Introduction
Comparative Oncology is an approach that has recently gained significant prominence in both the lay and scientific press.1-3 Comparative Oncology refers to the discipline that integrates the naturally occurring cancers seen in our veterinary patients into more general studies of cancer biology and therapy. This includes the study of cancer pathogenesis (i.e. the study of cancer-associated genes and proteins) and the study of new treatment options for the management of cancer.4 By nature this approach provides novel opportunities for current and future veterinary and human cancer patients. Although a number of veterinary species, including the cat, horse and ferret, develop cancers that are of comparative interest, the majority of both the scientific and clinical effort has thus far focused on the dog.5, 6 This is due to the strong anatomic and physiologic similarities between dogs and humans, their long use as a toxicological model in drug development and, most importantly, the sheer number of dogs that are diagnosed and managed with cancer annually.1, 4, 7-11 The state of the comparative oncology field will be outlined in this chapter with an emphasis on cancer in dogs.
Problem of Cancer in Dogs
The problem of cancer in dogs is a serious challenge that we face as veterinarians. It is estimated that one in four dogs greater than 2 years of age will die of cancer and certain very popular breeds are over-represented in terms of cancer incidence and mortality.1, 4, 7 The prevalence of cancer in dogs has increased in recent years. This may be the result of an actual increase in cancer incidence, an increase in the population of dogs at risk for the development of cancer, and/or the awareness and interest in the pet-owning community to pursue diagnostic and treatment options. Advances in the care of animals has allowed dogs to live longer due to better nutrition, vaccination for common infectious diseases, leash laws that limit automobile deaths and the availability of more sophisticated diagnostics and treatments for many ailments previously considered to be life-threatening. The improved general health of pets has resulted in an increase in age-related diseases, including cancer. Aware of the treatment options available for two-legged family members, pet owners now demand advanced care options for four-legged family members diagnosed with cancer. This includes all traditional treatment modalities such as surgery, radiation therapy, immunotherapy and chemotherapy and novel investigational drugs available through participation in clinical trials.4, 12-29
Comparative Advantage
Cancer in dogs shares with human cancer many features, including histological appearance, tumor genetics, molecular targets, biological behavior and response to conventional therapies (Figure 1).1-4, 18, 30-47 Significantly, cancer develops naturally in dogs within the environment they share with their human owners. Tumor initiation and progression are influenced by the similar factors including age, nutrition, sex, reproductive status, and environmental exposures.9, 48 The spectrum of cancers seen in dogs is as diverse as that seen in human patients. Some histologies of comparative interest include osteosarcoma, melanoma, non-Hodgkin’s lymphoma, leukemia, prostate carcinoma, mammary carcinoma, lung carcinoma, head and neck carcinomas, soft tissue sarcomas and bladder carcinoma.
Figure 1. Advantages of Comparative Oncology.
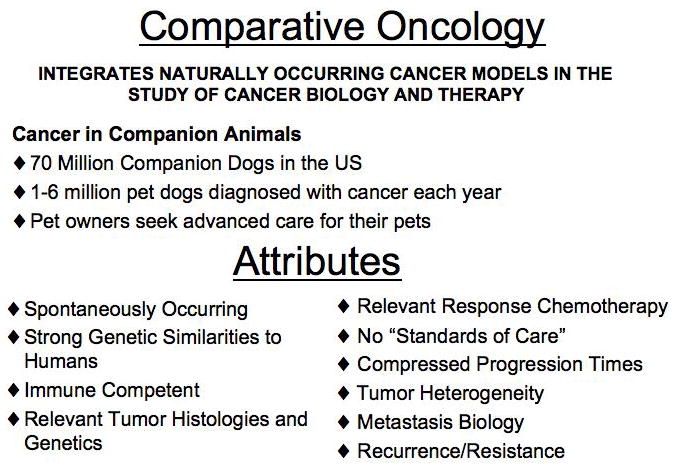
Cancer is a prevalent disease in dogs. The pet owning public is highly motivated to seek advanced care for their pets and are interested in both traditional and experimental therapies. Comparative Oncology aims to utilize the dog as a sophisticated model for the study cancer biology and therapy. Attributes of this opportunity are numerous. Cancer in dogs naturally share many of the genetic aberrations, oncogene over expression and tumor suppressor loss seen in the human disease. This provides a platform for the evaluation of target biology. Importantly pet dogs with cancer capture the complexity of cancer by representing tumor heterogeneity both within individual tumors and between patients with the same diagnosis, in a way impossible in traditional research models. Thus allowing for the study of metastasis biology, disease recurrence and resistance patterns in true clinical patients, corresponding to the key elements of the problem of cancer in man.
