Summary
Two-component signal transduction systems consisting of a sensor histidine kinase and a response regulator/transcription factor interpret a multitude of environmental and cellular signals and coordinate the expression of a wide array of genes in bacteria. Signal recognition by sensor histidine kinases is the province of a sensor complex consisting of several protein domains that together serve to augment or attenuate the activity of the histidine kinase and thereby of gene expression. Recent investigations have shown the diverse strategies bacteria use to assemble protein domains into the sensor complexes to accomplish signaling. Structural studies of such domains are leading to an understanding of the mechanisms by which sensor complexes recognize signals and regulate kinase activity.
Introduction
For a microbe, the key to successful exploitation of dynamic environmental variation is to recognize the signals of such change and rapidly adapt their gene expression profile to new situations. The predominant mechanism bacteria use to respond to changing environmental conditions is the two-component signal transduction system consisting of a signal sensing protein known as the sensor histidine kinase (SK) and a DNA-binding response protein known simply as the response regulator (RR). Signal recognition leads ultimately to alterations of the phosphorylation state of the RR, which differentially modulates the RRs’ propensity to activate or repress transcription of target genes (Fig. 1A). The average bacterium employs 10–50 such systems to sense environmental conditions. Several thousand sequences of these proteins are available in genome sequence databases (reviewed in [1]).
Fig. 1.

Two-component system concept and histidine kinase modular architecture (A) Two component signal transduction systems comprise a sensor histidine kinase that upon stimulus perception achieved by a sensor complex of domains autophosphorylates on a conserved histidine residue. The phosphoryl group is subject to transfer to an aspartate residue on the response regulator, which initiates the cellular response usually through regulation of the expression of target genes. (B) The sensor histidine kinase is a large modular protein consisting of the conserved catalytic core at the C-terminus and several optional N-terminal elements involved in signal sensing or transmission of a conformational change to the catalytic core. Any of these optional domains can occur as tandem repeats in histidine kinase proteins and their multiplicity comprises the sensor complex. Example structures for periplasmic domains are from left to right CitA, DcuS, PhoQ and LuxQ sensing domains; the example structure for the HAMP domain is from Archaeoglobus fulgidus protein AF1503; example structures for the cytoplasmic sensing domains from left to right are FixL, photoactive yellow protein (PYP), and bacteriophytochrome (BphP) sensing domains; the example structure for the catalytic core is of SK853 comprising HisKA and ATP-binding domains. (C) Venn diagram displaying the occurrence of the common cytoplasmic elements HAMP (Red), GAF (Yellow), and PAS (Blue) in combination with the histidine kinase HisKA (Gray) domain as recognized by the Smart Database. The majority (62%) of all HisKA domains are paired with at least one of these three elements.
Signal ligand binding to sensor domains of histidine kinases normally results in the induction of an ATP-dependent autophosphorylation of the HisKA domain in the catalytic core of the enzyme. Since the histidine kinase is mated to a unique response regulator through specific pairing interactions, gene expression changes are restricted to those genes required for dealing with the specific ligand. The expression of these genes can be fine tuned to ligand concentration by the ability of the catalytic domain to either phosphorylate or dephosphorylate the response regulator in response to ligand occupancy of the sensor domain. However not all histidine kinases can act as phosphatases and there are rare instances where genetic results indicate that ligands can induce phosphatase activity. The majority of SKs are transmembrane spanning proteins [2] which, in principle, allows for direct detection of extracellular signals and subsequent translation to intracellular response without the need to monitor intracellular concentration of signal ligands.
The catalytic domains of the SK, namely an ATP-binding domain at the C-terminus and the phosphorylatable HisKA domain are highly conserved in sequence and also in structure (for a recent structure see [3]). Several modular elements are commonly found N-terminal to the catalytic domains most of which are believed to be involved in stimulus perception forming a sensor complex (Fig. 1B–C) (for a recent review see [4]). The individual sensing domains within the complex are highly variable in sequence and this is particularly pronounced for domains that are flanked by transmembrane domains and localized outside the cytoplasm [1]. How the individual domains that comprise the sensor complex function to augment or attenuate the signal output in response to ligand binding has not been determined even for relatively simple sensor histidine kinases. It seems clear that the multiplicity of domains in the sensor complex allows multiple signals to affect signal output and may permit metabolic, cell cycle and other cellular processes to impinge on sensor histidine kinase activity and ultimately gene expression. It is reasonable to suppose that the diversity of signals detected by the sensing domains required the evolution of a large number of distinct folds.
Common cytoplasmic elements of the sensor complex include the PAS (PER, ARNT, SIM), GAF (c-GMP-specific and -stimulated phosphodiesterases, Anabaena adenylate cyclases and Escherichia coli FhlA) and HAMP (for histidine kinases, adenylyl cyclases, methyl-accepting proteins, and other prokaryotic signaling proteins) domains [5]. Whereas structures of the PAS and GAF domains have been known for some time the solution structure of a HAMP domain containing protein Af1503 from Archaeoglobus fulgidus has now been solved and will be further discussed below [6].
