Abstract
Temporal summation of second pain (TSSP) results from repetitive stimulation of peripheral C-fibers (> 0.33 Hz) and is thought to reflect summation mechanisms of dorsal horn neurons (i.e., windup). Both TSSP and windup result in short term enhancement of C fiber-evoked responses that decay rapidly after the end of stimulation. However, very low stimulus frequencies (0.17-0.08 Hz) can maintain this enhancement after TSSP and windup have occurred. This maintained enhancement is termed TSSP-maintenance (TSSP-M) and is indicative of central sensitization. TSSP-M may be especially relevant for chronic pain conditions such as fibromyalgia (FM) and may play an important role in its pathogenesis. Whereas TSSP-M of heat induced pain is well characterized in human subjects at spinal cord levels related to the upper body, TSSP-M at spinal levels related to the lower body has not been previously studied. The present study was designed to evoke TSSP-M at the upper and lower extremities of normal controls (NC) and FM patients and thus characterize their spatial distribution of central sensitization. Twenty-three NC and twenty-six FM patients were enrolled in this study. TSSP-M testing consisted of repetitive heat pain stimulation at the thenar eminences of the hands or feet. The subjects rated the pain intensity of repetitive heat stimuli as well as 15 sec and 30 sec pain aftersensations. The experiments demonstrated significant TSSP-M for both NC and FM patients. In contrast to NC, TSSP-M ratings of heat stimuli were increased in FM patients and their TSSP-aftersensations were prolonged. There was, however, no statistical difference between TSSP-M ratings or TSSP-aftersensations at the hands or feet in either NC or FM patients. These findings demonstrate that central sensitization of FM patients is widespread and similar along the spinal neuroaxis.
Perspective
The pain of FM seems to be accompanied by generalized central sensitization, involving the length of the spinal neuroaxis. Thus, widespread central sensitization appears to be a hallmark of FM and may be useful for the clinical case definition of this prevalent pain syndrome. In addition, measures of widespread central sensitization, like TSSP-M could also be used to assess treatment responses of FM patients.
Keywords: Temporal summation, Central Sensitization, Extremities, Fibromyalgia, Second Pain
Introduction
Characteristic features of FM patients include hyperalgesia and allodynia which represent clinically relevant manifestations of central sensitization and are related to increased excitability of spinal and supraspinal neurons. Central sensitization is associated with increased spontaneous activity of dorsal horn neurons, enhanced responsiveness to nociceptive and non-nociceptive stimuli, and enlarged receptive fields 6,10,10,33. The consequences of these neuronal changes include spontaneous pain as well as allodynia/hyperalgesia. Coding mechanism for these changes include temporal summation or ‘windup’ (WU) of dorsal horn neuron responses to repetitive C-fiber stimulation 8,14,19. Once WU occurs, dorsal horn nociceptive neurons maintain a state of increased responsiveness for long periods at much lower stimulus frequencies than would normally be necessary to induce WU 24. This state has been previously termed ‘WU-maintenance’ (WU-M) 40. WU-M is likely to be integrally related to central sensitization and persistent pain conditions, because it is accompanied by expanded receptive fields, enhanced responsiveness to nociceptive and non-nociceptive stimulation, and increased spontaneous activity of dorsal horn nociceptive neurons 6,9,10,10,24,33. Although WU and WU-M comprise important neural mechanisms related to the early phase of central sensitization 18 they can also be used to characterize long-term central sensitization of chronic pain patients 1,1,30,32,33,40,47. An important clinical implication of WU is that once it occurs, minimal tonic peripheral input is required to sustain enhanced responsiveness of dorsal horn neurons and thus persistent pain. Temporal summation of second pain (TSSP) and its maintenance (TSSP-M) represent psychophysical correlates to WU and WU-M in electrophysiological experiments 1,1,30,32,33,40,47.
Fibromyalgia (FM) is characterized by chronic widespread pain, involving many body areas, particularly the neck/shoulders and lower back 50. We have previously demonstrated that FM patients display enhanced TSSP compared to pain-free normal control (NC) subjects during repetitive heat stimulation of the upper extremities (UE) 43. In addition, TSSP-M is greatly increased in FM 40, suggesting that enhanced C-fiber pain is abnormally maintained in these patients. However, almost all evaluations of TSSP or TSSP-M in FM patients have been limited to the UE, thus excluding clinically relevant body areas with frequent pain complaints, including the lower extremities (LE) and lower back.
The pathogenesis of FM is unclear 38, but tonic impulse input from peripheral tissues seems to play an important role for pain and hyperalgesia in FM 41,44. Deep tissue hyperalgesia, however, has not only been described in painful body areas of FM patients but also in body locations without spontaneous pain 37. Similar finding have been reported in whiplash associated disorders, back pain, and osteoarthritis 2,3,11,21,35,45 suggesting that generalized hyperalgesia may be a common occurrence in these pain syndromes. In the current study we tested TSSP-M and TSSP-aftersensations of heat pain at the UE and LE to evaluate the extent of central sensitization in FM patients at both ends of the spinal neuroaxis. We hypothesized that these measures of central pain sensitivity would not only be abnormal but widespread in FM patients.
