Abstract
In previous studies, we have shown that cerebral hypoxia results in increased activity of caspase-9, the initiator caspase, and caspase-3, in the cytosolic fraction of the cerebral cortex of newborn piglets. The present study examines the mechanism of caspase-9 activation during hypoxia and tests the hypothesis that the ATP and cytochrome c-dependent activation of caspase-9 increases in the cytosol of the cerebral cortex of newborn piglets. Newborn piglets were divided into normoxic (Nx, n=4), and hypoxic (Hx, n=4) groups. Anesthetized, ventilated animals were exposed to an FiO2 of 0.21 (Nx) or 0.07 (Hx) for 60 min. Cerebral tissue hypoxia was documented biochemically by determining levels of ATP and phosphocreatine (PCr). Cytosolic fraction was isolated and passed through a G25-Sephadex column to remove endogenous ATP and cytochrome c. Fractions were collected and protein determined by UV spectrophotometry at 280 nm. Eluted high molecular weight samples from normoxic and hypoxic animals were divided into four subgroups: Subgroup 1 (control), incubated without added ATP and cytochrome c; Subgroup 2, incubated with added ATP; Subgroup 3, incubated with added cytochrome c; and Subgroup 4, incubated with added ATP and cytochrome c. The incubation was carried out at 37° C for 30 min. Following incubation, the protein was separated by 12% SDS PAGE and active caspase-9 was detected using specific active caspase-9 antibody. Protein bands were detected by enhanced chemilumenescence. Protein density was determined by imaging densitometry and expressed as absorbance (OD × mm2). ATP (μmoles/g brain) level was 4.7±0.18 in normoxic, as compared to 1.53±0.16 in hypoxic (p<0.05 vs Nx). PCr (μmoles/g brain) level was 4.03±0.11 in the normoxic and 1.1±0.3 in the hypoxic brain (p<0.05 vs Nx). In the normoxic preparations, active caspase-9 density increased by 9%, 4% and 20% in the presence of ATP, cytochrome c and ATP + cytochrome c, respectively. In the hypoxic preparations, active caspase-9 density increased by 30%, 45% and 60% in the presence of ATP, cytochrome c, and ATP + cytochrome c, respectively. These results show that incubation with ATP, cytochrome c and ATP +cytochrome c result in a significantly increased activation of caspase-9 in the hypoxic group (p<0.05). We conclude that the ATP and cytochrome c dependent activation of caspase-9 is increased during hypoxia. We propose that the ATP and cytochrome c sites of apoptotic protease activating factor I that mediate caspase-9 activation are modified during hypoxia.
Keywords: ATP, Cytochrome c, Caspase-9, Hypoxia, Newborn, Brain
Caspases are a group of cysteine proteases that are essential for initiating and executing programmed cell death [9, 13, 14, 25]. Capases contain a cysteine residue at their active site and act on their substrates after the aspartate residue. The activity of cysteine proteases is detected in cells undergoing programmed cell death, irrespective of their origin or death stimuli. Studies conducted on the nematode Caenorhbditis elegans (C. elegans) have provided considerable insights into the nature of programmed cell death and demonstrated that an aspartate specific cysteine protease is essential for programmed cell death of all somatic cells during development [7, 26]. Comparable homologous components of cell death machinery in mammalian systems have been identified. A group of genes including egl-1(egl, egg-laying defective), ced-3 (cell death abnormal), ced-4 and ced-9 are at the core of programmed cell death. Three protein components (Ced-3, Ced-4 and Egl-1) are required for cell death. These code for a caspase (Ced-3), an adopter protein (Ced-4) and a proapoptotic member of the Bcl-2 family of proteins (Egl-1). The Bcl-2 homolog Ced-9 is needed for cell survival. Protein –protein interactions between Ced -3, Ced-4, Ced-9 and Egl-1 provide a direct link between caspases as the effector arm of the programmed cell death pathway and Bcl-2 family proteins [3, 4, 23].
Apoptotic protease activating factor-1 (Apaf-1) serves as the adaptor protein [2, 25, 27, 29]. Antiapoptotic proteins Bcl-2 and Bcl-xl bind to Apaf-1 and this binding is essential for the antiapoptotic function of Bcl-2 family member proteins (27, 29). It has been shown that Apaf-1 acts upstream of caspases, and that the antiapoptotic proteins Bcl-2 or Bcl-xl act as inhibitors of Apaf-1. Apaf-1 can simultaneously bind to procaspase-9 (Ced-3 homolog), as well as the apoptotic proteins [5, 10]. In brief, the genetic components of programmed cell death have been identified and a possible activation sequence of these components in mammalian cells appears to be as follows: Bax→Bcl-2/Bcl-xl→ Apaf-1→ procaspase-9→caspase-9→procaspase-3→caspase-3→apoptosis.
