Abstract
The turtle spinal cord contains a central pattern generator (CPG) that produces rhythmic hindlimb motor patterns during a rostral scratch. This review describes evidence in support of the hypothesis that the turtle rostral scratch CPG has a modular structure similar to that described in the Unit-Burst-Generator hypothesis for cat locomotion by Grillner. During normal rostral scratch in turtle, activity bursts rhythmically alternate with quiescence for each motor neuron pool; agonist activity rhythmically alternates with antagonist activity at each degree of freedom, e.g., hip, knee; and a transition from knee-flexor to knee-extensor motor neuron activity occurs midway during each hip-flexor motor neuron burst. Hip-extensor deletions, knee-flexor deletions, and knee-extensor deletions are motor-pattern variations of rostral scratch. During each of these variations, agonist activity is rhythmic; antagonist activity and agonist quiescence are absent. Several classes of evidence during both normal and variation motor patterns support a modular organization of the turtle rostral scratch CPG: electroneurographic recordings from axons of motor neurons, intracellular recordings of synaptic potentials in motor neurons, and extracellular unit recordings from spinal interneurons. These data support the hypotheses that the knee-extensor module is different from the hip-extensor module and that the knee-flexor module is different from the hip-flexor module. Potential mechanisms for rhythmogenesis include reciprocal connections between agonist and antagonist modules at each degree of freedom, and agonist module rhythmogenesis. Additional tests of the modular hypothesis for turtle rostral scratch include unit recordings from knee-related interneurons during normal rostral scratch, as well as during knee-related deletions.
Section: Sensory and Motor Systems
Keywords: central pattern generator, deletion, fictive motor pattern, module, scratch reflex, spinal cord
1. Modular organization of central pattern generators
Central pattern generators (CPGs) are neuronal networks that generate motor patterns without movement-related sensory feedback (Grillner, 1981, 2006; Kiehn, 2006; Stein et al., 1997). This review discusses the modular organization of spinal cord CPGs that produce rhythmic motor output. Multiple lines of evidence support several distinct concepts of spinal modular organization (Tresch et al., 2002). My focus here is upon one particular point of view of spinal module, the concept described by Grillner (1981, 2006) in his Unit-Burst-Generator (UBG) hypothesis for control of cat hindlimb stepping: each module or UBG is a set of interneurons and motor neurons that control agonist musculature for a specific direction of a degree of freedom of body movement, e.g., hip flexion. The modular UBG concept has been very important in studies of other motor systems (Buschges, 2005; Grillner, 2003, 2006). In this review, I discuss a modular UBG hypothesis of the organization of the spinal neuronal network that generates the turtle rostral scratch (Robertson et al., 1985; Stein, 2005; Stein and Daniels-McQueen, 2002, 2003a, 2004).
According to the UBG concept (Fig. 1A), each agonist module consists of agonist excitatory interneurons (EINs), agonist inhibitory interneurons (IINs), and agonist motor neurons (MNs). Agonist EINs excite other members of the agonist module as well neurons in certain modules at other degrees of freedom. Agonist IINs inhibit members of the antagonist module at the same degree of freedom as well as neurons in certain modules at other degrees of freedom. There is a reciprocal inhibitory relationship between the agonist module and the antagonist module at each degree of freedom; for example, a reciprocal inhibitory relationship between the hip-flexor module and the hip-extensor module at the flexion-extension degree of freedom at the hip. This reciprocal inhibitory relationship serves as one potential mechanism for rhythm generation. Additional mechanisms for rhythm generation include the possibility that some agonist modules may activate motor rhythms even when the neurons in the antagonist module are quiet, i.e., some modules can be rhythmogenic and serve as burst generators.
Figure 1.
Schematic of a portion of the spinal neuronal network responsible for the production of the turtle rostral scratch as described in the modular unit-burst-generator (UBG) hypothesis. Only ipsilateral hip and knee UBGs are shown; only a subset of the possible synaptic connections are included in the sketch. EINs: excitatory interneurons; IINs: inhibitory interneurons; MNs: motor neurons. Reciprocal inhibition between agonist and antagonist UBGs at each degree of freedom is a fundamental characteristic of organization of this network. Proposed connections from hip modules to knee modules include mixed-synergy synaptic drives, e.g., hip-extensor EIN excitation of neurons in the knee-flexor module. Active neurons shown in black; quiet neurons shown in light blue. A: During the normal rostral scratch, all hip and knee UBGs are rhythmically active. B: During the hip-extensor deletion variation of rostral scratch, the hip-flexor, the knee-flexor, and the knee-extensor UBGs are rhythmically active. Neurons in the hip-extensor UBG are quiet. This schematic emphasizes that neurons in the hip-flexor UBG are rhythmic even when neurons in the hip-extensor UBG are quiet. This figure is a modification of an earlier version presented in a poster by Stein and Daniels-McQueen, 2003b.
