Abstract
Thirty-two African American and 23 non-Hispanic White women were compared for experimental pain threshold and tolerance to thermal, ischemic, and cold pressor pain. Approximately half of each group had prior mood disorders (17 African Americans, 13 non-Hispanic Whites), though all were free of current mood disturbance. Women with prior mood disorders were less sensitive to ischemic pain than women with no prior mood disorders (p<.05), while African Americans were more sensitive to ischemic pain than non-Hispanic Whites, though only at pain tolerance (p<.001). For cold pressor pain, the effects of race were only seen in women with prior mood disorders, since African Americans with prior mood disorders were more sensitive than non-Hispanic Whites with prior mood disorders (p<.05). These results indicate that experimental pain sensitivity in women is influenced by both race and histories of mood disorders.
Perspective:
We examined the association of race and histories of mood disorders with experimental pain sensitivity in an exclusively female sample. Our findings for racial differences in pain sensitivity may have implications for greater clinical pain in African American women. Persistent disturbance in pain modulatory mechanisms in women with a history of mood disorders may also have implications for the development of subsequent mood disturbances.
Keywords: Mood disorders, Race, Pain Tolerance
INTRODUCTION
It is well established that clinical pain and somatic symptoms are an associated feature of depression 16, 52. Since laboratory based methods to assess pain sensitivity are predictive of clinical pain 24 investigations of experimental pain sensitivity in individuals with depression and other mood disorders have been undertaken in order to elucidate the depression-pain relationship 3, 4, 5, 19, 20, 30, 42.
While results of these studies have been mixed 3, 4, 5, 19, 20, 30, 42, which may be due, in part, to the different pain modalities employed 3, 7, 28, 32, a systematic review and meta-analysis of studies comparing patients with current depression versus non-depressed controls for experimental pain perception concluded that experimental pain thresholds were higher (i.e. reduced sensitivity) in depressed individuals 19. However, only 2 of the 6 studies included in the meta-analysis assessed pain tolerance, which may be especially relevant for mood disorders, since pain tolerance reflects the affective experience of pain, while pain threshold reflects the sensory experience 43.
What is relatively understudied to date is whether euthymic individuals with a history of mood disorders experience alterations in pain sensitivity. This may be especially relevant to women since they experience significantly more clinical pain 55 and are twice as likely to experience lifetime depression relative to men 58. This is also of clinical relevance, given that a recent study in women with a history of depression showed that clinical pain (e.g. headaches, backaches) persists beyond the remission of a depressive episode 10.
The studies included in the meta-analysis on experimental pain sensitivity and current depression did not address racial differences in pain sensitivity. African Americans have more clinical pain 22, 34, 46 and experience more pain related disability 21 than primarily Caucasian samples. There is a growing and consistent literature that African Americans have lower experimental pain tolerance, though not pain thresholds, relative to primarily Caucasian samples 13, 23, 36, 50, 60. Recent work in our laboratory indicates that hyperalgesia to experimental pain stimuli in African Americans may be a result of alterations in endogenous pain regulatory mechanisms, including systolic blood pressure, since African Americans failed to show the expected blood pressure-related hypoalgesia that was seen in the primarily Caucasian sample 36. Moreover, it is important to emphasize that the studies that do exist on racial differences in pain sensitivity are based exclusively on men or on mixed gender samples with no gender-based analyses. Consequently, it is unknown whether these same racial differences in pain sensitivity would be evident in an exclusively female sample.
Although exceptions exist, there is also evidence that African Americans may have a higher prevalence of mood disorders, including depression 8, 56, 59. For example, a recent study documented that even after controlling for demographic factors, histories of depression , and other predictors, African American women were more than twice as likely to experience postpartum depressive symptoms than non-Hispanic White women 27. The greater rates of mental illness in African American populations have been attributed to greater psychosocial stress associated with poverty, crime, and racism 47, 48.
Consequently, the purpose of this study was to examine the association of race and a history of mood disorders with threshold and tolerance to experimental pain in women. Specifically, we hypothesized that African American women would show increased pain sensitivity compared to non-Hispanic White women, while women with histories of mood disturbance would show decreased pain sensitivity compared to women with no prior mood disorder. Moreover, since pain sensitivity in patients with depression is influenced by the pain modality 3, we employed a variety of noxious stimuli differing in the quality and sensation of the painful percept.
METHODS
Subjects
A total of 72 women were recruited via advertisements. A proportion of our advertisements specifically stated that African American women and women with histories of depression were needed for a research study. Approximately half of the subjects were African American (n=32), whereas the other half (n=30) included ‘Other’ racial groups (77% Non-Hispanic White, 10% Hispanic, 7% Asian, 3% Native American, and 3% Multi-racial heritage). One purpose of the study was to examine the association of psychosocial stress measures with cardiovascular and neuroendocrine stress reactivity in a multi-racial sample (to be reported elsewhere). An additional purpose of the study, which comprises the focus of this report, was to examine racial differences in the association of prior mood disorders with experimental pain sensitivity. Given the evidence that Hispanic populations differ in clinical pain symptoms 26 and are more sensitive to experimental pain than non-Hispanic Whites 31, and since the numbers of Hispanics (n = 3), Native Americans (n = 1), Asians (n = 2), and multi-racial women (n=1) in our study did not allow for valid analyses, the present report compares African Americans with non-Hispanic Whites only. Therefore, 32 African American women and 23 non-Hispanic White women are included in the present report.
