Abstract
The rapid evaluation of complex visual environments is critical for an organism's adaptation and survival. Previous studies have shown that emotionally significant visual scenes, both pleasant and unpleasant, elicit a larger late positive wave in the event-related brain potential (ERP) than emotionally neutral pictures. The purpose of the present study was to examine whether neuroelectric responses elicited by complex pictures discriminate between specific, biologically relevant contents of the visual scene and to determine how early in the picture processing this discrimination occurs. Subjects (n=264) viewed 55 color slides differing in both scene content and emotional significance. No categorical judgments or responses were required. Consistent with previous studies, we found that emotionally arousing pictures, regardless of their content, produce a larger late positive wave than neutral pictures. However, when pictures were further categorized by content, anterior ERP components in a time window between 200−600 ms following stimulus onset showed a high selectivity for pictures with erotic content compared to other pictures regardless of their emotional valence (pleasant, neutral, and unpleasant) or emotional arousal. The divergence of ERPs elicited by erotic and non-erotic contents started at 185 ms post-stimulus in the fronto-central midline regions, with a later onset in parietal regions. This rapid, selective, and content-specific processing of erotic materials and its dissociation from other pictures (including emotionally positive pictures) suggests the existence of a specialized neural network for prioritized processing of a distinct category of biologically relevant stimuli with high adaptive and evolutionary significance.
Keywords: pictures, visual processing, emotion, content selectivity, ERP
1. Introduction
In everyday life, organisms must make decisions based on a rapid analysis of the constantly changing visual environment. From an evolutionary perspective, the ability to rapidly determine the biological relevance of a visual scene is an important condition of survival. Since the main factors of natural selection are differences in fertility and mortality, it can be assumed that evolutionary processes have shaped neural mechanisms for efficient discrimination of visual stimuli directly relevant to reproduction (e.g. erotica) and/or survival (e.g. physical threat). Such mechanisms must involve a rapid automatic categorization of the contents of complex natural scenes that can vary tremendously with respect to their composition, complexity, and physical characteristics.
It has been argued recently that the use of natural, biologically relevant visual stimuli (in addition to “artificial,” easily parametrized stimuli) is vital for advancing our understanding of visual processing (Felsen and Dan, 2005). Other studies underscore the importance of using emotionally evocative natural images for the understanding of motivational and emotional processing in the human brain (Lang et al., 1998, Cuthbert et al., 2000). In humans, neural processing of motivationally relevant information has been studied using the presentation of slides depicting objects and scenes of different emotional and motivational content (e.g. Lang et al., 1998). Standard ratings of two fundamental dimensions of emotion - valence and arousal — have been obtained for a set of such pictures (Lang et al., 1999). Studies suggest that such images engage appetitive and aversive motivational systems and induce positive and negative emotions, as indicated by subjective reports, modification of the startle response, and autonomic responses (Lang et al., 1998). Within the groups of pleasant and unpleasant pictures, skin conductance and startle response changes paralleled arousal ratings (Bradley et al., 2001a). The largest modification of electrodermal and startle responses was produced by pictures of erotica and threat, i.e. the most highly arousing pleasant and unpleasant contents, that are directly related to the primary biological imperative of survival (Bradley et al., 2001a).
In an fMRI study, motivationally significant pictures (with both pleasant and unpleasant content) produced a greater activation of the visual cortex compared with neutral images (Bradley et al., 2003). Consistent with the fMRI data, studies using event-related potentials (ERPs) showed that emotionally arousing pleasant and unpleasant pictures elicited enlarged late positive potentials (LPPs) compared to neutral images (Cuthbert et al., 2000). These potentials developed at about 300 ms post-picture onset, lasted for several seconds, and were considered to reflect increased perceptual demands and allocation of attentional resources due to the intrinsic emotional relevance of such stimuli (Cuthbert et al., 2000, Schupp et al., 2004a). Keil et al. (2001) reported an enhancement of the N1 and P1 amplitudes for affectively arousing stimuli compared to neutral stimuli. Subsequent studies by this group using steady-state visual evoked potentials (Keil et al., 2005) and neuromagnetic responses (Moratti et al., 2004) have demonstrated the amplification of steady-state responses by affective stimuli compared to neutral stimuli, suggesting a modulation of spatiotemporal processing along the ventral visual stream by top-down influences from higher-order attentional networks. In other studies using briefly presented affective pictures (Schupp et al., 2003, Schupp et al., 2004b) and high-speed presentation of affective pictures (rapid serial visual processing paradigm, RSVP) (Junghofer et al., 2001) emotional pictures produced a negative ERP shift over temporo-occipital leads that started developing at 150 ms after picture onset. The maximum difference between emotional and neutral pictures was reached at about 300 ms. This effect, termed early posterior negativity (EPN), increased with the arousal level of the emotional pictures (Schupp et al., 2004b). It was suggested that the emotional significance of visual stimuli facilitates stimulus encoding at early levels of sensory processing due to implicit reflexive attention to such stimuli (Schupp et al., 2003). The strongest effect was found for pictures with erotic content among pleasant pictures and for pictures depicting physical threat among unpleasant pictures (Schupp et al., 2004a). Importantly, the responses to both pleasant and unpleasant images were larger than responses to neutral images, suggesting that both ERP effects (late positive wave and early posterior negativity) reflect the overall extent of emotional arousal, but not the valence of emotion (positive vs. negative). In summary, the results of ERP studies suggest that neuroelectric responses are sensitive to the motivational relevance of the stimulus, regardless of its emotional valence. These results were interpreted in terms of “motivated attention” to stimuli with high biological significance (Bradley et al., 2003). In addition, some (but not all) studies using affective pictures also noted that pleasant pictures produced a greater positivity at frontal sites than did neutral and unpleasant pictures (Diedrich et al., 1997, Palomba et al., 1997, Cuthbert et al., 2000, Amrhein et al., 2004), suggesting a possibility that emotional valence may also be reflected in the ERPs. However, across ERP studies using affective pictures, this finding was less consistent compared with the effects of emotional arousal. Amrhein et al. (2004) tentatively interpreted this finding as a valence effect, however, they noted that future studies have to systematically disprove alternative explanations, e.g. differences in picture characteristics other than valence.