Deletions are motor pattern variations that provide experimental support for the concepts that rhythmic spinal CPGs have modular organization and some UBGs are rhythmogenic. This review describes a set of turtle rostral scratch deletions, the long-studied hip-extensor deletion (first introduced by Stein and Grossman, 1980) and the recently-described knee-related deletions (Stein and Daniels-McQueen, 2004). Deletions observed during cat stepping and scratching provide additional support for the modularity of spinal CPGs and the rhythmicity of certain modules (Lafreniere-Roula and McCrea, 2005; Rybak et al., 2006; Turkin and Hamm, 2004).
The UBG hypothesis shares with the Half-Center hypothesis (Jankowska et al., 1967; Lundberg, 1981) the feature of reciprocal inhibition between agonist and antagonist modules. In the Half-Center hypothesis, however, all flexor neurons at hip, knee, and ankle belong to the same flexor half-center, and all extensor neurons at those degrees of freedom belong to the same extensor half-center. Furthermore, the Half-Center hypothesis predicts that there cannot be rhythmic activity of an agonist without alternating rhythmic activity of its antagonist. The Half-Center hypothesis fails to explain several characteristics of some spinal motor rhythms (Lafreniere-Roula and McCrea, 2005; Stein, 2005; Stein and Smith, 1997): it cannot explain motor patterns with mixed synergies such as the rostral scratch in turtle and the paw shake in cat; and it cannot explain motor-pattern deletions such as the hip-extensor deletion variation of rostral scratch in turtle and locomotion motor-pattern deletions in cat. The focus here is upon evidence from turtle rostral scratch that supports the Unit-Burst-Generator concepts that (1) the knee-extensor module is different from the hip-extensor module, and (2) the knee-flexor module is different from the hip-flexor module.
2. The hip-extensor deletion variation of turtle rostral scratch
The normal motor pattern for turtle rostral scratch (Fig. 2A) includes rhythmic alternation between bursts of hip-flexor and hip-extensor motor neuron activity; hip-extensor activity occurs during hip-flexor quiescence (Robertson et al., 1985; Stein, 2005; Stein and Daniels-McQueen, 2002). Normal cycles of rostral scratch also include rhythmic alternation between bursts of knee-flexor and knee-extensor motor neuron activity (Fig. 3A); knee-flexor activity occurs during knee-extensor quiescence (Stein and Daniels-McQueen, 2003a, 2004). Rostral scratch in turtle is an example of a mixed synergy (Stein and Smith, 1997): extensor motor neuron activity at one degree of freedom occurs during flexor motor neuron activity of another degree of freedom. During a normal rostral scratch cycle, knee-extensor motor neuron activity begins during the latter portion of each hip-flexor motor neuron burst, and knee-flexor motor neuron activity begins during each hip-extensor motor neuron burst (Robertson et al., 1985; Stein and Daniels-McQueen, 2003a, 2004).
Figure 2.