More important than histologic appearance, the basic biology of cancer in dogs is similar to human cancers. Most, if not all, of the cancer associated genetic alterations that influence cancer progression in humans have been identified in canine cancer.3, 7, 35, 37, 39, 41, 43-45, 49, 50 Many of the chemotherapy protocols used in veterinary medicine were originally co-opted from protocols used to treat human patients and have a similar activity spectrum.4 For example, the same chemotherapeutics that are active in canine lymphoma are those active in human lymphoma (i.e. vincristine, cyclophosphamide, doxorubicin, mitoxantrone, cytarabine arabinoside, methotrexate). 4 The opposite is also true; the drugs not as helpful in canine lymphoma are also not as helpful human lymphoma (i.e. gemcitabine, cisplatin, carboplatin).4 Similar parallels have been seen in investigative and targeted therapeutics. The biological complexity of cancers in pet animals mirrors that of the human disease based largely on the intra-tumoral (cell-to-cell) heterogeneity seen in these cancers. Natural consequences of this heterogeneity are the deadly features of all cancers, which include acquired resistance, recurrence, and metastasis.1, 2, 4, 10, 32, 51, 52 In these ways, companion animal cancers capture the “essence” of the problem of cancer in ways not seen in other animal model systems.
A Window of Opportunity
The opportunity to expand the scope of questions asked and answered through a comparative oncology approach has been the result of the completion of the recent canine genome sequence and resultant technologies generated using this genetic information. The efforts of the Canine Genome Project have resulted in the 2005 public release of a high-quality sequence covering 99% of the canine genome (2.5 billion base pairs).32, 34 Interrogation of the genome sequence suggests that all ~19,000 genes identified in the dog match to a similar or orthologous gene in the human genome.32, 34 Thus, the genome of the dog and human are similar enough to suggest that information learnt about one species can be transferred to and applicable to the other. The information provided by the canine genome sequence has become increasingly usable to veterinarians and research scientists through reductions in the costs for development of scientific tools.33, 53, 54 For example, oligonucleotide microarrays have been available for the study gene expression in human and murine tissues for several years. This technology allows the assessment of thousands and soon millions of gene segments within a single tissue in the matter of hours. The availability of a well-described canine genome has now led to the development of commercially available canine expression microarrays. Therefore veterinary cancers can be increasingly described in the same “language” as their human counterparts. This infrastructure provides the ability to conduct detailed and biologically intensive studies in canine cancer, not previously possible, which can evaluate target genes/proteins and pathways important in cancer biology, study their changes after exposure to new cancer therapies (described as pharmacodynamics) and connect the changes in these cancer targets to successful therapy.