All subjects were in good health, reported regular menstrual cycles, and were free of any current psychiatric Axis I disorder, as determined by structured interview (see below). Based on self report, subjects were not pregnant, nursing, or taking any prescription medication, including oral contraceptives. The protocol was approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board. Subjects provided written informed consent before participation and each received $100 compensation.
Procedures
During an initial screening session, blood pressure readings were obtained and a medical history questionnaire was administered. The Perceived Stress Scale 15(PSS;), the most widely used psychological instrument for measuring the degree to which situations in one's life are appraised as stressful, was also administered at this time, along with the Beck Depression Inventory 6 (BDI) and the Spielberger Trait Anxiety Scale 53 (STAI-Y2). The BDI is a self-rated scale to evaluate depressive symptoms (cognitive, behavioral, and somatic) 6, while the STAI-Y2 is a self-report assessment of long-term/chronic anxiety 53. Following, the Mini International Neuropsychiatric Interview 49 (M.I.N.I.), which is based on DSM-IV criteria for Axis I disorders, was administered by a trained interviewer (RK or BM). The reliability and validity of the MINI have been assessed in studies of psychiatric subjects 49, showing high inter-rater and retest reliability along with good diagnostic concordance of the MINI against the Structured Clinical Interview for DSM-III (SCID-P) 54 diagnoses. When compared with both the SCID-P and the Composite International Diagnostic Interview 61 (CIDI), the sensitivity of the MINI for major depression is 96% and specificity is 88% 49.
All diagnoses were based on a consensus diagnostic session with a psychiatrist (RB). In the present study, we defined prior mood disorders to include the diagnoses of major depressive disorder (MDD), minor depressive disorder, or a bipolar mixed episode, since patients with bipolar disorder do not differ from patients with major depression in thermal pain sensory discrimination and response criteria 20. No subject met criteria for current or past dysthymia. For prior mood disorders, 3 months in full remission was required before testing. For other Axis I disorders, 3 years in full remission was required for all subjects. Women classified as having no history of mood disorder met criteria for no lifetime major or minor depression, dysthymia, and bipolar disorder. Women with prior mood disorders did not differ from women with no prior mood disorders in proportions with history of anxiety disorders (4% vs. 2%), eating disorders (4% vs. 0%), or substance abuse disorders (4% vs. 3%).
Based on these criteria, 17 of the 32 (53%) African American women and 13 of the 23 (57%) non-Hispanic White women were classified with having a prior mood disorder. African Americans did not differ from non-Hispanic Whites in the proportion with prior MDD (34% vs. 39%), minor depression (9% vs. 13%) or bipolar mixed episode (9% vs. 4%). We operationally defined prior minor depression as having experienced 3 or 4 (as opposed to 5 or more) of the criterion symptoms for MDD and requiring the inclusion of either anhedonia or down/depressed mood. African Americans also did not differ from non-Hispanic Whites in months since full remission of the mood disorder (11 vs. 18 months, respectively), or in proportion with histories of anxiety disorders (6% vs. 4%), bipolar depression (0% vs. 0%), bipolar mania (0% vs. 0%), eating disorders (6% vs. 0%), or substance abuse disorders (9% vs. 0%).
Experimental procedures
All testing was conducted in the follicular phase of the menstrual cycle based on self-report (days 2-12 of the menstrual cycle). The experiment was conducted by a non-Hispanic white female experimenter. Studies on the effects of experimenter race have yielded inconsistent results regarding racial differences in pain sensitivity 57, 65. Subjects were asked to refrain from caffeine and all over-the-counter medications for 24 hours. After instrumentation for cardiovascular monitoring (results to be reported elsewhere), subjects were escorted to a sound-attenuated testing chamber and seated in a comfortable chair.
Pre-test rest
Immediately following cardiovascular instrumentation and administration of the Spielberger State Anxiety Scale 53, 5 minutes of quite rest ensued. Blood pressure and heart rates were taken at minutes 1, 3, and 5 and averaged to constitute baseline levels.
