Abstract
In food allergic individuals, exposure to food allergens by the oral route can trigger immediate (within minutes) local hypersensitivity reactions in the intestine followed by a late-phase inflammatory response. Previous work has shown that CD23 is constitutively expressed by human intestinal epithelial cells and mediates the uptake of allergen-IgE complexes. We hypothesized that allergen-IgE complexes could also signal via CD23 to trigger an inflammatory cascade in the local environment. Stimulation of polarized human intestinal epithelial cells (Caco-2) with IgE-Ag complexes triggered upregulation of the chemokines IL-8 and CCL20 at the mRNA and protein level. Allergen complexes induced phosphorylation of ERK and JNK, but not p38 MAP kinase or NF-κB, and resulted in AP-1 activation. Cross-linking of CD23 replicated the findings with IgE-Ag complexes, and silencing of CD23 expression abrogated the response to allergen-IgE complexes. Supernatant from IgE-Ag-stimulated epithelial cells was capable of recruiting immature human dendritic cells in a CCL20-dependent manner as shown by migration assays. Finally, immunostaining of duodenal biopsies from patients with intestinal manifestations of food allergy and controls demonstrated that CCL20 was constitutively expressed by villous epithelial cells. Thus, we hypothesize that signaling via epithelial CD23 is a critical step not only in delivery of allergen-IgE complexes to trigger immediate hypersensitivity reactions, but also may participate in the late-phase inflammatory response.
INTRODUCTION
Food allergy, defined as an immunologically-mediated adverse reaction to food proteins, is a growing clinical problem that has been estimated to affect approximately 6% of children and 3–4% of adults 1, 2. There is a paucity of information on the local gastrointestinal immune events that occur in humans in response to food allergens. Even in the absence of serum food-specific IgE, it has been shown that allergen ingestion is associated with an elevation of IgE in the duodenal mucosa, increased numbers of mast cells, eosinophils, T lymphocytes (including both CD4+ and CD8+) and upregulation of Th2-cytokine expression in the duodenal mucosa of subjects with food allergy 3. These changes are consistent with a “late-phase” allergic inflammatory response in the gastrointestinal mucosa 4–6.
In order for ingested allergen to induce either an immediate or delayed-type hypersensitivity response in the intestinal mucosa (or at distal sites such as lung or skin), food allergens must first cross the intestinal epithelial barrier. Tight junctions formed between intestinal epithelial cells prevent the passage of macromolecules such as food allergens. We have recently shown that the CD23a isoform of the low-affinity IgE receptor (FcεRII) is expressed constitutively on human intestinal epithelial cells, and together with food-specific IgE is found in the stool of subjects with IgE-mediated food allergies 7. We and others have shown that CD23 can function as an antigen-sampling mechanism by transporting intact allergen-IgE complexes across human intestinal epithelial cell monolayers 7–10. These complexes are then capable of inducing the degranulation of sub-epithelial effector cells 7.
In addition to their function as a barrier and absorptive/secretory surface, epithelial cells function as sentinel cells that transduce signals from the intestinal lumen to the mucosal immune system through the secretion of chemokines, cytokines, reactive oxygen species, and lipid metabolites 11–14. We hypothesized that activation of CD23 on human intestinal epithelial cells by binding of IgE-antigen complexes could trigger chemokine secretion to most efficiently deliver antigen to subepithelial immune cells. In this study we have analyzed the inflammatory signaling cascades induced by interaction of IgE-antigen complexes with human intestinal epithelial cells, and report that signaling through CD23 on human intestinal epithelial cells results in the induction of chemokines capable of recruiting cells of the innate and adaptive immune system to the gastrointestinal mucosa.
METHODS
Cell Culture
Caco-2 cells were grown in RPMI-1640 (Gibco, Grand Island, NY) containing 10% FCS and penicillin/streptomycin. For polarized monolayers, serum was reduced to 5%. Cells were seeded onto polyethylene terephthalate track etched membranes (0.4um) cell culture inserts (BD Falcon, Bedford, MA) at 105 cells per cm2 and grown for 7 to 10 days at which time cells reached a resistance of 400 ohms · cm2.
Stimulation with Allergen-IgE complexes
Polarized monolayers were stimulated by the addition of human anti-NP IgE-NP-BSA complexes at a concentration of 1 μg/ml of anti –NP IgE (Serotec, Raleigh, NC) and NP-BSA (Biosearch Technologies, Inc, Novato, CA). Complexes were pre-incubated for 1 hour prior to addition to cells.
IL-8 and CCL20 ELISA
Supernatants from the apical or basal wells of the transwells were removed 24 h after stimulation. IL-8 and CCL20 were detected by ELISA detection kit (Quantikine, R&D systems, Minneapolis, MN), according to the manufacturer’s guidelines. Optical density was measured at 450 nm.
IL-8 and CCL20 mRNA expression
Total RNA was extracted from polarized monolayers 6 hours after stimulation by using a Nucleospin RNA purification Kit (BD Biosciences, San Jose, CA). 50 ng of total RNA was used to do qRT-PCR on a Lightcycler (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. Primers were as follows: IL-8 forward: 5′GACCACACTGCGCCAACACA 3′; reverse: 5′TCTCAGCCCTCTTCAAAAACTTCT3′; CCL20 forward: 5′CGAATCAGAAGCAGCAAGCAACT3′, reverse: 5′TTTTTACTGAGGAGACGCACAAT-3′, β-actin forward: 5′-CATCGAGCACGGCATCGTCA-3′ reverse: 5′-TAGCACAGCCTGGATAGCAAC-3′. Data were expressed as fold change compared to unstimulated control after adjustment using the housekeeping gene β-actin (ΔΔCT method).
Immunoblotting
To assay for the phosphorylation of various proteins, cells were lysed in lysis buffer containing phosphatase inhibitor sets I and II (Calbiochem, San Diego, CA) and cell extracts were clarified by centrifugation. 30 μg of protein was used for immunoblotting. Applied antibodies included antibodies against p44/42 ERK, phospho-p44/42ERK(Thr202/Tyr204), SAPK/JNK, phospho-SAPK/JNK (Thr183/Tyr185), phospho-p38 (Thr180/Tyr182), phospho-IKBα (all from Cell Signaling, Beverly, MA); antibody against actin (Sigma). MAPK inhibitors used included JNK inhibitor II and JNK inhibitor II Negative Control, U0126 and U0124 (all from Calbiochem).
Electrophoretic mobility shift assay (EMSA)
Caco-2 cell nuclear extracts were prepared using a Nuclear Extraction Kit (Panomics, Fremont, CA). The NF-κB probe was prepared by annealing complementary single-stranded oligonucleotides with 5′-GATC overhangs (Genosys Biotechnologies, Inc., The Woodlands, TX) and were labeled by filling in with [(alpha)-32P]dGTP and [(alpha)-32P]dCTP using Klenow enzyme. Labeled probes were purified with Nuctrap purification columns (Roche). EMSAs were performed as described previously 15, using 105 cpm of probe and 5 μg of nuclear extracts per reaction. DNA-binding complexes were separated by electrophoresis on a 5% polyacrylamide-Tris/glycine-EDTA gel, which was dried and exposed to x-ray film.
AP-1 Reporter Assay
Caco-2 and CD23 shRNA Caco-2 cells were transiently transfected in 6-well plates using Superfect (Qiagen). For each transfection, 2.0 μg of pGL2-AP1 reporter plasmid 16 and 0.2μg pRL (Promega Co.) control reporter vector were mixed with 100 μl of Dulbecco’s modified Eagle’s medium (without serum and antibiotics) and 10 μl of Superfect reagent, incubated at room temperature for 10 min, mixed with 600 μl of Dulbecco’s modified Eagle’s medium complete medium, and immediately added to the cells in 6-well plates. After 24 hours, transfected cells were stimulated with EGF as positive control or Ag-IgE. After 10 h, cells were extracted with reporter lysis buffer (Promega), and 20 μl of extract was assayed for luciferase activity. Cell lysates were prepared and luciferase activity were detected by Dual-Luciferase Reporter Assay System (Promega Co.). Data were expressed as fold change compared to unstimulated cells.