Most extracytoplasmic sequences have not yet been grouped into families due to low sequence conservation. The few periplasmic elements recognized by the Pfam database [7] are the CHASE1-4 domains, the Cache1-2 domains and the NIT domain. Until only 5 years ago the only periplasmic ligand-binding domain structure solved was that of the chemotaxis receptor Tar, which formed a four-helix bundle structure [8]. In the last four years several cytoplasmic SK ligand binding structures have become available; those of Salmonella typhimurium PhoQ [9], the quorum sensor LuxQ of Vibrio harvei [10,11], and the two related CitA and DcuS sensing domains, citrate and fumarate sensors of Escherichia coli and Klebsiella pneumoniae, respectively [12,13]. Structures have revealed several interesting aspects both in regards to fold as well as signal recognition and transduction, which will be discussed in further detail in the following sections.
Lastly, the activities of some SKs are regulated by auxiliary proteins, which can be the primary ligand binding protein. In the last year new structures of proteins that regulate their associated SK have become available, including LuxP which regulates above mentioned LuxQ, the periplasmic membrane-tethered proteins YycH and YycI of Bacillus subtilis known to regulate the essential YycG SK [14,15] and the cytoplasmic proteins pXO1-118 and pXO2-61 of Bacillus anthracis which connect virulence with sporulation by regulating a sporulation SK [16].
The HAMP domain
The HAMP domain was originally discovered as a conserved sequence in a variety of signaling proteins. It has been suggested to be involved in the signal transduction process rather than in stimulus perception [17]. Some signal transduction proteins have a HAMP domain without an N-terminal sensor element suggesting that the HAMP domain is capable of acting in the sensor complex by interaction with other proteins with a sensing domain.
The Smart database recognizes HAMP domains in roughly 30 percent of all SKs (Fig. 1C) and the large majority of them occur just C-terminal to a transmembrane helix [17]. Therefore any conformational change upon ligand binding at a periplasmic domain has to be directly shuttled through this domain. The solution structure of such a HAMP domain, albeit not of a SK protein, has now been solved with potential implications on the nature of the conformational change that regulates SK activity [6]. The structure of A. fulgidus protein Af1503 revealed a dimeric architecture and a parallel four-helix coiled-coil where each monomer contributes two helices. Each helix in the monomer is of equivalent length offset by a single helical turn and connected via an extended linker (Fig. 2A).
Fig. 2.

The HAMP domain structure implies a rotational signal transduction mechanism. (A) The HAMP domain of Archaeoglobus fulgidus protein Af1503 displays a parallel four helical coiled-coil with each monomer contributing two helices. Interface residues are in red and blue. (B) Interface residues are arranged as knobs to knobs packing (left) which could convert to knobs into holes packing via a 26° rotation of all helices. The first of four layers of interacting residues is shown. Reprinted with permission from [6].
The coiled-coil exhibits an unusual knobs to knobs packing that can easily convert into the more common knobs into holes packing by simple 26° rotation of the individual helices (Fig. 2B). A single mutation A291V that was likely to favor the latter conformation was introduced and NMR experiments revealed that the HAMP mutant alternated between the two different packing forms. Two chimera proteins were constructed with two signaling proteins C-terminal to the Af1503 HAMP domain an adenylate cyclase and the chemotaxis receptor Tar, the latter related to two component SKs. Introduction of wild type and mutated HAMP into these proteins had opposite phenotypic effects suggesting that the proposed rotation is indeed capable of activating or inactivating signal transduction molecules.
The authors suggest that the actual function of the HAMP domain might be that of a converter that translates changes in upstream signaling elements into a downstream rotation since a rotation is not consistent with what has been observed upstream of the TM helices in E. coli chemotaxis signaling [18]. Mutations in the residue corresponding to A291V had been previously described for the two SK EnvZ and NarX resulting in biased kinase activity [19,20], therefore suggesting that true SK might also activate through rotation of the four-helix bundle.
The cytoplasmic sensing domains
Aside from the HAMP domain, several cytoplasmic sensing domains can be found in two component SKs. By far the most common and best described are the related PAS (recognized by the SMART database in > 33% of all histidine kinases) [21] and GAF domains (recognized in about 10% of all histidine kinases) [22], displaying a central five- or six-stranded anti-parallel β-sheet surrounded by several helices, respectively [23]. Structures of both domains individually have been known for some time.