Pain Testing Procedures
Immediately following the pre-test rest, each subject was exposed to three pain procedures. One of three task orders (i.e., 1. tourniquet, thermal, cold; 2. thermal, cold, tourniquet; or 3. cold, tourniquet, thermal) was randomly assigned to each subject, ensuring, however, that the number of subjects in each race and mood group having each of the three orders was roughly equivalent. The dominant arm was used for all pain testing. Since only three subjects were left-handed (all non-Hispanic White subjects, and two with histories of mood disorders), the likelihood that cerebral lateralization associated with handedness impacted the findings is minimal. Pain intensity and unpleasantness ratings were obtained for each of the three pain tasks. Subjects were instructed that at the point of tolerance for each pain test, they would be asked to rate the intensity and unpleasantness of their pain using separate visual analogue scales (0 – 100). Thus, immediately prior to deflating the tourniquet cuff, immediately prior to removal of the hand from the ice bath, and immediately after the third thermal tolerance temperature was delivered, the experimenter held up one visual analogue scale for intensity rating, with the 100 cm line anchored by the words ‘Not at all Intense’ and ‘The Most Intense Pain Imaginable’. Next, the experimenter held up the scale for unpleasantness, with the 100 cm line anchored by the words ‘Not at all Unpleasant’ and ‘The Most Unpleasant Pain Imaginable’.
The Submaximal Effort Tourniquet Procedure
In this procedure 40, a tourniquet cuff was positioned on the subject's dominant arm and the arm placed to the side. Before inflating the tourniquet cuff to 200 mm Hg (Hokanson E20 Rapid Cuff Inflator), the subject's arm was raised for 30 seconds to promote venous drainage, and then the cuff was inflated, the experimenter's stopwatch started, and the arm returned to the side. To promote forearm ischemia, subjects engaged in 20 handgrip exercises at 30% of their maximum force with an intersqueeze interval of 2 seconds. Subjects were instructed to indicate when the sensations in their arm first became painful (pain threshold) and when they were no longer willing or able to tolerate the pain (pain tolerance). After the subjects indicated their pain tolerance, but before the cuff was deflated, subjects indicated their pain intensity and unpleasantness ratings. A maximum time limit of 20 minutes was enforced, though subjects were not informed of this limit.
Hand Cold Pressor
The apparatus for the cold pressor consisted of a container filled with ice and water that was maintained at 4°C as recorded immediately before initiating the test. The use of a water circulator prevented the water from warming near the subject's hand. At the onset of the test, subjects were instructed to submerge their dominant hand to the marked line on their wrist and to remain still. Subjects were instructed to indicate to the experimenter when the sensations in their hand first became painful (pain threshold) and to also indicate when they were no longer willing or able to tolerate the pain by saying “stop” (pain tolerance). After the subjects indicated their pain tolerance, but before removing their hand from the ice water bath, subjects indicated their pain intensity and unpleasantness ratings. A maximum time limit of 5 minutes was imposed, though subjects were not informed of this limit.
Thermal Pain Testing
Thermal pain threshold and tolerance were determined by an ascending method of limits using a 1-cm-diameter contact thermode with the capability for a rise time of 10°C/second. The thermode was controlled by a personal computer, and thermal probe applied to the dominant volar forearm. During the pain testing, an adapting temperature of 38°C was maintained for 10 seconds. Then, the temperature increased directly to 41.5°C and from that point on, increased 0.5°C every 5 seconds until it reached 53°C or until the subject reached her tolerance. To determine thermal pain onset (threshold), subjects were instructed to press a mouse button (which terminated the stimulus) when the thermal percept first became painful. This was repeated three times and averaged to calculate thermal pain thresholds. Then, three series to determine average thermal pain tolerance were conducted by instructing the subject to press a mouse button when they were no long able to tolerate the pain. Immediately after the subjects indicated their pain tolerance on the third pain tolerance series, subjects indicated their pain intensity and unpleasantness ratings.
A 5 minute recovery period followed each pain procedure, since it has been suggested that 5 minutes should be the minimum amount of elapsed time between pain measurements 38.
Following pain testing, subjects were exposed to a mental stressor battery (results to be reported elsewhere).
DATA ANALYSIS
Group differences in demographic factors, psychiatric histories, months in full remission from the mood disorder, body mass index (BMI), pre-test rest systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), Perceived Stress Scale (PSS), Beck Depression Inventory (BDI), and State and Trait Anxiety were examined using a 2 (History of Mood Disorders: yes or no) × 2 (Race: Non-Hispanic White or African American) analysis of variance (ANOVA) or Chi-Square analyses.
Since African Americans had higher SBP levels (see Results), and since higher blood pressure is associated with reduced pain sensitivity 9, 11, 33, 35, 51, 64, we examined differences in pain intensity and unpleasantness ratings for each pain test, using a 2 (History of Mood Disorders) × 2 (Race) analysis of covariance (ANCOVA), with pre-test rest SBP as the covariate. Analyses did not indicate any relationship of BMI to any measure of pain sensitivity for any group. Thus, analyses were not adjusted for BMI. In addition, pain sensitivity was assessed using a 2 (History of Mood Disorders) × 2 (Race) repeated measures ANCOVA, with pre-test rest SBP as the covariate and time point (pain threshold and tolerance) as the repeated factor. Where significant interactions emerged, subsequent simple effects analyses were conducted in order to explore those effects.
RESULTS
Baseline and Demographic Factors (Table 1)
Table 1.