3.0 Materials and Methods
The University of Florida Institutional Review Board approved all procedures described in this report. Informed consent was obtained from all subjects and the study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
3.1 Study Subjects
NC subjects came from the University Health Science Center and the University Campus, Gainesville. Subjects who fulfilled the 1990 American College of Rheumatology (ACR) Criteria for FM were recruited from the Health Science Center Outpatient Clinics and from FM support groups. Prior to testing, all subjects underwent a clinical examination and were excluded from the study if they had abnormal findings unrelated to FM. Continuation of analgesics, including non-steroidal anti-inflammatory drugs (NSAID) and acetaminophen, was not allowed during the study. The subjects were asked to discontinue narcotic analgesics at least two weeks prior to study entry. Low dose cyclobenzaprine (<15 mg/day) was permissible during the study for treatment of insomnia. Other medications had to be discontinued for 5 half-lives prior to study entry.
3.2 Ratings of Clinical and Experimental Pain
For ratings of current clinical pain, a mechanical visual analogue scale (M-VAS) was used 29 ranging from 0 - 10. The scale was anchored on the left with “no pain at all” and on the right with “the most intense pain imaginable”.
A standardized numerical pain scale (NPS) was utilized for rating the magnitude of painful sensations produced by thermal stimulation as described previously 39,47. The scale ranged from 0 to 100, in increments of 5, with verbal descriptors at intervals of 10: 0 = no sensation, 10 = warm, 20 = a barely painful sensation (i.e. pain threshold), 30 = very weak pain, 40 = weak pain, 50 = moderate pain, 60 = slightly strong pain, 70 = strong pain, 80 = very strong pain, 90 = nearly intolerable pain, and 100 = intolerable pain. Previous experience with the scale has shown that increments of 5 provide appropriate resolution for discriminable levels of late sensation intensity from threshold to nearly intolerable levels 43,47. This numerical scale has been found to be particularly advantageous for pain ratings during series of repetitive stimuli 47.
3.3 Tender Point Testing
Nine paired tender points as defined by the American College of Rheumatology (ACR) Criteria 50 and two control points (at the center of the right forearm and the right thumbnail) were assessed by a trained investigator using a Fischer Dolorimeter (Pain Diagnostics, Great Neck, NY). The rubber tip of the Dolorimeter was 1 cm in diameter. The Dolorimeter was placed on the examination site, and pressure was gradually increased by 1 kg/sec. The subjects were instructed to report when the sensation at the examination site changed from pressure to pain. Pressure testing was stopped at that moment and the result recorded as positive (1) if maximal pressure was ≤ 4 kg. If no pain was elicited at ≥ 4 kg the test result was recorded as negative (0).
3.4 Testing Session
The subjects underwent one session of heat TSSP-M and TSSP-aftersensation testing at the UE and LE. The sequence of testing was counterbalanced. The location of the test stimuli always alternated between the right and left thenar of the hands and feet in counterbalanced order. There was at least 3 min between each trial or until all pain aftersensations had resolved.
3.5 TSSP Testing
All subjects were tested in a controlled environment providing ambient lighting and temperatures (25 ° C). They had at least 30 min to acclimatize prior to the testing procedures.
3.5.1 TSSP Ratings
TSSP was produced by computer-controlled thermal stimulation of the thenar eminence as described previously 43. All subjects were trained to attend to and rate late pain sensations evoked by repetitive thermal stimulation of the thenar surface of either hand or foot. These sites were chosen because it is well established that brief and intense heat stimulation of the glabrous surface results almost exclusively in delayed sensations associated with impulse conduction in C-fibers 5,47. Before application of repetitive heat stimuli, study subjects were told that they may or may not feel sensations of pain during contact of the thermal probe with the skin and to attend to a possible late sensation of pain beginning 1 to 2 sec after each contact. They were instructed to pay attention to and provide numerical ratings of the magnitude of the late sensation, which could increase or decrease with stimulus repetition. During at least one training session the subjects were asked to indicate the peak pain sensation associated with each repetitive heat pulse by saying “now”. If the time delay between each heat pulse and the subject’s response was clearly greater than 1sec, the events were considered to be consistent with second pain sensations. The subjects were also asked to provide ratings of heat sensations 15sec and 30sec after the last heat stimulus (aftersensations).
3.5.2 Apparatus Used for TSSP and TSSP-M
During the psychophysical testing session, a series of brief stimuli was presented to each hand or foot at a frequency of 0.33 Hz (contact time 0.7 sec; release time 2.3 sec), to evaluate sensitivity to stimulus frequency 9,20,31. During each test series, the subject placed the thenar eminence of one hand or foot on a Plexiglas surface with a designated area of palmar skin located over a 4.6 cm diameter hole. The thermode was positioned 8 mm below the Plexiglas surface when not in contact with the skin and was advanced to a position 2 mm above the surface by computerized activation of a solenoid. In each series, the preheated probe contacted the skin for 0.7 sec per contact. To prevent order effects, the stimulus location (right or left extremity) was counterbalanced between sessions. A three-minute interval separated each series. All stimulus parameters were controlled by custom-designed Labview software.