Wagner and colleagues recently solved the structure of the chromophore-binding domain of the Deinococcus radiodurans phytochrome BphP, a red light sensing SK [24,25]. As predicted the structure displays both a PAS and a GAF domain arranged in tandem and connected via a 10 amino acid long linker. The authors speculate that the PAS domain plays a role in protein-protein interaction as it displays a concave front surface similar to PAS domains known to interact with other proteins [26,27].
The GAF domain on the other hand contributes most of the chromophore-binding pocket. The 35 N-terminal mostly unstructured amino acids include a conserved cysteine residue that covalently binds the chromophore billiverdin. This N-terminus forms a highly unusual trefoil knot with a 32-residue loop insertion in the otherwise structurally conserved GAF domain. The importance of this knot for the stability of the protein is supported by the fact that a central hydrophobic residue I35 was previously shown to be essential for protein folding of two other phytochromes [28,29].
Aside from the interesting 3-dimensional features of the molecule the structure allowed for some speculation as to the nature of the light sensing mechanism. The chromophore biliverdin is covalently bound via the carbon C32. The A, B and C-rings all lie on a plane, whereas the D-ring is tilted 44° away from that plane. Photo-transformation of biliverdin from the red to the far-red form likely involves a 15Zanti to 15Eanti isomerization around the C15=C16 double bond [30,31]. The authors speculate that this might go along with a rotation of the D ring in respect to the other rings, supported by the fact that enough space appears to surround the D-ring that would allow for such a rotation.
Some suggestions of how the photo-conversion translates into altered SK activity are given. The most intriguing model involves a break of the structural bridge between the PAS and GAF domain in response to biliverdin rotation and subsequent signal transmission via the freed PAS domain. However no experimental evidence for such a model was presented.
Periplasmic sensing domains
Whereas the four-helix bundle structure of the chemotaxis receptor Tar sensing domain has been known for some time [8], the first periplasmic sensing domains of a true sensor SK were solved in 2003. The NMR structure of the fumarate sensing domain of E. coli DcuS and the crystal structure of the citrate sensing domain of Klebsiella pneumoniae CitA were published almost concurrently [12,13].
An interesting feature of these structures was that both displayed a fold similar to that of the common cytoplasmic PAS sensing domain mentioned above. This result was no surprise to Aravind and colleagues who described a group of periplasmic domains that they termed the Cache domain and predicted that the Cache domain might fold similarly to the PAS domain [32]. The CitA sensing domain was specifically mentioned as a permuted form of what is now known as the Cache1 domain. In the last two years two additional periplasmic sensing structures have become available which will be discussed in further detail below.
LuxQ sensing domain
V. harveii quorum sensing is controlled by a complicated regulatory circuit involving two SKs LuxN and LuxQ (reviewed in [33]). LuxQ does not directly bind its inactivation ligand autoinducer 2 (AI2) but interacts with a soluble periplasmic ligand binding protein (PBP) termed LuxP (reviewed in [34]). Co-crystal structures of LuxP in complex with the LuxQ sensing domain have been solved at first in the apo- and later in the AI2 bound halo-form with strong implications for the underlying mechanism by which AI2 inhibits the activity of the LuxQ kinase [10,11].
The structure of the soluble PBP LuxP displays a fold similar to other PBP’s that usually couple signal transduction to transport [35]. Other examples include the maltose binding protein, which promotes chemotaxis towards maltose via interaction with the chemotaxis receptor Tar [36]. The LuxQ sensing domain however proved different from the four-helix bundle structure of Tar chemotaxis receptor sensing domain [8] and instead displayed two domains, each domain adopting the PAS fold [11]. This supplied the first piece of evidence that PBP’s are capable of interacting with the PAS fold.
Upon AI2 binding the structure of the LuxQ sensing domain did not display any significant conformational change in contrast to the binding protein LuxP [11,37]. However the authors noted that the halo-LuxPQ complex forms an asymmetric dimer unlike the apo- form, which is monomeric, both in solution as well as in the crystal (Fig. 3) [10]. The authors argue that the asymmetric dimer formation might play a role in vivo in regulating the activity of SK LuxQ. Consistent with this notion the authors identified 12 mutants by alanine scanning mutagenesis of the dimer interface that desensitize the LuxPQ complex to its ligand AI2. The strongest phenotypes were attributed to mutations in aromatic residues at the dimer interface.
Fig. 3.