Establishment of shRNA vector-mediated stable CD23 RNAi cell line
The shRNAs were cloned into a LMP retroviral vector (OPEN Biosystems, Huntsville, AL) according to the manufacturer’s instructions. The oligonucleotide targeting CD23 had the following sequence: CGCTGAACAGCAGAGATTGAAA; the non-specificcontrol oligonucleotide’s sequence was as follows: CCCACGGATGCGGCCCCGTGCC. Human 293 EbnaT cells were transfected by using calcium phosphate with 2.5 μg of plasmid pMD. G encoding vesicular stomatitis virus G protein and 7.5 μg of plasmid pMD. OGP encoding gag-pol (both kindly provided by Adrian Ting, Mount Sinai School of Medicine), together with 10 μg of the LMP retroviral construct encoding either shRNA target CD23a or non-specific shRNA. Infected Caco-2 cells were cultured for 48 hours and then selected by using 2 μg/mL of puromycin for 10–14 days. The drug-resistant cells were maintained in selection medium.
AP-1 Assay
Nuclear extracts were prepared using the Nuclear Extraction Kit (Panomics, CA), 5 μg of extract was used to assay AP-1 activity with a TransBinding AP1 Assay Kit (Panomics, Fremont, CA) according the manufacturer’s instructions. AP1 activity was measured at OD450.
Generation of Dendritic Cells and Migration Assay
Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were prepared by Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ). 1.5×108 PBMCs were placed in a T75 flask with 25 ml supplemented RPMI1640, and allowed to adhere for 3 h prior to washing away non-adherent cells three times with 20 ml PBS. Cells were then grown in 25 ml supplemented RPMI 1640 containing 100 ng/ml IL-4 (PeproTech, Rocky Hill, NJ) and 50 ng/ml GM-CSF(Pepro Tech) for 6 days, with a change of media at day 3 to generate immature dendritic cells (DCs). CD11c and CD14 expression were checked by flow cytometry (antibodies from BD Biosciences). Cells were uniformly CD11c positive and CD14 negative at the time of the chemotaxis assay. Migration assays were performed with a 48-Well Micro Chemotaxis Chamber (5 μm pore size, Neuro Probe, Gaithersburg, MD). Briefly, 5000 DCs were added to the apical compartment of the filter and the basal compartment contained the medium to be tested. Cells were allowed to migrate for 3 h prior to removal of non-migrated cells by wiping the upper side of the filter, and the migrated cells were fixed and stained with 0.1% Crystal Violet. The number of migrated DCs on the filter was quantified by image analysis (ImageTool). Inhibition of migration was tested by pre-treating DCs with pertussis toxin (List Biologicals, Campbell, CA) at 200 ng/ml for 2 h, or by pre-treating supernatants with anti-CCL20 (R&D Systems, Minneapolis, MN) or control IgG.
Immunostaining of human duodenal biopsies
Sections were obtained from formalin-fixed paraffin-embedded duodenal biopsies from two groups of children who underwent esophagogastroduodenoscopy with biopsies for various gastrointestinal symptoms. The first group consisted of children with history of food sensitization as detected by a positive prick skin test and/or elevated serum food-specific IgE levels. Of the 12 patients in this group, 6 had duodenal inflammation with eosinophilia due to allergic eosinophilic gastroenteritis and 6 had normal duodenal histology. The second group (13 patients) consisted of subjects with gastrointestinal symptoms but no histologic evidence of duodenal inflammation or evidence of food sensitization by clinical history. 3 μm sections were cut, deparaffinized, and endogenous peroxidase quenched. Antigen retrieval was performed using antigen retrieval solution (pH 6.0) from Dako (Carpinteria, CA) and heating in a microwave. Primary antibody (monoclonal anti-human CCL20, clone 67310, R & D Systems, Minneapolis, MN) or mouse IgG1 (Dako) was incubated for 1 hour at room temperature, followed by detection with biotinylated secondary antibody, avidin-HRP, and DAB chromogen (all reagents from Dako). Sections were counterstained with hematoxylin and coverslipped.
RESULTS
Basolateral IgE-Antigen (IgE-Ag) Complexes Induce Polarized Chemokine Secretion from Caco-2 Cells
Caco-2 cells were chosen for this study as we had previously observed higher baseline CD23 expression in Caco-2 compared to T84 or HT-29 cells. Caco-2 cells were polarized on filter supports prior to addition of IgE-Ag complexes to the apical or basolateral side of the transwell. IL-8 and CCL20 are known to be produced by intestinal epithelial cells under pro-inflammatory conditions 17, 18, and IL-8 has been shown to be elevated in late-phase reactions in the airways and skin 19–21. We observed that Caco-2 cells responded to IgE-Ag complexes, but not antigen or IgE alone, with an upregulation of both IL-8 and CCL20 (Figure 1A and 1B). Although some upregulation was observed in response to apical stimulation, maximal chemokine upregulation was observed when complexes were added to the basolateral side of the transwell.
Figure 1. Polarized responsiveness of intestinal epithelial cells to IgE-Ag.
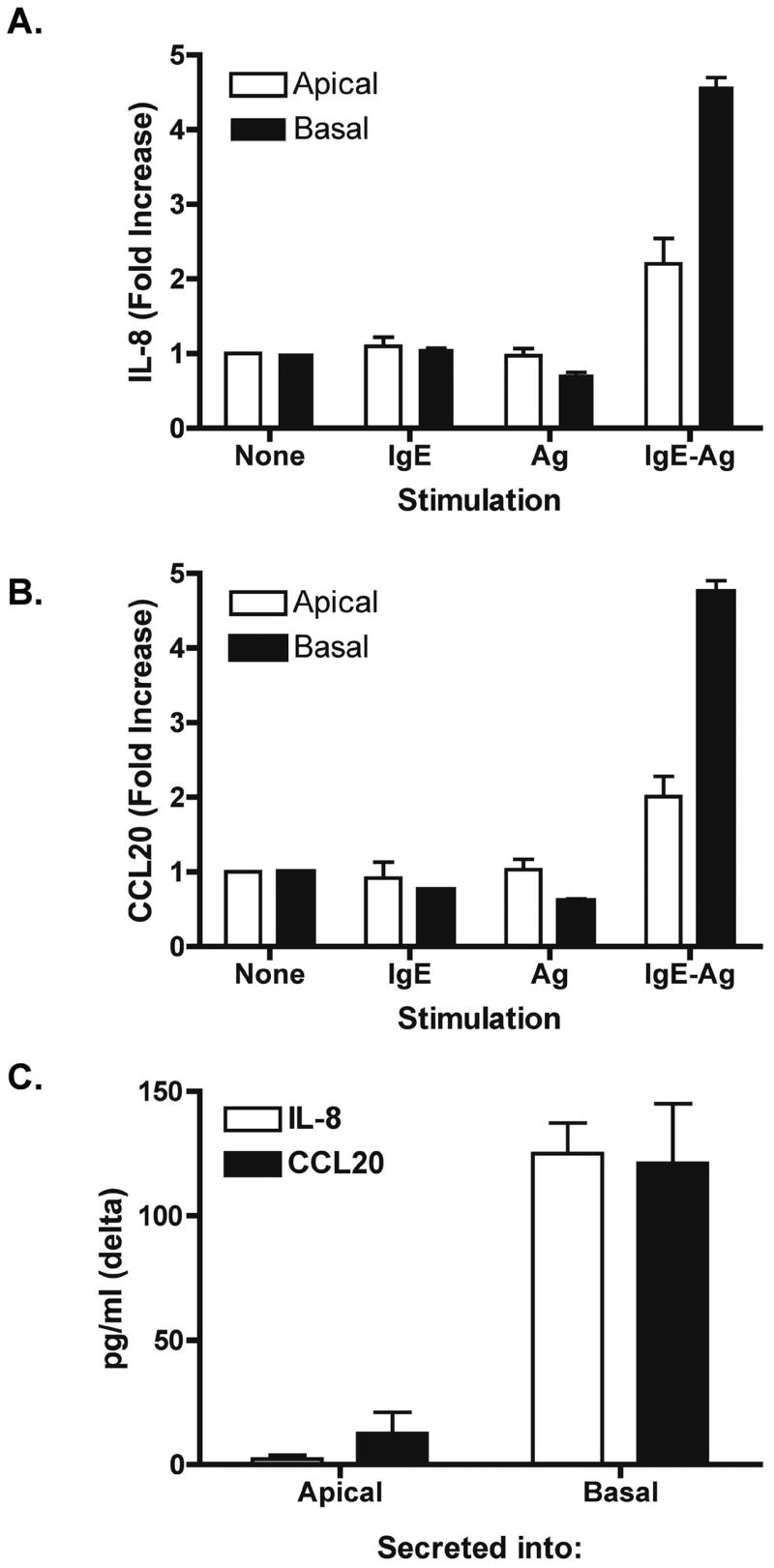
Caco-2 cells were polarized on filter supports and stimulated with anti-NP IgE (IgE), NP-BSA (Ag), or IgE-Ag complexes. Cells were stimulated either on the apical or basal side of the transwell, and IL-8 (A) and CCL20 (B) mRNA expression was assessed by real-time PCR after 6 hours. Data are expressed as fold increase compared to control, mean + SEM, n = 4/group. IL-8 and CCL20 chemokine secretion into the apical or basal transwell was measured 24 h after stimulation with IgE-Ag (C).