Another line of research has examined the speed of processing in the human visual system using. In tasks involving the categorization of complex natural scenes (e.g. by the presence or absence of an animal in the picture), human subjects responded quickly, and the ERP data indicated different processing of target and non-target pictures starting at 150 ms. (Thorpe et al., 1996, VanRullen and Thorpe, 2001a). This effect was most pronounced at frontal electrode sites. In subsequent studies, these findings were extended to tasks requiring the categorization of artificial objects such as cars vs. non-car means of transport, suggesting that there is no advantage for processing of natural biologically relevant stimuli (VanRullen and Thorpe, 2001b). An fMRI study suggested that this ERP response profile may be generated by activity in the fusiform gyrus and cingulate cortex (Fize et al., 2000). This study also found that the completely novel images were categorized nearly as quickly as images with which the subjects were highly familiar (Fabre-Thorpe et al., 2001). Furthermore, there appears to be no or little attentional cost in such a rapid visual categorization of complex natural scenes (Li et al., 2002).
Based on these findings, Thorpe et al. (2001) have suggested that complex visual processing (including scene categorization) can be achieved using largely automatic feed-forward pathways through the visual system, i.e. using bottom-up mechanisms, rather than iterative processing. However, other studies have indicated that a rapid flow of activation spreading from the visual system to parietal and prefrontal regions enables subsequent top-down feedback mechanisms to influence sensory processing (Foxe and Simpson, 2002). Initial signal transmission from primary visual areas to the frontal cortex can take as little as 30 ms, i.e. occurs earlier than it was commonly assumed, with activation of prefrontal cortex taking place at 85 ms post-stimulus onset (Foxe and Simpson, 2002). Although exact contribution of bottom-up and top-down mechanisms underlying rapid visual processing remains to be established, these studies suggest an important role of the prefrontal cortex in the early categorization of images and the regulation of further sensory processing. These studies also suggest that the complexity of the analyses within the 150−200 ms window is much greater than has been previously assumed (Fabre-Thorpe et al., 2001).
Based on the above evidence for the role of prefrontal cortex in early categorization of picture content and the evidence for facilitated processing of motivationally significant information, we hypothesized that neuroelectric activity recorded from anterior regions would show an increased sensitivity to biologically significant content, especially when such content was erotic or related to perceived physical threat. The overall goal of this study was to examine whether relatively early frontal electrocortical responses (i.e. within 150−200 ms post-stimulus) which have been implicated in rapid categorization of complex scenes, discriminate between distinct content categories. We hypothesized that neuroelectric activity recorded from anterior regions would show a selective early sensitivity to specific, biologically relevant contents, such as erotic content (reproduction-related) and threatening content (survival-related). This hypothesis receives partial support from a recent study showing that pictures of opposite sex nudes elicited stronger magnetoencephalographic (MEG) responses compared to neutral pictures, however, it was unclear whether this effect could be attributed to specific content or to other picture characteristics such as overall affective significance, the presence of human faces in the picture, or other aspects of the picture content (Costa et al., 2003). In the present study, we specifically examined whether ERP responses to erotic pictures were different from other picture categories and whether these differences could be attributed to other picture characteristics such as the presence of humans or human faces in the picture.
One of the intrinsic limitations of studies using emotionally significant pictures as stimuli is the limited number of pictures that can be presented in one experiment due to possible habituation or fatigue caused by the length of experiment. Averaging over a small number of pictures and small number of subjects leads to an unacceptably low signal-to-noise ratio in the resulting ERP. One solution is to use a rapid presentation of stimuli with short or no intervals between pictures. However, this increases the likelihood of carry-over effects. An efficient alternative to improve the reliability of ERP measurement without compromising important aspects of picture processing is to substantially increase the number of subjects. In this study we take advantage of a unique dataset collected as part of a twin study of brain function and cognition. This sample, which is unusually large for this type of study, provided us with sufficient power to compare responses to relatively small groups of pictures and even to test the significance of differential responding between individual pictures.
2. Results
2.1. Effects of picture category, time window, and scalp location
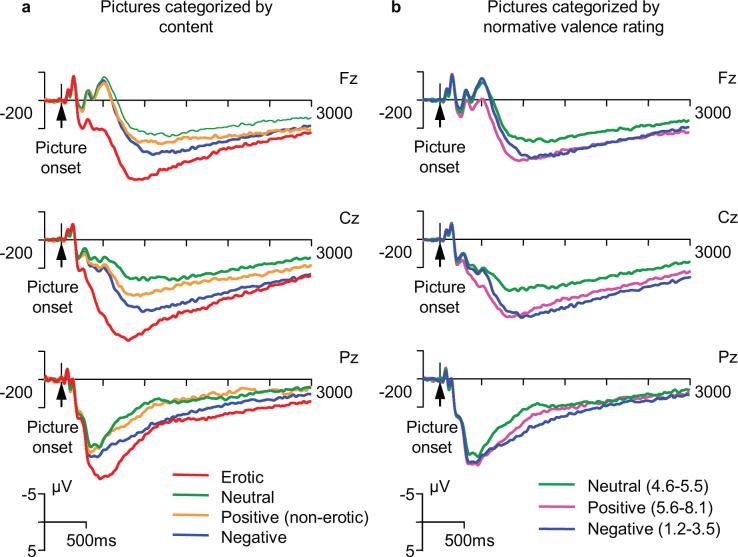
Grand-averaged waveforms for different picture categories over the entire 3000 ms period of ERP recording are shown in Fig.1 (see also Supplementary Fig. 1 for all scalp electrodes). All pictures elicited a large P300-like potential, however, distinct differences between picture categories can be observed. Analysis of variance (ANOVAs) results are presented in Table 1. The omnibus ANOVA including 3 time windows, 4 picture categories, and 19 electrodes showed that the main effect of picture category on ERP amplitude is highly significant (p<0.001). Furthermore, 2-way and 3-way interactions between picture, electrode, and time window (all significant at p<0.001) indicated that the effect of picture category depends on both scalp location and time window. The time course of ERP waveforms at different recording sites (Fig. 1 and Supplementary Fig. 1) shows that the pattern of differences between picture categories is different in the three latency ranges. To examine the effects of picture and scalp topography within each time window and to assess the contribution of anterior-posterior and laterality differences to the scalp topography effects, separate ANOVAs were conducted using a subset of 9 electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4). In each of the time windows, the results showed a highly significant main effect of the factor “Picture” and significant interactions between picture and repeated measures factors “Anterior-posterior” and “Laterality”. Within-subject contrasts showed that the effect of picture category was larger at frontal and central sites relative to parietal sites, and at midline sites relative to lateral sites. In addition, to test for hemispheric differences, the same analysis was repeated after the exclusion of midline electrodes. The interaction between picture category and laterality was no longer significant, indicating that picture effect do not differ in the left and right hemisphere. Therefore, to minimize the probability of Type 1 error, subsequent analyses, including pairwise comparisons between picture categories were restricted to the three most representative midline electrodes: Fz, Cz, and Pz.