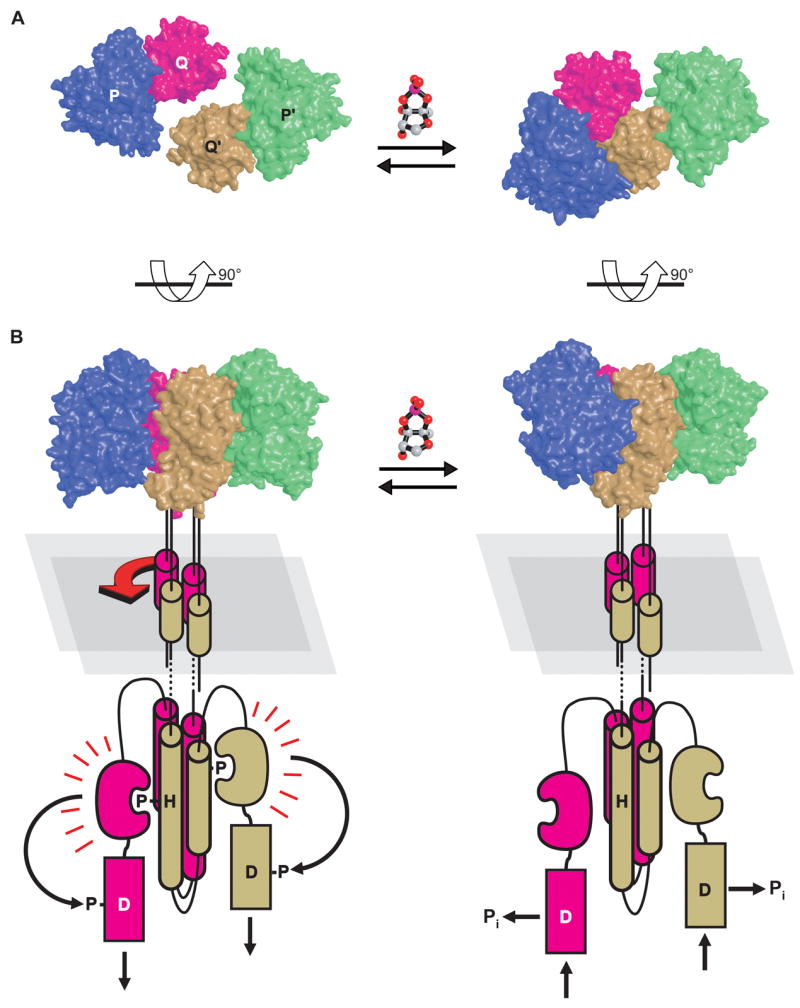
The LuxPQ structure implies a signal transduction mechanism involving asymmetric dimer formation. (A) LuxP and the sensing domain of LuxQ form a stable complex in apo- (left) and in halo-form (right). Upon ligand A2 binding the LuxPQ complex dimerizes along an asymmetric interface. (B) Model for histidine kinase inhibition via asymmetric dimerization. Reprinted with permission from [10].
How the formation of an asymmetric dimer translates into a downstream conformational change to lower SK activity is not clear at this point. We note however that the LuxQ kinase displays a putative HAMP domain and the above discussed HAMP structure study would predict that a rotation of the four-helix bundle might be involved.
PhoQ sensing domain
The S. typhimurium PhoQ sensing domain revealed yet another sensing domain with a structure similar to the PAS domain [9]. However, several aspects make this sensing domain particularly interesting. PhoQ activity is repressed by the presence of divalent cations such as Ca2+ and Mg2+ and activated by antimicrobial peptides [38,39]. The periplasmic sensing domain was shown to be essential for this sensing function since truncated mutants did not respond to either of those ligands [40–42]. The structure of the sensing domain now strongly suggests a mechanism of how this process is achieved [9,41].
Unlike conventional PAS domains that contain a binding cavity for small molecule ligands, the PhoQ sensing domain displays no such groove. Instead it features a helix-turn-helix insertion that contributes to a highly negatively charged flat surface. Positioning of the N- and C-terminal ends in the molecule suggests that this negatively charged surface faces the similarly negatively charged phospho-lipid-bilayer, an unusual feature (Fig. 4). It is this surface that coordinates at least three Ca2+ ions in the structure.
Fig. 4.

The PhoQ charge-repulsion sensing mechanism. The PhoQ periplasmic sensing domains form a dimer with calcium ions bound on one surface of the dimer (A). The N and C termini of the subunits are both attached to transmembrane helices that are proposed to protrude into the membrane from the calcium bound dimer surface. This surface is highly electro-negative (B) and would be repulsed from the similarly charged membrane surface were it not for the divalent cations bridging the two surfaces (C). The structure shown in (C) is proposed to be the “off” state of the kinase. Disruption of the cation bridging either through cation depletion, in vitro, or through intercalation in the membrane by host defense peptides, in vivo, is proposed to cause repulsion of the dimer/transmembrane surfaces with consequent conformational alteration of the PhoQ sensing domains and conversion of the kinase to the “on” state.
An intriguing mechanism has been proposed for signal transduction involving kinase activation by charge repulsion between the membrane and the sensing domain when cells are depleted for Ca2+, which otherwise offsets the charge between the electro-negative surfaces [9]. In line this model mutations in several Ca2+-coordinating residues render the protein less responsive to divalent cation repression [9].
