SUMMARY
Human papillomavirus (HPV) infects large numbers of women worldwide and is present in more than 99% of all cervical cancers. HPV E6 and E7 are two viral oncoproteins that are consistently expressed in HPV infections and HPV-associated malignancies. We have previously developed DNA vaccines encoding calreticulin (CRT) linked either to HPV type 16 (HPV-16) E6 or to HPV-16 E7, both of which generated significant antitumor effects against E6- and E7-expressing tumors. In this study, we demonstrate that simultaneous vaccination of C57BL/6 mice or HLA-A2 transgenic mice with both CRT/E6 and CRT/E7 DNA vaccines generates significant E6- and E7-specific T-cell immune responses in vaccinated mice. Furthermore, combined vaccination with both CRT/E6 and CRT/E7 DNA generates significantly better therapeutic antitumor effects against HPV E6- and E7-expressing tumors than vaccination with either CRT/E6 DNA or CRT/E7 DNA alone. Our data suggest that it may be desirable to combine DNA vaccines targeting E6 with DNA vaccines targeting E7 to develop effective immunotherapeutic strategies for control of HPV infection and HPV-associated lesions in a clinical setting.
Keywords: HLA-A2 transgenic mice, calreticulin, human papillomavirus (HPV), E6, E7, DNA vaccines
INTRODUCTION
DNA vaccines are considered a potentially valuable form of antigen-specific immunotherapy because of their safety, ease of production, and stability. The gene gun system can efficiently transduce DNA encoding antigen into professional antigen presenting cells (APCs), called Langerhans cells, that reside in the epidermis 1. Transduced APCs express the antigen encoded by the DNA vaccine and present peptides derived from this antigen via the major histocompatability complex (MHC) pathways. These APCs are capable of migrating from the site of vaccination to the draining lymph nodes, where they prime antigen-specific T cells 2.
Because the gene gun technique introduces DNA directly into APCs, strategies that target antigen to various subcellular compartments within APCs can be used to enhance antigen presentation by APCs. Previously, we developed several intracellular targeting strategies to enhance DNA vaccine potency against human papillomavirus (HPV), a virus that infects large numbers of women in the world and that is present in more than 99% of all cervical cancers. These targeting strategies enhanced MHC class I and/or MHC class II presentation of HPV E7 antigen by linking E7 to calreticulin (CRT) 3, to Mycobacterium tuberculosis heat shock protein 70 (HSP70) 4, to the translocation domain of Pseudomonas aeruginosa exotoxin A (ETA(dII)) 5, or to a sorting signal and the lysosome-associated membrane protein I (LAMP-1) 6. While each of these four strategies was capable of augmenting E7-specific T-cell responses and antitumor effects in vaccinated mice compared with E7 DNA alone, we observed in a head-to-head comparison that mice vaccinated with CRT linked to E7 generate the highest quantities of E7-specific CD8+ T-cell precursors as well as the highest numbers of E7-specific CD8+ memory T cells, resulting in potent tumor treatment and long-term tumor protection 7.
Our previous studies have focused on vaccinating against HPV type 16 (HPV-16) E7 antigen because HPV-16 is the HPV type most commonly associated with cervical cancer 8. Furthermore, HPV E7 is an intracellular protein that is consistently expressed throughout HPV infections and HPV-associated malignancies. While vaccination against HPV-16 E7 has been our focus, we have also recently developed a DNA vaccine against HPV-16 E6 9. HPV E6 represents a second potential target for therapeutic HPV vaccines. Like E7, E6 is an intracellular protein that is consistently expressed in HPV infections and HPV-associated malignances. Both E6 and E7 are required for the induction and maintenance of HPV-associated malignancies (for review, see 10), making antigen loss for evasion of an immune response to E6 or E7 vaccination highly unlikely for HPV-associated malignancies. Thus, vaccination targeting HPV-16 E6 is an alternate approach to eliciting HPV-specific antitumor effects. In addition, generation of both E6- and E7-specific immunity by vaccination with a combination of E6- and E7-expressing DNA may have a combined therapeutic effect greater than either E6 or E7 vaccination alone.
To test this hypothesis, C57BL/6 mice as well as HLA-A2 transgenic mice were vaccinated with combinations of CRT/E6 and CRT/E7 DNA vaccines and tested for generation of E6- and E7-specific T-cell responses. In addition, vaccination of mice with established E6- and E7-expressing tumors was used to assess tumor treatment effects. We showed that vaccination with CRT/E6 DNA vaccines and CRT/E7 DNA vaccines can generate simultaneous E6 and E7-specific CD8+ T cell immune responses in vaccinated mice. We also demonstrated that vaccination with the CRT/E6 and CRT/E7 DNA constructs generated the most potent antitumor effects in mice compared to vaccination with CRT/E7 or CRT/E6 DNA alone. The synergistic effects observed when CRT/E6 and CRT/E7 vaccines are combined may have implications for improving clinical HPV vaccine potency.