The mechanism of activation of caspase-9 during hypoxia that leads to initiation of programmed cell death in mammalian brain tissue is not known. Previous in vitro studies have indicated that the apoptotic caspase cascade is activated by cytochrome c and ATP. In vitro studies using 100,000 g supernatant (S-100) extracts of HeLa 60 cells demonstrated that incubation with dATP or ATP (1–2 mM) and cytochrome c (10 μM) together for 1 hr at 37°C resulted in cleaved products of poly-(ADP-ribose)polymerase (PARP) indicating activation of caspase-3 [16,17]. Cleaved active caspase-9 and caspase-3 were also demonstrated. In another in vitro study using caspase-9 deficient mice, it was shown that caspase -9 is needed for caspase-3 activation [13]. On the basis of these studies it was generally accepted that ATP and cytochrome c together activate caspase-9. However, several studies regarding the role of cytochrome c in programmed cell death are conflicting [6] and questions have been raised regarding the concentrations of ATP and cytochrome c and apoptosome formation, as well as caspase-9 activation [11, 21]. Recent studies also indicate that ATP-dependent regulation of caspase-9 activation may represent an additional level of control down stream from cytochrome c [25].
We have previously shown that cerebral hypoxia results in increased activity of caspase-9 and caspase-3 in the cerebral cortex of newborn piglets [12, 22]. The present study was designed to investigate the mechanism of activation of caspase-9 during hypoxia and tested the hypothesis that ATP and cytochrome c-dependent activation of caspase-9 is increased in the cerebral cortex of hypoxic newborn piglets. The results of the present study provide evidence that the increase in the activity of caspase-9 during hypoxia is mediated ATP and cytochrome c binding sites of Apaf-1..
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Drexel University. Studies were conducted on anesthetized, ventilated and instrumented 3–5 days old newborn piglets. Newborn piglets were divided into two groups: normoxic (Nx, n=4) and hypoxic (Hx, n=4). Anesthesia was induced with 4% halothane and lowered to 1% during surgery while allowing the animals to breathe spontaneously. Lidocaine 2% was injected locally before instrumentation for endotracheal tube insertion and femoral arterial and venous catheter insertion. After instrumentation, the use of halothane was discontinued, and anesthesia was maintained with nitrous oxide 79%, oxygen 21% and Fentanyl (50 μg/kg ) throughout the experiment. Tubocurarine (0.3 mg/kg) was administered after placing the animal on a volume ventilator. Arterial blood gases, heart rate and blood pressure were monitored in all animals throughout the study. Core body temperature was maintained at 38.5–39°C with a warming blanket. Baseline measurements were obtained in both groups for one hour after surgery to ensure normal arterial pressures and blood gas values. After stabilization following surgery, the piglets assigned to the hypoxic group were exposed to hypoxia (FiO2 = 0.06) for 1hr, while the piglets assigned to the normoxic group were ventilated at FiO2 of 0.21 for 1 hr. At the end of the experiment, the cortical brain tissue was removed and placed in buffer for the preparation of 100.000 g supernatant or frozen in liquid nitrogen within 5–7 sec for the analysis of high energy phosphates, ATP and phosphocreatine (PCr), to document cerebral tissue hypoxia.
Cerebral tissue ATP and phosphocreatine (PCr) concentrations were determined spectrophotometrically by the method of Lamprecht et al. [15]. Frozen cortical brain tissue was powdered under liquid nitrogen, extracted in 6 % (weight by volume) perchloric acid. The extract was thawed on ice and centrifuged at 2000 × g for 15 min at 4°C. The supernatant was neutralized to pH 7.6 by using 2.23 M K2CO3/0.5 M triethanolamine/50 mM EDTA buffer and then centrifuged at 2000 × g for 15 min at 4°C. 300 ul of supernatant was added to 1 ml of buffer (50 mM triethanolamine, 5 mM MgCl2, 2 mM EDTA, 2 mM glucose pH=7.6) and 20 μl NADP. Glucose 6-phosphate dehydrogenase (10μl) was added and the samples were incubated and read after 8 min. Hexokinase (10 μl) was then added, absorbance readings were taken until steady state was reached and ATP concentration was calculated from the increase in the absorbance at 340 nm. Next, ADP (20 μl) and creatine kinase (20 μl) were added to the solution. The samples were read every 5 min for 60 min and phosphocreatine concentrations were calculated from the increase in absorbance at 340 nm.