Mean (+SEM) Baseline and Demographic Factors as a Function of Prior Mood Disorder and Ethnicity
| Non-Hispanic White Women | African American Women | |||
|---|---|---|---|---|
| Prior Mood Disorder (n = 13) |
No Prior Mood Disorder (n = 10) |
Prior Mood Disorder (n = 17) |
No Prior Mood Disorder (n = 15) |
|
| A Age | 26.5 (1.2) | 23.2 (1.4) | 25.9 (1.0) | 24.0 (1.1) |
| B BMI | 23.0 (1.6) | 22.1 (1.9) | 27.2 (1.4) | 26.8 (1.5) |
| C Baseline Systolic Blood Pressure | 104.6 (2.2) | 105.5 (2.5) | 109.6 (1.9) | 108.9 (2.0) |
| Baseline Diastolic Blood Pressure | 65.9 (2.2) | 64.3 (2.5) | 71.5 (1.9) | 65.7 (2.1) |
| Baseline Heart Rate | 64.5 (2.7) | 65.3 (3.1) | 66.0 (2.4) | 68.3 (2.5) |
| D Perceived Stress Scale | 16.4 (1.9) | 14.4 (2.1) | 20.1 (1.7) | 19.5 (1.8) |
| Beck Depression Inventory | 4.08 (1.1) | 3.40 (1.2) | 5.76 (0.9) | 3.87 (1.0) |
| State Anxiety | 26.9 (1.8) | 27.1 (2.0) | 26.9 (1.5) | 30.2 (1.6) |
| E Trait Anxiety | 33.5 (2.3) | 31.3 (2.7) | 36.9 (2.0) | 29.6 (2.2) |
African Americans > Non-Hispanic Whites:
p<.01
p=.05
p<.05
Prior Mood Disorder > No Prior Mood Disorder:
p<.05
p<.05
Significant main effects of Race were seen for BMI (F(3,54) = 7.41, p<.01), SBP (F(3,54) = 3.88, p=.05), and PSS (F(3,54) = 5.48, p<.05), since African Americans had greater BMI, PSS, and SBP than non-Hispanic Whites. Significant main effects for a History of Mood Disorders were seen for Age (F(3,54) = 4.67, p<.05) and Trait Anxiety (F(3,54) = 5.26, p<.05), since women with prior mood disorders were older (by approximately 2.5 years) and had greater Trait Anxiety (an associated feature of depression) than women with no prior mood disorders. No significant main or interactive effects involving a History of mood disorders or Race were obtained for DBP, HR, BDI, or State Anxiety.
Pain Intensity and Unpleasantness Ratings
There were no differences between African Americans and non-Hispanic White women in pain intensity ratings (ranges = 10-95 and 5-100, respectively, ps > .10) and pain unpleasantness ratings (ranges = 0-100 and 5-100, respectively, ps > .10) for any pain test. Similarly, there were no differences between women with and without prior mood disorders in pain intensity ratings (ranges = 5-100 and 10-90, respectively, ps > .10) and pain unpleasantness ratings (ranges = 0-100 and 5-100, respectively, ps > .10) for any pain task. Analyses also failed to reveal any significant Race × Prior Mood Disorder interactions.
Associations Between Race, History of Mood Disorders, and Pain Threshold and Tolerance
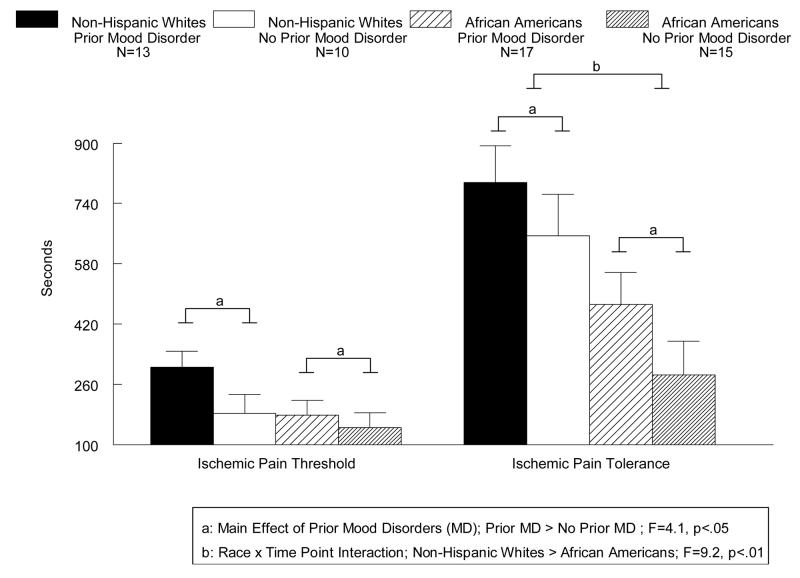
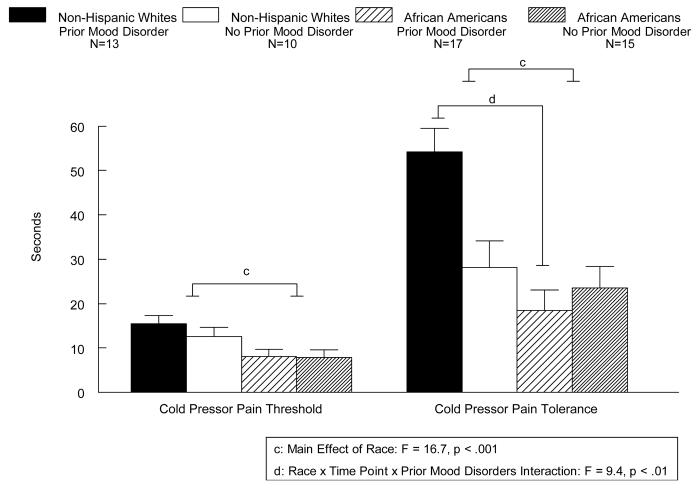
A main effect of Prior Mood Disorders was seen for ischemic pain (Figure 1) since, on average, women with prior mood disorders had higher threshold and tolerance to ischemic pain than women with no prior mood disorder (F(1,50) = 4.1, p<.05). A Race × Time Point interaction was also found (F(1,50) = 9.2, p<.01), since African American women had lower ischemic pain tolerance (p<.001), but not lower pain threshold (p=NS; Fig. 1) than non-Hispanic White women. As seen in Figure 2, a main effect of Race (F(1,50) = 16.7, p<.001) was seen for the cold pressor pain task, since on average, African American women had lower cold pressor threshold and tolerance than non-Hispanic White women. A Race × Prior Mood Disorder × Time Point interaction was also found (F(1,50) = 9.4, p<.01), since African Americans with prior mood disorders showed reduced tolerance to cold pressor pain than non-Hispanic Whites with prior mood disorders (p<.0001), while no ethnic differences in tolerance were evident in women with no history of mood disorders (see Fig. 2). While a similar pattern of effects was observed for thermal pain, with African Americans showing decreased pain tolerance than non-Hispanic Whites (48.3 and 49.3 degrees Celsius, respectively) and women with histories of mood disorders showing increased pain tolerance than women with no histories of mood disorders (49.4 and 48.2, degrees Celsius respectively), these differences did not reach statistical significance. Thus, no significant main effects or interactions were present involving thermal pain.