RESULTS
DC-1 cells transfected with pcDNA3-CRT/E6 or pcDNA3-CRT/E7 express CRT/E6 or CRT/E7, respectively
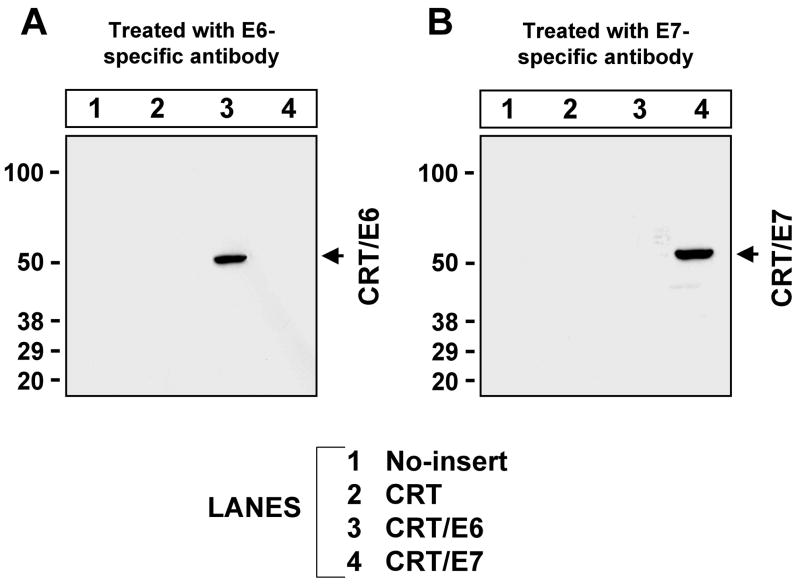
To verify that DC-1 cells transfected with pcDNA3-CRT/E6 or pcDNA3-CRT/E7 constructs are capable of expressing the encoded CRT/E6 or CRT/E7 fusion proteins, respectively, we transiently transfected DC-1 cells with pcDNA3, pcDNA3-CRT, pcDNA3-CRT/E6, or pcDNA3-CRT/E7. Protein extracts from these cells were separated by SDS-PAGE and incubated with either rabbit anti-HPV-16 E6 polyclonal antibody or mouse anti-HPV-16 E7 monoclonal antibody. As seen in Figure 1, Western blot analysis using anti-HPV-16 E6 antibody revealed a band with approximately the predicted size of CRT/E6 protein present in cell lysate from CRT/E6 transfected cells. Western blot analysis using anti-HPV-16 E7 antibody revealed a band with approximately the predicted size of CRT/E7 protein in cell lysate from CRT/E7 transfected cells. These data indicate that DC-1 cells transfected with CRT/E6 DNA or CRT/E7 DNA were capable of effectively expressing CRT/E6 or CRT/E7.
Figure 1. Western blot analysis to detect the expression of HPV-16 E6 and E7 antigens in DC-1 cells transfected with various DNA constructs.
DC-1 cells were transfected with pcDNA3-no insert, pcDNA3-CRT, pcDNA3-CRT/E6 or pcDNA3-CRT/E7. Western blot analysis was performed with 50μg of cell lysates 24 hours after transfection. E6 and E7 were detected using rabbit anti-HPV16-E6 or mouse anti-HPV16-E7 monoclonal antibody. (A) Detection of E6 expression. (B) Detection of E7 expression. The specific bands are indicated by arrows.
Vaccination of C57BL/6 mice with pcDNA3-CRT/E6 and/or pcDNA3-CRT/E7 generates E6- and/or E7-specific CD8+ T-cell immune responses in mice
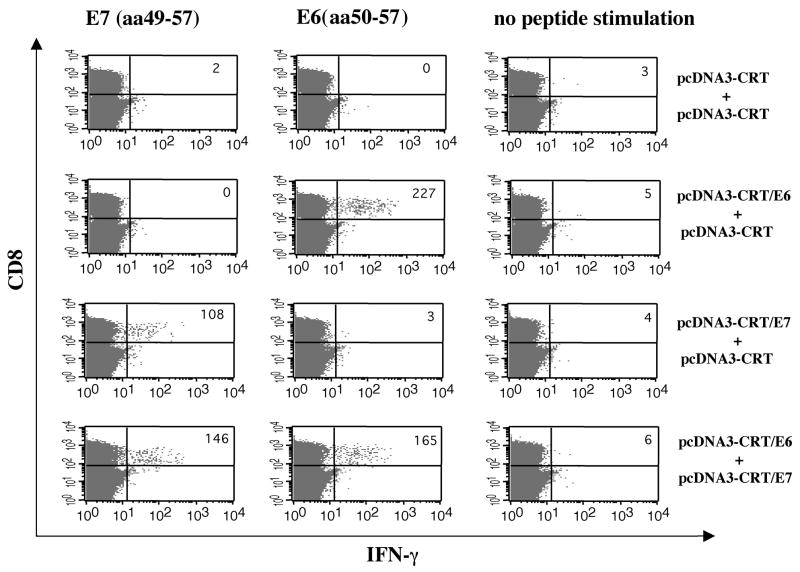
To explore whether mice vaccinated with pcDNA3-CRT/E6 and/or pcDNA3-CRT/E7 mount E6 and/or E7-specific CD8+ T cell immune responses, we used intracellular cytokine staining followed by flow cytometry analysis to determine the number of E6- and/or E7-specific IFN-γ-secreting CD8+ T cells present in vaccinated C57BL/6 mice. As shown in Figure 2, vaccination with pcDNA3-CRT/E6 and pcDNA3-CRT generated E6-specific IFN-γ-secreting CD8+ T cells only; vaccination with pcDNA3-CRT/E7 and pcDNA3-CRT generated E7-specific IFN-γ-secreting CD8+ T cells only; and vaccination with both pcDNA3-CRT/E6 and pcDNA3-CRT/E7 generated both E6- and E7-specific IFN-γ-secreting CD8+ T cells. The number of E6-specific T cells generated by mice vaccinated with the combination of pcDNA3-CRT/E6 and pcDNA3-CRT/E7 was similar to the number of E6-specific T cells generated by vaccination with CRT/E6 and CRT DNA constructs. Likewise, the number of E7-specific T cells generated by mice vaccinated with the combination of CRT/E6 DNA and CRT/E7 DNA was comparable to the number of E7-specific T cells generated by vaccination with CRT/E7 and CRT DNA constructs. Thus, simultaneous vaccination with the two antigen-specific DNA vaccines in two separate locations on the mouse did not diminish the antigen-specific immune response when compared to each of the DNA vaccines administered alone. Our data indicate that CRT/E6 DNA vaccines and CRT/E7 DNA vaccines may be used together without sacrificing the strength of the antigen-specific CD8+ T cell immune response generated by each of the DNA vaccines administered alone.