Fig. 1.
Event-related potentials elicited by complex visual scenes of different content at frontal (Fz), central (Cz), and parietal (Pz) midline recording sites. a. Pictures categorized by specific content (erotic pictures are in a separate category). b. Pictures categorized using normative ratings of affective valence (negative, positive, and neutral).
Table 1.
Effects of picture category, scalp location, and latency window on the ERP amplitude.
| Factors | df (factor, error) | F-value | p-value |
|---|---|---|---|
| Omnibus ANOVA (all time windows, all electrodes): | |||
| Picture | 3, 789 | 44.6 | <0.001 |
| Window | 2, 526 | 270.2 | <0.001 |
| Electrode | 18, 4734 | 91.6 | <0.001 |
| Picture X Electrode | 54, 14202 | 9.2 | <0.001 |
| Picture X Window | 6, 1578 | 19.8 | <0.001 |
| Picture X Window X Electrode | 108, 28404 | 5.4 | <0.001 |
| P2 window (200−375 ms): | |||
| Picture | 3, 789 | 68.0 | <0.001 |
| Picture X Anterior-posterior | 6, 1578 | 30.4 | <0.001 |
| Picture X Laterality | 6, 1578 | 7.1 | <0.001 |
| Picture X Anterior-posterior X Laterality | 12, 3156 | 4.9 | <0.001 |
| N4 window (375−600 ms): | |||
| Picture | 3, 789 | 241.1 | <0.001 |
| Picture X Anterior-posterior | 6, 1578 | 34.2 | <0.001 |
| Picture X Laterality | 6, 1578 | 12.1 | <0.001 |
| Picture X Anterior-posterior X Laterality | 12, 3156 | 4.7 | <0.001 |
| LPP (800−1800 ms): | |||
| Picture | 3, 789 | 71.0 | <0.001 |
| Picture X Anterior-posterior | 6, 1578 | 15.0 | <0.001 |
| Picture X Laterality | 6, 1578 | 2.1 | 0.07, n.s. |
| Picture X Anterior-posterior X Laterality | 12, 3156 | 3.2 | <0.01 |
Note: P-values are presented after Greenhouse-Geissler adjustment, when appropriate)
2.2. “Early” fronto-central response
The early ERP components recorded from the frontal midline region are shown in greater detail in Fig. 2a. Inspection of this figure reveals a clear difference between the waves associated with erotic pictures and all other categories. Responses to erotic pictures started diverging from responses to other pictures at approximately 160 ms after the picture onset. These differences reached significance (p<0.01) in the frontal and central midline regions at 185 and 195 ms, respectively, and in the parietal region at 274 ms (Fig. 3). As Fig. 1 shows, erotic pictures elicited a distinctive positive potential shift that led to amplification of the positive peak around 250 ms (P2), and to complete suppression of the negative peak at approximately 500 ms (N4) which was prominent for all other picture categories. Analysis of mean amplitude values in P2 (200−375 ms) and N4 (375−600 ms) latency windows (Fig.1a) revealed a significant main effect of picture category and a significant picture by electrode interaction (Table 1), indicating that differing responses to erotic content were most pronounced at midline frontal and central electrodes. All pairwise comparisons between erotic pictures and other categories (positive non-erotic, neutral, and negative) were highly significant in both latency windows (paired t-values from 8.4 to 16.3, p<0.001 in all cases; the differences remain highly significant after Bonferroni adjustment of the α-value). In sharp contrast, none of the non-erotic picture categories were significantly different.
Fig. 2.
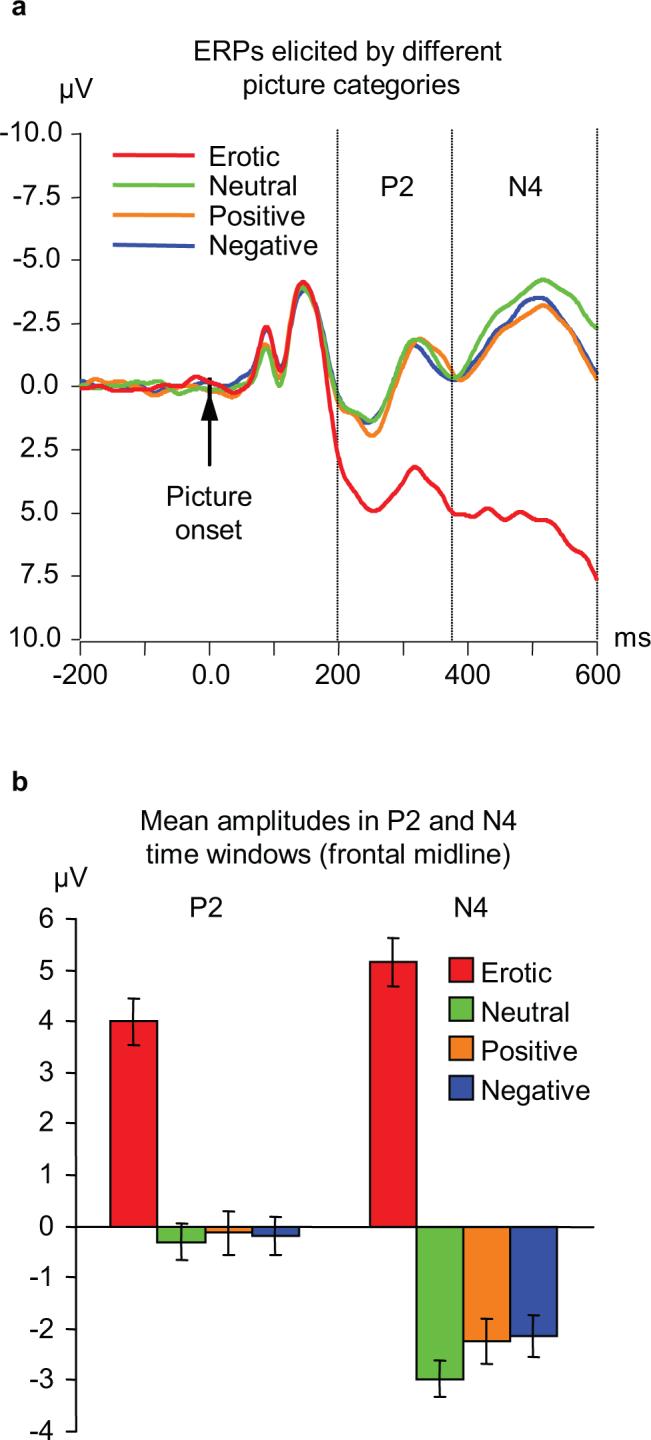
Frontal midline response to pictures of different content. a. ERP waveforms elicited at the frontal midline site (Fz) by four picture categories. Vertical lines indicate the two time windows for which quantitative analyses were conducted (200−375 ms and 375−600 ms). b. Mean amplitude values (±S.E.) in the time windows shown in panel a. Pairwise differences between erotic and each of the non-erotic categories are highly significant (t-values from 10.1 to 18.7; p<0.001 in all cases after Bonferroni correction). In both time windows, none of the comparisons among non-erotic categories (positive, negative, neutral) reached significance.