Fig. 1.
Mean (+SEM) ischemic pain tolerance (seconds) as a function of prior mood disorders and race in women.
Fig. 2.
Mean (+SEM) cold pressor pain tolerance (seconds) as a function of prior mood disorders and race in women.
DISCUSSION
The results of our study indicate that experimental pain threshold and tolerance are influenced by both race and histories of mood disorders in women. Our study is among the first to show that histories of mood disorders are associated with decreased sensitivity to experimental pain in euthymic women. Bar et al. 5 assessed thermal pain sensitivity in women who were in full clinical recovery from major depressive disorder and found significantly increased pain threshold and tolerance in women with prior depression compared to controls. However, most of the women in recovery from depression were taking anti-depressant medication, while our study included only women not taking any prescription medication. Nonetheless, similar findings emerged in our study, since all women with histories of mood disorders, regardless of race, showed increased pain threshold and tolerance to tourniquet ischemic pain, and only in women with prior mood disorders were the more pronounced racial differences in cold pressor pain tolerance evident.
Thus, our results, along with those of Bar et al. 5, suggest that persistent alterations in pain sensitivity may last beyond the remission of the mood disorder, and extend previous studies showing reduced sensitivity to experimental pain in subjects with current mood disorders 3, 4, 5, 19, 20, 30. Consistent with the meta-analysis and review by Dickens et al. 19 that showed increased pain thresholds in patients with current depression, our results indicate that in women in full remission from a previous mood disorder there is also increased pain threshold and tolerance to experimental pain. Several hypotheses have been proposed to explain the association between mood disorders and reduced pain sensitivity, such as the presence of a more stoic behavior or affective indifference in depression 17, a true sensory deficit in psychiatric patients 12, 14, and slower reaction time to experimental pain stimuli in depression 1. Although the mechanism(s) by which mood disorders influence pain sensitivity has not yet been identified, alterations in experimental pain sensitivity are likely to reflect alterations in autonomic and neuroendocrine mechanisms 18, disturbances associated with depression 41, 62, 63. Thus, persistent alterations in pain sensitivity in women with a history of mood disorders may have implications for risk for a subsequent mood disorder episode. Longitudinal studies will be needed to address this issue.
Despite the conclusions of the meta-analysis 19 and the results of the present report, it is important to point out that other studies have yielded opposing results for ischemic pain sensitivity, finding either no differences in ischemic pain threshold 42, or reduced ischemic pain threshold 3 and tolerance 3, 42 in women with current mood disorders compared to controls subjects. Although the reasons for the discrepancies in the literature remain unknown, one possibility is that women who recover from depression and other mood disorders may be fundamentally different from those who do not recover. The inclusion of women with current mood disorders as well as those with histories of mood disorders in future studies would shed light on this issue.