The exploratory tools now available for the study of canine cancer have also been useful in studying the causes of both human and canine caners. Unfortunately, dogs of all breeds, including the ever-popular mixed breed, develop cancer. However, it has been known for many years that there are some breeds that have a higher incidence of cancer. This has been an emphasis of research funding by the Morris Animal Foundation (MAF) and the American Kennel Club-Canine Health Foundation (AKCCHF).7, 51 Some over-represented breeds include the Boxer for mast cell tumors, Rottweilers and Greyhounds for osteosarcoma, Golden retrievers for lymphoma, Scottish terriers for transitional cell carcinoma of the bladder, Flat-coated retrievers and Bernese mountain dogs for histiocytic sarcomas and Chow Chows for melanoma.4, 32, 49, 55 Interestingly, decreased cancer incidence has also been reported in some breeds as well. Studying breeds with increased cancer incidence is potentially informative because the breed lines of most dogs are known with historically well-documented pedigrees. Predisposed breeds provide the platform to readily identify genes known to be linked to cancer development (i.e. oncogenes) and those whose loss trigger cancer development or progression (i.e. tumor suppressor genes). Several genetic alterations and molecular signaling pathways known to be important in human cancers have been defined and shown to be relevant in canine cancers.3, 7, 35, 37, 39, 41, 43-45, 50 The genetic similarities between dogs within a breed may allow more rapid progress in the identification of new cancer-associated genes than the study of human or mouse cancers alone.
Opportunities Provided to Pets by the Comparative Approach
Clinical trials in veterinary oncology are increasing in number and scope. Attributes of the comparative approach are a considerable reason for this increase as they provide a unique opportunity to integrate studies that include dogs with cancer into the development path of new cancer drugs.3, 13, 14, 16-19, 22, 24, 27, 38, 56 These new drugs may be used in dogs with cancer prior to or during their study in human patients. The ability to gather serial biopsies from tumors and repeated fluid collections (serum, plasma, whole blood, urine) from the same patient during exposure to an investigational agent can answer complex questions about how best to use drugs that cannot be answered from tumor measurements alone (Figure 2). This serial sampling allows for the identification of tumor and surrogate markers of drug activity or target modulation, pharmacodynamics endpoints, which can be uniquely correlated to response in ways that are often not feasible in traditional preclinical rodent studies or in human cancer trials.
Figure 2. Comparative Oncology Focused Clinical Trial Design.
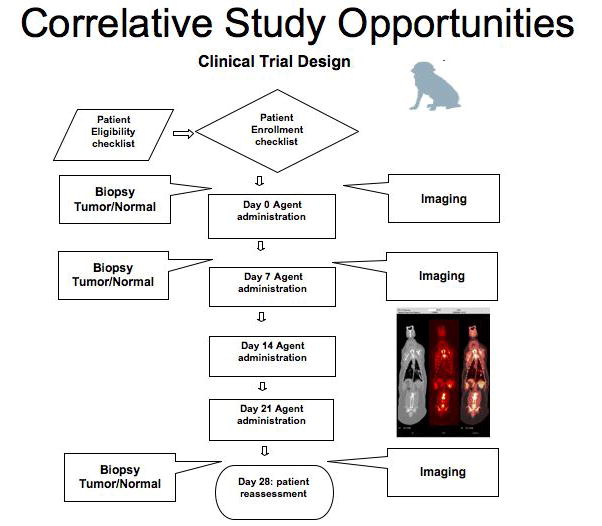
Comparative Oncology clinical trial designs focus on answering specific questions important for optimal drug development. The opportunity for serial biopsy of tumor, normal tissues, and other biofluids before, during and after exposure to new agents is readily incorporated into study designs. Biological evaluation of these tissues/samples can now include gene expression analysis, SNP and CGH array genomics, and all protein based analyses common in human studies. These evaluations can occur in parallel with advanced imaging studies including CT, MR, PET and PET/CT. Thus these studies allow for the unique correlation of drug exposure to tissue and fluid biomarkers and dynamic imaging endpoints. The anticipation is that studies in pet dogs will improve the efficiency of toxicological assessment and also have the potential to evaluate biology and activity in a natural occurring tumor model.
