Abstract
Intercellular communication between the somatic and germline cells is vital to development of the Drosophila egg chamber. One critical outcome of this communication is the polarization of the oocyte along the anterior-posterior axis, a process induced by an unknown signal from the somatic follicle cells to the oocyte. The existence of this signal has been inferred from several reports demonstrating that the differentiation and patterning of the follicle cells by the spatially restricted activation of certain cell-signaling pathways is necessary for axis formation in the oocyte. These reports have also provided a framework for understanding how these signaling pathways are integrated to generate the follicle-cell pattern, but the precise role of the follicle cells in anterior-posterior axis formation remains enigmatic. Research has identified several genes that appear to be involved in the polarizing communication from the follicle cells to the oocyte. Interestingly the proteins encoded by most of these genes are associated with the extracellular matrix, suggesting a pivotal role for this complex biological component in the polarizing communication between the follicle cells and oocyte. This review summarizes the findings in this area, and uses the the experimental analyses of these genes to evaluate various models describing the possible nature of the polarizing signal, and the role of these genes in it.
Keywords: polarity, cell signaling, cell adhesion, axis specification, microtubule repolarization, actin cytoskeleton, soma-germline interaction
Drosophila oogenesis has served as an excellent model system in cellular, molecular, and developmental biology for many years (reviewed in Deng and Bownes, 1998; Horne-Badovinac and Bilder, 2005; Huynh and St Johnston, 2004; Johnstone and Lasko, 2001; Muller, 2000; Riechmann and Ephrussi, 2001; Roth, 2001; Steinhauer and Kalderon, 2006; Tanentzapf et al., 2000). Over the last two decades particular attention has been given to the formation of the anterior-posterior (AP) and dorsal-ventral (DV) body axes, which are established during oogenesis. In Drosophila, axis formation is hierarchical in that AP axis formation precedes and is necessary for DV specification, highlighting the importance of proper AP axis formation. The AP axis is defined by the localization of several RNAs and proteins to distinct subcellular compartments of the oocyte; most notable among these are bicoid (bcd) RNA at the anterior cortex of the oocyte and oskar (osk) RNA at the posterior (Ephrussi et al., 1991; Kim-Ha et al., 1991; St Johnston et al., 1989).
During oogenesis, the majority of mRNAs and proteins present in the oocyte are provided by a cytoplasmically connected group of 15 other germline cells known as nurse cells. The 15 nurse cells, the oocyte, and a surrounding monolayer of somatically derived follicle cells together comprise the egg chamber. Research has shown that axis specification in the oocyte depends on the patterning of these follicle cells into discrete cell subtypes, which serve various functions in egg chamber development (Gonzalez-Reyes et al., 1995; Gonzalez-Reyes and St Johnston, 1998; Roth et al., 1995; Ruohola et al., 1991; Spradling, 1993; Xi et al., 2003). Proper patterning of the follicle cells is regulated by various cell-signaling events such as activation of the Notch, Epidermal Growth Factor Receptor (EGFR), and Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) pathways in follicle cells (Gonzalez-Reyes et al., 1995; Gonzalez-Reyes and St Johnston, 1998; Roth et al., 1995; Ruohola et al., 1991; Xi et al., 2003). These pathways must be properly activated both temporally and spatially for the correct patterning of the follicle cells and for the subsequent formation of the AP and DV axes in the oocyte. Our molecular and genetic understanding of the role of the follicle cells in DV axis formation is excellent, as is our knowledge of the processes within the oocyte that culminate in AP axis formation (Riechmann and Ephrussi, 2001; Steinhauer and Kalderon, 2006), but remarkably little is known of the precise role the follicle cells play in specifying the AP axis.
Because axis formation depends on the proper differentiation of the follicle cells in response to key signaling events, the follicle cells have been proposed to provide some polarizing signal(s) to the oocyte that initiate AP axis formation (Gonzalez-Reyes et al., 1995; Gonzalez-Reyes and St Johnston, 1998; Roth et al., 1995; Ruohola et al., 1991). During oogenesis, the oocyte lies at the posterior end of the developing egg chamber where it is in direct contact with a limited group of follicle cells referred to as the posterior follicle cells (PFC). These PFC develop a clear apicobasal polarity, with the apical region facing the inner germline cells and the basal sides facing out. Because of their unique contact with the oocyte (Fig. 1), the PFC are believed to be instrumental in generating this polarizing signal (Gonzalez-Reyes et al., 1995; Gonzalez-Reyes and St Johnston, 1998; Roth et al., 1995). Furthermore, experimental evidence has repeatedly demonstrated an important role for the PFC in AP axis formation, much of which will be presented here. The nature of the signal they produce is unknown, although recent research has identified several genes that must be functional in the follicle cells for axis formation to occur (Deng and Ruohola-Baker, 2000; Frydman and Spradling, 2001; Lee et al., 1997; MacDougall et al., 2001; Poulton and Deng, 2006). Of course, there are many genes required in the follicle cells as primary or regulatory components of the signaling pathways responsible for follicle-cell differentiation, while others operate downstream of differentiation and may therefore provide insight into the mechanistic nature of the polarizing signal. Of particular interest is the observation that of the five genes believed to function downstream of differentiation, three are either components or receptors of the extracellular matrix (ECM). In this review we synthesize the empirical research describing the role of the follicle cells in the communication that triggers AP axis specification in the oocyte, with special emphasis on the role of ECM-associated genes in this process. We will also use the phenotypic and genetic information available for these genes to evaluate various hypotheses about the nature of the polarizing signal, as well as how these genes may function in that communication.
Figure 1.
