Abstract
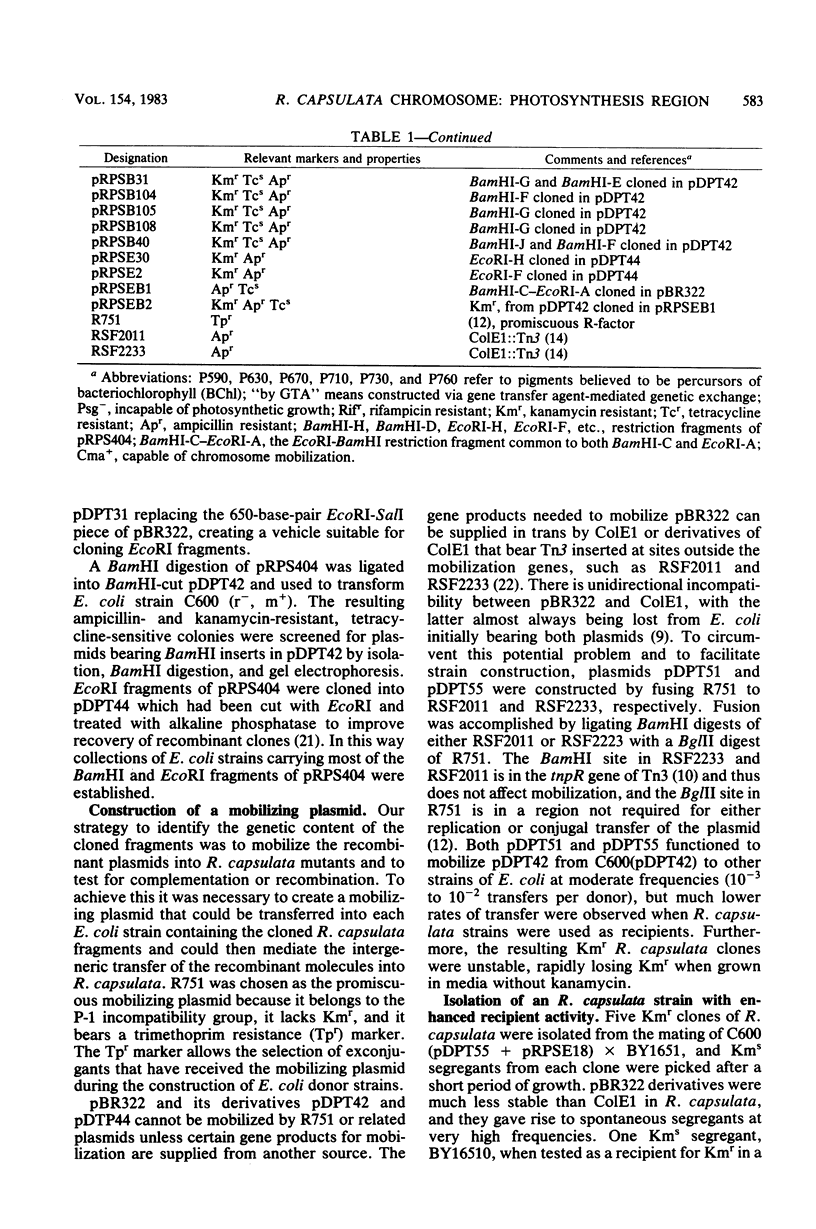
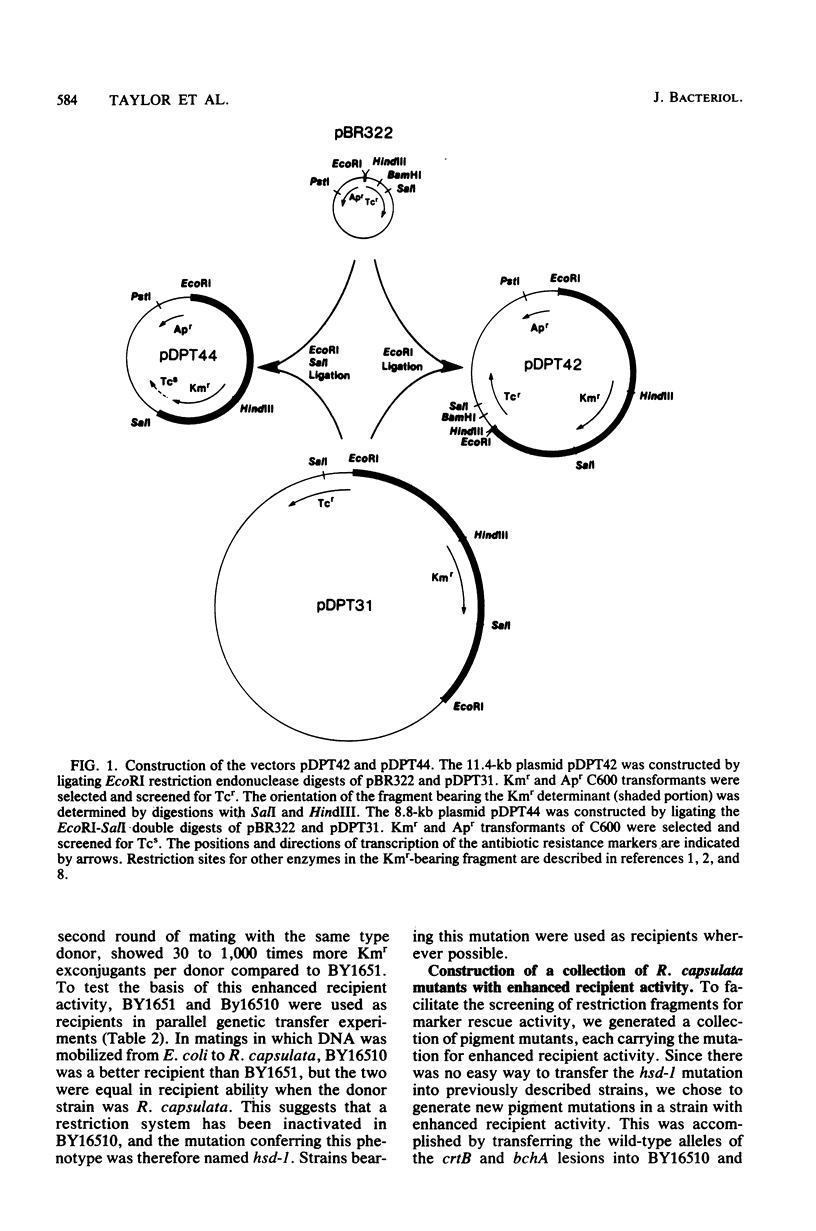
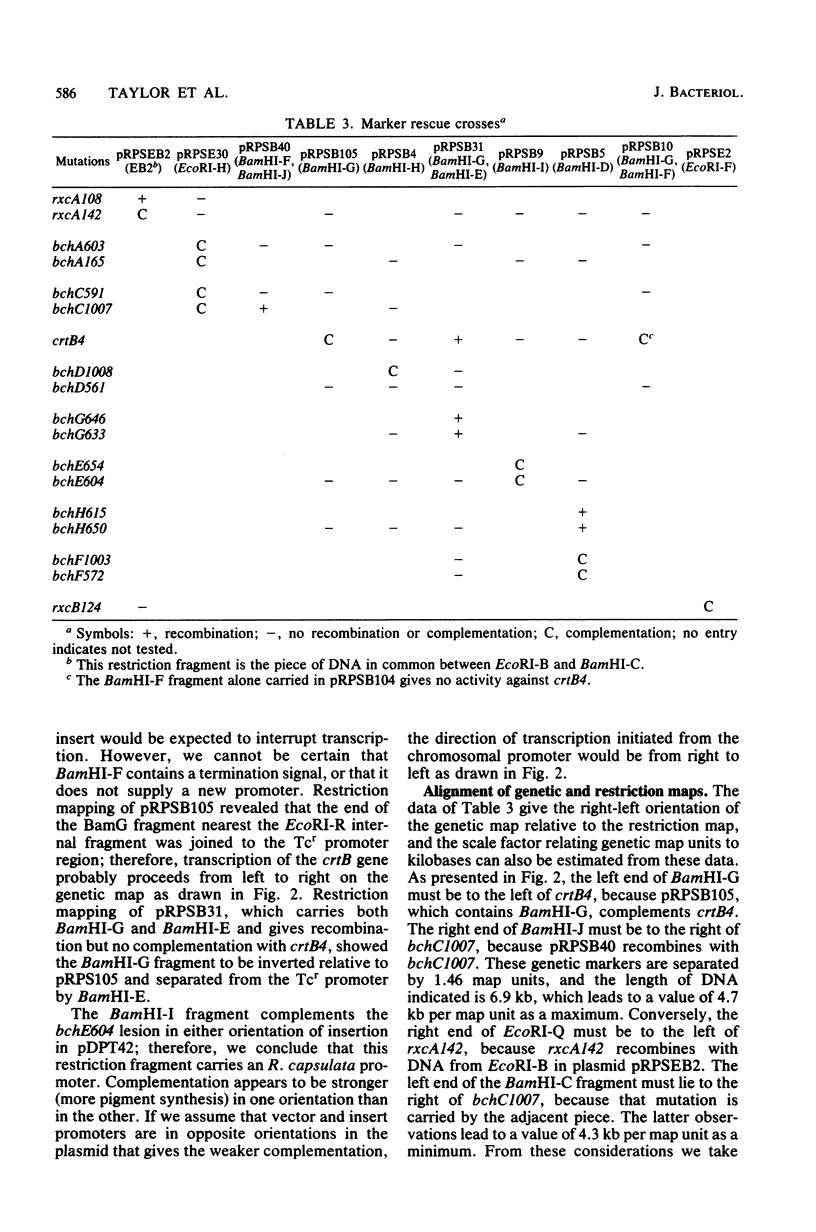
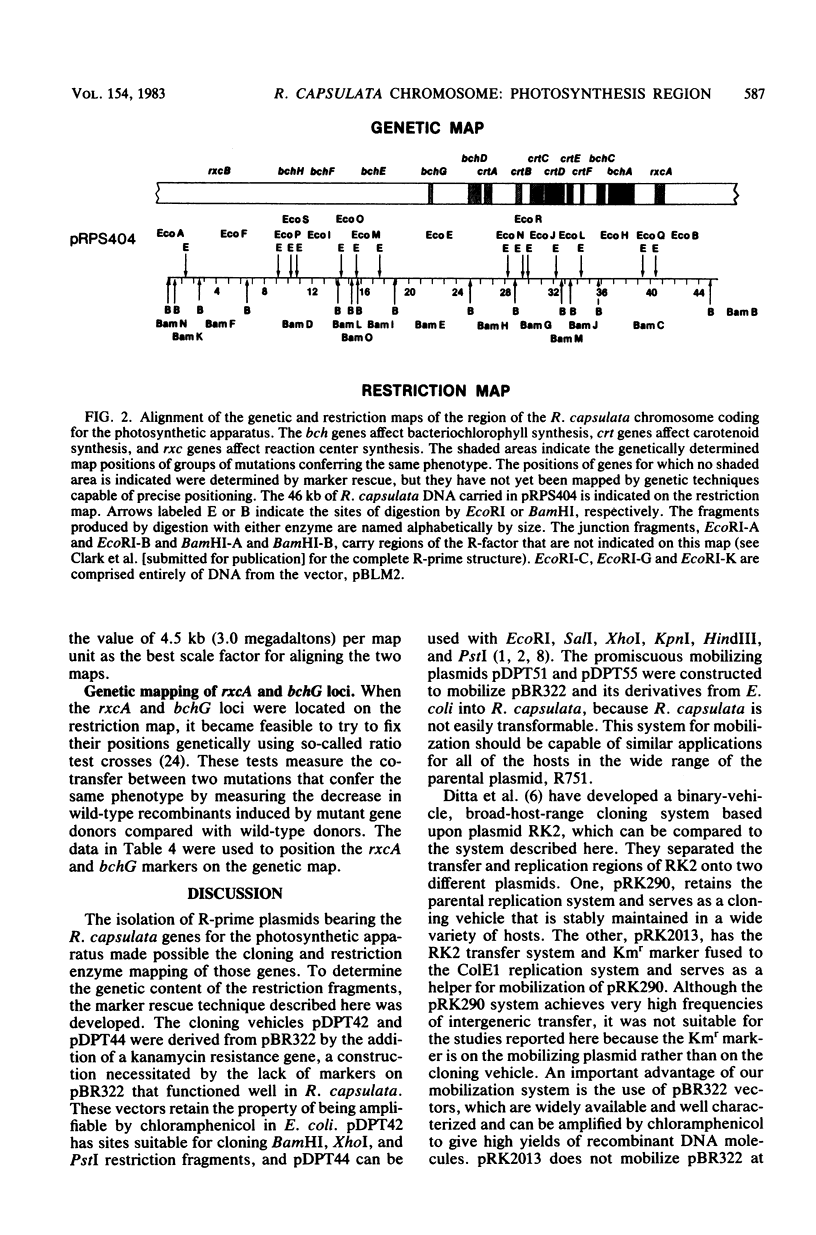
The restriction map of a 46-kilobase fragment of the Rhodopseudomonas capsulata chromosome was aligned with the genetic map of the photosynthesis region of that chromosome by a marker rescue technique. Marker rescue was effected by mobilization of vectors bearing fragments of R. capsulata DNA from Escherichia coli to a set of R. capsulata mutants. Plasmids pDPT51 and pDPT55 were constructed to mediate the intergeneric mobilization of pBR322 derivatives, and a mutant of R. capsulata with improved intergeneric recipient activity was isolated. Four previously unmapped genes affecting bacteriochlorophyll synthesis and two genes affecting photochemical reaction center synthesis have been located by marker rescue. Some of the fragments of R. capsulata DNA are capable of vector-independent complementation, implying that promoters are located on these fragments. Other fragments complement only in one orientation of insertion in the vector, implying transcription from promotors