Figure 2. Intracellular cytokine staining followed by flow cytometry analysis to determine E6 or E7-specific IFN-γ-secretingCD8+ T cells in vaccinated mice.
C57BL/6 mice (5 per group) were immunized and boosted intradermally via gene gun with the following vaccination groups: 1) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen), 2) pcDNA3-CRT/E7 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen) or 3) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT/E7 (2μg/mouse, left side of the abdomen). Splenocytes were harvested one week after the last vaccination and stimulated with either E7 (aa49-57) or E6 (aa50-57) peptide. Splenocytes without peptide stimulation were used as a negative control. The splenocytes were stained for both CD8 and intracellular IFN-γ. The numbers on the right upper corner represent the number E7-specific IFN-γ-secreting CD8+ T cells per 3×105 splenocytes acquired. The data presented in this figure are from one representative experiment of two performed.
Treatment with pcDNA3-CRT/E6 and pcDNA3-CRT/E7 leads to the greatest reduction of pulmonary tumor nodules in C57BL/6 mice
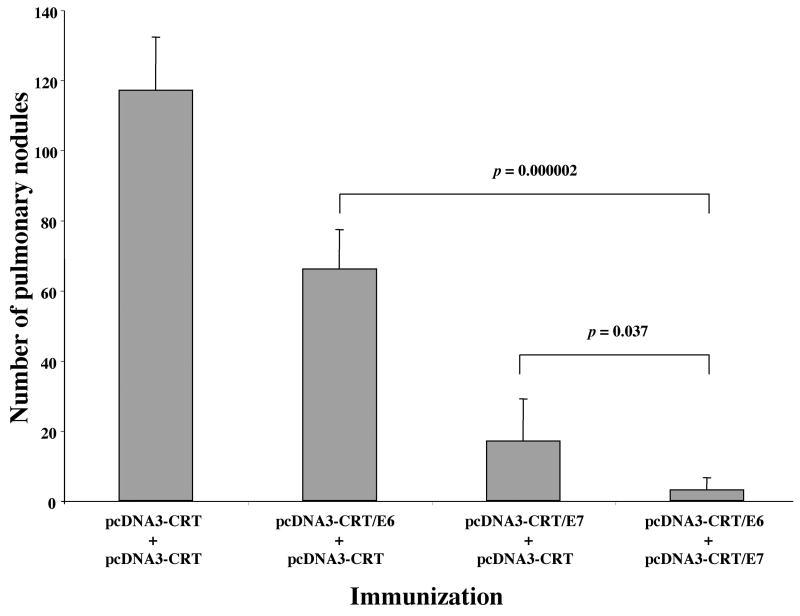
The therapeutic potential of combined pcDNA3-CRT/E6 and pcDNA3-CRT/E7 vaccination was assessed by performing an in vivo tumor treatment experiment using E6- and E7-expressing TC-1 tumor cells in a previously described lung hematogenous spread model 11. As seen in Figure 3, C57BL/6 mice treated with both CRT/E6 and CRT/E7 DNA constructs exhibited the lowest mean number of pulmonary tumor nodules (3 ± 4) compared to mice treated with CRT/E7 DNA and CRT DNA (17 ± 12), CRT/E6 DNA and CRT DNA (66 ± 11) or CRT DNA alone (117 ± 15) (t test, P < .05). Notably, the antitumor effects generated by mice vaccinated with CRT/E6 and CRT/E7 are significantly better than the antitumor effects generated by mice vaccinated with CRT/E7 alone (t test, P<.05). Mice treated with CRT/E6 DNA and CRT DNA and mice treated with CRT/E7 DNA and CRT DNA developed significantly fewer pulmonary tumor nodules than mice vaccinated with CRT DNA alone (t test, P < .01). Furthermore, mice treated with CRT/E7 DNA and CRT DNA exhibited a better therapeutic effect with than mice treated with CRT/E6 DNA and CRT DNA (t test, P < .01). These data demonstrate that vaccination with the CRT/E6 and CRT/E7 DNA constructs generated more potent antitumor effects in mice than vaccination with CRT/E7 or CRT/E6 DNA alone, supporting the rationale for simultaneous administration of both CRT/E6 and CRT/E7 DNA vaccines in the clinical arena.