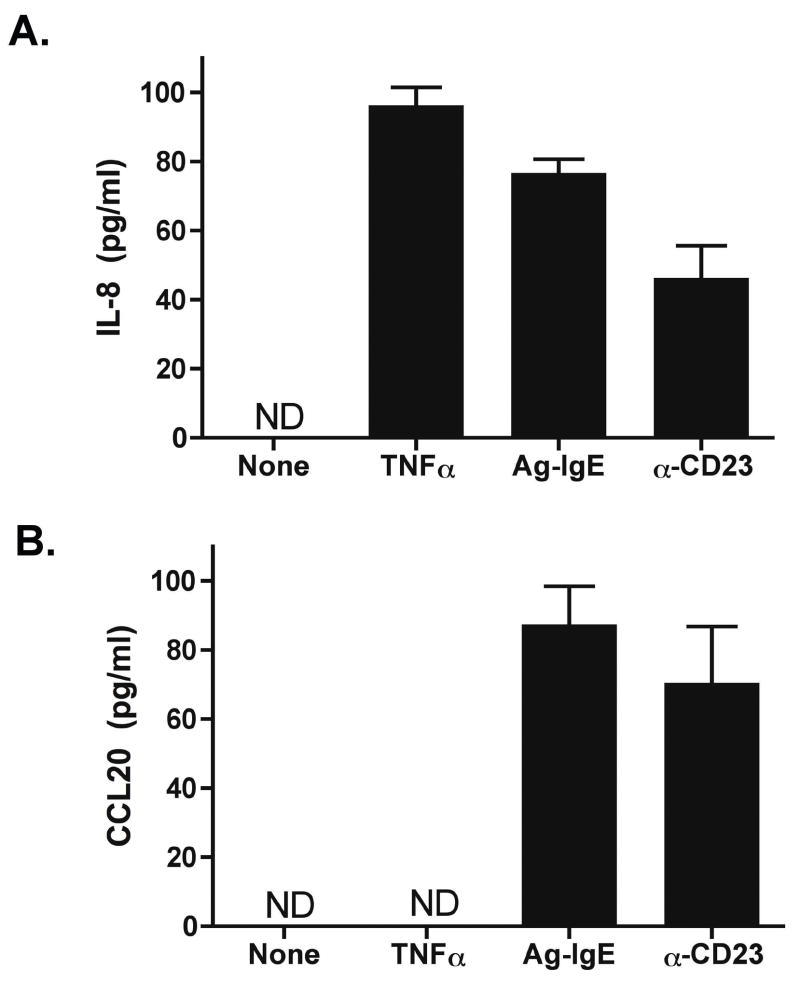
Secretion of IL-8 and CCL20 protein was measured by ELISA in transwell supernatants (Figure 1C). Addition of IgE-Ag complexes to the basolateral transwell induced a significant release of IL-8 and CCL20 into the basolateral, but not the apical, transwell. Thus, direction of chemokine secretion as well as site of responsiveness to IgE-Ag complexes is polarized in human intestinal epithelial cells. This is consistent with previous reports of polarized secretion of these chemokines 17, 18. Levels of IL-8 secreted by Caco-2 cells in response to IgE-Ag were on the same order of magnitude as those obtained using 20 ng/ml of TNFα as a positive control (Figure 4).
Figure 4. CD23 cross-linking induces epithelial chemokine secretion.
Caco-2 cells were polarized and stimulated with TNFα (20 ng/ml), Ag-IgE complexes, or anti-CD23 antibodies + anti-IgG to cross-link. IL-8 (A) and CCL20 (B) release into the basolateral supernatant was measured by ELISA. ND = none detected.
IgE-Ag Complexes Induce Activation of ERK and JNK MAP Kinase Pathways
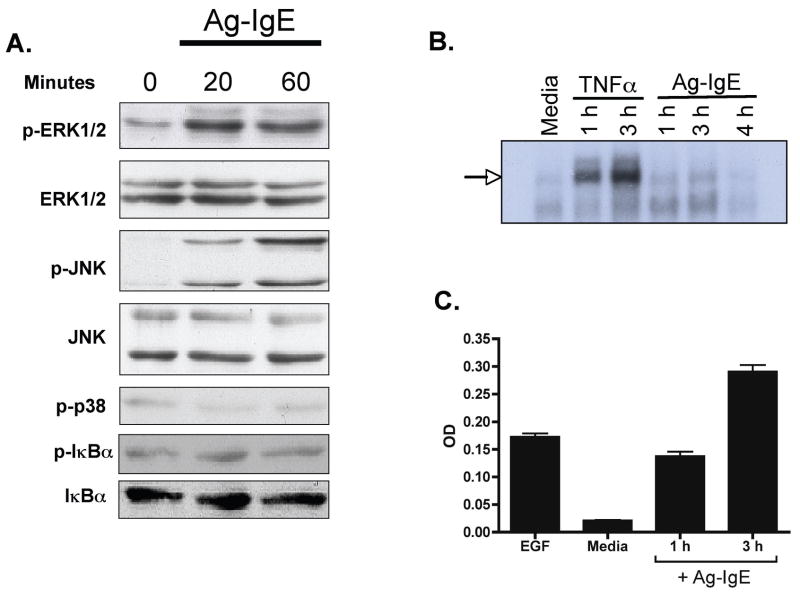
We examined signaling pathways upstream of IL-8 and CCL20, specifically the MAP kinase and NF-κB signaling pathways. Caco-2 cells were polarized on filter supports and stimulated basolaterally with IgE-Ag complexes. At various times after stimulation, cells were lysed and immunoblotting was performed on cell extracts (Figure 2A). Antibodies against phosphorylated and total ERK 1/2, JNK, p38, and IκBα were used. Neither phosphorylation nor degradation of IκBα was observed with allergen-IgE stimulation. In contrast, IgE-Ag stimulation resulted in phosphorylation of ERK and JNK, but not p38. ERK and JNK phosphorylation was observed starting 20 min after stimulation, and was sustained 60 min after stimulation with IgE-Ag. The lack of NF-κB activation was confirmed by EMSA (Fig 2B), indicating that IgE-Ag did not induce nuclear NF-κB binding activity. To determine if ERK and JNK phosphorylation resulted in AP-1 stimulation, we assessed AP-1 activation in nuclear extracts. Stimulation with IgE-Ag complexes induced a significant increase in nuclear activation of AP-1 compared to unstimulated cells (Fig 2C) that was sustained over the 3 h experimental period. This pattern was confirmed using an AP-1 reporter assay (Fig 5F), in which stimulation of Caco-2 cells with either EGF as positive control or Ag-IgE complexes induced AP-1-driven gene expression.
