Omega-3 fatty acids are being increasingly promoted as important dietary components for health and disease prevention.1,2 These fatty acids are naturally enriched in fatty fish like salmon and tuna and in fish-oil supplements. An increasing number of foods that are not traditional sources of omega-3 fatty acids, such as dairy and bakery products, are now being fortified with small amounts of these fatty acids.2 This recent promotion of omega-3 fatty acids has likely been driven by recommendations for omega-3 fatty acid consumption made by scientific groups such as the American Heart Association.3 The search for the molecular and cellular mechanisms by which omega-3 fatty acids affect health and disease has led to a large body of evidence which suggests that these dietary lipids modulate numerous processes, including brain and visual development, inflammatory reactions, thrombosis and carcinogenesis. An obvious question that someone unfamiliar with omega-3 fatty acids might ask is: How can these nutrients affect so many seemingly unrelated processes in different cell types and tissues? The goal of this review is not to comment on the extent to which dietary omega-3 fatty acids affect health and disease, but rather it is to give an overview of the nature of these dietary components and to present some of the mechanisms by which they may modulate cellular functions.
What are omega-3 fatty acids?
Our diet contains a complex mixture of fats and oils whose basic structural components are fatty acids. We generally consume at least 20 different types of fatty acids, which are classified as saturated, monounsaturated and polyunsaturated. Fatty acids have many fates in the body, including β-oxidation for energy, storage in depot fat or incorporation into phospholipids, which form the major structural components of all cellular membranes.
Not all dietary fatty acids are created equally. Because humans do not have the enzymatic machinery required to synthesize omega-3 fatty acids, they must be obtained from the diet (termed “essential fatty acids”). Even among dietary polyunsaturated fatty acids, there are different families of compounds, and this is at the heart of the difference between omega-3 fatty acids and other dietary lipids. Omega-3 fatty acids generally account for a small fraction of the total daily consumption of fatty acids in Western societies.2,4 Fish such as tuna, trout and salmon are especially rich sources of these fatty acids. Fish-oil supplements are also a rich source, as they typically contain 30%–50% omega-3 fatty acids by weight. Small quantities of omega-3 fatty acids are naturally present in meats like beef, pork and poultry. Despite containing small quantities of omega-3 fatty acids, meats contribute to the overall intake of these fatty acids simply because of the large amounts consumed in Western societies.4
Omega-3 fatty acids from fish and fish oils are not to be confused with those from plant sources, such as flax and canola oil. These plant oils are enriched in an omega-3 fatty acid called α-linolenic acid, which is a metabolic precursor of the omega-3 fatty acids found in fish and fish oils (Figure 1). Although we are able to convert dietary α-linolenic acid into eicosapentaenoic, docosapentaenoic and docosahexaenoic acids (which are found in fish and fish oils), this conversion is not efficient in people who consume a typical Western diet. Consequently, following the consumption of foods containing α-linolenic acid, our tissues are exposed to very little of the types of omega-3 fatty acids found in fish and fish oils. Some beneficial biological activity has been attributed to plant-derived omega-3 fatty acids; however, the associated health benefits are likely independent of the conversion of α-linolenic acid to the fatty acids found in fish. In addition, dietary oils that are rich in α-linolenic acid do not, for the most part, reproduce the biological activity associated with dietary fish oils.3 The balance of this review will address the types of omega-3 fatty acids typically found in fatty fish and fish-oil supplements.