Interest from the human cancer drug development industry is based on a need for more reliable ways to evaluate new cancer drugs and the strong similarities established between veterinary and human cancers.57, 58 Because there are no treatment standards for the management of cancer in dogs, there is an added opportunity to provide pet owners and their dogs with access to novel therapeutics earlier in the course of disease and before treatment with conventional chemotherapy as compared to human patients participating in early phase human cancer trials. Beyond the access to new and potentially effective treatments for cancer, most clinical trials involve targeted cancer treatments that are less likely to be associated with side effects than conventional treatment. Furthermore, trials often provide significant financial support to study participants. As such, studies are particularly appealing to populations of clients who would otherwise forego traditional cancer treatments. The naturally shorter life span of our patients also permits the more rapid completion of clinical trials of novel agents that can assess outcome within a 6-18 month window, again impossible in human cancer trials. The benefits of such clinical trials in dogs will include earlier assessment of drug activity and toxicity critical to the design of more informed future veterinary and human clinical trials.
A number of cooperative groups exist to study cancer in dogs. These organizations are made up of veterinary oncologists, surgeons, geneticists, basic scientists and general practitioners who wish to understand the causes of cancer in dogs, seek improved treatments and utilize the dog as a comparative model. The American College of Veterinary Internal Medicine (www.acvim.org) is the body responsible for training board-certified veterinary specialists in medical oncology; the American College of Veterinary Radiology (www.acvr.org) trains individuals to become boarded in radiation oncology. The Veterinary Cancer Society (VCS; www.vetcancersociety.org) founded in 1977 is an organization focused on education and the sharing of scientific knowledge within the veterinary oncology community. VCS along with the Veterinary Co-operative Oncology group (VCOG) have encouraged multi-center collaborative studies that have largely been retrospective in nature. In 2006 the Comparative Oncology and Genomics Consortium (CCOGC; ccr.cancer.gov/resources/cop/scientists/resource_genomics.asp) Inc., a new not-for-profit entity was established. The CCOGC consists of a broad representation of parties focused on the genetics and biology of cancer naturally in dogs. A primary effort of the CCOGC has been the development of a canine cancer biospecimen repository that can provide materials for large-scale studies of canine cancer biology.
The Comparative Oncology Program (COP; ccr.cancer.gov/resources/cop) of the National Cancer Institute was developed in 2003 and has established a multi-center collaborative network of academic comparative oncology programs known as the Comparative Oncology Trials Consortium (COTC). The COTC is made up of 14 veterinary teaching hospitals and the goal of this effort is to conduct well-organized and focused clinical trials that provide biologically rich answers to the cancer therapeutic development pathway. These trials emphasize pharmacokinetic and pharmacodynamic endpoints, correlating drug exposure to modulation of tumoral markers and defining their relationship to activity. The first two COTC trials were conducted at seven different institutions. COTC001 involved systemic delivery of a targeted phage carrying the gene for TNF-α, a known potent cytotoxic and antiangiogenic agent, that has been difficult to safely administer in the past. Data from a preliminary study showed that this novel delivery method could effectively target tumor vascular and spare normal organs while identifying a safely tolerable dose. This data was used to design a second study where the agent was given once weekly and its effect on response measured. COTC001 was illustrative of the benefits of the comparative approach as the drug’s target was unique to tumor vascular. Pet dogs with cancer were a necessary model to demonstrate the targeting specificity of this agent within a naturally heterogeneous tumor environment. This model provided the ability to fully evaluate the potential toxicities and efficacy of this drug, which would not have been equally achieved in more traditional research models. Information from this trial is currently directing the development path of this drug for human cancer patients. COTC003 involves the evaluation of Rapamycin in dogs with osteosarcoma, a drug which inhibits an important oncogenic pathway called mTOR that is upregulated in many tumor types. Again the approach in the first phase of study is to define a dose that may be safely administered to dogs that is capable of effectively inhibiting the activated mTOR pathway within the tumor and hopefully correlating to a secondary blood marker of this activity. A follow up study in dogs will measure Rapamycin’s benefit as a treatment for metastatic osteosarcoma. COTC003 provides an example of the benefits of serial tissue sampling allowing for evaluation of a target pathway before and after exposure to a new drug. This type of information is vital to designing more successful second phase treatment trials in canine and human cancer patients and is impossible to uniformly accomplish in trials in man. Also both phases of this study will be complete in less than 1 ½ years, much faster than the comparable human trials with analogues of this drug. This provides an opportunity for early and simultaneous reporting of canine data and subsequent integration of pertinent findings within the ongoing human clinical trials. If effective, this drug holds promise for future development in both species. As illustrated by both of these trials, information provided by COTC studies aims to improve the drug development pathway by answering critical questions in how best to use novel agents for the treatment of cancer in dogs and man.