Figure 3. In vivo tumor treatment experiment to compare the antitumor effect generated by various vaccination groups in mice.
C57BL/6 mice (5 per group) were challenged with 1×105 TC-1 tumor cells via tail vein injection. Three days later, the mice were immunized and boosted as described in the Materials and Methods section. Twenty one days after tumor challenge, the mice were sacrificed, and the lung tumor nodules were counted. The data are expressed as mean number of lung nodules ±S.D. The data shown represent one experiment of two performed.
Vaccination with pcDNA3-CRT/E6 and pcDNA3-CRT/mtE7(del aa49-57) generates HLA-A2-restricted E6 (aa29-38)- and E7 (aa11-20)-specific CD8+ T-cell immune responses in HLA-A2 transgenic mice
HLA transgenic mice may potentially be useful for evaluating HLA-restricted HPV antigen-specific CD8+ T cell immune responses and for correlating these responses with similar responses observed in humans12. We have recently employed HLA-A2 (AAD) transgenic mice to characterize immune responses to E7 DNA vaccines 13. Because HLA-A2 transgenic mice also express murine MHC class I molecules (H-2Db and Kb), when HLA-A2 transgenic mice were vaccinated with DNA encoding CRT linked to wild type E7, the vaccinated mice generated predominantly H-2Db-restricted E7-specific CD8+ T cell immune responses. We observed that the presence of the E7 49-57 amino-acid sequence within the E7 gene of the CRT/E7 DNA vaccine suppresses the efficient presentation of the E7 (aa11-20) epitope through the HLA-A2 molecule in HLA-A2 transgenic mice. In order to avoid this situation and accurately characterize the HLA-A2-restricted E7-specific CD8+ T cell immune response in HLA-A2 transgenic mice, we generated pcDNA3-CRT/mtE7 (del aa49-57), a DNA vaccine that deletes aa49-57 from the E7 gene. We found that vaccination with pcDNA3-CRT/E7(del aa49-57) generates HLA-A2-restricted E7 (aa11-20) peptide-specific CD8+ T cell immune response in HLA-A2 transgenic mice13.
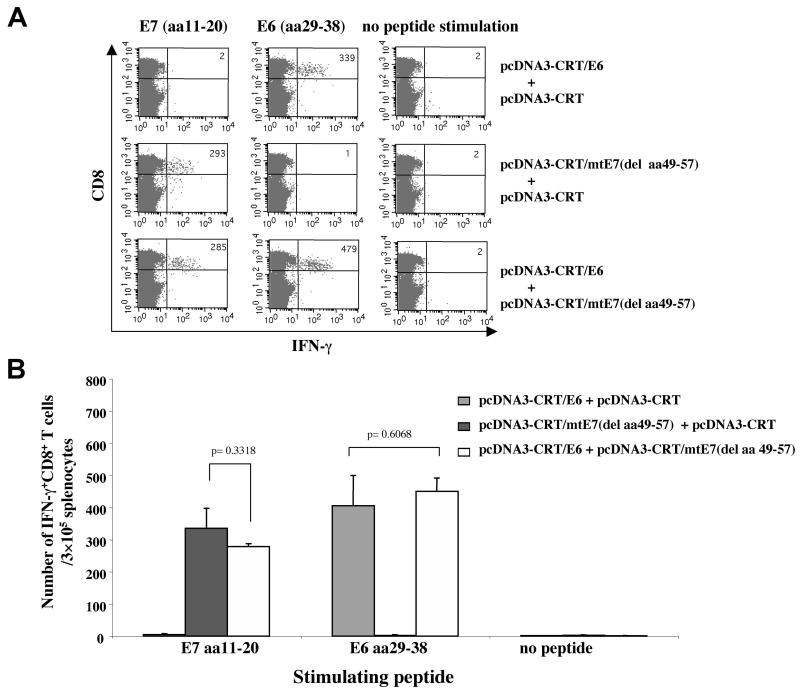
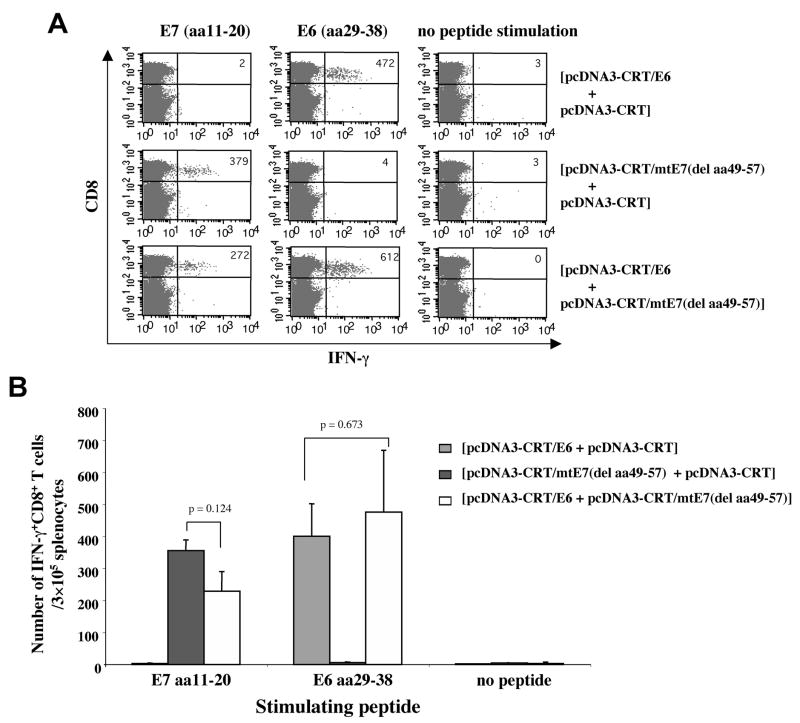
In the current study, we characterized the HLA-A2-restricted E6- and/or E7-specific CD8+ T cell immune responses generated by vaccination with pcDNA3-CRT/E6 and/or pcDNA3-CRT/mtE7(del aa49-57) in HLA-A2 transgenic mice by intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 4, vaccination with pcDNA3-CRT/E6 and pcDNA3-CRT/mtE7(del aa49-57) generated comparable numbers of HLA-A2-restricted E6 (aa29-38)-specific CD8+ T cells to vaccination with pcDNA3-CRT/E6 without pcDNA3-CRT/mtE7(del aa49-57). Similarly, vaccination with pcDNA3-CRT/E6 and pcDNA3-CRT/mtE7(del aa49-57) generated comparable numbers of HLA-A2-restricted E7 (aa11-20)-specific CD8+ T cells to vaccination with pcDNA3-CRT/mtE7(del aa49-57) without pcDNA3-CRT/E6. Our data indicate that both CRT/E6 DNA vaccines and CRT/mtE7(del aa49-57) DNA vaccines may be used together without compromising the strength of the HLA-A2-restricted antigen-specific CD8+ T cell immune response generated by each of the DNA vaccines administered alone.
Figure 4. Intracellular cytokine staining and flow cytometry analysis of E6 or E7-specific IFN-γ-secreting CD8+ T cells in vaccinated HLA-A*0201mice.