Figure 2. Signaling in intestinal epithelial cells in response to IgE-Ag.
Caco-2 cells were polarized on filter supports and stimulated with anti-NP IgE plus NP-BSA (Ag-IgE) on the basolateral side. A. Immunoblotting for phospho (p−) or total ERK 1/2, JNK, p38, and IκBα. Cell extracts were prepared from unstimulated cells or cells stimulated for 20 or 60 minutes with Ag-IgE. B. EMSA showing NF-κB binding activity in nuclear extracts prepared from unstimulated cells (media), or cells stimulated with TNFα (20 ng/ml) for 1 or 3 h, or IgE-Ag complexes for 1–4 hours. C. AP-1 binding in nuclear extracts prepared from cells that were untreated (media), treated with epidermal growth factor as positive control (EGF), or treated with Ag-IgE for 1 h or 3 h. AP-1 binding in nuclear extracts was determined by an ELISA-based technique.
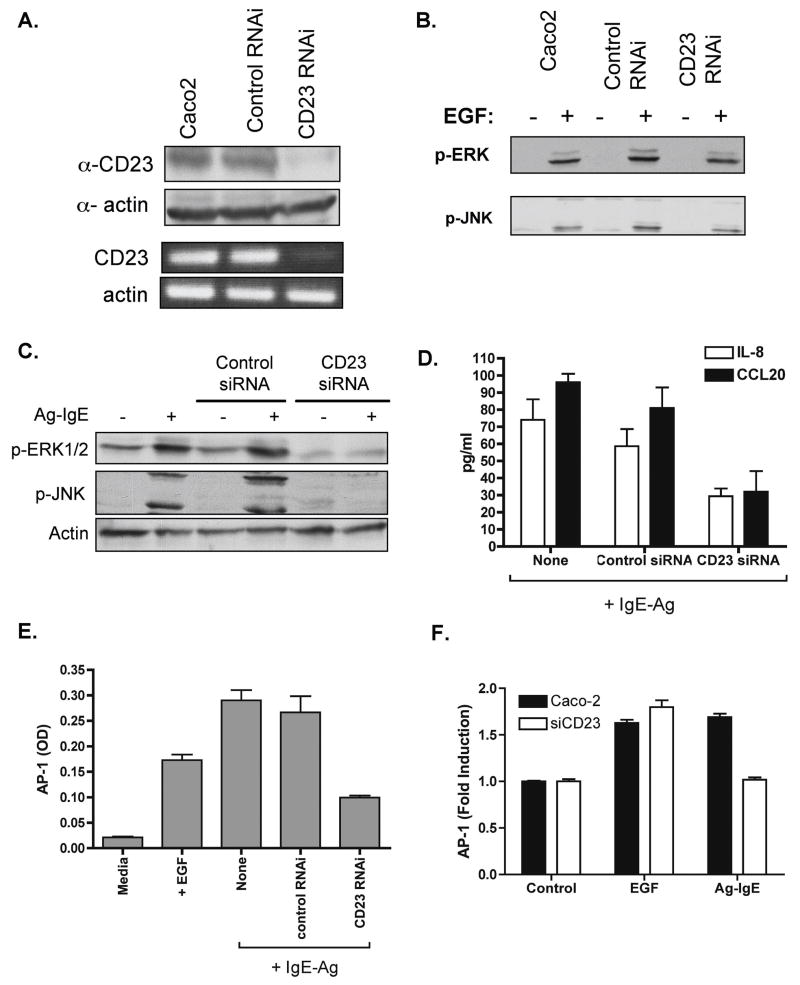
Figure 5. CD23 is required for IgE-Ag-induced activation of epithelial cells.
Caco-2 cells were stably transfected with shRNA targeting CD23, or with control shRNA or were left untransfected. Knockdown of CD23 expression was confirmed by western blotting and RT-PCR (A). Cells were stimulated with EGF and immunoblotting for phospho-ERK and phospho-JNK was performed to show that CD23 RNAi did not interfere with these pathways (B). Cells were then polarized on filter supports and stimulated with IgE-Ag complexes on the basolateral side as above. C. Immunoblotting for phospho (p−) ERK 1/2 and JNK or actin as a loading control. Lysates were prepared 60 min after stimulation. D. IL-8 and CCL20 secretion as measured in the basolateral supernatant 24 h after stimulation with IgE-Ag complexes. E. AP-1 binding activity as measured in cell extracts obtained 60 min after stimulation. F. AP-1 luciferase reporter assay.
IgE-Ag Complex-Induced Chemokine Secretion is ERK- and JNK-dependent
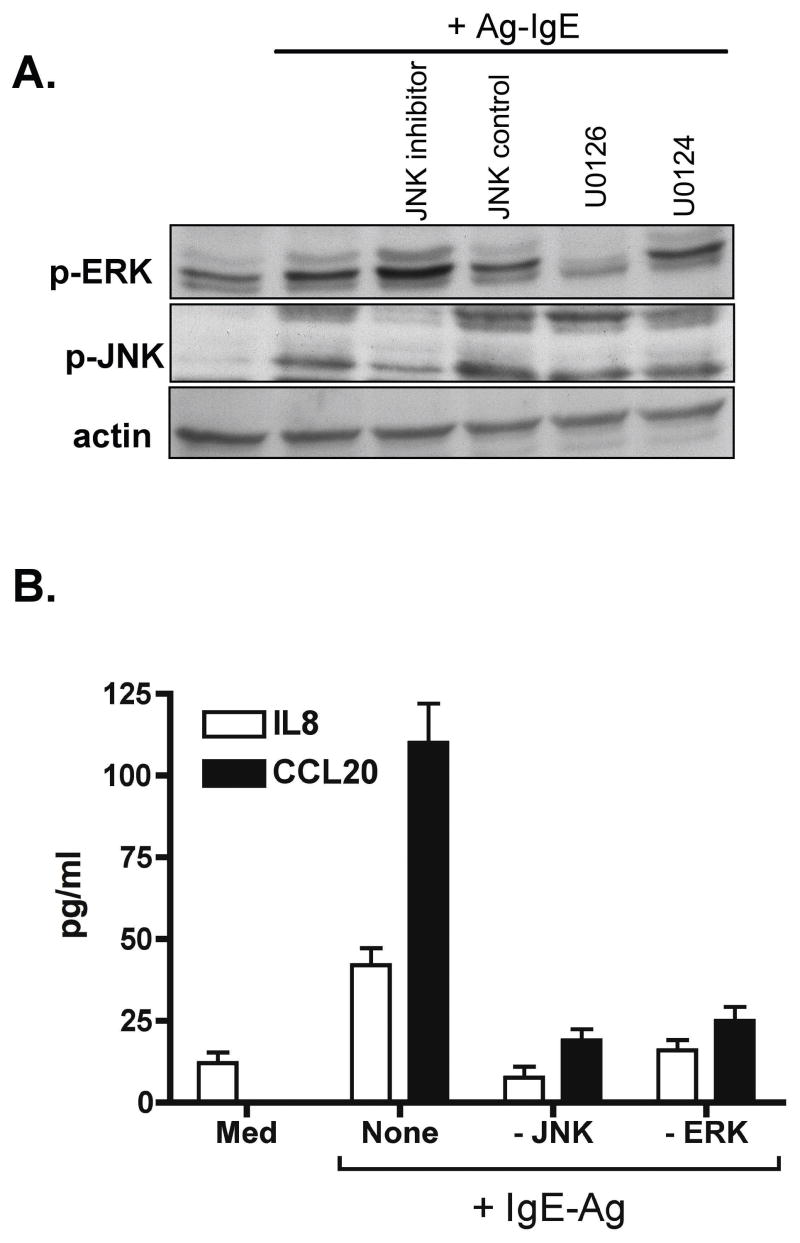
We hypothesized that activation of ERK and JNK signaling pathways was responsible for the upregulation in epithelial chemokine secretion observed after IgE-Ag stimulation. To test this, we used pharmacologic inhibitors of MEK 1/2 (upstream of ERK1/2, using U0126) and JNK (JNK inhibitor II). Pre-treatment of polarized Caco-2 cells with either MEK or JNK inhibitor (at 10 μM concentration) selectively abrogated the phosphorylation of ERK and JNK in response to IgE-Ag, respectively (Fig 3A), and significantly attenuated the secretion of IL-8 and CCL20 induced by IgE-Ag stimulation (Figure 3B).