Given the well documented evidence that women have increased clinical pain 55 and also show decreased experimental pain tolerance 45, 50, 60, it may seem paradoxical that women with prior mood disorders, who also have increased clinical pain 10, show increased experimental pain tolerance (i.e. reduced pain sensitivity). Lautenbacher and Krieg 29 have addressed this paradox of increased clinical pain complaints and reduced experimental pain sensitivity in depression, hypothesizing that diminished processing of painful stimuli could be responsible for both phenomena. The authors argue that reduced processing of nociceptive stimuli at both spinal and subcortical stages may cause hypoalgesia to phasic experimental pain, and at the same time cause hyperalgesia to clinical pain due to deficient activation of inhibitory systems 29. Although Lautenbacher et al. 30 failed to find a significant correlation between clinical pain complaints and pain threshold in depressed patients, this does not rule out the possibility that alterations in central and peripheral pain processing contribute to both phenomena. Additional studies on underlying mechanisms contributing to alterations in pain sensitivity in mood disordered individuals are clearly indicated.
Regarding the racial differences observed, African American women showed decreased experimental pain tolerance to the tourniquet ischemic and cold pressor pain tasks compared to non-Hispanic White women, even after controlling for racial differences in resting blood pressure. However, it is important to note that the racial differences in cold pressor pain tolerance were only seen in women with histories of mood disorders. Our results indicating reduced pain tolerance in African American women are consistent with other reports 13, 23, 36, 50, 60, though this is the first study to examine racial differences in pain sensitivity in an exclusively female sample. Although the mechanisms underlying racial differences in pain tolerance are unknown, the consistency of results across numerous studies underscores the robustness of the effect. Additionally, since pain intensity ratings reflect the sensory-discriminative aspect of pain, while pain unpleasantness ratings reflect the affective/emotional aspect of pain 44, our results revealing no significant ethnic differences in intensity and unpleasantness ratings for any of the pain tasks suggests that, for women, racial differences in pain sensitivity do not appear to be a function of either perceptual differences or affective interpretation of pain. While we did observe ethnic differences in perceived stress, with African American women reporting more stress in the month preceding testing then non-Hispanic White women, our study was not powered to examine whether perceived stress mediated the relationship between race and pain sensitivity. Future studies examining both biological and psychosocial factors that may contribute to racial differences in experimental and clinical pain are indicated, and may have implications for the racial disparities that exist in the management of clinical pain. Some of these studies are currently underway in our laboratory 36, 37.
Limitations to our study must be acknowledged. First, we did not assess chronic and current pain symptoms in our subjects, though all reported themselves to be in good health. Given that chronic pain has been shown to influence experimental pain sensitivity 39 and that African Americans 21, 34, 46 and individuals with depression 16, 52 have high rates of clinical pain, this would be important in future studies designed to address the paradox of increased clinical pain complaints but reduced experimental pain sensitivity in depression. Another limitation to our study is that we did not assess neuroendocrine measures, since alterations in plasma norepinephrine and hypothalamic-pituitary-adrenal axis factors are associated with both experimental pain sensitivity 2, 25 and depression 41, 62, 63. Thus, studies examining neuroendocrine factors and pain perception in women with mood disorders are indicated. Limitations in regards to the pain intensity and unpleasantness ratings also exist. Although ratings did not differ by race or history of mood disorders, subjects endorsed pain intensity and unpleasantness ratings as low as 0, 5, and 10 out of 100 during pain tolerance, a time when subjects were instructed to indicate when they were no longer willing or able to tolerate the pain. It is possible that the subjects giving these low ratings did not understand the instructions, or that they simply had no motivation to continue further with the pain tasks, regardless of the low levels of intensity and unpleasantness. Therefore, it may be beneficial for future studies to assess indices of motivation, as well as to ensure that subjects fully understand the pain rating scale. Finally, the possibility exists for the presence of stress-induced analgesia with respect to differential carryover effects from one pain test to another, despite randomizing order of pain testing.
In summary, our results suggest that experimental pain threshold and tolerance in women is influenced by both race and lifetime histories of mood disorders. Women with prior mood disorders, regardless of race, were less sensitive to ischemic pain than women with no history of mood disorders. On the other hand, African American women, regardless of psychiatric history, were more sensitive to ischemic pain than non-Hispanic White women. The interplay between race and prior mood disorders was most evident during cold pressor pain where racial differences in pain tolerance were only evident for women with histories of mood disorders.