The temperature of the thermode was controlled by circulating heated water at a rate of 7 liters/min from a Neslab, model RT-111 circulator through a 1.2 in. diameter copper tube (thermode). For the TSSP-M and TSSP-aftersensation experiments the thermode temperature was varied for each subject to achieve maximal TSSP ratings of 50 ± 5 (NPSmax) (see 3.8).
3.6 Testing of TSSP Sensitivity and Aftersensations
Pain sensations from heat stimuli vary as a function of each subject’s individual pain sensitivity as well as peripheral and/or central sensitization, which influences the rate of TSSP and TSSP decay. Because TSSP decay is dependent on the magnitude of TSSP, we needed to provide measures of TSSP sensitivity across all subjects as described in detail before 40. TSSP sensitivity was measured by the stimulus temperature required to evoke maximal TSSP pain of 50 ± 5 NPS units over 12 to 15 heat taps in each subject. TSSP-aftersensations were assessed by the pain intensity that remained 15 and 30 sec after the train of heat taps was presented. First, the subjects were tested with single 0.7 sec heat stimuli that resulted in painless ratings of heat (NPS < 20). The stimuli were alternatively applied to each thenar eminence in 0.5 min intervals. Testing was always started at 47 ° C, and the temperature was subsequently raised until pain was reported, then lowered in steps of 1-2 ° C until pain was absent, defining threshold. The stimulus temperature of threshold minus 0.5 ° C was then used for TSSP-aftersensation and TSSP-M testing. If subjects achieved NPS ratings of 50 ± 5 (NPSmax) within 15 taps, this stimulus intensity was used for subsequent testing of TSSP-M and TSSP-aftersensations. If NPSmax was > 55 on the last tap, the stimulus temperature was decreased in 0.5-1 °C steps until the target NPSmax (50 ± 5) was obtained. If, on the other hand, the subjects’ TSSP NPSmax rating was < 45 on the 15th tap, the stimulus temperature was increased in 0.5-1 ° C steps until target NPSmax was achieved or the rating of the 1st tap was >25. After target NPSmax (50 ± 5) was achieved, the subjects were asked to provide numerical ratings of TSSP-M sensations and TSSP-aftersensations, 15 sec and 30 sec after the last heat stimulus, using the NPS.
3.7 Testing of TSSP-M
After TSSP sensitivity was determined, the study subjects were tested twice with repetitive heat stimuli (0.33 Hz) that resulted in TSSP pain ratings of NPSmax 50 ± 5. After NPSmax was achieved, the frequency of the heat stimuli was changed to either 0.17 Hz or 0.08 Hz in counterbalanced order, while the contact time (0.7 sec) and temperature of the probe were maintained. The subjects were then asked to rate the magnitude of each of 10 taps at the current frequency using the NPS. Rest intervals of at least 3 min were used between tests of TSSP-M.
3.8 Diagram of Local Pain Areas
For identification of painful body areas the subjects were instructed to shade all areas that were painful at the time of the visit using a drawing depicting the front and back of the human body, as described before 42. This method has been previously used to determine the location of pain 15 and has satisfactory intra-subject and inter-rater reliability 26. Before statistical analysis the pain drawings were divided into 20 areas which included the 18 standardized TP. Body areas not containing TP included the abdomen. Body areas above and below to the umbilicus were considered upper and lower body areas, respectively.
4.0 Data analysis
Statistical analyses were calculated using SPSS 15.0 software (SPSS, Inc., Chicago, IL). A series of mixed model ANOVAs was used for testing of TSSP with diagnosis, tap, and stimulus modality as independent variables.
4.0 Results
4.1 Study Subjects
Twenty three NC and twenty six FM subjects were enrolled in this study. All subjects were female and right handed, and all were Caucasian except for two African American (both FM) and two Asian American females (one NC, one FM). The average age (SD) of the NC and FM subjects was 35.6 (14.1) years and 44.6 (15.9) years, respectively. An independent t-test showed that the age of NC and FM subjects was not significantly different (p > .05). FM subjects reported moderate overall clinical pain ratings of 3.3 (1.5) VAS units (see Table 1). The mean number of tender points was 4.5 and 16.6 for NC and FM subjects, respectively. The average duration of FM symptoms was 6.8 (5.9) years.
Table 1.
Characteristics of Study Participants
| NC mean (SD) | FM mean (SD) | |
|---|---|---|
| Age (years) | 35.6 (14.1) | 44.6 (15.9) |
| Pain VAS (0-10) | 0 | 3.3 (1.5) |
| Number of Painful Body Areas | ||
| - Upper Body (0-10) | 0 | 5.2 (1.8) |
| - Lower Body (0-10) | 0 | 4.5 (1.4) |
| Tender Points (0-18) | 4.5 | 16.6 |
4.2 Pain Ratings during Heat TSSP
Because there was no significant difference between TSSP ratings of the right or left side at the UE or LE (all p > .05), the averaged experimental pain ratings of UE or LE were used for the analysis.