Figure 3. Effect of JNK and ERK inhibitors on IL-8 and CCL20 release by IgE-Ag-stimulated intestinal epithelial cells.
Caco-2 cells were polarized on filter supports and pre-treated with the MEK inhibitor U0126 or its negative control U0124, or JNK inhibitor II or its negative control prior to stimulation with IgE-Ag complexes (IgE-Ag). A. Cell extracts were obtained 60 min after stimulation and immunoblotting performed for phospho-ERK, phospho-JNK, or actin as loading control. B. Chemokine secretion was measured by ELISA using the basolateral culture supernatant obtained 24 h after stimulation. Data are mean + SEM, n = 3–4/group.
IgE-Ag Complexes Induce Signaling via CD23
We have previously shown that Caco-2 cells constitutively express the CD23a isoform of CD23, and that CD23a transports IgE-Ag complexes intact across the epithelial barrier 7. We hypothesized that CD23 was also responsible for the transduction of signals into the epithelial cell. To test this, we first cross-linked CD23 by the use of anti-CD23 antibodies + anti-IgG. Cross-linking of CD23 induced similar levels of IL-8 (Figure 4A) and CCL20 (Figure 4B) secretion as stimulation with IgE-Ag complexes.
Next we inhibited CD23 expression with the use of shRNA. Stable transfectants were generated using either CD23 or control shRNA, and cells were polarized on filter supports. CD23 knockdown was confirmed by immunoblotting and RT-PCR (Fig 5A). IgE-Ag complex stimulation of cells transfected with control shRNA resulted in phosphorylation of ERK and JNK as was observed in control cells, but inhibition of CD23 expression resulted in complete abrogation of ERK or JNK phosphorylation (Figure 5C). Silencing of CD23 did not impair phosphorylation of ERK or JNK in response to the positive control (EGF, Fig 5B), showing that there was no non-specific disruption of this signaling pathway in response to shRNA targeting CD23. Furthermore, silencing of CD23 significantly attenuated activation of AP-1 (Fig 5E, F), as well as IL-8 and CCL20 secretion (Figure 5D). Therefore we conclude that IgE-Ag complexes activate epithelial cells through CD23.
Epithelial Cells Activated with IgE-Ag Recruit Immature Dendritic Cells
We have shown that IgE-Ag complexes can upregulate IL-8 and CCL20 expression in intestinal epithelial cells. CCL20 is a chemoattractant for immature dendritic cells, memory T lymphocytes, and B lymphocytes, while IL-8 is a chemoattractant for neutrophils and eosinophils, and has been shown to be increased in late-phase allergic inflammation 19–21. We hypothesized that one outcome of epithelial cell stimulation is recruitment of dendritic cells (DCs) that are capable of taking up transcytosed antigen and delivering that antigen for presentation in draining lymph nodes. To assess if stimulated epithelial cells could recruit DCs, we performed migration assays using monocyte-derived dendritic cells and supernatants from stimulated epithelial cells. Basolateral supernatants from IgE-Ag stimulated Caco-2 cells induced a significant (> 5-fold) increase in DC migration across filters of chemotaxis chambers compared to supernatants from unstimulated cells, or cells stimulated with IgE or allergen alone (Figure 6A).
Figure 6. Epithelial Cells Induce CCL20-Dependent Migration of Human Dendritic Cells after Stimulation with IgE-Ag.
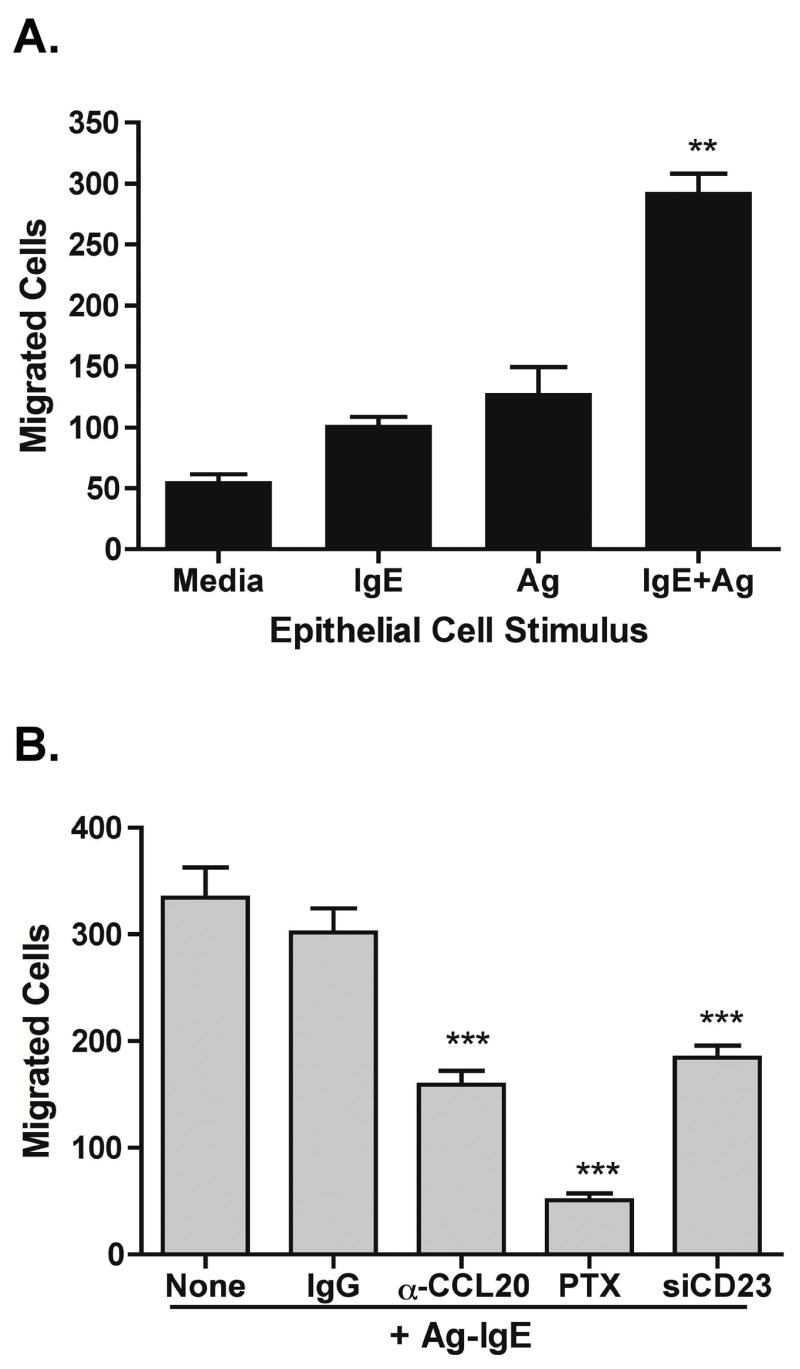
Dendritic cells (DCs) were generated from peripheral blood monocytes by culture with IL-4 and GM-CSF for 6 days. DCs were added to the top chamber of chemotaxis chambers, and supernatant from epithelial cell cultures was added to the basolateral chamber. A. Supernatant was derived from Caco-2 cells stimulated with nothing (media), IgE or antigen (Ag) alone, or IgE-Ag complexes (IgE+Ag). Data are mean + SEM of 6/group. ** p < 0.01 compared to control. B. Supernatant was obtained from Caco-2 cells or cells with CD23 knocked down (siCD23) 24 h after stimulation with Ag-IgE. Supernatant was pre-incubated with neutralizing anti-CCL20 antibody (α-CCL20) or isotype control (IgG). Pertussis toxin (PTX, 200 ng/ml) was added to DCs for 2 h prior to addition of cells to chemotaxis chambers. Data are mean + SEM of 4/group. *** p < 0.001 compared to control.