All of the multi-center efforts described emphasize collaborative science to further integrate comparative oncology into mainstream studies of cancer biology. Most clinical trials are conducted through academic veterinary teaching hospitals or referrals centers, but increasingly include direct involvement from general practitioners. Engaging general practitioners in the conduct of comparative oncology trials is essential to their success. This encourages more robust patient accrual, compliant client participation and more accurate outcome and toxicity reporting.
Conclusions
The value of Comparative Oncology has been increasingly recognized in the field of cancer research including, the identification of cancer-associated genes, the study of environmental risk factors, tumor biology and progression, and perhaps most importantly the valuation of novel cancer therapeutics. Like all innovations, it is important to define where the comparative oncology approach should and should not be used. This will continuously be defined on an agent or target basis and evaluated as this approach is employed in the future. It is expected that the fruits of this effort will be the creation of better and more specific drugs to benefit both veterinary and human cancer patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khanna C, Lindblad-Toh K, Vail D, et al. The dog as a cancer model. Nat Biotechnol. 2006 Sep;24(9):1065–1066. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- 2.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18(8):781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 3.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer Apr. 2004;40(6):858–880. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Withrow SJ, Vail DM. Withrow & MacEwen’s small animal clinical oncology. 4. St. Louis, Mo: Saunders Elsevier; 2007. [Google Scholar]
- 5.Zappulli V, De Zan G, Cardazzo B, Bargelloni L, Castagnaro M. Feline mammary tumours in comparative oncology. J Dairy Res. 2005;72:Spec No:98–106. doi: 10.1017/s0022029905001263. [DOI] [PubMed] [Google Scholar]
- 6.Antinoff N, Hahn K. Ferret oncology: diseases, diagnostics, and therapeutics. Vet Clin North Am Exot Anim Pract. 2004 Sep;7(3):579–625. vi. doi: 10.1016/j.cvex.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Olson PN. Using the canine genome to cure cancer and other diseases. Theriogenology. 2007 May 9; doi: 10.1016/j.theriogenology.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Naylor RJ, Rudd JA. Mechanisms of chemotherapy/radiotherapy-induced emesis in animal models. Oncology. 1996 Jun;53(Suppl 1):8–17. doi: 10.1159/000227634. [DOI] [PubMed] [Google Scholar]
- 9.Bukowski JA, Wartenberg D, Goldschmidt M. Environmental causes for sinonasal cancers in pet dogs, and their usefulness as sentinels of indoor cancer risk. J Toxicol Environ Health A. 1998 Aug 7;54(7):579–591. doi: 10.1080/009841098158719. [DOI] [PubMed] [Google Scholar]
- 10.Hahn KA, Bravo L, Adams WH, Frazier DL. Naturally occurring tumors in dogs as comparative models for cancer therapy research. In Vivo. 1994 Jan-Feb;8(1):133–143. [PubMed] [Google Scholar]
- 11.Mutsaers AJ, Widmer WR, Knapp DW. Canine transitional cell carcinoma. J Vet Intern Med. 2003 Mar-Apr;17(2):136–144. doi: 10.1892/0891-6640(2003)017<0136:ctcc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Rusk A, McKeegan E, Haviv F, Majest S, Henkin J, Khanna C. Preclinical evaluation of antiangiogenic thrombospondin-1 peptide mimetics, ABT-526 and ABT-510, in companion dogs with naturally occurring cancers. Clin Cancer Res. 2006 Dec 15;12(24):7444–7455. doi: 10.1158/1078-0432.CCR-06-0109. [DOI] [PubMed] [Google Scholar]