PhoQ—similar to the above-described LuxQ kinase—possesses a HAMP domain, and the distinct sensing mechanism proposed here could be converted into a downstream rotation at this domain.
Structures of auxiliary proteins
In several known instances an auxiliary protein regulates the activity of a sensor SK. The classic example is the PBP, which usually ties together signal sensing and transport of a small molecule ligand [35]. As mentioned above the LuxQ kinase interacts with such a PBP termed LuxP, which directly interacts with the small molecule ligand AI-2 (Fig. 5A) [11,37]. In the last year several structurally diverse auxiliary proteins that might function by different mechanisms have been solved and will be described below.
Fig. 5.
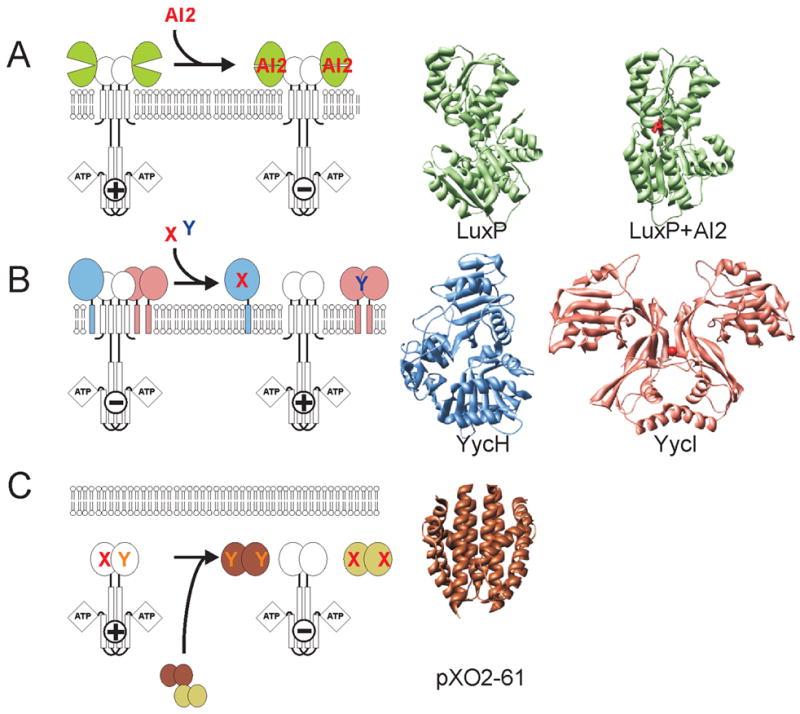
Diverse structures and mechanisms of auxiliary proteins. (A) Periplasmic binding proteins are soluble proteins, which regulate an associated kinase by direct interaction in response to ligand binding shown here utilizing the LuxPQ example of Vibrio harveii. Individual structures of Apo- and AI2 ligand-complexed LuxP are displayed (B) YycH and YycI are membrane tethered periplasmic proteins that negatively regulate the kinase YycG through direct interaction and might respond to unidentified ligands X and Y. The structures of YycH and YycI display a common fold over two domains. (C) The pXO2-61 protein of Bacillus anthracis display a myoglobin-like fold highly homologous in sequence and likely in structure to the sensing domain of the sporulation histidine kinase BA2291 and a second protein pXO1-118. Plasmid encoded proteins pXO2-61 and pXO1-118 are believed to compete with the associated kinase for unknown activation signals X and Y.
YycH and YycI
YycH and YycI are periplasmic proteins tethered to the cell membrane, each via a single N-terminal transmembrane helix. Both proteins were found to function as negative regulators of the essential YycFG two-component system of the Gram-positives [43,44]. Two-hybrid data showed strong interaction between the two proteins and weaker interaction with the sensor kinase YycG suggesting that these proteins function to regulate the activity of the YycG SK [43]. Interestingly, the structure of the periplasmic YycG domain was predicted with high probability to adopt the PAS fold by several structure prediction programs further underscoring the versatility of the PAS domain to interact with various protein folds [14].
The crystal structures of YycH and YycI revealed a related novel fold despite an absence of easily identifiable sequence homology between these proteins [14,15]. However, whereas YycH displays a three-domain architecture with each domain comprising an extensive central anti-parallel β-sheet, YycI shares only the two C-terminal domains. The N-terminal domain instead is displaced by a dimerization helix (Fig. 5B).