We next examined the contribution of CCL20 to this recruitment of DCs (Fig 6B). Pre-incubation of epithelial cell supernatants with neutralizing antibodies against CCL20, but not isotype control antibodies, significantly attenuated the migration of DCs in response to IgE-Ag-stimulated epithelial cells. In addition, supernatant from cells transfected with CD23 siRNA had significantly attenuated recruitment of DCs. Thus, CCL20 appears to be the major DC chemoattractant induced in a CD23-dependent manner. Pretreatment of DCs with pertussis toxin to inhibit signaling through all chemokine receptors abolished this migration down to baseline levels, indicating that there were chemokines in addition to CCL20 released by epithelial cells that could recruit DCs.
CCL20 Expression by Human Intestinal Epithelial Cells In Vivo
CCL20 is expressed under inflammatory conditions in human colonic epithelium and in human colonic epithelial cell lines, and has been reported to be constitutively expressed in epithelium overlying lymphoid follicles in the gastrointestinal tract 17, 22. We examined CCL20 expression by immunostaining in duodenal biopsies from subjects with IgE-sensitization to foods, with or without allergic eosinophilic gastroenteritis (AEG). Patients with gastrointestinal symptoms but histologically normal duodenal biopsies and no evidence of IgE sensitization were used as controls. We report CCL20 expression in histologically normal human duodenum. CCL20 expression in duodenal biopsies was patchy, and was restricted to epithelial cells in the villi and the base of the crypts (Figure 7). CCL20 staining was found in 6/12 patients with IgE sensitization (4/6 with AEG and 2/6 with no duodenal inflammation), and 10/13 non-allergic controls. Thus, at baseline conditions (in the absence of challenge to foods to which there is IgE-sensitization) there is evidence for constitutive (yet patchy) expression of CCL20 by duodenal epithelial cells. We hypothesize that acute exposure to allergen could increase this CCL20 expression further, as we observe in our in vitro system.
Figure 7. Expression of CCL20 by Human Duodenal Epithelial Cells In Vivo.
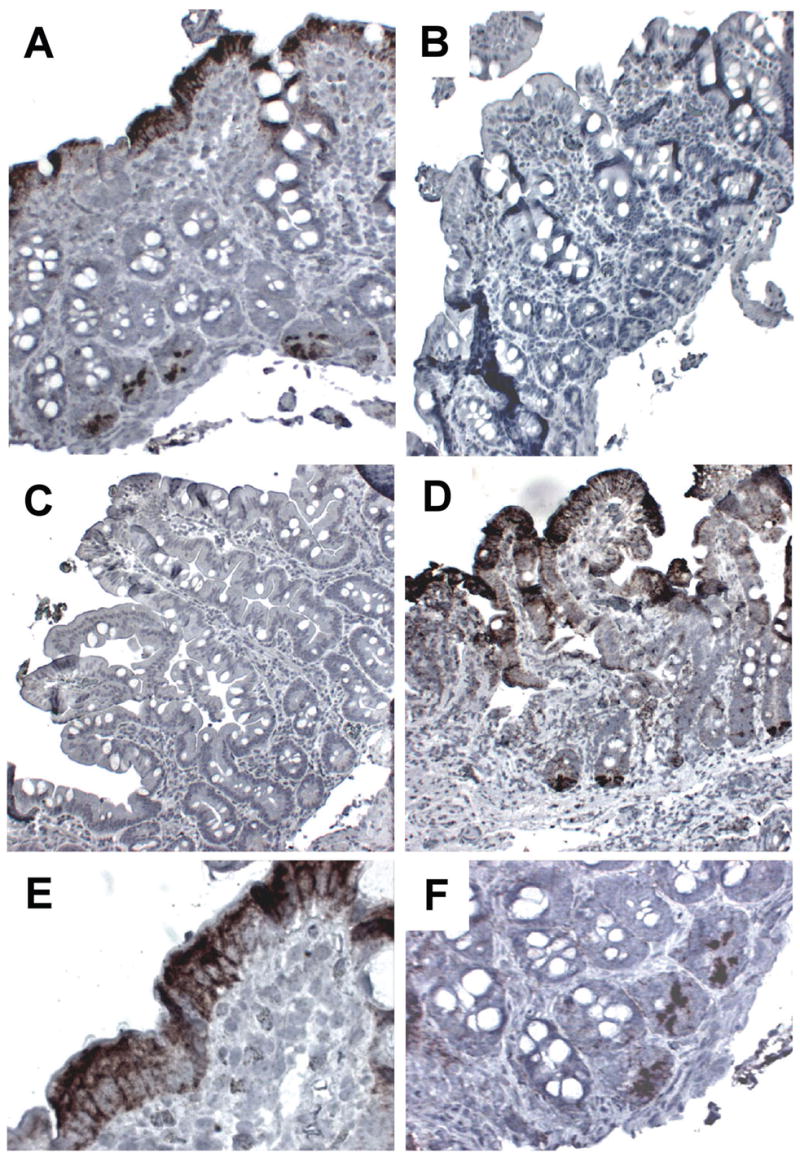
Sections from duodenal biopsies were stained with anti-CCL20 (A,C–F) or isotype control (B). A: Section from a subject with allergic eosinophilic gastroenteritis (AEG). Note the villous blunting, and the positive (brown) immunostaining in the epithelium and the crypts. Original magnification 100x. B: Section from the same biopsy as A, stained with isotype control (100x). C: Section from a non-inflamed control showing a complete absence of CCL20 staining (100x). D: Section from a non-inflamed control showing abundant CCL20 immunoreactivity in villous and crypt epithelium (100x). E: Higher power magnification of the section shown in A, showing detail of positive immunostaining in the surface epithelium (400x). F: Higher power magnification of section shown in A, showing detail of positive immunostaining in the crypt epithelium (200x).
DISCUSSION
We hypothesized that in addition to its function as an antigen capture mechanism, CD23 expressed on human intestinal epithelial cells could trigger upregulation of epithelial chemokines. We provide evidence that IgE-antigen complexes trigger CD23 on the basolateral membrane to induce MAP-kinase signaling and subsequent release of chemokines capable of recruiting DCs and potentially other inflammatory effector cells. We hypothesize that this is a critical second step in the antigen capture mechanism leading to a late-phase allergic response to food allergens in a sensitized individual.