Cerebral tissue was homogenized in 6.5 volumes of a medium containing 0.32 M sucrose, 20 mM Tris–HCl buffer (pH 7.0), 1 mM Na-EDTA, protease inhibitors cocktail and 1 mM dithiothreitol. The homogenate was centrifuged at 100,000 × g for 60 min to obtain the cytosolic fraction. All procedures were carried out at 0–4 °C. Cytosolic fraction was isolated and passed through a G25-Sephadex column to remove endogenous ATP and cytochrome c. Fractions were collected and protein determined by UV spectrophotometry at 280 nm. Eluted high molecular weight samples from normoxic and hypoxic animals were divided into four subgroups: Subgroup 1 (control), incubated without added ATP and cytochrome c; Subgroup 2, incubated with added ATP; Subgroup 3, incubated with added cytochrome c; and Subgroup 4, incubated with added ATP and cytochrome c. The incubation was carried out at 37° C for 30 min. Following incubation, the protein was separated by 12% SDS PAGE and active caspase-9 was detected using specific active caspase-9 antibody.
The expression of active caspase-9 protein was assessed by Western blot analysis. Equal amounts of each cytosolic sample were separated by 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to nitrocellulose membranes. The membranes were subsequently incubated with anti-active caspase-9 rabbit polyclonal antibodies (Santa Cruz Biotechnology, CA). Immunoreactivity was then detected by incubation with horseradish peroxidase conjugated anti-rabbit secondary antibody (Rockland, Gilbertsville, PA). Specific immunocomplexes were detected by enhanced chemiluminescence method using the ECL detection system (GE Healthcare, Buckinghamshire, England). The bands were analyzed by imaging densitometry (GS-700 densitometer, Bio-Rad) and expressed as autoradiographic values (OD × mm2) per immunoblot protein.
The statistical analysis of the data on ATP. PCr and caspase-9 density was performed using one way analysis of variance (ANOVA) and Dunn test for comparison among the groups. A p value <0.05 was considered significant.
The levels of tissue high energy phosphates in the cerebral cortex of normoxic and hypoxic piglets were determined. Cerebral tissue hypoxia was documented by decreases in the levels of high energy phosphates, ATP and phosphocreatine (PCr). ATP levels (μmoles/g brain) were 4.7±0.18 in the normoxic and 1.7 ± 0.16 in the hypoxic (p<0.05 vs Nx) group. PCr levels (μmoles/g brain) were 4.03±0.11 in the normoxic and 1.1 ± 0.3 in the hypoxic (p<0.05 vs Nx) group. These results demonstrate that cerebral tissue hypoxia was achieved in the hypoxic group of newborn piglets.
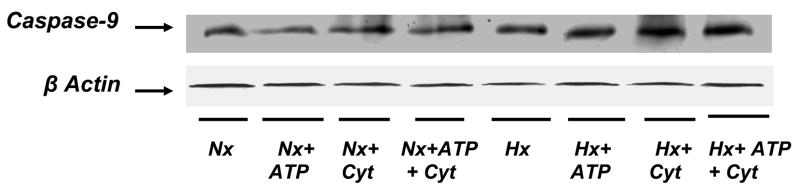
Representative immunoblots of active caspase-9 protein in the normoxic and hypoxic brain preparations are shown in Figure 1. In the normoxic preparation, the results show that the expression of active caspase-9 increased following incubation with ATP + cytochrome c. In the hypoxic preparation, however, the active caspase-9 protein increased following treatment with ATP, cytochrome c as well as with ATP + cytochrome C. The active caspase-9 protein density data expressed as % from all the animals is shown in Figure 2. In the normoxic brain preparations, the density of active caspase-9 protein increased by 9±4%, 4±5% and 20±5% in the presence of ATP, cytochrome c and ATP+cytochrome C, respectively. In the hypoxic brain preparations, the density of active caspase-9 increased by 30±5%, 45±4% and 60±6% in the presence of ATP, cytochrome C and ATP+cytochromec, respectively. The results show that incubation with ATP, cytochrome c and ATP+ cytochrome c result in a higher activation of caspase-9 in the hypoxic group. The data demonstrate that cerebral tissue hypoxia results in increased activation of caspase-9 by ATP and cytochrome c (p<0.05). The results indicate that that the ATP and cytochrome c sites of the apoptotic protease activating factor-1 that mediates caspase-9 activation are modified during hypoxia.
Figure 1.