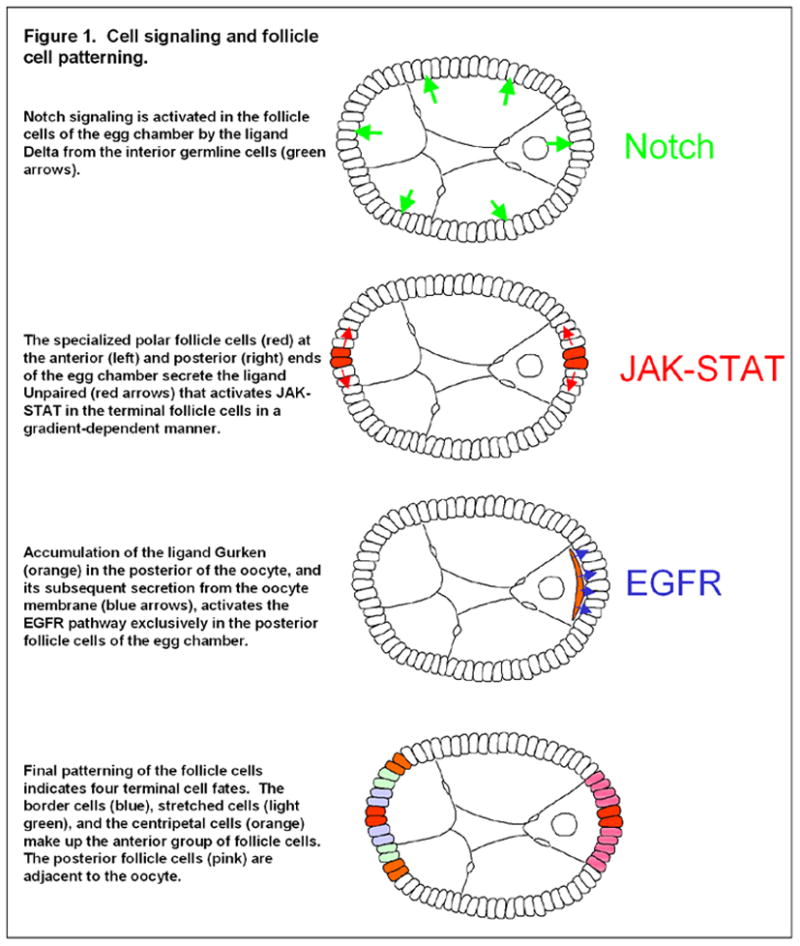
Cell signaling and follicle cell patterning.
Overview of Drosophila oogenesis and axis formation
Drosophila oogenesis is a highly amenable system to many questions in developmental biology partly because the temporal development of the egg chambers can be relatively easily divided into 14 distinct stages based on morphological characteristics (Spradling, 1993). For the purposes of this review, we will also refer to the broader classification of the timing of oogenesis into early (stages 1–6), mid- (stages 7–10a), and late (stages 10b–14) oogenesis.
The cellular processes occurring within the oocyte during axis formation have received a great deal of attention over the past two decades (Steinhauer and Kalderon, 2006). Several aspects of AP axis formation bear mention here, because their disruption may underlie many of the phenotypes observed in mutants of the follicle-cell genes that are the focus of our review. First, in early oogenesis a microtubule organizing center (MTOC) forms at the posterior of the oocyte, then at the onset of mid-oogenesis, and presumably as a consequence of the polarizing signal from the follicle cells, this MTOC is disassembled (Januschke et al., 2006; Theurkauf et al., 1992). Breakdown of the posterior MTOC appears to result from the posterior enrichment of the serine/threonine kinase Par-1N1 isoforms; this localization also requires the polarizing cue from the follicle cells, as well as the actin cytoskeleton (Doerflinger et al., 2006; Schulman et al., 2000). Disassembly of the posterior MTOC is believed to allow the migration of the oocyte nucleus to the anterior of the oocyte and formation of a gradient of microtubules (MT) from higher density at the anterior to few if any at the posterior (Cha et al., 2001; Januschke et al., 2006; Theurkauf et al., 1992). In addition, an overall reorganization of MT polarity within the oocyte takes place at this stage; the MT plus ends, as visualized by the localization of a protein fusion of the plus end MT motor Kinesin and β-Galactosidase (Kin:βGal) (Clark et al., 1994), accumulate in the posterior compartment, which had previously housed the MTOC. Because mutations in Kinesin or the minus end MT motor Dynein result in mislocalization of axis determinants, and because depolymerization of MT by chemical treatment can also inhibit axis formation, this MT reorganization appears to serve as the basis for the localization of axis-determining RNAs and proteins to their appropriate subcellular destinations around stage 9/10 (Brendza et al., 2000; Cha et al., 2001; Clark et al., 1994; Pokrywka and Stephenson, 1991; Pokrywka and Stephenson, 1995; Schnorrer et al., 2000).
The posterior localization of the Par-1N1 isoforms at stage 7 has emerged as a key early component of the cellular processes controlling the reorganization of MT polarity. In addition to its reported role in the disassembly of the posterior MTOC at stage 7 (Doerflinger et al., 2006), the initial localization of Par-1N1 to the posterior cortex of the oocyte has been suggested to recruit a small amount of MT plus ends to the posterior which then facilitate transport of a fraction of the oskar RNA pool located in the center of stage 8 oocytes to the posterior cortex, where translational repression of oskar RNA is relieved (Zimyanin et al., 2007). This small quantity of Oskar protein is then believed to recruit more Par-1N1 to the posterior, which serves to concentrate even more MT plus ends at the posterior, thereby establishing a positive feedback loop that will further localize the remaining oskar RNA to the posterior, as well as help form the overall MT polarity within the oocyte by accumulating the MT plus ends at the posterior of the oocyte.
The molecular machinery involved in the posterior transport of the MT plus ends and axis determinants is fully understood, however recent evidence implicates both the endocytosis and exocytosis pathways in this process. In terms of endocytic trafficking, Oskar protein has emerged as an important player in this process, as it appears to function at the posterior cortical membrane to promote endocytosis and the formation of F-actin projections in this region of the oocyte (Vanzo et al., 2007). Both endocytosis and these actin projections seem to be necessary for posterior determinant localization. Consistent with a role in endocytosis at the oocyte posterior, Oskar is also required for the accumulation of the endocytic recycling protein Rab11 at the posterior cortex, which is in turn required for proper localization of MT plus ends (Dollar et al., 2002; Jankovics et al., 2001). Together these studies demonstrate the importance of membrane trafficking in oocyte polarity. In addition to endocytosis, exocytosis, possibly via the trans-Golgi network, has also been implicated in the formation of AP polarity, as evidenced by research demonstrating that the GTPase Rab6 regulates aspects of the exocytic pathway and promotes localization of Staufen, oskar RNA, and MT plus ends to the posterior cortex (Coutelis and Ephrussi, 2007).
Follicle cell differentiation and axis formation
Although many signaling pathways are necessary for overall egg-chamber development (e.g. JNK, (Jordan et al., 2006; Suzanne et al., 2001); Dpp, (Deng and Bownes, 1997; Twombly et al., 1996); Hedgehog, (Forbes et al., 1996; Sun and Deng, 2007); and Insulin/Insulin-like, (Richard et al., 2005), to date, three classic signaling pathways have been identified that must be activated in the follicle cells in early to mid-oogenesis for the proper differentiation of the follicle cells and establishment of the body axes (Fig. 1): Notch, EGFR, and JAK-STAT (Gonzalez-Reyes et al., 1995; Roth et al., 1995; Ruohola et al., 1991; Xi et al., 2003). In fact, these signaling pathways are activated multiple times throughout oogenesis, but we will focus here on the signaling events most directly related to AP axis formation. Around stage 6 of oogenesis, Delta, a transmembrane ligand for the Notch receptor is up-regulated in the germline, activating Notch signaling in the surrounding follicle cells (Lopez-Schier and St Johnston, 2001). Notch activation in these cells has been shown to have two primary effects on the follicle cells: it initiates a transition from the mitotic cycle in early oogenesis to an endoreplication cycle during mid-oogenesis (Deng et al., 2001; Lopez-Schier and St Johnston, 2001), and it also serves as a differentiating signal in the follicle cells at stage 6, when they develop from an “immature” to “mature” fate (Lopez-Schier and St Johnston, 2001; Sun and Deng, 2005). Changes in the expression of various genes such as Fasciclin III (FasIII), Cut and Hindsight (Hnt) serve as effective markers of this differentiation (Lopez-Schier and St Johnston, 2001; Ruohola et al., 1991; Sun and Deng, 2005; Sun and Deng, 2007).