Fig. 3.
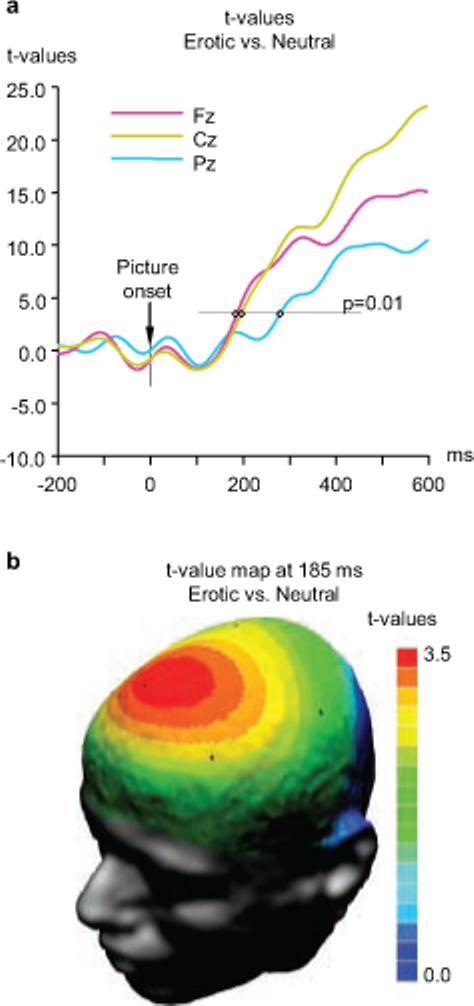
Onset of significant differences between erotic and neutral stimuli. a. Time course of significant differences at frontal, central, and parietal locations. Significance thresholds of t=3.33 for p<0.01 (2-tailed t-tests) is indicated. Significant differences between erotic and neutral pictures first emerge at frontal and central locations and are followed after a short delay by a significant difference at patietal locations. b. T-score map illustrating the scalp topography of significant differences at 185 ms post-stimulus (T-values of 3.33 and above are significant at p<0.01).
2.3. Late positive potential (LPP)
The late positive potential (time window 900 to 1800 ms) was larger for pictures with emotional content (erotic, positive non-erotic, negative) compared to neutral pictures as indicated by significant effect of factor picture (Table 1). All pairwise comparisons between the picture categories were highly significant (with Sidak adjustment for multiple testing). Furthermore, and again in agreement with previous studies, among positive pictures this effect was most pronounced for pictures with erotic content (Schupp et al., 2004a). To ensure a better comparability with previous studies, an additional analysis of LPP was conducted after collapsing erotic and non-erotic positive pictures into one category. The LPP amplitude showed a distinct V-shaped relationship with picture category, with both pleasant and unpleasant pictures showing larger LPP than neutral pictures (Supplementary Fig. 4). A repeated measures ANOVA showed a highly significant effect of the picture category (F2,592=197.6, p<0.001). This finding agrees with previous studies showing an increased late positive potential (LPP) for pictures with high emotional significance, regardless of their emotional valence. To further explore the relationship between the LPP amplitude and the dimensions of emotional valence and arousal, we computed the correlations across all 55 pictures between the grand-average ERPs for individual pictures (averaged across subjects for each picture) and normative picture ratings. As expected, mean amplitude of the late positive potential correlated significantly with normative arousal ratings of the picture (r=.45, .58, and .35 for Fz, Cz, and Pz, respectively, all p<0.01), but not with the valence ratings. In contrast, mean amplitudes in the two earlier time windows (200−375 and 375−600 ms) did not show any significant correlations with either arousal or valence ratings of the pictures.
2.4. Alternative picture groupings
To examine whether the observed differences between erotic and non-erotic pictures in the time windows from 200−375 and 375−600 ms could be explained by other picture characteristics, we performed additional post-hoc comparisons of alternative categories of non-erotic pictures. First, we tested whether ERP responses were modulated simply by the presence or absence of humans in the scene by comparing non-erotic pictures containing humans and non-erotic pictures without humans. There was no significant difference between ERPs elicited by these groups of pictures (t=−1.21, df=263, p=0.25), however, both groups differed significantly from the erotic pictures (p<0.001 in both windows). Next, to examine whether ERP differences associated with the processing of erotic pictures could in fact be caused by the presence of nude or partially nude humans in the picture, we selected 5 pictures from our set containing largely unclothed humans (4 males and 1 female) in emotionally positive contexts, such as recreational or athletic scenes. ERP responses to this group of pictures did not differ significantly from pictures without humans in the scene (paired t=0.82, df=263, p=0.41), but did differ significantly from erotic pictures (paired t=6.59, df=263, p<0.001). Finally, we performed comparisons within the emotionally negative category to determine whether images associated with direct threat (acts of violence, angry face, angry dog) differ from images with indirect threat (mutilated bodies, accident scenes). There was no significant difference among these groups of pictures.