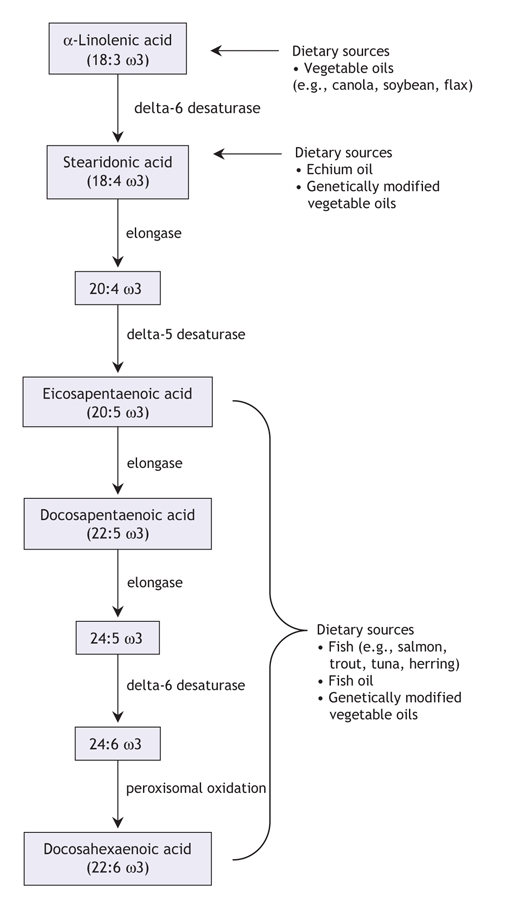
Figure 1: Metabolism and dietary sources of the omega-3 family of polyunsaturated fatty acids. Modified with permission from Annual Reviews (Annu Rev Nutr 2006;26:75-103).2
Following consumption, omega-3 fatty acids are incorporated into cell membranes in all tissues of the body (Figure 2). Whether the source of these omega-3 fatty acids is fish, fish-oil supplements or food products fortified with the appropriate omega-3 fatty acids, measurable changes in cellular membrane content occur within days of increasing the daily consumption of these fatty acids.5,6 Cellular membranes from some tissues (e.g, retina, brain, myocardium) are particularly enriched in these fatty acids. For example, about 30% of all fatty acids in the outer segment membrane of retinal photoreceptors are omega-3 fatty acids.7 The fact that these and other cells have developed the cellular machinery to preferentially incorporate these minor dietary components into their membranes suggests that these fatty acids play a role in the proper function of the cell. In fact, most cellular membranes accumulate omega-3 fatty acids in amounts that far outweigh their proportional content in the diet, and the content of these fatty acids in tissue membranes is generally indicative of our average daily intake.
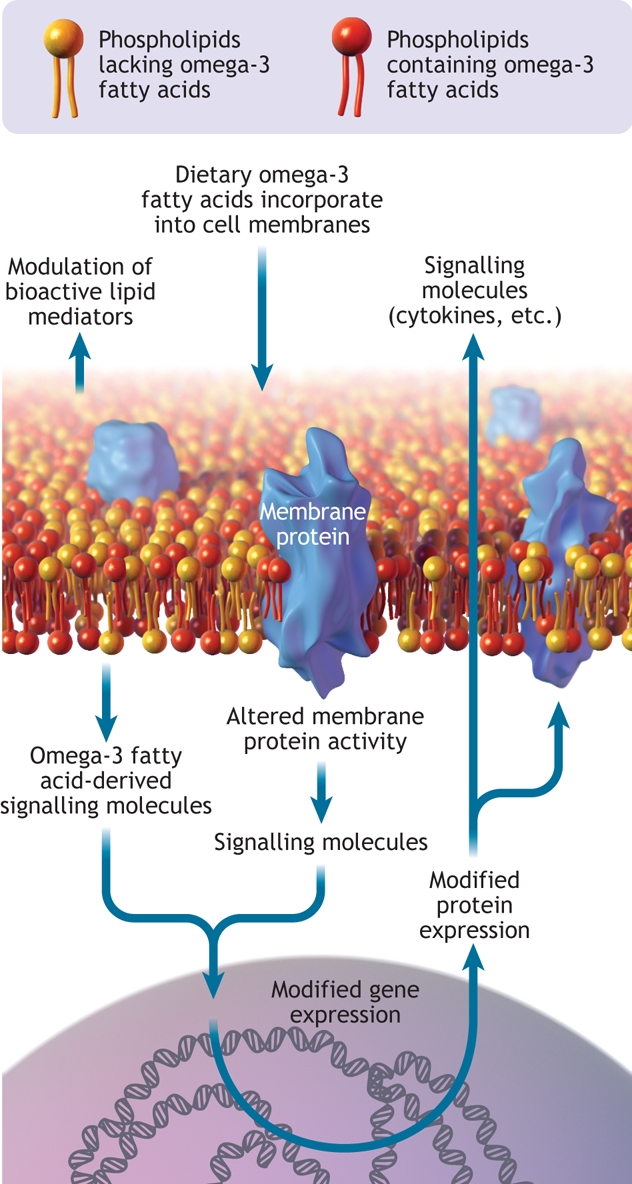
Figure 2: Cell membrane showing omega-3 fatty acids incorporated into the phospholipid bilayer. Omega-3 fatty acids can modify gene and protein expression, modulate membrane protein activity and act as a reservoir for bioactive molecules. Image by: Lianne Friesen and Nicholas Woolridge
How do omega-3 fatty acids function?