HLA-A*0201 transgenic mice (AAD, on C57BL/6 background) (5 per group) were immunized and boosted intradermally via gene gun with the following vaccination groups: 1) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen), 2) pcDNA3-CRT/mtE7(del aa49-57) (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen) or 3) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT/mtE7(del aa49-57) (2μg/mouse, left side of the abdomen). Splenocytes from vaccinated mice were harvested one week after the last boost and stimulated with either E7 (aa11-20) or E6 (aa29-38) peptides. Splenocytes without peptide stimulation were used as a negative control. The cells were then stained for both CD8 and intracellular IFN-γ. (A) Representative figure of the flow cytometry data. (B) Bar graph depicting the number of antigen-specific IFN-gamma-secreting CD8+ T-cell precursors/3 × 105 splenocytes (mean±S.D.).
Administration of CRT/E6 and CRT/mtE7(del aa49-57) DNA vaccines mixed in the same bullet generates similar numbers of E6- and E7 -specific CD8+ T-cells in HLA-A2 transgenic mice as administration of CRT/E6 and CRT/mtE7(del aa49-57) DNA vaccines in separate bullets
The administration of a DNA vaccine in a clinical setting requires simplicity. Thus, we explored whether vaccination with pcDNA3-CRT/E6 and pcDNA3-CRT/mtE7(del aa49-57) combined in a single bullet would generate E6- and E7-specific HLA-A2-restricted immune responses comparable to vaccination with pcDNA3-CRT/E6 and pcDNA3-CRT/mtE7(delaa49-57) administered in separate bullets. We used intracellular cytokine staining followed by flow cytometry analysis to determine the number of E6- and E7-specific IFN-γ-secreting CD8+ T cells present in vaccinated HLA-A2 transgenic mice. As shown in Figure 5, mice vaccinated with pcDNA3-CRT/E6 combined with pcDNA3-CRT/mtE7(del aa49-57) in the same bullet generated comparable numbers of E6- and E7-specific CD8+ T cells to mice vaccinated with pcDNA3-CRT/E6 or pcDNA3-CRT/mtE7(del aa49-57) alone. Taken together, Figures 4 and 5 indicate that administration of both CRT/E6 and CRT/mtE7(del aa49-57) DNA vaccines in one bullet generates comparable numbers of E6- and E7-specific CD8+ T-cells immune responses in HLA-A2 transgenic mice to administration of CRT/E6 and CRT/mtE7(del aa49-57) DNA vaccines in separate bullets.
Figure 5. Intracellular cytokine staining and flow cytometry analysis of HLA-A*0201 restricted E6 and E7-specific IFN-γ-secreting CD8+ T cells in vaccinated mice.
HLA-A*0201 transgenic mice (AAD, on C57BL/6 background) (5 per group) were immunized and boosted intradermally via gene gun with the following vaccination groups: 1) pcDNA3-CRT/E6 and pcDNA3-CRT, 2) pcDNA3-CRT/mtE7(del aa49-57) and pcDNA3-CRT or 3) pcDNA3-CRT/E6 and pcDNA3-CRT/mtE7(del aa49-57). For each vaccination, the two DNA constructs were mixed evenly in the same bullet and the dose for each construct was 2μg/mouse. Splenocytes were harvested one week after the last immunization and stimulated with either E7 (aa11-20) or E6 (aa29-38) peptides. The cells were then stained for both CD8 and intracellular IFN-γ. (A) Representative figure of the flow cytometry data. (B) Bar graph depicting the number of antigen-specific IFN-gamma-secreting CD8+ T-cell precursors/3 × 105 splenocytes (mean±S.D.).