Loss of either Notch in the follicle cells or Delta in the germline results in a severe disruption of AP axis formation. In addition, Notch/Delta defects result in overproliferation of the follicle cells caused by a failure to terminate mitosis at stage 6 (Lopez-Schier and St Johnston, 2001; Ruohola et al., 1991). The finding that Notch activity in the follicle cells was necessary for axis formation in the oocyte indicated that the follicle cells themselves may provide some signal to the germline that is necessary for AP axis formation (Ruohola et al., 1991). Further research has demonstrated that disruption of Notch activity inhibits differentiation of both anterior follicle cell (AFC) and PFC fates (Fig. 1) (Gonzalez-Reyes and St Johnston, 1998; Lopez-Schier and St Johnston, 2001). The requirement of Notch signaling for proper differentiation of both cell types indicates that Notch activity provides a basis for competency to respond appropriately to the JAK-STAT and EGFR signals that occur in the specific subsets of follicle cells.
JAK-STAT signaling occurs at both termini of the egg chamber and is initiated by secretion of the ligand Unpaired (Upd) from the polar cells, which creates an activity gradient, declining with distance from the polar cells (Xi et al., 2003). Depending on its location within the JAK-STAT signaling gradient, a given follicle cell will differentiate into one of the three currently identifiable AFC subtypes: border cell, stretched cell, or centripetal cell (Fig. 1). Border cells receive the highest levels of Upd, and centripetal the lowest (Xi et al., 2003). Because polar cells are present at both poles of the egg chamber, a mirror image of these three cell types can be generated at the posterior end of the egg chamber, however, subsequent activation of the EGFR pathway in the posterior cells masks the JAK-STAT–induced pattern at the posterior and serves as the third key signaling event in this process. Importantly, JAK-STAT activation must still occur in the PFC for proper EGFR-based differentiation (Xi et al., 2003). In this capacity, JAK-STAT is necessary for axis formation because it appears to act in conjunction with Notch signaling to make the PFC competent to respond to EGFR. The differentiation of the AFC by JAK-STAT does not appear necessary for axis formation but is still essential for differentiation of the three AFC types described, which have unique and important roles in other aspects of egg chamber development.
The EGFR pathway is activated in the PFC by secretion of the TGFα-like ligand Gurken (Grk) from the adjacent oocyte (Fig. 1). Although the exact timing of this activation is not known, it must be prior to stage 7, as EGFR-dependent events are apparent by this stage (Doerflinger et al., 2006; Gonzalez-Reyes et al., 1995; Roth et al., 1995). Among these events are up-regulation of various negative-feedback regulators, such as Argos (Zhao and Bownes, 1999), Pointed (Morimoto et al., 1996), Sprouty (Reich et al., 1999), and Kekkon (Ghiglione et al., 1999), the initiation of changes in follicle-cell morphology, and the initial oocyte-based aspects of axis formation (Doerflinger et al., 2006; Gonzalez-Reyes et al., 1995; Roth et al., 1995). Loss of EGFR activation in the follicle cells inhibits differentiation of the PFC fate, leading to a symmetrically organized epithelium in which markers induced by JAK-STAT signaling are visible at both termini (Gonzalez-Reyes et al., 1995; Gonzalez-Reyes and St Johnston, 1998; Roth et al., 1995). Furthermore, disruption of the EGFR pathway in the PFC causes severe defects in oocyte polarity, including failure to localize Par-1N1 at the posterior, defective reorganization of the MT cytoskeleton, and a failure to initiate anterior migration of the oocyte nucleus (Doerflinger et al., 2006; Gonzalez-Reyes et al., 1995; Roth et al., 1995). Although the processes taking place within the oocyte that lie downstream of MT reorganization are relatively well understood, the function of the PFC in initiating this process remains a great mystery. The remainder of our review will focus on the role of the follicle cells in AP axis formation.
Genes required in the PFC for AP axis specification
To date, several genes have been implicated in the polarizing cue from the PFC to the oocyte, but do not belong to the three aforementioned signaling pathways: α-Spectrin (α-Spec), Merlin, Laminin A (LanA), Dlar, and Dystroglycan (DG) (Deng and Ruohola-Baker, 2000; Frydman and Spradling, 2001; Lee et al., 1997; MacDougall et al., 2001; Poulton and Deng, 2006). The first two genes we will discuss, α-Spec and Merlin are known or proposed interactors with various cytoskeletal and signaling molecules, whereas the remaining three genes (LanA, Dlar, and DG) are associated with the ECM.
In PFC lacking α-Spec, Oskar protein does not accumulate at the posterior of the oocyte and the oocyte nucleus fails to migrate anteriorly after stage 7, indicating major defects in oocyte polarity (Lee et al., 1997). In these α-Spec clones (“clones” referring to a group of cells homozygous mutant for a particular gene in an otherwise heterozygous fly), Notch signaling seems to have been properly activated based on correct staining of FasIII in the PFC, however, PFC-specific markers have not been tested. Therefore it is not known whether mutations in α-Spec simply inhibit the differentiation of PFC, thus rendering them unable to generate the polarizing cue, or whether α-Spec acts downstream of differentiation and thus more directly in the communication back from the PFC to the oocyte. For this reason, we tentatively consider α-Spec one of the genes necessary for the polarizing communication from the PFC to the oocyte.
In addition to oocyte polarity defects, α-Spec clones were also reported to show disruption of apicobasal polarity and overproliferation (Lee et al., 1997). α-Spec is a main component of the spectrin-based membrane skeleton (SBMS) and has been shown to play a pivotal role in epithelial-cell apicobasal polarity (Zarnescu and Thomas, 1999). It was suggested that the loss of apicobasal polarity was responsible for follicle-cell overproliferation and oocyte polarity defects. This explanation for the overproliferation phenotype is supported by similar data from mutants of other genes in which defective follicle-cell polarity results in overproliferation of the follicle cells, and as in α-Spec clones, this phenotype is frequently exacerbated if not exclusive to the follicle cells at the poles of the egg chamber (Goode et al., 2005; MacDougall et al., 2001; Tanentzapf et al., 2000). In terms of oocyte polarity defects, because the apical surface of the PFC abuts the oocyte membrane, apicobasal polarity may be necessary for the apical targeting of the polarizing signal to the oocyte, however there is no conclusive evidence for this hypothesis.