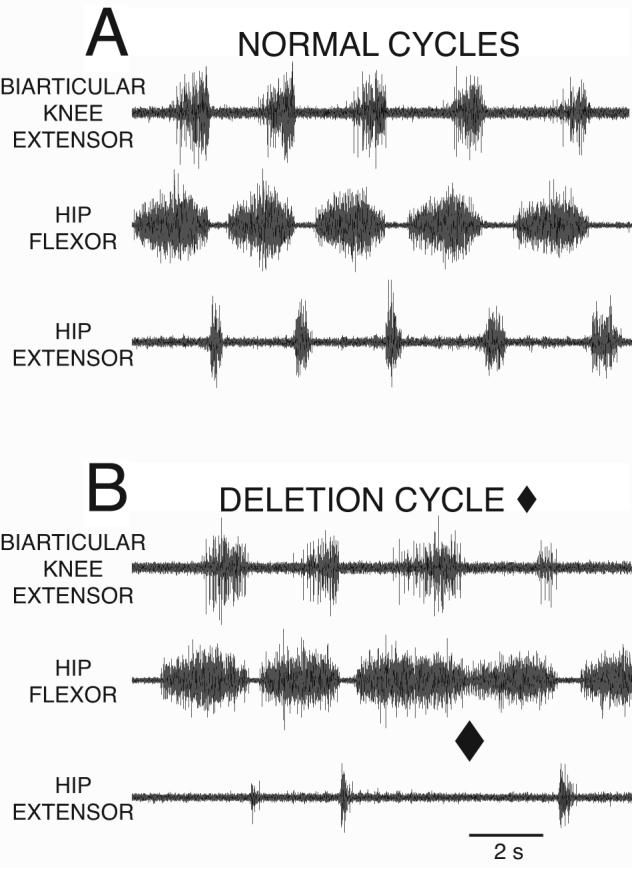
ENG recordings from the biarticular knee-extensor, the hip-flexor, and the hip-extensor motor nerves during fictive rostral scratch in the turtle. A: Normal rostral scratch with rhythmic alternation between hip-flexor activity and quiescence. Hip-extensor activity occurs during hip-flexor quiescence. Knee-extensor activity occurs during the latter portion of hip-flexor activity. B: One cycle of a hip-extensor deletion with the end of the cycle marked with filled diamond. At the filled diamond, there is no hip-extensor activity, and no quiescent period between the end of the hip-flexor burst and the start of the next hip-flexor burst. The other cycles in B are examples of normal rostral scratch. From Stein and Daniels-McQueen, J Neuroscience 22: 6800-6809, 2002; used with permission of and copyright 2002 by the Society for Neuroscience.
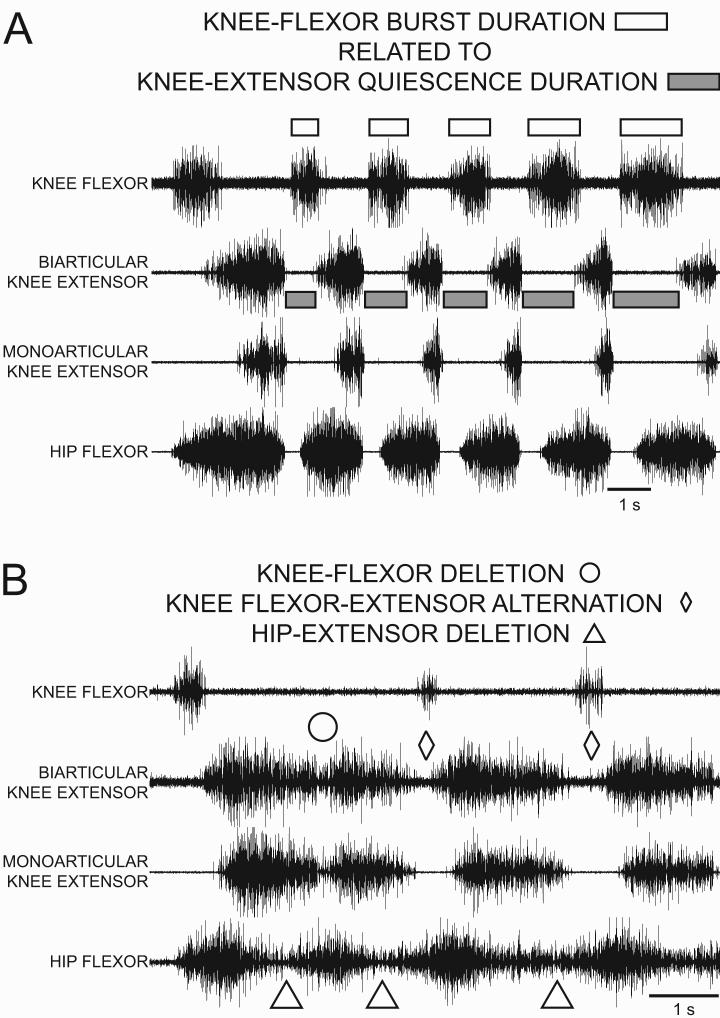
Figure 3.
ENG recordings from the knee-flexor, the biarticular knee-extensor, the monoarticular knee-extensor, and the hip-flexor motor nerves during fictive rostral scratch in the turtle. A: Normal rostral scratch with rhythmic alternation between knee-extensor activity and quiescence. Knee-flexor activity (marked with unfilled rectangles) occurs during knee-extensor quiescence (biarticular knee-extensor quiescence marked with gray-filled rectangles). The transition from knee-flexor activity to knee-extensor activity occurs during hip-flexor activity. B: Unfilled triangles mark hip-extensor deletion variations of rostral scratch. Unfilled circle marks a knee-flexor deletion. During the knee-flexor deletion, there is no quiescent period between the end of one knee-extensor burst and the start of the next knee-extensor burst. Unfilled diamonds mark knee flexor-extensor alternation: knee-flexor activity occurs during knee-extensor quiescence. From Stein and Daniels-McQueen, J Neurophysiol, 91: 2380-2384, 2004; used with permission of and copyright 2004 by the American Physiological Society.