The precise mechanism by which these two proteins regulate the YycG kinase remains to be elucidated. The structure of YycI revealed a deep 20 Å long pocket at the dimer interface occupied by a chloride ion. This pocket resembles a ligand-binding site, suggesting a potential role in ligand binding and regulation of YycG. Furthermore not unlike the PhoQ sensing domain described above, YycI displays a negatively charged surface, likely to face the lipid bilayer that could regulate the YycG kinase by a similar charge-repulsion mechanism.
pXO1-118 and pXO2-61
Expression of anthrax toxin and progression of Anthrax disease by B. anthracis is dependent on genes located on the plasmids pXO1 and pXO2 [45]. Both pathogenicity plasmids pXO1 and pXO2 encode for individual proteins, pXO1-118 and pXO2-61, that are highly homologous to the sensing domain of a major sporulation sensor histidine kinase BA2291 [16,46]. In vivo analysis revealed that these sensing domains negatively regulate sporulation by out-competing the sporulation kinase for its activation signal (Fig. 5C).
Structures of the two sensing proteins have now been solved and not surprisingly revealed a similar fold, strongly suggesting that the associated SK sensing domain resembles a similar structure (G. Stranzl and R. Liddington, unpublished data). Interestingly the sensing domains showed similarity to the myoglobin fold, but the heme-binding residues were not conserved. The structure allowed for speculation of a possible ligand as pXO1-118 co-crystallized with a ligand that appeared to be a fatty acid. A heme-binding domain has been described as the sensing domain of the chemotaxis receptor HemAT [47,48].
Conclusions
Structures of sensing domains of SKs have contributed to an initial understanding of the mechanism of two-component signal transduction. An emerging common theme is the ubiquity of the PAS domain. Recent results indicate that the PAS domain can no longer be considered just a cytoplasmic sensing domain. Fold prediction programs such as the Ffas server [49] suggest that the majority of extracellular sensing domains of sensor histidine kinases adopt a PAS-like fold including the CACHE1-2 domains, the Chase1 and the Chase4 domains (unpublished).
Despite the apparent reduction in structural diversity of SK sensing domains, the underlying conformational changes that promote altered kinase activity in response to signals remain mysterious at this point. Models of PhoQ and LuxQ conformational changes in response to ligand binding differ substantially [9,10]. The structure of the HAMP domain suggests that it might serve as an adapter that translates diverse upstream conformational changes into a singular downstream effect, i.e a rotation [6]. It remains to be seen whether the proposed rotation mechanism encapsulates the important input/output characteristics of the HAMP domain, and if so whether any rotation arrives as such at the HisKA domain. Such mechanistic mysteries would be resolved with structures of a full-length kinase both in the absence and presence of ligand that has eluded, so far, our group.
Acknowledgments
This manuscript was written with support from grant GM019416 from the National Institute of General Medicine Sciences and grant AI055860 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USPHS. R. A. W. is supported by fellowship DBI-0532925 from the National Science Foundation.
This is manuscript No. 19071 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
of special interest (•)
of outstanding interest (••)
- 1.Hoch JA. Two-component and phosphorelay signal transduction. Curr Opin Microbiol. 2000;3:165–170. doi: 10.1016/s1369-5274(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 2.Galperin MY. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 2005;5:35. doi: 10.1186/1471-2180-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(••).Marina A, Waldburger CD, Hendrickson WA. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. Embo J. 2005;24:4247–4259. doi: 10.1038/sj.emboj.7600886. The first crystal structure of a full-length histidine kinase catalytic core. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascher T, Helmann JD, Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev. 2006;70:910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- 6.(••).Hulko M, Berndt F, Gruber M, Linder JU, Truffault V, Schultz A, Martin J, Schultz JE, Lupas AN, Coles M. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell. 2006;126:929–940. doi: 10.1016/j.cell.2006.06.058. Description of the first structure of the HAMP domain found in about 30% of all histidine kinases suggests a rotational signal transduction mechanism for kinase activation or inactivation. [DOI] [PubMed] [Google Scholar]