Merlin, the Drosophila homologue of the tumor suppressor Neurofibromatosis-2, was identified from a screen for temperature-sensitive (ts) mutations which disrupted oocyte polarity (MacDougall et al., 2001). When the PFC are mutant for Merlints, the AP axis is severely disrupted and the PFC show a double-layering phenotype. These oocyte polarity defects were reported to lie in the signaling from the PFC to the oocyte and not in the differentiation of the PFC, as Merlints mutant PFC have normal expression of the PFC fate marker pointed-lacZ (Deak, P., Glover, D., Bownes, M., Deng, W.-M., Pathirana, S., Gonzalez-Reyes, A., and St Johnston, D. unpublished data; MacDougall et al., 2001; Micklem et al., 1997). It should be noted, however, that the use of a temperature-sensitive allele leaves open the possibility that Merlin could still be involved in cell differentiation, such that enough function is retained in Merlints to allow sufficient levels of activity in the signaling pathways responsible for pointed-lacZ expression, while not allowing the full activation needed for generating the polarizing signal to the oocyte.
Merlin is related to the Moesin, Ezrin, and Radixin family of proteins, which have been shown to link actin to transmembrane proteins (Turunen et al., 1998). Interestingly, a series of recent reports suggest a diverse range of functions for Merlin, including acting with Expanded in the Hippo tumor suppressor pathway (Hamaratoglu et al., 2006; MacDougall et al., 2001; Maitra et al., 2006; McCartney et al., 2000; Pellock et al., 2007). Because the cellular function of Merlin is the center of much ongoing research, and due to the uncertainty regarding PFC fate specification in Merlints mutants, it is difficult to speculate as to Merlin’s precise function in the process of follicle cell-oocyte communication.
The ECM was first identified as a key player in axis formation with the discovery that loss of LanA in the PFC causes mislocalizations of posterior markers such as Stau, Osk, Dynein, and Kin:βGal. Occasionally, bcd RNA is mislocalized from the oocyte anterior, and a less frequent failure of the oocyte nucleus to migrate from posterior to anterior after stage 6 is observed in LanA PFC clones (Deng and Ruohola-Baker, 2000). Laminin is a heterotrimeric protein consisting of α, β1, and β2/γ1 subunits, with the α-subunit primarily responsible for receptor binding (Brown, 2000). Loss of LanA can disrupt oocyte polarity, but it appears to require large PFC clones to do so because the presence of some wild-type follicle cells within a larger clone of LanA mutant PFC remain capable of correctly forming the AP axis (Deng and Ruohola-Baker, 2000). The function of LanA in axis formation appears to be in the signaling from the PFC to the oocyte and not in the Grk-induced activation of EGFR, as LanA clones are not defective in their expression of PFC markers, nor do anterior LanA clones lead to defects in DV polarity, which is also initiated by Grk-EGFR signaling between the oocyte and anterior follicle cells. In addition, loss of LanA does not result in defects in the apicobasal polarity of the follicle cells themselves (Deng and Ruohola-Baker, 2000). Although the mechanistic role of LanA is unknown, the finding that the ECM is necessary for the signaling from the PFC to the oocyte is important when considering the potential mechanistic role of other known players in this communication.
Dlar, a receptor protein tyrosine phosphatase, is also required in the PFC for oocyte polarity (Frydman and Spradling, 2001). Dlar has also been shown to be involved in axon pathfinding and may be involved in regulating the actin cytoskeleton (Baum et al., 2000; Lanier and Gertler, 2000). Importantly, the mammalian homologue of Dlar is able to bind Lan (O’Grady et al., 1998), and as in LanA clones, PFC clones of Dlar also cause oocyte polarity defects without disrupting apicobasal polarity. However, differences between the AP axis phenotypes generated by Dlar clones and those of LanA clones requires careful consideration. First, although LanA mutations can apparently disrupt the MT-dependent localization of both anterior and posterior determinants in the oocyte (Deng and Ruohola-Baker, 2000), PFC mutations of Dlar appear to only affect posterior polarity markers, such as Osk localization, whereas anterior markers such as bcd RNA and oocyte nucleus migration are normal (Frydman and Spradling, 2001). In addition, Dlar clones create a type of cell-autonomous defect that we have referred to elsewhere as the clone adjacent mislocalization (CAM) phenotype, in which posterior polarity markers fail to accumulate at the oocyte cortex directly adjacent to the clone cells but localize normally over the remaining wild-type cells (Poulton and Deng, 2006), a phenotype not generated by similarly positioned LanA clones (Deng and Ruohola-Baker, 2000). Interestingly, this phenotype has also been reported for smaller clones of genes in both the EGFR and JAK-STAT pathways (Poulton and Deng, 2006; Xi et al., 2003). Although the processes governing this phenotype in the oocyte are not known, it does indicate that some sharply defined positional information is being relayed between the PFC and oocyte. On the basis of these phenotypic dissimilarities, if the effects of Dlar mutations on oocyte polarity are mediated by disruption of Lan in the ECM, they are clearly less severe than those caused by a complete loss of LanA. Mechanistically, Dlar has been proposed to be specifically activated on the basal surface of the PFC by Lan, which is basally restricted in the follicle cells in mid-oogenesis (Fig. 2B) (Frydman and Spradling, 2001; Gutzeit et al., 1991; Poulton and Deng, 2006).
Figure 2.
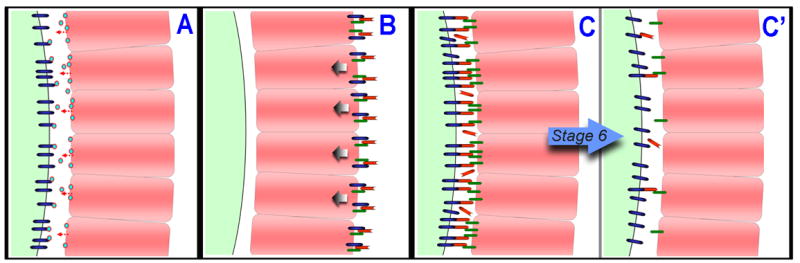
Models depicting the possible nature of the polarizing signal from the posterior follicle cells (PFC, pink) to the oocyte (light green on left side of figures). (A) The signal could be a secreted molecule (blue and red circle) released from the PFC that activates some receptor (dark blue) on the surface of the oocyte. (B) A complex of proteins, possibly including DG, Dlar, and Lan, is localized to the basal surface of the PFC, a process that may be necessary for the generation and/or transduction of the polarizing signal toward the apical surface of the PFC (arrows) (Frydman and Spradling, 2001; Poulton and Deng, 2006). Mislocalizations of DG and Lan to other membrane surfaces of the PFC could then dilute the targeting of the polarizing signal away from the apical surface (not shown). (C) The polarizing signal may operate through either an increase or decrease in PFC-oocyte adhesion. This model depicts a decrease in cell adhesion in which, prior to stage 6, PFC receptors (dark green) interact with oocyte receptors (dark blue), perhaps via the ECM (red). (C′) After stage 6, these proteins are largely cleared from the apical surface and become basally localized (not shown), which is in fact the observed localization pattern for DG and Lan. This change in the composition of the ECM and associated receptors at the apical surface of the PFC could be a key component of the polarizing signal.