During a cycle of the hip-extensor deletion motor pattern variation of rostral scratch (end of cycle marked with filled diamond in Fig. 2B), previously termed B-phase deletion (Stein and Grossman, 1980) and HR-KF deletion (Robertson and Stein 1988), there is no hip-extensor motor neuron activity and no quiescence between successive hip-flexor motor neuron bursts (Stein et al., 1995, 1998; Stein and Daniels-McQueen, 2002). In addition, knee-extensor motor neuron quiescence occurs during the initial portion of each hip-flexor motor neuron burst, and knee-extensor motor neuron activity occurs during the latter portion of each hip-flexor motor neuron burst. This pattern of knee-extensor quiescence and activity also occurs in a normal rostral scratch during each hip-flexor motor neuron burst. Stein and Daniels-McQueen (2004) observed rhythmic alternation of knee-flexor and knee-extensor activity during some hip-extensor deletions: knee-flexor motor neuron activity occurred during knee-extensor motor neuron quiescence (marked with unfilled diamonds in Fig 3B). This establishes that rhythmic bursts of agonist activity without antagonist activity can occur at one degree of freedom while rhythmic alternation between agonist and antagonist can occur at another degree of freedom (Fig. 1B). This result supports the hypothesis that the hip-flexor module is rhythmogenic, i.e., the hip-flexor UBG is a “burst generator” that can produce a rhythm even when the antagonist hip-extensor UBG is not active.
Our observations of motor patterns during hip-extensor deletion variations of rostral scratch support the concept that the neurons in the knee-extensor module are members of a different population than the neurons in the hip-extensor module (Fig. 1B). This concept is a key component of Grillner’s Unit-Burst-Generator hypothesis and the modular hypothesis for turtle rostral scratch. This concept conflicts with the assumption of the Half-Center hypothesis that all hindlimb extensor neurons have similar activity patterns and belong to the same extensor half-center.
3. Hip-extensor interneurons are quiet during the hip-extensor deletion variation of turtle rostral scratch
Two classes of evidence support the concept that the interneurons of the hip-extensor module are quiet when hip-extensor motor neurons are quiet during the hip-extensor deletion variation of rostral scratch (Fig. 1B): 1) intracellular recordings of synaptic potentials in hip-extensor and in hip-flexor motor neurons; and 2) extracellular unit recordings from priopriospinal interneurons with descending axons (Fig. 4). In contrast, knee-extensor motor neurons (Figs. 2B, 3B, 4A) and candidate interneuronal members of the knee-extensor module (“ON-units,” see Section 5 below) are rhythmically active during hip-extensor deletions.
Figure 4.
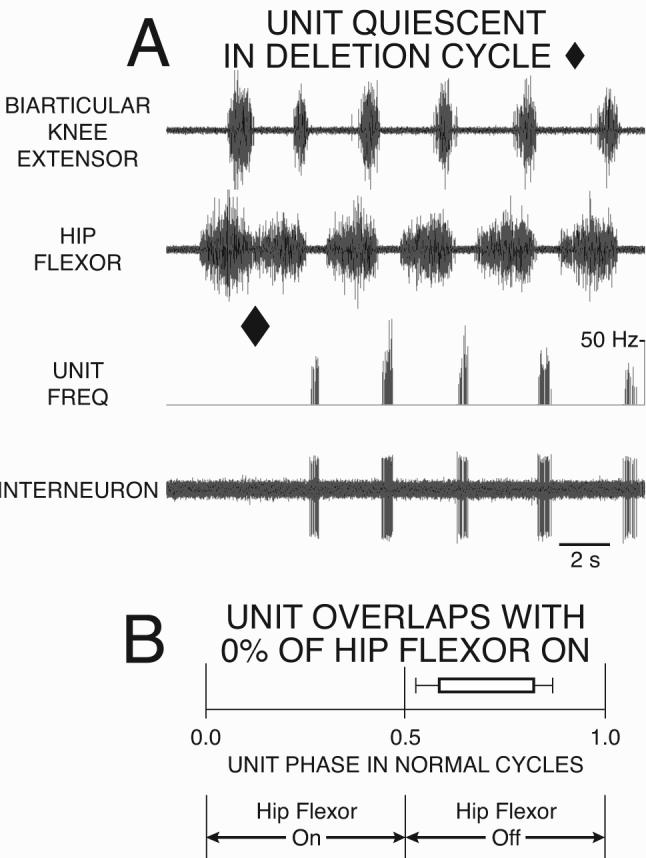
Hip-extensor interneuron characterized by its 0% overlap with the hip-flexor burst during fictive rostral scratch in the turtle. The hip-extensor interneuron is active in a burst during the hip-flexor quiesence of normal rostral scratch, and is quiet during the hip-extensor deletion variation of rostral scratch. A: ENG recordings of the biarticular knee-extensor and the hip-flexor motor nerves (top two traces), unit instantaneous frequency (third trace), extracellular single-unit interneuronal recording (bottom trace). The first cycle is an example of a hip-extensor deletion (marked with filled diamond at end of cycle): the unit is not active in this cycle. The other 5 cycles are examples of normal rostral scratch: the unit fires during hip-flexor quiescence. B: Unit phase during normal cycles of rostral scratch. The start-phase and the end-phase of the unit’s burst occur during hip-flexor quiescence. Note that hip-extensor motor neurons are also active during hip-flexor quiescence (Fig. 2A). From Stein and Daniels-McQueen, J Neuroscience 22: 6800-6809, 2002; used with permission of and copyright 2002 by the Society for Neuroscience.