- 13.Rusk A, Cozzi E, Stebbins M, et al. Cooperative activity of cytotoxic chemotherapy with antiangiogenic thrombospondin-I peptides, ABT-526 in pet dogs with relapsed lymphoma. Clin Cancer Res. 2006 Dec 15;12(24):7456–7464. doi: 10.1158/1078-0432.CCR-06-0110. [DOI] [PubMed] [Google Scholar]
- 14.Thamm DH, Kurzman ID, King I, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res. 2005 Jul 1;11(13):4827–4834. doi: 10.1158/1078-0432.CCR-04-2510. [DOI] [PubMed] [Google Scholar]
- 15.Buchholz J, Kaser-Hotz B, Khan T, et al. Optimizing photodynamic therapy: in vivo pharmacokinetics of liposomal meta-(tetrahydroxyphenyl)chlorin in feline squamous cell carcinoma. Clin Cancer Res. 2005 Oct 15;11(20):7538–7544. doi: 10.1158/1078-0432.CCR-05-0490. [DOI] [PubMed] [Google Scholar]
- 16.Vail DM, Amantea MA, Colbern GT, Martin FJ, Hilger RA, Working PK. Pegylated liposomal doxorubicin: proof of principle using preclinical animal models and pharmacokinetic studies. Semin Oncol. 2004 Dec;31(6 Suppl 13):16–35. doi: 10.1053/j.seminoncol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 17.London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003 Jul;9(7):2755–2768. [PubMed] [Google Scholar]
- 18.Khanna C, Vail DM. Targeting the lung: preclinical and comparative evaluation of anticancer aerosols in dogs with naturally occurring cancers. Curr Cancer Drug Targets. 2003 Aug;3(4):265–273. doi: 10.2174/1568009033481903. [DOI] [PubMed] [Google Scholar]
- 19.Khanna C, Prehn J, Hayden D, et al. A randomized controlled trial of octreotide pamoate long-acting release and carboplatin versus carboplatin alone in dogs with naturally occurring osteosarcoma: evaluation of insulin-like growth factor suppression and chemotherapy. Clin Cancer Res. 2002 Jul;8(7):2406–2412. [PubMed] [Google Scholar]
- 20.MacEwen EG, Kurzman ID, Vail DM, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide, and granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999 Dec;5(12):4249–4258. [PubMed] [Google Scholar]
- 21.Hershey AE, Kurzman ID, Forrest LJ, et al. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res. 1999 Sep;5(9):2653–2659. [PubMed] [Google Scholar]
- 22.Khanna C, Waldrep JC, Anderson PM, et al. Nebulized interleukin 2 liposomes: aerosol characteristics and biodistribution. J Pharm Pharmacol. 1997 Oct;49(10):960–971. doi: 10.1111/j.2042-7158.1997.tb06024.x. [DOI] [PubMed] [Google Scholar]
- 23.Vail DM, MacEwen EG, Kurzman ID, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: a randomized multi-institutional clinical trial. Clin Cancer Res. 1995 Oct;1(10):1165–1170. [PubMed] [Google Scholar]
- 24.Kurzman ID, MacEwen EG, Rosenthal RC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. 1995 Dec;1(12):1595–1601. [PubMed] [Google Scholar]
- 25.Kurzman ID, Cheng H, MacEwen EG. Effect of liposome-muramyl tripeptide combined with recombinant canine granulocyte colony-stimulating factor on canine monocyte activity. Cancer Biother. 1994 Summer;9(2):113–121. doi: 10.1089/cbr.1994.9.113. [DOI] [PubMed] [Google Scholar]