Representative immunoblots of active caspase-9 protein from normoxic and hypoxic brain preparations are shown. Cytosolic fraction was isolated and passed through a G25-Sephadex column to remove endogenous ATP and cytochrome c. Eluted high molecular weight samples from normoxic and hypoxic animals were divided into four subgroups: Subgroup 1 (control), incubated without added ATP and cytochrome c; Subgroup 2, incubated with added ATP (1 mM); Subgroup 3, incubated with added cytochrome c (100μM); and Subgroup 4, incubated with added ATP ( 1mM)and cytochrome c (100μM). The incubation was carried out at 37° C for 30 min. Following incubation, the protein was separated by 12% SDS PAGE and active caspase-9 was detected using specific active caspase-9 antibody.
Figure 2.
Density of active caspase-9 protein in the normoxic and hypoxic brain preparations following incubations without and with added ATP and cytochrome c as described above (Figure 1). The protein density is expressed as percent of normoxic or hypoxic controls following incubations without added ATP and cytochrome c. The data are presented here from four independent experiments from normoxic (n=4) and hypoxic (n=4) brain preparations. Significance is indicated as p<0.05.
There are several potential mechanisms for caspase-9 activation during hypoxia. We have previously shown that hypoxia results in increased generation of nitric oxide free radicals and nitration of several proteins including the N-mehtyl-D-aspartate receptor, a transcription-independent pathway. We have also shown that hypoxia results in (a) increased nuclear high affinity Ca++-ATPase activity, (b) increased nuclear Ca++-influx and increased Ca++/calmodulin-dependent protein kinase IV activity in neuronal nuclei [6, 18, 31], (c). increased phosphorylation of CREB protein, (d) increased expression of proapoptotic protein Bax [19, 24, 30] and increased activity of caspase-9. We have demonstrated that administration of a selective inhibitor of neuronal nitric oxide synthase (nNOS), 7-Nitro-indazole, prevented the hypoxia-induced increased activation of caspase-9 in the cerebral cortex of newborn piglets [22]. Furthermore we have demonstrated that nNOS inhibitor prevent the hypoxia-induced increased activation caspase-3 and poly-ADP-ribose polymerase (PARP) and increased nuclear DNA fragmentation [20, 22, 30]. These studies demonstrate the pivotal role of nitric oxide in caspase-9 activation and subsequent cascade of hypoxic neuronal death.
Furthermore, we demonstrated that administration of nNOS inhibitors prevented the hypoxia-induced decrease in protein tyrosine phosphatase activity and mitogen activated protein kinase phosphatase-1 and -3 activities as well as and the hypoxia-induced increase in protein tyrosine kinase activity, ERK and JNK activities. Thus, NO can mediated nitration as well as phosphorylation-dependent modification of Apaf-1 during hypoxia, a transcription-independent mechanism.
Increased NO generation during hypoxia in neurons may activate caspase-9 through a transcription-dependent mechanism. We have demonstrated that hypoxia-induced increase in nuclear high affinity Ca++-ATPase, nuclear Ca++-influx and the expression of proapoptotic protein Bax [24,30]. The hypoxia-induced increase in Bax is prevented by administration of nNOS inhibitor. The NO-mediated increase in proapoptotic proteins such as Bax and Bad during hypoxia may lead to apoptotic protease activating factor-1 (Apaf-1) activation resulting in conversion of procaspase-9 to active caspase-9 and subsequent activation of caspase-3.
In summary,: we have shown that the incubation with ATP, cytochrome and ATP+cytochrome c results in higher activation of caspase-9 in the hypoxic brain preparation as compared to normoxic brain. We conclude that the ATP and cytochrome c –dependent activation of caspase-9 is increased during hypoxia and the hypoxia –induced modification of ATP and cytochrome c binding sites of Apaf-1 is the potential mechanism of caspase-9 activation. We propose that the mechanism of caspase-9 activation during hypoxia in the newborn brain is due to NO-mediated transcription-independent as well as transcription-dependent mechanisms.