DISCUSSION
In this study, we investigated the possibility of producing potent antitumor effects against HPV-associated neoplasms by simultaneous vaccination with multiple HPV tumor antigens, E6 and E7. Since both HPV E6 and E7 are responsible for malignant transformation and are consistently co- expressed in HPV-associated malignancies, a combination of vaccines targeting E6 and E7 may potentially generate better antitumor effects compared to vaccines targeting either E6 or E7 alone. However, such a notion has never been rigorously tested in the past. The current manuscript presents the first demonstration that combined vaccination with both CRT/E6 and CRT/E7 DNA generates more potent therapeutic antitumor effects against HPV E6- and E7-expressing tumors than vaccination with either CRT/E6 DNA or CRT/E7 DNA alone. Given the promising results of this study, the strategy of targeting multiple tumor antigens may be of use with other tumor antigens and other tumor models for enhancing the potency of therapeutic tumor vaccines. Our current study serves as an important foundation for future HPV DNA vaccine trials combining both the CRT-E6 and the CRT-E7 DNA vaccines in patients with HPV-associated lesions.
In our current experiments, we observed no significant antitumor treatment effects in CRT DNA-vaccinated mice compared to naïve mice in the lung hematogenous spread model (Figure 3). Previously, however, we demonstrated that CRT DNA vaccination alone generated significant antitumor effects in mice using the lung hematogenous spread model with TC-1 cells 3. The different results observed in these two experiments can be accounted for by the dose dependent nature of the antitumor effects of CRT. It has been shown that higher doses of CRT can lead to greater antitumor effects14. In our previous experiments, a total of 64 μg of CRT DNA was used to generate the antitumor effect observed. However, in the experiments presented in this paper, only a total of 4μg of CRT DNA was used for control vaccination. This amount was not enough to generate significant antitumor treatment effects in mice. Thus, our data remains consistent when the dose-dependent nature of CRT’s antitumor effect is taken into account.
The results of our study show that vaccination with CRT/E7 DNA alone elicits significantly better antitumor responses in C57BL/6 mice than vaccination with CRT/E6 DNA alone (Figure 3). While the true reasons for this effect remain unclear, several factors could potentially account for the different antitumor effects of CRT/E6 and CRT/E7 DNA vaccination. First, E7-specific T cells derived from vaccination with CRT/E7 DNA could differ from E6-specific T cells generated from vaccination with CRT/E6 DNA. For example, the E7-specific T cells could have a higher avidity for E7 antigen presented by TC-1 tumor cells, making them more effective CTLs. Second, TC-1 tumor cells themselves could be responsible for the effect. More efficient presentation of HPV type 16 E7 antigen through MHC class I molecules in TC-1 cells could contribute to the stronger antitumor effects observed after CRT/E7 DNA vaccination. Third, factors unrelated to the specific immune interaction between T cells and tumor cells, such as different cytokine milieux generated by CRT/E6 and CRT/E7 DNA vaccination, could be responsible for the different antitumor effects of these vaccines. It will be of interest to explore the mechanisms underlying these observed phenomena in the future.
For clinical translation, safety will become an important issue. Some concerns have been raised about the possibility that vaccination with DNA encoding CRT could cause autoimmune reactions to develop. These concerns are based on the observation that anti-CRT antibodies have been identified in approximately 40% of patients with the autoimmune disease systemic lupus erythematosus (SLE) and in a small percentage of patients with other autoimmune diseases (for review, see 15). However, it remains unclear whether CRT is the cause of SLE or simply a molecule against which antibodies are generated as a result of SLE. Additionally, extensive observations from our lab of mice vaccinated with CRT containing DNA vaccines have identified no evidence of autoimmune reactions in the tissues or organs of vaccinated mice (data not shown). Thus, while concerns about CRT seem warranted, our observations indicate that CRT does not induce autoantibodies or autoimmune reactions in mice.
Another concern is the potential for oncogenicity associated with the use of E6 and E7 proteins in DNA vaccines. To alleviate concerns of oncogenicity associated with administration of E6 and E7 into the body it will be important to use attenuated versions of E6 and E7 that have been mutated. It has been demonstrated that a mutation at E7 position 24 and/or 26 will disrupt the Rb binding site of E7, abolishing the capacity of E7 to transform cells16. Furthermore, mutation at E6 positions 63 or 106 has been shown to destroy several HPV-16 E6 functions, preventing the mutated E6 protein from immortalizing human epithelial cells17,18. Furthermore, clinical translation may preclude the use of the pcDNA3 vector, because it contains an ampicillin resistance gene. We have recently used the pNGVL4a vector for E7 DNA vaccine development 19. This vector has been previously used for human clinical trials, and would therefore be suitable for clinical translation of E6 DNA vaccines.