3.1. Synaptic potentials in hip motor neurons
During hip-extensor motor neuron activity in a normal cycle of rostral scratch, EPSPs activate action potentials in hip-extensor motor neurons and corresponding IPSPs inhibit action potentials in hip-flexor motor neurons (Robertson and Stein, 1988). During the hip-extensor deletion variation of turtle rostral scratch, there are no EPSPs in hip-extensor motor neurons and no corresponding IPSPs in hip-flexor motor neurons (Robertson and Stein, 1988). These motor neuron intracellular recordings support the hypothesis that there is a population of spinal interneurons active during the hip-extensor phase of normal rostral scratching that are quiet during the hip-extensor deletion variation of rostral scratching. Moreover, these data support the hypothesis that there is linkage between excitation of hip-extensor motor neurons and inhibition of hip-flexor motor neurons (Fig. 1A).
3.2. Unit recordings from spinal interneurons
Stein and Daniels-McQueen (2002) recorded single-unit extracellular action potentials from rhythmic spinal interneurons during normal cycles of rostral scratch and during cycles of the hip-extensor deletion variation of rostral scratch. They characterized the start-phase and end-phase of each unit’s burst during normal rostral scratch (Stein and Daniels-McQueen, 2002). Units that fire only during hip-flexor quiescence of normal rostral scratch are termed 0%-overlap interneurons since their burst has no overlap with hip-flexor motor neuron activity (Fig. 4). 0%-overlap interneurons are 1) active during hip-extensor motor neuron activity of normal rostral scratch (last 5 cycles in Fig. 4A) and 2) quiet during the hip-extensor deletion variation of rostral scratch (end of cycle marked with filled diamond in Fig. 4A).
These data are consistent with the hypothesis that 0%-overlap interneurons are hip-extensor interneurons and members of the hip-extensor module. From this point of view, the interneurons and the motor neurons in the hip-extensor module 1) are all active during the hip-extensor phase of normal rostral scratching (Figs. 1A, 2A; last 5 cycles in Fig. 4A) and 2) are all quiet during hip-extensor deletions, i.e., they act in concert as a module (marked with light blue in Fig. 1B; marked with filled diamonds in Figs. 2B, 4A).
4. The knee-flexor deletion and the knee-extensor deletion variations of turtle rostral scratch
During normal rostral scratch (Figs. 2A, 3A, 5A), knee-extensor motor neurons are active during the latter portion of each hip-flexor motor neuron burst (Robertson et al., 1985; Stein, 2005) and knee-flexor motor neurons (Fig. 3A) are active during knee-extensor quiescence (Stein and Daniels-McQueen, 2003a, 2004). Stein and Daniels-McQueen (2004) observed three distinct types of knee motor neuron activities during rostral scratch variations in which hip-extensor motor neurons were quiet. They observed some hip-extensor deletions with knee flexor-extensor alternation (marked with open diamonds in Fig. 3B). They also observed other cycles in which there were knee motor-pattern variations along with deletions of hip-extensor activity.
Figure 5.