- 26.Withrow SJ, Thrall DE, Straw RC, et al. Intra-arterial cisplatin with or without radiation in limb-sparing for canine osteosarcoma. Cancer. 1993 Apr 15;71(8):2484–2490. doi: 10.1002/1097-0142(19930415)71:8<2484::aid-cncr2820710810>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.LaRue SM, Withrow SJ, Powers BE, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc. 1989 Dec 15;195(12):1734–1744. [PubMed] [Google Scholar]
- 28.Elmslie RE, Dow SW. Genetic immunotherapy for cancer. Semin Vet Med Surg (Small Anim) 1997 Aug;12(3):193–205. doi: 10.1016/s1096-2867(97)80033-3. [DOI] [PubMed] [Google Scholar]
- 29.Dow SW, Potter TA. Expression of bacterial superantigen genes in mice induces localized mononuclear cell inflammatory responses. J Clin Invest. 1997 Jun 1;99(11):2616–2624. doi: 10.1172/JCI119450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutter NB, Bustamante CD, Chase K, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007 Apr 6;316(5821):112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick DJ, Fitzgerald SD, Sesterhenn IA, Davis CJ, Kiupel M. Classification of canine urinary bladder urothelial tumours based on the World Health Organization/International Society of Urological Pathology consensus classification. J Comp Pathol. 2006 Nov;135(4):190–199. doi: 10.1016/j.jcpa.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Ostrander EA, Giger U, Lindblad-Toh K. The dog and its genome. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2006. [Google Scholar]
- 33.Thomas R, Scott A, Langford CF, et al. Construction of a 2-Mb resolution BAC microarray for CGH analysis of canine tumors. Genome Res. 2005 Dec;15(12):1831–1837. doi: 10.1101/gr.3825705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005 Dec 8;438(7069):803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 35.Lee CH, Kim WH, Lim JH, Kang MS, Kim DY, Kweon OK. Mutation and overexpression of p53 as a prognostic factor in canine mammary tumors. J Vet Sci. 2004 Mar;5(1):63–69. [PubMed] [Google Scholar]
- 36.Jones CL, Grahn RA, Chien MB, Lyons LA, London CA. Detection of c-kit mutations in canine mast cell tumors using fluorescent polyacrylamide gel electrophoresis. J Vet Diagn Invest. 2004 Mar;16(2):95–100. doi: 10.1177/104063870401600201. [DOI] [PubMed] [Google Scholar]
- 37.Thomas R, Smith KC, Ostrander EA, Galibert F, Breen M. Chromosome aberrations in canine multicentric lymphomas detected with comparative genomic hybridisation and a panel of single locus probes. Br J Cancer. 2003 Oct 20;89(8):1530–1537. doi: 10.1038/sj.bjc.6601275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pryer NK, Lee LB, Zadovaskaya R, et al. Proof of target for SU11654: inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. 2003 Nov 15;9(15):5729–5734. [PubMed] [Google Scholar]
- 39.Lingaas F, Comstock KE, Kirkness EF, et al. A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German Shepherd dog. Hum Mol Genet. 2003 Dec 1;12(23):3043–3053. doi: 10.1093/hmg/ddg336. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki K, Yamagami T, Nomura K, Narama I. Mast cell tumors of the gastrointestinal tract in 39 dogs. Vet Pathol. 2002 Sep;39(5):557–564. doi: 10.1354/vp.39-5-557. [DOI] [PubMed] [Google Scholar]
- 41.Lee CH, Kweon OK. Mutations of p53 tumor suppressor gene in spontaneous canine mammary tumors. J Vet Sci. 2002 Dec;3(4):321–325. [PubMed] [Google Scholar]
- 42.Wakui S, Muto T, Yokoo K, et al. Prognostic status of p53 gene mutation in canine mammary carcinoma. Anticancer Res. 2001 Jan-Feb;21(1B):611–616. [PubMed] [Google Scholar]