It should also be noted that in the case of Dlar, there remains some uncertainty as to the differentiated state of the PFC in Dlar mutant egg chambers. The expression of specific PFC markers were not reported in Dlar mutants, allowing for the possibility that Dlar may be necessary for PFC differentiation. The Notch-associated marker FasIII was examined in Dlar mutants, and did not indicate any defects in Notch activation in the follicle cells, although its expression in one to several additional cells around the polar cells suggests that Dlar mutant egg chambers possess ectopic polar cells.
EGFR activation in the PFC is essential for the repolarization of the MT cytoskeleton and AP axis formation. Although the exact role of EGFR activation in this process is not known, we have recently found that EGFR activation in the PFC leads to the down-regulation of Dystroglycan (DG), and this down-regulation is necessary for AP polarity in the oocyte (Poulton and Deng, 2006). DG is a transmembrane protein that serves as a receptor for a number of ECM proteins, including Lan and Perlecan (Pcan), and functions as a link between the ECM and the actin cytoskeleton (Brancaccio, 2005; Deng et al., 2003; Schneider et al., 2006). In addition to the requirement for DG down-regulation in the specification of the AP axis, both EGFR activation and DG down-regulation appear necessary for the basal restriction of Lan in the PFC. Failure to down-regulate DG also results in mislocalization of the MT plus ends from the posterior cortex of the oocyte, as indicated by Kin:βGal staining, as well as similar mislocalizations of the posterior polarity markers Staufen and Vasa. Interestingly, anterior polarity markers in the oocyte appear to behave normally in the presence of ectopic DG in the PFC, suggesting that DG down-regulation alone cannot serve as the signal to polarize the oocyte. The observation that the polarity defects caused by ectopic DG are limited to posterior polarity markers in the oocyte is very similar to that found in Dlar mutant egg chambers (Frydman and Spradling, 2001).
Frydman and Spradling originally proposed a model in which basally localized Lan interacts with Dlar at the basal surface of the PFC (Frydman and Spradling, 2001). This interaction is then necessary to generate some signal or possibly to regulate some key biophysical properties of the PFC, such as cell adhesion, that are in turn necessary for the generation or transduction of the polarizing signal. If this model is true, we can now extend this model to include DG based on our findings that DG expression patterns in the PFC can regulate Lan localization and oocyte polarity (Poulton and Deng, 2006). Specifically, the stage 6 downregulation and basal restriction of DG in the PFC mediates localization of Lan to the basal surface where it may then interact with Dlar. This interaction at the basal membrane can then initiate some signaling process that is transduced to the apical surface, or cause some physical modification of the PFC that is necessary for the proper interface between the apical surface of the PFC and oocyte (Fig. 2B). Thus, in cases of ectopic DG in the PFC, as seen in EGFR pathway mutants, the resulting apical and lateral accumulations of Lan may dilute Lan in the basal ECM, or dilute the apically-targeted signal itself, resulting in insufficient levels of signal at the apical surface. It is possible that Merlin is involved in the apical targeting of this signal because Merlin is apically localized in the follicle cells (McCartney and Fehon, 1996). In addition, α-Spec could also be incorporated into such a model as it is a cytoskeletal component reported to be involved in the apical-basal polarity of these cells (Lee et al., 1997), therefore α-Spec may be required to mediate the effects of these ECM-associated proteins on the polarizing signal, particularly if those effects are manifested in physical properties of the PFC. This model demonstrates the importance of investigating the state of the ECM in many of these mutants as well as in mutations of genes that may be identified in the future as part of this process. One could test this model by knocking down Lan levels, perhaps by RNAi, while simultaneously overexpressing DG to see if reducing overall Lan levels may allow the residual basal Lan to interact with basal Dlar and rescue the polarity defects associated with ectopic DG. However, this model did not consider that Lan and DG are localized at both the apical and basal surfaces of the follicle cells prior to stage 7 (Deng et al., 2003; Poulton and Deng, 2006). We will incorporate these findings into some additional models which are detailed in the following section.
PFC function in AP axis formation
Models representing the role of the PFC in AP axis formation can be divided into two categories based on their description of: (1) the molecular nature of the polarizing signal, specifically at the PFC-oocyte interface, or (2) the complexity of the signal(s) provided by the PFC.
The first set of models focuses on the potential molecular nature of the polarizing signal provided by the PFC, and how mutations in these PFC genes may play into disrupting that signaling process. First, the PFC may provide a secreted type of molecule to the oocyte that serves as the trigger for AP axis formation. Secretion of an inductive signal is an attractive conceptual basis for the polarizing signal from the PFC to the oocyte (Fig. 2A), but as yet no direct evidence supports this model. We previously reported that RNAi-based down-regulation of the transmembrane protein DG can rescue the CAM phenotype but not the cell-fate defects caused by Ras clones (Poulton and Deng, 2006); Ras is a key component of the EGFR pathway (Lee and Montell, 1997). This finding demonstrates that simply correcting the defect in DG expression and localization is sufficient to allow the MT plus ends to accumulate at the posterior cortex adjacent to the Ras clones, given that a portion of the PFC cells are still wild type. Failure to rescue cell fate in this experiment suggests that any other signals typically produced by the PFC (e.g., some form of secreted signal induced by EGFR activity) should remain absent in the Ras clones, yet the AP axis can form normally in these egg chambers once the ectopic DG defect is corrected. These results may be compatible with a secreted type of polarizing signal when one takes into account the fact that we also found defects in Lan localization in Ras mutant clones (Poulton and Deng, 2006), which, together with the need for Lan in establishing oocyte polarity (Deng and Ruohola-Baker, 2000), implicates ectopic Lan as having a role in generating the CAM phenotype in Ras clones, as well as when DG is simply overexpressed. We previously proposed that these ectopic Lan accumulations in the apical ECM might preclude diffusion of a secreted signal from the adjacent wild-type cells to the region of the oocyte posterior next to the clone cells (Poulton and Deng, 2006). Therefore, when Lan is properly restricted to the basal side of the PFC in the DG RNAi rescue of Ras clones, the secreted molecule from the wild-type cells can then permeate the extracellular space between the clone and oocyte membranes. Determining whether such a mechanism might also operate in JAK-STAT and Dlar mutants, which are each capable of generating the CAM phenotype, is difficult because the state of the ECM in those cases has not been described (Frydman and Spradling, 2001; Xi et al., 2003). Note that ectopic DG expression in the follicle cells can also lead to apical accumulations of another ECM protein, Pcan (Schneider et al., 2006). Similar to Lan, Pcan is normally restricted to the basal surface of the follicle cells during mid-oogenesis. Pcan was previously demonstrated to be involved in Drosophila neuroblast proliferation, probably through sequestration of ligand molecules in the ECM (Voigt et al., 2002), therefore the apical accumulations of Pcan and/or Lan resulting from ectopic DG in the follicle cells could be responsible for preventing proper signaling from the PFC in DG overexpression experiments. Whether Pcan is mislocalized in Ras mutant PFC clones, as is the case for Lan, has not yet been determined.