4.3. TSSP Using Adjusted Heat Stimuli
For the analysis shown in Figures 1 and 2, it was first necessary to determine the thermal stimulation parameters that resulted in equal rates of TSSP for FM and NC subjects. This required using individually tailored stimulus temperatures for all subjects. Thus TSSP sensitivity could be measured and analyzed in terms of differences in stimulus temperature necessary to produce TSSP at similar rates for FM and NC subjects. A mixed model ANOVA of TSSP pain ratings with heat tap (2) and location (2) as within subjects’ factors and diagnosis (2) as between subjects’ factor showed a significant main effect for heat tap (F(3,123) = 428.9, p < .001) and non-significant effects for diagnosis (F(3,123) = 43.3; p > .05) and location (F(3,123) = 0.7; p > .05), indicating TSSP and that the adjusted TSSP rates at the hands and feet were similar for FM and NC subjects. This is clearly shown in the TSSP induction phase in Figures 1 and 2. The mean (SD) stimulus temperature of the heat probe that produced maximal TSSP pain ratings (NPSmax = 50 ± 5) was lower for FM compared to NC subjects at the hands [49.9 (2.7) ° C and 52.2 (1.1) ° C] and feet [50.0 (2.8) ° C and 53.4 (1.4) ° C], respectively (t=3.6, df 44; p = .001). Thus, FM subjects had greater TSSP sensitivity than NC subjects, consistent with our previously published results 34,43,48.
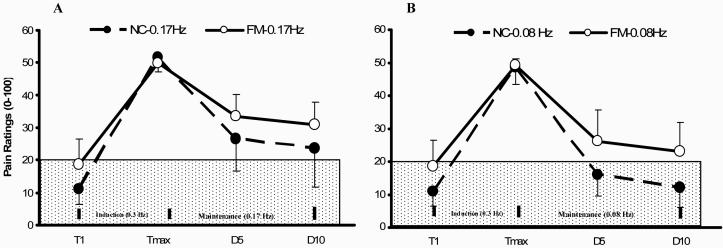
Figure 1.
Mean ratings (SD) of TSSP and TSSP-M of NC and FM subjects at the hands. After maximal WU pain ratings of 50 NPS units were achieved (Induction) the subjects underwent TSSP-M testing with 10 heat stimuli at 0.17 Hz (Panel A) and 0.08 Hz (Panel B) in counterbalanced order (Maintenance). The ratings of the 1st (D1), 5th (D5) and 10th (D10) TSSP-M tap are shown. Stimuli at 0.17 Hz resulted in greater TSSP-M pain ratings compared to 0.08 Hz in NC (p < .001) and FMS subjects (p < .001). (T = heat tap; TSSP = temporal summation of second pain; TSSP-M = TSSP maintenance; Tmax = maximal heat pain ratings during the last TSSP stimulus).
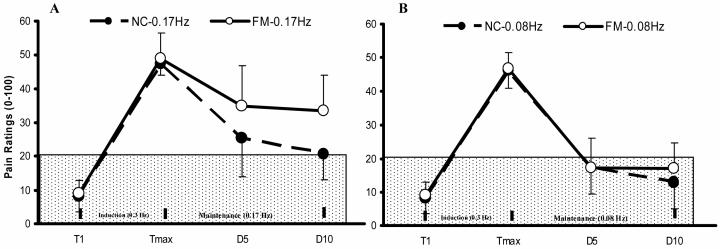
Figure 2.
Mean ratings (SD) of TSSP and TSSP-M of NC and FM subjects at the feet. After maximal TSSP pain ratings of 50 NPS units were achieved (Induction) the subjects underwent TSSP-M testing with 10 heat stimuli at 0.17 Hz (Panel A) and 0.08 Hz (Panel B) in counterbalanced order (Maintenance). The ratings of TSSP-M tap 1, 5, and 10 are shown. Stimuli at 0.17 Hz resulted in greater experimental pain ratings compared to 0.08 Hz in NC and FMS subjects (p = .01). (T = heat tap; TSSP = temporal summation of second pain; TSSP-M = TSSP maintenance; Tmax = maximal heat pain ratings during the last TSSP stimulus).
4.4 TSSP-M Analysis
In order to illustrate the impact of the different stimulus frequencies on experimental pain ratings, TSSP-M was analyzed as a function of heat tap number (Figures 1 and 2).
4.4.1 TSSP-M Testing at Hands of NC and FM Subjects
Similarly to our previous report 40 we analyzed all subjects’ TSSP-M ratings comparing sensitivity adjusted Tmax, TSSP-M tap 5 (D5) and TSSP-M tap 10 (D10) heat tap ratings during subsequent TSSP-M trials at 0.17 Hz and 0.08 Hz at the hands (Figure 1). A mixed model ANOVA of TSSP-M ratings with stimulus frequency (2), tap (3), and diagnosis (2) as independent factors showed significant main effects for stimulus frequency (F(1,19)=53.0; p < .001), tap (F(2,38) = 248.0; p < .001) and diagnosis (F(1,19) = 5.9; p = .04). There were significant interaction effects of diagnosis × tap (F(2,38) = 8.2; p = .001) and frequency × tap (F(2,38)= 18.2; p < .001) noted, indicating that FM subjects rated TSSP-M stimuli higher than NC at either frequency.