Acknowledgements
This research was support by NIH grant DA13705.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Adler G, Gattaz W. Pain perception threshold in major depression. Biol Psychiatry. 1993;34:687–689. doi: 10.1016/0006-3223(93)90041-b. [DOI] [PubMed] [Google Scholar]
- 2.al'Abasi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 3.Bar KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117:87–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Bar KJ, Brehm S, Boettger MK, Wagner G, Boettger S, Sauer H. Decreased sensitivity to experimental pain in adjustment disorder. Eur J Pain. 2006;10:467–471. doi: 10.1016/j.ejpain.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Bar KJ, Greiner W, Letzsch A, Kobele R, Sauer H. Influence of gender and hemispheric lateralization on heat pain perception in major depression. J Psychiatr Res. 2003;37:345–353. doi: 10.1016/s0022-3956(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 6.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 7.Bhalang K, Sigurdsson A, Slade GD, Maixner W. Associations among four modalities of experimental pain in women. J Pain. 2005;6:604–611. doi: 10.1016/j.jpain.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Bolden L, Wicks MN. Length of stay, admission types, psychiatric diagnoses, and the implications of stigma in African Americans in the nationwide inpatient sample. Issues Ment Health Nurs. 2005;26:1043–1059. doi: 10.1080/01612840500280703. [DOI] [PubMed] [Google Scholar]
- 9.Bragdon EE, Light KC, Girdler SS, Maixner W. Blood pressure, gender, and parental hypertension are factors in baseline and poststress pain sensitivity in normotensive adults. Int J Behav Med. 1997;4:17–38. doi: 10.1207/s15327558ijbm0401_2. [DOI] [PubMed] [Google Scholar]
- 10.Bromberger JT, Kravitz HM, Wei HL, Brown C, Youk AO, Cordal A, Powell LH, Matthews KA. History of depression and women's current health and functioning during midlife. Gen Hosp Psychiatry. 2005;27:200–208. doi: 10.1016/j.genhosppsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Bruehl S, Carlson C, McCubbin J. Cardiovascular reactivity to psychological stress may enhance subsequent pain sensitivity. Pain. 1997;69:237–244. doi: 10.1016/S0304-3959(96)03289-7. [DOI] [PubMed] [Google Scholar]
- 12.Buchsbaum M, Davies GC, Goodwin FK, Murphy DL, Post RM. Psychophysical pain judgments and somatosensory evoked potentials in patients with affective illness and in normal adults. Adv Biol Psychiatry. 1980;4:63–72. [Google Scholar]
- 13.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in response to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Clark WC, Yang JC, Janal MN. Altered pain and visual sensitivity in humans: Effects of acute and chronic stress. Ann N Y Acad Sci. 1986;467:116–129. doi: 10.1111/j.1749-6632.1986.tb14623.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 16.Corruble E, Guelfi JD. Pain complaints in depressed patients. Psychopathology. 2000;33:307–309. doi: 10.1159/000029163. [DOI] [PubMed] [Google Scholar]
- 17.Davis GC, Buchsbaum MS. Pain sensitivity and endorphins in functional psychoses. Mod Probl Pharmacopsychiatry. 1981;17:97–108. doi: 10.1159/000402408. [DOI] [PubMed] [Google Scholar]
- 18.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: A systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–375. doi: 10.1097/01.psy.0000041622.69462.06. [DOI] [PubMed] [Google Scholar]
- 20.Dworkin RH, Clark CW, Lipsitz JD. Pain responsivity in major depression and bipolar disorder. Psychiatry Res. 1995;56:173–181. doi: 10.1016/0165-1781(95)02501-7. [DOI] [PubMed] [Google Scholar]
- 21.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001a;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001b;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris M. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain. 1996;12:260–269. doi: 10.1097/00002508-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–85. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez A, Sachs-Ericsson N. Ethnic differences in pain reports and the moderating role of depression in a community sample of Hispanic and Caucasian participants with serious health problems. Psychosom Med. 2006;68:121–128. doi: 10.1097/01.psy.0000197673.29650.8e. [DOI] [PubMed] [Google Scholar]
- 27.Howell EA, Mora PA, Horowitz CR, Leventhal H. Racial and ethnic differences in factors associated with early postpartum depressive symptoms. Obstet Gynecol. 2005;105:1442–1450. doi: 10.1097/01.AOG.0000164050.34126.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janal MN, Glusman M, Kuhl JP, Clark WC. On the absence of correlation between responses to noxious heat, cold, electrical and ischemic stimulation. Pain. 1994;58:403–411. doi: 10.1016/0304-3959(94)90135-X. [DOI] [PubMed] [Google Scholar]
- 29.Lautenbacher S, Krieg JC. Pain perception in psychiatric disorders: a review of the literature. J Psychiatr Res. 1994;28:109–122. doi: 10.1016/0022-3956(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 30.Lautenbacher S, Spernal J, Schreiber W, Krieg JC. Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. Psychosom Med. 1999;61:822–827. doi: 10.1097/00006842-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Lawlis GF, Achterberg J, Kenner L, Kopetz K. Ethnic and sex differences in response to clinical and induced pain in chronic spinal pain patients. Spine. 1984;9:751–754. doi: 10.1097/00007632-198410000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Lynn B, Perl ER. A comparison of four tests for assessing the pain sensitivity of different subjects and test areas. Pain. 1977;3:353–365. doi: 10.1016/0304-3959(77)90065-3. [DOI] [PubMed] [Google Scholar]