Taken together, these additional post-hoc comparisons indicate that the observed effects are highly specific to the erotic content of the picture and cannot be explained by other picture characteristics.
3. Discussion
The main finding of this study is a clear divergence of neuroelectric response to erotic pictures from other picture categories, regardless of their emotional valence or arousal. The present findings suggest that the human brain is able to discriminate rapidly between specific contents of complex visual scenes and that neural representation of the scene meaning can be achieved very early in visual processing. Importantly, this discrimination occurred spontaneously, during free viewing, and in the absence of external cues or explicit response demands (the subjects were not required to make any judgments or responses). The results provide the first evidence for rapid and automatic discriminations of distinct content categories of complex visual scenes in the absence of external cues.
Our results differ from previous studies using complex picture stimuli in several important respects. First, and most important, different neuroelectric responses in our study were associated with a distinct content category (i.e. “eroticism”), rather than with the more general dimensions of emotional valence and arousal that have served as the theoretical framework for previous ERP studies of affective pictures. Second, relatively early content-specific ERP effects were observed over frontal regions, whereas previous studies involving emotional pictures focused mainly on posterior regions of the scalp. Third, this fronto-central activity occurred significantly earlier than the late positive potentials described in previous studies. Finally, in this study, the discrimination of content categories occurred during passive viewing of the pictures and was spontaneous and internally driven, since the subjects were not asked to make evaluative judgments and/or motor responses to specific categories of pictures.
Importantly, the pictures were discriminated by the meaning (biological and/or social) of the depicted situation, rather than merely by the presence or absence of a certain class of objects. It is reasonable to assume that discrimination between erotic and non-erotic scenes should involve even more complex processing compared to other possible types of categorization, such as presence the of humans or faces in the picture, etc. It remains to be seen whether erotic content is unique in eliciting such a distinct neural response. A somewhat puzzling finding of the present study is that emotionally negative pictures (or their specific subcategories) were not discriminated from neutral and positive non-erotic pictures, despite their apparent biological relevance. It is reasonable to speculate that the rapid processing of negative pictures involves, at least partially, different neural circuits. In particular, the fast subcortical visual pathway involving the amygdala (Pessoa, 2005) may be more involved in the processing of threat-related scenes and the activation of this circuit may not be readily detected by the scalp-recorded ERP.
The present findings challenge the widespread view that ERP responses to affective pictures reflect the overall level of emotional arousal. In our study, early ERP components elicited by erotic scenes were clearly dissociated from other positive emotional pictures, suggesting that early frontal positivity is related to specific content, rather than to positive emotional valence of the picture. The normative female valence ratings for erotic pictures (Lang et al., 1999) were actually lower than for positive non-erotic pictures (6.1 and 7.6, respectively). These differences cannot be explained by emotional arousal either, since erotic and positive non-erotic pictures did not differ with respect to their normative arousal ratings (average of 5.9 in both categories). Moreover, negative pictures had the highest arousal ratings (7.0) but did not differ significantly from neutral pictures (3.2) with respect to the anterior ERP response in the 200−600 ms window.
A direct comparison of our results concerning the early anterior effects with previous ERP studies is complicated by the fact that most of these studies used the traditional picture categorization by emotional valence. Very few ERP studies that distinguished specific content categories, including erotica focused on responses in posterior brain regions (Schupp et al., 2004a, Schupp et al., 2004b). Nevertheless, the present findings are in line with the observation made in some previous studies that pleasant pictures elicited a larger electrocortical positivity at frontal sites relative to negative and neutral pictures (Diedrich et al., 1997, Palomba et al., 1997, Cuthbert et al., 2000, Amrhein et al., 2004). Cuthbert et al. (2000) interpreted this finding in terms of arousal, since in their sample (predominantly male) positive pictures also elicited greater skin conductance responses (SCR). However, other authors (Amrhein et al., 2004) did not find such difference in SCR and tentatively interpreted this finding as a valence effect, but noted that alternative picture characteristics other than valence need to be ruled out. The present study using alternative categorizations of pictures (by contents vs. by valence) helps to resolve this important issue. When picture were categorized by valence ratings, i.e. erotic and non-erotic positive pictures were in the same category, our results were in good agreement with the above studies (Fig. 1b, Supplementary Fig. 2). Although the most prominent ERP effect was an LPP enhancement for both positive and negative pictures relative to neutral ones, a small but significant (p<0.001) early positive shift was observed for positive pictures relative to negative and neutral pictures. However, further categorization of pictures by content (Fig 1a) clearly shows that the early anterior effect is produced entirely by erotic pictures, whereas no significant differences were observed among positive non-erotic, neutral, and negative pictures.
The results obtained for the late positive potential (time window 900−1800 ms) replicate previous findings (Cuthbert et al., 2000): mean amplitudes for positively and negatively valenced pictures were significantly larger than for the neutral pictures. Consistent with Schupp et al. (2004a), this effect was most pronounced for erotic pictures.
Overall, our findings suggest that ERP response to complex visual scenes reflect at least two major stages of processing. The first stage includes a rapid and phasic response that is content-specific and reflects the discrimination of the contextual meaning of the scene. This stage is followed by a more tonic response that is likely to reflect attention allocation during sustained viewing of motivationally relevant information (Cuthbert et al., 2000, Schupp et al., 2000, Amrhein et al., 2004). The existence of functionally distinct modes of processing within relatively narrow time windows underscores the utility of electrophysiology methods for the detection of transient, short-lived, but fundamentally important stages of neural processing. Our findings indicate a rapid, selective, and content-specific processing of erotic materials which can be dissociated from other picture categories regardless of their emotional valence and arousal. One possible interpretation of this finding could be the existence of specialized neural networks for preferential processing of specific categories of stimuli with high adaptive and evolutionary significance.