Acknowledgments
This study was supported by grants from the National Institutes of Health, NIH-HD- 38079 (O.P.M) and NIH-HD-20337 (M.D-P). The authors wish to express their thanks to Ms. Joanna Kubin for her expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashraf QM, Haider HS, Katsetos CD, Delivoria-Papadopoulos M, Mishra OP. Nitric oxide mediated alterations of protein tyrosine phosphatase activity and expression during hypoxia in the cerebral cortex of newborn piglets. Neurosci Lett. 2004;362:108–112. doi: 10.1016/j.neulet.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 2.Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- 3.Chang HY, Yang X. Proteases for cell suicide: Functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinnaiyan AM. The apoptosome: Heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnaiyan AM, O’Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 6.Delivoria-Papadopoulos M, Akhter W, Mishra OP. Hypoxia-induced Ca++-influx in cerebral cortical neuronal nucleiof newborn piglets. Neurosci Lett. 2003;342:119–123. doi: 10.1016/s0304-3940(03)00256-8. [DOI] [PubMed] [Google Scholar]
- 7.Ellis RE, Yuan J, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 8.Finkel E. The mitochrondion: is it central to apoptosis? Science. 2001;292:624–626. doi: 10.1126/science.292.5517.624. [DOI] [PubMed] [Google Scholar]
- 9.Grutter MG. Caspases: key players in Programmed cell death. Curr Opin Strl Biol. 2000;10:649–655. doi: 10.1016/s0959-440x(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 10.Hengartner MO, Horvitz HR. C. elegans survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene Bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Kim HE, Shu H, et al. Distintive roles of PHAP proteins and prothymosin-α in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 12.Khurana P, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on caspase-3, -8 and –9 activity and expression in the cerebral cortex of newborn piglets. Neurochem Res. 2002;27:931–938. doi: 10.1023/a:1020347732741. [DOI] [PubMed] [Google Scholar]
- 13.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MSS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase-9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 15.Lamprecht W, Stein P, Heinz F, Weisser H. Creatine phosphate. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Vol. 4. 1974. pp. 1777–1781. [Google Scholar]
- 16.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri E, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 18.Mishra OP, Delivoria-Papadopoulos M. Effect of graded hypoxia on high affinity Ca++-ATPase activity in cortical neuronal nuclei of newborn piglets. Neurochem Res. 2002;25:1559–1565. doi: 10.1023/a:1014205702905. [DOI] [PubMed] [Google Scholar]
- 19.Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. Phosphorylation of cAMP response element binding (CREB) protein during hypoxia in cerebral cortex of newborn piglets and the effect of nitric oxide synthase inhibition. Neurosci. 2002;115:985–991. doi: 10.1016/s0306-4522(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 20.Mishra OP, Akhter W, Ashraf QM, Delivoria-Papadopoulos M. Hypoxia-induced modification of poly (ADP-ribose) polymerase and DNA polymerase β activity in cerebral cortical nuclei of newborn piglets; Role of nitric oxide. Neurosci. 2003;119:1023–1032. doi: 10.1016/s0306-4522(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 21.Mishra OP, Delivoria Papadopoulos M. ATP and cytochrome c –dependent inhibition of caspase-9 activity in the cerebral cortex of newborn piglets. Neurosci Lett. 2004;364:119–123. doi: 10.1016/j.neulet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Mishra OP, Delivoria-Papadopoulos M. Effect of neuronal nitric oxide synthase inhibition on caspase-9 activity during hypoxia in the cerebral cortex of newborn piglets. Neurosci Lett. 2006;401:81–85. doi: 10.1016/j.neulet.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 23.Ranganath RM, Nagashree NR. Role of programmed cell death in development. Int Rev Cytol. 2001;202:59–242. doi: 10.1016/s0074-7696(01)02005-8. [DOI] [PubMed] [Google Scholar]
- 24.Ravishankar S, Ashraf QM, Fritz KI, Mishra OP, Delivoria-Papadopoulos M. Expression of Bax and Bcl-2 proteins during hypoxia in cerebral cortical neuronal nuclei of newborn piglets: Effect of administration of magnesium sulfate. Brain Res. 2001;901:23–29. doi: 10.1016/s0006-8993(01)02109-6. [DOI] [PubMed] [Google Scholar]
- 25.Riedl SJ, Salvesen GS. The apoptosome: Signalling platform for cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 26.Xue D, Shaham S, Horvitz HR. The Caenorhabditis elagans cell death protein CED-3 is a cysteine protease with substrate specificities similar to those of human CPP32 protease. Genes Dev. 1996;10:1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- 27.Yuan JY, Horvitz RH. The C. elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- 28.Zanelli SA, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neurosci. 2002;112:869–877. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- 29.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 30.Zubrow AB, Delivoria-Papodopoulos M, Ashraf QM, Mishra OP. Nitric oxide-mediated expression of Bax protein and DNA fragmentation during hypoxia in neuronal nuclei from newborn piglets. 2002a;954:60–67. doi: 10.1016/s0006-8993(02)03342-5. [DOI] [PubMed] [Google Scholar]
- 31.Zubrow AB, Ashraf QM, Delivoria-Papodopoulos M, Mishra OP. Effect of nitric oxide synthase inhibition on nuclear Ca++/calmodulin dependent protein kinase IV (CaM kinase) activity during hypoxia in neuronal nuclei of newborn piglets. Neurosci Lett. 2002b;335:5–8. doi: 10.1016/s0304-3940(02)01138-2. [DOI] [PubMed] [Google Scholar]