In summary, our data suggest that combined vaccination with both CRT/E6 and CRT/E7 DNA generates significantly better therapeutic antitumor effects against HPV E6- and E7-expressing tumors than vaccination with either CRT/E6 DNA or CRT/E7 DNA alone. The preclinical observations suggest that it may be desirable to consider clinical translation of HPV vaccine strategies incorporating DNA vaccines targeting E6 and DNA vaccines targeting E7.
MATERIALS AND METHODS
Plasmid DNA constructs and preparation
The generation of pcDNA3-CRT3, pcDNA3-CRT/E69, pcDNA3-CRT/E73, and pcDNA3-CRT/mtE7(del 49-57) 13 have been described previously. All plasmid constructs were confirmed by DNA sequencing. DNA for vaccination was prepared using an endotoxin-free kit (Qiagen, Valencia, CA).
Peptides
Peptides representing defined HPV-16 E7 CTL epitopes, including H-2Db-restricted E7 aa49-57 (RAHYNIVTF)20, HLA-A*0201 restricted E7 aa11-20 (YMLDLPETT)21, and HPV-16 E6 CTL epitopes, including H-2Kb-restricted E6 aa50-57 (YDFAFRDL)9, and HLA-A*0201 restricted E6 aa29-38 (TIHDIILECV)21 were synthesized by Macromolecular Resources (Denver, Colorado, USA) at a purity of ≥70%.
Cells
TC-1 cells were generated as previously described 22. DC-1 cells were generated from the dendritic cell line 23 provided by Dr. Kenneth Rock at the University of Massachusetts. With continued passage, we have generated subclones of DCs (DC-1) that can be easily transfected 24. All cells were maintained in RPMI medium (Invitrogen, Carlsbad, CA) supplemented with 2mM glutamine, 1mM sodium pyruvate, 20mM HEPES, 50μM β-mercaptoethanol, 100 IU/ml penicillin, 100μg/ml streptomycin and 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA).
Western blot analysis
DC-1 cells were transiently transfected with pcDNA3-no insert, pcDNA3-CRT, pcDNA3-CRT/E6 and pcDNA3-CRT/E7 respectively using Lipofectamine 2000 (Gibco, USA). 24 hours after transfection, transfected DC-1 cells were lysed in M-PER Mammalian protein extraction reagent (PIERCE, Rockford, IL, USA) based on manufacturer’s instruction. Equal amounts of protein (50 μg) were loaded and separated by SDS-PAGE using a 10% polyacrylamide gel and blotted onto a PVDF membrane. After blocking, the membrane was incubated with either rabbit anti-HPV-16 E6 polyclonal antibody or mouse anti-HPV-16 E7 polyclonal antibody for 2 hours at room temperature, washed, and incubated with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit Ig (for E6) (Amersham Biosciences, England), or horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG(H+L) (for E7) (ZYMED, San Francisco, Carlifornia, USA). The blot was then washed and detected using chemilluminescence (ECL kit, Amersham, Arlington Heights, IL, USA).
Mice
C57BL/6 mice (5- to 8-week-old) were purchased from the National Cancer Institute. HLA-A*0201/Dd (AAD) transgenic female C57BL/6 mice, 6–8 weeks of age, were kindly provided by Dr. Victor Engelhard at the University of Virginia Health Sciences Center 25. These transgenic mice express a chimeric HLA class I molecule, composed of the α-1 and α-2 domains of HLA-A*0201 and the α-3 transmembrane and cytoplasmic domain of H-2Dd. This allows the murine CD8 molecule on the murine CD8+ T cells to interact with the syngenic α-3 domain of the chimeric MHC class I molecule. All animals were maintained under specific-pathogen free condition, and all procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
DNA vaccination
Preparation of DNA-coated gold particles and gene gun particle-mediated DNA vaccination was performed using a helium-driven gene gun (BioRad Laboratories Inc., Hercules, CA) according to a protocol described previously 4. For the immunological assays and in vivo tumor treatment experiment in C57BL/6 mice, mice (5 per group) were immunized and boosted intradermally via gene gun with the following vaccination groups: 1) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen), 2) pcDNA3-CRT/E7 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen) or 3) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT/E7 (2μg/mouse, left side of the abdomen). For the immunological assays in HLA-A2 (AAD) transgenic mice, mice (5 per group) were immunized and boosted intradermally via gene gun with two different regimens. The first regimen included the following vaccination groups: 1) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen), 2) pcDNA3-CRT/mtE7(del aa49-57) (2μg/mouse, right side of the abdomen) and pcDNA3-CRT (2μg/mouse, left side of the abdomen) or 3) pcDNA3-CRT/E6 (2μg/mouse, right side of the abdomen) and pcDNA3-CRT/mtE7(del aa49-57) (2μg/mouse, left side of the abdomen). The second vaccination regimen in HLA-A2 transgenic mice included the following vaccination groups: 1) pcDNA3-CRT/E6 and pcDNA3-CRT, 2) pcDNA3-CRT/mtE7(del aa49-57) and pcDNA3-CRT or 3) pcDNA3-CRT/E6 and pcDNA3-CRT/mtE7(del aa49-57). For this regimen, in each vaccination group, the two DNA constructs were mixed in the same bullet and the dose for each construct was 2μg/mouse. DNA-coated gold particles were delivered to the shaved abdominal region of mice using a helium-driven gene gun (BioRad Laboratories Inc., Hercules, CA) with a discharge pressure of 400 psi.