- 7.Finn RD, Mistry J, Schuster-Bockler B, Griffiths-Jones S, Hollich V, Lassmann T, Moxon S, Marshall M, Khanna A, Durbin R, et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34:D247–251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milburn MV, Prive GG, Milligan DL, Scott WG, Yeh J, Jancarik J, Koshland DE, Jr, Kim SH. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991;254:1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- 9.(••).Cho US, Bader MW, Amaya MF, Daley ME, Klevit RE, Miller SI, Xu W. Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J Mol Biol. 2006;356:1193–1206. doi: 10.1016/j.jmb.2005.12.032. Describes the structure of the PhoQ sensing domain and proposes an intriguing mechanism for signal sensing and transduction involving charge-repulsion between the phospholipid bilayer and a negatively charged surface of the sensing domain. [DOI] [PubMed] [Google Scholar]
- 10. (••).Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, Bassler BL, Hughson FM. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. The structure of the apo- and halo-forms of the LuxPQ sensing complex is the first to propose a signal transduction mechanism that involves formation of an asymmetric dimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Reinelt S, Hofmann E, Gerharz T, Bott M, Madden DR. The structure of the periplasmic ligand-binding domain of the sensor kinase CitA reveals the first extracellular PAS domain. J Biol Chem. 2003;278:39189–39196. doi: 10.1074/jbc.M305864200. [DOI] [PubMed] [Google Scholar]
- 13.Pappalardo L, Janausch IG, Vijayan V, Zientz E, Junker J, Peti W, Zweckstetter M, Unden G, Griesinger C. The NMR structure of the sensory domain of the membranous two-component fumarate sensor (histidine protein kinase) DcuS of Escherichia coli. J Biol Chem. 2003;278:39185–39188. doi: 10.1074/jbc.C300344200. [DOI] [PubMed] [Google Scholar]
- 14. (•).Santelli E, Liddington RC, Mohan MA, Hoch JA, Szurmant H. The crystal structure of Bacillus subtilis YycI reveals a common fold for two members of an unusual class of sensor histidine kinase regulatory proteins. J Bacteriol. 2007;189:3290–3295. doi: 10.1128/JB.01937-06. Describes structure of two novel auxiliary proteins that control the activity of the essential YycG kinase conserved in the Gram-positives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szurmant H, Zhao H, Mohan MA, Hoch JA, Varughese KI. The crystal structure of YycH involved in the regulation of the essential YycFG two-component system in Bacillus subtilis reveals a novel tertiary structure. Protein Sci. 2006;15:929–934. doi: 10.1110/ps.052064406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White AK, Hoch JA, Grynberg M, Godzik A, Perego M. Sensor domains encoded in Bacillus anthracis virulence plasmids prevent sporulation by hijacking a sporulation sensor histidine kinase. J Bacteriol. 2006;188:6354–6360. doi: 10.1128/JB.00656-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aravind L, Ponting CP. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol Lett. 1999;176:111–116. doi: 10.1111/j.1574-6968.1999.tb13650.x. [DOI] [PubMed] [Google Scholar]
- 18.Ottemann KM, Xiao W, Shin YK, Koshland DE., Jr A piston model for transmembrane signaling of the aspartate receptor. Science. 1999;285:1751–1754. doi: 10.1126/science.285.5434.1751. [DOI] [PubMed] [Google Scholar]
- 19.Appleman JA, Stewart V. Mutational analysis of a conserved signal-transducing element: the HAMP linker of the Escherichia coli nitrate sensor NarX. J Bacteriol. 2003b:89–97. doi: 10.1128/JB.185.1.89-97.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokishita S, Kojima A, Mizuno T. Transmembrane signal transduction and osmoregulation in Escherichia coli: functional importance of the transmembrane regions of membrane-located protein kinase, EnvZ. J Biochem (Tokyo) 1992;111:707–713. doi: 10.1093/oxfordjournals.jbchem.a123823. [DOI] [PubMed] [Google Scholar]
- 21.Ponting CP, Aravind L. PAS: a multifunctional domain family comes to light. Curr Biol. 1997;7:R674–677. doi: 10.1016/s0960-9822(06)00352-6. [DOI] [PubMed] [Google Scholar]
- 22.Aravind L, Ponting CP. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem Sci. 1997;22:458–459. doi: 10.1016/s0968-0004(97)01148-1. [DOI] [PubMed] [Google Scholar]
- 23.Ho YS, Burden LM, Hurley JH. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor. Embo J. 2000;19:5288–5299. doi: 10.1093/emboj/19.20.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner JR, Zhang J, Brunzelle JS, Vierstra RD, Forest KT. High resolution structure of deinococcus bacteriophytochrome yields new insights into phytochrome architecture and evolution. J Biol Chem. 2007;282:12298–12309. doi: 10.1074/jbc.M611824200. [DOI] [PubMed] [Google Scholar]
- 25. (•).Wagner JR, Brunzelle JS, Forest KT, Vierstra RD. A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature. 2005;438:325–331. doi: 10.1038/nature04118. The Structure of the phytochrome light sensing domain gives insight into the light sensing mechanism. [DOI] [PubMed] [Google Scholar]