- 43.Setoguchi A, Sakai T, Okuda M, et al. Aberrations of the p53 tumor suppressor gene in various tumors in dogs. Am J Vet Res. 2001 Mar;62(3):433–439. doi: 10.2460/ajvr.2001.62.433. [DOI] [PubMed] [Google Scholar]
- 44.Reguera MJ, Rabanal RM, Puigdemont A, Ferrer L. Canine mast cell tumors express stem cell factor receptor. Am J Dermatopathol. 2000 Feb;22(1):49–54. doi: 10.1097/00000372-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 45.London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp Hematol. 1999 Apr;27(4):689–697. doi: 10.1016/s0301-472x(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 46.Van Leeuwen IS, Hellmen E, Cornelisse CJ, Van den Burgh B, Rutteman GR. P53 mutations in mammary tumor cell lines and corresponding tumor tissues in the dog. Anticancer Res. 1996 Nov-Dec;16(6B):3737–3744. [PubMed] [Google Scholar]
- 47.London CA, Kisseberth WC, Galli SJ, Geissler EN, Helfand SC. Expression of stem cell factor receptor (c-kit) by the malignant mast cells from spontaneous canine mast cell tumours. J Comp Pathol. 1996 Nov;115(4):399–414. doi: 10.1016/s0021-9975(96)80074-0. [DOI] [PubMed] [Google Scholar]
- 48.Hayes HM, Jr, Fraumeni JF., Jr Epidemiological features of canine renal neoplasms. Cancer Res. 1977 Aug;37(8 Pt 1):2553–2556. [PubMed] [Google Scholar]
- 49.Modiano JF, Breen M, Burnett RC, et al. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005 Jul 1;65(13):5654–5661. doi: 10.1158/0008-5472.CAN-04-4613. [DOI] [PubMed] [Google Scholar]
- 50.Kiupel M, Webster JD, Kaneene JB, Miller R, Yuzbasiyan-Gurkan V. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. 2004 Jul;41(4):371–377. doi: 10.1354/vp.41-4-371. [DOI] [PubMed] [Google Scholar]
- 51.Olson PN. Fighting Cancer in Dogs. JAVMA. 2007 May 1;230(9):1280–1297. [Google Scholar]
- 52.Porrello A, Cardelli P, Spugnini EP. Oncology of companion animals as a model for humans. an overview of tumor histotypes. J Exp Clin Cancer Res. 2006 Mar;25(1):97–105. [PubMed] [Google Scholar]
- 53.Lana S, Plaza S, Hampe K, Burnett R, Avery AC. Diagnosis of mediastinal masses in dogs by flow cytometry. J Vet Intern Med. 2006 Sep-Oct;20(5):1161–1165. doi: 10.1892/0891-6640(2006)20[1161:dommid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Lana SE, Jackson TL, Burnett RC, Morley PS, Avery AC. Utility of polymerase chain reaction for analysis of antigen receptor rearrangement in staging and predicting prognosis in dogs with lymphoma. J Vet Intern Med. 2006 Mar-Apr;20(2):329–334. doi: 10.1892/0891-6640(2006)20[329:uopcrf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Proschowsky HF, Rugbjerg H, Ersboll AK. Mortality of purebred and mixed-breed dogs in Denmark. Prev Vet Med. 2003 Apr 30;58(12):63–74. doi: 10.1016/s0167-5877(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 56.MacEwen EG, Kurzman ID, Helfand S, et al. Current studies of liposome muramyl tripeptide (CGP 19835A lipid) therapy for metastasis in spontaneous tumors: a progress review. J Drug Target. 1994;2(5):391–396. doi: 10.3109/10611869408996814. [DOI] [PubMed] [Google Scholar]
- 57.Kamb A, Wee S, Lengauer C. Why is cancer drug discovery so difficult? Nat Rev Drug Discov. 2007 Feb;6(2):115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 58.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004 Aug;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]