Previous studies suggest a top-down regulation of sensory processing based on early categorization of visual scenes (e.g. Foxe and Simpson, 2002). One can speculate that erotic content engages a highly selective neuronal network starting at about 180 ms after picture onset. The activity of this network modulates subsequent processing of the picture, which is reflected in a distinct positive shift of the scalp potential. This modulation may involve suppression of the processing of other, competitive visual information in order to ensure facilitated processing of information of high biological significance.
The specific neural substrates underlying this rapid spontaneous discrimination of picture content remain to be determined. The divergence of neuroelectric responses to different picture categories starts in anterior regions of the scalp, suggesting an involvement of the prefrontal cortex in the discrimination of specific contents. The prefrontal cortex receives projections from the inferior temporal cortex responsible for higher-level object processing. It has been shown recently that the prefrontal cortex of monkeys contains neurons that respond differently to specific categories of complex visual images regardless of their physical similarity (Freedman et al., 2003). These data, as well as data from neuroimaging studies of visual scene categorization (Fize et al., 2000) and processing of erotic content (Karama et al., 2002, Stark et al., 2005) suggest that a selective, content-specific processing of erotic material is likely to engage areas in the prefrontal and anterior cingulate cortex. Moreover, one of these fMRI studies indicated that early differential ERP responses related to the categorization of natural images can be generated by activity in the fusiform gyrus and cingulate cortex (Fize et al., 2000). Our finding that the earliest discrimination between picture categories occurs in the frontal midline region is in good agreement with the above studies implicating prefrontal and anterior cingulate cortices in the processing of complex object categories and erotic contents in particular. However, the limited spatial resolution of the EEG and the modest number of electrodes used in this study precludes further speculations about the cortical localization of the effects reported here.
Studies using affective pictures noted stronger subjective and autonomic response to positive, particularly erotic, stimuli in men than women, whereas women were more reactive to threatening stimuli than men (Bradley et al., 2001b). A recent neuroimaging study (Sabatinelli et al., 2004) showed that erotic pictures elicited a greater activity in extrastriate visual cortex in men compared with women, suggesting a male visual bias for erotic stimuli. Our findings, however, indicate a strong selectivity of ERP responses to erotic contents in women, suggesting that “erotic bias” can exist in both genders, although the extent to which it is expressed may depend on research methods, in particular their temporal and spatial resolution. Potential gender differences of the effects reported here should be a matter of future studies. Our preliminary data from another study including 18 males and using the same experimental paradigm but slightly different recording parameters indicate a similar frontal positivity effect, the size of which is comparable to the effect reported here for female subjects.
Several alternative explanations of our findings, other than content-specific processing of erotic material, should be considered. First, there is a possibility that the differing ERP responses described here can be caused by systematic differences between erotic and non-erotic pictures with respect to picture characteristics unrelated to their erotic vs. non-erotic content. Pictures may differ with respect to low-level, physical properties such as shape, orientation, brightness, and overall complexity. In our experiments, pictures were equated with respect to the color resolution and the color palette. Careful inspection of images in different content categories does not suggest any systematic differences between erotic and non-erotic pictures with respect to physical properties, overall complexity, or composition. It should be noted that due to multidimensionality of physical and statistical properties of complex natural pictures it is practically impossible to match pictures with respect to such properties.
Apart from low-level features, ERP differences can be attributed to higher-level features such as the presence of humans in the scene or, more specifically, the presence of nude or partially nude humans in the picture. However, post-hoc analyses using alternative picture groupings did not support this hypothesis. There was no significant difference between ERP responses to non-erotic pictures containing images of largely unclothed humans and non-human images. However, this alternative hypothesis needs to be tested more systematically in future studies by comparing responses elicited by erotic scenes with responses produced by images of nude bodies of the same and opposite sex, including both erotically attractive and non-attractive images.
Another possibility is that differing responses to a distinct stimulus category can be related to selective attention directed to this category. Recent studies suggest that the processing of emotionally significant stimuli is not completely automatic and may be subject to top-down attentional modulation (reviewed in Pessoa, 2005). However, this explanation also seems unlikely, since both positive and negative emotional pictures can draw attentional resources, which is believed to be reflected in a larger late positive potential elicited by such stimuli (Cuthbert et al., 2000). It is also important that processing of images in this study was spontaneous and unrestricted. Participants viewed the pictures freely and were not instructed to categorize, name, match, or evaluate the pictures, or to make any responses. Thus, no categorical templates were imposed, in contrast to studies using object categorization tasks, where the processing of the target objects could be primed by an instruction-induced internal template.
It should be noted that while the group data presented here reflect overall trends, large individual differences in responses were observed. The nature and significance of these individual differences should be a matter of future analyses. The present findings may also have practical implications, since ERP modulation by erotic content may provide an objective measure of the responsiveness of sex-related motivational systems, dysfunction of which is present in some neuropsychiatric disorders, such as major depression and substance use disorders. Studies conducted using patient groups could determine whether ERPs elicited by erotic and other contents can assist in the diagnosis and treatment evaluation of such disorders.
Several limitations of the present study need to be acknowledged. First, analyses reported here are based solely on a female sample. Our preliminary results from a sample of males tested in another study using the same experimental paradigm are in good agreement with the results reported here, however, direct quantitative comparison of the samples is problematic due to some differences in EEG recording parameters. Therefore, further research is needed to examine possible gender differences in the reported effects. Second, it is not entirely clear whether the observed effect is specific to the erotic context of the depicted social interaction, or it is caused by erotically appealing nude or partially nude bodies of the opposite or same gender. Although our post-hoc analyses suggest that partial nudity alone does not produce the same kind of effect as observed for erotic pictures, more systematic comparisons of pictures are needed in order to elucidate the relative contribution of specific factors, such as nudity, gender, physical attractiveness, and dyadic interaction. For example, future studies should compare responses produced by the same vs. opposite gender nudes, attractive vs. non-attractive nudes, single nudes vs. erotic pairs, etc. Next, the subjects were not required to evaluate each picture on valence and arousal scales in order to ensure that picture viewing is not biased by externally imposed categorical templates. Therefore, normative ratings were used for categorization of pictures based on valence and arousal. Given the large sample size used in this study, it is unlikely that average ratings in our sample would deviate from normative ratings in any significant way, especially since the origin, gender, and age range of our sample was very similar to the sample on which the normative ratings were obtained (Lang et al., 1999). Furthermore, significant correlations between normative arousal (but not valence) ratings and the late positive potential indicated that the normative ratings applied very well to this sample. Finally, the relatively small number of EEG electrodes used in this study precludes an accurate localization of the sources of electrical activity.
