di Perri G, Danzi MC, DeChecchi G. Nosocomial epidemic of active tuberculosis among HIV-infected patients. Lancet. 1989;2:1502–04. [PubMed] [Google Scholar]
- 20.Reider HL, Snider DE, Cauthen GM. Extrpulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141:347–51. doi: 10.1164/ajrccm/141.2.347. [DOI] [PubMed] [Google Scholar]
- 21.Shafer RW, Kim DS, Weiss JP, et al. Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection. Medicine. 1991;70:384–97. doi: 10.1097/00005792-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Autran B. Effects of antiretroviral therapy on immune reconstitution. Antivir Ther. 1999;4S:3–6. [PubMed] [Google Scholar]
- 23.Thwaites GE, Chau TT, Caws M, et al. Isoniazid resistance, mycobacterial genotype and outcome in Vietnamese adults with tuberculous meningitis. Int J Tuberc Lung Dis. 2002;6:865–71. [PubMed] [Google Scholar]
- 24.Thwaites GE, Lan NT, Dung NH, et al. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J Infect Dis. 2005;192:79–88. doi: 10.1086/430616. [DOI] [PubMed] [Google Scholar]
- 25.Clark WC, Metcalf JC, Muhlbauer MS, Dohan FC, Robertson JH. Mycobacterium tuberculosis meningitis: a report of twelve cases and a literature review. Neurosurgery. 1986;18:604–10. doi: 10.1227/00006123-198605000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Wallace RC, Burton EM, Barrett FF, Leggiadro RJ, Gerald BE, Lasater OE. Intracranial tuberculosis in children: CT appearance and clinical outcome. Pediatr Radiol. 1991;21:241–6. doi: 10.1007/BF02018612. [DOI] [PubMed] [Google Scholar]
- 27.Gupta RK, Gupta S, Singh D, Sharma B, Kohil A, Gujral RB. MR imaging and angiography in tuberculous meningitis. Neuroradiology. 1994;6:87–92. doi: 10.1007/BF00588066. [DOI] [PubMed] [Google Scholar]
- 28.Bullock MRR, Welchman JM. Diagnostic and prognostic features of tuberculous meningitis on CT scanning. J Neurol Neurosurg Psychiatry. 1982;45:1098–101. doi: 10.1136/jnnp.45.12.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman PK, Cumming WJK, Foster JB. Hydrocephalus and tuberculous meningitis in adults. J Neurol Neurosurg Psychiatry. 1980;43:188–90. doi: 10.1136/jnnp.43.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang KW, Chang WN, Chang HW, Wang HC, Lu CH. Clinical relevance of hydrocephalus in bacterial meningitis in adults. Surg Neurol. 2005;64:61–5. doi: 10.1016/j.surneu.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Arsura EL, Johnson R, Penrose J, et al. Neuroimaging as a guide to predict outcomes for patients with coccidioidal meningitis. Clin Infect Dis. 2005;40:624–7. doi: 10.1086/427215. [DOI] [PubMed] [Google Scholar]
- 32.Ramos JM, Esteban J, Fernandez-Guerrero ML, Soriano F. Tuberculous meningitis: prognostic aspects of 22 microbiologically confirmed cases. Enferm Infecc Microbiol Clin. 1995;13:12–6. [PubMed] [Google Scholar]
- 33.Tan EK, Chee MW, Chan LL, Lee YL. Culture positive tuberculous meningitis: clinical indicators of poor prognosis. Clin Neurol Neurosurg. 1999;101:157–60. doi: 10.1016/s0303-8467(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 34.Schoeman J, Donald P, van Zyl L, Keet M, Wait J. Tuberculous hydrocephalus: a comparison of different treatments with regard to ICP, ventricular size and clinical outcome. Dev Med child Neurol. 1991;33:396–405. doi: 10.1111/j.1469-8749.1991.tb14899.x. [DOI] [PubMed] [Google Scholar]
- 35.Mathew JM, Rajshekhar V, Chandy MJ. Shunt surgery in poor grade patients with tuberculous meningitis and hydrocephalus: effects of response to external ventricular drainage and other variables on long term outcome. J Neurol Neursurg Psychiatry. 1998;65:115–8. doi: 10.1136/jnnp.65.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–51. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 37.Thwaites GE, Macmullen-Price J, Tran TH, et al. Serial MRI to determine the effect of dexamethasone in the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol. 2007;6:230–6. doi: 10.1016/S1474-4422(07)70034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710–8. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 39.Kain KP, Haley CA, Armstrong LM, et al. Tuberculosis among foreign-born persons in the United States: achieving tuberculosis elimination. Am J Respir Crit Care Med. 2007;175:75–9. doi: 10.1164/rccm.200608-1178OC. [DOI] [PubMed] [Google Scholar]