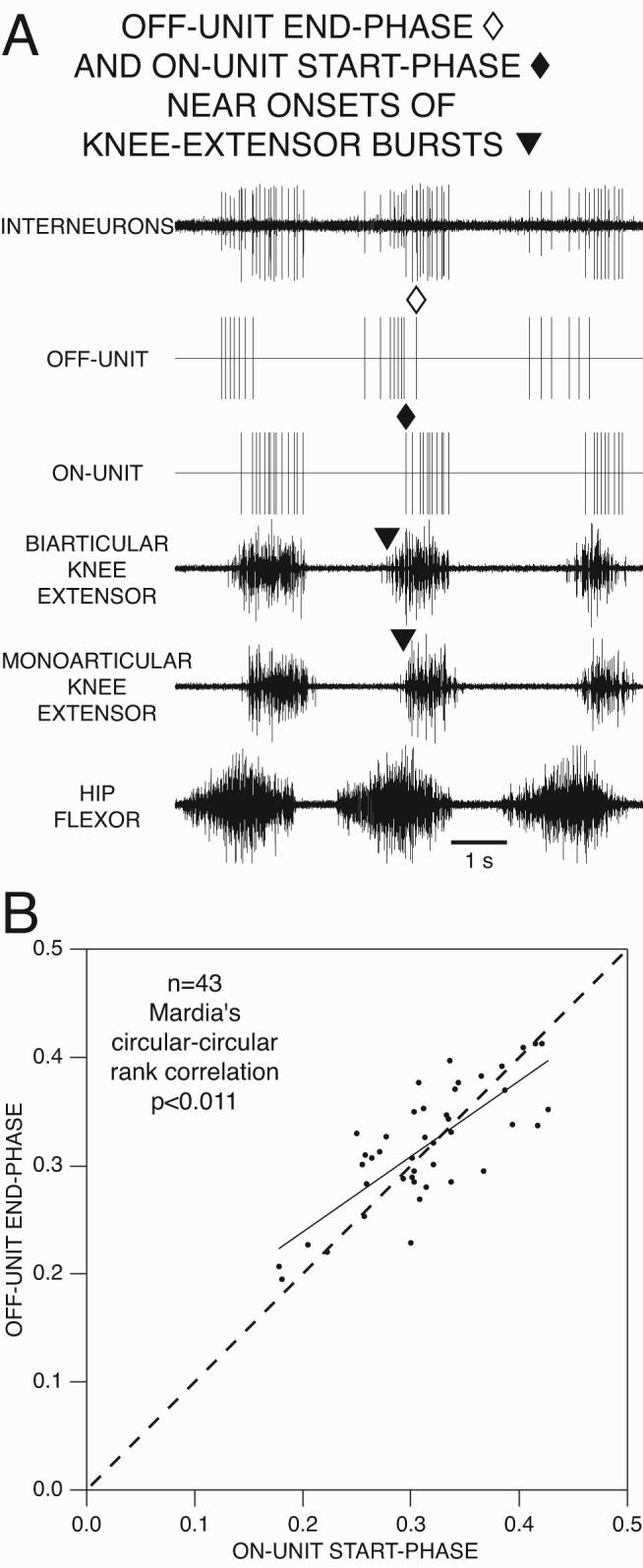
A: Simultaneous recording of 2 interneurons, an ON-unit and an OFF-unit, during 3 cycles of normal fictive rostral scratch in the turtle. Extracellular single-unit interneuronal recordings in the top trace; vertical lines in second trace represent timing of action potentials of the OFF-unit, the second-largest unit in the top trace; vertical lines in the third trace represent timing of action potentials of the ON-unit, the largest unit in the top trace; fourth, fifth, and sixth traces are ENG recordings from the biarticular knee-extensor, the monoarticular knee-extensor, and the hip-flexor motor nerves, respectively. The end of OFF-unit burst (marked with unfilled diamond) occurs near the start of ON-unit burst (marked with filled diamond) and near the start of knee-extensor bursts (marked with filled triangles). B: Plot of OFF-unit end-phase as a function of ON-unit start-phase. OFF-units are candidate members of the knee-flexor UBG; ON-units are candidate members of the knee-extensor UBG. From Stein and Daniels-McQueen, J Neurophysiol 90: 3585-3593, 2003a; used with permission of and copyright 2003 by the American Physiological Society.
During a knee-flexor deletion, there is no quiet period between successive knee-extensor motor neuron bursts and no knee-flexor motor neuron activity (marked with open circle in Fig 3B). This result supports the hypothesis that the knee-extensor module is rhythmogenic, i.e., the knee-extensor UBG is a “burst generator” that can produce a rhythm even when the antagonist knee-flexor UBG is not active.