Diet-induced changes in the polyunsaturated fatty acid composition of a cell membrane have an impact on the cell's function, partly because these fatty acids represent a reservoir of molecules that perform important signaling or communication roles within and between cells. In particular, dietary omega-3 fatty acids compete with the omega-6 family of dietary polyunsaturated fatty acids for incorporation into all cell membranes.8,9 Arguably, the most important of all cellular polyunsaturated fatty acids is the omega-6 family member arachidonic acid. When cells are activated by external stimuli, arachidonic acid is released from cell membranes and is transformed into powerful cellular mediators such as thromboxanes, prostaglandins and leukotrienes.10 These compounds possess a range of activities, including activation of leukocytes and platelets, regulation of gastric secretions, induction of bronchoconstriction and signaling of pain in nerve cells. The importance of these compounds in health and disease is evident by the range of pharmaceutical products that target their biosynthesis or action.11 Indeed, arachidonic acid metabolism is the target of nonsteroidal anti-inflammatory drugs (e.g., acetylsalicylic acid, ibuprofen), cyclooxygenase-2 (COX-2) inhibitors (e.g., rofecoxib, celecoxib12) and leukotriene antagonists (e.g., montelukast, zafirlukast).13 Dietary omega-3 fatty acids directly affect arachidonic acid metabolism because they displace arachidonic acid from membranes and compete with arachidonic acid for the enzymes that catalyze the biosynthesis of thromboxanes, prostaglandins and leukotrienes.8 Thus, the net effect of consuming foods enriched in omega-3 fatty acids is a diminished potential for cells like monocytes, neutrophils and eosinophils to synthesize these powerful arachidonic acid–derived mediators of inflammation and a diminished potential for platelets to produce the prothrombotic agent thromboxane A2.
Inflammation is part of the body's immediate response to infection or injury, but uncontrolled inflammation damages tissues. Indeed, uncontrolled inflammation plays an important role in the pathology of diseases such as asthma, rheumatoid arthritis and atherosclerosis. The ability of omega-3 fatty acids to interfere with arachidonic acid metabolism is at the heart of their proposed anti-inflammatory effects. However, enriching cells or tissues with omega-3 fatty acids also modulates the expression of adhesion proteins (Figure 2), such as selectins and vascular cell adhesion molecule-1, which participate in leukocyte–endothelium interactions.14 Omega-3 fatty acids exert this effect by modulating the intracellular signaling pathways associated with the control of transcription factors (e.g., nuclear factor-κB) and gene transcription.15,16 Omega-3 fatty acids can also directly bind to nuclear receptors, such as the retinoid X receptor, that operate as transcription factors.17 Similarly, the enrichment of monocyte membranes with omega-3 fatty acids results in the synthesis and secretion of reduced quantities of cytokines (e.g., tumour necrosis factor-α, interleukin-1β) that are involved in the amplification of the inflammatory response.16,18 Therefore, at the cellular level, omega-3 fatty acids from fish oils can directly or indirectly modulate a number of cellular activities associated with inflammation. It should be emphasized that these effects of omega-3 fatty acids should not be compared with the powerful ability of pharmacologic agents to inhibit individual targets, but rather viewed as a gentle shift in mediator production, cell signaling and gene expression toward a phenotype of lessened reactivity against environmental stimuli.
In addition to acting as reservoirs of bioactive molecules within cell membranes, omega-3 fatty acids can also affect the function of membrane-associated proteins that are in direct contact with the lipid bilayer of cell membranes. The retinal protein rhodopsin is an example of this phenomenon.19 The conformational changes that this transmembrane protein undergoes in response to light are much more efficient in membranes highly enriched in omega-3 fatty acids. This translates into differences in electroretinogram waveforms, a measure of retinal function, that vary based on membrane omega-3 fatty acid content.20 The special requirement of a particular fatty acid composition in these cells results in the very efficient incorporation of omega-3 fatty acids. Ion channels are another example of membrane-associated proteins whose activity is modulated by omega-3 fatty acids.1,21 Sodium and calcium channels control voltage-gated sodium and calcium currents respectively. These currents are critical for the excitation of heart cells and contraction of the heart. Omega-3 fatty acids inhibit the activity of a number of cardiac ion-channel proteins, and this has been proposed to be partially responsible for their antiarrhythmic properties. Although this is likely not the only mechanism by which omega-3 fatty acids affect arrhythmia, such mechanisms may explain the fast onset of the protective effects on coronary heart disease mortality reported in clinical trials.22,23
The inclusion of omega-3 fatty acids in the diet has a rapid effect on the composition of cellular membranes in all tissues. Given the fact that fatty acids act as reservoirs for potent biologically-active molecules and that they regulate the environment of membrane-bound proteins, it is not surprising that they affect many tissues and their functions. Altogether, the general shift to a phenotype of reduced responses and reactivity in cells and tissues enriched with these lipids may explain the general health-promoting properties of these dietary fats.