An alternative model for the nature of the polarizing signal is that changes in cell adhesion or simply cell surface protein interactions could act as the cue to establish the AP axis (Fig. 2C,C′). The induction of MT reorganization may be based on an increase or decrease in cell adhesion between the oocyte and PFC, which then disrupts the MT cytoskeleton either directly or possibly through an intermediary such as the actin cytoskeleton. The ability of changes in cell adhesion to regulate the actin cytoskeleton has been established in a variety of organisms and cell types (Drubin and Nelson, 1996; Noritake et al., 2005; Tsukita et al., 1992; Watanabe et al., 2005), as has the ability of the actin cytoskeleton to regulate MTs (Manseau et al., 1996; Noritake et al., 2005; Theurkauf, 1994; Watanabe et al., 2005). The Drosophila oocyte possesses an enriched cortical actin cytoskeleton that plays several important roles in oocyte development and polarity. Although some of the actin-related proteins known to be involved in proper axis formation do not appear to affect this MT reorganization (Baum, 2002; Erdelyi et al., 1995; Jankovics et al., 2002; Manseau et al., 1996; Manseau and Schupbach, 1989; Polesello et al., 2002; Theurkauf, 1994), the actin-binding protein Capulet has been shown to disrupt the MT reorganization, leading to severe disruptions of osk and bcd RNA localization, as well as Kin:βGal (Baum et al., 2000). Furthermore, the actin cytoskeleton is specifically required in the Drosophila oocyte for localization of Par-1N1 to the oocyte posterior in response to the polarizing signal (Doerflinger et al., 2006). Therefore, a change in oocyte-PFC adhesion, either through direct receptor-receptor interactions or possibly through a “receptor to ECM to receptor” adhesive complex (Fig. 2C,C′), could serve as the polarizing signal by modifying the oocyte cortical actin cytoskeleton, which then initiates the MT reorientation at stage 7 of oogenesis.
Support for such a model can be found in the known role of DG and Lan in an adhesive complex in muscle cells (Brancaccio, 2005) in conjunction with our previous work on the role of DG and Lan in AP axis formation. Prior to the activation of the EGFR pathway in the PFC around stage 6, DG and Lan are both present to some degree on all surfaces of the follicle cells. After stage 6, DG is down-regulated in the PFC, and becomes restricted to the basal surface of the follicle cells. Furthermore, this down-regulation and reorganization of DG within the PFC appears to mediate the basal restriction of Lan that occurs around this same time (Poulton and Deng, 2006). Because we have shown that this down-regulation of DG is necessary for localization of posterior markers in the adjacent oocyte, it is possible that in early oogenesis, the DG/Lan complex establishes some adhesive interaction with proteins on the oocyte surface that are then lost or remodeled following the down-regulation and basal restriction of DG/Lan after stage 6 (Fig. 2C,C′). Although our previous findings do not support a role for DG down-regulation as the single cue to initiate AP axis formation, they do demonstrate the capacity for an adhesion molecule, not previously identified in cell-cell communication, to at least mediate if not directly contribute to the signaling from the PFC to the oocyte. These observations indicate that changes in cell adhesion type molecules could be a mechanism for this communication.
The final hypothesis we propose suggests that the PFC serve multiple functions with respect to formation/maintenance of the AP axis. The need for this model arises from the ability to experimentally uncouple the processes occurring in the anterior and posterior regions of the oocyte during AP axis formation. The most obvious example of this uncoupling is the capacity for some mutations to generate severe polarity defects affecting both anterior and posterior marker localization as well as overall MT polarity (e.g. EGFR, Notch, LanA, Merlin) (Deng and Ruohola-Baker, 2000; Gonzalez-Reyes et al., 1995; MacDougall et al., 2001; Roth et al., 1995; Ruohola et al., 1991), whereas other mutants appear to have a milder phenotype that is limited to posterior polarity markers (e.g., Dlar and ectopic DG) (Frydman and Spradling, 2001; Poulton and Deng, 2006). One explanation for these observations is that the PFC transmit one signal to the oocyte that initiates the global reorganization of MT polarity in the oocyte, upon which localization patterns throughout the oocyte are governed, but then a separate process of communication between the PFC and oocyte functions exclusively in directing/maintaining the accumulation of the MT plus ends at the posterior cortex. This model could explain the range of phenotypic defects observed for various mutants, and can also explain the CAM phenotype in that the wildtype cells adjacent to the clones are competent to provide the initial MT repolarizing cue, however the clone cells lack the ability to perform this secondary function in terms of localizing the MT plus ends at the posterior cortex. Consistent with this model, previous findings have uncovered an actin-based anchoring mechanism functioning at the posterior cortex of the oocyte that is needed to maintain posterior axis determinants at the posterior cortex (Baum, 2002; Jankovics et al., 2002; Polesello et al., 2002).