In conclusion, our findings suggest that, in the absence of explicit instruction, the brain is capable of a rapid spontaneous discrimination of complex visual scenes on the basis of their specific biological and/or social meaning.
4. Experimental Procedure
1.1. Subjects
The sample consisted of 272 young adult females (age 18−28) who were recruited through a twin registry for participation in a twin study of brain function and behavior. Subjects with known neurological disorders, a history of serious head trauma, and those currently taking psychoactive medications were excluded at the initial screening stage. Four subjects did not complete the experiment due to fatigue, sleepiness, or their request to abort the experiment, and data from further 4 subjects were excluded because of poor recording due to excessive electromyographic activity and movement-related artifacts. Thus, a total of 264 subjects were included in the final analysis. A written informed consent was obtained from all subjects, and the experimental protocol was approved by Washington University Institutional Review Board.
1.2. Stimuli
Stimuli were 55 color slides selected from the International Affective Pictures System (IAPS) (Lang et al., 1999), a standardized set of color photographs for experimental studies of emotion and attention developed by the NIMH Center for Emotion and Attention (CSEA) at the University of Florida. These photographs depict a broad range of scenes and objects varying in their emotional significance, and normative subjective ratings of picture valence and arousal (on the scale of 1 to 9) are available for different populations. Selection of pictures for this study was based on the normative valence ratings for young females provided by Lang et al. (1999). The goal of the selection was to maximize the differences in normative valence ratings between pleasant and unpleasant pictures, while keeping their arousal ratings as close as possible. We selected 3 groups of pictures with non-overlapping valence ratings: 18 emotionally positive (valence ratings from 1.2 to 3.0), 19 neutral (valence ratings from 4.7 to 5.7) and 18 negative (valence ratings from 5.9 to 8.1). As a result of this classification based on normative ratings, 7 erotic pictures fell in to the positive and 2 into the neutral category. The pictures were further classified with respect to their content by placing erotic pictures into a separate category This category included pictures defined as “erotic couples” in the IAPS manual and showing fully or partially unclothed heterosexual couples in erotic embraces .This reclassification yielded a group of 9 erotic pictures, 11 positive non-erotic pictures (happy people in festive scenes, sports and entertainment scenes, and pleasant objects, such an appetizing ice-cream cone, a mass of banknotes), 17 neutral non-erotic pictures (people in neutral scenes, household objects), and 18 negative pictures (aimed gun, attacks with weapon, angry crowd, mutilated bodies, accidents). The following pictures from the IAPS set were used (by content category) - erotic: 4607,4608, 4611, 4651, 4652, 4660, 4664, 4666, 4680; positive non-erotic: 4510, 5470, 7330, 8080, 8170, 8190, 8200, 8461, 8470, 8490, 8501; neutral: 1616, 2214, 2220, 2495, 2516, 2570, 2749, 2880, 5510, 7002, 7004, 7010, 7035, 7090, 7150, 7175, 7211; negative 1300, 2120, 3000, 3010, 3053, 3060, 3500, 3530, 3266, 9252, 6313, 6230, 6260, 6540, 6560, 9410, 9910, 9921. To control for possible differences in photographic quality of the pictures, all images were reduced to 256 colors (8 bit), and a common palette (computed using Quant software) was applied to all images.
1.3. Procedure
The experimental procedure was very similar to previous studies using affective pictures (Cuthbert et al., 2000). During the experiments, the subjects sat in a comfortable reclining chair located in a semi-darkened chamber in front of a 19-inch high resolution color monitor (distance to the subject's eyes 110 cm, visual angle 14°). The subject was asked to keep their gaze on the fixation cross in the center of the screen and attend to the pictures the entire time they were displayed. Importantly, the subjects were not required to make any judgments about the pictures (such as picture ratings or categorizations). Each picture was presented for 6s with an inter-picture interval varying from 12 to 18 s, during which a blank screen with a fixation cross in the center was presented. We used this relatively long and variable interval to reduce the predictability of picture onset and to control for potential spill-over effects. Pictures of different content were presented in a fixed pseudo-random order. During two-thirds of the pictures, an acoustic startle stimulus (a 40 ms burst of white noise) was presented at 3, 4, or 5 s after the picture onset, thus allowing at least 3s of undisturbed picture viewing (startle data are not presented here).
1.4. ERP recording and analysis
The electroencephalogram (EEG) was recorded continuously from 19 electrodes of the International 10−20 System using Synamps bioamplifiers (Compumedics-Neuroscan) with a bandpass of 0.05 to 70 Hz and digitized at 1000 Hz. Electrode impedances were kept below 5 KΩ. Vertical and horizontal electro-oculograms (EOG) were recorded to monitor eye movements. A left mastoid reference was used for recording, and data were re-referenced offline to averaged mastoids. A correction for ocular artifacts was performed using a regression procedure (Semlitsch et al., 1986). Epochs containing neuroelectric activity from −200 to 3000 ms relative to picture onset were extracted from the continuous recording and baseline-corrected using the −200 to 0 ms baseline. For each individual picture and each individual subject, mean amplitude was measured within three time windows: 200 to 375, 375 to 600, and 900 to 1800 ms. The first two time windows were selected according to the distinct peaks observed on grand-averaged ERP waveform at frontal electrodes, and the third window was used to capture the late positive wave described in previous studies. To obtain grand-averaged waveforms, single epochs were averaged across 264 individuals and across pictures within each picture category.