4.4.2 TSSP-M Testing s at Feet of NC and FM Subject
Similarly to TSSP-M testing at the hands, we analyzed all subjects’ TSSP-M ratings at the feet by comparing sensitivity adjusted Tmax, D5 and D10 heat tap ratings after maximal TSSP during subsequent TSSP-M trials at 0.17 Hz and 0.08 Hz (Figure 2). A mixed model ANOVA with stimulus frequency (2), diagnosis (2), and tap (3) as independent factors showed significant main effects for stimulus frequency (F(1,47) = 53.9; p < .001), diagnosis (F(1,47) = 12.5; p = .001), and tap (F(2,94) = 461.8; p < .001). There was a significant interaction effect of stimulus frequency × diagnosis (F(1,47) = 6.7; p = .01), stimulus frequency × tap (F(2,94) = 25.1; p < .001), and diagnosis × tap (F(2,94) = 7.7; p < .001) noted. These results indicate that during TSSP-M FM subjects’ experimental pain ratings decreased significantly less rapidly than NC and this decrease was dependent on TSSP-M stimulus frequency. When simple contrasts were used to decompose the observed interaction effects, they demonstrated that NC and FM subjects rated TSSP-M experimental pain ratings as significantly different at each time point (F(1,16) = 4.5; p < .05) during 0.17 Hz but not during 0.08 Hz maintenance heat pulses (F(1,47) = 1.2; p > .05).
4.4.3 TSSP-M Comparisons at Hands and Feet
A mixed model ANOVA of TSSP-M ratings during 0.17 Hz stimuli at the hands or feet of NC and FM subjects showed significant main effects for taps (F(2,32)= 74.0; p < .001) and diagnosis (F(1,16)= 5.8; p = .03). However, the interactions of location × tap (F(2,32)=1.1; p > .05) and location × diagnosis (F(2,32)= 1.7; p > .05) were not statistically significant. These findings indicate that FM subjects rated TSSP-M stimuli significantly higher than NC at the hands and feet, but these TSSP-M ratings were not statistically different across the two locations.
4.4.4 TSSP-Aftersensations at the Hands and Feet
Pain ratings of TSSP-aftersensations (AS) were obtained 15sec and 30sec after sensitivity adjusted Tmax at the hands and feet of NC and FM subjects (Figure 3). A mixed model ANOVA of TSSP-AS with time (3) and diagnosis (2) as independent factors indicated significant main effects for time (F(2,60) = 365.8; p < .001) and diagnosis (F(1,30) = 6.9; p = .01). A significant interaction effect of time × diagnosis (F(2,60) = 6.7; p = .002) revealed that TSSP-AS declined more slowly for FM subjects compared to NC (Figure 3A).
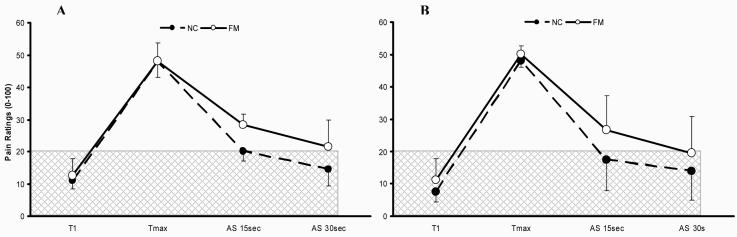
Figure 3.
Ratings of TSSP and TSSP-AS of NC and FM subjects at the hands (Panel A) and feet (Panel B). After maximal TSSP pain ratings of 50 NPS units (Tmax) were achieved the subjects rated TSSP-AS 15 sec and 30 sec after the last heat pulse.
Panel A: TSSP-AS of NC and FM subjects after heat stimuli at the hands. For TSSP, 15 heat stimuli adjusted to each individual’s pain sensitivity were applied to the hands at 0.33 Hz.
Panel B: Similarly, TSSP-AS of NC and FM subjects after repetitive heat stimuli to the feet. For TSSP, 15 heat stimuli adjusted to each individual’s pain sensitivity were applied to the feet at 0.33 Hz. TSSP-AS were more prolonged in FM subjects compared to NC at the hands (p = .002) and feet (p = .03) indicating central sensitization.
Similarly, ratings of TSSP-AS 15 sec and 30 sec were obtained after the last heat stimulus of a train of 15 pulses at the feet in NC and FM subjects (Figure 3B). A mixed model ANOVA of TSSP-AS with time (3) and diagnosis (2) as independent factors showed significant main effects for time (F(2,66) = 217.1; p < .001) and diagnosis (F(1,33) = 8.7; p = .01). A significant interaction effect of time × diagnosis (F(2,66) = 3.6; p = .03) indicated that TSSP-AS declined more slowly for FM subjects compared to NC (Figure 3B).