During a knee-extensor deletion, there is no quiet period between successive knee-flexor motor neuron bursts and no knee-extensor motor neuron activity. This result supports the hypothesis that the knee-flexor module is rhythmogenic, i.e., the knee-flexor UBG is a “burst generator” that can produce a rhythm even when the antagonist knee-extensor UBG is not active.
5. Candidate knee-flexor interneurons and knee-extensor interneurons during turtle rostral scratch
Stein and Daniels-McQueen (2003a) developed criteria to identify spinal interneurons recorded during normal cycles of rostral scratch that are candidate members of the knee-related modules. The knee-flexor motor neuron burst ends and the knee-extensor motor neuron burst starts midway through the hip-flexor motor neuron burst (Fig. 3A). There is a strong positive correlation between knee-flexor motor neuron end-phases and knee-extensor motor neuron start-phases. Thus a critical transition of knee motor activity occurs during the hip-flexor motor neuron burst of normal rostral scratch.
They analyzed spinal interneurons with end-phases close to this critical transition (Fig. 5). For some interneurons, termed OFF-units, there is a strong positive correlation between the unit’s end-phases and knee-extensor motor neuron start-phases. OFF-units are candidate members of the knee-flexor module. They also analyzed other spinal interneurons with start-phases close to this critical transition. For some interneurons, termed ON-units, there is a strong positive correlation between the unit’s start-phases and knee-extensor motor neuron start-phases. ON-units are candidate members of the knee-extensor module.
5.1. OFF-units and ON-units fire in bursts during hip-extensor deletions
OFF-units end midway through the hip-flexor burst of a normal rostral scratch. These units fired in bursts during hip-extensor deletions (Stein and Daniels-McQueen, 2003a). ON-units start midway through the hip-flexor burst. These units mainly fired in bursts during hip-extensor deletions (Stein and Daniels-McQueen, 2003a). OFF-units and ON-units have intermediate levels of overlap with hip-flexor motor neuron bursts. Stein and Daniels-McQueen (2002) found that most units with intermediate levels of overlap fired in bursts during hip-extensor deletions.
Both OFF-units (candidate members of the knee-flexor module) and ON-units (candidate members of the knee-extensor module) continue to be active during hip-extensor deletions, in contrast to 0%-overlap units (candidate members of the hip-extensor module) which are quiet during hip-extensor deletions (Stein and Daniels-McQueen, 2002, 2003a). This provides strong support for the UBG concept applied to turtle rostral scratch (Fig. 1B). The Half-Center hypothesis fails to explain the rhythmic activity of knee-extensor interneurons and the quiescence of hip-extensor interneurons during hip-extensor deletions.
5.2. Tests of the hypotheses that knee-flexor interneurons are quiet during knee-flexor deletions and that knee-extensor interneurons are quiet during knee-extensor deletions
Stein and Daniels-McQueen (2004) predicted that OFF-units, candidate members of the knee-flexor module, will be quiet during knee-flexor deletions. They also predicted that ON-units, candidate members of the knee-extensor module, will be quiet during knee-extensor deletions. We are currently testing these predictions in our laboratory.
6. Modular organization of turtle spinal cord central pattern generators
Grillner (1981) states in his Unit-Burst-Generator hypothesis that each UBG “can burst by itself or produce tonic output.” It is an experimental question to determine the rhythmicity of each module. Work with the hip-extensor deletion of rostral scratch demonstrated that the interneurons and motor neurons in the hip-flexor module are rhythmic even when the interneurons and motor neurons in the hip-extensor module are quiet (Stein and Daniels-McQueen, 2002). Work in progress is examining the rhythmicity of knee-related interneurons in knee modules during knee-related deletions.
This review summarizes evidence in turtle rostral scratch that (1) the knee-extensor module is different from the hip-extensor module, and (2) the knee-flexor module is different from the hip-flexor module. Turtle spinal cord can produce a variety of different motor rhythms (Berkowitz, 2002, 2005; Berkowitz et al., 2006; Earhart and Stein, 2000; Juranek and Currie, 2000; Stein, 2005). It is a question for future work to characterize the extent to which the modular structure of the turtle rostral scratch CPG is also a characteristic of CPGs for other motor rhythms, e.g., pocket scratch, caudal scratch, forward swim, backward swim, and forward step.
Acknowledgments
I thank Dr. Ari Berkowitz for editorial comments. Research in the Stein Laboratory is supported by NIH Grant NS30786 to PSGS.