Although the past two decades have yielded a wealth of information regarding axis determination in the Drosophila oocyte, we continue to lack a thorough understanding of the critical role the PFC play in initiating axis formation. The obvious trend in the functions of the PFC genes identified to date indicates that the ECM serves a vital role in the communication between the PFC and oocyte. As future discoveries inevitably help provide answers to some of the questions surrounding the precise function of the PFC and surrounding ECM, we will be interested to see which facets of these various models are supported or ruled out. Future research will also answer the exciting question of whether regulation of cell polarity in other systems and cell types involves mechanisms of communication similar to those at work in AP axis formation in Drosophila.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum B. Drosophila oogenesis: generating an axis of polarity. Curr Biol. 2002;12:R835–7. doi: 10.1016/s0960-9822(02)01346-5. [DOI] [PubMed] [Google Scholar]
- Baum B, Li W, Perrimon N. A cyclase-associated protein regulates actin and cell polarity during Drosophila oogenesis and in yeast. Curr Biol. 2000;10:964–73. doi: 10.1016/s0960-9822(00)00640-0. [DOI] [PubMed] [Google Scholar]
- Brancaccio A. Alpha-dystroglycan, the usual suspect? Neuromuscul Disord. 2005;15:825–8. doi: 10.1016/j.nmd.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Brendza RP, Serbus LR, Duffy JB, Saxton WM. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000;289:2120–2. doi: 10.1126/science.289.5487.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NH. Cell-cell adhesion via the ECM: integrin genetics in fly and worm. Matrix Biol. 2000;19:191–201. doi: 10.1016/s0945-053x(00)00064-0. [DOI] [PubMed] [Google Scholar]
- Cha BJ, Koppetsch BS, Theurkauf WE. In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell. 2001;106:35–46. doi: 10.1016/s0092-8674(01)00419-6. [DOI] [PubMed] [Google Scholar]
- Clark I, Giniger E, Ruohola-Baker H, Jan LY, Jan YN. Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr Biol. 1994;4:289–300. doi: 10.1016/s0960-9822(00)00068-3. [DOI] [PubMed] [Google Scholar]
- Coutelis JB, Ephrussi A. Rab6 mediates membrane organization and determinant localization during Drosophila oogenesis. Development. 2007;134:1419–30. doi: 10.1242/dev.02821. [DOI] [PubMed] [Google Scholar]
- Deak P, Glover D, Bownes M, Deng W-M, Pathirana S, Gonzalez-Reyes A, St Johnston D. unpublished data. Unpublished data. [Google Scholar]
- Deng WM, Althauser C, Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–46. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- Deng WM, Bownes M. Two signalling pathways specify localised expression of the Broad-Complex in Drosophila eggshell patterning and morphogenesis. Development. 1997;124:4639–47. doi: 10.1242/dev.124.22.4639. [DOI] [PubMed] [Google Scholar]
- Deng WM, Bownes M. Patterning and morphogenesis of the follicle cell epithelium during Drosophila oogenesis. Int J Dev Biol. 1998;42:541–52. [PubMed] [Google Scholar]
- Deng WM, Ruohola-Baker H. Laminin A is required for follicle cell-oocyte signaling that leads to establishment of the anterior-posterior axis in Drosophila. Curr Biol. 2000;10:683–6. doi: 10.1016/s0960-9822(00)00514-5. [DOI] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–84. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Torres IL, Zwart MF, St Johnston D. Drosophila anterior-posterior polarity requires actin-dependent PAR-1 recruitment to the oocyte posterior. Curr Biol. 2006;16:1090–5. doi: 10.1016/j.cub.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Dollar G, Struckhoff E, Michaud J, Cohen RS. Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development. 2002;129:517–26. doi: 10.1242/dev.129.2.517. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–44. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Erdelyi M, Michon AM, Guichet A, Glotzer JB, Ephrussi A. Requirement for Drosophila cytoplasmic tropomyosin in oskar mRNA localization. Nature. 1995;377:524–7. doi: 10.1038/377524a0. [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–35. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Frydman HM, Spradling AC. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within drosophila ovarian follicles. Development. 2001;128:3209–20. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- Ghiglione C, Carraway KL, 3rd, Amundadottir LT, Boswell RE, Perrimon N, Duffy JB. The transmembrane molecule kekkon 1 acts in a feedback loop to negatively regulate the activity of the Drosophila EGF receptor during oogenesis. Cell. 1999;96:847–56. doi: 10.1016/s0092-8674(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, Elliott H, St Johnston D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 1995;375:654–8. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, St Johnston D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development. 1998;125:2837–46. doi: 10.1242/dev.125.15.2837. [DOI] [PubMed] [Google Scholar]
- Goode S, Wei J, Kishore S. Novel spatiotemporal patterns of epithelial tumor invasion in Drosophila discs large egg chambers. Dev Dyn. 2005;232:855–64. doi: 10.1002/dvdy.20336. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO, Eberhardt W, Gratwohl E. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J Cell Sci. 1991;100(Pt 4):781–8. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–74. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- Huynh JR, St Johnston D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr Biol. 2004;14:R438–49. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Jankovics F, Sinka R, Erdelyi M. An interaction type of genetic screen reveals a role of the Rab11 gene in oskar mRNA localization in the developing Drosophila melanogaster oocyte. Genetics. 2001;158:1177–88. doi: 10.1093/genetics/158.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovics F, Sinka R, Lukacsovich T, Erdelyi M. MOESIN crosslinks actin and cell membrane in Drosophila oocytes and is required for OSKAR anchoring. Curr Biol. 2002;12:2060–5. doi: 10.1016/s0960-9822(02)01256-3. [DOI] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Gillet L, Keryer G, Bornens M, Guichet A. The centrosome-nucleus complex and microtubule organization in the Drosophila oocyte. Development. 2006;133:129–39. doi: 10.1242/dev.02179. [DOI] [PubMed] [Google Scholar]
- Johnstone O, Lasko P. Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu Rev Genet. 2001;35:365–406. doi: 10.1146/annurev.genet.35.102401.090756. [DOI] [PubMed] [Google Scholar]
- Jordan KC, Schaeffer V, Fischer KA, Gray EE, Ruohola-Baker H. Notch signaling through tramtrack bypasses the mitosis promoting activity of the JNK pathway in the mitotic-to-endocycle transition of Drosophila follicle cells. BMC Dev Biol. 2006;6:16. doi: 10.1186/1471-213X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Lanier LM, Gertler FB. From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility. Curr Opin Neurobiol. 2000;10:80–7. doi: 10.1016/s0959-4388(99)00058-6. [DOI] [PubMed] [Google Scholar]
- Lee JK, Brandin E, Branton D, Goldstein LS. alpha-Spectrin is required for ovarian follicle monolayer integrity in Drosophila melanogaster. Development. 1997;124:353–62. doi: 10.1242/dev.124.2.353. [DOI] [PubMed] [Google Scholar]
- Lee T, Montell DJ. Multiple Ras signals pattern the Drosophila ovarian follicle cells. Dev Biol. 1997;185:25–33. doi: 10.1006/dbio.1997.8537. [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall N, Lad Y, Wilkie GS, Francis-Lang H, Sullivan W, Davis I. Merlin, the Drosophila homologue of neurofibromatosis-2, is specifically required in posterior follicle cells for axis formation in the oocyte. Development. 2001;128:665–73. doi: 10.1242/dev.128.5.665. [DOI] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–9. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Manseau L, Calley J, Phan H. Profilin is required for posterior patterning of the Drosophila oocyte. Development. 1996;122:2109–16. doi: 10.1242/dev.122.7.2109. [DOI] [PubMed] [Google Scholar]