We have previously shown that CD23 is constitutively expressed on human intestinal epithelial cells isolated from colonic resections 7. In addition, Kaiserlian et al reported constitutive CD23 expression by immunostaining in human duodenum and colon 23. The function of CD23 has been most extensively studied on B cells, where it has been shown to be involved in IgE-mediated antigen capture and facilitated presentation to T cells 24, 25. There is a paucity of information on the link between engagement of CD23 on the cell surface and the functional outcome, although the cytoplasmic tail of the CD23a isoform has been shown to be critical for trafficking of IgE 10, 26. Related C-type lectins such as CLEC-2 and Dectin-1 activate signaling pathways through a YXXL/I motif in the cytoplasmic tail, a motif that is also found in CD23a 27. We first tested the effect of IgE-antigen complexes on chemokine expression by the human intestinal epithelial cell line Caco-2 that expresses constitutive levels of CD23a. We observed that complexes on the basolateral side of monolayers preferentially induced the upregulation (and basolateral secretion) of the chemokines IL-8 (IL-8) and CCL20. These chemokines can recruit both inflammatory cells (neutrophils and eosinophils for IL-8) and cells of the adaptive immune system (immature dendritic cells, memory T cells, and B lymphocytes for CCL20). IL-8 and CCL20 (also known as MIP-3α) are expressed by intestinal epithelial cells and are known to be regulated by pro-inflammatory stimuli including pro-inflammatory cytokines and interaction with bacterial pathogens 14, 17. The receptor for CCL20 is CCR6 and is expressed on a range of cell types including immature dendritic cells, B lymphocytes, memory T cells, and epithelial cells. Under baseline conditions CCR6 expression on dendritic cells has been shown to be restricted to the Peyer’s patch in the mouse intestine 28. However, immunostaining for CCR6 in human colonic biopsies indicates positive cells in the subepithelial zone of non-follicular areas of the lamina propria 22. CCL20, and its receptor CCR6, are upregulated in lesional skin of patients with atopic dermatitis 29, although other groups have reported a decrease in CCL20 in atopic dermatitis 30. IL-8 has also been shown to be upregulated in the nasal mucosa and skin following local allergen provocation 19–21. We hypothesize that release of chemokines including IL-8 and CCL20 from intestinal epithelial cells may contribute to late-phase inflammation in the gastrointestinal tract by recruitment of both effector cells and antigen presenting cells.
Polarization of responsiveness is a common feature of intestinal epithelial cells. Toll-like receptors (TLRs) are also polarized for maximal responsiveness at the basolateral surface 31, 32, which is likely to be an effective means of preventing uncontrolled immune responsiveness to luminal contents that cannot breach the epithelial barrier. We have previously shown that CD23 and food-specific IgE are present in the stool of patients with food allergies 7, demonstrating the availability of both IgE and receptor for the uptake of food allergens from the gastrointestinal lumen. Once the IgE-antigen complexes have breached the epithelial barrier, these can then induce the release of chemokines from the epithelium. The molecular basis for this polarization remains to be addressed as we have shown that CD23 is expressed on the apical side of intestinal epithelial cells, and therefore the polarization may reflect access to appropriate signaling molecules at the basolateral but not apical membrane.
We examined MAPK and NF-κB signaling pathways after IgE-antigen stimulation of human intestinal epithelial cells. We observed activation of ERK and JNK, but not p38 or NF-κB by immunoblotting. EMSA assays also showed that NF-κB was not activated in response to IgE-Ag. Although CD23 cross-linking has been shown to activate NF-κB in monocytes and monocytic cell lines 33, 34, these cells express the CD23b isoform that has a different cytoplasmic domain than the CD23a isoform expressed in epithelial cells and B cells. We confirmed that ERK and JNK activation were necessary for downstream chemokine release through the use of pharmacologic inhibitors of MEK and JNK. Therefore the signaling pathways appear to be different than those activated by stimuli such as infection with bacterial pathogens. This is consistent with our finding that known NF-κB target genes such as HBD-2 11 and TSLP 35 are not regulated by IgE-antigen complexes (Li and Berin, unpublished observations). This makes physiological sense as the appropriate response to IgE-antigen (i.e. in the context of helminth infection) would require different effector cells than those recruited to fight bacterial infection.
Epithelial cells express a number of Fc receptors, including but not limited to pIgR and FcRn 36. To show that IgE-antigen complexes were signaling specifically through CD23 we used two approaches. First, we replicated our findings with IgE-Ag stimulation using cross-linked anti-CD23 antibodies. Anti-CD23 induced chemokine secretion when cross-linked at the basolateral surface. Secondly, we inhibited CD23 expression using shRNA. Inhibition of CD23 expression by shRNA resulted in complete abrogation of ERK and JNK phosphorylation in response to IgE-antigen, and significant inhibition of IL-8 and CCL20 secretion. Therefore we conclude that the effects we observe on human intestinal epithelial cells are mediated via CD23 on the cell surface.
We have previously shown that antigen-IgE complexes that are transcytosed across human intestinal epithelial cells can trigger the degranulation of effector cells (we used rat basophil leukemia cells transfected with the human high affinity IgE receptor as a model of mast cells) 7. We hypothesize that antigen is also delivered to antigen presenting cells such as DCs for presentation to T lymphocytes, as has been shown for transepithelial antigen sampling by IgG and FcRn 37–39. Human DCs express the high-affinity IgE receptor and can use this receptor for IgE-mediated antigen focusing 40. We show that epithelial cells release functional quantities of chemoattractants for immature DCs in response to IgE-antigen complexes, and that the major DC chemoattractant released by epithelial cells is CCL20. We speculate that this may be an important step in the amplification of adaptive immune responses to allergen ingestion, or may be a critical step in the initiation of a local late-phase inflammatory response.
We examined CCL20 expression in biopsies from children with history of IgE-sensitization to food proteins with or without allergic eosinophilic gastroenteritis. As controls, we used biopsies from children without history of food allergies or duodenal inflammation. CCL20 was constitutively expressed (in a patchy manner) in the duodenal epithelium, similar to reports of heterogeneous expression in non-inflamed colonic epithelium 17. CCL20 expression was not noticeably elevated in those with IgE sensitization. This is however not surprising given the fact that patients who have known IgE-sensitization to foods are advised to strictly avoid those foods. Our results do however demonstrate that human villous and crypt epithelial cells of the duodenum express CCL20 protein in vivo, which we hypothesize would be further upregulated following acute allergen exposure.
Thus, we have now shown the presence in vivo in food allergic patients of: (1) epithelial CD23 expression 7 (2) luminal food-specific IgE 7, and (3) epithelial CCL20 expression. Our in vitro studies demonstrate that allergen-IgE complexes acting on CD23 upregulate CCL20, and CCL20 can then recruit immature dendritic cells. Together with our previous findings7, these data support a paradigm in which food-specific IgE that is present in the gut lumen of individuals with food allergy can capture antigen, and together with CD23 can traffic the antigen across the epithelium to the lamina propria. Once it reaches the lamina propria, IgE-Ag complexes can trigger hypersensitivity reactions and also signal back to the epithelium to trigger chemokine release to support the influx of a new wave of inflammatory and adaptive immune cells into the gastrointestinal mucosa. We therefore speculate that CD23 is a critical receptor in the initiation of allergic responses in human food allergic disorders.
Acknowledgments
The authors would like to thank Oksana Yershov for excellent technical assistance with immunostaining.