1.5. Statistical analysis
Statistical distributions of quantitative variables (mean amplitude values in different time windows) did not deviate significantly from a normal distribution, as indicated by nonsignificant Kolmogorov-Smirnov tests. Significance of differences among picture categories was tested using repeated-measures analysis of variance (RM ANOVA) including all picture categories, followed by planned pairwise comparisons. First, we conducted an omnibus ANOVA including 3 time windows, 4 picture categories, and 19 electrodes to test whether the effect of picture category on ERP amplitude is significant and whether this effect depends on the latency range and scalp location. Second, to determine whether modulation of picture category effect by scalp topography is due to anterior-posterior or lateral (e.g. hemispheric) differences, or both, we conducted separate ANOVAs within each time window using a subset of 9 electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4) and tested for the interactions between the factor “Picture” (including 4 levels corresponding to the 4 picture categories) and repeated measures factors “Anterior-posterior” (3 levels) and “Laterality” (3 levels). In all ANOVAs, evaluation of significance (p-values) was performed using Greenhouse-Geisser adjusted degrees of freedom to control for deviations from sphericity. Third, we conducted pairwise comparisons among individual picture categories using paired t-tests at selected scalp locations suggested by the ANOVAs and the inspection of scalp topography. A Bonferroni correction of the α-value to was used to evaluate the significance of differences. In addition, to control for non-independence of observations within twin pairs, we applied multilevel modeling using the HLM (v.6) software (Raudenbush et al., 2004) that partitioned within- and between-pair variance and yielded standard errors that were not biased by the clustering of individual observations. Finally, we also checked for possible effects of the paired observations by repeating all analyses twice using only one twin from each pair. The results of these parallel analyses were virtually identical to each other and to the results obtained using the whole sample.
Acknowledgements
This work was supported by the grant DA00421 from the National Institute on Drug Abuse. The authors thank Angela Ralano and Erin Myers for their role in the data collection.
Sources of support: This work was supported by the grant DA00421 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: Cognitive and Behavioral Neuroscience
Supplementary Material
Supplementary Fig. 1. Event-related potentials (ERPs) elicited by four categories of picures at 19 scalp locations.
Supplementary Fig. 2. Late positive potential (LPP) elicited by emotionally positive, neutral, and negative pictures (mean amplitude in the time window of 800−1800 ms). Note that positive pictures include both erotic and non-erotic contents.
References
- Amrhein C, Muhlberger A, Pauli P, Wiedemann G. Modulation of event-related brain potentials during affective picture processing: a complement to startle reflex and skin conductance response? Int J Psychophysiol. 2004;54:231–240. doi: 10.1016/j.ijpsycho.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001a;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001b;1:300–319. [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behav Neurosci. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Costa M, Braun C, Birbaumer N. Gender differences in response to pictures of nudes: a magnetoencephalographic study. Biol Psychol. 2003;63:129–147. doi: 10.1016/s0301-0511(03)00054-1. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Diedrich O, Naumann E, Maier S, Becker G, Bartussek D. A frontal positive slow wave in the ERP associated with emotional slides. Journal of Psychophysiology. 1997;11:71–84. [Google Scholar]
- Fabre-Thorpe M, Delorme A, Marlot C, Thorpe S. A limit to the speed of processing in ultra-rapid visual categorization of novel natural scenes. J Cogn Neurosci. 2001;13:171–180. doi: 10.1162/089892901564234. [DOI] [PubMed] [Google Scholar]
- Felsen G, Dan Y. A natural approach to studying vision. Nat Neurosci. 2005;8:1643–1646. doi: 10.1038/nn1608. [DOI] [PubMed] [Google Scholar]
- Fize D, Boulanouar K, Chatel Y, Ranjeva JP, Fabre-Thorpe M, Thorpe S. Brain areas involved in rapid categorization of natural images: an event-related fMRI study. Neuroimage. 2000;11:634–643. doi: 10.1006/nimg.2000.0585. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp Brain Res. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghofer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38:175–178. [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Moratti S, Sabatinelli D, Bradley MM, Lang PJ. Additive effects of emotional content and spatial selective attention on electrocortical facilitation. Cereb Cortex. 2005;15:1187–1197. doi: 10.1093/cercor/bhi001. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin Neurophysiol. 2001;112:2057–2068. doi: 10.1016/s1388-2457(01)00654-x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4). The Center for Research in Psychophysiology. University of Florida; 1999. International affective picture system (IAPS):instruction manual and affective ratings. [Google Scholar]
- Li FF, VanRullen R, Koch C, Perona P. Rapid natural scene categorization in the near absence of attention. Proc Natl Acad Sci U S A. 2002;99:9596–9601. doi: 10.1073/pnas.092277599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratti S, Keil A, Stolarova M. Motivated attention in emotional picture processing is reflected by activity modulation in cortical attention networks. Neuroimage. 2004;21:954–964. doi: 10.1016/j.neuroimage.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. Int J Psychophysiol. 1997;27:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin Neurobiol. 2005;15:188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM. Scientific Software International; Lincolnwood, IL: 2004. [Google Scholar]
- Sabatinelli D, Flaisch T, Bradley MM, Fitzsimmons JR, Lang PJ. Affective picture perception: gender differences in visual cortex? Neuroreport. 2004;15:1109–1112. doi: 10.1097/00001756-200405190-00005. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition & Emotion. 2004a;18:593–611. [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychol Sci. 2003;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004b;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Girod C, Walter B, Kirsch P, Blecker C, Ott U, Schafer A, Sammer G, Zimmermann M, Vaitl D. Erotic and disgust-inducing pictures--differences in the hemodynamic responses of the brain. Biol Psychol. 2005;70:19–29. doi: 10.1016/j.biopsycho.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Fabre-Thorpe M. Neuroscience. Seeking categories in the brain. Science. 2001;291:260–263. doi: 10.1126/science.1058249. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Thorpe SJ. Is it a bird? Is it a plane? Ultra-rapid visual categorisation of natural and artifactual objects. Perception. 2001a;30:655–668. doi: 10.1068/p3029. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Thorpe SJ. The time course of visual processing: from early perception to decision-making. J Cogn Neurosci. 2001b;13:454–461. doi: 10.1162/08989290152001880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Event-related potentials (ERPs) elicited by four categories of picures at 19 scalp locations.
Supplementary Fig. 2. Late positive potential (LPP) elicited by emotionally positive, neutral, and negative pictures (mean amplitude in the time window of 800−1800 ms). Note that positive pictures include both erotic and non-erotic contents.