Abbreviations
- CPG
central pattern generator
- EIN
excitatory interneuron
- ENG
electroneurogram
- EPSP
excitatory postsynaptic potential
- IIN
inhibitory interneuron
- IPSP
inhibitory postsynaptic potential
- MN
motor neuron
- UBG
unit burst generator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Berkowitz A. Both shared and specialized spinal circuitry for scratching and swimming in turtles. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2002;188:225–234. doi: 10.1007/s00359-002-0297-7. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Physiology and morphology indicate that individual spinal interneurons contribute to diverse limb movements. J. Neurophysiol. 2005;94:4455–4470. doi: 10.1152/jn.00229.2005. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Yosten GL, Ballard RM. Somato-dendritic morphology predicts physiology for neurons that contribute to several kinds of limb movements. J. Neurophysiol. 2006;95:2821–2831. doi: 10.1152/jn.01246.2005. [DOI] [PubMed] [Google Scholar]
- Buschges A. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J. Neurophysiol. 2005;93:1127–1135. doi: 10.1152/jn.00615.2004. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Stein PSG. Step, swim, and scratch motor patterns in the turtle. J. Neurophysiol. 2000;84:2181–2190. doi: 10.1152/jn.2000.84.5.2181. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB, editor. Handbook of Physiology, Sect. 1, The Nervous System, Vol. 2, Motor Control. American Physiological Society; Bethesda, Maryland: 1981. pp. 1179–1236. [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol. Scand. 1967;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Juranek J, Currie SN. Electrically evoked fictive swimming in the low-spinal immobilized turtle. J. Neurophysiol. 2000;83:146–155. doi: 10.1152/jn.2000.83.1.146. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J. Neurophysiol. 2005;94:1120–1132. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Half-centres revisited. Adv. Physiol. Sci. 1981;1:155–167. [Google Scholar]
- Robertson GA, Stein PSG. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J. Physiol. (London) 1988;404:101–128. doi: 10.1113/jphysiol.1988.sp017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GA, Mortin LI, Keifer J, Stein PSG. Three forms of the scratch reflex in the spinal turtle: central generation of motor patterns. J. Neurophysiol. 1985;53:1517–1534. doi: 10.1152/jn.1985.53.6.1517. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J. Physiol. (London) 2006;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PSG. Neuronal control of turtle hindlimb motor rhythms. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005;191:213–229. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- Stein PSG, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J. Neurosci. 2002;22:6800–6809. doi: 10.1523/JNEUROSCI.22-15-06800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PSG, Daniels-McQueen S. Timing of knee-related spinal neurons during fictive rostral scratching in the turtle. J. Neurophysiol. 2003a;90:3585–3593. doi: 10.1152/jn.00762.2003. [DOI] [PubMed] [Google Scholar]
- Stein PSG, Daniels-McQueen S. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington DC: 2003b. Knee-flexor motor activity during fictive rostral scratching in the turtle. Program Number 188.5. [Google Scholar]
- Stein PSG, Daniels-McQueen S. Variations in motor patterns during fictive rostral scratching in the turtle: knee-related deletions. J. Neurophysiol. 2004;91:2380–2384. doi: 10.1152/jn.01184.2003. [DOI] [PubMed] [Google Scholar]
- Stein PSG, Grossman ML. Central program for scratch reflex in turtle. J. Comp. Physiol. A. 1980;140:287–294. [Google Scholar]
- Stein PSG, Smith JL. Neural and biomechanical control strategies for different forms of vertebrate hindlimb motor tasks. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks, and Motor Behavior. MIT Press; Cambridge, MA: 1997. pp. 61–73. [Google Scholar]
- Stein PSG, Victor JC, Field EC, Currie SN. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J. Neurosci. 1995;15:4343–4355. doi: 10.1523/JNEUROSCI.15-06-04343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks, and Motor Behavior. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- Stein PSG, McCullough ML, Currie SN. Reconstruction of flexor/extensor alternation during fictive rostral scratching by two-site stimulation in the spinal turtle with a transverse spinal hemisection. J. Neurosci. 1998;18:467–479. doi: 10.1523/JNEUROSCI.18-01-00467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, d’Avella A, Bizzi E. Coordination and localization in spinal motor systems. Brain Res. Rev. 2002;40:66–79. doi: 10.1016/s0165-0173(02)00189-3. [DOI] [PubMed] [Google Scholar]
- Turkin VV, Hamm TM. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington DC: 2004. Changes in locomotor drive potentials and cycle characteristics associated with deletions during fictive locomotion. Program Number 883.14. [Google Scholar]