on the vectors and thereby fixing the direction of transcription for those fragments. Still other fragments of DNA show rescue only via recombination between homologous plasmid-borne DNA fragments and chromosomal mutations. The physical dimensions of the genetic map are 3.0 megadaltons per map unit, which agrees with previous estimates based on the size of the R. capsulata gene transfer agent.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong K. A., Hershfield V., Helinski D. R. Gene cloning and containment properties of plasmid Col E1 and its derivatives. Science. 1977 Apr 8;196(4286):172–174. doi: 10.1126/science.322277. [DOI] [PubMed] [Google Scholar]
- Beckingham K. A plasmid cloning vector for Kpnl-cleaved DNA. Plasmid. 1980 Nov;4(3):354–356. doi: 10.1016/0147-619x(80)90074-8. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Haselkorn R. Protein components of bacterial photosynthetic membranes. J Mol Biol. 1972 Jul 14;68(1):97–105. doi: 10.1016/0022-2836(72)90265-3. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G., Oelze J. Organization and differentiation of membranes of phototrophic bacteria. Adv Microb Physiol. 1981;22:1–92. doi: 10.1016/s0065-2911(08)60325-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Franklin F. C., Nordheim A., Timmis K. N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981 Dec;16(1-3):227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Timmis K. N. Incompatibility properties of Col E1 and pMB1 derivative plasmids: random replication of multicopy replicons. Cell. 1981 Jan;23(1):229–238. doi: 10.1016/0092-8674(81)90287-7. [DOI] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. J., Shapiro J. A. Genetic organization of the broad-host-range IncP-1 plasmid R751. J Bacteriol. 1980 Sep;143(3):1362–1373. doi: 10.1128/jb.143.3.1362-1373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. L., West R. W., Heyneker H. L., Bolivar F., Boyer H. W. Characterizing wild-type and mutant promoters of the tetracycline resistance gene in pBR313. Nucleic Acids Res. 1979 Jul 25;6(10):3267–3287. doi: 10.1093/nar/6.10.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P. A., Walker M. A., Marrs B. L. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J Biol Chem. 1980 Mar 25;255(6):2427–2432. [PubMed] [Google Scholar]
- Solioz M., Marrs B. The gene transfer agent of Rhodopseudomonas capsulata. Purification and characterization of its nucleic acid. Arch Biochem Biophys. 1977 May;181(1):300–307. doi: 10.1016/0003-9861(77)90508-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J., Lascelles J. Coupling between bacteriochlorophyll and membrane protein synthesis in Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):799–803. doi: 10.1073/pnas.70.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N. Structural and functional analysis of cloned DNA segments containing the replication and incompatibility regions of a miniplasmid derived from a copy number mutant of NR1. J Bacteriol. 1979 Jan;137(1):92–104. doi: 10.1128/jb.137.1.92-104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Warren G. J., Saul M. W., Sherratt D. J. ColE1 plasmid mobility: essential and conditional functions. Mol Gen Genet. 1979 Feb 16;170(1):103–107. doi: 10.1007/BF00268585. [DOI] [PubMed] [Google Scholar]
- Yen H. C., Hu N. T., Marrs B. L. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J Mol Biol. 1979 Jun 25;131(2):157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