Dietary intake and future sources of omega-3 fatty acids
Among people who consume typical Western diets, the average intake of the types of omega-3 fatty acids found in fish is about 150 mg per day. This is equivalent to consuming about 1 fish meal every 10 days.24 This falls well below the combined intake of dietary eicosapentaenoic and docosahexaenoic acids (650 mg/d) recommended at a workshop held in 1999 on essential fatty acids at the US National Institutes of Health.25Similarly, the International Society for the Study of Fatty Acids and Lipids recommends an intake of at least 500 mg daily.26 The American Heart Association recommends that people without coronary heart disease have 2 fish meals each week (at least 300 mg of omega-3 fatty acids daily), and they recommend that patients with documented coronary heart disease receive 1000 mg daily.3 In addition, the US Food and Drug Administration recommends that the average daily intake of omega-3 fatty acids from fish should not exceed 3000 mg because of possible adverse effects related to glycemic control, increased bleeding tendencies and elevations in low-density lipoprotein cholesterol associated with very high intake of omega-3 fatty acids.27 Whether these concerns are warranted remains to be determined; however, it is not clear if these higher doses would provide additional health benefits.
Although the recommendations for the intake of omega-3 fatty acids are based on extensive literature, in practice, these intakes have been difficult to achieve because dietary habits are well entrenched and are difficult to change. Consequently, most people are reluctant to regularly include several weekly servings of fish in their diets. Additionally, concerns exist that fish contain environmental contaminants such as heavy metals, methyl mercury and organochlorides.3 The consumption of dietary fish-oil supplements is an effective way to increase omega-3 fatty acid intake without changing dietary habits; however, compliance is a problem because 1–3 fish-oil capsules must be taken daily to achieve the recommended intake.
Despite these obstacles, the demand for fatty acids has been increasing. In recent years, an increasing number of foods have become available that are fortified with omega-3 fatty acids mostly from fish oils. Overall, the increased awareness of omega-3 fatty acids from fish and fish oils raises the question of whether fish-oil producers will be able to meet the impending worldwide demand. Consequently, a number of alternative sources of omega-3 fatty acids have been or are being developed. For example, oils naturally enriched in docosahexaenoic acid are being extracted from cultured microorganisms like the algae Crypthecodinium cohnii and are now being used to fortify a number of products, including baby formula. Similarly, novel plants like Echium plantagineum are now being cultivated because their seed oil naturally contains stearidonic acid, an intermediate in the metabolism of omega-3 fatty acids (Figure 1). This fatty acid is typically found in very small quantities in the diet, but it appears to be metabolized much more efficiently than α-linolenic acid, resulting in an enrichment of tissues with eicosapentaenoic and docosapentaenoic acid following its consumption.28 Finally, a number of companies have developed transgenic varieties of common plants such canola, soybean and safflower that can produce seed oils that are highly enriched with stearidonic, eicosapentaenoic and docosahexanoic acids.
The increased diversity and availability of sources of omega-3 fatty acids will likely allow the continued expansion of food products fortified with these fatty acids, a trend that may result in the attainment of the recommended dietary intake of these nutrients. Given the extensive literature supporting the health-promoting effects of these dietary components, the gentle shift to reduced cell and tissue reactivity may be accompanied by a subtle, but relevant, shift in the overall health and well-being of the population.
@ See related articles, pages 150, 157 and 181
Key points of the article
• Omega-3 fatty acids are essential nutrients that cannot be synthesized in the body and must be obtained from the diet.
• Dietary omega-3 fatty acids are incorporated into cellular membranes of all tissues. The extent of incorporation into tissue membranes is dependent on dietary intake.
• The enrichment of membranes with omega-3 fatty acids can modulate cellular signalling events, membrane protein function and gene expression.
• Consumption of recommended intakes of omega-3 fatty acids may lead to a general increase in the overall health and well-being of the population.