Funding: Funding was provided by NIH grants AI044236 and DK071576 (to MCB)
Footnotes
Conflicts: No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–65. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 3.Lin XP, Magnusson J, Ahlstedt S, Dahlman-Hoglund A, Hanson LL, Magnusson O, Bengtsson U, Telemo E. Local allergic reaction in food-hypersensitive adults despite a lack of systemic food-specific IgE. J Allergy Clin Immunol. 2002;109:879–87. doi: 10.1067/mai.2002.123238. [DOI] [PubMed] [Google Scholar]
- 4.Yang PC, Berin MC, Yu L, Perdue MH. Mucosal pathophysiology and inflammatory changes in the late phase of the intestinal allergic reaction in the rat. Am J Pathol. 2001;158:681–90. doi: 10.1016/S0002-9440(10)64010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wershil BK, Furuta GT, Wang ZS, Galli SJ. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996;110:1482–90. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- 6.Furuta GT, Schmidt-Choudhury A, Wang MY, Wang ZS, Lu L, Furlano RI, Wershil BK. Mast cell-dependent tumor necrosis factor alpha production participates in allergic gastric inflammation in mice. Gastroenterology. 1997;113:1560–9. doi: 10.1053/gast.1997.v113.pm9352858. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Nowak-Wegrzyn A, Charlop-Powers Z, Shreffler W, Chehade M, Thomas S, Roda G, Dahan S, Sperber K, Berin MC. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology. 2006;131:47–58. doi: 10.1053/j.gastro.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Tu Y, Perdue MH. CD23-mediated transport of IgE/immune complexes across human intestinal epithelium: role of p38 MAPK. Am J Physiol Gastrointest Liver Physiol. 2006;291:G532–8. doi: 10.1152/ajpgi.00524.2005. [DOI] [PubMed] [Google Scholar]
- 9.Tu Y, Salim S, Bourgeois J, Di Leo V, Irvine EJ, Marshall JK, Perdue MH. CD23-mediated IgE transport across human intestinal epithelium: inhibition by blocking sites of translation or binding. Gastroenterology. 2005;129:928–40. doi: 10.1053/j.gastro.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Montagnac G, Molla-Herman A, Bouchet J, Yu LC, Conrad DH, Perdue MH, Benmerah A. Intracellular trafficking of CD23: differential regulation in humans and mice by both extracellular and intracellular exons. J Immunol. 2005;174:5562–72. doi: 10.4049/jimmunol.174.9.5562. [DOI] [PubMed] [Google Scholar]
- 11.O’Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–24. [PubMed] [Google Scholar]
- 12.Witthoft T, Eckmann L, Kim JM, Kagnoff MF. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am J Physiol. 1998;275:G564–71. doi: 10.1152/ajpgi.1998.275.3.G564. [DOI] [PubMed] [Google Scholar]
- 13.Eckmann L, Rudolf MT, Ptasznik A, Schultz C, Jiang T, Wolfson N, Tsien R, Fierer J, Shears SB, Kagnoff MF, Traynor-Kaplan AE. D-myo-Inositol 1,4,5,6-tetrakisphosphate produced in human intestinal epithelial cells in response to Salmonella invasion inhibits phosphoinositide 3-kinase signaling pathways. Proc Natl Acad Sci U S A. 1997;94:14456–60. doi: 10.1073/pnas.94.26.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckmann L, Kagnoff MF, Falco MT. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994;82:505. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Kong HJ, Li H, Huang B, Yang M, Zhu C, Bogunovic M, Zheng F, Mayer L, Ozato K, Unkeless J, Xiong H. IRF-8/interferon (IFN) consensus sequence-binding protein is involved in Toll-like receptor (TLR) signaling and contributes to the cross-talk between TLR and IFN-gamma signaling pathways. J Biol Chem. 2006;281:10073–80. doi: 10.1074/jbc.M507788200. [DOI] [PubMed] [Google Scholar]
- 16.Hedin KE, Bell MP, Huntoon CJ, Karnitz LM, McKean DJ. Gi proteins use a novel beta gamma- and Ras-independent pathway to activate extracellular signal-regulated kinase and mobilize AP-1 transcription factors in Jurkat T lymphocytes. J Biol Chem. 1999;274:19992–20001. doi: 10.1074/jbc.274.28.19992. [DOI] [PubMed] [Google Scholar]
- 17.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–9. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann L, Jung HC, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–97. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 19.KleinJan A, Dijkstra MD, Boks SS, Severijnen LA, Mulder PG, Fokkens WJ. Increase in IL-8, IL-10, IL-13, and RANTES mRNA levels (in situ hybridization) in the nasal mucosa after nasal allergen provocation. J Allergy Clin Immunol. 1999;103:441–50. doi: 10.1016/s0091-6749(99)70469-0. [DOI] [PubMed] [Google Scholar]
- 20.Zweiman B, Kaplan AP, Tong L, Moskovitz AR. Cytokine levels and inflammatory responses in developing late-phase allergic reactions in the skin. J Allergy Clin Immunol. 1997;100:104–9. doi: 10.1016/s0091-6749(97)70201-x. [DOI] [PubMed] [Google Scholar]
- 21.Erin EM, Zacharasiewicz AS, Nicholson GC, Tan AJ, Higgins LA, Williams TJ, Murdoch RD, Durham SR, Barnes PJ, Hansel TT. Topical corticosteroid inhibits interleukin-4, -5 and -13 in nasal secretions following allergen challenge. Clin Exp Allergy. 2005;35:1608–14. doi: 10.1111/j.1365-2222.2005.02381.x. [DOI] [PubMed] [Google Scholar]
- 22.Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn’s disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–7. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiserlian D, Lachaux A, Grosjean I, Graber P, Bonnefoy JY. Intestinal epithelial cells express the CD23/Fc epsilon RII molecule: enhanced expression in enteropathies. Immunology. 1993;80:90–5. [PMC free article] [PubMed] [Google Scholar]
- 24.van der Heijden FL, Joost van Neerven RJ, van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993;150:3643–50. [PubMed] [Google Scholar]
- 25.Getahun A, Hjelm F, Heyman B. IgE enhances antibody and T cell responses in vivo via CD23+ B cells. J Immunol. 2005;175:1473–82. doi: 10.4049/jimmunol.175.3.1473. [DOI] [PubMed] [Google Scholar]
- 26.Yokota A, Kikutani H, Tanaka T, Sato R, Barsumian EL, Suemura M, Kishimoto T. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988;55:611–8. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]
- 27.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type Lectin Receptors CLEC-2 and Dectin-1, but Not DC-SIGN, Signal via a Novel YXXL-dependent Signaling Cascade. J Biol Chem. 2007;282:12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, McCormick BA, Pazos MA, Vella AT, Lefrancois L, Reinecker HC, McSorley SJ. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–32. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama T, Fujisawa R, Yamada H, Horikawa T, Kawasaki H, Hieshima K, Izawa D, Fujiie S, Tezuka T, Yoshie O. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13:95–103. doi: 10.1093/intimm/13.1.95. [DOI] [PubMed] [Google Scholar]
- 30.Kim BE, Leung DY, Streib JE, Boguniewicz M, Hamid QA, Howell MD. Macrophage inflammatory protein 3alpha deficiency in atopic dermatitis skin and role in innate immune response to vaccinia virus. J Allergy Clin Immunol. 2007;119:457–63. doi: 10.1016/j.jaci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–36. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 33.Ten RM, McKinstry MJ, Trushin SA, Asin S, Paya CV. The signal transduction pathway of CD23 (Fc epsilon RIIb) targets I kappa B kinase. J Immunol. 1999;163:3851–7. [PubMed] [Google Scholar]
- 34.Ten RM, McKinstry MJ, Bren GD, Paya CV. Signal transduction pathways triggered by the FcepsilonRIIb receptor (CD23) in human monocytes lead to nuclear factor-kappaB activation. J Allergy Clin Immunol. 1999;104:376–87. doi: 10.1016/s0091-6749(99)70382-9. [DOI] [PubMed] [Google Scholar]
- 35.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 36.Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–55. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, Blumberg RS. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida M, Masuda A, Kuo TT, Kobayashi K, Claypool SM, Takagawa T, Kutsumi H, Azuma T, Lencer WI, Blumberg RS. IgG transport across mucosal barriers by neonatal Fc receptor for IgG and mucosal immunity. Springer Semin Immunopathol. 2006;28:397–403. doi: 10.1007/s00281-006-0054-z. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–83. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 40.von Bubnoff D, Novak N, Kraft S, Bieber T. The central role of FcepsilonRI in allergy. Clin Exp Dermatol. 2003;28:184–7. doi: 10.1046/j.1365-2230.2003.01209.x. [DOI] [PubMed] [Google Scholar]