- 33.Maixner W. Interactions between cardiovascular and pain modulatory systems: physiological and pathophysiological implications. Journal of Cardiovascular Electrophysiology. 1991;2:S3–12. [Google Scholar]
- 34.McCracken LM, Matthews AK, Tang TS, Cuba SL. A comparison of blacks and whites seeking treatment for chronic pain. Clin J Pain. 2001;17:249–255. doi: 10.1097/00002508-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 35.McCubbin JA, Bruehl S. Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain. 1994;57:63–7. doi: 10.1016/0304-3959(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 36.Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;6:948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- 37.Mechlin B, Morrow AL, Maixner W, Girdler SS. The relationship of allopregnanolone immunoreactivity and HPA-axis measures to experimental pain sensitivity: Evidence for ethnic differences. Pain. 2007 Feb 8; doi: 10.1016/j.pain.2006.12.027. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menegazzi J. Measuring Pain at Baseline and Over Time. Ann Emerg Med. 1996;27:433–435. doi: 10.1016/s0196-0644(96)70224-x. [DOI] [PubMed] [Google Scholar]
- 39.Merskey H. The effect of chronic pain upon the response to noxious stimuli by psychiatric patients. J Psychiatr Res. 1965;8:405–419. doi: 10.1016/0022-3999(65)90083-8. [DOI] [PubMed] [Google Scholar]
- 40.Moore PA, Duncan GH, Scott DS, Gregg JM, Ghia JN. The submaximal effort tourniquet test: its use in evaluating experimental and chronic pain. Pain. 1979;6:375–382. doi: 10.1016/0304-3959(79)90055-1. [DOI] [PubMed] [Google Scholar]
- 41.O'Keane V, Dinan TG, Scott L, Corcoran C. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58:963–968. doi: 10.1016/j.biopsych.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 42.Pinerua-Shuhaibar L, Prieto-Rincon D, Ferrer A, Bonilla E, Maixner W, Suarez-Roca H. Reduced tolerance and cardiovascular response to ischemic pain in minor depression. J Affect Disord. 1999;56:119–126. doi: 10.1016/s0165-0327(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 43.Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Molecular Interventions. 2002;2:392–403. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- 44.Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. [DOI] [PubMed] [Google Scholar]
- 45.Riley JL, III, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 46.Riley JL, III, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. Pain. 2002;100:291–298. doi: 10.1016/S0304-3959(02)00306-8. [DOI] [PubMed] [Google Scholar]
- 47.Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: Findings from the nation health and nutrition examination survey III. Am J Public Health. 2005;95:998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachs-Ericsson N, Plant EA, Blazer DG. Racial differences in the frequency of depressive symptoms among community dwelling elders: The role of socioeconomic factors. Aging MentHealth. 2005;9:201–209. doi: 10.1080/13607860500114480. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–57. [PubMed] [Google Scholar]
- 50.Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med. 2000;62:517–523. doi: 10.1097/00006842-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Sheps DS, Bragdon EE, Gray TF, Ballenger M, Usedom JE, Maixner M. Relation between systemic hypertension and pain perception. Am J Cardiol. 1992;70:3F–5F. doi: 10.1016/0002-9149(92)90181-w. [DOI] [PubMed] [Google Scholar]
- 52.Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study on the relation between somatic symptoms and depression. N Engl J Med. 1999;341:1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- 53.Spielberger CD. Manual for the State–Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 54.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSMIII-R—Patient Version (SCID-P) New York State Psychiatric Institute, Biometrics Research; New York: 1989. [Google Scholar]
- 55.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 56.U.S. Department of Health and Human Services Substance Abuse and Mental Health Services Administration; Rockville, MD: Mental health: A report of the Surgeon General. 1999
- 57.Weisse CS, Foster KK, Fisher EA. The influence of experimenter gender and race on pain reporting: Does racial or gender concordance matter? Pain Med. 2005;6(1):80–87. doi: 10.1111/j.1526-4637.2005.05004.x. [DOI] [PubMed] [Google Scholar]
- 58.Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 59.Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse, and mental health care. Am J Psychiatry. 2001;15:2027–2032. doi: 10.1176/appi.ajp.158.12.2027. [DOI] [PubMed] [Google Scholar]
- 60.Woodrow KM, Friedman GD, Siegelaub AB, Collen MF. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548–556. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization . Composite International Diagnostic Interview (CIDI), Version 1.0. World Health Organization; Geneva, Switzerland: 1990. [Google Scholar]
- 62.Young EA, Carlson NE, Brown MB. Twenty-four hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 63.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 64.Zamir N, Shuber E. Altered pain perception in hypertensive humans. Brain Res. 1980;201:471–474. doi: 10.1016/0006-8993(80)91055-0. [DOI] [PubMed] [Google Scholar]
- 65.Zatzick DF, Dimsdale JE. Cultural variations in response to painful stimuli. Psychosom Med. 1990;52(5):544–57. doi: 10.1097/00006842-199009000-00007. [DOI] [PubMed] [Google Scholar]