Acknowledgments
Marc Surette is supported by the Canadian Institutes for Health Research and the Canada Research Chairs Program.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Marc Surette, Département de Chimie et Biochimie, Université de Moncton, Moncton NB E1A 3E9; fax 506 858-4541; marc.surette@umoncton.ca
REFERENCES
- 1.Lee KW, Lip GY. The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. QJM 2003;96:465-80. [DOI] [PubMed]
- 2.Whelan J, Rust C. Innovative dietary sources of n-3 fatty acids. Annu Rev Nutr 2006;26:75-103. [DOI] [PubMed]
- 3.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747-57. [DOI] [PubMed]
- 4.Taber L, Chiu CH, Whelan J. Assessment of the arachidonic acid content in foods commonly consumed in the American diet. Lipids 1998;33:1151-7. [DOI] [PubMed]
- 5.Zuijdgeest-van Leeuwen SD, Dagnelie PC, Rietveld T, et al. Incorporation and washout of orally administered n-3 fatty acid ethyl esters in different plasma lipid fractions. Br J Nutr 1999;82:481-8. [DOI] [PubMed]
- 6.Surette ME, Koumenis IL, Edens MB, et al. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin Ther 2003;25:948-71. [DOI] [PubMed]
- 7.Bazan HE, Bazan NG, Feeney-Burns L, et al. Lipids in human lipofuscin-enriched subcellular fractions of 2 age populations. Comparison with rod outer segments and neural retina. Invest Ophthalmol Vis Sci 1990;31:1433-43. [PubMed]
- 8.Calder PC. n-3 polyunsaturated fatty acids, inflammation and inflammatory diseases. Am J Clin Nutr 2006;83:1505S-19S. [DOI] [PubMed]
- 9.Healy DA, Wallace FA, Miles EA, et al. Effect of low-to-moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids 2000;35:763-8. [DOI] [PubMed]
- 10.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001;294:1871-5. [DOI] [PubMed]
- 11.Celotti F, Durand T. The metabolic effects of inhibitors of 5-lipoxygenase and of cyclooxygenase 1 and 2 are an advancement in the efficacy and safety of anti-inflammatory therapy. Prostaglandins Other Lipid Mediat 2003;71:147-62. [DOI] [PubMed]
- 12.Loewen PS. Review of the selective COX-2 inhibitors celecoxib and rofecoxib: focus on clinical aspects. CJEM 2002;4:268-75. [DOI] [PubMed]
- 13.Riccioni G, Bucciarelli T, Mancini B, et al. Antileukotriene drugs: clinical application, effectiveness and safety. Curr Med Chem 2007;14:1966-77. [DOI] [PubMed]
- 14.De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol 2005;206:103-16. [DOI] [PubMed]
- 15.Weber C, Erl W, Pietsch A, et al. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol 1995;15:622-8. [DOI] [PubMed]
- 16.Novak TE, Babcock TA, Jho DH, et al. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol 2003;284:L84-9. [DOI] [PubMed]
- 17.de Urquiza AM, Liu S, Sjoberg M, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 2000;290:2140-4. [DOI] [PubMed]
- 18.Caughey GE, Mantzioris E, Gibson RA, et al. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 1996;63:116-22. [DOI] [PubMed]
- 19.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 2005;24:87-138. [DOI] [PubMed]
- 20.Jeffrey BG, Weisinger HS, Neuringer M, et al. The role of docosahexaenoic acid in retinal function. Lipids 2001;36:859-71. [DOI] [PubMed]
- 21.Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol 2005;206:141-54. [DOI] [PubMed]
- 22.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897-903. [DOI] [PubMed]
- 23.Siscovick DS, Lemaitre RN, Mozaffarian D. The fish story: a diet-heart hypothesis with clinical implications: n-3 polyunsaturated fatty acids, myocardial vulnerability and sudden death. Circulation 2003;107:2632-4. [DOI] [PubMed]
- 24.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 2000;71:179S-88S. [DOI] [PubMed]
- 25.Simopoulos AP, Leaf A, Salem N Jr. Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab 1999;43:127-30. [DOI] [PubMed]
- 26.Cunnane S, Drevon CA, Harris W, et al. Recommendations for intakes of polyunsaturated fatty acids in healthy adults. ISSFAL Newsl 2004;11:12-25.
- 27.Department of Health and Human Services. US Food and Drug Administration. Substances affirmed as generally recognized as safe: menhaden oil. Washington: Federal Register; 1997. 1997;62:30751–7 (21 CFR Part 184 [Docket No. 86G-0289]). Available: http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=1997_register&docid=fr05jn97-5 (accessed 2007 Nov 26).
- 28.Surette ME, Edens M, Chilton FH, et al. Dietary echium oil increases plasma and neutrophil long-chain (n-3) fatty acids and lowers serum triacylglycerols in hypertriglyceridemic humans. J Nutr 2004;134:1406-11. [DOI] [PubMed]