- Manseau LJ, Schupbach T. cappuccino and spire: two unique maternal-effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes Dev. 1989;3:1437–52. doi: 10.1101/gad.3.9.1437. [DOI] [PubMed] [Google Scholar]
- McCartney BM, Fehon RG. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol. 1996;133:843–52. doi: 10.1083/jcb.133.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–24. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- Micklem DR, Dasgupta R, Elliott H, Gergely F, Davidson C, Brand A, Gonzalez-Reyes A, St Johnston D. The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr Biol. 1997;7:468–78. doi: 10.1016/s0960-9822(06)00218-1. [DOI] [PubMed] [Google Scholar]
- Morimoto AM, Jordan KC, Tietze K, Britton JS, O’Neill EM, Ruohola-Baker H. Pointed, an ETS domain transcription factor, negatively regulates the EGF receptor pathway in Drosophila oogenesis. Development. 1996;122:3745–54. doi: 10.1242/dev.122.12.3745. [DOI] [PubMed] [Google Scholar]
- Muller HA. Genetic control of epithelial cell polarity: lessons from Drosophila. Dev Dyn. 2000;218:52–67. doi: 10.1002/(SICI)1097-0177(200005)218:1<52::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–92. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- O’Grady P, Thai TC, Saito H. The laminin-nidogen complex is a ligand for a specific splice isoform of the transmembrane protein tyrosine phosphatase LAR. J Cell Biol. 1998;141:1675–84. doi: 10.1083/jcb.141.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellock BJ, Buff E, White K, Hariharan IK. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev Biol. 2007;304:102–15. doi: 10.1016/j.ydbio.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrywka NJ, Stephenson EC. Microtubules mediate the localization of bicoid RNA during Drosophila oogenesis. Development. 1991;113:55–66. doi: 10.1242/dev.113.1.55. [DOI] [PubMed] [Google Scholar]
- Pokrywka NJ, Stephenson EC. Microtubules are a general component of mRNA localization systems in Drosophila oocytes. Dev Biol. 1995;167:363–70. doi: 10.1006/dbio.1995.1030. [DOI] [PubMed] [Google Scholar]
- Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat Cell Biol. 2002;4:782–9. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- Poulton JS, Deng WM. Dystroglycan down-regulation links EGF receptor signaling and anterior-posterior polarity formation in the Drosophila oocyte. Proc Natl Acad Sci U S A. 2006;103:12775–80. doi: 10.1073/pnas.0603817103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich A, Sapir A, Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–47. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, Zhou Y, Partridge L, Harshman LG. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J Insect Physiol. 2005;51:455–64. doi: 10.1016/j.jinsphys.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 2001;11:374–83. doi: 10.1016/s0959-437x(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Roth S. Drosophila oogenesis: coordinating germ line and soma. Curr Biol. 2001;11:R779–81. doi: 10.1016/s0960-9822(01)00469-9. [DOI] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schupbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–78. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66:433–49. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–15. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JM, Benton R, St Johnston D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localisation to the posterior pole. Cell. 2000;101:1–20. doi: 10.1016/s0092-8674(00)80848-x. [DOI] [PubMed] [Google Scholar]
- Schnorrer F, Bohmann K, Nusslein-Volhard C. The molecular motor dynein is involved in targeting swallow and bicoid RNA to the anterior pole of Drosophila oocytes. Nat Cell Biol. 2000;2:185–90. doi: 10.1038/35008601. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1993. [Google Scholar]
- St Johnston D, Driever W, Berleth T, Richstein S, Nusslein-Volhard C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development. 1989;107(Suppl):13–9. doi: 10.1242/dev.107.Supplement.13. [DOI] [PubMed] [Google Scholar]
- Steinhauer J, Kalderon D. Microtubule polarity and axis formation in the Drosophila oocyte. Dev Dyn. 2006;235:1455–68. doi: 10.1002/dvdy.20770. [DOI] [PubMed] [Google Scholar]
- Sun J, Deng WM. Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development. 2005;132:4299–308. doi: 10.1242/dev.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Deng WM. Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev Cell. 2007;12:431–42. doi: 10.1016/j.devcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzanne M, Perrimon N, Noselli S. The Drosophila JNK pathway controls the morphogenesis of the egg dorsal appendages and micropyle. Dev Biol. 2001;237:282–94. doi: 10.1006/dbio.2001.0384. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Smith C, McGlade J, Tepass U. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J Cell Biol. 2000;151:891–904. doi: 10.1083/jcb.151.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE. Premature microtubule-dependent cytoplasmic streaming in cappuccino and spire mutant oocytes. Science. 1994;265:2093–6. doi: 10.1126/science.8091233. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 1992;115:923–36. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992;4:834–9. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Turunen O, Sainio M, Jaaskelainen J, Carpen O, Vaheri A. Structure-function relationships in the ezrin family and the effect of tumor-associated point mutations in neurofibromatosis 2 protein. Biochim Biophys Acta. 1998;1387:1–16. doi: 10.1016/s0167-4838(98)00103-4. [DOI] [PubMed] [Google Scholar]
- Twombly V, Blackman RK, Jin H, Graff JM, Padgett RW, Gelbart WM. The TGF-beta signaling pathway is essential for Drosophila oogenesis. Development. 1996;122:1555–65. doi: 10.1242/dev.122.5.1555. [DOI] [PubMed] [Google Scholar]
- Vanzo N, Oprins A, Xanthakis D, Ephrussi A, Rabouille C. Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev Cell. 2007;12:543–55. doi: 10.1016/j.devcel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Voigt A, Pflanz R, Schafer U, Jackle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev Dyn. 2002;224:403–12. doi: 10.1002/dvdy.10120. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kaibuchi K. Roles of IQGAP1 in cell polarization and migration. Novartis Found Symp. 2005;269:92–101. discussion 101–5, 223–30. [PubMed] [Google Scholar]
- Xi R, McGregor JR, Harrison DA. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev Cell. 2003;4:167–77. doi: 10.1016/s1534-5807(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Zarnescu DC, Thomas GH. Apical spectrin is essential for epithelial morphogenesis but not apicobasal polarity in Drosophila. J Cell Biol. 1999;146:1075–86. doi: 10.1083/jcb.146.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Bownes M. Misexpression of argos, an inhibitor of EGFR signaling in oogenesis, leads to the production of bicephalic, ventralized, and lateralized Drosophila melanogaster eggs. Dev Genet. 1999;25:375–86. doi: 10.1002/(SICI)1520-6408(1999)25:4<375::AID-DVG11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Zimyanin V, Lowe N, St Johnston D. An oskar-dependent positive feedback loop maintains the polarity of the Drosophila oocyte. Curr Biol. 2007;17:353–9. doi: 10.1016/j.cub.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]