4.4.5 TSSP-AS Comparisons between Hands and Feet
A mixed model ANOVA of 15 sec and 30 sec TSSP-AS ratings at the hands or feet of NC and FM subjects showed significant main effects for time (F(2,26) = 110.6; p < .001). However, the interactions of location × time (F(1,13) = 0.7; p > .05) and location × diagnosis (F(1,13) = 0.1; p > .05) were not statistically significant. These findings indicate that the ratings of NC and FM subjects of TSSP-AS ratings at 15 sec and 30 sec were not statistically different at the hands or feet.
4.5 Correlation of Pain Areas with TSSP-M
The analysis of FM subjects’ pain drawings indicated clinical pain in an average (SD) of 9.7 (1.5) out of 20 standardized body areas. The number of FM pain areas located in the upper and lower body were 5.2 (1.8) and 4.5 (1.4), respectively (Table 1). A paired t-test showed no significant difference between the number of upper and lower body pain areas (p > .05). The body areas most frequently described as painful included the shoulders (75.2%), arms (66.9%), lower back (64.3%), and thighs (63.1%). Pearson’s moment product correlations of number of pain areas with TSSP-M difference scores were not significant at 0.17 Hz and 0.08 Hz (all p > .05).
5.0 Discussion
Our experiments demonstrate that once TSSP has occurred in NC and FM patients, only very low stimulus frequencies are necessary for its maintenance at the hands and feet. Specifically, TSSP could be maintained in both groups at stimulus frequencies that are ineffective in initiating this phenomenon. Compared to NC, TSSP-M of FM patients was enhanced during repetitive heat pulses to the hands and feet providing evidence for increased central sensitization in these chronic pain patients. Thus, our current study not only confirms abnormal TSSP-M during repetitive heat stimuli to the hand of FM patients 40, but also extends theses findings to the feet. In addition, sensitivity adjusted heat stimuli to both the hands and feet resulted in significantly prolonged TSSP-AS of FM patients providing additional evidence for abnormal central pain sensitivity in this pain population. Because TSSP and TSSP-M represent psychophysical correlates of slow temporal summation of dorsal horn neurons, these results clearly demonstrate enhanced central pain sensitivity in FM patients at both ends of the spinal neuroaxis and therefore widespread central sensitization.
5.1 TSSP and Central Sensitization
Slow temporal summation of pain is dependent on C-fiber input and normally occurs only if the nociceptive stimulus frequency is equal or greater than 0.33 Hz 7,24,30,30,49. The neurotransmitters involved in TSSP include glutamate/aspartate, tachykinins, and substance P (SP), which are released from C-fiber terminals and lead to progressive activation of N-methyl-D-aspartate (NMDA) receptors in dorsal horn neurons by removal of the Mg-block from the ion channel. This spinal cord mechanism results in temporal summation of neuronal responses and pain. TSSP and TSSP-M are central nervous system phenomena 28,30. Both reflect the early stages of central sensitization, which are interfaced with later stages in which cascades of intracellular signaling events lead to activation of kinases and subsequent phosphorylation of ion channels and receptors 6,10,17,33,52,53. The latter are associated with long-term neuroplastic changes including transcription and translation of genes which can change receptor expression or phenotype of the neuron thus leading to increased excitability and central sensitization.
5.2 TSSP-M
Animal experiments have shown that spinal cord neurons, conditioned by repetitive C-fiber stimuli, require only minimal nociceptive input to maintain a sensitized state termed windup (WU) 24. Without repetitive nociceptive input following WU, dorsal horn WDR neuronal activity returns rapidly (within seconds) to baseline. Li et al. (1999) showed that WU-M could not be attributed to prolonged afterdischarges of WDR neurons, because such discharges rapidly became undetectable following WU. Thus WU-M likely relies on early central sensitization events that occur rapidly during nociceptive stimulation and result in amplification of central input to the spinal cord by synaptic modulators like SP, intracellular kinases, nitric oxide, and cyclooxygenase-2 (COX-2) 51,53. Psychophysical testing of WU in healthy human subjects has also shown that thermal TSSP pain decays rapidly and becomes undetectable within six seconds after termination of nociceptive stimulation 43,47. In the present study TSSP was produced by repetitive nociceptive thermal stimuli at a rate of 0.33 Hz. Subsequently experimental pain ratings of NC declined rapidly and frequency dependent for TSSP-M stimuli of identical intensity delivered at 0.08 Hz or 0.16 Hz.
5.3 Abnormal TSSP-M of FM Patients
Patients with FM complain of widespread pain and fatigue without consistent evidence of peripheral tissue abnormalities 36,36. Also, no peripheral nervous system abnormalities have been associated with FM. However, FM patients show evidence of hyperalgesia for mechanical cutaneous/muscle stimulation and for electrical as well as thermal stimuli 13,13,16,22,23,37. In addition, central nervous system pain processing abnormalities, including increased TSSP, have been consistently demonstrated in this chronic pain population 12,12,27,27,34,43. The stimulus frequencies necessary to produce TSSP are well established for NC and include repeated heat stimuli at ≥ 0.3 Hz 28,30,30. FM patients, however, demonstrate TSSP at lower stimulus frequencies (< 0.2 Hz) and intensities than NC 43. To adjust for this group difference between NC and FM subjects, heat stimulus intensities were adjusted according to each individual subject’s TSSP sensitivity. This manipulation provided comparable starting points for subsequent TSSP-M testing of NC and FM subjects at stimulus frequencies that do not result in TSSP. Additionally, TSSP-M may have reduced possible rating bias because the subjects could not anticipate heat tap related changes in pain intensity or aftersensations.