Intracellular cytokine staining and flow cytometry analysis
Pooled splenocytes from the vaccinated mice were harvested 1 week after the last vaccination and incubated overnight with 1 μg/ml of indicated peptide at the presence of GolgiPlug (BD Pharmingen, San Diego, CA) (1 μl/ml). The peptides tested included Db-restricted E7 (aa49-57), HLA-A2-restricted E7 (aa11-20), Kb-restricted E6 (aa50-57) and HLA-A2-restricted E6 (aa29-38) peptides. The stimulated splenocytes were then washed once with FACScan buffer and stained with phycoerythrin-conjugated monoclonal rat antimouse CD8a (clone 53.6.7). Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer’s instruction (BD Pharmingen, San Diego, CA). Intracellular IFN-γ was stained with FITC-conjugated rat antimouse IFN-γ. All antibodies were purchased from BD Pharmingen. Flow cytometry analysis was performed using FACSCalibur with CELLQuest software (BD biosciences, Mountain View, CA).
In vivo tumor treatment experiment
For the in vivo tumor treatment experiment, 1×105 TC-1 tumor cells were injected into 5~8 weeks old C57BL/6 mice (5 per group) via tail vein. Three days later, the mice were immunized with the DNA vaccines as described above. One week later, these mice were boosted once with the same immunization regimen. Twenty one days after tumor challenge, the mice were sacrificed, and the lung tumor nodules were counted. Data are expressed as mean number of lung nodules ±SE. The data shown here represent one of the two experiments performed.
Statistical Analysis
All data expressed as means ± Standard Deviation (SD) are representative of at least two different experiments. Data for intracellular cytokine staining with flow cytometry analysis and tumor treatment experiments were evaluated by analysis of variance (ANOVA). Comparisons between individual data points were made using a student’s t-test. All p values < 0.05 were considered significant.
Acknowledgments
We thank Drs. Drew M. Pardoll, Robert J. Kurman, and Richard Roden for helpful discussions. We would also like to thank Drs. Ralph Hruban and Ken-Yu Lin for critical review of the manuscript. We thank Mr. Bruno Macaes for the preparation of the manuscript. This work was supported by the National Cancer Institute.
References
- 1.Condon C, et al. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 2.Porgador A, et al. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng WF, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CH, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 5.Hung C-F, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Research. 2001;61:3698–3703. [PubMed] [Google Scholar]
- 6.Wu T-C, et al. Engineering an intracellular pathway for MHC class II presentation of HPV-16 E7. Proc Natl Acad Sci. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JW, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004 doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 9.Peng S, et al. Development of a DNA vaccine targeting HPV-16 oncogenic protein E6. J Virol. 2004;78:8468–8476. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–538. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Ji H, et al. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int J Cancer. 1998;78:41–45. doi: 10.1002/(sici)1097-0215(19980925)78:1<41::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Wentworth PA, et al. Differences and similarities in the A2. 1-restricted cytotoxic T cell repertoire in humans and human leukocyte antigen-transgenic mice. Eur J Immunol. 1996;26:97–101. doi: 10.1002/eji.1830260115. [DOI] [PubMed] [Google Scholar]
- 13.Peng S, et al. Characterization of HLA-A2-restricted HPV-16 E7-specific CD8+ T Cell Immune Responses Induced by DNA Vaccines in HLA-A2 Transgenic Mice. Gene Ther (submitted) 2004 doi: 10.1038/sj.gt.3302607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao F, et al. A gene therapy for cancer based on the angiogenesis inhibitor, vasostatin. Gene Ther. 2002;9:1207–1213. doi: 10.1038/sj.gt.3301788. [DOI] [PubMed] [Google Scholar]
- 15.Eggleton P, Llewellyn DH. Pathophysiological roles of calreticulin in autoimmune disease. Scand J Immunol. 1999;49:466–473. doi: 10.1046/j.1365-3083.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 16.Munger K, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 17.Dalal S, Gao Q, Androphy EJ, Band V. Mutational analysis of human papillomavirus type 16 E6 demonstrates that p53 degradation is necessary for immortalization of mammary epithelial cells. J Virol. 1996;70:683–688. doi: 10.1128/jvi.70.2.683-688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Q, et al. Human papillomavirus type 16 E6-induced degradation of E6TP1 correlates with its ability to immortalize human mammary epithelial cells. J Virol. 2001;75:4459–4466. doi: 10.1128/JVI.75.9.4459-4466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trimble C, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 20.Feltkamp MC, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 21.Ressing ME, et al. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 22.Lin K-Y, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Research. 1996;56:21–26. [PubMed] [Google Scholar]
- 23.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 24.Kim TW, et al. Enhancement of DNA vaccine potency by coadministration of a tumor antigen gene and DNA encoding serine protease inhibitor-6. Cancer Res. 2004;64:400–405. doi: 10.1158/0008-5472.can-03-1475. [DOI] [PubMed] [Google Scholar]
- 25.Newberg MH, et al. Importance of MHC class 1 alpha2 and alpha3 domains in the recognition of self and non-self MHC molecules. J Immunol. 1996;156:2473–2480. [PubMed] [Google Scholar]