- 26.Razeto A, Ramakrishnan V, Litterst CM, Giller K, Griesinger C, Carlomagno T, Lakomek N, Heimburg T, Lodrini M, Pfitzner E, et al. Structure of the NCoA-1/SRC-1 PAS-B domain bound to the LXXLL motif of the STAT6 transactivation domain. J Mol Biol. 2004;336:319–329. doi: 10.1016/j.jmb.2003.12.057. [DOI] [PubMed] [Google Scholar]
- 27.Yildiz O, Doi M, Yujnovsky I, Cardone L, Berndt A, Hennig S, Schulze S, Urbanke C, Sassone-Corsi P, Wolf E. Crystal structure and interactions of the PAS repeat region of the Drosophila clock protein PERIOD. Mol Cell. 2005;17:69–82. doi: 10.1016/j.molcel.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Karniol B, Wagner JR, Walker JM, Vierstra RD. Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors. Biochem J. 2005;392:103–116. doi: 10.1042/BJ20050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhoo SH, Hirano T, Jeong HY, Lee JG, Furuya M, Song PS. Phytochrome Photochromism Probed by Site-Directed Mutations and Chromophore Esterification. J Am Chem Soc. 1997;119:11717–11718. [Google Scholar]
- 30.Inomata K, Hammam MA, Kinoshita H, Murata Y, Khawn H, Noack S, Michael N, Lamparter T. Sterically locked synthetic bilin derivatives and phytochrome Agp1 from Agrobacterium tumefaciens form photoinsensitive Pr- and Pfr-like adducts. J Biol Chem. 2005;280:24491–24497. doi: 10.1074/jbc.M504710200. [DOI] [PubMed] [Google Scholar]
- 31.Fodor SP, Lagarias JC, Mathies RA. Resonance Raman analysis of the Pr and Pfr forms of phytochrome. Biochemistry. 1990;29:11141–11146. doi: 10.1021/bi00502a018. [DOI] [PubMed] [Google Scholar]
- 32.Anantharaman V, Aravind L. Cache - a signaling domain common to animal Ca(2+)-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem Sci. 2000;25:535–537. doi: 10.1016/s0968-0004(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 33.Milton DL. Quorum sensing in vibrios: complexity for diversification. Int J Med Microbiol. 2006;296:61–71. doi: 10.1016/j.ijmm.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Pappas KM, Weingart CL, Winans SC. Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signalling. Mol Microbiol. 2004;53:755–769. doi: 10.1111/j.1365-2958.2004.04212.x. [DOI] [PubMed] [Google Scholar]
- 35.Quiocho FA, Ledvina PS. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol Microbiol. 1996;20:17–25. doi: 10.1111/j.1365-2958.1996.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 36.Manson MD, Kossmann M. Mutations in tar suppress defects in maltose chemotaxis caused by specific malE mutations. J Bacteriol. 1986;165:34–40. doi: 10.1128/jb.165.1.34-40.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 38.Soncini FC, Garcia Vescovi E, Solomon F, Groisman EA. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bader MW, Navarre WW, Shiau W, Nikaido H, Frye JG, McClelland M, Fang FC, Miller SI. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol Microbiol. 2003;50:219–230. doi: 10.1046/j.1365-2958.2003.03675.x. [DOI] [PubMed] [Google Scholar]
- 40.Castelli ME, Garcia Vescovi E, Soncini FC. The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J Biol Chem. 2000;275:22948–22954. doi: 10.1074/jbc.M909335199. [DOI] [PubMed] [Google Scholar]
- 41.Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 42.Montagne M, Martel A, Le Moual H. Characterization of the catalytic activities of the PhoQ histidine protein kinase of Salmonella enterica serovar Typhimurium. J Bacteriol. 2001;183:1787–1791. doi: 10.1128/JB.183.5.1787-1791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szurmant H, Mohan MA, Imus PM, Hoch JA. YycH and YycI interact to regulate the essential YycFG two-component system in Bacillus subtilis. J Bacteriol. 2007;189:3280–3289. doi: 10.1128/JB.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szurmant H, Nelson K, Kim EJ, Perego M, Hoch JA. YycH regulates the activity of the essential YycFG two-component system in Bacillus subtilis. J Bacteriol. 2005;187:5419–5426. doi: 10.1128/JB.187.15.5419-5426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 46.Brunsing RL, La Clair C, Tang S, Chiang C, Hancock LE, Perego M, Hoch JA. Characterization of sporulation histidine kinases of Bacillus anthracis. J Bacteriol. 2005;187:6972–6981. doi: 10.1128/JB.187.20.6972-6981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Phillips GN., Jr Structure of the oxygen sensor in Bacillus subtilis: signal transduction of chemotaxis by control of symmetry. Structure. 2003;11:1097–1110. doi: 10.1016/s0969-2126(03)00169-2. [DOI] [PubMed] [Google Scholar]
- 48.Hou S, Larsen RW, Boudko D, Riley CW, Karatan E, Zimmer M, Ordal GW, Alam M. Myoglobin-like aerotaxis transducers in Archaea and Bacteria. Nature. 2000;403:540–544. doi: 10.1038/35000570. [DOI] [PubMed] [Google Scholar]
- 49.Jaroszewski L, Rychlewski L, Li Z, Li W, Godzik A. FFAS03: a server for profile--profile sequence alignments. Nucleic Acids Res. 2005;33:W284–288. doi: 10.1093/nar/gki418. [DOI] [PMC free article] [PubMed] [Google Scholar]