5.4 Widespread Central Sensitization in FM Patients
Mechanical hyperalgesia of FM patients is not restricted to TP but also present in other body areas 37,46. Our study is the first to systematically examine central sensitization at both ends of the spinal neuroaxis in FM patients. Our data suggest that central sensitization of FM patients is increased but similar along multiple spinal cord levels. Although central sensitization along the spinal neuroaxis of FM patients is not unexpected, the uniformity of central pain hypersensitivity is surprising. An important factor contributing to this hypersensitivity is tonic impulse input from peripheral tissues. This peripheral input may have been widespread and similar in the upper and lower body areas of our FM cohort as indicated by their body pain drawings (see 4.7).
Similar to TSSP and TSSP-M, other measures including thermal and mechanical hyperalgesia have been used to assess central hypersensitivity in FM 22,37. Evidence for widespread mechanical hyperalgesia, however, has not only been found in FM but other musculoskeletal pain syndromes including chronic whiplash injury 21. Here, muscular hyperalgesia was not only detectable in the neck and shoulder region, but also in more distant body areas in which the patients did not normally experienced pain. Similarly, increased mechanical pain sensitivity of the fingers has been found in tension-type headache patients compared to NC subjects 4. Although the exact pathogenesis of central hyperexcitability of FM patients is currently unknown it may be the result of several factors, including (1) longlasting expansion of receptive fields; (2) decrease in the efficacy of descending antinociceptive systems; or (3) heterotopic facilitation caused by active nociceptive fibers outside the receptive fields 25.
6.0 Conclusions
The maintenance of thermal TSSP at the hands and feet of NC and FM subjects was compared following a series of heat stimuli at 0.33 Hz that produced comparable magnitudes of second pain summation for both groups of subjects. For NC and FM subjects, pain ratings declined rapidly and in inverse proportion to the rate of stimulation during TSSP-M (0.17 or 0.08 Hz). FM subjects, however, reported TSSP-M pain ratings as much higher and much more prolonged than NC except during 0.08 Hz stimuli to the feet. At this frequency and location, TSSP-M ratings of NC and FM subjects returned quickly to baseline and were not different between groups, most likely related to floor effects. Thus our current study not only confirms previously reported evidence for central sensitization of the cervical neuroaxis in FM 40 but extends these findings to lumbo-sacral segments of the spinal cord. Because this abnormal responsiveness to TSSP and TSSP-M stimuli was similar at both ends of the spinal neuroaxis, our results provide evidence for widespread central sensitization in FM patients. Thus widespread central sensitization is associated with body areas that are spontaneously painful as well as those which are not.
ACKNOWLEDGMENTS
Supported by NIH grants NS38767, AR053541 and supported in part by Clinical Research Center grant RR00082.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- 1.Arendt-Nielsen L. In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z, editors. Induction and assessment of experimental pain from human skin, muscle, and viscera; Proceedings of the 8th World Congress on Pain; Seattle, IASP Press. 1997.pp. 393–425. [Google Scholar]
- 2.Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93:107–114. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 3.Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Bendtsen L, Jensen R, Olesen J. Decreased pain detection and tolerance thresholds in chronic tension-type headache. Arch Neurol. 1996;53:373–376. doi: 10.1001/archneur.1996.00550040113021. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Res. 1983;266:203–208. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- 6.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 7.Cuellar JM, Montesano PX, Antognini JF, Carstens E. Application of nucleus pulposus to L-5 dorsal root ganglion in rats enhances nociceptive dorsal horn neuronal windup. J Neurophysiol. 2005;94:35–48. doi: 10.1152/jn.00762.2004. [DOI] [PubMed] [Google Scholar]
- 8.Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28:633–638. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- 9.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty PM, Palecek J, Paleckova V, Sorkin LS, Willis WD. The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. J Neurosci. 1992;12:3025–3041. doi: 10.1523/JNEUROSCI.12-08-03025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giesecke T, Gracely RH, Grant MAB, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 12.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 13.Graven-Nielsen T, Sorensen J, Henriksson KG, Bengtsson M, Arendt-Nielsen L. Central hyperexcitability in fibromyalgia. J Musculoskelet Pain. 1999;7:261–271. [Google Scholar]
- 14.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 15.Hunt IM, Silman AJ, Benjamin S, McBeth J, Macfarlane GJ. The prevalence and associated features of chronic widespread pain in the community using the ‘Manchester’ definition of chronic widespread pain. Rheumatology. 1999;38:275–279. doi: 10.1093/rheumatology/38.3.275. [DOI] [PubMed] [Google Scholar]
- 16.Hurtig IM, Raak RI, Kendall SA, Gerdle B, Wahren LK. Quantitative sensory testing in fibromyalgia patients and in healthy subjects: Identification of subgroups. Clin J Pain. 2001;17:316–322. doi: 10.1097/00002508-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 19.Jonakait GM, Ni L, Walker PD, Hart RP. Development of substance P (SP)-containing cells in the central nervous system: consequences of neurotransmitter co-localization. Prog Neurobiol. 1991;36:1–21. doi: 10.1016/0301-0082(91)90034-x. [DOI] [PubMed] [Google Scholar]
- 20.Kellstein DE, Price DD, Hayes RL, Mayer DJ. Evidence that substance P selectively modulates C-fiber-evoked discharges of dorsal horn nociceptive neurons. Brain Res. 1990;526:291–298. doi: 10.1016/0006-8993(90)91234-8. [DOI] [PubMed] [Google Scholar]
- 21.Koelbaek-Johansen M, Graven-Nielsen T, Schou OA, Arendt-Nielsen L. Generalised muscular hyperalgesia in chronic whiplash syndrome. Pain. 1999;83:229–234. doi: 10.1016/s0304-3959(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 22.Kosek E, Ekholm J, Hansson P. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain. 1996;68:375–383. doi: 10.1016/s0304-3959(96)03188-0. [DOI] [PubMed] [Google Scholar]
- 23.Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. Pain. 1994;59:45–53. doi: 10.1016/0304-3959(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 25.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- 26.Ohnmeiss DD. Repeatability of pain drawings in a low back pain population. Spine. 2000;25:980–988. doi: 10.1097/00007632-200004150-00014. [DOI] [PubMed] [Google Scholar]
- 27.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 28.Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol. 1972;37:371–387. doi: 10.1016/0014-4886(72)90081-7. [DOI] [PubMed] [Google Scholar]
- 29.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 31.Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 32.Price DD, Mao J, Mayer DJ. Central neural mechanisms of normal and abnormal pain states. In: Fields HL, Liebeskind JC, editors. Pharmacological approaches to the treatment of pain: new concepts and critical issues. I.A.S.P. Press; Seattle: 1994. pp. 61–84. [Google Scholar]
- 33.Price DD, Mao J, Mayer DJ. In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z, editors. Central consequences of persistent pain states; Proceedings of the 8th World Congress on Pain, Progress in Pain Research and Management; Seattle, I.A.S.P. Press. 1997.pp. 155–184. [Google Scholar]
- 34.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon RL, Vierck CJ., Jr. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 35.Scott D, Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clin J Pain. 2005;21:175–181. doi: 10.1097/00002508-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Simms RW. Fibromyalgia is not a muscle disorder. Am J Med Sci. 1998;315:346–350. doi: 10.1097/00000441-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Sorensen J, Graven-Nielsen T, Henriksson KG, Bengtsson M, Arendt-Nielsen L. Hyperexcitability in fibromyalgia. J Rheumatol. 1998;25:152–155. [PubMed] [Google Scholar]
- 38.Staud R. Future perspectives: Pathogenesis of chronic muscle pain. Best Pract Res Clin Rheumatol. 2007 doi: 10.1016/j.berh.2007.02.013. doi:10.1016/j.berh.2007.02.013:1-16. [DOI] [PubMed] [Google Scholar]
- 39.Staud R, Price DD, Fillingim RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (wind-up) J Pain. 2006;7:575–582. doi: 10.1016/j.jpain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–696. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Staud R, Price DD, Robinson ME, Vierck CJ. Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain. 2004;5:338–343. doi: 10.1016/j.jpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Staud R, Robinson ME, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 43.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 44.Staud R, Vierck CJ, Robinson ME, Price DD. Overall fibromyalgia pain is predicted by ratings of local pain and pain related negative affect: possible role of peripheral tissues. Rheumatology. 2006;45:1409–1415. doi: 10.1093/rheumatology/kel121. [DOI] [PubMed] [Google Scholar]
- 45.Sterling M, Jull G, Vicenzino B, Kenardy J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain. 2003;104:509–517. doi: 10.1016/S0304-3959(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 46.Vecchiet L, Giamberardino MA, de Bigontina P, Dragani L. Comparative sensory evaluation of parietal tissues in painful and nonpainful areas in fibromyalgia and myofascial pain syndrome. In: Gebhardt GF, Hammond DL, Jensen TS, editors. Progress in Pain Research and Management. IASP Press; Seattle: 1994. pp. 177–185. [Google Scholar]
- 47.Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 48.Vierck CJ, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 49.Whitsel BL, Tommerdahl M, Kohn A, Vierck CJ, Favorov O. The S1 response to noxious skin heating by optical intrinsic signal imaging. In: Casey KL, Bushnell MC, editors. Pain Imaging, Progress in Pain Research and Managment. IASP Press; Seattle: 2000. pp. 47–93. [Google Scholar]
- 50.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 51.Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 52.Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 53.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]